Peptidoglycan Structural Dynamics during Germination of Bacillus subtilis 168 Endospores (original) (raw)
Abstract
Peptidoglycan structural dynamics during endospore germination of Bacillus subtilis 168 have been examined by muropeptide analysis. The first germination-associated peptidoglycan structural changes are detected within 3 min after the addition of the specific germinant l-alanine. We detected in the spore-associated material new muropeptides which, although they have slightly longer retention times by reversed-phase (RP)-high-pressure liquid chromatography (HPLC) than related ones in dormant spores, show the same amino acid composition and molecular mass. Two-dimensional nuclear magnetic resonance (NMR) analysis shows that the chemical changes to the muropeptides on germination are minor and are probably limited to stereochemical inversion. These new muropeptides account for almost 26% of the total muropeptides in spore-associated material after 2 h of germination. The exudate of germinated spores of B. subtilis 168 contains novel muropeptides in addition to those present in spore-associated material. Exudate-specific muropeptides have longer retention times, have no reducing termini, and exhibit a molecular mass 20 Da lower than those of related reduced muropeptides. These new products are anhydro-muropeptides which are generated by a lytic transglycosylase, the first to be identified in a gram-positive bacterium. There is also evidence for the activity of a glucosaminidase during the germination process. Quantification of muropeptides in spore-associated material indicates that there is a heterogeneous distribution of muropeptides in spore peptidoglycan. The spore-specific residue, muramic δ-lactam, is proposed to be a major substrate specificity determinant of germination-specific lytic enzymes, allowing cortex hydrolysis without any effect on the primordial cell wall.
The extreme heat resistance of dormant bacterial endospores has made them an important problem in the production of safe foodstuffs (3). The spore cell wall peptidoglycan is considered to play a major role in the maintenance of heat resistance and dormancy (6). Bacillus subtilis spore peptidoglycan is composed of two layers. A thin, inner layer called the primordial cell wall retains the basic vegetative cell peptidoglycan structure. The primordial cell wall represents 2 to 4% of the total endospore peptidoglycan, is not digested during germination, and serves as the initial cell wall during outgrowth (2, 5, 25, 29). The outer thick layer of peptidoglycan, known as the cortex, is characterized by several unique spore-specific features. Approximately 50% of the muramic acid residues in the glycan strands are present in the δ-lactam form (2, 24). Muramic acid side chains are composed of 26 and 23% of tetrapeptide and single l-alanine, respectively (2).
Despite their extreme dormancy and thermostability, bacterial endospores retain an alert sensory mechanism enabling them to respond within minutes to the presence of specific germinants. Spores of B. subtilis respond to at least two different types of germinative stimuli: (i) l-alanine and (ii) a combination of l-asparagine, glucose, fructose, and KCl (AGFK) (34). The germination response is initiated by the interaction of a receptor protein with specific germinants which triggers the loss of spore-specific properties and the transformation of a dormant resistant bacterial spore into a metabolically active vegetative cell. The germination process is characterized by sequential, interrelated biochemical events. The specific hydrolysis of peptidoglycan in the spore cortex layer is an essential event in germination (2, 25). Its degradation removes the physical constraints of the cortex and allows core expansion and outgrowth (9, 25). As a consequence of cortex hydrolysis, peptidoglycan fragments can be detected in the germination exudate (13, 33).
A number of bacterial spore germination-specific cortex-lytic enzymes (GSLEs) have been reported to be involved in cortex hydrolysis (9, 18–20). A gene homologous to that encoding the GSLE from Bacillus cereus has been identified and inactivated in B. subtilis, and the resulting mutant germinates more slowly than the wild type (22). Recently a germination-specific muramidase isolated from a germination extract of Clostridium perfringens S40 has been purified and characterized (4).
GSLEs have a high substrate specificity, requiring intact spore cortex for activity (9, 23). The muramidase from C. perfringens S40, however, hydrolyzes cortical fragments but has a strict requirement for the presence of the muramic δ-lactam residues (4). Thus, the GSLEs are highly specialized and may exist as proforms which are specifically activated during germination (9).
Very little is known about the mechanism by which the cortex is hydrolyzed during germination and the autolytic enzymes involved. Muropeptide analysis provides a method for fine chemical structural determination of spore cortex (2, 24, 25). In this paper, we report the use of muropeptide analysis to determine the peptidoglycan structural dynamics which occur during spore germination of B. subtilis 168 and the evidence for a number of different enzyme activities.
MATERIALS AND METHODS
Bacterial strains and sporulation conditions.
All B. subtilis 168 strains used in this study are in the HR background (2). Specific mutations were transferred into HR by transformation with donor chromosomal DNA (1). Spores were prepared and stored as previously described (2).
Spore germination.
Purified spores were heat activated in distilled water at 70°C for 45 min. Activated spores were quickly cooled in ice and used within 1 h for germination experiments. Spores were suspended at a final concentration of 9 to 11 mg/ml in 30 mM potassium phosphate buffer (pH 7) and prewarmed for 15 min to 37°C before addition of l-alanine to a final concentration of 1 mM. Continuous monitoring of germination was carried out by recording the decrease of _A_600 (9).
Determination of the loss of heat resistance during germination.
Germinating spore samples (100 μl) were added immediately to 900 μl of 10 mM d-alanine and incubated for 25 min at 70°C. After cooling, viability was measured by serial dilution and plate counting on nutrient agar (8).
Preparation of spore-associated peptidoglycan.
Germinating spore samples (3 ml) were added directly to 6 ml of propan-1-ol (prewarmed to 80°C) and incubated for 15 min at 80°C to stop germination. Spores were recovered by centrifugation (14,000 × g, 8 min, room temperature), and resuspended in 1 ml of 50 mM Tris-HCl (pH 7)–4% (wt/vol) sodium dodecyl sulfate–30 mM dithiothreitol–2 mM EDTA, boiled for 16 min, and then incubated at 37°C for 40 min. Peptidoglycan-containing insoluble material was recovered by centrifugation (14,000 × g, 8 min, room temperature) and washed by repeated resuspension and centrifugation with warm (37°C) distilled water until free of sodium dodecyl sulfate. Samples were finally resuspended in MilliQ water (18 M/Ω/cm) and stored at −20°C.
Preparation of germination exudate.
For the analysis of the germination exudate, 3-ml aliquots of germinating spore samples were centrifuged (14,000 × g, 8 min, room temperature), and the supernatant was treated for 3 min at 100°C to inactivate the cortex lytic enzyme(s). The supernatant was freeze-dried, resuspended in 1 ml of MilliQ water, and stored at −20°C.
