3′-Azido-3′-Deoxythymidine (AZT) Mediates Cross-Resistance to Nucleoside Analogs in the Case of AZT-Resistant Human Immunodeficiency Virus Type 1 Variants (original) (raw)
Abstract
Difficulties in deciphering the mechanisms of 3′-azido-3′-deoxythymidine (AZT)-resistance by human immunodeficiency virus type 1 (HIV-1) variants are due in part to an inability to reconstitute resistance in vitro using AZT-resistant reverse transcriptases. We decided to characterize mechanisms of AZT resistance in tissue culture infections by studying the ability of drug-resistant viruses to synthesize viral DNA in the presence or absence of drug. Through use of PCR amplifications, we discovered an AZT-mediated stimulation of reverse transcription by AZT-resistant viruses carrying the M41L and T215Y mutations that can apparently override the inhibitory effects of AZT-5′-triphosphate. In addition, the presence of AZT also causes viruses containing the M41L and T215Y substitutions to have diminished sensitivity to other nucleoside analogs (i.e., ddC, ddI, and d4T). This AZT-mediated cross-resistance may help to explain the virological failure of treatment regimens that included ddI plus AZT or ddC plus AZT in situations in which the T215Y and/or M41L mutations were present (F. Brun-Vézinet, C. Boucher, C. Loveday, D. Descamps, V. Fauveau, J. Izopet, D. Jeffries, S. Kaye, C. Krzyanowski, A. Nunn, R. Schuurman, J. M. Seigneurin, C. Tamalet, R. Tedder, J. Weber, and G. J. Weverling, Lancet 350:983–990, 1997). Our results suggest that the use of AZT may be contraindicated in those patients for whom resistance to this compound (M41L and/or T215Y) has been demonstrated.
Resistance to antiretroviral drugs is a formidable obstacle in the treatment of human immunodeficiency virus-positive (HIV+) patients. Development of new antiretroviral drugs, such as HIV type 1 (HIV-1) protease inhibitors and nonnucleoside reverse transcriptase (RT) inhibitors, and use of new treatment strategies, e.g., triple-drug-combination therapy, has not eliminated the emergence of drug-resistant isolates (24). In addition, the problems of drug cross-resistance and the potential for transmission of drug-resistant viruses in new infections are of great concern. In the developed world, treatment with 3′-azido-3′-deoxythymidine (zidovudine) (AZT) or transmission of AZT-resistant virus has resulted in an increase in the HIV+ population carrying AZT-resistant HIV-1 (4). AZT remains the drug most commonly included in combination therapies, even though we lack a solid understanding of the mechanisms responsible for AZT resistance (4).
AZT, shown in 1985 to inhibit HIV-1 replication (36), was the first antiretroviral agent administered to HIV-infected patients (13). Even with the extensive development and analysis of new nucleoside analogs such as 3TC (2′,3′-dideoxy-3′-thiacytidine) and 1592U89 [(−)-(1S,4R)-1-4-[2-amino-6-(cyclo- propyl-amino)-9H-purin-9-yl]-2-cyclopentene-1-methanol], these drugs are still less effective than AZT in blocking HIV replication in tissue culture; concentrations responsible for 50% inhibition (IC50s) for these drugs are at least 10- to 100-fold more than that for AZT (0.001 to 0.01 μM) (10, 11, 44). However, virological response (e.g., the drop in viral load) is generally more pronounced in HIV+ individuals receiving 3TC or 1592U89 than in those receiving AZT monotherapy (20). In almost all cases of nucleoside analog monotherapy, drug-resistant HIV-1 variants, which result from specific mutations in the coding region of HIV-1 reverse transcriptase (RT), will dominate the HIV-1 population during the course of therapy (30, 43).
HIV-1 RT is a heterodimer composed of the polymerase-RNase H active 66-kDa subunit and the inactive but structurally significant 51-kDa subunit (4). High-resolution crystal structures of HIV-1 RT have modeled the p66 subunit as a human right hand (27). The “fingers” and “thumb” subdomains are involved in substrate binding (i.e., deoxynucleosidetriphosphate [dNTP] and primer-template), whereas the “palm” subdomain contains the polymerase active site (27). With most nucleoside analogs, resistance is conferred by a single mutation in the RT coding region of HIV-1 that is stably maintained throughout therapy. A cluster of nucleoside analog-resistant mutations are found in a region (residues 65 to 77) of the fingers subdomain (β3-β4 hairpin) thought to be involved in dNTP and/or primer-template binding (4, 7, 47). We and others have previously shown that resistance to ddI (2′,3′-dideoxyinosine) and ddC (2′,3′-dideoxycytidine) conferred by mutations L74V and K65R, respectively, is the result of decreased binding by the mutant RT to the analog and increased selectivity for the native nucleoside (17, 19, 33). This effect may be related to possible alterations in the primer-template binding site. With an extended template modeled into the original crystal structure of HIV-1 RT complexed with an 18/19-nucleotide (nt) template/primer, it appears that residues L74V and E89G may interact with the template strand (7).
Treatment with AZT will also select for a mutation (K70R) in this region of RT, but for reasons yet unclear, the K70R HIV-1 is not stable and is eventually outgrown by another AZT-resistant HIV-1 containing a T215Y/F change (12). This mutation and the rarer M41L, K219E/Q, and L210W AZT-resistant mutations (21, 26, 29) are found outside the polymerase-active site in the palm subdomain, a region of unknown function. With the exception of a modest increase in processivity during DNA synthesis by an AZT-resistant RT (9), no differences have been confirmed between the wild-type or AZT-resistant RT in competitive inhibition by AZT-5′-triphosphate (AZT-TP), incorporation of AZT-TP, RNase H activity, and fidelity (28).
In this study, we have employed a tissue culture infection system to study AZT-resistant mechanisms, rather than an in vitro assay, so as not to omit any cellular or viral factors. Amounts of proviral DNA synthesized by various AZT-resistant clinical isolates, AZT-resistant clones, and wild-type HIV-1 were measured in the presence or absence of AZT. An obvious trend was apparent from these analyses, i.e., AZT appears to stimulate reverse transcription in cells infected with AZT-resistant viruses containing the M41L and/or T215Y mutations. No such stimulation was observed in cells exposed to wild-type virus or to HIV-1 carrying only the K70R mutation. Thus, we hypothesize that an AZT anabolite (e.g., AZT-5′-monophosphate) (AZT-MP) may mediate a stimulation of reverse transcription by AZT-resistant, M41L and T215Y HIV-1 to overcome any inhibitory effects of AZT-TP. Considering that all triphosphorylated nucleoside analogs inhibit HIV-1 reverse transcription in a similar fashion, we examined whether AZT could also mediate cross-resistance by AZT-resistant viruses to other nucleoside analogs. We found that M41L and T215Y HIV-1 was resistant to each of 2′,3′-didehydro-2′,3′-deoxythymidine (d4T), ddI, and ddC, but only in the presence of low concentrations of AZT.
