Regulation of monocyte differentiation by specific signaling modules and associated transcription factor networks (original) (raw)
Abstract
Monocyte/macrophages are important players in orchestrating the immune response as well as connecting innate and adaptive immunity. Myelopoiesis and monopoiesis are characterized by the interplay between expansion of stem/progenitor cells and progression towards further developed (myelo)monocytic phenotypes. In response to a variety of differentiation-inducing stimuli, various prominent signaling pathways are activated. Subsequently, specific transcription factors are induced, regulating cell proliferation and maturation. This review article focuses on the integration of signaling modules and transcriptional networks involved in the determination of monocytic differentiation.
Keywords: Monocytes, Macrophages, Signaling, Transcription, Differentiation
Monocytic differentiation and function
Monocytes are bone marrow-derived mononuclear cells giving rise to macrophages and dendritic cells (DC) [1]. Together, these leukocytes are part of the cellular arm of the innate immune system representing the mononuclear phagocyte system [1, 2]. In addition, cells of the monocytic lineage support the activation of the adaptive immune system by antigen presentation, thus linking innate and adaptive immunity.
Hematopoietic stem cells (HSC)
The monocytic development, which comprises several myeloid and premonocytic stages, is initiated in the bone marrow by multipotent, self-renewing HSC (Fig. 1). The stem cell status of HSC is maintained in response to a variety of extracellular signals (e.g., angiopoietin, thrombopoietin, stem cell factor (SCF), and CXCL12) [3] regulating a network of transcription factors that suppress the initiation of cellular differentiation and lineage commitment [4]. In particular, transcriptional regulators such as Gfi1, Bmi1, signal transducer and activator of transcription (STAT) 5, and Homeobox family members (e.g., Hox B4, A4, A9, and B6) have been identified to be decisively involved in bone marrow homing, maintenance of the undifferentiated cell status, cell cycle regulation, and HSC expansion [5–7]. In the course of maturation, the composition of the relevant transcription factor network changes in response to differentiation-supporting stimuli, thus effectively modifying the overarching transcription factor architecture within the cells, which is discussed later. To provide both the maintenance of their multipotent/self-renewal potential and the formation of initially differentiated, lineage-committing progenies, HSC are able to undergo asymmetrical cell divisions, resulting in the formation of two deviating daughter cells [8]. Thus, the number of HSC in the stem cell pool is preserved [6] and the development of a series of myeloid and lymphoid progenitors without self-renewal potential, but characterized by (decreasing) proliferative and (increasing) differentiating capacity, is enabled [9]. In consequence, asymmetrical division of HSC and their progenies provides the cellular basis for the creation and maintenance of the whole hematopoietic system [3]. Although the mechanisms regulating hematopoietic homeostasis at the stem cell level are not fully understood, it is generally accepted that these processes are regulated by environmental signals in the bone marrow stem cell niche [3, 8]. Dependent on the particular conditions to which a single HSC is exposed, the cell may take different cell fates, including asymmetrical division and the release of early progenitor cells to external micro-environments for further maturation [3, 10]. To date, the molecular basis for a cell-intrinsic polarity of HSC is still unclear. However, it has been reported that unbalanced levels of Notch signaling, which is involved in the regulation of cell fate decisions during developmental processes, are present in the daughter cells of asymmetrically dividing HSC, presumably induced by segregation of Numb, a regulator of Notch signaling [8].
Fig. 1.
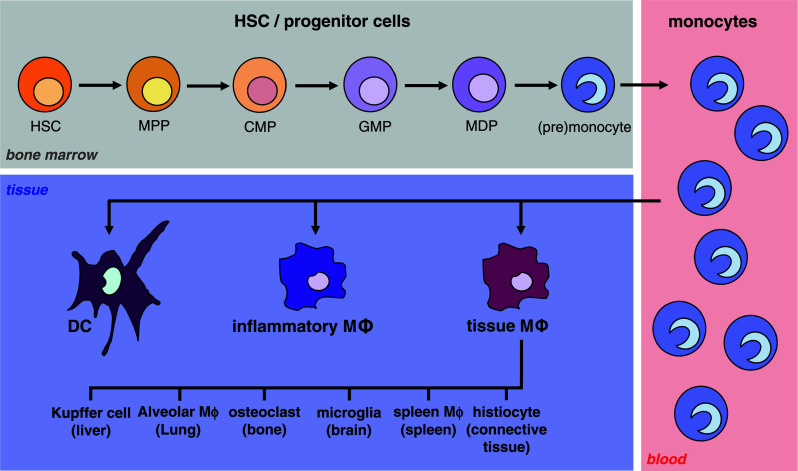
The development of monocytic cells. The scheme shows the different myeloid and premonocytic stages of monocytic maturation beginning with self-renewing HSC in the bone marrow via several myeloid progenitors resulting in the formation of blood monocytes. Further developmental steps yield the generation of macrophages and DC in different tissues. Further details are described in the text (HSC hematopoietic stem cell, MPP multipotent progenitor, CMP common myeloid progenitor, GMP granulocyte/monocyte progenitor, MDP monocyte/macrophage and dendritic cell progenitor, MΦ macrophage)
Progenitor stages
The first, but still non-specific developmental step towards monocytes, is represented by multipotent progenitors (MPP), common progenitors of all myeloid and lymphoid cells, which have lost the stem cell-specific self-renewal capacity [9]. Then, common myeloid progenitors (CMP) are formed, which give rise to all myeloid cells, followed by the granulocyte/monocyte progenitor (GMP) as the source of both granulocytes as well as the monocytic lineage [9]. Further differentiation of GMP creates the monocyte/macrophage and dendritic cell progenitor (MDP), which has lost the ability to generate granulocytes but provides the basis for monocytic lineage development, including monoblasts, promonocytes, monocytes, macrophages, and DC [1]. The complex network of differentiating progenitors illustrates the differentiation plasticity of hematopoietic cells and reflects their ability to undergo lineage switches even in more differentiated developmental stages [11]. Due to the imperative of producing large amounts of mature cells from a limited number of progenitors, monopoiesis occurs within the interplay of proliferation and differentiation. While early myeloid progenitor cells exhibit a significant proliferative potential even during differentiation [1], later stages are characterized by increasingly limited duplication and a deceleration or arrest of the cell cycle, thus indicating progressing terminal differentiation [12].
Blood monocytes
Mature monocytes translocate to the bloodstream where they patrol for a few days on the endothelial cell layer in a transient inactive state [1, 13], screening for indication of infections or cell damage [14]. Subsequently, they extravasate or undergo apoptosis. Three subsets of human blood monocytes have been identified to date: classical CD14++CD16− monocytes, intermediate CD14++CD16+ monocytes, and non-classical CD14+CD16++ monocytes [15]. The emigration from the bloodstream into adjacent tissues includes processes like capture, rolling, arrest, adhesion, crawling, and transendothelial migration [14].
Macrophages and DC
Tissue-directed monocytes may physiologically differentiate into resident macrophages and DC, thus contributing especially to tissue homeostasis (Fig. 1) [1]. Prominent representatives of tissue-specific macrophages are alveolar macrophages (lung), Kupffer cells (liver), subcapsular sinusoidal and medullary macrophages (lymph nodes), white-pulp and metallophilic macrophages (spleen), intestinal macrophages (small intestine), intraocular macrophages (eye), and microglia (central nervous system) [1, 13, 16, 17]. Similarly, DC may stably reside in several organs and tissues, e.g., epidermis (Langerhans cells), intestine, respiratory mucosa, spleen, and bone marrow [18]. During the process of differentiation, monocytes and their further-developed descendants gain and diversify their distinct molecular, morphological, and functional properties [2, 19, 20]. This also includes the formation of a characteristic macrophage- or DC-like phenotype by cytoskeletal rearrangements [21], modulation of nuclear architecture [22], and changes in the cellular distribution of adhesion molecules [23].
Morphological changes
During monopoiesis, the progenitor stages are characterized by the formation of round, non-adherent cells containing large nuclei and low amounts of cytoplasm. In contrast to the morphologically unobtrusive, relatively small stem and early progenitor cells containing generally round nuclei [24, 25], the monoblasts, promonocytes, and monocytes represent larger cell types with increasingly indented nuclei [26, 27]. Further developmental steps yield the generation of clearly shaped macrophages and DC [28]. Macrophages are adherent cells with a flattened, spindle-shaped, amoeboid, and/or polygonal morphology [21] and a large, indented nucleus, also possessing an increased cytoplasmatic volume and—especially following activation—an enhanced number of mitochondria, Golgi apparatus components, lipid droplets, and acid phosphatase-positive granules [29, 30]. DC also represent an adherent cell type characterized by a flattened, granular body, a large nucleus, and stellate/dendritical cytoplasmic extensions [28].
Immunological functions and pathophysiological aspects
Especially in exposed tissues such as the intestinal lumen and the bronchoalveolar space, which are important gateways for infections, monocyte/macrophage populations adopt important functions in surveillance and primary host defense [31]. In response to chemokines and other granule proteins which may be produced by polymorphonuclear leukocytes following tissue injury or infections, monocytes may also invade inflamed or damaged tissue [32]. Under these conditions, monocytic cells contribute to host defense by mediating early proinflammatory and antimicrobial events [31] as well as clearing of apoptotic cells, cell debris, and toxic molecules [13]. Subsequently, these monocytes may further differentiate within hours into inflammatory macrophages and DC [1, 13], which amplify the local immune response by cytokine/chemokine production, phagocytosis as well as antigen presentation [13, 33]. In addition, the formation of alternatively activated macrophages is possible, which are involved in tissue remodeling, wound repair, and immunomodulation [1]. Hyperactivated and dysregulated as well as degenerated or transformed monocyte-derived cells may be involved in numerous diseases, e.g., sepsis [34], chronic inflammatory diseases such as rheumatoid arthritis and atherosclerosis [35–38], obesity [39], leukemia [9] as well as persistence of bacterial and viral infections such as tuberculosis or HIV-1 [31, 40].
Focus of the review
Pathophysiological mechanisms underlying infections, inflammation, leukemia, and hyperproliferation have been extensively reviewed elsewhere [31, 34, 41]. This review focuses on the integration of signaling modules and transcriptional networks involved in physiological aspects of progenitor cell expansion and myelomonocytic differentiation, and aspects of monocyte function are mentioned throughout the text.
Differentiation-associated signaling modules
Monocytic differentiation initially depends on the interaction of differentiation-inducing stimuli with their cognate receptors [42]. Subsequently, distinct signaling pathways are induced, resulting in the expression and activation of the variety of differentiation-associated transcription factors [42, 43]. In the following, the most important stimuli are specified. Afterwards, the respective signal transduction pathways are described in detail and summarized in Fig. 2.
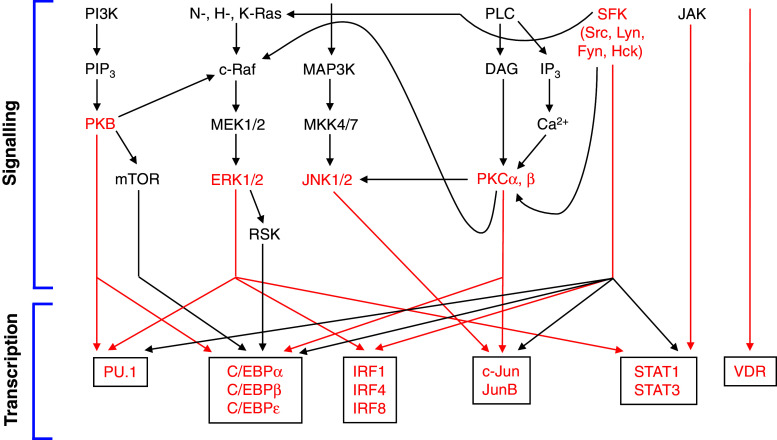
Fig. 2.
Signaling modules and associated transcription factor networks contributing to monocytic differentiation. A simplified flow diagram is shown summarizing the most important signaling modules involved in monopoiesis by orchestrating progenitor cell expansion and maturation of (myelo)monocytic cells. Furthermore, the most important connections between signaling cascades and transcriptional networks are depicted. Potential key molecules and the most prominent associations are indicated in red. Beyond regulation of transcriptional networks, several signaling molecules may also directly influence additional regulatory levels and cellular functions during monocyte/macrophage differentiation, which are not shown in this figure (for further details, see text)
Differentiation-inducing stimuli
In cells of the myelomonocytic lineage, differentiation is induced in response to a variety of different stimuli.
