Lactobacillus casei 64H Contains a Phosphoenolpyruvate-Dependent Phosphotransferase System for Uptake of Galactose, as Confirmed by Analysis of ptsH and Different gal Mutants (original) (raw)
Abstract
Galactose metabolism in Lactobacillus casei 64H was analyzed by genetic and biochemical methods. Mutants with defects in_ptsH_, galK, or the tagatose 6-phosphate pathway were isolated either by positive selection using 2-deoxyglucose or 2-deoxygalactose or by an enrichment procedure with streptozotocin.ptsH mutations abolish growth on lactose, cellobiose,N_-acetylglucosamine, mannose, fructose, mannitol, glucitol, and ribitol, while growth on galactose continues at a reduced rate. Growth on galactose is also reduced, but not abolished, in_galK mutants. A mutation in galK in combination with a mutation in the tagatose 6-phosphate pathway results in sensitivity to galactose and lactose, while a galK mutation in combination with a mutation in ptsH completely abolishes galactose metabolism. Transport assays, in vitro phosphorylation assays, and thin-layer chromatography of intermediates of galactose metabolism also indicate the functioning of a permease/Leloir pathway and a phosphoenolpyruvate-dependent phosphotransferase system (PTS)/tagatose 6-phosphate pathway. The galactose-PTS is induced by growth on either galactose or lactose, but the induction kinetics for the two substrates are different.
Many procaryotes can utilized-galactose as a carbon source. Often there are several different uptake systems for d-galactose within a cell; e.g., for Escherichia coli, seven systems that belong to different types of transport systems have been described. All release unmodified galactose into the cytoplasm (13, 26); the galactose is subsequently metabolized by the Leloir pathway. Operons coding for the enzymes of the Leloir pathway in different organisms, including Lactobacillus casei 64H (3), have been characterized.
Two different galactose phosphates can be detected if L. casei 64H accumulates galactose intracellularly: galactose 1-phosphate, the first intermediate of the Leloir pathway, and galactose 6-phosphate (7). Galactose 6-phosphate was first identified as the hydrolysis product of lactose in Staphylococcus aureus. Lactose is transported and phosphorylated in this organism by the lactose-phosphoenolpyruvate-dependent phosphotransferase system (PTS) to lactose 6-phosphate and is subsequently cleaved by a phospho-β-galactosidase to glucose and galactose 6-phosphate (15–18). Further metabolism of galactose 6-phosphate was found to involve as intermediates tagatose 6-phosphate, tagatose 1,6-biphosphate, glyceraldehyde 3-phosphate, and dihydroxyacetone phosphate, whose interconversion is facilitated by the enzymes galactose 6-phosphate isomerase, tagatose 6-phosphate kinase, and tagatose 1,6-bis-phosphate aldolase (4). The absence of the enzymes of the Leloir pathway and the inducibility of the enzymes of this tagatose 6-phosphate pathway by galactose as well as lactose led to the hypothesis that galactose is also a PTS substrate in S. aureus. The PTS-derived galactose 6-phosphate would then also be metabolized by the tagatose 6-phosphate pathway. While this pathway seems to be the only route for galactose metabolism in S. aureus, it was found that group N streptococci express the enzymes for both pathways of galactose metabolism (5). The genes coding for the enzymes of the tagatose 6-phosphate pathway have been found to be part of the lac operon together with the genes coding for the lactose-PTS and the phospho-β-galactosidase in_Lactococcus lactis_, S. aureus, and_Streptococcus mutans_ (27, 28, 34). The exception is L. casei, in which the genes encoding the enzymes of the tagatose 6-phosphate pathway are not part of the lac operon (1, 2, 12). Similar to the uptake of lactose, it has been suggested that in L. casei 64H, galactose is taken up by a specific galactose-PTS, which results in the formation of galactose 6-phosphate. This hypothesis is supported by phosphorylation studies using cell extracts (7). The experiments of Chassy and Thompson indicated the existence of a high-affinity uptake system for galactose in L. casei 64H. Its Km (15 μM) lies within the range of the _Km_s of other PTSs characterized so far but exceeds by far the value determined for the galactose-PTS of Streptococcus lactis (1 mM) (32).
The Leloir pathway of L. casei 64H has recently be characterized by cloning of the corresponding genes and determination of enzyme activities (3). Although there are many hints for the existence of a PTS specific for the uptake of galactose in L. casei 64H, the existence of such a system is not yet proven. The aim of our work was therefore to further investigate the uptake and metabolism of galactose in L. casei 64H and to provide new evidence for the galactose-PTS. In contrast to previous efforts, we chose to achieve this by isolating and analyzing ptsH and_gal_ mutants.
MATERIALS AND METHODS
Strains and media.
L. casei subsp. _casei_64H (10) was used for analysis of galactose metabolism. Cultures were grown in Lactobacillus carrying medium (LCM) (9) supplemented with 0.5% of carbohydrate. Cells were plated on LCM solidified with 1.4% (wt/vol) agar. Some growth experiments were done in carbohydrate test medium (CTM), a derivative of LCM (3a); this medium contained 0.5 g of Trypticase peptone, 0.25 g of yeast extract, 0.15 g of tryptone, 3 g of sodium dihydrogen phosphate, 3 g of disodium hydrogen phosphate, 2 g of ammonium citrate, 1 g of sodium acetate, and 0.2 g of l-cysteine in 900 ml of H2O. After autoclaving, 100 ml of a solution containing 10% (wt/vol) sodium dihydrogen phosphate and 10% (wt/vol) disodium hydrogen phosphate as well as 5 ml of a salt solution containing 11.5 g of MgSO4 · 6H2O, 0.68 g of Fe(II)SO4 · 7H2O, and 2.4 g of Mn(II)SO4 · 1H2O in 100 ml of H2O were added to the cold medium. The medium was stored in the dark at 4°C.