RP-HPLC, amino acid analysis, and MS.
Spore-associated peptidoglycan was digested with Cellosyl and reduced with sodium borohydride as previously described (2). Germination exudate was reduced with sodium borohydride (3.3 mg/ml) after Cellosyl digestion. Reverse-phase high-pressure liquid chromatography (RP-HPLC), desalting, amino acid analysis, and mass spectrometry (MS) were performed as previously reported (2).
Gel filtration of germination exudate samples.
Freeze-dried germination exudate samples were resuspended in MilliQ water and applied to a TSK SW2000 gel filtration column (7.8 mm by 30 cm). The column was eluted with 10 mM sodium phosphate (pH 6.5) at 0.3 ml/min. The eluate was then desalted and analyzed as described above.
Nuclear magnetic resonance (NMR) analysis of muropeptides.
Samples of ca. 1 mM muropeptide were prepared in 90% H2O–10% D2O, and studied at 19 to 35°C on a Bruker DRX-500 spectrometer. Spectra were assigned by using two-dimensional (2D) correlated spectroscopy (COSY), total correlated spectroscopy (TOCSY), and rotating frame nuclear Overhauser effect spectroscopy (ROESY), which were acquired by using spectral widths of 12,500 Hz in _t_2 and 5,000 Hz in _t_1 over 256 complex points with quadrature detection using the States-TPPI scheme. Mixing times for both TOCSY and ROESY were 100 ms. Spectra were processed by using Felix 97.0 (Molecular Simulations, Inc., San Diego, Calif.).
RESULTS
Changes in spore-associated peptidoglycan structure during germination.
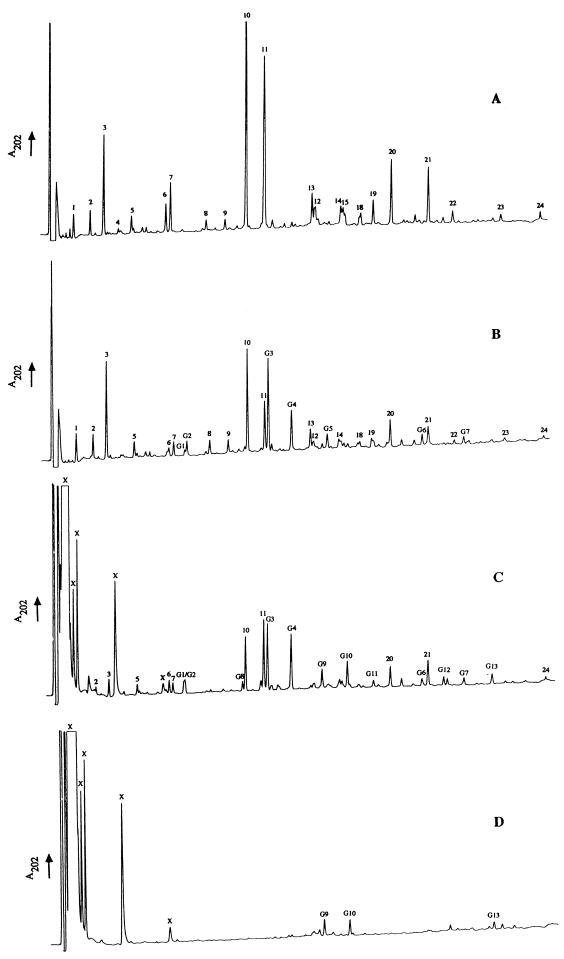
To avoid possible loss of muropeptides from germinated spores during spore extraction, only the first detergent treatment of the previously derived protocol was used (2). After this extraction, almost 97% of the peptidoglycan was solubilized after Cellosyl digestion. The RP-HPLC profiles of muropeptides from dormant and germinated spore-associated material (2 h after addition of l-alanine) are shown in Fig. 1A and B. During germination, >60% of the original _A_600 was lost by the spore population over 2 h. The major germination-associated changes in muropeptide profile comprised a decrease in the muramic δ-lactam-containing muropeptides, which are characteristic of the spore cortex (e.g., muropeptides 6, 7, 10, and 11), and the appearance of seven novel muropeptides (Fig. 1B, muropeptides G1 to G7).
FIG. 1.
Analysis of muropeptides by RP-HPLC during germination (120 min) of B. subtilis 168 HR spores. Muropeptide-containing samples were separated by RP-HPLC, and the _A_202 of the eluates was monitored. (A) Dormant spore-associated material; (B) germinated spore-associated material; (C) germination exudate; (D) germination exudate (no Cellosyl digestion or reduction).
RP-HPLC analysis of the germination exudate.
The RP-HPLC profile of the germination exudate, after Cellosyl digestion, revealed the appearance of several potential muropeptides (Fig. 1C). Nearly all the spore-associated muropeptides were also found in the exudate (e.g., muropeptides 6, 7, 10, and 11 [Fig. 1B and C]). However, G9, G10, G11, G12, and G13 are germination exudate-specific products. Approximately the same amounts of products labeled X were found in the germination exudate whether digested with Cellosyl or not (Fig. 1C and D). The resolved X peaks are not peptidoglycan derived since they do not contain amino acids or amino sugars (results not shown). The novel exudate-specific products G9, G10, and G13 were also resolved without Cellosyl digestion (Fig. 1D), but their amounts increased following digestion (Fig. 1C). Omission of borohydride reduction did not affect the peak shapes or retention times of products G9, G10, G12, and G13 (Fig. 1C and D and results not shown).
Molecular weight determination of native peptidoglycan fragments in the germination exudate.
The profiles of the germination exudate with (Fig. 1C) or without (Fig. 1D) Cellosyl digestion revealed that most of the peptidoglycan is released in the form of fragments too large to be resolved by RP-HPLC. Gel filtration was used to purify the native fragments (results not shown). Peptidoglycan-derived material was shown to consist of several molecular species, ranging from m/z 1,758 to 5,537.5.
Germination by AGFK and the role of peptidoglycan and protein biosynthesis.
Germination in the presence of AGFK led to muropeptide flux comparable to that in l-alanine (results not shown). Also, the addition of chloramphenicol (100 μg/ml) or penicillin G (100 μg/ml) to the germination mix had no significant effect on the muropeptide profiles (results not shown). Therefore, cortex modification and hydrolysis are common to different germinants and are not due to the synthesis of new enzymes or peptidoglycan during germination.
Characterization of the novel spore-associated muropeptides.