Fms-like tyrosine kinase 3 ligand (FLT3L)
During myelo- and monopoiesis, especially during the initial steps, FLT3L represents an important stimulus sustaining cell survival and driving the expansion of hematopoietic progenitors [44]. FLT3L binds to the Fms-like tyrosine kinase 3 receptor (FLT3) and may activate several signaling pathways. FLT3L may also support (pre)monocytic differentiation under certain conditions [44]. Its key feature, however, remains the maintenance and expansion of immature progenitor cells.
Colony-stimulating factors (CSF)
CSF exhibit both pro-proliferative and differentiation-inducing qualities (for a detailed review, see [45]). The CSF family consists of granulocyte/macrophage CSF (GM-CSF), macrophage CSF (M-CSF), granulocyte CSF (G-CSF), and interleukin (IL)-3 (also denoted multi-CSF). Like FLT3L, GM-CSF and IL-3 are crucial factors affecting survival and expansion of early hematopoietic progenitors [45]. In addition, they are able to induce initial steps of progenitor cell maturation. M-CSF and G-CSF are important inducers of monocyte and granulocyte development, respectively [45]. They are involved in regulating survival and proliferation of monocyte/granulocyte precursors and their maturation towards monocyte/macrophages or neutrophil granulocytes, thus also driving lineage commitment decisions [46]. A combined treatment with IL-3 and M-CSF, for example, efficiently differentiates embryonic stem cell lines (KCL001, KCL002, and HUES-2) towards homogenous monocytic cells [47].
Deltanoids
Deltanoids, i.e., vitamin D3 (VitD3), its biologically active form 1,25-dihydroxy vitamin D3 (1,25(OH)2D3), and other derivatives and analogues are prominent physiological agents inducing monocytic differentiation [48, 49]. 1,25(OH)2D3-induced gene expression contributes to the inhibition of proliferation, differentiation of cells of the monocytic lineage, and modulation of differentiation-related functions, e.g., the immune response [49, 50]. The effect of 1,25(OH)2D3 may be antagonized by lipopolysaccharide (LPS), a repressor of vitamin D receptor (VDR) expression [51].
Retinoids
Retinoids such as retinoic acid (RA) or all-trans retinoic acid (ATRA) also have been described as inducers of myeloid differentiation [43]. Stimulation of human (pre)monocytic cell lines such as THP-1 and HL-60 with RA or ATRA has been shown to provoke inhibition of proliferation [52, 53] and the expression of maturation-associated genes (e.g., peroxisome proliferator activated receptor γ1) [54]. Moreover, differentiation of THP-1 towards a macrophage-like phenotype can be induced with high concentrations of RA and ATRA [55, 56]. In the presence of additional agents such as bile acids or catalase, lower retinoid concentrations may also contribute to the differentiation of THP-1 and HL-60 cells [57, 58]. In addition, retinoids are able to enhance the differentiation-inducing effect of 1,25(OH)2D3 as demonstrated in U937 cells [59]. However, within the myeloid lineage, retinoids appear to predominantly induce the formation of granulocytes [43] as shown for HL-60 cells, normal or chronic myelogenous leukemia (CML)-derived GMP, and myelodysplastic syndrome-derived primary human bone marrow cells [60–62].
Cytokines and other growth factors
IL-1β stimulation yields the differentiation of murine WEHI-3B JCS myelomonocytic leukemia cells towards macrophage-like cells [63]. Growth inhibition and monocyte/macrophage differentiation was also induced by tumor necrosis factor (TNF) in ML-1 human myelogenous leukemia cells [64] and WEHI-3B JCS cells [65], an effect which is further enhanced in the presence of IL-1α, IL-1β [63], and IL-4 [66]. Stimulation of primary human monocytes with IL-4 or IL-15 results in the development of macrophages [67]. In murine M1 myeloid leukemia cells, monocyte/macrophage formation has been demonstrated to be induced by IL-6, leukemic inhibitory factor, and oncostatin M (OSM) [68]. IL-32 treatment of primary human monocytes and THP-1 cells induces monocyte/macrophage differentiation and reversed GM-CSF/IL-4-induced DC development resulting in the formation of macrophages [69]. Other factors supporting myelomonocytic differentiation and restricting the proliferation of hematopoietic cells, are members of the transforming growth factor (TGF)-β [42] and interferon (IFN) families [70]. TGF-β restricts proliferation of stem and progenitor cells by cell cycle inhibition and enhances monocytic/granulocytic differentiation [42]. IFN-γ influences myelomonocytic differentiation, lineage commitment decisions, and development of monocyte/macrophage functions [70, 71].
Microbial products
An infection of primary human HSC with Listeria monocytogenes or Yersinia enterocolitica predominantly induced the expression of monocyte differentiation markers [72]. In guinea pigs, the injection of killed Mycobacterium tuberculosis results in monocyte/macrophage differentiation in vivo [73]. The _Mycoplasma fermentans_-derived protein P48 is able to induce growth arrest and monocytic differentiation of M1, HL-60, and U937 cells [74]. Furthermore, HIV-1 infection also induces monocytic differentiation of U937 and human myelomonoblastic PLB-985 cells [75, 76].
Further agents
Monocytic differentiation can also be effectively initiated using phorbol esters such as phorbol-12-myristate 13-acetate (PMA) [77]. PMA-induced delay/inhibition of proliferation [78] and differentiation towards monocyte/macrophages have been demonstrated in a variety of leukemia-derived cell lines such as HL-60, U937, and THP-1 [79–81]. Monocytic maturation may be induced or supported by a variety of further substances. For instance, in several leukemia cell lines, an inhibition of proliferation and/or the differentiation towards a monocytic phenotype can be provoked by vitamin E (VitE)-succinate [82], adhesion molecules (e.g., laminin, fibronectin) [82, 83], and triterpenoids such as ursolic acid (UA) [84] or 2-cyano-3,12-dioxooleana-1,9-dien-28-oic acid (CDDO) [85].
Fms-like tyrosine kinase 3 (FLT3) pathway
The FLT3 receptor system is a major contributor to proliferation and expansion of hematopoietic stem and progenitor cells in response to FLT3L [44]. FLT3 is a member of the receptor tyrosine kinase class III family predominantly expressed on hematopoietic stem and progenitor cells, but also premonocytic cells [86]. Moreover, FLT3 has been suggested to play an important role in the pathogenesis of acute myeloid leukemia (AML) [44]. FLT3 directly activates several signal cascades, e.g., mitogen-activated protein kinase (MAPK)- and phosphoinositide-3-kinase (PI3K)-involving pathways (see below) [44].
FLT3-dependent effects in myelomonocytic cells
FLT3 is expressed in primitive hematopoietic progenitor cells and its expression is increased and maintained during (pre)monocytic differentiation [87]. Following activation, FLT3 promotes proliferation of early hematopoietic progenitor cells [88]. Thus, the amount of available progenitors, especially GMP, is expanded [88, 89]. In the absence of further pro-proliferative agents, FLT3 appears to support differentiation of early hematopoietic progenitors towards a monocytic phenotype [88, 89]. Treatment of HSC with FLT3L, thrombopoietin, and SCF has also been demonstrated to result in monocytic differentiation [90]. Interestingly, FLT3 deficiency seems not to affect myelomonocytic development severely as shown by virtually normal hematopoiesis in FLT3ko mice [91]. However, in irradiated mice, a combined transplantation of two hematopoietic progenitor cell populations expressing either an inactive FLT3 variant or the FLT3wt receptor reveals a predominant reconstitution of the bone marrow by FLT3wt-positive cells [91], indicating the positive (but at least not essential) influence of FLT3 on hematopoietic cell proliferation/replenishment [44]. FLT3 presumably supports proliferation of hematopoietic/myelomonocytic cells by inducing C/EBPβ-liver-enriched inhibitory protein (LIP) and decreasing the liver-enriched activating protein (LAP)/LIP ratio [92]. Moreover, an increased expression of C/EBPα and purine-rich box 1 (PU.1) has been described in FLT3L-stimulated FLT3wt cells [93].
Phosphoinositide-3-kinase (PI3K) pathway
PI3K-dependent signaling is initiated in response to ligand-induced receptor activation by cytokines and growth factors [94] and renders significant contributions to hematopoietic cell survival [42]. The PI3K family consists of three subfamilies (class I-III) of which class I has been shown to contribute to the regulation of hematopoiesis [95]. Class I includes G protein-coupled receptor (GPCR)- and receptor tyrosine kinase (RTK)-activated class IA PI3K with a regulatory subunit (p85α or β, p50α, p55α or γ) and a catalytic subunit (p110α, β, or δ) as well as selectively GPCR-activated class IB PI3K (regulatory subunit: p101, catalytic subunit p110γ). Following activation and recruitment to the cell membrane, class I PI3K are able to phosphorylate membrane-bound phosphoinositide-4,5-bisphosphate resulting in the formation of phosphoinositide-3,4,5-trisphosphate (PIP3) [95]. PIP3 may serve as an anchor molecule for the pleckstrin homology domain of protein kinase B (PKB) (isoforms: α, β, γ) thus recruiting PKB to the cell membrane. Following activation, PKB is able to translocate to the cytoplasm and to phosphorylate its substrates.
PI3K-dependent effects
PI3K/PKB activation has been demonstrated during 1,25(OH)2D3-induced monocytic differentiation of HL-60 cells [96] and sRAGE-induced monocyte-to-macrophage differentiation [97]. PI3K has also been shown to be involved in monocytic actin reorganization and adhesion [98, 99].
PKB-dependent transcription factors
Downstream, a variety of PKB substrates may be negatively regulated including glycogen synthase kinase 3 (GSK3), FoxO1/3/4, and TSC1/2 [95]. PI3K/PKB may support proliferation, survival, and differentiation of myelomonocytic progenitors since it has been suggested that GSK3 is involved in maintaining HSC and restricting neutrophil development, at least in part via negatively regulating C/EBPα [95]. In addition, inactivation of GSK3 leads to the induction of β-catenin as shown in K562 CML cells [100]. FoxO proteins negatively regulate both HSC proliferation/expansion and myeloid lineage commitment [95]. PKB also supports activation of Rheb, a mammalian target of rapamycin (mTOR)-activating Ras family GTPase, by inhibiting TSC1/2, which is a negative regulator of Rheb. Consequently, PKB positively influences mTOR activity [101]. Subsequently, activated mTOR appears to enhance HSC and progenitor cell proliferation [95] presumably by upregulating pro-proliferative C/EBPα-p30 and C/EBPβ-LIP [102, 103]. It has also been shown that M-CSF-induced and spliceosome complex protein THOC5-mediated increase in C/EBPα, C/EBPβ, and PU.1 expression also involves PIP3 [104], suggesting a role of PI3K/PKB signaling within this context. Moreover, PKB appears to be involved in distinct lineage commitment decision, since it has been shown that transduction of constitutively active PKB induces neutrophil and monocyte formation [42].
Mitogen-activated protein kinase (MAPK) pathway
MAPK signaling is a crucial mechanism controlling fundamental processes such as growth, proliferation, survival, motility, and migration and involves numerous variations of participating signaling mediators such as cytokines, growth factors, and PMA [105, 106]. In addition, differentiation and hematopoiesis may also be regulated by MAPK pathways, especially involving extracellular signal-regulated kinase (ERK) 1/2, and/or c-Jun N-terminal kinase (JNK) [107].