Only the AZT-resistant T215Y HIV-1 or a multinucleoside analog-resistant HIV-1 (but not the K70R virus or viruses resistant to ddI or ddC) was isolated from HIV+ patients treated with AZT plus ddI or AZT plus ddC (8, 23, 25, 38, 42). Thus, it is possible that the T215Y mutation, in the presence of AZT, may confer multinucleoside analog resistance. It is important that we understand these mechanisms of AZT resistance, since a large proportion of HIV+ individuals, many of whom are AZT experienced, are now being treated with multiple-drug regimens that commonly include a protease inhibitor as well as AZT.
MATERIALS AND METHODS
Cell culture.
Jurkat (CD4+ T lymphocytic tumor cell line) and MT4 (human T-cell leukemia virus type 1 transformed CD4+ T lymphocytes) cells, obtained from the American Type Culture Collection (Rockville, Md.), were grown in 1× RPMI 1640 supplemented with 10% fetal calf serum, l-glutamine (2 mM), penicillin (100 U/ml), and streptomycin (100 μg/ml) at 37°C and 5% CO2.
Viruses and infection studies.
Peripheral blood was drawn from HIV+ patients treated with AZT for 3 to 12 months. Virus was propagated from patient peripheral blood mononuclear cells (PBMCs) in the presence of low AZT concentrations (0.01 μM) as described previously (15). These AZT-resistant isolates were used to infect MT4 cells in soft agar-containing, RPMI-supplemented media. Individual AZT-resistant isolates were purified from single plaques (identified by syncytium formation and cytopathic effects) and propagated on MT4 cells. The RT-coding region was PCR amplified from proviral DNA found in infected MT4 lysates, cloned into M13, and sequenced. Almost all plaques, produced by infectious AZT-resistant viruses isolated from each patient sample, harbored HIV-1 isolates with the same AZT-resistance mutations. The AZT-resistant HIV-1 clones containing the K70R or M41L and T215Y mutations, kindly provided by Brendan Larder (Glaxo-Wellcome, Beckenham, United Kingdom), were also propagated on MT4 cells. Culture fluids containing purified AZT-resistant HIV-1 clones or patient isolates (ARI-1, ARI-2, ARI-3, ARI-4, ARI-5, and ARI-6) were passed through a 0.45-μm-pore-size filter, DNase I-treated, and stored at −70°C. The absence of viral DNA in the supernatant after DNase I treatment was verified by PCR analysis (1). HIV DNA was found in less than 0.1% of virions used in this infection study (2).
The same 50% tissue culture infective dose for each virus was used to infect Jurkat or MT4 cells, pretreated for 8 h with AZT (0.001 to 0.1 μM) (Moravek Biochemicals, Brea, Calif.). After 2 h of viral adsorption, the cultures were washed twice in phosphate-buffered saline and resuspended in RPMI 1640 containing 10% fetal calf serum. Cultures were incubated for a further 12 h in this medium containing drug as indicated, following which 106 cells were washed four times in phosphate-buffered saline and then lysed in Hirt buffer (0.6% sodium dodecyl sulfate; 10 mM Tris-HCl, pH 7.4; 10 mM EDTA, pH 8.0) (1, 3). These experiments were repeated three times in Jurkat cells to obtain the standard deviation shown in Fig. 1B.
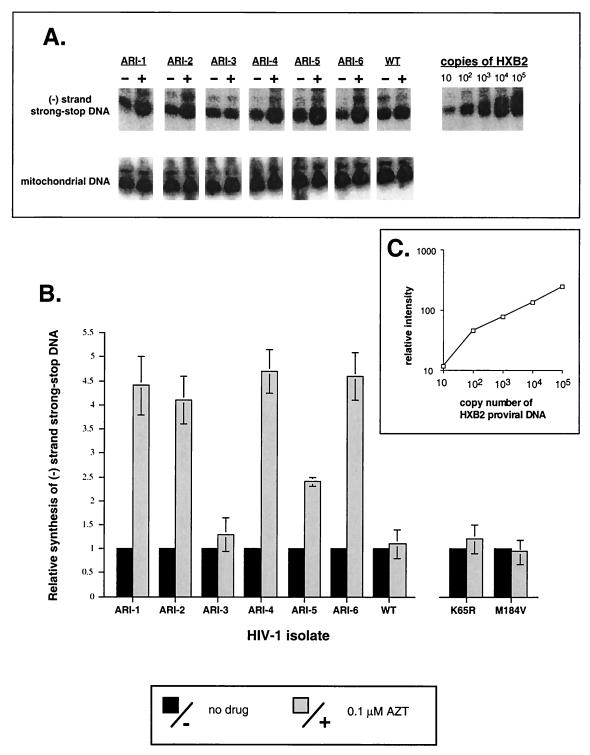
FIG. 1.
Synthesis of minus-strand strong-stop DNA by wild-type and AZT-resistant HIV-1 isolates. Jurkat cells were exposed to wild-type or AZT-resistant isolates (ARI-1 through ARI-6; Table 1) in the presence or absence of AZT (0.1 μM). Minus-strand strong-stop HIV-1 DNA and mitochondrial DNA were PCR amplified from cellular extracts of LMW DNA and then electrophoresed on 5% denaturing polyacrylamide gels (A). Using phosphor-imaging analysis, amount of minus-strand strong-stop DNA produced in the presence of AZT with one viral isolate is presented relative to the amount of mitochondrial DNA in that sample and to the amount of minus-strand strong-stop DNA produced in the absence of drug (B). PCR-amplified minus-strand strong-stop DNA from cells exposed to K65R HIV-1 or M184V HIV-1 are not shown in panel A, but quantitations of three independent experiments with these viruses as well as the ARI viruses are presented in panel B.
DNA isolation, purification, and PCR amplification.
Low-molecular-weight (LMW) DNA was isolated as described previously (1). The LMW DNA (5 μl) was then added to a 100-μl reaction mixture containing 100 pmol of unlabeled sense primer and [γ-32P]-end-labelled antisense primer, 0.2 mM concentrations of the four dNTPs, 50 mM KCl, 10 mM Tris-HCl (pH 8.3), 2.5 mM MgCl2, and 2 U of Taq polymerase (Boehringer Mannheim, Indianapolis, Ind.) and subjected to 27 amplification cycles as described previously (1, 3). The following primer pairs were employed: A13 (635 to 614)-S1 (496 to 516) to amplify a 140-bp segment in the U5 region of the long terminal repeat; A2 (410 to 389)-S2 (221 to 242) to amplify a 207-bp segment in the U3 region of the long terminal repeat; and AG4 (804 to 784)-SG4 (709 to 730) for a 95-bp segment upstream of the gag gene (Fig. 2A) (1). The MTA (390 to 370)-MTS (260 to 280) primer pair was used to amplify a 130-bp segment in the noncoding region of human mitochondrial DNA (1). The plasmid pHλHXB2 (approximately 13 kbp) was linearized by restriction endonuclease digestion with _Xho_I and serially diluted (1:10); each dilution was subjected to PCR amplification as a quantitation control (Fig. 1C). The addition of Hirt LMW extracts from 107 cells had no effect on the amplification efficiency of linearized plasmid and was therefore not included as a control. The PCR amplifications (1:20) were electrophoresed on 7% denaturing polyacrylamide gels, which were dried, autoradiographed, and analyzed by phosphor-imaging (Molecular Dynamics Inc., Sunnyville, Calif.).