Cells for transport assays or mutagenesis procedures were washed with the minimal medium (MM) as described by Tanaka et al. (31).
Growth tests and mutant selections.
Marker tests were performed in CTM supplemented with 0.5% carbohydrate to be tested. Five milliliters of prewarmed medium was inoculated with 10 μl of an overnight culture in LCM without additional carbohydrate. The cultures were incubated at 37°C for 2 days, and growth was determined by measuring the optical density at 420 nm (OD420).
To determine doubling times, cells from an overnight culture in LCM without additional carbohydrate were diluted to an OD420 of 0.1 in prewarmed CTM supplemented with the carbohydrate to be tested. The cultures were incubated at 37°C, and growth was monitored by measurement of OD420 (OD420 of 1 ≅ 5 × 108 cells per ml in a Shimadzu model UV-1202 spectrophotometer [Shimadzu Europe, Duisburg, Germany]).
To determine sensitivities, cell growth was measured in LCM supplemented with 0.5% mannitol at 37°C. After the cells had reached exponential growth, galactose or lactose was added to 0.5%. Growth was monitored by measurement of OD600 (OD600 of 1 ≅ 109 cells per ml in the Shimadzu UV-1202).
Spontaneous mutants resistant to 2-deoxyglucose (2DGlc) were isolated by plating 109 cells from an overnight culture in LCM on LCM agar plates supplemented with 0.5% ribose and 10 mM 2DGlc. After incubation for 2 to 4 days at 37°C, resistant colonies were obtained and purified twice on the same medium and once on LCM with 0.5% ribose.
Selection with 2-deoxygalactose was performed as described by Thompson and Chassy (33). One-tenth milliliter of cells from a mid-log culture in LCM with 0.5% lactose was mixed with 3 ml of LCM soft agar (0.6% [wt/vol])–0.5% lactose and poured over the surface of an LCM plate supplemented with 0.5% lactose. When the top agar was solidified, a plug was removed from the center of the plate and 0.5 to 1 ml of 10 mM 2-deoxygalactose was added to the well. The plates were incubated at 37°C.
EMS mutagenesis and streptozotocin selection.
Mutagenesis with ethyl methanesulfonate (EMS) was carried out by a variation of the protocol of Lin et al. (24). Cells from an overnight culture in LCM were diluted to 5 × 107 cells per ml in LCM supplemented with 0.5% glucose and incubated at 37°C. After the culture reached a density of 5 × 108 cells per ml, 5 ml of cells was harvested by centrifugation, washed twice with MM, and resuspended in 2.5 ml of MM. EMS (7.5 μl per ml of cell suspension) was added, and the culture was incubated at 37°C for 2 h. The cells were then washed twice with MM, resuspended in LCM, and grown overnight.
Selection with streptozotocin was done according to the method of Lengeler (22) by a protocol optimized for lactobacilli. This selection procedure is based on the principle that only energized cells are able to take up the toxic N_-acetylglucosamine analogue streptozotocin. EMS-mutagenized cells from an overnight culture of_L. casei PG4 in LCM supplemented with 0.5%_N_-acetylglucosamine were diluted into fresh medium to 8 × 107 cells per ml. After the culture reached early exponential growth, 5 ml was washed twice in MM and resuspended in 5 ml of LCM supplemented with 0.5% galactose. After cells had again reached early exponential growth at 37°C, streptozotocin was added to 50 μg per ml and the culture was incubated at 37°C for a further 90 min. Cells mutated in galactose uptake or metabolism are not energized because of lack of carbohydrate; therefore, they are not able to take up streptozotocin and will survive. In contrast, wild-type cells able to grow with galactose take up streptozotocin and are killed. After incubation, the cells were washed in MM, sonicated to break up the cell chains, and plated on LCM supplemented with 0.5% galactose. The plates were incubated at 37°C.
Test for membrane-dependent in vitro phosphorylation of carbohydrates.
To measure membrane-dependent phosphorylation of galactose or lactose, tests for PTS-dependent in vitro phosphorylation were performed as described by Lengeler (23). Membranes or membrane-free cell extracts (supernatants) from cells of L. casei 64H or the mutant strains were used. Mixes containing 20 μl of 50 mM phosphoenolpyruvate, 20 μl of 0.5 mM MgCl2, 90 μl of membrane-free cell extract, 10 μl of membranes, and 0.1 M Tris-HCl (pH 7.6) to a final volume of 180 μl were equilibrated at 28°C for 5 min. The reaction was started by adding [14C]galactose to 7.5 μM or [14C]lactose to 15 μM. After 30, 60, and 90 s, aliquots of 50 μl were removed and spotted on anion-exchange filters (Whatman DE81), and the reaction was stopped in ethanol (80%). The filters were washed three times in water and dried, and the amount of radioactivity on each filter was determined.
Test for galactokinase-dependent phosphorylation of galactose.
For measuring enzyme activities, extracts were prepared from L. casei 64H which had been induced with 0.5% galactose for 3 h. Cells were harvested in mid-logarithmic phase and broken by shaking with zirconia beads in a Retsch mill MM2 (Retsch, Haan, Germany). Extracts were centrifuged at 15,000 ×g for 5 min. Protein concentrations were determined by the bicinchoninic acid method of Smith et al. (30).
