Deletion of a CD2-Like Gene, 8-DR, from African Swine Fever Virus Affects Viral Infection in Domestic Swine (original) (raw)
Abstract
An African swine fever virus (ASFV) gene with similarity to the T-lymphocyte surface antigen CD2 has been found in the pathogenic African isolate Malawi Lil-20/1 (open reading frame [ORF] 8-DR) and a cell culture-adapted European virus, BA71V (ORF EP402R) and has been shown to be responsible for the hemadsorption phenomenon observed for ASFV-infected cells. The structural and functional similarities of the ASFV gene product to CD2, a cellular protein involved in cell-cell adhesion and T-cell-mediated immune responses, suggested a possible role for this gene in tissue tropism and/or immune evasion in the swine host. In this study, we constructed an ASFV 8-DR gene deletion mutant (Δ8-DR) and its revertant (8-DR.R) from the Malawi Lil-20/1 isolate to examine gene function in vivo. In vitro, Δ8-DR, 8-DR.R, and the parental virus exhibited indistinguishable growth characteristics on primary porcine macrophage cell cultures. In vivo, 8-DR had no obvious effect on viral virulence in domestic pigs; disease onset, disease course, and mortality were similar for the mutant Δ8-DR, its revertant 8-DR.R, and the parental virus. Altered viral infection was, however, observed for pigs infected with Δ8-DR. A delay in spread to and/or replication of Δ8-DR in the draining lymph node, a delay in generalization of infection, and a 100- to 1,000-fold reduction in virus titers in lymphoid tissue and bone marrow were observed. Onset of viremia for Δ8-DR-infected animals was significantly delayed (by 2 to 5 days), and mean viremia titers were reduced approximately 10,000-fold at 5 days postinfection and 30- to 100-fold at later times; moreover, unlike in 8-DR.R-infected animals, the viremia was no longer predominantly erythrocyte associated but rather was equally distributed among erythrocyte, leukocyte, and plasma fractions. Mitogen-dependent lymphocyte proliferation of swine peripheral blood mononuclear cells in vitro was reduced by 90 to 95% following infection with 8-DR.R but remained unaltered following infection with Δ8-DR, suggesting that 8-DR has immunosuppressive activity in vitro. Together, these results suggest an immunosuppressive role for 8-DR in the swine host which facilitates early events in viral infection. This may be of most significance for ASFV infection of its highly adapted natural host, the warthog.
African swine fever (ASF) is a highly lethal and economically significant disease of domestic pigs for which there is no vaccine or disease control strategy other than animal quarantine and slaughter. The causative agent of ASF, a large enveloped double-stranded DNA virus (ASFV), is the sole member of an unnamed family of animal viruses (4, 12, 15). ASFV genomic organization and its cytoplasmic replication strategy suggest some relationship to the Poxviridae (17, 37, 54).
ASFV is the only known DNA arbovirus (4, 12, 15). In nature, the perpetuation and transmission of this virus involve the cycling of virus between two highly adapted hosts, Ornithodoros ticks and wild pig populations (warthogs and bushpigs) in sub-Saharan Africa (41, 42, 57, 63). In the warthog host, ASFV infection is subclinical, characterized by low-titer viremias (44, 56). An important aspect of this natural virus-host interaction is persistent infection, where virus persists in both ticks and wild pigs following infection (7, 13, 14, 51, 57).
In domestic pigs, ASF occurs in several disease forms, ranging from highly lethal to subclinical infections, depending on contributing viral and host factors which remain poorly understood (11, 27). ASFV infects cells of the mononuclear-phagocytic system, including highly differentiated fixed-tissue macrophages and reticular cells; affected tissues show extensive damage after infection with highly virulent viral strains (11, 24, 25, 27, 31). ASFV strains of lesser virulence also appear to infect these cell types, but the degree of tissue involvement and resulting tissue damage are much less severe (20, 27, 28). The abilities of ASFV to replicate and induce marked cytopathology in these cell types in vivo appear to be critical factors in ASFV virulence. Two ASFV genes, NL-S and UK, with functions involving virulence and host range have been identified in the European pathogenic isolate E70 (65, 66). While these genes are necessary for ASFV virulence, they alone are not sufficient, indicating that other viral determinants must play significant roles in viral virulence (65, 66).
An ASFV gene encoding a protein with similarity to the T-lymphocyte surface antigen CD2 has been found in the pathogenic African isolate Malawi Lil-20/1 (open reading frame [ORF] 8DR) and a cell culture-adapted European virus, BA71V (ORF EP402R) (3, 48, 64). The CD2 protein, which is expressed late in infection, has been shown to be both necessary (3, 48) and sufficient (3, 50) for mediating hemadsorption of swine erythrocytes to ASFV-infected cells. Deletion of the gene from the BA71V virus did not affect viral growth on Vero cell cultures (48).
CD2, a nonpolymorphic surface glycoprotein present on the surface of T lymphocytes and natural killer cells, plays an important role in augmenting both antigen-dependent and antigen-independent T-cell activation and in natural killer cell activity (1, 2, 26, 35, 59). The natural ligands for CD2 are CD58 (LFA-3), a surface glycoprotein present on most cell types which is responsible for the rosetting of sheep erythrocytes on the surface of T cells, and CD59, a membrane protein which inhibits cell lysis by human complement (47, 62). The CD2 protein has been shown to play an important physiological role in facilitating adhesion between T cells and antigen-presenting cells (APC) by specifically interacting with LFA-3, thus promoting T-cell recognition of foreign antigens presented by the major histocompatibility complex on APC (30). Blocking of this CD2-LFA3 interaction by free ligand, anti-CD2 antibodies, or soluble CD2 resulted in inhibition of a variety of T-cell functions (19, 26, 29, 35, 38, 46, 47, 52, 59).
The structural and functional similarities of the ASFV 8-DR gene product to CD2, a cellular protein involved in cell-cell adhesion and T-cell-mediated immune responses, suggested a possible role for this gene in tissue tropism and/or immune evasion in the swine host.
MATERIALS AND METHODS
Viruses and cell cultures.
The pathogenic African ASFV isolate Malawi Lil-20/1 was obtained from L. Dixon (Institute of Animal Health, Pirbright Laboratory, Woking, Surrey, United Kingdom). Primary porcine macrophage cell cultures were prepared from heparinized swine blood as previously described (16, 33).
Construction of ASFV recombinant virus Δ8-DR and its revertant 8-DR.R.