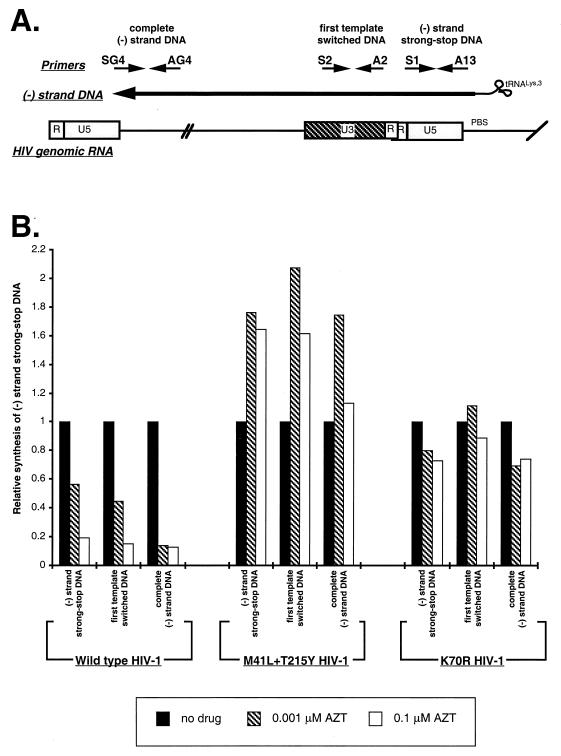
FIG. 2.
Synthesis of minus-strand DNA in AZT-treated MT4 cells infected by wild-type HIV-1 or AZT-resistant HIV-1 clones. MT4 cells were untreated or treated with AZT (0.001 and 0.1 μM) and then exposed to wild-type HIV-1, K70R HIV-1, or M41L plus T215Y HIV-1. Minus-strand strong-stop DNA (immediately upstream of initiation of reverse transcription), first template switched DNA (200 nt upstream of initiation), and complete minus-strand DNA (∼9,000 nt upstream of initiation) was PCR amplified from LMW DNA extracts. These products are schematically illustrated in panel A. Each bar (B) represents the amount of PCR-amplified minus-strand DNA product relative to the amount of PCR-amplified mitochondrial DNA in the same sample and the amount of PCR-amplified minus-strand DNA product in the absence of drug (adjusted to 1).
Drug sensitivity assays.
MT4 cells, untreated or treated for 8 h with 0.001, 0.01, or 0.1 μM AZT, were exposed to wild-type HIV-1, ddC-resistant (K65R) HIV-1, K70R HIV-1, or M41L and T215Y HIV-1 for 2 h. Washed cells were then plated into microtiter plates containing 10-fold dilutions (0.0001 to 100 μM) of different nucleoside analogs (AZT, ddI, ddC, d4T, and 3TC; Sigma Chemical Co., St. Louis, Mo., or Moravek Biochemicals). Upon initial detection of cytopathic effects, supernatants were harvested and used for RT assays (10). RT activity was then plotted against drug concentration for determination of IC50s directly from the graph or by using the PROBIT program (46).
RESULTS
Effects of AZT on reverse transcription by AZT-resistant clinical isolates of HIV-1.
Although resistance to AZT has been well documented in tissue culture infections by using virus isolated from AZT-treated HIV+ patients (4), no one has examined proviral DNA synthesis by AZT-resistant viruses in the presence of this drug. We, therefore, determined the amount and extent of proviral DNA synthesis in CD4+ T lymphocytic cells exposed to wild-type HIV-1 or to various AZT-resistant clinical isolates in the presence or absence of AZT. AZT-resistant clinical isolates were propagated on PBMCs and plaque purified. Sequencing of the RT coding region of these isolates revealed several AZT resistance mutations (e.g., M41L, K70R, and T215Y) (Table 1) as well as other nonspecific changes not associated with any drug-resistant phenotype. Sensitivity to AZT varied with each clinical isolate but virus containing both the M41L and T215Y mutations (ARI-1, ARI-4, and ARI-6) showed consistently higher IC50s for AZT than did viruses containing only the K70R mutation (ARI-3) (Table 1).
TABLE 1.
Mutational analysis of AZT-resistant clinical isolates of HIV-1
| Isolate | IC50 (μM) for AZT | Mutations (residue in RT) | |||
|---|---|---|---|---|---|
| 41 | 70 | 215 | 219 | ||
| Wild type | 0.0025 ± 0.0007 | Met | Lys | Thr | Lys |
| ARI-1 | 0.069 ± 0.008 | Leu | Tyr | ||
| ARI-2 | 0.027 ± 0.004 | Tyr | |||
| ARI-3 | 0.018 ± 0.005 | Arg | |||
| ARI-4 | 0.038 ± 0.004 | Leu | Tyr | ||
| ARI-5 | 0.020 ± 0.005 | Arg | Ile | ||
| ARI-6 | 0.042 ± 0.006 | Leu | Tyr |
In this set of experiments, untreated or AZT (0.1 μM)-treated Jurkat cells were exposed to virus for 2 h and then incubated for a further 20 h prior to cell harvest and lysis. LMW DNA was subjected to PCR amplification by using HIV-1 DNA-specific and mitochondrial DNA-specific primer pairs (see Materials and Methods). As previously described, nucleoside analogs (e.g., AZT) did not inhibit synthesis of minus-strand DNA in Jurkat cells exposed to wild-type HIV-1 (Fig. 1A and B), due to high intracellular dNTP concentrations (1). Instead, nucleoside analogs were preferentially incorporated after the first template switch, a significant pause site during reverse transcription (1). Similar results were obtained in experiments employing phytohemaglutinin-stimulated PBMCs. However, the total amount of reverse-transcribed HIV-1 DNA was at least 10-fold less in PBMCs than in Jurkat cells (3).
Thus, Jurkat cells provide an excellent system in which to study an AZT-mediated effect, other than inhibition, during minus-strand strong-stop DNA synthesis. In fact, we observed a nearly fivefold increase in amounts of minus-strand strong-stop DNA produced by AZT-resistant clinical isolates (ARI-1, ARI-2, ARI-4, ARI-5, and ARI-6) in the presence of AZT (0.1 μM) as compared to the absence of drug (Fig. 1A and B). AZT-mediated stimulation of minus-strand strong-stop DNA was not observed with either the ARI-3 AZT-resistant isolate (containing the K70R), a ddC-resistant (K65R) HIV-1, a 3TC-resistant (M184V) HIV-1, or wild-type HIV-1 (Fig. 1B). Interestingly, there was a slight AZT-mediated stimulation of minus-strand strong-stop DNA synthesis by the ARI-5 virus, found to contain both the K70R and T215I mutations. The significance of the T215I mutation is currently under study. Figure 1C shows a direct linear correlation between the amount of PCR-amplified minus-strand strong-stop DNA and the copy number of HXB2 proviral DNA. This plot was used to quantify the amounts of PCR-amplified sample DNA shown in Fig. 1A.