Galactokinase activity was determined according to the method of Sherman (29) and a protocol described by Lengeler et al. (20). Probes were taken at 30, 60, and 90 s after the start of the reaction.
Tests for uptake of galactose in L. casei 64H.
Uptake of carbohydrates in cells of L. casei 64H and derivatives was determined by the method of Lengeler (21) as follows. Cells from a culture in LCM were diluted in LCM to 5 × 107 cells per ml and incubated at 37°C until early exponential growth phase was reached. For induction, 0.5% carbohydrate was added, and cells were further incubated at 37°C. For transport activity tests, cells were harvested 3 h after induction. The cells were washed twice in an equal amount of ice-cold MM to remove carbohydrate, resuspended in MM to 1 × 108 to 3 × 108 cells per ml, and kept on ice for no longer than 2 h before use; then 180 μl of cell suspension was equilibrated at 28°C for 5 min. The reaction was started by adding 20 μl of14C-labeled substrate. [14C]galactose was added to 7.5 μM, and [14C]lactose was added to 15 μM (final concentrations). After incubation for 30, 60, and 90 s, aliquots were removed and deposited on membrane filters (0.6-μm pore size), and cells were washed with cold MM. Transport activity was determined by measuring the amount of radioactivity retained on each filter.
Preparation of membranes and soluble cell fractions.
For preparation of extracts, cells from an overnight culture in LCM were diluted in 400 ml of LCM supplemented with 20 mM (final concentration)dl-threonine to 108 cells per ml and incubated at 37°C. After the cells had reached early exponential growth, 0.5% carbohydrate was added and cells were incubated for a further 3 h at 37°C. Cells were then harvested, washed twice in 1% NaCl, and frozen at −20°C. After thawing, the cells were resuspended in 0.1 M Tris-HCl (pH 7.6) to a density of 2 × 1011 cells per ml, and the suspension was sonicated (Branson Sonifier B12, microtip, setting 7, 75 W) for a total sonication time of 8 to 10 min at intervals of 30 s. During sonication, extracts were cooled in an ice water-ethanol bath. Efficiency was controlled by observation of extracts under a microscope. After most cells were broken, extracts were separated from whole cells and cell debris by centrifugation at 15,000 × g for 20 min. Extracts were separated into membranes and soluble cell fraction (supernatants) by ultracentrifugation at 200,000 × g for 2 h. Supernatants (membrane-free cell extracts) were frozen in aliquots at −20°C, and membranes (the pellet after ultracentrifugation) were washed in 0.1 M Tris-HCl (pH 7.6) and again centrifuged for 2 h at 200,000 × g. The pellet was resuspended in 0.5 ml of 0.1 M Tris-HCl (pH 7.6) and kept at −80°C.
Thin-layer chromatography.
Cells of L. casei were grown in 10 ml of LCM–0.5% ribose to 5 × 108 cells per ml at 37°C. d-Galactose was added to 0.5%, and the cells were incubated for a further 2 h. The cells were then harvested by centrifugation, washed twice, and resuspended in 0.5 ml of MM. Afterwards, the cells were equilibrated at 25°C for 5 min, and 50 μl of 10 mM [14C]galactose (0.1 μCi/μl) was added. After a short incubation for approximately 30 s, the cells were harvested and the supernatant was drawn off with a pipette. The cells were immediately resuspended in 50 μl of hot water, and the cups were incubated in boiling water for 2 min. Cell debris was pelleted by centrifugation, and 10 μl of the supernatant was used for thin-layer chromatography.
The supernatants and reference substances were separated by chromatography on Silica Gel 60 plates (Merck, Darmstadt, Germany). For separation, a mixture of 1 M ammonium acetate (pH 5)–98% ethanol–0.1 M EDTA (pH 8) (70:30:1, vol/vol/vol) was used. After the solvent front reached the top, the plates were dried. For analysis, autoradiograms were taken by exposure for 3 to 4 days. Reference substances were detected by spray coating with 0.5% potassium permanganate in 1 N NaOH.
RESULTS
Isolation of pleiotropic mutants.
The glucose analogue 2DGlc has been used to isolate Pts− mutants from different bacteria, including Lactobacillus sake and_Streptococcus salivarius_ (11, 19). Such mutants display a pleiotropic phenotype because uptake of all PTS substrates is abolished. We used 2DGlc to isolate pleiotropic mutants from L. casei 64H. The selection was done twice, and from each selection procedure 48 resistant colonies were tested. From each experiment, one pleiotropic mutant (KB6417 and KB6419) was obtained. For phenotypic characterization of KB6417 and KB6419, growth tests were performed in CTM supplemented with different carbohydrates. The mutants grew withd-galactose, d-glucose, d-maltose,d-xylose, and d-ribose, while growth on lactose, cellobiose, d-mannose,_N_-acetylglucosamine, d-fructose, mannitol, ribitol, and glucitol was abolished. This pleiotropic phenotype indicated that both might be Pts− mutants.
We tried to isolate from KB6417 and KB6419 mutants that regained growth on one, some, or all of these carbohydrates. To isolate such mutants, the bacteria were spread on different plates supplemented with 0.5% each carbohydrate. From KB6417 we isolated mutants, possibly revertants, that displayed the same phenotype as _L. casei_64H, but no mutants displaying a different phenotype were obtained. It was not possible to isolate any mutant from KB6419, indicating that in contrast to KB6417, KB6419 does not revert to Pts+. Although displaying similar phenotypes, the mutants differ from each other at the molecular level.
Analysis of the mutation present in KB6419.