ASFV recombinant viruses were generated by homologous recombination between ASFV genomes and engineered recombination transfer vectors following infection/transfection of primary swine macrophages (33, 65). Flanking genomic regions mapping to the left (1,013 bp) and right (1,034 bp) of 8-DR were amplified by PCR using Malawi Lil-20/1 genomic DNA as a template. The left flanking region was amplified by using a primer pair (forward primer, 5′-ATTATTGCATGCTTGGTGCTATTACTC-3′; reverse primer, 5′-TTATTATCTCGAGATGCACATATGTTTT-3′) that introduced _Sph_I and _Xho_I restriction sites (underlined) at the 5′ and 3′ ends, respectively, of the fragment. The right flanking region was amplified by using a primer pair (forward primer, 5′-CATTACTCGAGCTTTCAAGTCGGT-3′; reverse primer, 5′-TAGCGGGCTGAATTCTAGGCC-3′) that introduced _Xho_I and _Eco_RI restriction sites at the 5′ and 3′ ends, respectively, of the fragment. Amplified fragments were digested with appropriate restriction enzymes, cloned into pUC19 to give pUC.1, and sequenced to verify ASFV sequences. The nucleotide sequences of cloned ASFV flanking regions were identical over their entire lengths to that of the template DNA used, purified Malawi Lil-20/1 genomic DNA. A reporter gene cassette containing the β-glucuronidase (GUS) gene with the ASFV p72 late gene promoter, p72GUS (33), was inserted into _Xho_I-digested pUC.1 to yield the transfer vector p72GUS Δ8-DR. This construction removed the complete 8-DR ORF with the exception of 9 bp at the amino terminus. Macrophage cell cultures were infected with Malawi Lil-20/1 and transfected with p72GUS Δ8-DR as described previously (33, 65). GUS-expressing recombinant viruses were detected in a plaque assay by overlaying cultures with 0.5% agarose containing 100 μg of X-Gluc (5-bromo-4-chloro-3-indolyl-β-d-glucuronic acid) per ml. Recombinant viruses were purified by six to eight rounds of plaque assay on macrophages and verified as products of a double-crossover recombination event using PCR and Southern blot analysis. A recombinant, Δ8-DR, was selected for further study. A 2,217-bp region of the Δ8-DR genome containing the genomic regions flanking the 8-DR gene deletion was amplified by PCR using purified viral genomic DNA, cloned into the TA cloning vector pCR II (Invitrogen, San Diego, Calif.), and sequenced to confirm the integrity of sequences surrounding the gene deletion. The left flanking region of 1,115 bp was amplified by using the forward primer 5′-TAGGCGCGGCAACATGTACTACTC-3′ (position −28 bp from _Sph_I site) and reverse primer 5′-TGACACGCTCTTGCTAGCAGA-3′ (position +74 bp from _Xho_I site). The right flanking region of 1,102 bp was amplified by using the forward primer 5′-TATCGCGCGCGGTGTCATCTATGT-3′ (position −36 bp from _Xho_I site) and reverse primer 5′-GTGCAATGGCTGCGTTGTAGCGAG-3′ (position +32 bp from _Eco_RI site). Three independent clones of each flanking region were sequenced in their entirety with an Applied Biosystems Inc. model 370A automated DNA sequencer as previously described (65).
A Δ8-DR revertant virus, 8-DR.R, was constructed in a similar fashion. Macrophages were infected with the deletion mutant Δ8-DR and then transfected with a recombinant plasmid containing a 3.07-kbp Malawi genomic fragment that included the intact 8-DR gene and adjacent flanking regions. Hemadsorption-positive revertant viruses were selected, purified in macrophage cell cultures by eight rounds of limited dilution, and characterized as described above.
Animal infections.
Yorkshire pigs (30 to 35 kg) were inoculated intramuscularly in the left rear leg with 102 50% tissue culture infective doses (TCID50) of the deletion mutant Δ8-DR, the revertant virus 8-DR.R, or the parental virus Malawi Lil-20/1. Three to five animals from each group were monitored throughout the disease course. Clinical signs of ASF infection, i.e., fever (a rectal temperature of greater than 40°C), anorexia, lethargy, shivering, cyanosis, and recumbency, were monitored daily. Blood samples were collected every other day for the course of the experiment. Virus isolation and titration of ASFV in blood or tissue samples were performed as previously described (36). Heparinized blood samples were fractionated into plasma, leukocyte, and erythrocyte components on Ficoll-Hypaque gradients and adjusted to the original volume prior to titration. Other randomly selected animals were euthanized at various times postinfection (p.i.) (three animals/time point/group), and tissue samples were collected for virus titration and in some cases in situ hybridization and histopathology. Tissues removed at necropsy were weighed and immediately frozen at −70°C. Tissue homogenates (10% suspensions in Dulbecco modified Eagle medium containing 10% fetal bovine serum) were clarified by low-speed centrifugation and then titrated on porcine macrophage cell cultures. Tissue samples for histopathology were fixed in 10% neutral buffered formalin, processed by standard paraffin procedures, and stained with hematoxylin and eosin.
In situ hybridization.
In situ hybridization was performed essentially as described previously (55). Tissue samples were fixed in periodate-lysine-paraformaldehyde fixative for 24 h at 4°C and embedded in paraffin. Tissue sections were deparaffinized with xylene, rehydrated in graded ethanols, pretreated with 0.2 N HCl for 20 min, and treated with proteinase K (10 μg/ml) in 10 mM Tris (pH 7.4)–2 mM CaCl2 for 20 min at 37°C. After incubation in 0.2 M dithiothreitol for 20 min, the sections were treated with 0.1 M iodoacetamide in acetic anhydride-triethanolamine buffer (pH 8.2) for 30 min at 37°C. Prior to hybridization, DNA was denatured by heating to 65°C in deionized formamide in 0.1× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) for 15 min and then quenched in ice-cold 0.1× SSC. Hybridization was performed at 42°C for 48 h by using 35S-labeled ASFV cosmid DNA probes (5 × 106 cpm) in a hybridization solution containing 2× SSC, 45% formamide, 10% dextran sulfate, 10 mM EDTA, 1× Denhardt solution (0.02% bovine serum albumin, 0.02% polyvinylpyrrolidone, 0.02% Ficoll), and 0.5 mg of calf thymus DNA per ml. After hybridization, sections were washed in 2× SSC–45% formamide–10 mM Tris (pH 7.4)–1 mM EDTA for 1 day at room temperature, coated with Kodak NTB-2 emulsion, exposed for 5 days at 4°C, developed, and stained with hematoxylin and eosin by using standard procedures. The number of positive cells in a tissue section was estimated by counting those found in 20 random fields at a magnification of ×250.
Lymphocyte proliferation assay.
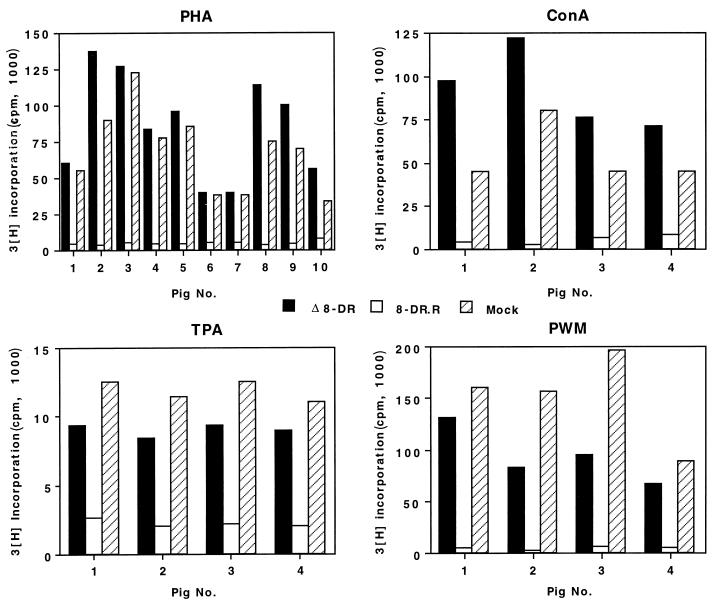
Peripheral blood mononuclear cells (PBMC) from naive pigs (n = 4 to 10) were obtained by Ficoll-Hypaque gradient centrifugation, washed twice in Dulbecco modified Eagle medium, and seeded in 96-well plates at a concentration of 105 cells/well. Cells were then immediately infected with Δ8-DR or 8-DR.R (multiplicity of infection [MOI] = 10), and treated with one of the following mitogens: phytohemagglutinin (PHA; 0.25 μg/ml), concanavalin A (ConA; 2.5 μg/ml), pokeweed mitogen (PWM; 0.1 μg/ml), and _O_-tetradecanoylphorbol-13-acetate (TPA; 0.01 μg/ml). At 72 h, cultures were pulse-labeled overnight with 1 μCi of [3H]thymidine per well and automatically harvested. Radioactivity incorporated was expressed as 103 cpm per well.