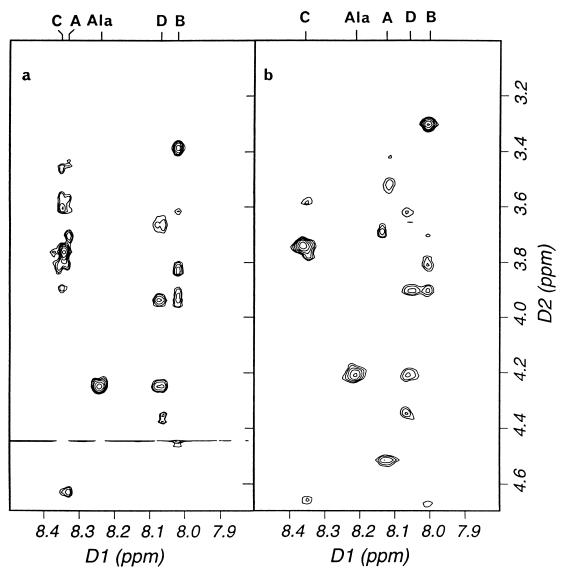
All of the germination-specific muropeptides (Fig. 1B) were purified, characterized by amino acid analysis and MS (Table 1), identified, and quantified (Table 2). All are peptidoglycan derived and have the same basic composition as dormant spore muropeptides (Table 2). Muropeptides G1 to G7 all have their equivalents in the dormant spore, to which they are ostensibly identical in terms of amino acids and MS (Tables 1 and 2, muropeptides 6, 7, 10, 11, 13, 20, and 21, respectively) (2). The germination-specific muropeptides all, however, show a characteristic increase in retention times over their dormant spore counterparts (Fig. 1A and B). The germination-specific muropeptides all have reducing termini and are unaffected by HF (48% [vol/vol], 24 h, 0°C), HCl (9 M, 15 min, 35°C), or desalting treatment prior to separation by RP-HPLC compared to the equivalent dormant spore muropeptides (results not shown). One-dimensional NMR clearly showed the absence of amidation in the novel muropeptides (results not shown); amidation would cause a mass change of only one mass unit and thus be hard to detect by MS. Further analysis by 2D NMR showed that corresponding pairs of normal and germination-specific muropeptides have very similar chemical shifts and ROESY spectra (Fig. 2 and Table 3), indicating that the covalent structures of the novel muropeptides are very similar to those of their parent muropeptides. In particular, nuclear Overhauser enhancements between sugars confirmed that there is no alteration in linkage on germination. Thus, the germination-associated change is a subtle modification that does not affect the gross structure and is most likely a change in the stereochemistry at one or more chiral centers. After 2 h of germination, the novel muropeptides (G1 to G7) constitute 25.8% of the total spore-associated material.
TABLE 1.
Calculated and observed m/z values for sodiated and deprotonated molecular ions of new muropeptides identified during B. subtilis 168 HR germination
| Muropeptidea | Ion | m/z | Δ_m_ (Da)b | Error (%)c | Muropeptide compositiond | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Observed | Calculated | Glc | Mur | δ-Mur | Glu | Ala | Dmp | ||||
| G1 | [M + Na]+ | 996.4 | 1,011.0 | −14.6 | 2 | 1 | 1 | 0 | 1 | 0 | |
| [M − H] | 972.5 | 987.0 | −14.5 | ||||||||
| G2 | [M + Na]+ | 1,369.6 | 1,383.3 | −13.7 | 2 | 1 | 1 | 1 | 2 | 1 | |
| [M − H]− | 1,345.7 | 1,359.3 | −13.6 | ||||||||
| G3 | [M + Na]+ | 1,384.4 | 1,383.3 | 1.1 | 0.07 | 2 | 1 | 1 | 1 | 2 | 1 |
| [M − H]− | 1,359.6 | 1,359.3 | 0.3 | 0.02 | |||||||
| G4 | [M + Na]+ | 1,009.6 | 1,011.0 | −1.4 | −0.13 | 2 | 1 | 1 | 0 | 1 | 0 |
| [M − H]− | 986.3 | 987.0 | −0.7 | −0.07 | |||||||
| G5 | [M + Na]+ | 2,307.5 | 2,307.2 | −0.3 | −0.01 | 3 | 2 | 1 | 2 | 4 | 2 |
| [M − H]− | 2,283.1 | 2,283.2 | −0.1 | −0.004 | |||||||
| G6 | [M + Na]+ | 1,802.5 | 1,801.7 | −0.8 | −0.04 | 3 | 1 | 2 | 1 | 2 | 1 |
| [M − H]− | 1,778.3 | 1,777.7 | −0.6 | −0.03 | |||||||
| G7 | [M + Na]+ | 1,429.9 | 1,429.4 | 0.5 | 0.03 | 3 | 1 | 2 | 0 | 1 | 0 |
| [M − H]− | 1,405.5 | 1,405.4 | 0.1 | 0.007 | |||||||
| G8 | [M + Na]+ | 1,178.4 | 1,383.3 | −204.9 | 1 | 1 | 1 | 1 | 2 | 1 | |
| [M − H]− | 1,155.3 | 1,359.3 | −204.0 | ||||||||
| G9 | [M + Na]+ | 1,363.4 | 1,383.3 | −19.9 | 2 | 1 | 1 | 1 | 2 | 1 | |
| [M − H]− | 1,339.1 | 1,359.3 | −20.2 | ||||||||
| G10 | [M + Na]+ | 990.5 | 1,011.0 | −20.5 | 2 | 1 | 1 | 0 | 1 | 0 | |
| [M − H]− | 966.6 | 987.0 | −20.4 | ||||||||
| G11 | [M + Na]+ | 990.4 | 1,011.0 | −20.6 | 2 | 1 | 1 | 0 | 1 | 0 | |
| [M − H]− | 966.1 | 987.0 | −20.9 | ||||||||
| G12 | [M + Na]+ | 1,781.3 | 1,801.7 | −20.4 | 3 | 1 | 2 | 1 | 2 | 1 | |
| [M − H]− | 1,757.1 | 1,777.7 | −20.6 | ||||||||
| G13 | [M + Na]+ | 1,409.3 | 1,429.4 | −20.1 | 3 | 1 | 2 | 0 | 1 | 0 | |
| [M − H]− | 1,385.1 | 1,405.4 | −20.3 |
TABLE 2.