To prove that KB6417 and KB6419 are indeed Pts− mutants, we performed phosphorylation studies with lactose, the only carbohydrate thus far characterized as an exclusive PTS substrate in L. casei 64H. Since the lac operon is regulated by antitermination (1), the lac genes should be expressed constitutively in a Pts− background; this eliminates problems with induction that might arise in Pts− mutants. The results of the phosphorylation studies are given in Table1. Combinations of membranes and membrane-free supernatants of the wild-type strain 64H and mutant strain KB6419 showed that a cytoplasmic component of KB6419 is affected by the mutation. Since the activity of the supernatants of KB6419 was restored by the addition of purified HPr from Streptococcus faecalis, KB6419 was characterized as HPr−. We have no data on the exact nature of the mutation; however, in all bacteria so far characterized, ptsH and ptsI constitute operons or are part of operons where the gene order is ptsH ptsI. Because enzyme I (EI) is still expressed, it is not likely that the mutation of KB6419 is located in a regulatory gene which affects the expression of the operon. For this reason, until further characterization of the mutation, KB6419 is regarded as being_ptsH_.
TABLE 1.
Characterization of KB6419 by membrane-dependent lactose phosphorylation.
| Source of membranes | Source of supernatants | Addition of: | Sp acta (nmol/mg of membrane protein/min) |
|---|---|---|---|
| 64H (Lacb) | 64H (Lac) | 2.1 | |
| KB6419 (Rbs) | KB6419 (Rbs) | 0 | |
| 64H (Lac) | KB6419 (Rbs) | 0 | |
| KB6419 (Rbs) | 64H (Lac) | 1.2 | |
| 64H (Lac) | KB6419 (Rbs) | 5 μg of EI | 0 |
| 64H (Lac) | KB6419 (Rbs) | 5 μg of HPr | 0.9 |
| 64H (Lac) | KB6419 (Rbs) | 5 μg of EI + 5 μg of HPr | 1.0 |
| 64H (Lac) | 5 μg of EI | 0 | |
| 64H (Lac) | 5 μg of HPr | 0 |
Because KB6417 has been cured from pLZ64 (data not shown), which carries the lac operon, it was not possible to characterize it in the lactose phosphorylation test. Based on the similarity of the phenotypes of KB6417 and KB6419, we consider KB6417 to be Pts− too. However, the different reversion rates indicate that KB6417 carries a mutation that is distinct from that in KB6419.
Analysis of the high-affinity galactose uptake system in Pts− and Pts+ backgrounds.
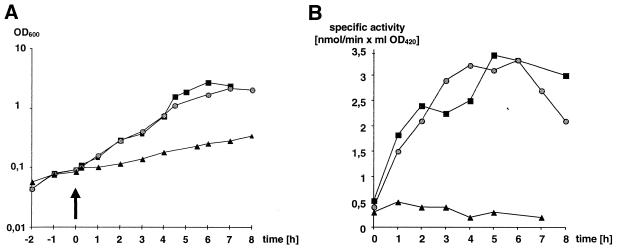
As the high-affinity uptake of galactose most likely reflects the activity of the galactose-PTS, induction of the galactose-PTS can be analyzed by measuring induction of galactose transport. As expected for a system specific for galactose, it was induced by galactose. Interestingly, it was also induced by lactose in the medium (Fig.1). The kinetics of induction, however, displayed remarkable differences between the two carbohydrates. Induction by galactose was biphasic, with peaks after 2 and 5 h, while induction by lactose displayed only one peak, after 4 h. The induction kinetics confirm that the system is a galactose-specific uptake system.
FIG. 1.
Induction of the galactose-PTS, detetmined for growth on either galactose or lactose. (A) Growth curves. The arrow indicates the time of addition of the carbohydrates. (B) Curves of specific activity. ▴, uninduced; ■, induced by galactose; Created by potrace 1.16, written by Peter Selinger 2001-2019 , induced by lactose.
To analyze the effect of the ptsH mutation of KB6419 and of the proposed Pts− mutation of KB6417 on uptake of galactose, we measured the high-affinity uptake of [14C]galactose in these strains (Table2). The data show that the high-affinity uptake of galactose could no longer be detected in these strains, further confirming that the system is PTS dependent and represents the galactose-PTS. Both strains have also lost the ability to take up lactose. KB6417 lost the lac operon with the curing of pLZ64 and is therefore Lac−; however, KB6419 also shows a Lac− phenotype, as expected for a Pts−mutant. The loss of the high-affinity uptake system for galactose is also reflected by slower growth of KB6417 and KB6419 than of 64H on galactose (Table 2).
TABLE 2.
Characterization of strains 64H, KB6417, and KB6419
| Strain | Activity | Doubling timea (h) | ||||
|---|---|---|---|---|---|---|
| Transportb (nmol/min/5 × 108 cells) | Galactokinase (nmol/mg of protein/min) | |||||
| Galactose | Lactose | Galactose | Lactose | Glucose | ||
| 64H | 3.4 | 3.5 | 150 | 2.2 | 2.2 | 1.8 |
| KB6417 | 0 | 0 | 240 | 3.2 | ∞ | 3.2 |
| KB6419 | 0 | 0 | 413 | 3.4 | ∞ | 3.4 |
To further ensure that the PTS-dependent system measured in L. casei 64H is specific for galactose, its substrate specificity was determined by competition experiments (Table3). Of the carbohydrates tested,d-mannose, d-glucose, and 2DGlc inhibited uptake of galactose, indicating that these carbohydrates could be substrates of the galactose-PTS. An extremely weak competition was also detectable with d-xylose. Nevertheless, the relatively high concentrations needed to inhibit galactose uptake indicate that the system is a galactose-specific uptake system.