Ultrastructural analysis of Δ8-DR- and 8-DR.R-infected peripheral PBMC cultures.
PHA-treated PBMC cultures were infected with Δ8-DR or 8.DR.R (MOI = 10). At various times p.i., infected cells were gently scraped from culture dishes and resuspended in 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.4) for 1 h at 4°C. Cells were then washed twice with 0.1 M sodium cacodylate buffer (pH 7.4) containing 10% sucrose, postfixed (1% osmium tetroxide followed by 1% tannic acid), and stained in block overnight at 4°C with 2% aqueous uranyl acetate. The fixed cell pellet was embedded in 2% agarose, dehydrated in ethanol, and embedded in EM 812 epoxy resin (Electron Microscopy Sciences, Fort Washington, Pa.). Thin sections were collected on Formvar-coated, carbon-stabilized slot grids or uncoated 200 mesh grids and examined and photographed with a Philips 410 electron microscope operating at 80 kV.
RESULTS
Construction and analysis of Δ8-DR and 8-DR.R.
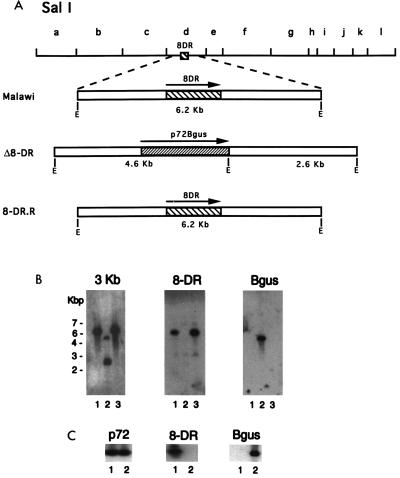
An ASFV 8-DR gene deletion mutant, Δ8-DR, was constructed from the pathogenic African isolate Malawi Lil-20/1 by homologous recombination between the parental genome and an engineered recombination transfer vector following infection/transfection of primary swine macrophage cell cultures as described in Materials and Methods. Sequence analysis of Δ8-DR indicated that the deletion introduced into the Malawi Lil-20/1 genome removed 1,146 bp which included the complete 8-DR ORF with the exception of 9 bp at the amino terminus and 30 bp of 3′ flanking sequence and inserted in its place a 2.4-kb p72GUS reporter gene cassette. No other nucleotide changes were found in flanking genomic regions of Δ8-DR. A revertant of Δ8-DR, 8-DR.R, was constructed as described in Materials and Methods. Genomic DNA from Δ8-DR and 8-DR.R was analyzed by Southern and PCR analysis. Viral DNA purified from infected macrophage cell cultures was digested with _Eco_RI, gel electrophoresed, Southern blotted, and hybridized with 32P-labeled DNA probes. As expected, an 8-DR gene probe failed to hybridize with genomic DNA from Δ8-DR (Fig. 1B, lane 2). Novel _Eco_RI fragments with predicted sizes of 4.6 and 2.6 kbp were observed for Δ8-DR when probed with a 3.0-kbp genomic fragment of Malawi Lil-20/1 which included the 8-DR gene region and flanking sequences (Fig. 1B, lane 2). A GUS gene probe hybridized only with DNA from Δ8-DR, recognizing the novel 4.6-kb _Eco_RI fragment (Fig. 1B, lane 2). The revertant virus 8-DR.R exhibited the parental genomic structure (Fig. 1B, lanes 1 and 3). PCR analysis failed to detect any parental virus in Δ8-DR virus stocks (Fig. 1C, lane 2). As expected, and unlike macrophages infected with 8-DR.R or the parental virus, macrophages infected with Δ8-DR failed to hemadsorb swine erythrocytes (data not shown). These data indicate the deletion mutant Δ8-DR and its revertant 8-DR.R were of the expected genomic structure and free of contaminating parental virus.
FIG. 1.
Characterization of Δ8-DR and 8-DR.R. (A) Diagram of the 8-DR gene regions in the parental Malawi Lil-20/1 isolate, the deletion mutant virus Δ8-DR, and its revertant 8-DR.R. (B) Southern blot analysis of Malawi Lil-20/1 (lane 1), Δ8DR (lane 2), and 8-DR.R (lane 3). Purified viral DNAs were digested with _Eco_RI, electrophoresed, blotted, and hybridized with a 3.0-kbp probe including 8-DR gene sequences and flanking regions on either side (3 kb), an 8-DR gene probe (8-DR), and a GUS gene probe (Bgus). (C) PCR analysis of 8-DR.R (lane 1) and Δ8-DR (lane 2) viral DNAs for p72, 8-DR, and GUS sequences.
8-DR is nonessential for growth of ASFV in porcine macrophage cell cultures in vitro.
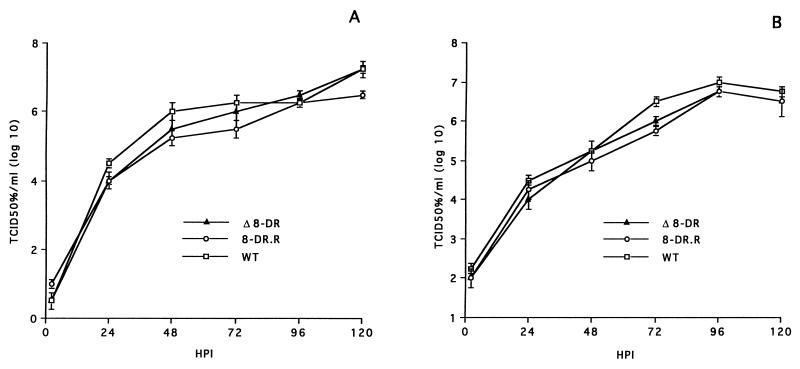
Growth characteristics of Δ8-DR were compared to those of 8-DR.R and parental Malawi Lil-20/1 by infecting primary macrophage cultures (MOI = 0.01) and determining titers both cell-associated and extracellular virus at various times p.i. The three viruses exhibited indistinguishable growth kinetics and viral yields (Fig. 2).
FIG. 2.
Growth characteristics of ASFV Malawi Lil-20/1, Δ8-DR, and 8-DR.R viruses in primary swine macrophages infected with each virus at an MOI of 0.01. At indicated times, duplicate samples were collected and titrated for intracellular (A) and extracellular (B) virus yield. Data represent means and standard errors of two independent experiments.
8-DR affects ASFV infection in the domestic swine host.
To examine the role of 8-DR in viral pathogenesis and virulence, Yorkshire pigs were inoculated intramuscularly with 102 TCID50 of either Δ8-DR or wild-type Malawi Lil-20/1 (experiment 1) or Δ8-DR and 8-DR.R (experiment 2) (102 TCID50 of Malawi Lil-20/1 represents a challenge dose of between 10 and 100 100% lethal doses). Results of these experiments are shown in Table 1. No differences in disease onset, disease course, or mortality were observed for groups of animals infected with the parental or recombinant virus; all animals presented with clinical disease 3 to 4 days p.i. (dpi) and died 7 to 11 dpi. Δ8-DR-infected animals did, however, exhibit altered patterns of viremia (Table 1). With Δ8-DR-infected pigs, onset of detectable viremia was significantly delayed, by 2 to 5 days (P = 0.004). Mean viremia titers for Δ8-DR-infected animals were reduced significantly, approximately 10,000-fold at 5 dpi and 30- to 100-fold at all later time points. Fractionation of infected blood into erythrocytes, leukocytes, and plasma prior to virus titration revealed that unlike 8-DR.R, where approximately 90 to 99% of virus was erythrocyte associated, Δ8-DR was equally distributed among the three fractions (Fig. 3). This result indicates that 8-DR is necessary for the erythrocyte-associated viremia seen with ASFV infection.