As discussed earlier, the K70R mutation is located in a region of RT thought to be involved in dNTP and/or primer-template binding (4). Other drug-resistant mutations in this region (e.g., K65R and L74V), result in decreased binding affinity to the nucleoside analog (15, 16, 30), suggesting that the K70R mutation may result in a similar phenotype. In contrast, the M41L and T215Y mutations are not found in regions of known enzymatic or structural function. Although results from Fig. 1 suggest that the M41L and/or T215Y mutations are involved in this AZT-mediated stimulation, other mutations in the RT coding region may also contribute to this effect. In addition, there is no direct evidence that the clinical isolates, containing the M41L, K70R, and/or T215Y mutations, are resistant to the inhibitory effects of AZT-TP since minus-strand strong-stop DNA synthesis is not affected by AZT in Jurkat cells. Therefore, we employed AZT-resistant HIV-1HXB2 clones (Table 2), containing the K70R mutation or the M41L and T215Y mutations, to infect MT4 cells (a human T-cell leukemia virus type 1-transformed T-lymphocyte cell line) in the absence or presence of AZT (0.001 and 0.1 μM). Unlike Jurkat cells or phytohemagglutinin-stimulated peripheral blood lymphocytes (1, 3), MT4 cells contain lower dNTP concentrations and support nucleoside analog inhibition of early proviral DNA products, i.e., minus-strand strong-stop DNA (Fig. 2B). In these experiments, we examined three successive products of minus-strand DNA synthesis by quantitative PCR, i.e., minus-strand strong-stop DNA, first-template-switched DNA, and complete minus-strand DNA by using the PCR primers described in Materials and Methods (Fig. 2A) (1, 2). Similar results on the activity of nucleoside analogs have been obtained in quiescent PBMCs but the total amount of reverse-transcribed DNA was about 10-fold less than that in MT4 cells (1, 3). For quantitative analyses, we have utilized these tumor cell lines in the following studies.
TABLE 2.
Effect of AZT on the inhibition of wild-type and resistant viruses by ddC in MT4 cells
| HIV-1 strain | Mutations in RT | IC50 for AZT (μM) | IC50 for ddC (μM) | IC50s for ddC (μM)a | ||
|---|---|---|---|---|---|---|
| 0.001 μM AZT | 0.01 μM AZT | 0.1 μM AZT | ||||
| Wild-type HIV | No mutations | 0.0025 ± 0.0004 | 0.025 ± 0.01 | NAb | NA | NA |
| AZT-resistant 1241 HIV-1 | K70R | 0.016 ± 0.007 | 0.038 ± 0.004 | 0.040 ± 0.007 | 0.043 ± 0.004 | 0.011 ± 0.007 |
| AZT-resistant HIV-1 clone | M41L and T215Y | 0.060 ± 0.01 | 0.043 ± 0.004 | 0.25 ± 0.07 (sixfold)c | 0.13 ± 0.04 (threefold) | 0.093 ± 0.01 (twofold) |
| ddC-resistant HIV-1 clone | K65R | 0.0030 ± 0.0006 | 0.161 ± 0.04 | NA | NA | NA |
Although AZT-mediated stimulation of minus-strand strong-stop DNA synthesis by the M41L and T215Y HIV-1 was evident in MT4 cells, this increase was reduced from that observed in Jurkat cells (compare Fig. 2B with 1B). In MT4 cells, this reduction in stimulation may be related to increased AZT inhibition of minus-strand strong-stop DNA synthesis by wild-type HIV-1. In the presence of AZT, there was nearly a fivefold increase in the amount of minus-strand strong-stop DNA produced by an AZT-resistant, M41L plus T215Y HIV-1HXB2 clone over that produced by wild-type HIV-1 (Fig. 2B). Taking AZT inhibition into account, this AZT-mediated stimulation is similar to that observed with AZT-resistant clinical isolates (i.e., ARI-1, ARI-2, ARI-4, ARI-5, and ARI-6) in Jurkat cells. In addition, only the M41L and/or T215Y mutations and not other changes appear to be responsible for this phenotype. As will be discussed, AZT-mediated stimulation of reverse transcription by M41L plus T215Y HIV-1 may override inhibition by AZT-TP. This stimulation effect was maintained throughout minus-strand DNA synthesis by M41L and T215Y HIV-1 but decreased with increasing AZT concentrations (0.001 to 0.1 μM) (Fig. 2B). Since AZT inhibition of minus-strand strong-stop DNA synthesis by wild-type HIV-1 was seen in MT4 cells, we could now verify the AZT resistance phenotype exhibited by the K70R virus (Fig. 2B). In MT4 cells exposed to K70R HIV-1, minus-strand DNA synthesis was largely unaffected by both inhibitory and stimulatory activities of AZT. Interestingly, the K70R HIV-1 exhibits a similar resistance phenotype to AZT as does K65R HIV-1 to ddC. In vitro, the K65R mutation in HIV-1 RT results in increased selectivity for dCTP and decreased binding to ddCTP (17, 19).
AZT mediates resistance by AZT-resistant HIV-1 to other nucleoside analogs.
The results described above suggest that two mechanisms, encoded by different RT mutations (e.g., K70R versus T215Y), may be responsible for AZT resistance. In HIV+ patients treated with AZT, the T215Y HIV-1, conferring high level resistance to AZT, is eventually selected over the K70R virus showing weak AZT resistance (12). However, only the T215Y virus, and not the K70R HIV-1 or ddC- or ddI-resistant virus, was isolated from HIV+ patients failing AZT plus ddI or AZT plus ddC therapy (8, 25, 38). A multinucleoside analog-resistant HIV-1 was identified in <20% of these patients (23, 42). These clinical findings suggest that the AZT-resistant T215Y virus may also be less susceptible to ddI or ddC, yet previous studies detected no such cross-resistance (4).
Considering that all triphosphorylated nucleoside analogs (ddNTPs) inhibit HIV-1 reverse transcription in a similar manner (4), an AZT-mediated stimulation of reverse transcription by M41L and T215Y HIV-1 may override inhibition by AZT-TP and other ddNTPs. To test this hypothesis, we treated MT4 cells with 0.001, 0.01, and 0.1 μM AZT prior to infection with wild-type, K70R, M41L and T215Y, and ddC-resistant (K65R) viruses. IC50s were then determined for ddC with each virus in the presence or absence of AZT. Due to AZT inhibition, IC50s for ddC could not be determined in the case of MT4 cells exposed to wild-type or K65R HIV-1 in the presence of AZT (Table 2). Increased sensitivity to ddC was observed with the K70R and wild-type HIV-1 when treated with AZT (Table 2), suggesting an additive inhibition of AZT and ddC on these viruses. In contrast, addition of low AZT concentrations to MT4 cells resulted in a sixfold decreased sensitivity to ddC by M41L and T215Y HIV-1 (Table 2). Resistance to ddC by the M41L and T215Y virus diminished with increasing AZT concentrations suggesting inhibition by AZT-TP and ddCTP may occur independent of AZT-mediated stimulation. This decrease in ddC sensitivity by M41L plus T215Y HIV-1 in the presence of 0.001 μM AZT was similar to that observed with the K65R HIV-1 in the absence of AZT. The K65R substitution in HIV-1 is directly associated with ddC failure in vivo (4).