TABLE 3.
Competition of high-affinity galactose uptake activity by various carbohydratesa
| Inhibitor | 100 μM inhibitor | 50 μM inhibitor | ||
|---|---|---|---|---|
| Sp act of uptake of [14C]galactose (nmol/5 × 107 cells/min) | Inhibition (%) | Sp act of uptake of [14C]galactose (nmol/5 × 107cells/min) | Inhibition (%) | |
| None | 3.10 | 0 | 3.5 | 0 |
| d-Galactose | 0.12 | 96.2 | 0.3 | 91.3 |
| d-Mannose | 1.19 | 62.0 | 3.0 | 15.7 |
| d-Glucose | 1.59 | 48.9 | 3.2 | 9.5 |
| 2-Deoxygalactose | 1.67 | 46.3 | 3.3 | 7.0 |
| d-Xylose | 2.40 | 22.6 | 3.5 | 1.4 |
Measurement of membrane-dependent phosphorylation of galactose in_L. casei_ 64H.
PTS-dependent phosphorylation of carbohydrates uses phosphoenolpyruvate as the phosphate donor and needs membranes as well as the soluble cell fraction. We measured this type of galactose phosphorylation in L. casei 64H and the mutant strains. As the system is inducible, we combined membranes and soluble cell fractions from induced and uninduced cells. The high-affinity uptake of galactose can be induced either by galactose or by lactose; however, in supernatants prepared from cells induced by galactose, a relatively high activity for phosphorylation of galactose was detectable. This is most probably due to the activity of galactokinase. Therefore, we used cells induced with lactose, where this background activity is less pronounced. Membrane-dependent phosphorylation of galactose could be detected in L. casei 64H if membranes as well as the soluble cell fraction from induced cells were used. Specific phosphorylation activities of 1.3 nmol of galactose per mg of membrane protein per min were measured. As no galactose phosphorylation could be detected if membranes or soluble proteins from uninduced cells were used, the galactose-PTS should possess at least one soluble substrate-specific domain. Neither membranes nor soluble cell fractions of mutants KB6417 and KB6419 showed any galactose phosphorylation activity. This is in contrast to lactose phosphorylation, where membranes prepared from KB6419 were still active (Table 1). This difference might be due to differences in induction. Possibly in a Pts− background the galactose-PTS cannot be induced because the required inducer cannot be synthesized, while the lactose-PTS is constitutive in a Pts− background, as noted above.
Characterization of mutants with altered galactose metabolism.
L. casei 64H appears to have two distinct pathways for the degradation of galactose (7), an observation confirmed by the Gal+ phenotype of the ptsH mutants. It should be possible to isolate mutants that are defective in the second pathway and hence totally dependent on the galactose-PTS for growth.
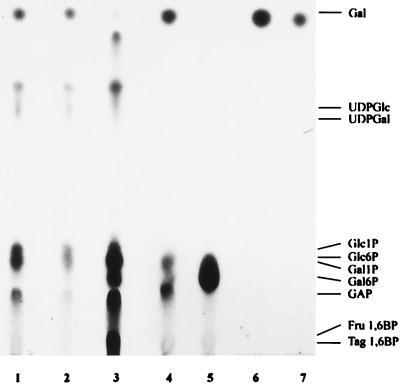
2-Deoxygalactose selection was used to isolate resistant mutants. One of these mutants grew more slowly than the wild type on galactose but was not affected in growth on other carbohydrates. No galactokinase activity could be detected in extracts prepared from this mutant, which was named PG4 and used for further characterization (Table4). In addition, in PG4 the high-affinity uptake system for galactose was no longer detectable whereas lactose uptake remained unaffected, indicating that HPr and EI are still active. Thin-layer chromatography of extracts of PG4 prepared from cells incubated with [14C]galactose showed a pattern of metabolites different from that for L. casei 64H. In PG4, intermediates resulting from metabolism of galactose via the Leloir pathway (for example, UDP-glucose and UDP-galactose) cannot be detected (Fig. 2). This finding correlates with the lack of galactokinase activity. The metabolites seen in PG4 as well as in 64H most probably represent intermediates of the tagatose 6-phosphate pathway. PG4 apparently carries a mutation that reduces uptake activity of the galactose-PTS as shown by the uptake assays, but the mutation does not completely block phosphorylation of galactose to galactose 6-phosphate.
TABLE 4.
PTS transport and galactokinase activities of strains PG4 and STZB1
| Strain | Doubling time (h) on galactose | Activity | ||
|---|---|---|---|---|
| PTS transport (nmol/5 × 107 cells/min) | Galactokinase (nmol/min/mg of protein) | |||
| Galactose | Lactose | |||
| 64H | 2.2 | 3.4 | 3.5 | 150 |
| PG4 | 4.2 | 0 | 2.7 | 0 |
| STZB1 | ∞ | 0 | 2.8 | 0 |
| PG41 | ∞ | 0 | 0 | 0 |
| PG45 | ∞ | 0 | 0 | 0 |
FIG. 2.
Analysis of intermediates of galactose metabolism in different mutants. The autoradiogram shows extracts of [14C]galactose-metabolizing cells which were separated by thin-layer chromatography. Lane 1, KB6419; lane 2, KB6417; lane 3, 64H; lane 4, PG4; lane 5, STZB1; lane 6, PG41; lane 7, PG45. Positions of reference substances are indicated at the right. GAP, glyceraldehyde 3-phosphate; Fru, fructose; Tag, tagatose.