TABLE 1.
Swine survival, viremia, and fever response following infection with Malawi Lil-20/1, Δ8DR, and 8-DR.R
| Virus | Days to death | Fever, days to onset | Viremia (no. of viremic animals)a | |||||
|---|---|---|---|---|---|---|---|---|
| Days to onset | Mean titer log10/TCID50/ml at dpi: | |||||||
| 1 | 3 | 5 | 7 | 9 | ||||
| Expt 1 (n = 3) | ||||||||
| Malawi | ||||||||
| Lil-20/1 | 7.0 ± 0.0 | 3.0 ± 0.0 | 3.0 ± 0.0 | Negative | 7.4 ± 0.4 (3) | 8.8 ± 0.3 (3) | ||
| Δ8-DR | 8.3 ± 0.3 | 4.3 ± 0.9 | 5.0 ± 0.0 | Negative | Negative | 4.1 ± 0.4(a) (3) | 6.1 ± 0.8 (3) | 8.3 ± 0.0 (1) |
| Expt 2 (n = 5) | ||||||||
| 8-DR.R | 9.6 ± 0.8 | 2.8 ± 0.6 | 2.2 ± 0.5 | 3.1 ± 0.4 (2) | 4.9 ± 0.5 (5) | 6.9 ± 0.1 (5) | 7.3 ± 0.1 (4) | 7.0 ± 0.5 (2) |
| Δ8-DR | 10.3 ± 0.3 | 3.4 ± 0.7 | 6.2 ± 0.5(b) | Negative | Negative | 3.4 ± 0.1(c) (2) | 4.6 ± 0.2(c) (5) | 5.6 ± 0.3(d) (4) |
FIG. 3.
8-DR is necessary for erythrocyte-associated viremia. Shown are titers (mean values from five animals) of Δ8-DR and 8-DR.R viruses in whole blood, plasma, PBMC, and erythrocytes (RBC) from pigs at various times.
For a more detailed comparison of infection with Δ8-DR and 8-DR.R, randomly selected animals infected with 102 TCID50 were euthanized at 2, 4, 6, and 8 dpi (three animals/time point/group), and tissue samples were collected for virus titration and in some cases in situ hybridization and histopathology. Virus titration results from this experiment are shown in Table 2. At 2 dpi, Δ8-DR was isolated only from the draining internal iliac lymph node at titers that were 1,000-fold lower than those observed for 8-DR.R-infected animals. At this time, generalization of infection had already occurred in all three 8-DR.R-infected animals; virus was isolated from blood, spleen, and liver. By 4 dpi, generalized infection was observed for Δ8-DR-infected animals. At this and all later time points, Δ8-DR titers in spleen, submandibular lymph node, and bone marrow were significantly (approximately 100-fold) lower than 8-DR.R values. At 6 and 8 dpi, viral titers of liver, lung, and, interestingly, iliac lymph node were comparable for the two groups.
TABLE 2.
Viral titers in tissues following infection with Δ8-DR and 8-DR.R
| Tissue | dpi | Log10/TCID50/g | P | |
|---|---|---|---|---|
| Δ8-DR | 8-DR.R | |||
| Blood | 2 | <2.0 | 3.0 ± 0.1 | |
| 4 | 2.8 ± 0.3 | 6.7 ± 0.1 | 0.0002 | |
| 6 | 3.6 ± 0.4 | 7.4 ± 0.7 | 0.008 | |
| 8 | 4.8 ± 0.3 | 7.0 ± 0.0 | 0.006 | |
| Iliac lymph node | 2 | 3.1 ± 0.5 | 6.3 ± 0.1 | 0.0004 |
| 4 | 4.0 ± 1.0 | 5.4 ± 0.1 | ||
| 6 | 6.7 ± 0.5 | 7.1 ± 0.2 | ||
| 8 | 6.7 ± 0.3 | 6.8 ± 0.6 | ||
| Spleen | 2 | <2.0 | 4.1 ± 0.2 | 0.0004 |
| 4 | 4.9 ± 0.4 | 6.8 ± 0.2 | 0.015 | |
| 6 | 5.3 ± 0.3 | 6.9 ± 0.2 | 0.012 | |
| 8 | 5.7 ± 0.2 | 7.8 ± 0.3 | 0.005 | |
| Lung | 2 | <2.0 | <2.0 | |
| 4 | 3.3 ± 0.8 | 5.8 ± 0.2 | 0.027 | |
| 6 | 5.6 ± 0.5 | 5.8 ± 0.2 | ||
| 8 | 4.8 ± 0.4 | 6.1 ± 0.1 | ||
| Liver | 2 | <2.0 | 2.3 ± 0.3 | |
| 4 | 4.0 ± 1.0 | 6.5 ± 0.6 | ||
| 6 | 5.4 ± 0.3 | 6.1 ± 0.0 | ||
| 8 | 5.3 ± 0.1 | 6.3 ± 0.3 | ||
| Submandibular lymph node | 2 | <2.0 | <2.0 | |
| 4 | 2.9 ± 0.5 | 5.0 ± 0.3 | 0.02 | |
| 6 | 5.6 ± 0.2 | 5.6 ± 0.2 | ||
| 8 | 5.5 ± 0.0 | 7.5 ± 0.0 | 0.002 | |
| Bone marrow | 2 | <2.0 | <2.0 | |
| 4 | 2.8 ± 0.8 | 5.2 ± 0.2 | 0.03 | |
| 6 | 3.2 ± 0.2 | 5.8 ± 0.4 | 0.018 | |
| 8 | 4.3 ± 0.3 | 5.9 ± 0.1 | 0.03 |
There was no significant difference between histologic changes present in sections of internal iliac lymph node and spleen from pigs infected with Δ8-DR and 8-DR.R. Changes in the iliac lymph node at 4 dpi included distention of sinusoids, hypocellularity with clear spaces surrounding most cells of the cortex, an increased number of large lymphocytes and mitotic figures in the cortex, and scattered foci of necrosis. These observations were more pronounced at later time points. In agreement with the virus titration data, large numbers of macrophage-like cells positive by in situ hybridization for ASFV were present in the medulla and paracortical regions (490 ± 285 positive cells per sampled area), with a few positive cells in follicles and perifollicular regions of 8-DR.R-infected nodes at 2 dpi, but were virtually absent from nodes of Δ8-DR-infected animals (3 ± 3 positive cells per sampled area) (Fig. 4). Highly variable numbers of positive cells were present in medullary and paracortical regions of both Δ8-DR- and 8-DR.R-infected nodes at later time points. In spleen, a generally progressive depletion of mononuclear cells in the red pulp was evident for both viruses. Small foci of loss of cellular detail with pyknosis and karyorrhexis were present in mononuclear cells of the red pulp and within sheathed arterioles and germinal centers on 6 to 8 dpi. Cells positive by in situ hybridization for ASFV first appeared in small numbers in the red pulp at 2 dpi for 8-DR.R and at 4 dpi for Δ8-DR. Numbers of positive cells in the red pulp increased progressively to 8 dpi, with distinctly more positive cells in spleens from 8-DR.R-infected pigs.