Ras, Raf, MAPK/ERK kinase (MEK), and ERK
The mammalian Ras family of small GTPases comprises a variety of proteins (H-, K-, N-, E-, R-, M-Ras, TC21, RalA/B, Rap1A/B, Rap2A/B/C, Rit1/2, Rheb/L1) acting as activators of Raf family kinases [108]. An activation of Ras-dependent signaling results in the increased expression and activation of activator protein (AP)-1 transcription factors, e.g., c-Jun, JunB, and Fos-related antigen (Fra)-1/2. In general, Ras proteins are strongly involved in regulating proliferation or tumorigenesis/malignancy [109] and Ras signaling-associated antiproliferative capacity is an important feature by which Ras contributes to monocytic differentiation. Ras has been shown to negatively regulate cell cycle progression in several cell types by inducing the expression and/or activation of cell cycle inhibitors such as p15, p16 [110], p19 [111], p21, and p53 [112]. In U937 and K562 cells transduced with constitutively active N-Ras, Ras-dependent antiproliferative effects appear to be mediated via interferon regulatory factor (IRF)-1 [113]. Consequently, Ras-mediated growth arrest may render myeloid cells susceptible to differentiation-promoting agents [42]. The level of active Ras is also increased during PMA-induced differentiation of HL-60 cells [79] and Ras suppression results in an altered terminal monocytic differentiation and accumulation of atypical monocytes in the blood of Ras suppressor-transduced mice [114]. In CMP and GMP, constitutively active H-Ras and N-Ras variants favor the formation of the monocytic lineage [115]. Human progenitor cells or murine myeloid FDC-P1 cells transduced with constitutively active H-Ras exhibit an enhanced monocyte/macrophage differentiation in vitro, but also tumorigenesis in mice [115, 116]. Comparably, constitutively active K-Ras induces a transient proliferative advantage as well as myelomonocytic differentiation in primary human hematopoietic stem and progenitor cells [117]. Downstream of K-Ras, pro-proliferative effects appear to depend predominantly on ERK, whereas differentiation-supporting effects are mediated via p38 [117]. Transduction of 32D cells with H-Ras or K-Ras induces differentiation towards a monocytic phenotype [118] and primary murine bone marrow-derived cells developed to terminal differentiated macrophages (despite exhibiting an enhanced self-renewal capacity) following H-Ras transduction [119]. Accordingly, bone marrow-depleted mice transplanted with H-Ras-transduced bone marrow cells are characterized by development of (pre-) T and B cell lymphomas but enhanced monocyte and macrophage differentiation [120, 121] whereas N-Ras-transduced mice exhibited hyperproliferation of myeloid cells [122]. In addition, a constitutively active H-Ras variant enhanced susceptibility of human P39 AML cells to ATRA-induced differentiation towards granulocytes and monocytes, presumably in a Raf-independent but protein kinase C (PKC)-dependent manner [123].
Ras-activated Raf family kinases (c-Raf, Raf-A, Raf-B), especially c-Raf, may further contribute to the transduction of both proliferation- and differentiation-supporting signals [107]. For example, it has been shown that c-Raf is involved in proliferation of hematopoietic progenitor cells [124] and the expansion of myeloid progenitors as demonstrated via the formation of myeloid colonies by IL-3- and GM-CSF-treated early hematopoietic progenitor cells [125]. During PMA-induced monocytic differentiation of HL-60 cells, c-Raf is activated [126]. In PMA-resistant HL60 cells undergoing differentiation towards monocytes in response to okadaic acid, c-Raf/MAPK signaling is also enhanced, presumably via an inhibition of serine-threonine phosphatases 1 and 2A [126]. Overexpression of c-Raf enhances both ATRA-dependent granulocyte formation and 1,25(OH)2D3-dependent monocyte formation of HL-60 cells [127]. Moreover, Raf plays a role in later stages of 1,25(OH)2D3-induced monocytic differentiation of HL-60 cells by activating the 90-kDa ribosomal protein S6 kinase in a MEK/ERK-independent manner [128].
Raf subsequently activates MEK1/2, serine and threonine phosphorylating members of the MEK family of kinases predominantly targeting ERK1/2 [107]. In response to pro-proliferative stimuli such as IL-3, MEK-ERK is only transiently activated in 32D cells [129]. In contrast, MEK1/2 and ERK1/2 are perpetually activated in a variety of hematopoietic or leukemia cell lines during granulocytic and monocytic maturation [129, 130]. Inhibition of MEK1/2 suppressed the differentiation of primary human bone marrow-derived cells, 32D, M1, HL-60, and U937 cells towards monocytes in response to several stimuli (e.g., PMA, 1,25(OH)2D3, CDDO, sRAGE, UA) [84, 85, 97, 130–132] as well as macrophage formation of PMA-treated human myeloid leukemia TF-1a cells [133]. ERK1ko mice are characterized by a slight increase in CMP, but a significant decrease in GMP, indicating that ERK1 plays an important role in CMP-to-GMP development [134]. Macrophage formation and function, however, were not impaired. In U937 cells treated with a combination of M-CSF and TNF, a sustained ERK activation correlates with the induction of differentiation [135]. Interestingly, during 1,25(OH)2D3-induced differentiation of HL-60 cells, ERK1/2 are highly activated in a first phase of monocyte development, which is characterized by retained proliferation in combination with the expression of initial differentiation markers [136]. In a second phase, ERK1/2 activation decreased while p27 levels increased, resulting in a block of cell cycle progression and presumably enabling terminal differentiation [136]. The p21- and retinoblastoma protein (Rb) hypophosphorylation-associated inhibition of proliferation during VitE-succinate-induced monocytic differentiation of HL-60 cells also depends on ERK activity [137]. Equivalent results have been observed in HL-60 cells treated with VitD3 derivatives [138]. Mechanistically, differentiation-supporting effects of the Ras-Raf-MEK-ERK cascade are mediated via activation and/or induction of several transcription factors as shown for PU.1, STAT3 [129], C/EBPβ [139], C/EBPα, and c-Fos [140].
MAPK kinase kinase (MAP3K), MAPK kinase (MKK), and JNK
Myelopoiesis and monopoiesis may also be affected by another MAPK pathway, i.e., the MAP3K-MKK-JNK pathway [106]. JNK is predominantly activated by upstream kinases MKK4/7 [106], which in turn are mainly activated by a variety of MAP3K [141]. Effects of JNK family members and their isoforms (p46 isoforms: JNK1α1/β1, JNK2α1/β1, JNK3α1; p54 isoforms: JNK1α2/β2, JNK2α2/β2, JNK3α2) on myelomonocytic differentiation are not comprehensively characterized [42, 141]. However, it is known that JNK is activated during monocytic differentiation of PMA-treated THP-1 and U937 cells [142, 143] or 1,25(OH)2D3-/carnosic acid-treated HL-60 cells [144]. JNK1β1, JNK2α1, and JNK2α2 are predominantly expressed and phosphorylated in LPS-stimulated THP-1-derived monocyte/macrophages [142]. Inhibition of JNK leads to (i) decreased monocytic differentiation of 1,25(OH)2D3-treated HL-60 cells [144], (ii) a reduction of the protein tyrosine phosphatase (PTP) Shp2-induced increased monocytic precursor cell formation in Shp2-transduced murine hematopoietic progenitor cells [145], and (iii) to a markedly reduced formation of macrophages in response to M-CSF in primary murine bone marrow cells [146]. A sustained activation of JNK by HPK1 contributes to cell survival and differentiation of M-CSF-treated primary murine hematopoietic progenitor cells, even in the absence of IL-3 [147]. Recently, it has been shown that JNK activation, e.g., in response to GM-CSF, is also involved in inducing autophagy, a process which is essential for maturation and survival of differentiating primary human blood monocytes [148]. Mechanistically, JNK activity may contribute to differentiation by activating transcription factors of the AP-1 family [144], especially c-Jun, JunB, and JunD [149].
Protein kinase C (PKC) pathway
PKC family kinases are divided into three subgroups: classical/conventional (PKCα, βI, βII, γ), novel (PKCσ, δ, ε, η, θ), and atypical PKC (PKCζ, ι, λ) exhibiting distinct as well as opposing features [150]. A variety of fundamental cellular processes such as proliferation, cell cycle control, and differentiation are controlled by signaling pathways which are (in)directly regulated by PKC family members [150] and PKC represents one of the central molecules involved in myeloid and monocytic differentiation [42].
PKC-dependent signaling
PKC-dependent signaling cascades are classically initiated by GPCR, RTK, or non-receptor tyrosine kinases in response to a variety of stimuli (e.g., CSF, PMA, growth factors, or cytokines) leading to the activation of phospholipase C isoforms (e.g., PLCβ/γ) and the production of diacylglycerol (DAG) and Ca2+-releasing inositol trisphosphate [150]. DAG activates conventional and novel PKC in a Ca2+-dependent (conventional PKC) or -independent (novel PKC) manner whereas atypical PKC are Ca2+/DAG-independent, but respond to ceramide (PKCζ) or activation by src family kinases (SFK) (PKCι/λ). Subsequently, PKC are able to effect further signaling mediators, e.g., GSK3β or Raf1. Thus, several PKC variants may be involved in influencing central cellular features [150] and PKCα and β appear to be the most important isoforms for lineage commitment decisions and maturation of myelomonocytic cells [42]. Following PMA or GM-CSF treatment, PKC, especially PKCα/β, rapidly translocate from the cytosol to the cell membrane in U937 and primary human monocytes and persisting membrane-associated PKC activity is induced [151–153]. During 1,25(OH)2D3-induced monocytic differentiation of HL60 cells, protein expression and activation of PKCα and PKCβ are enhanced [154, 155]. Although PKC activity typically depends on a localization at the cell membrane [156], high PKC activation levels [157] and nuclear PKC localization [158] have been reported to be associated with monocyte/macrophage maturation of GMP, whereas low levels [157] and cytosolic PKC distribution [159] appear to be associated with granulocyte formation. In early hematopoietic precursor cells, however, it has been demonstrated that high PKC activity may favor eosinophil formation, while low PKC activity induces myelomonocytic differentiation [160]. Moreover, during PMA-induced differentiation of U937 cells, PKC appears to contribute to increased cell adherence by inducing integrin hyposialylation in a Ras- and ERK-dependent manner [161]. On the (post)transcriptional level, PKC-dependent effects may be mediated via c-Jun, which is transcriptionally induced following PMA-dependent PKC activation [80], C/EBP proteins that are directly phosphorylated by PKC in vitro [162], or differentiation-associated miRNAs such as miR22 [163].
PKCα- and PKCβ-dependent effects
Selective PKCα activation yields the enhanced maturation of primary human monocyte towards macrophages [152], whereas selective inhibition of PKCα/βI results in the reduced differentiation of primary human monocyte towards macrophages in response to PMA [153]. The transduction of primary GMP with a constitutively active variant of PKCα leads to the development of macrophages even in the presence of granulocyte formation-stimulating agents [164] and PKCα overexpression in 32D cells supports PMA-driven macrophage formation [165]. These effects may be mediated at least in part by PKCα-driven phosphorylation of JNK2 [166] and the subsequent activation of Jun proteins. PKC activation is also associated with proliferation arrest and monocyte differentiation in PMA-stimulated U937 cells [80]. These effects appear to be particularly mediated via PKCβ since PKCβ-deficient U937 cells [167] and PMA-resistant HL-60 cells exhibiting low PKCβ levels [168] are unable to undergo monocytic differentiation in the presence of PMA, an effect which could be resolved by ectopic PKCβ overexpression [167]. Similar effects can be observed in HL60 cells in the presence of a PKCβ-specific inhibitor [169]. Moreover, it has been suggested that macrophage differentiation is associated with PKCβ-dependent expression of extracellular matrix proteins [170].
Src family kinase (SFK) pathway
SFK (Src, Lyn, Fyn, Yes, Fgr, Hck, Lck, Blk, Yrk) are non-receptor protein tyrosine kinases normally attached to the cell membrane and responding to a variety of stimuli, including growth factors, cytokines, and steroids (for review see [171] and [172]). While the majority of SFK is expressed in various cell types including myeloid cells [42], Fgr and Hck appear to be predominantly expressed in cells of the myeloid lineage [173]. Following activation by several types of receptors (e.g., either directly or indirectly by RTK and GPCR) or intracellular kinases such as focal adhesion kinase (FAK), SFK are able to regulate function and activity of a variety of substrates [171, 172]. Thus, SFK may control (i) cell morphology by cytoskeletal rearrangements, (ii) cell motility and adhesion via regulating proteins at focal adhesions or cell–cell contacts, or (iii) translation, metabolism, and proliferation/cell survival via regulating docking/adapter proteins, guanine-nucleotide-exchange factors, or further kinases [42, 171].
Signal integration
One of the most important features of SFK activity is their ability to activate or enhance alternative signaling cascades [42]. For instance, SFK may directly activate STAT proteins in several cell types [174, 175] or indirectly (by recruiting several adapter proteins) the Ras pathway [176], thus potentially driving PU.1, C/EBPα/β, or c-Fos activation. PKC-dependent pathways also appear to be prone to SFK-mediated modulation, since PKC may be activated by Fyn in THP-1 [177] and PKCδ has been shown to be activated by Hck and Lyn in U937 and peripheral blood monocytes [178]. In addition, in various models, it has been reported that SFK are able to phosphorylate RTK (e.g., epidermal growth factor receptor and SCF receptor) [179, 180].