Using EMS mutagenesis and selection with streptozotocin, we tried to isolate Gal− mutants from PG4. Cells from the selection procedure were plated on LCM supplemented with 0.5% galactose. After 3 days of incubation at 37°C, colonies of different sizes were visible. As Gal− mutants are expected to produce small colonies, these mutants were used for further testing. About 20% of the colonies tested were Gal−. Since all independent mutants isolated by this procedure displayed the same phenotype, only one of them, named STZB1, is described further. Growth analysis of STZB1 showed that this mutant cannot grow with galactose (Table 4) or lactose (data not shown) as the sole carbohydrate. Moreover, growth of the mutant on mannitol slowed after addition of galactose or lactose, which is characteristic for a sensitive phenotype. This phenotype could be due to a mutation in one of the genes coding for the enzymes of the tagatose 6-phosphate pathway. The accumulation of galactose 6-phosphate in STZB1 supports this hypothesis (Fig. 2). Analysis of STZB1 therefore confirms the idea that the same enzymes are used for the metabolism of both galactose and lactose in L. casei 64H.
We were able to also isolate pleiotropic mutants from PG4 by again using 2DGlc selection. These mutants, PG41 and PG45, derived from separate selection procedures, showed the same phenotype as KB6417 and KB6419 except that they were also Gal−. Thin-layer chromatography showed that no metabolites of galactose can be detected in extracts of these mutants (Fig. 2). By using the test system described for the characterization of KB6419, PG45 was also characterized as a ptsH mutant (data not shown). As expected, uptake of galactose as well as of lactose could no longer be measured in these mutants (Table 4).
DISCUSSION
We were able to confirm the existence of a specific galactose-PTS in L. casei 64H by isolation and characterization of_ptsH_ mutants and of mutants with altered galactose metabolism. Analyses of these mutants also confirmed the existence of two distinct pathways for galactose in L. casei 64H.ptsH mutants from L. casei 64H showed reduced growth on galactose, and high-affinity uptake of galactose could not be measured. Remaining growth of the Pts− mutants on galactose indicates the existence of a non-PTS uptake system for galactose coupled to the Leloir pathway. The unidentified uptake system should have a Km clearly higher than that for the PTS, as it could not be detected in uptake studies with 7.5 μM galactose. The existence of the Leloir pathway in _L. casei_64H has been demonstrated (3) and confirmed by thin-layer chromatography (Fig. 2).
The PTS-dependent uptake and metabolism of galactose are most clearly shown in PG4 and its ptsH derivatives, PG41 and PG45. PG4 grows well with galactose as the sole carbon source. Growth is possible only by metabolism via the tagatose 6-phosphate pathway, as the Leloir pathway is blocked by a galK mutation. Introduction of a_ptsH_ mutation in PG4, as in strains PG41 and PG45, resulted in a Gal− phenotype. These mutants were, in addition, unable to take up and phosphorylate galactose via the PTS. No galactose 6-phosphate could be detected in the ptsH mutants. Galactose 6-phosphate is therefore the specific product of the PTS-dependent phosphorylation of galactose.
The PTS-dependent phosphorylation of galactose could be measured only if membranes as well as the soluble cell fraction were prepared from cells induced with galactose or lactose, confirming the data for induction studies using transport assays. Inducibility by galactose indicates again that a system specific for galactose was measured. The need for soluble extracts from induced cells shows that the galactose-PTS possesses at least one galactose-specific soluble protein, possibly a EIIAGal, as postulated by Chassy and Thompson (7).
Judging from the competition studies, the galactose-PTS is specific for galactose, as only mannose, glucose, and 2-deoxygalactose displayed significant effects on galactose uptake. These carbohydrates are also substrates of other galactose systems, such as the galactose permease and the methylgalactoside systems of E. coli (13, 14,26). Chassy and Thompson (7) obtained different results for similar experiments, possibly because they used higher concentrations not only of galactose but also of the competing substances, which in turn may have caused an elevated level of contaminating galactose, which would certainly skew the results, as was noted by the authors themselves.
The galactose-PTS is induced by galactose or lactose in the medium. Its induction differs from the induction of the galactokinase, which had been shown to be induced by galactose but not by lactose in the medium (3), reflecting the two distinct pathways for galactose metabolism in L. casei 64H. As the inducer of the galactose-PTS is synthesized during growth on lactose, galactose 6-phosphate or a metabolite of the tagatose 6-phosphate pathway must be the molecular inducer. These metabolites should be produced during lactose and galactose metabolism as well (7). Interestingly, the induction kinetics of the galactose-PTS for galactose and lactose differ from each other, possibly due to a regulation which is caused by free intracellular galactose, or one of the products of the Leloir pathway, but not by lactose. It is also interesting that the plateau in the galactose-dependent induction of the galactose-PTS occurs at the time when galactokinase is fully induced. This might indicate a regulation mechanism that coordinates expression of the galactose-PTS/tagatose 6-phosphate pathway and the galactose permease/Leloir pathway. It will be interesting to further elucidate the regulatory mechanisms.
The ptsH mutants described in this work are the first to be isolated from L. casei. Besides the characterization of galactose metabolism, they also allow the characterization of PTS substrates in L. casei 64H and information about catabolite repression in this organism. Judging from the phenotype of KB6417 and KB6419, the carbohydrates lactose, d-mannose, mannitol,_N_-acetylglucosamine, d-fructose, ribitol, cellobiose, glucitol, and d-galactose are substrates of the PTS in L. casei 64H. Our results are in agreement with those reported by different authors who identified lactose and pentitols, including ribitol, as PTS substrates of L. casei (6,25).