FIG. 4.
Detection of Δ8-DR and 8-DR.R viruses in the draining internal iliac lymph node at 2 dpi by in situ hybridization. Magnification, ×450.
These data indicate that infection with Δ8-DR is characterized by a delay in spread to and/or replication of Δ8-DR in the draining lymph node, a delay in generalization of infection, and a 100- to 1,000-fold reduction in virus titers in lymphoid tissue and bone marrow.
8-DR inhibits mitogen-dependent lymphocyte proliferation in virus-infected swine PBMC cultures.
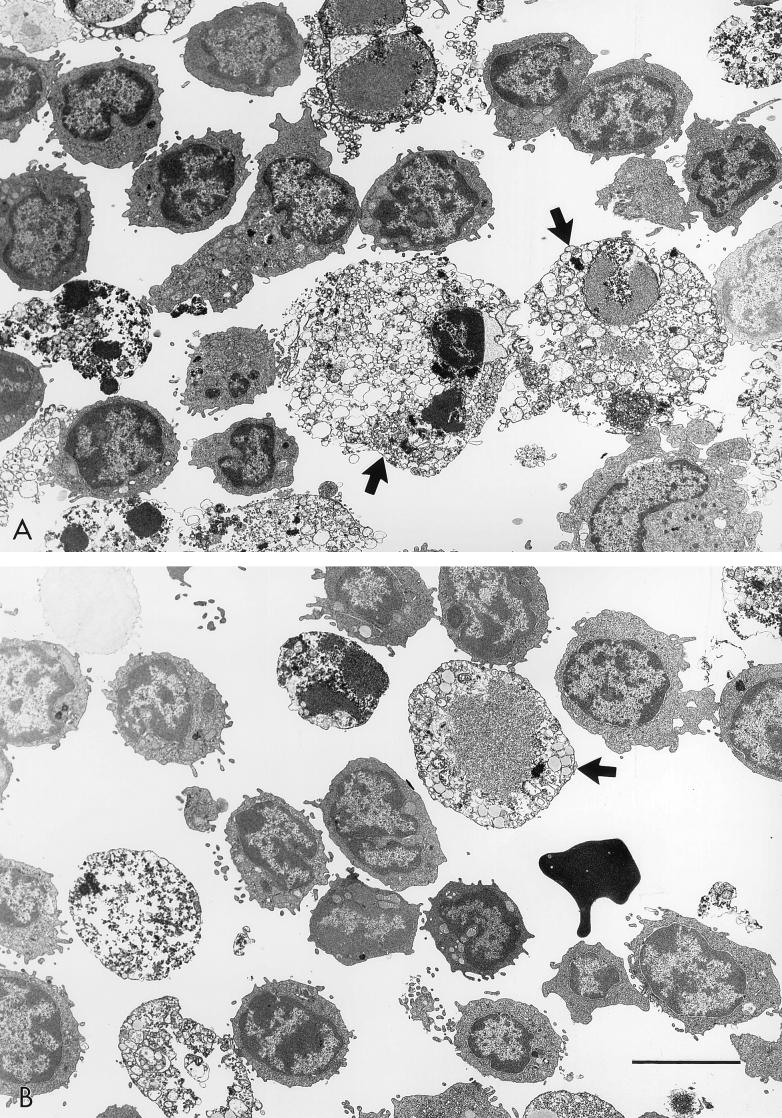
The observations described above for Δ8-DR-infected pigs together with the known function of CD2 in T-cell-mediated immune responses suggested that 8DR may have an immune evasion function in the swine host. To examine this, we performed mitogen-dependent lymphocyte proliferation assays of PBMC cultures infected with Δ8-DR and 8-DR.R. The mitogens PHA, ConA, PWM, and TPA were used. PHA, ConA, and PWM stimulate T-cell proliferation (PWM also induces proliferation of B cells under some conditions) and require the presence of macrophages in the culture to provide an accessory cell function (22, 23), while TPA, although still influenced by accessory cell function, acts more directly on the lymphocyte. Representative results of five to seven independent experiments are shown in Fig. 5. PBMC cultures infected with 8-DR.R exhibited a 90 to 95% reduction in mitogen-induced proliferation, while proliferative activity in Δ8-DR-infected cultures was unaffected and indistinguishable from that in mock-infected mitogen-stimulated PBMC cultures. This inhibition was observed with all tested mitogens in 8-DR.R-infected cultures. PHA-stimulated PBMC cultures infected with Δ8-DR and 8-DR.R were examined by electron microscopy at 48, 72, and 96 h p.i. Consistent with the in vitro growth characteristics of Δ8-DR and 8-DR.R described above, there was no difference between the viruses in kinetics of replication as judged by the appearance of virus factories, and the percentages of infected cells at all time points were comparable. Approximately 100% of all monocyte/macrophage cells showed evidence of virus infection at 24 h p.i., with extensive cytopathology evident at 72 h p.i. (Fig. 6). Ultrastructural evidence of virus infection and/or replication in lymphocytes was not observed in cultures infected with either virus.
FIG. 5.
Mitogen-induced proliferation in PBMC cultures infected with Δ8-DR and 8-DR.R. PBMC (105/ml) from 4 to 10 naive swine were either infected (MOI = 10) with Δ8-DR or 8-DR.R or mock infected in the presence of the mitogen PHA, ConA, PWM, or TPA for 3 days and then pulsed with 1 μCi of [3H]thymidine per ml for 16 h. Bars indicate mean values of five replicates.
FIG. 6.
Electron micrographs of PHA-treated PBMC cultures infected with 8-DR.R (A) and Δ8-DR (B) at 72 h p.i. Note the extensive macrophage cytopathology (arrows) and the normal appearance of lymphocytes in cultures infected with both viruses. The bar represents 5 μm.
Thus, 8-DR is necessary for inhibition of mitogen-dependent lymphocyte proliferation observed for ASFV-infected PBMC cultures, and this effect does not appear to involve altered viral replication and/or cell tropism.
DISCUSSION
Here, we have shown that deletion of 8-DR from the ASFV Malawi Lil-20/1 genome did not affect disease onset, disease course, or mortality (Table 1). This finding indicates that the gene is nonessential for acute disease and viral virulence in domestic swine. Consistent with this observation are prior data indicating a lack of correlation between hemadsorption and pig virulence; relatively avirulent hemadsorbing isolates and nonhemadsorbing pathogenic ASFV isolates have been described elsewhere (9, 10, 39, 60).
An altered pattern of viral infection was, however, observed for Δ8-DR-infected animals. A delay in spread to and/or replication in the draining iliac lymph node, a delay in generalization of infection with viremia no longer erythrocyte-associated, and a 100- to 1,000-fold reduction in virus titers in lymphoid tissue and bone marrow were observed for pigs infected with the 8-DR deletion mutant Δ8-DR. It is unlikely that the decreased level of Δ8-DR replication in tissues is due to alteration of cell tropism. Δ8-DR exhibited normal growth characteristics in macrophages in vitro (Fig. 2), and there was a similar pattern, although at reduced levels, of tissue involvement as evidenced by histopathology and localization of ASFV-infected cells by in situ hybridization. The most plausible explanation for this defect is a block which prevents efficient virus replication in these tissues. The observed delay in generalization of Δ8-DR infection is most likely a direct consequence of this early replication defect. What role, if any, an erythrocyte-associated viremia may play in increasing virus half-life in blood or virus infectivity in tissues is unknown. While viremia with many pathogenic ASFV isolates is largely erythrocyte associated (90 to 99% of viremia), there are examples of highly pathogenic hemadsorbing viruses where relatively high titer viremias are maintained without this high degree of erythrocyte association (40), as well as examples of pathogenic nonhemadsorbing ASFV isolates (10, 39).