Progenitor cell differentiation
SFK appear to be involved in maintaining cell survival and in the expansion of immature myeloid progenitor cells even under differentiating conditions [42]. In addition, differentiation and differentiation-associated cellular processes such as cytoskeletal rearrangements, adhesion, and migration, may also be effected by SFK [42]. Src, which is an activator of STAT3 and (via FAK/JNK) Jun proteins [172], is significantly induced and activated during PMA-induced monocytic differentiation of U937 and HL-60 cells [181]. Fgr is induced by M-CSF in primary murine monocytic cells and appears to be especially expressed during later developmental stages, whereas Src has been detected during all developmental steps [182]. Hck or Fyn are induced in differentiating THP-1 cells in response to PMA or Bryostatin 1, respectively [177, 183]. Interestingly, overexpression of an inactive Hck variant enhances adherence in U937 cells, whereas constitutively active Hck enhances cell migration [184]. During monocyte/macrophage differentiation of HL-60 cells, SFK expression appears to be stimulus-dependent, since Lyn and Fgr are induced in response to VitD3, whereas Lyn and Fyn are induced in response to PMA [185]. In this context, Lyn and Fyn have been shown to be associated with tubulin phosphorylation and reorganization of microtubules [186] and Lyn has been also suggested to be involved in regulating actin filament polymerization [187]. Lynko mice exhibit increased amounts of immature myeloid progenitor cells (including GMP, promyelocytes, and multilineage progenitors) [188] and susceptibility towards monocyte/macrophage-derived leukemia development [189]. This suggests a role of Lyn in controlling myeloid progenitor proliferation. SFK-dependent effects may be mediated by IRF1, IRF8, and/or c-Jun since it has been demonstrated in DC that these transcription factors can be activated (in)directly by Src family kinases [190]. Lyn has been described to be an activator of PU.1 expression and activity in chicken B cell line DT40 and primary murine splenocytes [191]. In RAW 264.7 cells, SFK such as Hck have been shown to be involved in the activation and binding activity of C/EBPβ, c-Jun, JunB, JunD, and NF-κB subunits c-Rel and p50 [192]. In addition, STAT3 can act as a substrate of Src, Hck, Lyn, and Fyn as demonstrated in SF9 insect cells [193].
Differentiation-associated transcription networks
In the following, prominent transcription factors contributing to progenitor cell expansion and monocyte/macrophage differentiation during different stages of maturation are described (Table 1).
Table 1.
Predominant roles of relevant transcription factors during monocytic differentiation
| Transcription factor | Predominant role during monopoiesis |
|---|---|
| PU.1 | Initiation and maintenance of monopoiesis |
| C/EBPα-p30 | Expansion of progenitor cells, initial differentiation towards GMP |
| C/EBPβ-LIP | Expansion of progenitor cells |
| STAT1/3 | Restriction of proliferation, progenitor stage transitions |
| C/EBPα-p42 | Restriction of proliferation, MPP-CMP-GMP-MDP transition |
| c-Jun | MPP-CMP-GMP-MDP-monocyte transition |
| JunB | Restriction of proliferation |
| VDR | Restriction of proliferation MPP-CMP-GMP-MDP-monocyte transition |
| IRF1/4 | Restriction of proliferation, GMP-MDP-monocyte transition |
| C/EBPβ-LAP*/LAP | Restriction of proliferation, GMP-MDP-monocyte transition |
| IRF8 | Restriction of proliferation, GMP-MDP-monocyte transition, terminal differentiation |
| C/EBPε | Terminal differentiation |
Purine-rich box 1 (PU.1)
The transcription factor PU.1 is a member of the Ets transcription factor family [194] especially expressed in cells of the myeloid lineage. Although there is no master regulator solely determining myeloid lineage commitment [195], PU.1 appears to be the major regulator of monocytic differentiation [196].
Dose-dependent influence on differentiation
Mice deficient in functional PU.1 are unable to generate maturated cells of the myeloid lineage and die at a late gestational stage or quickly after their birth [197–199]. Expression of PU.1 is increased during early steps of monocyte maturation [200] and essential for CMP formation [201, 202]. PU.1 favors the formation of the myeloid lineage in early developmental steps by inhibiting GATA-1-mediated generation of the erythroid lineage [203, 204] by direct protein–protein interaction [205], whereas PU.1-dependent downregulation of GATA2 gene expression induces macrophage formation and a block of mast cell development [206]. Myeloid progenitor cells transduced with a 4HT-inducible PU.1/ER differentiated towards MDP but not granulocytes in response to 4HT when combined with GM-CSF or IL-3/IL-6/SCF [207]. The impact of PU.1 on myeloid cell maturation appears to be dose dependent, since PU.1-retransduced PU.1ko progenitor cells develop towards monocyte/macrophages in the presence of high PU.1 concentrations, while low PU.1 levels support the formation of B lymphocytes [208]. In this context, high PU.1 expression may be supported by EDAG, which is highly expressed in early stage bone marrow cells and can act as a potent inducer of PU.1, thus favoring myelopoiesis and blocking lymphopoiesis [209]. In addition, high PU.1 amounts in GMP yield the development of monocytes, a phenomenon which may be supported by a positive autoregulatory feedback loop [210], while low levels result in the formation of promyelocytes as precursors of granulocytes [211, 212]. The latter effect could be shown by selective knock out of one PU.1 allele [211], reduction of PU.1 expression by deletion of its distal enhancer [213], or PU.1 elimination in adult mice [201]. Equivalently, in PU.1wt/wt, PU.1wt/ko, and PU.1wt/kd bone marrow-derived myeloid progenitor cells, C/EBPα-induced PU.1 expression yields the formation of monocytic cells, while impaired C/EBPα-induced PU.1 expression in PU.1kd/kd cells (also lacking the distal enhancer) results in the development of granulocytic cells [214]. In an alternative murine model, in which PU.1 expression was considerably reduced by mutation of two (out of three alternative) PU.1 start codons, lymphoid and myeloid cell development was impaired including an expansion and infiltration of immature myeloid cells, e.g., in the spleen of the respective mice [215]. An induction of PU.1 in combination with IRF8 and the initiation of monopoiesis but repression of granulopoiesis can also be created in GMP responding to IFN-γ [216]. Actually, a cooperation of PU.1 and proteins of the IRF family for differentiation-associated gene expression has been demonstrated in promoters containing different Ets/IRF binding sites [217]. During terminal differentiation towards macrophages and granulocytes, however, PU.1 levels are further enhanced in both lineages [200, 211].
Lineage commitment and lineage conversion
Following PU.1 transduction, the expression of high amounts of PU.1 in both hematopoietic progenitors and PU.1ko myeloid progenitors results in the formation of macrophages [208, 218]. Vice versa, PU.1ko HSC exhibit an inhibited CMP development and monocytic differentiation is blocked in PU.1ko CMP or GMP [202]. Murine embryos exclusively expressing inactive variants of PU.1 are characterized by an absence of monocytes in combination with a diminished formation of neutrophil granulocytes [197, 198]. In PU.1ko 503 hematopoietic liver cells, neither the monocytic nor the granulocytic lineage can be formed even in the presence of other differentiation-supporting transcription factors such as C/EBPα [214]. Moreover, it has been shown that PU.1 transduction enables the conversion of B and T lymphocytes into monocytes or macrophages [219–221]. Interestingly, even fibroblasts can be converted into macrophage-like cells if transduced with PU.1 in combination with C/EBPα or C/EBPβ [222].
Potential substitution by other Ets proteins
In vitro cultured PU.1ko murine embryonic stem cells and myeloid progenitors exhibit expression of selected myeloid mRNA species (e.g., GM-CSFR, G-CSFR, and myeloperoxidase) [223] or monocytic differentiation markers (e.g., Ly6C, CD11b, CD18, CD31) [224], respectively. This supports the assumption that other Ets family members, presumably in combination with other differentiation-supporting transcription factors, may be able to compensate (at least in part) for a loss of PU.1 under these conditions [225]. The Ets transcription factor FL-1, for example, plays a role within myeloid lineage commitment decisions since Friend leukemia integration (FLI1)ko mice exhibit (amongst other hematopoietic abnormalities) an increase in monocytic and a decrease in granulocytic progenitors [226] whereas a knock out of the multimeric Ets family member GABP results in a reduced overall amount of myeloid cells [227]. For terminal maturation towards monocytes and granulocytes, however, PU.1 is indispensable [223].
Downstream effects
In part, PU.1 may mediate its differentiation-inducing features by activation of its downstream target KLF4 which is able to initiate monocyte formation in HSC, CMP, and even PU.1ko fetal liver cells, while KLF4 deficiency increased the formation of granulocytes [228]. In proleukemic HSC, PU.1kd is associated with downregulation of c-Jun and JunB as well as the appearance of a myelomonocytic differentiation block, indicating a positive influence of PU.1 on c-Jun and/or JunB expression and potential c-Jun/JunB-mediated effects [229]. Furthermore, a variety of miRNAs is induced or repressed by PU.1 [230]. For instance, PU.1 positively regulates the transcription of miR146a and the miR23a cluster [230, 231]. The latter, coding for miR23a, miR27a, and miR24-2, supports myelopoiesis and inhibits B lymphopoiesis even under B cell formation-promoting conditions [231] whereas ectopic miR146a expression directs adult hematopoietic stem cells selectively to functional peritoneal macrophages in a murine transplantation approach [230]. Moreover, in a zebrafish model, it could be shown that miR146a is also required for myeloid development and macrophage formation during embryogenesis [230].
CCAAT/enhancer binding protein (C/EBP) family
The six members of the C/EBP family (C/EBPα, β, γ, δ, ε, and ξ) are transcription factors of the bZIP type (for review see [232]). While C/EBPγ, δ, and ξ are unique proteins, several isoforms exist of C/EBPα, β, and ε, differing in size and function [232]. C/EBPα-p42 has a higher transactivation capacity than the smaller C/EBPα-p30 variant, which is able to inhibit p42 transactivation potential. Full-length C/EBPβ, i.e., C/EBPβ-LAP*, and the only slightly smaller C/EBPβ-LAP exhibit transactivation activity, whereas C/EBPβ-LIP is transcriptionally inactive and may serve as a dominant-negative inhibitor of LAP*/LAP. Four C/EBPε isoforms with decreasing transactivating capacity are known: p32, p30, p27, and p14 [232]. As homo- or heterodimers, C/EBP family proteins play a relevant role within the process of hematopoiesis and (myelo)monocytic differentiation [43]. C/EBP family members—especially C/EBPα, β, and δ—are strongly expressed in the myeloid lineage and different C/EBP factors appear to play distinct roles during selected stages of myelomonocytic differentiation [43].