Muropeptide identities and quantificationa
| Muropeptide | Identity | Mol% | ||
|---|---|---|---|---|
| DM | SAM | GE | ||
| 1 | Disaccharide tripeptide | 3.3 | 6.1 | |
| 2 | Disaccharide alanine | 5 | 7 | 2.3 |
| 3 | Disaccharide tetrapeptide | 13.8 | 18.9 | 5.7 |
| 4 | Tetrasaccharide alanine with open lactam | 1.0 | ||
| 5 | Tetrasaccharide tetrapeptide with open lactam | 2.6 | 5.0 | 3.0 |
| 6 | Tetrasaccharide alanine with a reduced lactam | 4.6 | 1.3 | 4 |
| 7 | Tetrasaccharide tetrapeptide with a reduced lactam | 4.8 | 2 | 1.7 |
| 8 | Disaccharide tripeptide disaccharide tetrapeptide | 0.8 | 1.3 | |
| 9 | Disaccharide tetrapeptide disaccharide tetrapeptide | 0.7 | 1.0 | |
| 10 | Tetrasaccharide tetrapeptide | 20.1 | 9.8 | 8.8 |
| 11 | Tetrasaccharide alanine | 22.0 | 8.5 | 13.8 |
| 12 | Hexasaccharide tetrapeptide with one reduced lactam | 1.5 | 0.6 | |
| 13 | Disaccharide tetrapeptide tetrasaccharide tetrapeptide | 2 | 1.7 | |
| 14 | Hexasaccharide alanine with one reduced lactam | 0.8 | 0.6 | |
| 15 | Hexasaccharide alanine with one reduced lactam | 1.1 | ||
| 18 | Hexasaccharide alanine with three acetylations and one reduced lactam | 1.6 | 1.9 | |
| 19 | Tetrasaccharide tetrapeptide tetrasaccharide tetrapeptide | 0.9 | 0.6 | |
| 20 | Hexasaccharide tetrapeptide | 6 | 3.3 | 4.6 |
| 21 | Hexasaccharide alanine | 5.6 | 3.5 | 5.6 |
| 22 | Tetrasaccharide tetrapeptide hexasaccharide tetrapeptide | 0.5 | 0.3 | |
| 23 | Octasaccharide tetrapeptide | 0.6 | 0.4 | |
| 24 | Octasaccharide alanine | 0.7 | 0.5 | 0.4 |
| G1 | Tetrasaccharide alanine with a reduced lactam | 1.1 | 1.4 | |
| G2 | Tetrasaccharide tetrapeptide with a reduced lactam | 3 | 2.2 | |
| G3 | Tetrasaccharide tetrapeptide | 9.8 | 10.7 | |
| G4 | Tetrasaccharide alanine | 8.0 | 12.5 | |
| G5 | Disaccharide tetrapeptide tetrasaccharide tetrapeptide | 0.9 | ||
| G6 | Hexasaccharide tetrapeptide | 1.5 | 1.3 | |
| G7 | Hexasaccharide alanine | 1.4 | 1.8 | |
| G8 | Trisaccharide tetrapeptide | 1.4 | ||
| G9 | Anhydro-tetrasaccharide tetrapeptide | 5 | ||
| G10 | Anhydro-tetrasaccharide alanine | 8.5 | ||
| G11 | Anhydro-tetrasaccharide alanine | 1.5 | ||
| G12 | Anhydro-hexasaccharide tetrapeptide | 1.4 | ||
| G13 | Anhydro-hexasaccharide alanine | 2.4 |
FIG. 2.
Portions of the ROESY spectra of the corresponding dormant and germination-associated tetrasaccharide alanine muropeptides 11 and G4 (a and b, respectively). The spectra show nuclear Overhauser enhancements between the 2′-amide protons (and alanine amide proton) and other protons in the muropeptides. The protons are labeled at the top with the identity of the saccharide unit (from A at the nonreducing end to D at the reducing end). Chemical shift assignments for these muropeptides are given in Table 3.
TABLE 3.
NMR chemical shift assignments for the tetrasaccharide alanine muropeptides 11 and G4 (dormant and germinating spore-associated, respectively) (1 mM, 30°C)
| Proton | NMR chemical shift assignmenta (ppm) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | Ala | ||||||
| 11 | G4 | 11 | G4 | 11 | G4 | 11 | G4 | 11 | G4 | |
| 1′ | 4.63 | 4.52 | 4.73 | 4.84 | 4.69 | 4.66 | 3.68, 3.73 | 3.73, 3.67 | ||
| 2′ | 3.70 | 3.71 | 3.38 | 3.31 | 3.76 | 3.75 | 4.36 | 4.41 | ||
| 3′ | 3.56 | 3.42 | 3.63 | 3.65 | 3.43 | 3.58 | 3.94 | 3.96 | ||
| Others | 3.45 | 3.53 | 3.83 | 3.86 | 3.59 | 3.77 | 3.88 | 3.84 | ||
| 3.80 | 3.88b | 3.91 | 4.13 | 3.93 | 3.91 | 3.65 | 3.67 | |||
| 3.91 | 3.88b | 3.70 | 3.70 | 3.61 | 3.64 | 3.80 | 3.84 | |||
| NH | 8.33 | 8.12 | 8.00 | 8.01 | 8.33 | 8.35 | 8.05 | 8.05 | 8.23 | 8.21 |
| Methyl | 2.07 | 2.04 | 1.45 | 1.44 | 2.07 | 2.06 | 2.06 | 1.98 | 1.40 | 1.37 |
| CH | 4.45 | 4.39 | 4.22 | 4.19 | ||||||
| Mur Me | 1.40 | 1.37 | ||||||||
| Mur CH | 4.22 | 4.19 |
The novel germination-associated muropeptides are not the result of alanine racemase activity, as they still appeared during germination of B. subtilis 1A288 (amyE dal-1 metB5 sacA321), which strictly requires d-alanine for growth. Also, the addition of _O_-carbamyl-d-serine (a potent inhibitor of alanine racemase) (26) at 100 μg/ml had no effect on germination kinetics or muropeptide modification.
Characterization of the novel germination exudate-specific products.