Interestingly, and unlike infection with 8-DR.R, infection of PBMC cultures with Δ8-DR had no inhibitory effect on mitogen-dependent lymphocyte proliferation (Fig. 5). This lack of effect was not the result of altered virus replication in macrophages or altered lymphocyte tropism. 8-DR.R and Δ8-DR demonstrated similar patterns of virus replication in macrophages (Fig. 2 and 6), and neither virus appeared to infect and/or replicate in lymphocytes present in PBMC cultures (Fig. 6). The lack of lymphocyte susceptibility to ASFV infection has been described previously: in vitro, resting or mitogen-stimulated B and T cells were not susceptible to infection (8), and no evidence of lymphocyte infection was observed in lymph nodes of ASFV-infected swine (32). These observations suggest that 8-DR mediates this inhibition via an immunosuppressive mechanism.
Significant inhibition of lectin-dependent lymphocyte proliferation in PBMC cultures infected with ASFV or those incubated in the presence of virus-free infected cell extracts has been previously described (5, 6, 18, 61). While the nature of the responsible inhibitory factor(s) is unknown, it appears to be a soluble protein with a molecular mass estimated to be between 40 and 80 kDa (6, 18). Notably, a soluble hemagglutinin, made up of 51-kDa monomers, has been identified in the medium of cultures infected with some ASFV isolates (49). 8-DR, with a predicted molecular mass of 42 kDa, may be both the soluble inhibitory factor and the hemagglutinin identified previously in ASFV-infected PBMC cultures (18, 49).
Although CD2 functions only as an adhesion molecule on T cells, interacting with its natural ligand LFA-3, blocking this interaction with soluble ligand, anti-CD2 antibodies, or soluble CD2 resulted in inhibition of a variety of T-cell functions, including antigen-specific cytotoxicity, mitogen-induced and antigen-specific lymphocyte proliferation, interleukin-2 secretion, and interleukin-2 receptor expression (19, 26, 29, 35, 38, 46, 47, 52, 59). Soluble CD2 effectively inhibited T-cell proliferative responses to several bacterial and viral antigens as well as inhibiting reactivity to alloantigens in mixed lymphocyte cultures (46). A secreted or released form of ASFV 8-DR from infected cells could conceivably mimic or compete with host CD2, resulting in inhibition of T-cell function. Additionally, 8-DR could conceivably have an immunomodulatory role that effectively involves sequestration of LFA-3 molecules within the infected macrophage that prevents membrane presentation, thus interfering with macrophage (or other APC)-T cell interactions.
In nature, perpetuation of ASFV involves the cycling of virus between two highly adapted hosts, Ornithodoros ticks and warthogs or bushpigs, in sub-Saharan Africa (41, 57, 63). In the warthog host, acute ASFV infection is subclinical and characterized by low-titer viremias (44, 56). This high degree of virus-host adaptation may necessitate viral immune evasion strategies that will ensure that sufficient levels of viral replication occur in the warthog and that resulting viremias are high enough to infect new populations of feeding ticks. Given that 8-DR gene sequences have been selected for under these natural conditions and that the gene is conserved among field isolates, it is possible that the protein performs a host range function involving immune evasion in the warthog host. Additionally, persistent infection of warthogs with ASFV has been reported. In ASFV enzootic areas adult warthogs are typically nonviremic, although most are seropositive and virus can usually be isolated only from lymph nodes (21, 41, 43, 53, 58). Conceivably, 8-DR could have an immune evasion function here that permits continued virus replication and persistence.
Interestingly, the ASFV genome contains a second gene that also may be involved in immune modulation and evasion in the warthog. A highly conserved gene, 5-EL, with similarity to the gene for cellular inhibitor of NF-κB, has been described and shown to be capable of downregulating NF-κB-regulated gene expression in vitro (45). We have recently demonstrated, using a 5-EL gene deletion mutant of Malawi Lil-20/1, that the gene does not affect acute disease or viral virulence in domestic swine (34).
In summary, the results reported here suggest an immunosuppressive role for 8-DR in the swine host which facilitates early events in viral replication and generalization of infection. This effect may be of most significance for ASFV infection of its highly adapted natural host, the warthog.
ACKNOWLEDGMENTS
We thank R. Mireles, A. Zsak, J. R. Emmanuelli, and the PIADC animal care staff for excellent technical assistance.
REFERENCES
- 1.Beyers A D, Barclay A N, Law D A, He Q, Williams A F. Activation of T lymphocytes via monoclonal antibodies against rat cell surface antigens with particular reference to CD2 antigen. Immunol Rev. 1989;111:59–77. doi: 10.1111/j.1600-065x.1989.tb00542.x. [DOI] [PubMed] [Google Scholar]
- 2.Bierer B E, Burakoff S J. T-lymphocyte activation: the biology and function of CD2 and CD4. Immunol Rev. 1989;111:267–294. doi: 10.1111/j.1600-065x.1989.tb00549.x. [DOI] [PubMed] [Google Scholar]
- 3.Borca M V, Kutish G F, Afonso C L, Irusta P, Carrillo C, Brun A, Sussman M, Rock D L. An African swine fever virus gene with similarity to the T-lymphocyte surface antigen CD2 mediates hemadsorption. Virology. 1994;199:463–468. doi: 10.1006/viro.1994.1146. [DOI] [PubMed] [Google Scholar]
- 4.Brown F. The classification and nomenclature of viruses: summary of results of meetings of the International Committee on Taxonomy of Viruses in Sendai, September 1984. Intervirology. 1986;25:141–143. doi: 10.1159/000150091. [DOI] [PubMed] [Google Scholar]
- 5.Canals A, Alonso F, Tomillo J, Domínguez J. Analysis of T lymphocyte subsets proliferating in response to infective and UV-inactivated African swine fever virus. Vet Microbiol. 1992;33:117–127. doi: 10.1016/0378-1135(92)90040-z. [DOI] [PubMed] [Google Scholar]
- 6.Canals A, Domínguez J, Tomillo J, Babín M, Alonso F. Inhibition of IL-2R and SLA class II expression on stimulated lymphocytes by a suppressor activity found in homogenates of African swine fever virus infected cultures. Arch Virol. 1995;140:1075–1085. doi: 10.1007/BF01315416. [DOI] [PubMed] [Google Scholar]
- 7.Carrillo C, Borca M V, Afonso C L, Onisk D V, Rock D L. Long-term persistent infection of swine monocytes/macrophages with African swine fever virus. J Virol. 1994;68:580–583. doi: 10.1128/jvi.68.1.580-583.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Casal I, Enjuanes L, Viñuela E. Porcine leukocyte cellular subsets sensitive to African swine fever virus in vitro. J Virol. 1984;52:37–46. doi: 10.1128/jvi.52.1.37-46.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coggins L. Segregation of a nonhemadsorbing African swine fever virus in tissue culture. Cornell Vet. 1968;58:12–20. [PubMed] [Google Scholar]
- 10.Coggins L, Moulton J E, Colgrove G S. Studies with hinde attenuated African swine fever virus. Cornell Vet. 1968;58:525–540. [PubMed] [Google Scholar]
- 11.Colgrove G S, Haelterman E O, Coggins L. Pathogenesis of African swine fever in young pigs. Am J Vet Res. 1969;30:1343–1359. [PubMed] [Google Scholar]
- 12.Costa J V. African swine fever virus. In: Darai G, editor. Molecular biology of iridoviruses. Norwell, Mass: Kluwer Academic Publishers; 1990. pp. 247–270. [Google Scholar]
- 13.DeKock G, Robinson E M, Keppel J J G. Swine fever in South Africa. Onderstepoort J Vet Sci Anim Ind. 1994;14:31–93. [Google Scholar]
- 14.DeTray D E. Persistence of viremia and immunity in African swine fever. Am J Vet Res. 1957;18:811–816. [PubMed] [Google Scholar]
- 15.Dixon, L. K., D. L. Rock, and E. Viñuela. 1995. African swine fever-like viruses. Arch. Virol. 10(Suppl.)**:**92–94.