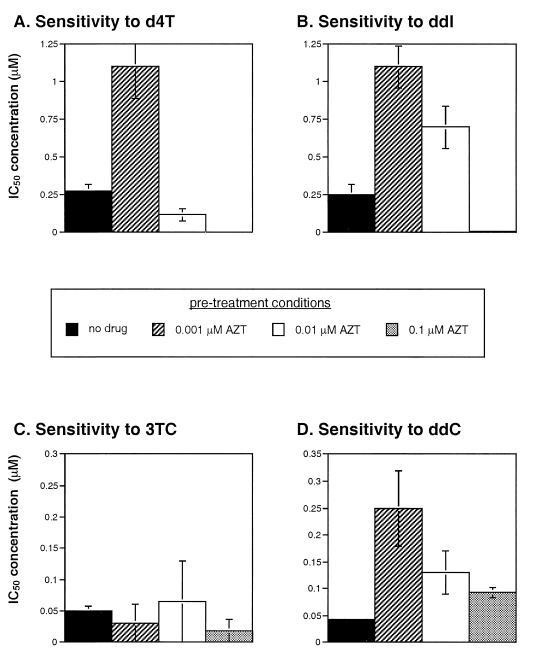
Considering that AZT may mediate cross-resistance to ddC, in the case of an AZT-resistant (M41L and T215Y) virus, we also determined if such cross-resistance extended to other nucleoside analogs. By using only the M41L and T215Y virus in the absence or presence of AZT (0.001, 0.01, and 0.1 μM), IC50s were calculated for ddI, 3TC, and 2′,3′-didehydro-2′-deoxythymidine (d4T or stavudine) (Fig. 3). In the presence of 0.001 μM AZT, M41L and T215Y HIV-1 showed decreased sensitivity to ddI and d4T (Fig. 3). Sensitivity of M41L and T215Y HIV-1 to ddI, d4T, and ddC increased with increasing concentrations of AZT (0.001 to 0.1 μM), suggesting an additive inhibition by AZT and the other nucleoside analogs independent of AZT-mediated cross-resistance (Fig. 3). In contrast, no significant cross-resistance to 3TC was found with the M41L and T215Y virus in the presence or absence of AZT (Fig. 3). It is also important to note that other nucleoside analogs (i.e., ddC, ddI, or d4T) or a nucleoside reverse transcriptase inhibitor (i.e., nevirapine) could not mediate stimulation of reverse transcription or cross-resistance by the M41L and T215Y virus (data not shown). Instead, these drugs efficiently inhibited the M41L plus T215Y virus even in the absence of other nucleoside analogs. When IC50s were calculated for ddI or d4T with the M41L and T215Y virus, treatment with low concentrations of other drugs with the exception of AZT resulted in additive inhibition and an increased sensitivity to these drugs (data not shown).
FIG. 3.
Sensitivity of AZT-resistant (M41L and T215Y) virus to other nucleoside analogs in the presence or absence of AZT. MT4 cells were untreated or pretreated with 0.001, 0.01, and 0.1 μM AZT prior to addition of the M41L and T215Y virus. IC50s were then determined for d4T (A), ddI (B), 3TC (C), and ddC (D).
DISCUSSION
Although HIV-1 resistance to AZT was first described by Larder et al. in 1989 (30), mechanisms responsible for this resistance are still not understood. In contrast, mechanisms for resistance to other nucleoside analogs (e.g., 3TC, ddI, and ddC) have been well characterized due to the relative ease in reconstituting resistance to these drugs in vitro by using recominant, mutant HIV-1 RTs (17, 23, 33). Most of these nucleoside analog-resistant mutations (e.g., K65R conferring resistance to ddC) are found clustered in a region of RT thought to be involved in both dNTP and primer-template binding (7, 47). However, it should be noted that the K65R substitution is found infrequently in patients treated with ddC (18, 48), whereas the K70R mutation is readily identified in AZT-treated patients (4, 12, 30). Another amino acid residue at position 184, changed from Met to Val upon selection with 3TC or another oxathiolated cytosine, is found in the polymerase active site of RT (5, 15, 40, 45). Nucleoside analog-resistant mutations in both of these regions result in decreased RT affinity for the analog and increased selectivity for the native nucleoside (17, 19, 33, 45). Treatment with AZT generally selects for two mutually exclusive mutations, i.e., the initial K70R mutation located in the same dNTP or primer-template binding site as described above and the dominant T215Y mutation found in an RT region of unknown structure or function (12).
Our studies reveal that K70R HIV-1 displays a similar resistance phenotype to AZT in tissue culture, as does K65R HIV-1 to ddC, suggesting a similar mechanism for resistance. Recently, Sharma et al. have demonstrated that recombinant HIV-1 RT containing the K70R mutation has a reduced affinity for AZT-TP as well as ddATP (41). In contrast, reverse transcription by HIV-1 containing the M41L and T215Y mutations was stimulated up to fivefold in the presence of AZT. This stimulation was reduced with increasing AZT concentrations, suggesting that the AZT-mediated effect may be independent of inhibition by AZT-TP. Unlike other nucleoside analogs, there is an intracellular accumulation of the monophosphate form of AZT due to a weak affinity by thymidylate kinase for AZT-MP and slow formation of AZT-TP, the active antiretroviral form of AZT (14, 32). Does this AZT-MP accumulation contribute to a unique selection of the T215Y mutation in HIV-1? We are currently attempting to answer this question by selecting for AZT-resistant HIV-1 in cells displaying increased efflux of AZT-MP.
Based on our results, it appears that AZT may stimulate reverse transcription via the M41L and/or T215Y mutations in HIV-1 to override the inhibitory effects of AZT-TP. Drug-stimulated or drug-mediated resistance is a common mechanism for microbial drug resistance. For example, β-lactamase expression is induced in many gram-positive bacteria by β-lactam drugs (e.g., penicillin G) to compensate for the thick peptidoglycan layer and absence of a periplasmic space (34). Drug-mediated resistance is also selected by mutants of poliovirus type 3 (Sabin strain) treated with WIN 51711, an uncoating inhibitor (37). In fact, some viral mutants became dependent on this drug for efficient replication (37). However, the drug resistance mechanism most analogous to that proposed for AZT is found in Escherichia coli resistance to kirromycin, where selection occurs for mutants that require kirromycin release from bacterial elongation factor (EF-Tu) for polypeptide chain elongation during translation (35). Thus, precedents exist in other microbial systems for the type of situation described here in which an AZT anabolite (i.e., AZT-MP, AZT-5′-diphosphate-, or AZT-TP) may stimulate reverse transcription to overcome the inhibitory effects of AZT-TP.