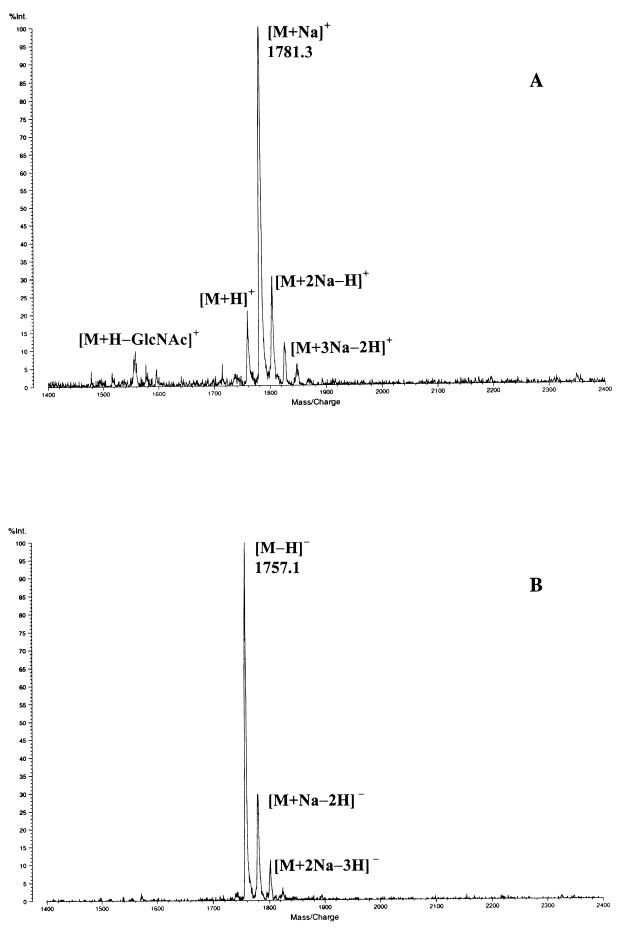
All products labeled in Fig. 1C are peptidoglycan derived except those lettered X, which are also found in the exudate without Cellosyl digestion (Fig. 1D). Germination exudate-specific muropeptides G9 to G13 (Fig. 1C) have the same amino acid analysis but a characteristic mass deviation of −20 Da determined by matrix-assisted laser desorption-ionization (MALDI) reflector time-of-flight MS compared to dormant spore muropeptides 10, 11, 20, and 21, respectively (Tables 1 and 2) (2). As G11 has a longer retention time than G10, it may be derived from the germination-specific spore-associated muropeptide G4 (Fig. 1C). The germination exudate-specific muropeptides (G9 to G13) all have longer retention times than their related spore muropeptides (Fig. 1C). Omission of sodium borohydride reduction prior to RP-HPLC led to loss of resolution and alterations in retention time of all muropeptides apart from G9 to G13 (results not shown). All of the features of G9 to G13 suggest that they have a 1-6 anhydro-muramic acid moiety. The positive- and negative-ion MALDI mass spectrum of muropeptide G12, which is the largest mass spectrometrically determined anhydro-muropeptide in B. subtilis, is shown in Fig. 3. The peak at m/z 1,781.3 corresponds to the [M + Na]+ molecular ion (Fig. 3A). Several satellite peaks were detected and corresponded to [M + H]+, [M + 2Na − H]+ and [M + 3Na − H]+ molecular ions. Further, in the positive-ion mode an intense fragment ion at m/z 1,558.2 ([M + H − GlcNAc]+) was determined. In the negative-ion mode, the base peak at m/z 1,757.1 corresponded to the molecular ion [M − H]− (Fig. 3B). The lack of 20 Da corresponds to the loss of one molecule of water between carbon 1 and carbon 6 of the _N_-acetylmuramic acid and the two hydrogens which would have been gained by sodium borohydride reduction. Anhydro-muropeptides have been found in gram-negative bacteria and are known for their hydrophobic character and acid lability (11, 12). These muropeptides are produced by the action of a lytic transglycosylase (12). G9 to G13 account for almost 19% of the total muropeptides in the germination exudate (Table 2). Interestingly, almost 55% of the dominant anhydro-muropeptides G9, G10, and G13 are also present in the exudate without Cellosyl digestion (Fig. 1D).
FIG. 3.
Positive (A)- and negative (B)-ion MALDI mass spectrum of muropeptide G12 (Tables 1 and 2; anhydro-hexasaccharide tetrapeptide) obtained in the reflector mode.
Muropeptide G8 is a trisaccharide tetrapeptide (Fig. 1C; Tables 1 and 2); the missing 204 Da corresponds to an _N_-acetylglucosamine moiety. G8 is likely to have been generated by the activity of an _N_-acetylglucosaminidase during germination. G8 accounts for only 1.4% of total exudate muropeptides, and the glucosaminidase activity is therefore minor compared to the lytic transglycosylase activity.
Muramidase activity during germination?
To determine whether a germination-specific muramidase is involved in cortex hydrolysis, as reported for C. perfringens (4), the germination exudate RP-HPLC profiles were examined after various treatments. Only anhydro-muropeptides were detected by RP-HPLC when non-Cellosyl-digested exudate was separated with or without sodium borohydride reduction (Fig. 1D and results not shown). When the germination exudate was reduced, digested with Cellosyl, and analyzed by RP-HPLC, an increase in anhydro-muropeptides and the appearance of nonreduced tetrasaccharide alanine and tetrasaccharide tetrapeptide were noted (the nonreduced muropeptides have retention times different from those of the reduced forms). However, when this sample was reduced again after Cellosyl digestion, the RP-HPLC profile was comparable to that in Fig. 1C. This clearly indicates that there is not a significant amount of muramic acid residues with free reducing termini in the native germination exudate (which would result from muramidase activity). Thus, it is unlikely that gross muramidase activity is involved in B. subtilis cortex hydrolysis during germination.
Kinetics of peptidoglycan structural dynamics, and other biochemical events, during germination.
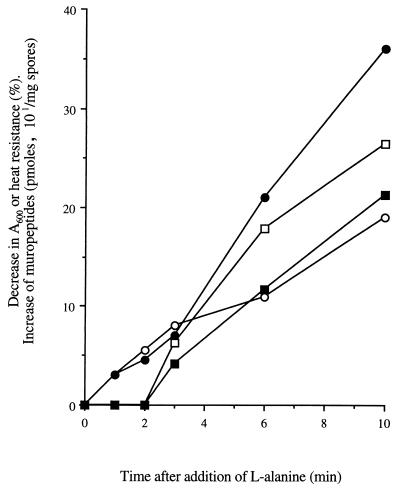
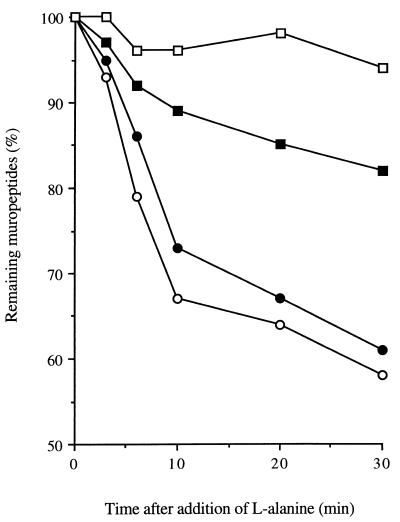
The kinetics of biochemical events occurring during germination were examined to determine their sequential interrelationships. The dominant germination-associated muropeptides, G3 and G4 (Fig. 1B; Table 2), were detected 3 min after addition of l-alanine and increased throughout germination (Fig. 4). However, loss of heat resistance and absorbance were measurable within 1 min.