- 16.Genovesi E V, Villinger F, Gerstner D J, Whyard T C, Knudsen R C. Effect of macrophage-specific colony-stimulating factor (CSF-1) on swine monocyte/macrophage susceptibility to in vitro infection by African swine fever virus. Vet Microbiol. 1990;25:153–176. doi: 10.1016/0378-1135(90)90074-6. [DOI] [PubMed] [Google Scholar]
- 17.González A, Talavera A, Almendral J M, Viñuela E. Hairpin loop structure of African swine fever virus DNA. Nucleic Acids Res. 1986;14:6835–6844. doi: 10.1093/nar/14.17.6835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.González S, Mendoza C, Sánchez-Vizcaino J M, Alonso F. Inhibitory effect of African swine fever virus on lectin-dependent swine lymphocyte proliferation. Vet Immunol Immunopathol. 1990;26:71–80. doi: 10.1016/0165-2427(90)90133-d. [DOI] [PubMed] [Google Scholar]
- 19.Guckel B, Berek C, Lutz M, Altevogt P, Schirrmacher V, Kyewski B A. Anti-CD2 antibodies induce T cell unresponsiveness in vivo. J Exp Med. 1991;174:957–967. doi: 10.1084/jem.174.5.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hess W R. African swine fever: a reassessment. Adv Vet Sci Comp Med. 1982;25:39–69. [PubMed] [Google Scholar]
- 21.Heuschele W P, Coggins L. Epizootiology of African swine fever in warthogs. Bull of Epizoot Dis Afr. 1969;17:179–183. [PubMed] [Google Scholar]
- 22.Kern J A, Daniele R P, Nowell P C. Accessory cells provide more than one signal for lectin mitogen-stimulated proliferation of human lymphocytes. J Leukocyte Biol. 1985;38:495–507. doi: 10.1002/jlb.38.4.495. [DOI] [PubMed] [Google Scholar]
- 23.Kern J A, Reed J C, Daniele R P, Nowell P C. The role of the accessory cell in mitogen-stimulated human T cell gene expression. J Immunol. 1986;137:764–769. [PubMed] [Google Scholar]
- 24.Konno S, Taylor W D, Dardiri A H. Acute African swine fever. Proliferative phase in lymphoreticular tissue and the reticuloendothelial system. Cornell Vet. 1971;61:71–84. [PubMed] [Google Scholar]
- 25.Konno S, Taylor W D, Hess W R, Heuschele W P. Liver pathology in African swine fever. Cornell Vet. 1971;61:125–150. [PubMed] [Google Scholar]
- 26.Krensky A M, Sanchez-Madrid F, Robbins E, Nagy J A, Springer T A, Burakoff S J. The functional significance, distribution, and structure of LFA-1, LFA-2, and LFA-3: cell surface antigens associated with CTL-target interactions. J Immunol. 1983;131:611–616. [PubMed] [Google Scholar]
- 27.Mebus C A. African swine fever. Adv Virus Res. 1988;35:251–269. doi: 10.1016/s0065-3527(08)60714-9. [DOI] [PubMed] [Google Scholar]
- 28.Mebus C A, McVicar J W, Dardiri A H. Comparison of the pathology of high and low virulence African swine fever virus infections. In: Wilkinson P J, editor. Proceedings of CEC/FAO expert consultation in African swine fever research, Sardinia, Italy, September 1981. Luxemburg, Belgium: Commission of the European Communities; 1981. pp. 183–194. [Google Scholar]
- 29.Miller G T, Hochman P S, Meier W, Tizard R, Bixler S A, Rosa M D, Wallner B P. Specific interaction of lymphocyte function-associated antigen 3 with CD2 can inhibit T cell responses. J Exp Med. 1993;178:211–222. doi: 10.1084/jem.178.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moingeon P, Chang H-C, Wallner B P, Stebbins C, Frey A Z, Reinherz E L. CD2-mediated adhesion facilitates T lymphocyte antigen recognition function. Nature (London) 1989;339:312–314. doi: 10.1038/339312a0. [DOI] [PubMed] [Google Scholar]
- 31.Moulton J, Coggins L. Comparison of lesions in acute and chronic African swine fever. Cornell Vet. 1968;58:364–388. [PubMed] [Google Scholar]
- 32.Moura Nunes J F, Nunes-Petisca J L. Replication of African swine fever virus in lymph nodes of experimentally infected swine. In: Wilkinson P J, editor. ASF, EUR 8466 EN, proceedings of CEC/FAO Research Seminar, Sardinia, Italy, September 1981. Luxemburg, Belgium: Commission of the European Communities; 1983. pp. 132–142. [Google Scholar]
- 33.Neilan J G, Lu Z, Kutish G F, Zsak L, Burrage T G, Borca M V, Carrillo C, Rock D L. A BIR motif containing gene of African swine fever virus, 4CL, is nonessential for growth in vitro and viral virulence. Virology. 1997;230:252–264. doi: 10.1006/viro.1997.8481. [DOI] [PubMed] [Google Scholar]
- 34.Neilan J G, Lu Z, Kutish G F, Zsak L, Lewis T L, Rock D L. A conserved African swine fever virus IκB homolog, 5EL, is nonessential for growth in vitro and virulence in domestic pigs. Virology. 1997;235:377–385. doi: 10.1006/viro.1997.8693. [DOI] [PubMed] [Google Scholar]
- 35.O’Flynn K, Russul-Saib M, Ando I, Wallace D L, Beverley P C L, Boylston A W, Linch D C. Different pathways of human T-cell activation revealed by PHA-P and PHA-M. Immunology. 1986;57:55–60. [PMC free article] [PubMed] [Google Scholar]
- 36.Onisk D V, Borca M V, Kutish G, Kramer E, Irusta P, Rock D L. Passively transferred African swine fever virus antibodies protect swine against lethal infection. Virology. 1994;198:350–354. doi: 10.1006/viro.1994.1040. [DOI] [PubMed] [Google Scholar]
- 37.Ortin J, Enjuanes L, Viñuela E. Cross-links in African swine fever virus DNA. J Virol. 1979;31:579–583. doi: 10.1128/jvi.31.3.579-583.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Palacios R, Martinez-Maza O. Is the E receptor on human T lymphocytes a “negative signal receptor”? J Immunol. 1982;129:2479–2485. [PubMed] [Google Scholar]
- 39.Pini A, Wagenaar G. Isolation of a non-haemadsorbing strain of African swine fever (ASF) virus from a natural outbreak of the disease. Vet Rec. 1974;94:2. doi: 10.1136/vr.94.1.2. [DOI] [PubMed] [Google Scholar]
- 40.Plowright W, Parker J, Staple R F. The growth of a virulent strain of African swine fever virus in domestic pigs. J Hyg (Cambridge) 1968;66:117–134. doi: 10.1017/s0022172400040997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Plowright W, Parker J, Pierce M A. The epizootiology of African swine fever in Africa. Vet Rec. 1969;85:668–674. [PubMed] [Google Scholar]
- 42.Plowright W, Parker J, Pierce M A. African swine fever virus in ticks (Ornithodoros moubata, Murray) collected from animal burrows in Tanzania. Nature (London) 1969;221:1071–1073. doi: 10.1038/2211071a0. [DOI] [PubMed] [Google Scholar]