Future studies on this AZT-mediated resistance will focus on the effects of altered AZT metabolism on cross-resistance by AZT-resistant HIV-1 to other nucleoside analogs. AZT metabolism will be manipulated through the use of thymidine kinase/thymidylate kinase inhibitors or mutant T-lymphocyte cell lines with altered nucleoside anabolism (39). We suspect that the small increases in processivity observed with an AZT-resistant RT containing the D67N, K70R, T215Y, and K219E substitutions (9) may be even greater when using an RT containing the clinically relevant M41L and T215Y mutations and the correct AZT anabolite. Both the M41L and T215Y substitutions are located in a region of RT which may be involved in primer-template interactions. Although the interrelationship between these RT activities are still being investigated, it appears that an increased primer-template binding by RT is associated with increased processivity, increased fidelity, and decreased pausing (3–6, 22). In addition, we have previously shown that increased pausing by RT during RNA-dependent DNA synthesis contributes to increased incorporation of triphosphorylated nucleoside analogs (1, 3, 4). A direct or indirect interaction of an AZT anabolite with the M41L and T215Y RT may switch on this mechanism, i.e., increased primer-template binding leading to reduced antiviral activity by a nucleoside analog. An AZT-mediated switch as opposed to constitutive resistance may be selected by these AZT-resistant viruses due to the possible detrimental effects of this mechanism on viral fitness. Interestingly, the M41L and/or T215Y substitutions are quite stable in the virus population in the absence of AZT treatment. In contrast, many drug-resistant viruses (e.g., 3TC-resistant [M184V] HIV-1) are outgrown by the wild type or possibly revert when drug pressure is removed (5).
Previous studies have shown that AZT-resistant HIV-1 isolates do not show cross-resistance to other nucleoside analogs (4). This finding is consistent with our data as cross-resistance to ddI, ddC, and d4T by AZT-resistant viruses was only observed in the presence of AZT. Furthermore, an AZT-mediated cross-resistance was limited to those viruses containing the M41L and T215Y mutations. As mentioned above, treatment of HIV+ patients with AZT and ddI or ddC resulted in an eventual failure of both drugs and a corresponding emergence of AZT-resistant HIV-1 harboring the M41L and T215Y mutations (8, 38). In contrast, monotherapy with ddI or ddC selects for specific ddI-resistant (e.g., L74V) (43) and ddC-resistant (e.g., K65R) (18, 48) viruses, mutations not found when these drugs were combined with AZT (8, 38). It appears that AZT plus ddI or AZT plus ddC combination therapy selects for two resistant genotypes: a multinucleoside analog-resistant virus containing a combination of five mutations (A62V, V75I, F77L, F116Y, and Q151M) found in only 5 to 20% of the treated patients (23, 42) and a virus containing the M41L and/or T215Y mutation found in the vast majority of patients (8, 38)).
AZT did not mediate cross-resistance to 3TC. Several factors, including augmented antiviral activity of 3TC over other nucleoside analogs, may contribute to the success of AZT plus 3TC treatment. For example, the combination of the M184V and T215Y mutations can restore sensitivity to AZT in the face of 3TC resistance (31). However, these findings do not explain why AZT mediates cross-resistance by the M41L and T215Y HIV-1 to most nucleoside analogs and not to 3TC. Since 3TC appears to be efficiently phosphorylated to 3TC-5′-triphosphate (3TC-TP) (16), the AZT-mediated stimulation effect may not suffice to overcome the inhibitory effects of high 3TC-TP concentrations. With the exception of 3TC, we have shown in this study that AZT will mediate a cross-resistance by AZT-resistant (M41L and T215Y) HIV-1 to ddI, ddC, and d4T. These results suggest that prior treatment with AZT and the appearance of the M41L and/or T215Y substitutions in patient virus may hamper future combination therapies involving AZT and another nucleoside analog.
ACKNOWLEDGMENTS
This research was supported by developmental funds from the Center for AIDS Research grant (NIH A1-36219) at Case Western Reserve University (E.J.A.). Research performed in the laboratory of M. A. Wainberg was supported by grants from Health and Welfare Canada, and Medical Research Council of Canada.
REFERENCES
- 1.Arts E J, Wainberg M A. Preferential incorporation of nucleoside analogs after template switching during human immunodeficiency virus reverse transcription. Antimicrob Agents Chemother. 1994;38:1008–1016. doi: 10.1128/aac.38.5.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arts E J, Mak J, Kleiman L, Wainberg M A. DNA found in human immunodeficiency virus type 1 particles may not be required for infectivity. J Gen Virol. 1994;75:1605–1613. doi: 10.1099/0022-1317-75-7-1605. [DOI] [PubMed] [Google Scholar]
- 3.Arts E J, Marois J P, Gu Z, Le Grice S F J, Wainberg M A. Effects of 3′-deoxynucleoside 5′-triphosphate concentrations on chain termination by nucleoside analogs during human immunodeficiency virus type-1 reverse transcription of minus-strand strong-stop DNA. J Virol. 1996;70:712–720. doi: 10.1128/jvi.70.2.712-720.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arts E J, Wainberg M A. Mechanisms of nucleoside analog antiviral activity and resistance during human immunodeficiency virus reverse transcription. Antimicrob Agents Chemother. 1996;40:527–540. doi: 10.1128/aac.40.3.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Back N K, Nijhuis M, Keuken W, Boucher C A, Oude Essink B O, Kuilenburg A B, Gennip A H, Berkhout B. Reduced replication of 3TC-resistant HIV-1 variants in primary cells due to a processivity defect of the reverse transcriptase enzyme. EMBO J. 1996;15:4040–4049. [PMC free article] [PubMed] [Google Scholar]
- 6.Bebenek K, Abbotts J, Wilson S H, Kunkel T A. Error-prone polymerization by HIV-1 reverse transcriptase: contribution of template-primer misalignment, miscoding and termination probability to mutational hot spots. J Biol Chem. 1993;268:10324–10334. [PubMed] [Google Scholar]
- 7.Boyer P L, Tantillo C, Jacobo-Molina A, Nanni R G, Ding J, Arnold E, Hughes S H. Sensitivity of wild-type human immunodeficiency virus type 1 reverse transcriptase to dideoxynucleotides depends on template length; the sensitivity of drug-resistant mutants does not. Proc Natl Acad Sci USA. 1994;91:4882–4886. doi: 10.1073/pnas.91.11.4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brun-Vézinet F, Boucher C, Loveday C, Descamps D, Fauveau V, Izopet J, Jeffries D, Kaye S, Krzyanowski C, Nunn A, Schuurman R, Seigneurin J M, Tamalet C, Tedder R, Weber J, Weverling G J. HIV-1 viral load, phenotype, and resistance in a subset of drug-naive participants from the Delta trial. Lancet. 1997;350:983–990. doi: 10.1016/s0140-6736(97)03380-1. [DOI] [PubMed] [Google Scholar]