FIG. 4.
Kinetics of biochemical events during germination of B. subtilis 168 HR spores. •, percent loss of heat resistance; ○, percent loss of _A_600; ■, amount of muropeptide G3; □, amount of muropeptide G4.
Spore-associated muropeptides were quantified throughout germination. The percentage decreases of total muropeptides containing hexasaccharides and tetrasaccharides were 42 and 39%, respectively, within 30 min (Fig. 5). Disaccharide alanine- and disaccharide tetrapeptide-containing muropeptides decreased at a lower rate; only 18% of the initial amount was lost over the same period (Fig. 5). The loss of disaccharide tripeptide-containing material (muropeptides 1 and 8) during germination was minimal (Fig. 5). The trends in muropeptide dynamics continued over 2 h of germination (data not shown). Muropeptide quantification of the germination exudate (Table 2) confirms the differential muropeptide loss from the germinating spores. Indeed, tetrasaccharide- and hexasaccharide-containing muropeptides constitute the major products found in the exudate (Table 2). The relative percentage increase of disaccharide-containing material in the spore-associated peptidoglycan during germination (Table 2) is due not to biosynthesis of these muropeptides but rather to the greater relative decrease in the muropeptides containing muramic δ-lactam residues (Fig. 5).
FIG. 5.
Differential muropeptide release during germination of B. subtilis 168 HR. Amounts are calculated as a percentage of the dormant spore value. ○, hexasaccharide-containing muropeptides; •, tetrasaccharide-containing muropeptides; ■, disaccharide-containing muropeptides with alanine or tetrapeptide side chains; □, disaccharide-containing muropeptides with tripeptide side chains.
Germination of cwlD and other germination mutants.
Germination of AA107 (cwlD) resulted in a 40% decrease in _A_600 over 2 h, but no structural alterations of the spore peptidoglycan occurred over this time period. The cortex of this strain has no muramic δ-lactam residues (2, 25). Spores of strains AA114 (gerD [32]) and AA115 (gerB [21]) had dormant spore peptidoglycan structures comparable to that of HR (wild type) except that muropeptides with single l-alanine substituents were present at lower levels in AA115 (gerB). AA115 (gerB) germinated in l-alanine showed the same peptidoglycan dynamics as HR (wild type) and no changes in the presence of AGFK (as expected, as the mutant cannot respond specifically to the AGFK germinants). AA114 (gerD [32]) germinated slowly with 10 mM l-alanine and 10 mM KCl (35% loss of _A_600 after 4 h) and showed the same structural changes as HR (wild type) but at a lower rate.
DISCUSSION
Specific cortex hydrolysis by the action of a GSLE is an essential step during endospore germination, as its removal allows spore core expansion and outgrowth (10, 13, 30). This finding is corroborated by the fact that the cwlD mutant has an altered spore cortex structure and is unable to outgrow and form a colony on a plate (2, 25, 28). This observation led to the suggestion that the muramic δ-lactam residues (missing in cwlD) are part of the substrate recognition profile of the GSLE (2, 9, 25). However, the mechanism of cortex hydrolysis during germination and the number of enzymes involved have remained obscure.
Cortex modification as reflected by changes in peptidoglycan structure is initiated within 3 min of addition of the germinant l-alanine. The modification is stable and does not arise from amidation or hydrolytic cleavage, although it is possible that the modified muropeptides are then marked for hydrolysis by ensuing autolytic enzymes. Alternatively, the modification may not be essential for germination but rather has a more subtle role. It is clear that the cortex modification is not essential for loss of absorbance or heat resistance, because these changes precede the modification (Fig. 4). Furthermore, spores of the cwlD mutant lose heat resistance and partial absorbance on germination, even though cortex modification does not occur (25, 28). Modified disaccharide-containing muropeptides are not apparent, which suggests that the alteration may occur on the δ-lactam moiety. However, the δ-lactams in the modified muropeptides are still able to be reduced, and acid hydrolysis (2) results in its conversion to muramic acid. Also, 2D NMR spectra did not reveal any alterations in δ-lactam stereochemistry. Similar modifications occur to muropeptides with tetra- or hexasaccharides and containing either a single l-alanine or tetrapeptide as the side chain, implying that the change occurs close to the muramyl alanine and may be an alteration in stereochemistry. As the modification occurs only on muropeptides containing the δ-lactam moiety, it is likely that this moiety is required for the activity of the enzyme responsible for the modification. Such requirement for the presence of the δ-lactam moiety for cortex-active enzymes has been previously demonstrated (4, 9). It is possible that epimerase activity can result in a stable alteration in the stereochemistry of the muramic acid residues.
The characteristics of the novel germination exudate-specific muropeptides match the properties of anhydro-muropeptides, suggesting the involvement of a lytic transglycosylase in germination (12). This is the first evidence in gram-positive bacteria for lytic transglycosylase activity. There are a number of lytic transglycosylases in Escherichia coli which have been characterized at the molecular level (27). The recently released B. subtilis genome sequence has revealed the presence of a gene (yjbJ) which encodes a putative protein showing high identity (33% over 148 amino acids) to Slt, the major lytic transglycosylase of E. coli (7). The possible involvement of YjbJ in germination is currently being investigated.
The anhydro-muropeptides are almost entirely specific to the germination exudate, although muropeptide G9 is just detectable in spore-associated material (eluted between muropeptide 12 and G5 [Fig. 1B]). The presence of anhydro-muropeptides predominantly in the exudate suggests that the lytic transglycosylase acts mostly on released material or at least that which has been previously cleaved by the GSLE (which would result in relaxation of the stress-bearing properties of the polymer). In E. coli, the products of lytic transglycosylase activity are also mostly found as soluble material (15).