C/EBPα
C/EBPα-p42 may facilitate (myelo)monocytic differentiation in an initial step by inhibiting cell cycle progression [233, 234] via inhibition of pro-proliferative factors (e.g., c-Myc) [235], induction of cell cycle inhibitors (e.g., p21) [236], and direct protein–protein interaction with Rb, E2F, and cyclin-dependent kinase (CDK)2/4 [233]. Although it has been reported that C/EBPα-p30 retains the ability to slow myeloid progenitor proliferation despite its limited transactivation potential [237], p42 appears to be required for effective control of myeloid progenitor proliferation [238]. C/EBPα expression is upregulated during early differentiation stages towards the GMP in humans and mice [43, 212] and equivalent results have been obtained in Xenopus [239]. Irradiated mice showed a significant increase in myeloid cells but a decrease in erythroid cells following transplantation of C/EBPα overexpressing bone marrow cells [240]. C/EBPα overexpression in murine erythroleukemia cells induced the expression of myeloid genes and the development of a myeloid-like phenotype [240]. Furthermore, C/EBPα caused a lineage switch towards the myeloid lineage in megakaryocyte-erythroid progenitors [240, 241]. This supports the assumption that C/EBPα suppresses erythroid lineage formation but promotes the determination of the myeloid lineage [240]. Primary murine bone marrow-derived mononuclear cells which were transduced with an estradiol-inducible C/EBPα fusion protein and stimulated with estradiol and myeloid cytokines (IL-3, IL-6, SCF, and/or GM-CSF) exhibited increased numbers of monocytic cells in combination with a reduced amount of granulocytic cells [207]. This effect may be mediated via C/EBPα-induced PU.1 expression as well as the interaction with other transcription factors, e.g., c-Jun and c-Fos [207, 212]. Moreover, it could be shown that the presence of C/EBPα inhibits a further determination of the lymphoid lineage in common lymphoid progenitors (CLP) and (pro-) B cells by antagonizing Pax5, thus promoting the subsequent reprogramming of these cells and the development of pluripotent cells [242] or GMP [219, 243]. In addition, transduction of lymphoid cells like CLP, B cells, and T cells with C/EBPα results in their reprogramming towards monocyte/macrophages [219, 221, 241, 244]. Several knock-out models illustrated that C/EBPαko mice are characterized by an inhibited differentiation of CMP towards GMP, resulting in a reduced number of GMP, MDP, monocytes, and macrophages as well as granulocytes in combination with an accumulation of immature myeloid progenitors [245–247] and cells of the erythroid lineage [240]. Accordingly, inhibition of the intrinsic transactivation capacity of C/EBP family members by application of an inducible, dominant-negative C/EBP inhibitor protein inhibits the formation of GMP, MDP, and granulocyte progenitors in mice as well as the differentiation of murine 32Dcl3 cells towards granulocytes [248]. Interestingly, C/EBPα-p42 does not appear to be essential for CMP-to-GMP transition, since the presence of p30 is sufficient for GMP formation [238]. However, in contrast to p42, p30 presumably posses no further differentiation potential since p30 overexpression attenuated G-CSF-induced differentiation of murine 32D hematopoietic cells in a dose-dependent manner [249]. Moreover, in mice, expression of p30 in the absence of p42 or overexpression of a C/EBPα-p42 variant carrying a mutation in the bZIP region yields the formation of hyperproliferative myeloid progenitors and progression to acute myeloid leukemia [238, 249]. In an alternative study, the enhanced expression of p30 results in a block of myeloid differentiation in human but not in murine primary hematopoietic progenitor cells [250]. In human hematopoietic cells transduced with p30, it has also been shown that p30 is able to provoke the initial steps in myelopoiesis, resulting in the accumulation of myeloblasts and myelocytes, but fails to promote terminal steps of monocytic or granulocytic differentiation [251]. These data also indicate that C/EBPα contributes to myeloid lineage commitment in early and central developmental stages. In later stages, C/EBPα is able to contribute to further differentiation of myelocytes towards monocytes as represented by upregulated C/EBPα levels during monocytic differentiation of mixed lineage leukemia (MLL) fusion gene-positive myelomonoblastic cell lines (i.e., THP-1, MOLM-14, HF-6) [252]. Moreover, in HF-6 and primary AML cells, C/EBPα transduction results in reduced proliferation and enhanced monocytic differentiation [252, 253]. For terminal monocyte maturation, however, C/EBPα appears to be dispensable since Cre recombinase-directed C/EBPα deletion in GMP did not alter the distribution of mature macrophages or granulocytes [246].
C/EBPβ
During myelo-/monopoiesis, different C/EBPβ isoforms appear to regulate multifaceted features in early versus late developmental stages [254]. LIP promotes proliferation in early progenitor cells, as reflected by FLT3L-mediated LIP induction in combination with a decreased LAP/LIP ratio in FLT3wt receptor-transduced 32D hematopoietic cells [92]. These effects can also be found in premonocytic and/or leukemia cells possessing constitutively activating FLT3 mutations such as internal tandem duplications [92]. In contrast, LAP* and LAP suppress (pre)monocytic proliferation by inhibition of c-Myc, E2F1, and cyclin D1/E expression, induction of p27, and interaction with Rb and E2F [255]. In developmental stages providing the formation of both the granulocytic and the monocytic lineage, presence of LAP*/LAP appears to promote the differentiation towards granulocytes since C/EBPβ overexpression in murine bone marrow cells yields an increased formation of granulocytes at the expense of myeloid progenitors which are present in reduced amounts [256, 257]. However, during maturation of (pre)monocytic cells such as MDP, promonocytes, monocytes, and macrophages, LAP* and LAP are prominently expressed [254]. Then, the activation of the larger C/EBPβ isoforms is directly associated with monocytic maturation including the expression of differentiation markers [254], morphological changes [84, 255], and an increasing antimicrobial activity [84]. For instance, in PMA-stimulated NB4 cells, ERK-mediated C/EBPβ expression is involved in inducing Pyk2, a kinase involved in regulating macrophage morphology and migration [258]. For the establishment of the (myelo)monocytic lineage, however, C/EBPβ seems not to be absolutely essential, since C/EBPβko mice are able to produce macrophage-like cells [259, 260]. Immortalized C/EBPβko macrophages derived from C/EBPβko mice, however, exhibit an impaired functionality as reflected by a defective activation in response to activators such as LPS and bacteria [259, 261], impaired induction of differentiation markers [260], and an incomplete development of macrophage morphology [255]. Moreover, the respective mice exhibit an enhanced susceptibility to microbial infections [259, 261]. In addition, formation of myeloid colonies and the amount of generated cells were decreased in C/EBPβko bone marrow-derived progenitor cells [256].
C/EBPε
Another C/EBP family member, C/EBPε, is expressed in both myeloid and lymphoid tissues [262] and may be transcriptionally induced by PU.1 [263]. C/EBPε-p32 has also been shown to negatively regulate the cell cycle and myeloid progenitor proliferation, e.g., by downregulation of c-Myc, CDK4/6, and cyclin A/D2/E in combination with induction of cell cycle inhibitor p27 [264, 265]. Although the influence of different C/EBPε isoforms on (myelo)monocytic differentiation has not been completely elucidated, p32 and p30 appear to be the variants most influencing differentiation processes since they are coexpressed during different myeloid progenitor stages and p30 is specifically upregulated during retinoid-induced differentiation of NB4 cells [266]. Human hematopoietic progenitor cells transduced with different C/EBPε isoforms are able to generate GMP regardless of the respective isoform when treated with SCF, GM-CSF, IL-3, and IL-5 [267]. In addition, the induction of myeloid-specific genes and markers of differentiation (e.g., neutrophil elastase, M-CSFR) may be mediated by p32 and/or p30 in hematopoietic cells, either basal or in response to activating stimuli (e.g., LPS) [266]. Elevated levels of C/EBPε occur during granulocytic versus monocytic lineage determination [268]. Furthermore, C/EBPε-deficient mice demonstrated an enhanced population of actively proliferating bone marrow cells [269], incomplete terminal neutrophil granulocyte differentiation, and impaired macrophage functions [43]. On the other hand, C/EBPε appears to also play a role within the terminal maturation of monocyte/macrophages, since C/EBPε is upregulated during differentiation of MLL-positive myelomonoblasts towards a monocytic phenotype and C/EBPε transduction of HF-6 cells leads to growth arrest and monocyte formation [252]. In addition, other C/EBPεko mouse models solely produce immature and functionally impaired macrophages [270, 271] or an immature intermediate myeloid phenotype exhibiting characteristics of both monocytes and granulocytes [268]. In addition, monocyte/macrophages derived from neutrophil-specific granule deficiency patients carrying homozygous recessive C/EBPε loss-of-function mutations are abnormal in terms of granularity and migration [272]. Consequently, C/EBPε may act as a factor possessing general importance for myeloid lineage determination and terminal differentiation of myeloid cell types [273].
Vitamin D receptor (VDR)
The VDR, which is constitutively expressed in primary human monocytes, but downregulated following differentiation towards macrophages [274], is a member of the nuclear receptor superfamily [275]. Following association with VitD3 derivatives such as 1,25(OH)2D3 [49], VDR is able to directly act together with a coreceptor (retinoic X receptor; RXR) as a heterodimeric transcription factor influencing myeloid differentiation and supporting monocytic lineage commitment [275]. Within the cell, VDR is located in the cytoplasm, the nucleus, or partitioned between both compartments [276]. Upon activation, VDR/RXR heterodimers are able to directly interact with VDRE in the promoters of a variety of VDR-responsive target genes [49, 277].
VDR-mediated inhibition of proliferation
Although it has been reported that 1,25(OH)2D3 is able to promote monocyte proliferation at low concentrations [278] and that HL-60 cells proliferate and expand rapidly in response to 1,25(OH)2D3 [279], alternative vitamin D compounds/analogues as well as higher concentrations of 1,25(OH)2D3 have been shown to inhibit proliferation of NB4, HL-60, U937, and THP-1 cells in a VDR-dependent manner [49, 280, 281]. VDR repression, in turn, significantly decreases antiproliferative effects of 1,25(OH)2D3 [282], which are presumably based on the presence of VDRE in the promoters of cell cycle regulators such as p21, Rb, or p53 [49]. In addition, reduced proliferation of 1,25(OH)2D3-treated HL-60 cells was associated with an increase in VDR but a decrease in c-Myc expression [283]. Accordingly, 1,25(OH)2D3-resistant HL-60 cells were not able to reduce their proliferation and the expression of both genes remained unaffected [283]. The occurrence of 1,25(OH)2D3-induced inhibition of proliferation in combination with cellular differentiation is reflected by enhanced expression of C/EBPβ and Rb in differentiating HL-60 cells [49]. Comparably, monocytic differentiation of U937 cells and increased expression of differentiation markers (e.g., CD14) in response to 1,25(OH)2D3 [282] are associated with VDR-dependent p21 expression [284]. In consequence, 1,25(OH)2D3 and its derivatives are regarded as possible therapeutic tools for the treatment of hyperproliferative disorders such as leukemia [285].
VDR-mediated monocytic differentiation
In 1,25(OH)2D3- or VitD3 derivative-treated HL-60 and U937 cells, VDR-associated reduction of proliferation was accompanied by differentiation towards a monocyte-like phenotype [283, 286]. Treatment of primary human mononuclear blood cells, normal or leukemic primary human myeloid stem/progenitor cells, and HL-60 cells with 1,25(OH)2D3 or fluorinated analogues/derivatives induced their differentiation towards monocytes [287] and macrophages [288, 289]. Stimulation of human bone marrow-derived mononuclear cells, U937, or HL-60 cells with a combination of 1,25(OH)2D3 and ATRA [290–292] or GM-CSF [286, 293] yields monocytic cells but not granulocytes. This phenomenon is presumably mediated by VDR/RXR via the repression of retinoic acid receptor (RAR)/RXR-dependent genes and activation of VDR/RXR-dependent genes [292, 294]. Like granulopoiesis, monocyte-derived DC formation and function are also inhibited by 1,25(OH)2D3 and its analogues in humans and mice [295–297]. In contrast, VDR appears to play a positive role in TGF-β-induced Langerhans cell development [298]. In NB4 cells, it has been shown that differentiation-supporting effects of 1,25(OH)2D3 and other VitD3 derivatives may also be mediated in a VDR-independent manner, i.e., without VDR induction and activation, which suggests an influence of deltanoids on alternative signaling pathways, at least in promyelocytic leukemia cells [299]. VDRko mice are characterized by normal amounts of monocytes and granulocytes, which indicates that myelopoiesis may be induced via alternative pathways under these conditions [300]. Thus, 1,25(OH)2D3-induced VDR activity is not essential for terminal monocyte/granulocyte development in vivo [49]. Nonetheless, functional processes are impaired in VDRko macrophages as demonstrated by decreased IL-18 production [300].
Interferon regulatory factor (IRF) family
The IRF family of transcription factors consists of nine proteins (IRF1-9) (for a detailed review see [217] and [301]). IRF are constitutively expressed in a variety of cell types including myeloid cells, but may be induced in response to a variety of stimuli, e.g., IFN-α/β/γ [217], PMA [302], ATRA [303], GM-CSF, IL-4 [304], or TGF-β [305]. Following activation by phosphorylation, IRF dimerize and translocate to the nucleus, where they can directly act as transcription factors in cooperation with CBP/p300. IRF are involved in regulating immune response and host defense, apoptosis, and tumor suppression, but also cellular differentiation of immune cells. Different combinations of IRF (especially IRF1/4/8) contribute to the formation of myeloid cells, especially granulocytes, macrophages, and DC [217].
Proliferation
IRF family members appear to act as negative regulators of proliferation in myeloid cells [217]. It has been shown in several cell types that IRF1 transactivates p21 expression and cell cycle arrest in cooperation with p53 [306, 307] and induces apoptosis under certain conditions (e.g., in response to IFN-γ) [308]. Overexpression of IRF4 or IRF8 in murine Tot2 myeloid progenitor cells and IRF7 in U937 cells has been demonstrated to induce cell cycle arrest [309–311]. In myeloid (progenitor) cells, IRF8 is able to induce cell cycle inhibitor p15 [312], repressors of c-Myc expression (i.e., BLIMP1 and METS) [313], and neurofibromin 1, a repressor of Ras-associated proliferation [314]. Correspondingly, IRF8-deficient mice are characterized by myeloproliferative disorders [315].