- 9.Caliendo A M, Savara A, An D, DeVore K, Kaplan J C, D’Aquila R T. Effects of zidovudine-selected human immunodeficiency virus type 1 reverse transcriptase amino acid substitutions on processive DNA synthesis and viral replication. J Virol. 1996;70:2146–2153. doi: 10.1128/jvi.70.4.2146-2153.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coates J A V, Cammack N, Jenkinson H J, Jowett A J, Jowett M I, Pearson B A, Penn C R, Rouse P L, Viner K C, Cameron J M. (−)-2′-deoxy-3′-thiacytidine is a potent, highly selective inhibitor of human immunodeficiency virus type 1 and type 2 replication in vitro. Antimicrob Agents Chemother. 1992;36:733–739. doi: 10.1128/aac.36.4.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daluge S M, Good S S, Faletto M B, Miller W H, St. Clair M H, Boone L R, Tisdale M, Parry N R, Reardon J E, Dornsife R E, Averett D R, Krenitsky T A. 1592U89, a novel carbocylic nucleoside analog with potent, selective anti-human immunodeficiency virus activity. Antimicrob Agents Chemother. 1997;41:1082–1093. doi: 10.1128/aac.41.5.1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Jong M D, Veenstra J, Stilianakis N I, Schuurman R, Lange J M, de Boer R J, Boucher C A. Host-parasite dynamics and outgrowth of virus containing a single K70R amino acid change in reverse transcriptase are responsible for the loss of human immunodeficiency virus type 1 RNA load suppression by zidovudine. Proc Natl Acad Sci USA. 1996;93:5501–5506. doi: 10.1073/pnas.93.11.5501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fischl M A, Richman D D, Grieco M H, Gottlieb M S, Volberding P A, Laskin O L, Leedom J M, Groopman J E, Mildvan D, Schooley R T, Hirsch M S, Jackson G G, Track D T, Nusinoff-Lehrman S the AZT Collaborative Working Group. The efficacy of azidothymidine (AZT) in the treatment of patients with AIDS and AIDS-related complex. N Engl J Med. 1987;317:185–191. doi: 10.1056/NEJM198707233170401. [DOI] [PubMed] [Google Scholar]
- 14.Furman P A, Fyfe J A, St. Clair M H, Weinhold K, Rideout J L, Freeman G A, Lehrman S N, Bolognesi D P, Broder S, Mitsuya H, Barry D W. Phosphorylation of 3′-azido-3′-deoxythymidine and selective interaction of the 5′-triphosphate with human immunodeficiency virus reverse transcriptase. Proc Natl Acad Sci USA. 1986;83:8333–8337. doi: 10.1073/pnas.83.21.8333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao Q, Gu Z, Parniak M A, Cameron J, Cammack N, Boucher C, Wainberg M A. The same mutation that encodes low-level human immunodeficiency virus type-1 resistance to 2′,3′-dideoxyinosine and 2′,3′-dideoxycytidine confers high-level resistance to the (−) enantiomer of 2′,3′-dideoxy-3′-thiacytidine. Antimicrob Agents Chemother. 1993;37:1390–1392. doi: 10.1128/aac.37.6.1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gray N M, Marr C L, Penn C R, Cameron J M, Bethel R C. The intracellular phosphorylation of (−)-2′-deoxy-3′-thiacytidine (3TC) and the incorporation of 3TC 5′-monophosphate into DNA by HIV-1 reverse transcriptase and human DNA polymerase gamma. Biochem Pharmacol. 1995;50:1043–1051. doi: 10.1016/0006-2952(95)96620-a. [DOI] [PubMed] [Google Scholar]
- 17.Gu Z, Arts E J, Parniak M A, Wainberg M A. Mutated K65R recombinant reverse transcriptase of human immunodeficiency virus type 1 shows diminished chain termination in the presence of 2′,3′-dideoxycytidine 5′-triphosphate and other drugs. Proc Natl Acad Sci USA. 1995;92:2760–2764. doi: 10.1073/pnas.92.7.2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gu Z, Gao Q, Fang H, Salomon H, Parniak M A, Goldberg E, Cameron J, Wainberg M A. Identification of a mutation at codon 65 in the IKKK motif of reverse transcriptase that encodes human immunodeficiency virus resistance to 2′,3′-dideoxycytidine and 2′,3′-dideoxy-3′-thiacytidine. Antimicrob Agents Chemother. 1994;38:275–281. doi: 10.1128/aac.38.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gu Z, Fletcher R S, Arts E J, Wainberg M A, Parniak M A. The K65R mutant reverse transcriptase of HIV-1 cross-resistant to 2′,3′-dideoxycytidine, 2′,3′-dideoxy-3′-thiacytidine, and 2′,3′-dideoxyinosine shows reduced sensitivity to specific dideoxynucleoside triphosphate inhibitors in vitro. J Biol Chem. 1994;269:28118–28122. [PubMed] [Google Scholar]
- 20.Hammer S M, Katzenstein D A, Hughes M D, Gundacker H, Schooley R T, Haubrich R H, Henry W K, Lederman M M, Phair J P, Niu M, Hirsch M S, Merigan T C. A trial comparing nucleoside monotherapy with combination therapy HIV-infected adults with CD4 cell counts from 200 to 500 per cubic millimeter. N Engl J Med. 1996;335:1081–1090. doi: 10.1056/NEJM199610103351501. [DOI] [PubMed] [Google Scholar]
- 21.Harrigan P R, Kinghorn I, Bloor S, Kemp S D, Najera I, Kohll A, Larder B A. Significance of amino acid variation at human immunodeficiency virus type-1 reverse transcriptase residue 210 for zidovudine susceptibility. J Virol. 1996;70:5930–5934. doi: 10.1128/jvi.70.9.5930-5934.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huber H E, McCoy J M, Seehra J S, Richardson C C. Human immunodeficiency virus 1 reverse transcriptase: template binding, processivity, strand displacement synthesis, and template switching. J Biol Chem. 1989;262:4669–4678. [PubMed] [Google Scholar]
- 23.Iversen A K, Shafer R W, Wehrly K, Winters M A, Mullins J I, Chesebro B, Merigan T C. Multidrug-resistant human immunodeficiency virus type 1 strains resulting from combination antiretroviral therapy. J Virol. 1996;70:1086–1090. doi: 10.1128/jvi.70.2.1086-1090.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson V A. Combination therapy for HIV-1 infection—overview: preclinical and clinical analysis of antiretroviral combinations. Antivir Res. 1996;29:35–39. doi: 10.1016/0166-3542(95)00912-4. [DOI] [PubMed] [Google Scholar]
- 25.Katzenstein D A, Hammer S M, Hughes M D, Gundacker H, Jackson J B, Fiscus S, Rasheed S, Elbeik T, Reichman R, Japour A, Merigan T C, Hirsch M S. The relation of virologic and immunologic markers to clinical outcomes after nucleoside therapy in HIV-infected adults with 200 to 500 CD4 per cubic millimeter. N Engl J Med. 1996;335:1091–1098. doi: 10.1056/NEJM199610103351502. [DOI] [PubMed] [Google Scholar]