- 43.Plowright W. African swine fever. In: Davis J W, Karstad L H, Trainer D O, editors. Infectious diseases of wild mammals. 2nd ed. Ames, Iowa: Iowa State University Press; 1981. pp. 178–190. [Google Scholar]
- 44.Plowright W, Thomson G R, Neser J A. African swine fever. In: Coetzer J A W, Thomson G R, Tustin R C, editors. Infectious diseases in livestock with special reference to South Africa. Vol. 1. Capetown, South Africa: Oxford University Press; 1994. pp. 568–599. [Google Scholar]
- 45.Powell P P, Dixon L K, Parkhouse R M. An IκB homolog encoded by African swine fever virus provides a novel mechanism for downregulation of proinflammatory cytokine responses in host macrophages. J Virol. 1996;70:8527–8533. doi: 10.1128/jvi.70.12.8527-8533.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rabin E M, Gordon K, Knoppers M H, Luther M A, Neidhardt E A, Flynn J F, Sardonini C A, Sampo T M, Concino M F, Recny M A, Reinherz E L, Dwyer D S. Inhibition of T cell activation and adhesion functions by soluble CD2 protein. Cell Immunol. 1993;149:24–38. doi: 10.1006/cimm.1993.1133. [DOI] [PubMed] [Google Scholar]
- 47.Reed J C, Tadmori W, Kamoun M, Koretzky G, Nowell P C. Suppression of interleukin 2 receptor acquisition by monoclonal antibodies recognizing the 50 KD protein associated with the sheep erythrocyte receptor on human T lymphocytes. J Immunol. 1985;134:1631–1639. [PubMed] [Google Scholar]
- 48.Rodríguez J M, Yáñez R J, Almazán F, Viñuela E, Rodriguez J F. African swine fever virus encodes a CD2 homolog responsible for the adhesion of erythrocytes to infected cells. J Virol. 1993;67:5312–5320. doi: 10.1128/jvi.67.9.5312-5320.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ruíz-Gonzalvo F, Coll J M. Characterization of a soluble hemagglutinin induced in African swine fever virus-infected cells. Virology. 1993;196:769–777. doi: 10.1006/viro.1993.1534. [DOI] [PubMed] [Google Scholar]
- 50.Ruíz-Gonzalvo F, Rodríguez F, Escribano J M. Functional and immunological properties of the baculovirus-expressed hemagglutinin of African swine fever virus. Virology. 1996;218:285–289. doi: 10.1006/viro.1996.0193. [DOI] [PubMed] [Google Scholar]
- 51.Sanchez-Botija C. Reservorios del virus de la peste porcina africana. Investigación del virus de la P.P.A. en los artropodos mediante la prueba de la hemoadsorción. Bull Off Int Epizoot. 1963;60:895–899. [Google Scholar]
- 52.Sanchez-Madrid F, Krensky A M, Ware C F, Robbins E, Strominger J L, Burakoff S J, Springer T A. Three distinct antigens associated with human T-lymphocyte-mediated cytolysis: LFA-1, LFA-2, and LFA-3. Proc Natl Acad Sci USA. 1982;79:7489–7493. doi: 10.1073/pnas.79.23.7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Simpson V R, Drager N. African swine fever antibody detection in warthogs. Vet Rec. 1979;105:61. doi: 10.1136/vr.105.3.61. [DOI] [PubMed] [Google Scholar]
- 54.Sogo J M, Almendral J M, Talavera A, Viñuela E. Terminal and internal inverted repetitions in African swine fever virus DNA. Virology. 1984;133:271–275. doi: 10.1016/0042-6822(84)90394-5. [DOI] [PubMed] [Google Scholar]
- 55.Stroop W G, Rock D L, Fraser N W. Localization of herpes simplex virus in the trigeminal and olfactory systems of the mouse central nervous system during acute and latent infections by in situ hybridization. Lab Invest. 1984;51:27–38. [PubMed] [Google Scholar]
- 56.Thomson G R, Gainaru M D, Van Dellen A F. Experimental infection of warthog (Phacochoerus aethiopicus) with African swine fever virus. Onderstepoort J Vet Res. 1980;47:19–22. [PubMed] [Google Scholar]
- 57.Thomson G R, Gainaru M, Lewis A, Biggs H, Nevill E, Van Der Pypekamp M, Gerbes L, Esterhuysen J, Bengis R, Bezuidenhout D, Condy J. The relationship between ASFV, the warthog and Ornithodoros species in southern Africa. In: Wilkinson P J, editor. ASF, EUR 8466 EN, proceedings of CEC/FAO Research Seminar, Sardinia, Italy, September 1981. Luxemburg, Belgium: Commission of the European Communities; 1983. pp. 85–100. [Google Scholar]
- 58.Thomson G R. The epidemiology of African swine fever: the role of free-living hosts in Africa. Onderstepoort J Vet Res. 1985;52:201–209. [PubMed] [Google Scholar]
- 59.Van Wauwe J, Goossens J, Decock W, Kung P, Goldstein G. Suppression of human T-cell mitogenesis and E-rosette formation by the monoclonal antibody OKT11A. Immunology. 1981;44:865–871. [PMC free article] [PubMed] [Google Scholar]
- 60.Vigario J D, Terrinha A M, Moura Nunes J F. Antigenic relationships among strains of African swine fever virus. Arch Gesamte Virusforsch. 1974;45:272–277. doi: 10.1007/BF01249690. [DOI] [PubMed] [Google Scholar]
- 61.Wardley R C. Effect of African swine fever on lymphocyte mitogenesis. Immunology. 1982;46:215–220. [PMC free article] [PubMed] [Google Scholar]
- 62.Whitlow M B, Iida K, Stefanova I, Bernard A, Nussenzweig V. H19, a surface membrane molecule involved in T-cell activation, inhibits channel formation by human complement. Cell Immunol. 1990;126:176–184. doi: 10.1016/0008-8749(90)90310-n. [DOI] [PubMed] [Google Scholar]
- 63.Wilkinson P J. African swine fever virus. In: Pensaert M B, editor. Virus infections of porcines. Amsterdam, The Netherlands: Elsevier Science Publishers; 1989. pp. 17–35. [Google Scholar]
- 64.Yáñez R J, Rodri“guez J M, Nogal M L, Yuste L, Enríquez C, Rodriguez J F, Viñuela E. Analysis of the complete nucleotide sequence of African swine fever virus. Virology. 1995;208:249–278. doi: 10.1006/viro.1995.1149. [DOI] [PubMed] [Google Scholar]
- 65.Zsak L, Lu Z, Kutish G F, Neilan J G, Rock D L. An African swine fever virus virulence-associated gene NL-S with similarity to the herpes simplex virus ICP34.5 gene. J Virol. 1996;70:8865–8871. doi: 10.1128/jvi.70.12.8865-8871.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zsak L, Caler E, Lu Z, Kutish G F, Neilan J G, Rock D L. A nonessential African swine fever virus gene UK is a significant virulence determinant in domestic swine. J Virol. 1998;72:1028–1035. doi: 10.1128/jvi.72.2.1028-1035.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]