Monocytic differentiation
Maturation of macrophages and DC appears to be influenced particularly by IRF1, 4, and 8 [217]. IRF1 expression is upregulated during monocytic maturation [316] and the expression of several macrophage differentiation markers (e.g., iNOS, Cox2, or CIITA) is IRF1-sensitive [317–319]. IRF1 appears to mediate essential maturation-associated features, since IRF1 repression inhibits PMA-induced monocytic differentiation of U937 cells [302] and IRF1ko bone marrow cells are characterized by increased amounts of immature granulocyte precursor cells [320]. Moreover, their ability to generate further differentiated cells in response to differentiation-inducing stimuli (i.e., M-CSF or G-CSF) is impaired [320]. Mechanistically, this may be connected to reduced expression levels of PU.1, C/EBPα, and C/EBPε which were found in IRF1ko bone marrow cells [320]. Although IRF4ko mice do not exhibit obvious abnormalities in the myeloid lineage, IRF4 appears to contribute to monopoiesis, especially by inhibiting granulocyte formation and during macrophage formation as shown using Tot2 cells [309]. Mice exhibiting both IRF4ko and IRF8ko are characterized by the development of more severe splenomegaly and CML-like leukemia than in IRF8ko mice, including an enhanced proportion of granulocytes [309]. However, since IRF4 is not as strongly expressed as IRF8 in GMP, IRF8 appears to be the more important regulator of monocyte/macrophage maturation [309]. In humans and mice, IRF8 plays a decisive role in concurrently initiating macrophage and DC maturation and suppressing the formation of granulocytes [310, 321, 322], e.g., in response to IFN-γ during inflammation-induced myelopoiesis [216]. This has also been confirmed in alternative models such as zebrafish [323]. Accordingly, IRF8 is significantly expressed in murine hematopoietic progenitor cells during differentiation towards macrophages and further persists in mature macrophages, whereas its expression is reduced in the granulocytic lineage [321]. IRF8 also appears to be regulated by redox signaling, since it has been shown that 12/15-lipoxygenase deficiency leads to impaired IRF8 transactivation activity and reduced monopoiesis in mice [324]. In part, IRF8-dependent effects during monocyte/macrophage differentiation may be mediated via the promyelocytic leukemia protein [325]. IRF8ko mice exhibit reduced amounts of MDP and/or macrophages in combination with increased amounts of immature granulocyte precursor cells and/or neutrophil granulocytes [315, 326] culminating in the development of CML-like blast crisis [315]. In normal mice, injection of IRF8ko blasts yields the development of AML [315], an effect which may be aggravated by constitutive active variants of Shp2 [327]. Moreover, IRF8ko progenitor cells tended to differentiate towards granulocytes even in the presence of M-CSF [326]. This is also reflected in the bone marrow of IRF8ko mice, which is characterized by decreased amounts of cells of the monocytic lineage [326]. However, transduction of IRF8 into IRF8ko bone marrow cells counteracts IRF8ko-dependent effects as represented by increasing monocyte/macrophage formation at the expense of granulocyte formation [321]. IRF8 regulates distinct transcriptional programs in lymphoid and myeloid progenitors [322] and several IRF8-dependent genes adopt a pivotal role for macrophage function, e.g., a variety of enzymes (cathepsin C, lysozyme, cystatin C), cytokines/chemokines (IL-8, IL-12p35/p40, IL-18), and receptors (Toll-like receptor 4/9, FcγR) [217]. During IFN-γ-induced differentiation of U937 cells, IRF8 also mediates protective effects against genotoxic stress by inducing Fanconi F, a protein involved in the repair of cross-linked DNA [328]. IRF1 and especially IRF8 appear to be positive regulators of macrophage maturation, whereas neutrophil formation depends on IRF1 but is inhibited by IRF8 [217].
Signal transducer and activator of transcription (STAT) family
In response to a variety of stimuli, e.g., IFN family members, SCF, M-CSF [329], G-CSF [330], leukemia inhibitory factor (LIF), IL-6, OSM [331], IL-13 [332], and IL-3 [333], (myelo)monocytic differentiation is induced following receptor oligomerization [42] via activation of receptor-bound members of the Janus kinase (JAK) family consisting of JAK1, JAK2, JAK3, and Tyk2 [334]. Following activation, JAK are able to directly activate transcription factors of the STAT family (STAT1, 2, 3, 4, 5A, 5B, 6) by tyrosine phosphorylation.
JAK-dependent effects
JAK1 and Tyk2 appear to play a role for monopoiesis since PTPεC inhibited IL-6- and LIF-induced monocytic differentiation of M1 cells presumably via JAK1 and Tyk2 dephosphorylation [335]. JAK2 has been identified as a positive mediator during GMP formation of the murine multipotent hematopoietic cell line EML in response to IL-3 [336]. JAK3 is upregulated and/or activated during IL-6-induced monocytic differentiation of M1 cells [337]. Furthermore, terminal maturation appears to be JAK3-dependent as shown in JAK3ko mice, which are characterized by increased amounts of immature granulocytic and monocytic progenitor cells [338]. Conversely, JAK3 overexpression yields accelerated macrophage differentiation in GM-CSF-treated primary murine bone marrow cells [337].
STAT1/3-dependent effects
It has been shown that STAT1 is activated during spontaneous or adhesion molecule (laminin, fibronectin)-induced differentiation of primary human monocytes [83] and IFN-γ-induced monocytic differentiation of U937 cells [339]. Interestingly, in this context, differentiation-supporting STAT1 activity appears to depend on JAK-independent serine phosphorylation since tyrosine- and serine-phosphorylated STAT1 is able to induce differentiation marker in human monocytes [83], whereas even constitutive STAT1 tyrosine phosphorylation by Tyk2 overexpression did not enhance STAT1 target gene transcription in U937-derived monocytes [340]. However, in U937 cells constitutively expressing tyrosine phosphorylation-defective STAT1, proliferation arrest and differentiation are inhibited, indicating that STAT1 tyrosine phosphorylation is also of importance, presumably for dimerization and nuclear translocation [341]. During monocyte-to-macrophage differentiation, STAT1 nuclear translocation appears to be specifically mediated by the nuclear shuttle protein nucleolin [342]. Stimulation of human monocytes with the secreted tumor suppressor protein Dickkopf-3 induced STAT1/3 activation and resulted in the differentiation towards a DC-like phenotype [343]. An activation of STAT1, 3, and 5 in murine bone marrow-derived cells is accompanied by an expansion of myeloid cells and an enhanced expression of macrophage differentiation marker [344]. STAT3 appears to be involved in cell cycle arrest by induction of p27 [345] and p19 [346]. During IL-6- and/or LIF-driven growth arrest of M1 cells and differentiation towards macrophages, STAT3 is significantly induced, whereas a dominant-negative STAT3 variant inhibits proliferation stop and differentiation [347, 348]. STAT3 already plays a role during early steps of hematopoiesis as demonstrated by JAK2 and STAT3 activation in erythropoietin-treated murine HSC [349]. Moreover, suppression of STAT3 activity by PTPεC-dependent inhibition of JAK1 and Tyk2 impaired IL-6- and LIF-induced monocytic differentiation of M1 cells [335]. Mice possessing STAT3ko bone marrow cells are characterized by an accumulation of mature (at the expense of immature) neutrophil granulocytes suggesting that STAT3 may act as a restrictor of granulopoiesis [350, 351]. However, neutrophil mobilization in response to G-CSF and chemotactic response to MIP-2 were severely impaired in STAT3ko neutrophils [351] and a reduced anti-inflammatory capacity in STAT3ko macrophages and neutrophils could be determined [352]. STAT3 also induces expression of C/EBPα [353], JunB, and IRF1 [346], thus potentially enhancing its antiproliferative and differentiation-supporting effects.
Activator protein 1 (AP-1) family
AP-1 is a bZIP type transcription factor classically consisting of homo- or heterodimers of the Jun (c-Jun, JunB, JunD) and Fos (c-Fos, FosB, Fra-1, Fra-2) protein families (for review see [354] and [355]). Nonetheless, an interaction with other bZIP transcription factor families, such as JDP (JDP1, JDP2), ATF (ATF1, ATF3, B-ATF), and Maf (c-Maf, Maf-A, -B, -F, -G, -K, Nrl) is also possible [356].
Regulation of proliferation
Overexpression c-Jun in U937 cells or chicken BM2 monoblasts contributes to reduced proliferation rates [357, 358] and equivalent results were obtained in c-Jun and c-Fos expressing U937 or HL-60 cells in response to PMA [151, 359] or tanshinone derivative E278 [360]. In an alternative study, inhibition of c-Jun by antisense approaches was associated with reduced proliferation suggesting a pro-proliferative role of c-Jun in PMA-stimulated U937 cells [361]. In addition, ectopic overexpression of c-Jun in HL-60 cells led to enhanced proliferation, whereas inhibited expression of c-Jun was associated with cell cycle arrest, increased expression of p21, reduced activity of CDK2/4, and attenuated Rb phosphorylation [362]. Therefore, one might speculate that c-Jun mediates pro- and antiproliferative effects under certain but different conditions. JunB mRNA expression is also rapidly and strongly induced in PMA-treated HL-60, THP-1, and U937 cells [363]. JunB or c-Fos overexpression in M1 cells resulted in reduced proliferation rates [364]. Moreover, mice with a myeloid lineage-specific JunBko are characterized by the development of myeloproliferative disease, a phenotype which could be reverted by ectopic JunB expression [365, 366]. The antiproliferative features of JunB may be based on a positive regulation of cell cycle inhibitors (e.g., p16) and/or negative regulation of proliferation-inducing genes (e.g., cyclin D1), either directly or indirectly, as suggested by results obtained in bone marrow-derived mast cells [367]. In proleukemic PU.1kd HSC, it has been shown that a restoration of (originally downregulated) JunB expression is able to suppress leukemic self-renewal capacity and to prevented the development of leukemia in PU.1kd HSC-transplanted NOD-SCID mice [229]. In consequence, JunB may act as a restrictor of excessive myeloid progenitor expansion [366]. Although the influence of JunD on myelomonocytic cell proliferation has not been extensively addressed, it has been shown recently that JunD may also adopt proliferation-restricting functions, since JunD silencing promotes the expansion of 32D cells [368].
Monocytic differentiation
Early it has been shown that Jun proteins are involved in monocytic differentiation since the levels of Jun and Fos proteins are rapidly increased during PMA-induced maturation of U937 and/or HL-60 cells [151, 357], whereas dexamethasone-induced inhibition of U937 differentiation is associated with decreased c-Jun and c-Fos gene expression [369]. Consequently, during differentiation, the amount of functional AP-1 complexes is increased [357] and AP-1 DNA binding activity is enhanced [370]. Besides inhibition of proliferation, overexpression of c-Jun induced monocytic differentiation and function (e.g., phagocytic capacity, nitroblue tetrazolium reduction activity) of myeloid cells such as U937 and HL-60 cells, murine bone marrow-derived cells or myelomonocytic WEHI-3B D + cells [357, 371, 372]. Conversely, Bcr/Abl-mediated inhibition of c-Jun expression resulted in enhanced granulocyte formation as demonstrated in primary human CML granulocytes and KCL22 and K562 CML cell lines, while monocytic differentiation could be induced by c-Jun upregulation [373]. An enhanced myeloid differentiation was also observed in JunB- or c-Fos-transduced M1 cells [364]. An increased granulocyte formation has also been demonstrated in mice with myeloid lineage-specific JunBko [365] but it has been proposed that this might rather reflect the antiproliferative features of JunB [43]. Downregulation of JunD, however, appears to suppress differentiation of 32D cells towards granulocytes even in the presence of G-CSF, suggesting differentiation-supporting characteristics [368].