The anhydro-muropeptides represent 18.8% of the total muropeptides released after Cellosyl digestion, 55% of which were found free as single-unit muropeptides in the exudate without digestion. The free muropeptides are likely to have been cleaved from the ends of the glycan strands, and thus the lytic transglycosylase is an exoenzyme, processively hydrolyzing the peptidoglycan. Anhydro-muropeptides represent 60 to 80% of cell wall degradation products released from E. coli during autolysis triggered by cephaloridine or trichloroacetic acid (17). In E. coli, anhydro-muropeptides are involved in peptidoglycan recycling and gene regulation (14, 15, 16). The cortex material released during germination is likely to be recycled during the biosynthesis of new peptidoglycan in outgrowing cells (31). Thus, the anhydro-muropeptides may be recycled and/or form part of a signalling mechanism to initiate new peptidoglycan biosynthesis. We are currently investigating the fate of the germination exudate muropeptides during spore outgrowth.
The dormancy-maintaining function of the cortex could be relieved solely by the action of the lytic transglycosylase. However, its products are not found in significant levels associated with the germinated spores. It has been suggested that GSLEs may be amidases whose activity would lead to depolymerization of the cortex (10, 23). The remarkably low cross-linking of the spore cortex peptidoglycan (2.9% per muramic acid) would facilitate this process (2). Our study does not reveal direct evidence for amidase activity during germination in the form of amidase products. However, although the amount of cross-linked cortex material decreases during germination (70% of tetrasaccharide tetrapeptide tetrasaccharide tetrapeptide [muropeptide 19] is lost over 2 h), very low amounts are released in the germination exudate. Therefore, it is possible that amidase activity is occurring. The appearance of trisaccharide tetrapeptide suggests the activity of an _N_-acetylglucosaminidase during germination, although at a very low level. Such an activity has been previously shown to be associated with broken spores of B. subtilis (33), B. megaterium (13), and B. cereus (33). Although a germination-associated muramidase from C. perfringens has been characterized (4), there is no evidence for such an activity in B. subtilis. To determine the true hydrolytic bond specificity of the GSLE(s), it will be necessary to use purified enzyme and to monitor muropeptide changes associated with its activity on decoated, inactivated spores.
From the analysis of the dynamics of cortex structure during germination, it can be seen that cortex muropeptides containing muramic δ-lactam residues are lost from the spores at a higher rate than those without. Thus, the distribution of muropeptides in the cortex is likely to be heterogeneous. It may be that the muramic δ-lactam residue concentration is greatest in the outer regions of the cortex and thus hydrolysis would be initiated from this area, as the GSLE requires δ-lactam for its activity.
Muropeptides 1 and 8 are disaccharide tripeptide and disaccharide tripeptide disaccharide tetrapeptide, respectively, and their levels remain fairly constant throughout germination. They have been proposed to be part of the primordial cell wall which remains intact during germination, to become the basis of the new vegetative cell wall during outgrowth (2). It is possible that the primordial cell wall contains single l-alanine or tetrapeptide substitutions, but this has not been demonstrated. The primordial cell wall is more cross-linked (20%) than the cortex (2.9%), but it is the absence of the muramic δ-lactam residues which renders this polymer resistant to hydrolysis by GSLE(s), which cannot hydrolyze peptidoglycan without this determinant (2, 25).
Muropeptide analysis has revealed a hitherto unexpected degree of complexity in the mechanism of cortex hydrolysis during germination of B. subtilis endospores. We are currently studying structural dynamics during germination of endospores of other species to determine if the mechanism is generic. Identification of the enzymes responsible for the observed activities will allow their role, and how they are regulated as part of the germination trigger mechanism, to be determined.
ACKNOWLEDGMENTS
We thank A. Moir for provision of strains and R. Marquardt for the gift of Cellosyl.
This work was supported by the BBSRC (A.A.), the Royal Society (S.J.F.), the Fonds zur Förderung der wissenschaftlichen Forschung (MALDI MS, grant 11183 to G.A.), the European Community (HCM grant ERB CHRX CT940425), and the ARC Programme (UK/Austria travel fund).
REFERENCES
- 1.Anagnostopoulos C, Spizizen J. Requirements for transformation in Bacillus subtilis. J Bacteriol. 1961;81:741–746. doi: 10.1128/jb.81.5.741-746.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atrih A, Zöllner P, Allmaier G, Foster S J. Structural analysis of Bacillus subtilis 168 endospore peptidoglycan and its role during differentiation. J Bacteriol. 1996;178:6173–6183. doi: 10.1128/jb.178.21.6173-6183.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown K L. Spore resistance and ultra heat treatment processes. J Appl Bacteriol. 1994;76:67S–80S. doi: 10.1111/j.1365-2672.1994.tb04359.x. [DOI] [PubMed] [Google Scholar]
- 4.Chen Y, Miyata S, Makino S, Moriyama R. Molecular characterization of a germination-specific muramidase from Clostridium perfringens S40 spores and nucleotide sequence of the corresponding gene. J Bacteriol. 1997;179:3181–3187. doi: 10.1128/jb.179.10.3181-3187.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cleveland E F, Gilvarg C. Selective degradation of peptidoglycan from Bacillus megaterium spores during germination. In: Gerhardt P, Costilow R N, Sadoff H L, editors. Spores VI. Washington, D.C: American Society for Microbiology; 1975. pp. 458–464. [Google Scholar]
- 6.Ellar D J. Spore specific structures and their function. Symp Soc Gen Microbiol. 1978;28:295–334. [Google Scholar]
- 7.Engel H, Kazemier B, Keck W. Murein-metabolizing enzymes from Escherichia coli: sequence analysis and controlled overexpression of the slt gene, which encodes the soluble lytic transglycosylase. J Bacteriol. 1991;173:6773–6782. doi: 10.1128/jb.173.21.6773-6782.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foster S J, Johnstone K. The use of inhibitors to identify early events during Bacillus megaterium KM spore germination. Biochem J. 1986;237:565–870. doi: 10.1042/bj2370865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foster S J, Johnstone K. Purification and properties of a germination-specific cortex-lytic enzyme from spores of Bacillus megaterium KM. Biochem J. 1987;242:573–579. doi: 10.1042/bj2420573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foster S J, Johnstone K. Pulling the trigger, the mechanism of bacterial spore germination. Mol Microbiol. 1990;4:137–141. doi: 10.1111/j.1365-2958.1990.tb02023.x. [DOI] [PubMed] [Google Scholar]