- 26.Kellam P, Boucher C A, Larder B A. Fifth mutation in human immunodeficiency virus type 1 reverse transcriptase contributes to the development of high-level resistance to zidovudine. Proc Natl Acad Sci USA. 1992;89:1934–1938. doi: 10.1073/pnas.89.5.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kohlstaedt L A, Wang J, Friedman J M, Rice P A, Steitz T A. Crystal structure at 3.5 A resolution of HIV-1 reverse transcriptase complexed with an inhibitor. Science. 1992;256:1783–1790. doi: 10.1126/science.1377403. [DOI] [PubMed] [Google Scholar]
- 28.Lacey S F, Reardon J E, Furfine E S, Kunkel T A, Bebenek K, Eckert K A, Kemp S D, Larder B A. Biochemical studies on the reverse transcriptase and RNase H activities from human immunodeficiency virus strains resistant to 3′-azido-3′-deoxythymidine. J Biol Chem. 1992;267:15789–15794. [PubMed] [Google Scholar]
- 29.Larder B A, Kemp S D. Multiple mutations in HIV-1 reverse transcriptase confer high-level resistance to zidovudine (AZT) Science. 1989;246:1155–1158. doi: 10.1126/science.2479983. [DOI] [PubMed] [Google Scholar]
- 30.Larder B A, Darby G, Richman D D. HIV with reduced sensitivity to zidovudine (AZT) isolated during prolonged therapy. Science. 1989;243:1731–1734. doi: 10.1126/science.2467383. [DOI] [PubMed] [Google Scholar]
- 31.Larder B A, Kemp S D, Harrigan P R. Potential mechanism for sustained antiretroviral efficacy of AZT-3TC combination therapy. Science. 1995;269:696–699. doi: 10.1126/science.7542804. [DOI] [PubMed] [Google Scholar]
- 32.Lavie A, Vetter I R, Konrad M, Goody R S, Reinstein J, Schlichting I. Structure of thymidylate kinase reveals the cause behind the limiting step in AZT activation. Nature Struct Biol. 1997;4:601–604. doi: 10.1038/nsb0897-601. [DOI] [PubMed] [Google Scholar]
- 33.Martin J L, Wilson J E, Haynes R L, Furman P A. Mechanism of resistance of human immunodeficiency virus type 1 to 2′,3′-dideoxyinosine. Proc Natl Acad Sci USA. 1993;90:6135–6139. doi: 10.1073/pnas.90.13.6135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Medeiros A A. Evolution and dissemination of β-lactamases accelerated by generations of β-lactam antibiotics. Clin Infect Dis. 1997;24:S19–S45. doi: 10.1093/clinids/24.supplement_1.s19. [DOI] [PubMed] [Google Scholar]
- 35.Mesters J R, Zeef L A, Hilgenfeld R, de Graaf J M, Kraal B, Bosch L. The structural and functional basis for the kirromycin resistance of mutant EF-Tu species in Escherichia coli. EMBO J. 1994;13:4877–4885. doi: 10.1002/j.1460-2075.1994.tb06815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mitsuya H, Weinhold K J, Furman P A, St. Clair M H, Lehrman S N, Gallo R C, Bolognesi D, Barry D W, Broder S. 3′-Azido-3′-deoxythymidine (BWA509V): an antiretroviral agent that inhibits the infectivity and cytopathic effects of human T-lymphotropic virus type III/lymphadenopathy-associated virus in vitro. Proc Natl Acad Sci USA. 1985;82:7096–7100. doi: 10.1073/pnas.82.20.7096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mosser A G, Rueckert R R. WIN 51711-dependent mutants of poliovirus type 3: evidence that virions decay after release from cells unless drug is present. J Virol. 1993;67:1246–1254. doi: 10.1128/jvi.67.3.1246-1254.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rey D, Pi T, Diehl L J, Hughes M, Merigan T C, Katzenstein D. Abstracts of the Fifth International Workshop on HIV Drug Resistance 1996, Whistler, British Columbia, Canada. 1996. Do plasma codon 215 mutations explain the failure of ZDV in ACTG 175, abstr., 38. [Google Scholar]
- 39.Robbins B L, Greenhaw J, Connelly M C, Fridland A. Metabolic pathways for activation of the antiviral agent 9-(2-phosphonylmethoxyethyl)adenine in human lymphoid cells. Antimicrob Agents Chemother. 1995;39:2304–2308. doi: 10.1128/aac.39.10.2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schinazi R F, Lloyd R M, Jr, Nguyen M-H, Cannon D L, McMillan A, Ilksoy N, Chu C K, Liotta D C, Bazmi H Z, Mellors J W. Characterization of human immunodeficiency viruses resistant to oxathiolane-cytosine nucleosides. Antimicrob Agents Chemother. 1993;37:875–881. doi: 10.1128/aac.37.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sharma P L, Chatis P A, Dogon A L, Mayers D L, McCutchan F E, Page C, Crumpacker C S. AZT-related mutation Lys70Arg in reverse transcriptase of human immunodeficiency virus type 1 confers decrease in susceptibility to ddATP in in vitro RT inhibition assay. Virology. 1996;223:365–369. doi: 10.1006/viro.1996.0488. [DOI] [PubMed] [Google Scholar]
- 42.Shirasaka T, Kavlick M F, Ueno T, Gao W-Y, Kojima E, Alcaide M L, Chokekijchai S, Roy B M, Arnold E, Yarchoan R, Mitsuya H. Emergence of human immunodeficiency virus type 1 variants with resistance to multiple dideoxynucleosides in patients receiving therapy with dideoxynucleosides. Proc Natl Acad Sci USA. 1995;92:2398–2402. doi: 10.1073/pnas.92.6.2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.St. Clair M H, Martin J L, Tudor-Williams G, Bach M C, Vavro C L, King D M, Kellam P, Kemp S D, Larder B A. Resistance to ddI and sensitivity to AZT induced by a mutation in HIV-1 reverse transcriptase. Science. 1991;253:1557–1559. doi: 10.1126/science.1716788. [DOI] [PubMed] [Google Scholar]
- 44.St. Clair M H, Richards C A, Spector T, Weinhold K J, Miller W H, Langlois A J, Furman P A. 3′-Azido-3′-deoxythymidine triphosphate as an inhibitor and substrate of purified human immunodeficiency virus reverse transcriptase. Antimicrob Agents Chemother. 1987;31:1972–1977. doi: 10.1128/aac.31.12.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wainberg M A, Drosopoulos W C, Salomon H, Hsu M, Borkow G, Parniak M, Gu Z, Song Q, Manne J, Islam S, Castriota G, Prasad V R. Enhanced fidelity of 3TC-selected mutant HIV-1 reverse transcriptase. Science. 1996;271:1282–1285. doi: 10.1126/science.271.5253.1282. [DOI] [PubMed] [Google Scholar]
- 46.Wallis R S. PROBIT: a computer program analysis. J Immunol Meth. 1991;145:267–268. doi: 10.1016/0022-1759(91)90338-g. [DOI] [PubMed] [Google Scholar]
- 47.Wu J, Amandoron E, Li X, Wainberg M A, Parniak M A. Monoclonal antibody-mediated inhibition of HIV-1 reverse transcriptase polymerase activity. Interaction with a possible deoxynucleoside triphosphate binding domain. J Biol Chem. 1993;268:9980–9985. [PubMed] [Google Scholar]
- 48.Zhang D, Caliendo A M, Eron J J, DeVore K M, Kaplan J C, Hirsch M S, D’Aquila R T. Resistance to 2′,3′-dideoxycytidine conferred by a mutation in codon 65 of the human immunodeficiency virus type 1 reverse transcriptase. Antimicrob Agents Chemother. 1994;38:282–287. doi: 10.1128/aac.38.2.282. [DOI] [PMC free article] [PubMed] [Google Scholar]