Network architecture
Although no unique monocyte-forming transcription factor has been identified so far and most of the monopoiesis-inducing factors appear dispensable or replaceable, an essential transcription factor network may be recognized (Fig. 3). The major inducer of monocytic differentiation is PU.1, since it is the first relevant transcription factor appearing in this process [1, 9, 43]. In the course of monocytic differentiation, PU.1 induces other differentiation-supporting factors (e.g., c-Jun, JunB, and C/EBPε) and its presence during monopoiesis may be maintained by positive autoregulation as well as transcription factors such as C/EBPα-p42. Moreover, the effect of several transcription factors is mediated at least in part via cooperation with PU.1 (e.g., C/EBPα, C/EBPβ, IRF proteins, c-Jun, JunB) reflecting the central role of PU.1 in monocyte maturation. Thus, PU.1 facilitates the transition of progenitor cells from one progenitor state to another. Throughout the different stages of monopoiesis, STAT family proteins contribute to PU.1-induced differentiation by preventing excessive progenitor cell expansion (early stages), facilitating progenitor stage transitions, and increasingly inhibiting proliferation of further differentiated progenitors (intermediate/late stages) [42]. However, in each stage, additional factors may provide further transcriptional ‘input’ [9, 43]. Especially in the early stem and progenitor stages (HSC, MPP), factors such as C/EBPα-p30 and C/EBPβ-LIP trigger the required cell expansion, but also the transition towards the directly following progenitors (CMP, GMP). However, for the establishment of the myeloid lineage (CMP), predominantly differentiation-supporting regulators like C/EBPα-p42, VDR, and c-Jun/JunB are required. The formation of pre- and promonocytic cell types as well as monocytes depends on the accessory contribution of C/EBPβ-LAP and proteins of the IRF and STAT families. Finally, terminal differentiation towards macrophages and DC appears to predominantly driven by C/EBPε and IRF8 [9].
Fig. 3.
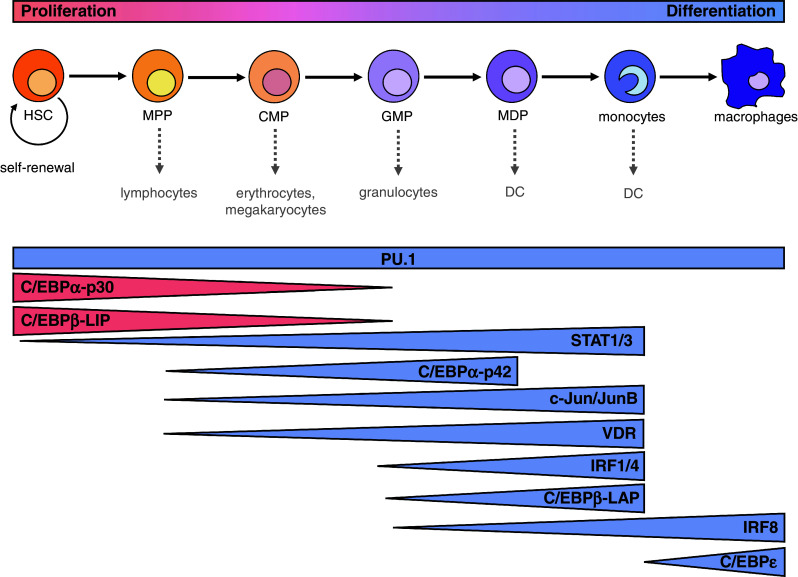
The influence of key transcription factors on monopoiesis. The figure illustrates the most important transcription factors contributing to progenitor cell expansion (red) and monocyte/macrophage differentiation (blue) during each step of maturation (see text for further details)
Concluding remarks
The present review summarizes specific signaling modules and associated transcription factor networks contributing to (myelo)monocytic differentiation and orchestrating the interplay of proliferation and maturation (Figs. 2, 3). Several signaling modules are involved in mediating proliferation- and/or differentiation-supporting signals, especially PI3K, MAPK, PKC, SFK, JAK, and VDR signaling pathways. Most of them represent powerful, but also general signaling cascades, e.g., MAPK, PI3K, and PKC, regulating a variety of cellular features. Other pathways, e.g., SFK and VDR signaling, cover more specific functionalities such as changes in morphology or expression of effector molecules. Mutual activation of different signaling modules and integration of the variety of complementing or contradictory signals are central aspects affecting monopoiesis. On the transcriptional level, monocyte development is positively or negatively regulated by a network of transcription factors. In the early phase, expansion of immature stem/progenitor cells appears to be governed by C/EBPα-p30 and C/EBPβ-LIP. In the further development, proliferation is progressively restricted and the progenitor cells undergo increasing differentiation, also including the transition towards further differentiated precursors (driven by STAT1/3, C/EBPα-p42, c-Jun/JunB, VDR, IRF1/4/8, and C/EBPβ-LAP). Within the variety of differentiation-supporting transcription factors, PU.1 is the initial and the most powerful mediator of definite monocytic differentiation and represents the key initiator and regulator of monopoiesis. Only for the terminal differentiation towards macrophages and DC, IRF8 and C/EBPε appear to be more influential than PU.1. Together, these findings indicate that the composition of maturation-supporting complexes varies in different developmental steps. It should also be mentioned that beyond regulating transcriptional networks, signaling molecules (e.g., PKC or SFK) may also contribute to monocytic differentiation by directly accessing other regulatory levels or cellular functions which have not been addressed in detail in this article. Due to the complexity and the insufficiently determined composition and precise function of the integrated signaling molecules/transcription factors participating in monocyte/macrophage formation, further studies are necessary to characterize (the combination of) specific conditions and genes/proteins inducing monopoiesis which will presumably elucidate general principles of cellular differentiation. Superior signaling principles and transcription networks may lead to the identification of specific differentiation-associated molecular processes potentially providing further molecular tools for improved diagnostic and therapeutic approaches to assess and/or control inflammatory processes as well as leukemia.
Acknowledgements
We thank Kerstin Püllmann for helpful discussions, Sharon Page for critical reading of the manuscript, and apologize to all authors/working groups whose work has not been considered for this review. This work was supported by the Stiftung für Pathobiochemie und Molekulare Diagnostik der Deutschen Gesellschaft für Klinische Chemie und Laboratoriumsmedizin (DGKL) and by the Deutsche Forschungsgemeinschaft (DFG; SFB 566).
Abbreviations
1,25(OH)2D3
1,25-dihydroxy vitamin D3
4HT
4-hydroxytamoxifen
AML
Acute myeloid leukemia
AP
Activator protein
ATF
Activating transcription factor
ATRA
All-trans retinoic acid
Bcr/Abl
Breakpoint cluster region/Abelson murine leukemia viral oncogene homolog fusion protein
BLIMP
B lymphocyte-induced maturation protein
Bmi
Homolog of B cell-specific Moloney murine leukemia virus integration site
bZIP
Basic leucine zipper
CIITA
Class II major histocompatibility complex transactivator
CBP
CREB binding protein
CD
Cluster of differentiation
CDDO
2-cyano-3,12-dioxooleana-1,9-dien-28-oic acid
CDK
Cyclin-dependent kinase
C/EBP
CCAAT/enhancer binding protein
CLP
Common lymphoid progenitor
CML
Chronic myelogenous leukemia
CMP
Common myeloid progenitor
c-Myc
Avian myelocytomatosis viral oncogene homolog
Cox
Cyclooxygenase
c-Rel
Avian reticuloendotheliosis viral oncogene homolog
CREB
CAMP response element-binding protein
CSF
Colony-stimulating factor
CSFR
CSF receptor
CXCL
C-X-C-motif chemokine ligand
DAG
Diacylgylcerol
DC
Dendritic cell
EDAG
Erythroid differentiation-associated gene
ER
Estrogen receptor
ERK
Extracellular signal-regulated kinase
Ets
E26 transformation-specific
FAK
Focal adhesion kinase
FcγR
Fcγ receptor
FLT3
Fms-like tyrosine kinase 3
FLT3L
FLT3 ligand
Fos
v-Fos murine osteosarcoma viral oncogene homolog
FoxO
Forkhead box protein O
Fra
Fos-related antigen
GABP
GA-binding protein
GATA
GATA-binding factor
G-CSF
Granulocyte CSF
Gfi
Growth factor-independent
GM-CSF
Granulocyte/macrophage CSF
GMP
Granulocyte/monocyte progenitor
GPCR
G protein-coupled receptor
GSK
Glycogen synthase kinase
HIV
Human immunodeficiency virus
Hox
Homeobox
HPK
Hematopoietic progenitor kinase
HSC
Hematopoietic stem cell
IFN
Interferon
IL
Interleukin
iNOS
Inducible nitric oxide synthase
IRF
Interferon regulatory factor
JAK
Janus kinase
JDP
Jun dimerization protein
JNK
c-Jun N-terminal kinase
kd
Knock down
KLF
Krueppel-like factor
ko
Knock out
LAP
Liver-enriched activating protein
LIF
Leukemia inhibitory factor
LIP
Liver-enriched inhibitory protein
LPS
Lipopolysaccharide
Ly6C
Lymphocyte antigen 6 complex locus C
Maf
Musculoaponeurotic fibrosarcoma oncogene homolog
MAPK
Mitogen-activated protein kinase
MAP3K
MAPK kinase kinase
M-CSF
Macrophage-colony stimulating factor
MDP
Monocyte/macrophage and dendritic cell progenitor
MEK
MAPK/ERK kinase
MEP
Megakaryocyte-erythroid progenitor
METS
Mitogenic Ets transcriptional suppressor
MIP
Macrophage inflammatory protein
MKK
MAPK kinase
MLL
Mixed-lineage leukemia
MPP
Multipotent progenitor
mTOR
Mammalian target of rapamycin
NF-κB
Nuclear factor κ-light-chain-enhancer of activated B cells
NOD
Non-obese diabetic
Numb
Homolog of Drosophila Numb
OSM
Oncostatin M
Pax
Paired box protein
PI3K
Phosphoinositide-3-kinase
PIP3
Phosphoinositide-3,4,5-trisphosphate
PKB
Protein kinase B
PKC
Protein kinase C
PMA
Phorbol-12-myristate 13-acetate
PTP
Protein tyrosine phosphatase
PU.1
Purine-rich box 1
PU.1/ER
PU.1/estrogen receptor fusion protein
Pyk
Proline-rich tyrosine kinase
RA
Retinoic acid
Raf
Rapidly accelerated fibrosarcoma
RAR
Retinoic acid receptor
Ras
Rat sarcoma
Rb
Retinoblastoma protein
Rheb
Ras homolog enriched in brain
RSK
90-kDa ribosomal protein S6 kinase
RTK
Receptor tyrosine kinase
RXR
Retinoic X receptor
SCF
Stem cell factor
SCID
Severe combined immunodeficiency
Shp
Src homology 2 domain-containing protein tyrosine phosphatase
SFK
Src family kinase
sRAGE
Soluble receptor for advanced glycation end products
Src
v-Src avian sarcoma viral oncogene homolog
STAT
Signal transducer and activator of transcription
TGF
Transforming growth factor
THOC
THO complex
TNF
Tumor necrosis factor
TSC
Tuberous sclerosis protein
Tyk
Tyrosine kinase
UA
Ursolic acid
VDR
Vitamin D receptor
VDRE
Vitamin D responsive element
Vit
Vitamin
wt
Wild type
References
- 1.Auffray C, Sieweke MH, Geissmann F. Blood monocytes: development, heterogeneity, and relationship with dendritic cells. Annu Rev Immunol. 2009;27:669–692. doi: 10.1146/annurev.immunol.021908.132557. [DOI] [PubMed] [Google Scholar]
- 2.Chow A, Brown BD, Merad M. Studying the mononuclear phagocyte system in the molecular age. Nat Rev Immunol. 2011;11:788–798. doi: 10.1038/nri3087. [DOI] [PubMed] [Google Scholar]
- 3.Wang LD, Wagers AJ. Dynamic niches in the origination and differentiation of haematopoietic stem cells. Nat Rev Mol Cell Biol. 2011;12:643–655. doi: 10.1038/nrm3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.He S, Nakada D, Morrison SJ. Mechanisms of stem cell self-renewal. Annu Rev Cell Dev Biol. 2009;25:377–406. doi: 10.1146/annurev.cellbio.042308.113248. [DOI] [PubMed] [Google Scholar]
- 5.Mercer EM, Lin YC, Murre C. Factors and networks that underpin early hematopoiesis. Semin Immunol. 2011;23:317–325. doi: 10.1016/j.smim.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walasek MA, van Os R, de Haan G. Hematopoietic stem cell expansion: challenges and opportunities. Ann NY Acad Sci. 2012;1266:138–150. doi: 10.1111/j.1749-6632.2012.06549.x. [DOI] [PubMed] [Google Scholar]