The reversion studies showed that no carbohydrate-dependent repression is active in KB6417 and KB6419, as expected for ptsH mutants on the basis of the present model of catabolite repression in gram-positive bacteria (8).
The phenotype of the Pts− mutants indicates that_N_-acetylglucosamine is a PTS substrate in L. casei. This suggests that the selection by streptozotocin used for the isolation of STZB1 follows the same principle as in E. coli. The results obtained with the isolation of STZB1 demonstrate the feasibility of the streptozotocin selection method with lactobacilli, which should be applicable for the isolation of a variety of other metabolic mutations as well.
In summary, we could demonstrate a specific PTS for the uptake of galactose in L. casei 64H, whose product is galactose 6-phosphate. The galactose-PTS has at least one soluble, specific domain. Galactose 6-phosphate produced by the PTS is metabolized via the tagatose 6-phosphate pathway using the same enzymes and genes used in lactose metabolism. Therefore, we could confirm the existence of a galactose-PTS as well as the existence of two distinct pathways for metabolism of galactose within one cell by genetic and biochemical analysis.
ACKNOWLEDGMENTS
The constant advice and generous support of Joseph W. Lengeler during this work are gratefully acknowledged.
This research was supported by grant SFB171 TP C17 from Deutsche Forschungsgemeinschaft.
REFERENCES
- 1.Alpert C-A, Siebers U. The lac operon of Lactobacillus casei contains lacT, a gene coding for a protein of the BglG family of transcriptional antiterminators. J Bacteriol. 1997;179:1555–1562. doi: 10.1128/jb.179.5.1555-1562.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alpert C-A, Chassy B M. Molecular organization of the plasmid encoded lactose-PTS of Lactobacillus casei. FEMS Microbiol Rev. 1989;63:157–166. doi: 10.1016/0168-6445(89)90020-x. [DOI] [PubMed] [Google Scholar]
- 3.Bettenbrock K, Alpert C-A. The gal genes for the Leloir pathway of Lactobacillus casei64H. Appl Environ Microbiol. 1998;64:2013–2019. doi: 10.1128/aem.64.6.2013-2019.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3a.**Bettenbrock, K., and C.-A. Alpert.**Unpublished data.
- 4.Bisset D L, Anderson R L. Lactose and d-galactose metabolism in Staphylococcus aureus: pathway of d-galactose 6-phosphate degradation. Biochem Biophys Res Commun. 1973;52:641–647. doi: 10.1016/0006-291x(73)90761-4. [DOI] [PubMed] [Google Scholar]
- 5.Bisset D L, Anderson R L. Lactose and d-galactose metabolism in group N streptococci: presence of enzymes of both the d-galactose 1-phosphate and d-tagatose 6-phosphate pathways. J Bacteriol. 1974;117:313–320. doi: 10.1128/jb.117.1.318-320.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chassy B M, Thompson J. Regulation of lactose-phosphoenolpyruvate-dependent phosphotransferase system and β-d-phospho-galactoside galactohydrolase activities in Lactobacillus casei. J Bacteriol. 1983;154:1195–1203. doi: 10.1128/jb.154.3.1195-1203.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chassy B M, Thompson J. Regulation and characterization of the galactose-phosphoenolpyruvate-dependent phosphotransferase system in Lactobacillus casei. J Bacteriol. 1983;154:1204–1214. doi: 10.1128/jb.154.3.1204-1214.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deutscher J, Fischer C, Charrier V, Galinier A, Lindner C, Darbon E, Dossonnet V. Regulation of carbon metabolism in Gram-positive bacteria by protein phosphorylation. Folia Microbiol. 1997;42:171–178. doi: 10.1007/BF02818974. [DOI] [PubMed] [Google Scholar]
- 9.Efthymiou C, Hansen P A. An antigenic analysis of Lactobacillus acidophilus. J Infect Dis. 1962;110:258–267. doi: 10.1093/infdis/110.3.258. [DOI] [PubMed] [Google Scholar]
- 10.Gasser F, Mandel M. Deoxyribonucleic acid base composition of the genus Lactobacillus. J Bacteriol. 1968;96:580–588. doi: 10.1128/jb.96.3.580-588.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gauthier L, Thompson S, Gagnon G, Frenette M, Trahan L, Vadeboncoeur C. Positive selection for resistance to 2-deoxyglucose gives rise, in Streptococcus salivarius, to seven classes of pleiotropic mutants, including ptsH and ptsImissense mutants. Mol Microbiol. 1994;13:1101–1109. doi: 10.1111/j.1365-2958.1994.tb00501.x. [DOI] [PubMed] [Google Scholar]
- 12.Gosalbes M J, Monedero V, Alpert C-A, Pérez-Martinez G. Establishing a model to study regulation of the lactose operon in Lactobacillus casei. FEMS Microbiol Lett. 1997;148:83–89. doi: 10.1111/j.1574-6968.1997.tb10271.x. [DOI] [PubMed] [Google Scholar]
- 13.Henderson P J F, Giddens R A, Jones-Mortimer M C. Transport of galactose, glucose and their analogues by Escherichia coliK12. Biochem J. 1977;162:309–320. doi: 10.1042/bj1620309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henderson P J F, Baldwin S A, Cairns M T, Charalambous B M, Dent H C, Gunn F, Liang W-J, Lucas V A, Martin G E, McDonald T P, McKeown B J, Muiry J A R, Petro K R, Roberts P E, Shatwell K P, Smith G, Tate C G. Sugar cation symport systems in bacteria. Int Rev Cytol. 1992;137A:149–208. [PubMed] [Google Scholar]