- 11.Glauner B J. Separation and quantification of muropeptides with high-performance liquid chromatography. Anal Biochem. 1988;172:451–664. doi: 10.1016/0003-2697(88)90468-x. [DOI] [PubMed] [Google Scholar]
- 12.Höltje J V, Mirelman D, Sharon N, Schwarz U. Novel type of murein transglycosylase in Escherichia coli. J Bacteriol. 1975;124:1067–1076. doi: 10.1128/jb.124.3.1067-1076.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hsieh L K, Vary J C. Germination and peptidoglycan solubilization in Bacillus megaterium spores. J Bacteriol. 1975;123:463–470. doi: 10.1128/jb.123.2.463-470.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jacobs C, Frere J M, Normark S. Cytosolic intermediates for cell wall biosynthesis and degradation control inducible β-lactam resistance in Gram-negative bacteria. Cell. 1997;88:823–832. doi: 10.1016/s0092-8674(00)81928-5. [DOI] [PubMed] [Google Scholar]
- 15.Jacobs C, Huang L J, Bartowsky E, Normark S, Park J T. Bacterial cell wall recycling provides cytosolic muropeptides as effectors for β-lactamase induction. EMBO J. 1994;13:4684–4694. doi: 10.1002/j.1460-2075.1994.tb06792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacobs C, Joris B, Jamin M, Klarsov K, van Heijenoort J, Park J T, Normark S, Frere J M. AmpD, essential for both β-lactamase regulation and cell wall recycling, is a novel cytosolic N-acetylmuramyl-l-alanine amidase. Mol Microbiol. 1995;15:553–559. doi: 10.1111/j.1365-2958.1995.tb02268.x. [DOI] [PubMed] [Google Scholar]
- 17.Kitano K, Tuomanen E, Tomasz A. Transglycosylase and endopeptidase participate in the degradation of murein during autolysis of Escherichia coli. J Bacteriol. 1986;167:759–765. doi: 10.1128/jb.167.3.759-765.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Makino S, Ito N, Inoue T, Miyata S, Moriyama R. A spore-lytic enzyme released from Bacillus cereus spores during germination. Microbiology. 1994;140:1403–1410. doi: 10.1099/00221287-140-6-1403. [DOI] [PubMed] [Google Scholar]
- 19.Miyata S, Moriyama R, Miyahara N, Makino S. A gene (sleC) encoding a spore cortex-lytic enzyme from Clostridium perfringens S40 spore; cloning sequence analysis and molecular characterization. Microbiology. 1995;141:2643–2650. doi: 10.1099/13500872-141-10-2643. [DOI] [PubMed] [Google Scholar]
- 20.Miyata S, Moriyama R, Sugimoto K, Makino S. Purification and partial characterization of a spore cortex-lytic enzyme of Clostridium perfringens S40 spores. Biosci Biotechnol Biochem. 1995;59:514–515. doi: 10.1271/bbb.59.514. [DOI] [PubMed] [Google Scholar]
- 21.Moir A, Smith D A. The genetics of bacterial spore germination. Annu Rev Microbiol. 1990;44:531–553. doi: 10.1146/annurev.mi.44.100190.002531. [DOI] [PubMed] [Google Scholar]
- 22.Moriyama R, Hattori A, Miyata S, Kudoh S, Makino S. A gene (sleB) encoding a spore-lytic enzyme from Bacillus subtilis and response of the enzyme to l-alanine-mediated germination. J Bacteriol. 1996;178:6059–6063. doi: 10.1128/jb.178.20.6059-6063.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moriyama R, Kudoh S, Miyata S, Nonobe S, Hattori A, Makino S. A germination-specific spore cortex-lytic enzyme from Bacillus cereus spores: cloning and sequencing of the gene and molecular characterization of the enzyme. J Bacteriol. 1996;178:5330–5332. doi: 10.1128/jb.178.17.5330-5332.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Popham D L, Helin J, Costello C E, Setlow P. Analysis of the peptidoglycan structure of Bacillus subtilis endospores. J Bacteriol. 1996;178:6451–6458. doi: 10.1128/jb.178.22.6451-6458.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Popham D L, Helin J, Costello C E, Setlow P. Muramic lactam in peptidoglycan of Bacillus subtilis spores is required for spore outgrowth but not spore dehydration or heat resistance. Proc Natl Acad Sci USA. 1996;93:15405–15410. doi: 10.1073/pnas.93.26.15405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Preston R A, Douthit H A. Germination of Bacillus cereus spores—critical control by dl-alanine racemase. J Gen Microbiol. 1984;130:3123–3133. doi: 10.1099/00221287-130-5-1041. [DOI] [PubMed] [Google Scholar]
- 27.Romeis T, Vollmer W, Höltje J V. Characterization of three different lytic transglycosylases in Escherichia coli. FEMS Microbiol Lett. 1993;111:141–146. doi: 10.1111/j.1574-6968.1993.tb06376.x. [DOI] [PubMed] [Google Scholar]
- 28.Sekiguchi J, Akeo K, Yamamoto H, Khasanov F K, Alonso J C, Kuroda A. Nucleotide sequence and regulation of a new putative cell wall hydrolyase gene, cwlD, which affects germination in Bacillus subtilis. J Bacteriol. 1995;177:5582–5589. doi: 10.1128/jb.177.19.5582-5589.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tipper D J, Linnett P E. Distribution of peptidoglycan synthetase activities between sporangia and forespores in sporulating cells of Bacillus sphaericus. J Bacteriol. 1976;126:213–221. doi: 10.1128/jb.126.1.213-221.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Venkatasubramanian P, Johnstone K. Biochemical analysis of the Bacillus subtilis 1604 spore germination response. J Gen Microbiol. 1989;135:2723–2733. doi: 10.1099/00221287-135-10-2723. [DOI] [PubMed] [Google Scholar]
- 31.Vinter V. Commencement of synthetic activities of germinating bacterial spores and changes in vulnerability of cells during outgrowth. In: Campbell L L, Halvorson H O, editors. Spores III. Washington, D.C: American Society for Microbiology; 1965. pp. 25–37. [Google Scholar]
- 32.Warburg R J, Moir A, Smith D A. Influence of alkali metal cations on the germination of spores of wild-type and gerD mutants of Bacillus subtilis. J Gen Microbiol. 1985;131:221–230. [Google Scholar]
- 33.Warth A D. Action of spore lytic enzymes on the cortex. In: Halvorson H O, Hanson R, Campbell L L, editors. Spores V. Washington, D.C: American Society for Microbiology; 1972. pp. 28–34. [Google Scholar]
- 34.Wax R, Freese E. Initiation of the germination of Bacillus subtilis spores by a combination of compounds in place of l-alanine. J Bacteriol. 1968;95:433–438. doi: 10.1128/jb.95.2.433-438.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]