- 15.Hengstenberg W, Egan J B, Morse M L. Carbohydrate transport in Staphylococcus aureus. V. The accumulation of phosphorylated carbohydrate derivatives, and evidence for a new enzyme splitting lactose phosphate. Proc Natl Acad Sci USA. 1967;58:274–279. doi: 10.1073/pnas.58.1.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hengstenberg W, Penberthy W K, Morse M L. The phosphotransferase system of Staphylococcus aureus. Fed Proc. 1968;27:643. doi: 10.1128/jb.99.2.383-388.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kennedy E P, Scarborough G A. Mechanism of hydrolysis of o-nitrophenyl-β-galactoside in Staphylococcus aureusand its significance for theories of sugar transport. Proc Natl Acad Sci USA. 1967;58:225–228. doi: 10.1073/pnas.58.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laue P, MacDoald R E. Identification of thiomethyl-beta-d-galactoside 6-phosphate accumulated by Staphylococcus aureus. J Biol Chem. 1968;243:680–682. [PubMed] [Google Scholar]
- 19.Lauret R, Morel-Deville F, Berthier F, Champomier-Verges M, Postma P, Ehrlich S D, Zagorec M. Carbohydrate utilization in Lactobacillus sake. Appl Environ Microbiol. 1996;62:1922–1927. doi: 10.1128/aem.62.6.1922-1927.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lengeler J, Herman K O, Unsöld H J, Boos W. The regulation of the β-methylgalactoside transport system and of the galactose binding protein of Escherichia coliK12. Eur J Biochem. 1971;19:457–470. doi: 10.1111/j.1432-1033.1971.tb01336.x. [DOI] [PubMed] [Google Scholar]
- 21.Lengeler J W. Mutations affecting the transport of the hexitols d-mannitol, d-glucitol, and galactitol in Escherichia coliK-12: isolation and mapping. J Bacteriol. 1975;124:26–38. doi: 10.1128/jb.124.1.26-38.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lengeler J W. Streptozotozin, an antibiotic superior to penicillin in the selection of rare bacterial mutations. FEMS Microbiol Lett. 1979;5:417–429. [Google Scholar]
- 23.Lengeler J W. Polyhydric alcohol transport by bacteria. Methods Enzymol. 1986;125:473–485. doi: 10.1016/s0076-6879(86)25037-5. [DOI] [PubMed] [Google Scholar]
- 24.Lin E C C, Lerner S A, Jorgensen S E. A method for isolating constitutive mutants for carbohydrate-catabolizing enzymes. Biochim Biophys Acta. 1962;60:422–424. doi: 10.1016/0006-3002(62)90423-7. [DOI] [PubMed] [Google Scholar]
- 25.London J, Chase N M. New pathway for the metabolism of pentitols. Proc Natl Acad Sci USA. 1977;74:4296–4300. doi: 10.1073/pnas.74.10.4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rotman B, Ganesan A K, Guzman R. Transport systems for galactose and galactosides in Escherichia coli. II. Substrate and inducer specificities. J Mol Biol. 1968;36:247–260. doi: 10.1016/0022-2836(68)90379-3. [DOI] [PubMed] [Google Scholar]
- 27.Rosey E L, Stewart G C. Nucleotide and deduced amino acid sequences of the lacR, lacABCD, and lacF genes encoding the repressor, tagatose 6-phosphate gene cluster, and sugar-specific phosphotransferase system components of the lactose operon of Streptococcus mutans. J Bacteriol. 1992;174:6159–6170. doi: 10.1128/jb.174.19.6159-6170.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosey E L, Oskouian B, Stewart G C. Lactose metabolism by Staphylococcus aureus: characterization of lacABCD, the structural genes of the tagatose 6-phosphate pathway. J Bacteriol. 1991;173:5992–5998. doi: 10.1128/jb.173.19.5992-5998.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sherman J R. Rapid enzyme assay technique utilizing radioactive substrate, ion-exchange paper and scintillation counting. Anal Biochem. 1963;5:548. doi: 10.1016/0003-2697(63)90075-7. [DOI] [PubMed] [Google Scholar]
- 30.Smith P K, Krohn R I, Hermahnson G T, Mallia A K, Gartner F H, Provenzano M D, Fujimoto M K, Goeke N M, Olson B J, Klenk D C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 31.Tanaka S, Lerner S A, Lin E C C. Replacement of a phosphoenolpyruvate-dependent phosphotransferase by a nicotinamide adenine dinucleotide linked dehydrogenase for the utilization of mannitol. J Bacteriol. 1967;93:642–648. doi: 10.1128/jb.93.2.642-648.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thompson J. Galactose transport systems in Streptococcus lactis. J Bacteriol. 1980;144:683–691. doi: 10.1128/jb.144.2.683-691.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thompson J, Chassy B M. Novel phosphoenolpyruvate-dependent futile cycle in Streptococcus lactis: 2-deoxy-d-glucose uncouples energy production from growth. J Bacteriol. 1982;151:1454–1465. doi: 10.1128/jb.151.3.1454-1465.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Rooijen R J, van Schalkwijk S, de Vos W M. Molecular cloning, characterization and nucleotide sequence of the tagatose 6-phosphate gene cluster of the lactose operon of Lactococcus lactis. J Biol Chem. 1991;266:7176–7181. [PubMed] [Google Scholar]