- 7.Aggarwal R, Lu J, Pompili VJ, Das H. Hematopoietic stem cells: transcriptional regulation, ex vivo expansion and clinical application. Curr Mol Med. 2012;12:34–49. doi: 10.2174/156652412798376125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knoblich JA. Mechanisms of asymmetric stem cell division. Cell. 2008;132:583–597. doi: 10.1016/j.cell.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 9.Rosenbauer F, Tenen DG. Transcription factors in myeloid development: balancing differentiation with transformation. Nat Rev Immunol. 2007;7:105–117. doi: 10.1038/nri2024. [DOI] [PubMed] [Google Scholar]
- 10.Wilson A, Trumpp A. Bone-marrow haematopoietic-stem-cell niches. Nat Rev Immunol. 2006;6:93–106. doi: 10.1038/nri1779. [DOI] [PubMed] [Google Scholar]
- 11.Graf T. Differentiation plasticity of hematopoietic cells. Blood. 2002;99:3089–3101. doi: 10.1182/blood.v99.9.3089. [DOI] [PubMed] [Google Scholar]
- 12.Friedman AD. Runx1, c-Myb, and C/EBPalpha couple differentiation to proliferation or growth arrest during hematopoiesis. J Cell Biochem. 2002;86:624–629. doi: 10.1002/jcb.10271. [DOI] [PubMed] [Google Scholar]
- 13.Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327:656–661. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swirski FK. The spatial and developmental relationships in the macrophage family. Arterioscler Thromb Vasc Biol. 2011;31:1517–1522. doi: 10.1161/ATVBAHA.110.221150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ziegler-Heitbrock L, Ancuta P, Crowe S, Dalod M, Grau V, Hart DN, Leenen PJ, Liu YJ, MacPherson G, Randolph GJ, Scherberich J, Schmitz J, Shortman K, Sozzani S, Strobl H, Zembala M, Austyn JM, Lutz MB. Nomenclature of monocytes and dendritic cells in blood. Blood. 2010;116:e74–e80. doi: 10.1182/blood-2010-02-258558. [DOI] [PubMed] [Google Scholar]
- 16.Yona S, Jung S. Monocytes: subsets, origins, fates and functions. Curr Opin Hematol. 2010;17:53–59. doi: 10.1097/MOH.0b013e3283324f80. [DOI] [PubMed] [Google Scholar]
- 17.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11:723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bonasio R, von Andrian UH. Generation, migration and function of circulating dendritic cells. Curr Opin Immunol. 2006;18:503–511. doi: 10.1016/j.coi.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 19.Lawrence T, Natoli G. Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nat Rev Immunol. 2011;11:750–761. doi: 10.1038/nri3088. [DOI] [PubMed] [Google Scholar]
- 20.Dale DC, Boxer L, Liles WC. The phagocytes: neutrophils and monocytes. Blood. 2008;112:935–945. doi: 10.1182/blood-2007-12-077917. [DOI] [PubMed] [Google Scholar]
- 21.Lehto VP, Hovi T, Vartio T, Badley RA, Virtanen I. Reorganization of cytoskeletal and contractile elements during transition of human monocytes into adherent macrophages. Lab Invest. 1982;47:391–399. [PubMed] [Google Scholar]
- 22.Bertagnolo V, Brugnoli F, Grassilli S, Nika E, Capitani S. Vav1 in differentiation of tumoral promyelocytes. Cell Signal. 2012;24:612–620. doi: 10.1016/j.cellsig.2011.11.017. [DOI] [PubMed] [Google Scholar]
- 23.Gaidano G, Bergui L, Schena M, Gaboli M, Cremona O, Marchisio PC, Caligaris-Cappio F. Integrin distribution and cytoskeleton organization in normal and malignant monocytes. Leukemia. 1990;4:682–687. [PubMed] [Google Scholar]
- 24.Williams DE, Lu L, Broxmeyer HE. Characterization of hematopoietic stem and progenitor cells. Immunol Res. 1987;6:294–304. doi: 10.1007/BF02935524. [DOI] [PubMed] [Google Scholar]
- 25.Rowley SD, Sharkis SJ, Hattenburg C, Sensenbrenner LL. Culture from human bone marrow of blast progenitor cells with an extensive proliferative capacity. Blood. 1987;69:804–808. [PubMed] [Google Scholar]
- 26.Goud TJ, Schotte C, van Furth R. Identification and characterization of the monoblast in mononuclear phagocyte colonies grown in vitro. J Exp Med. 1975;142:1180–1199. doi: 10.1084/jem.142.5.1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bainton DR, Golde DW. Differentiation of macrophages from normal human bone marrow in liquid culture: electron microscopy and cytochemistry. J Clin Invest. 1978;61:1555–1569. doi: 10.1172/JCI109076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Satpathy AT, Wu X, Albring JC, Murphy KM. Re(de)fining the dendritic cell lineage. Nat Immunol. 2012;13:1145–1154. doi: 10.1038/ni.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cohn ZA, Hirsch JG, Fedorko ME. The in vitro differentiation of mononuclear phagocytes. IV: the ultrastructure of macrophage differentiation in the peritoneal cavity and in culture. J Exp Med. 1966;123:747–756. doi: 10.1084/jem.123.4.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Daigneault M, Preston JA, Marriott HM, Whyte MK, Dockrell DH. The identification of markers of macrophage differentiation in PMA-stimulated THP-1 cells and monocyte-derived macrophages. PLoS ONE. 2010;5:e8668. doi: 10.1371/journal.pone.0008668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Serbina NV, Jia T, Hohl TM, Pamer EG. Monocyte-mediated defense against microbial pathogens. Annu Rev Immunol. 2008;26:421–452. doi: 10.1146/annurev.immunol.26.021607.090326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Soehnlein O, Lindbom L, Weber C. Mechanisms underlying neutrophil-mediated monocyte recruitment. Blood. 2009;114:4613–4623. doi: 10.1182/blood-2009-06-221630. [DOI] [PubMed] [Google Scholar]
- 33.Tam MA, Rydstrom A, Sundquist M, Wick MJ. Early cellular responses to Salmonella infection: dendritic cells, monocytes, and more. Immunol Rev. 2008;225:140–162. doi: 10.1111/j.1600-065X.2008.00679.x. [DOI] [PubMed] [Google Scholar]
- 34.Stearns-Kurosawa DJ, Osuchowski MF, Valentine C, Kurosawa S, Remick DG. The pathogenesis of sepsis. Annu Rev Pathol. 2011;6:19–48. doi: 10.1146/annurev-pathol-011110-130327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Szekanecz Z, Koch AE. Macrophages and their products in rheumatoid arthritis. Curr Opin Rheumatol. 2007;19:289–295. doi: 10.1097/BOR.0b013e32805e87ae. [DOI] [PubMed] [Google Scholar]
- 36.Weber C, Zernecke A, Libby P. The multifaceted contributions of leukocyte subsets to atherosclerosis: lessons from mouse models. Nat Rev Immunol. 2008;8:802–815. doi: 10.1038/nri2415. [DOI] [PubMed] [Google Scholar]
- 37.Ingersoll MA, Platt AM, Potteaux S, Randolph GJ. Monocyte trafficking in acute and chronic inflammation. Trends Immunol. 2011;32:470–477. doi: 10.1016/j.it.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145:341–355. doi: 10.1016/j.cell.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dalmas E, Clement K, Guerre-Millo M. Defining macrophage phenotype and function in adipose tissue. Trends Immunol. 2011;32:307–314. doi: 10.1016/j.it.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 40.Noursadeghi M, Katz DR, Miller RF. HIV-1 infection of mononuclear phagocytic cells: the case for bacterial innate immune deficiency in AIDS. Lancet Infect Dis. 2006;6:794–804. doi: 10.1016/S1473-3099(06)70656-9. [DOI] [PubMed] [Google Scholar]
- 41.Tenen DG. Disruption of differentiation in human cancer: AML shows the way. Nat Rev Cancer. 2003;3:89–101. doi: 10.1038/nrc989. [DOI] [PubMed] [Google Scholar]
- 42.Miranda MB, Johnson DE. Signal transduction pathways that contribute to myeloid differentiation. Leukemia. 2007;21:1363–1377. doi: 10.1038/sj.leu.2404690. [DOI] [PubMed] [Google Scholar]
- 43.Friedman AD. Transcriptional control of granulocyte and monocyte development. Oncogene. 2007;26:6816–6828. doi: 10.1038/sj.onc.1210764. [DOI] [PubMed] [Google Scholar]
- 44.Stirewalt DL, Radich JP. The role of FLT3 in haematopoietic malignancies. Nat Rev Cancer. 2003;3:650–665. doi: 10.1038/nrc1169. [DOI] [PubMed] [Google Scholar]
- 45.Barreda DR, Hanington PC, Belosevic M. Regulation of myeloid development and function by colony-stimulating factors. Dev Comp Immunol. 2004;28:509–554. doi: 10.1016/j.dci.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 46.Robb L. Cytokine receptors and hematopoietic differentiation. Oncogene. 2007;26:6715–6723. doi: 10.1038/sj.onc.1210756. [DOI] [PubMed] [Google Scholar]
- 47.Karlsson KR, Cowley S, Martinez FO, Shaw M, Minger SL, James W. Homogeneous monocytes and macrophages from human embryonic stem cells following coculture-free differentiation in M-CSF and IL-3. Exp Hematol. 2008;36:1167–1175. doi: 10.1016/j.exphem.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carlberg C, Molnar F, Mourino A. Vitamin D receptor ligands: the impact of crystal structures. Expert Opin Ther Pat. 2012;22:417–435. doi: 10.1517/13543776.2012.673590. [DOI] [PubMed] [Google Scholar]
- 49.Gombart AF, Luong QT, Koeffler HP. Vitamin D compounds: activity against microbes and cancer. Anticancer Res. 2006;26:2531–2542. [PubMed] [Google Scholar]
- 50.Di Rosa M, Malaguarnera M, Nicoletti F, Malaguarnera L. Vitamin D3: a helpful immuno-modulator. Immunology. 2011;134:123–139. doi: 10.1111/j.1365-2567.2011.03482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pramanik R, Asplin JR, Lindeman C, Favus MJ, Bai S, Coe FL. Lipopolysaccharide negatively modulates vitamin D action by down-regulating expression of vitamin D-induced VDR in human monocytic THP-1 cells. Cell Immunol. 2004;232:137–143. doi: 10.1016/j.cellimm.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 52.Miyaura C, Abe E, Suda T, Kuroki T. Alternative differentiation of human promyelocytic leukemia cells (HL-60) induced selectively by retinoic acid and 1 alpha,25-dihydroxyvitamin D3. Cancer Res. 1985;45:4244–4248. [PubMed] [Google Scholar]
- 53.Wagsater D, Sirsjo A, Dimberg J. Down-regulation of ID2 by all-trans retinoic acid in monocytic leukemia cells (THP-1) J Exp Clin Cancer Res. 2003;22:471–475. [PubMed] [Google Scholar]
- 54.Zhu L, Gong B, Bisgaier CL, Aviram M, Newton RS. Induction of PPARgamma1 expression in human THP-1 monocytic leukemia cells by 9-cis-retinoic acid is associated with cellular growth suppression. Biochem Biophys Res Commun. 1998;251:842–848. doi: 10.1006/bbrc.1998.9567. [DOI] [PubMed] [Google Scholar]
- 55.Hemmi H, Breitman TR. Induction of functional differentiation of a human monocytic leukemia cell line (THP-1) by retinoic acid and cholera toxin. Jpn J Cancer Res. 1985;76:345–351. [PubMed] [Google Scholar]
- 56.Nakamura T, Hemmi H, Aso H, Ishida N. Variants of a human monocytic leukemia cell line (THP-1): induction of differentiation by retinoic acid, interferon-gamma, and T-lymphocyte-derived differentiation-inducing activity. J Natl Cancer Inst. 1986;77:21–27. [PubMed] [Google Scholar]
- 57.Zimber A, Chedeville A, Abita JP, Barbu V, Gespach C. Functional interactions between bile acids, all-trans retinoic acid, and 1,25-dihydroxy-vitamin D3 on monocytic differentiation and myeloblastin gene down-regulation in HL60 and THP-1 human leukemia cells. Cancer Res. 2000;60:672–678. [PubMed] [Google Scholar]
- 58.Ding Q, Jin T, Wang Z, Chen Y. Catalase potentiates retinoic acid-induced THP-1 monocyte differentiation into macrophage through inhibition of peroxisome proliferator-activated receptor gamma. J Leukoc Biol. 2007;81:1568–1576. doi: 10.1189/jlb.1106672. [DOI] [PubMed] [Google Scholar]
- 59.Taimi M, Chateau MT, Cabane S, Marti J. Synergistic effect of retinoic acid and 1,25-dihydroxyvitamin D3 on the differentiation of the human monocytic cell line U937. Leuk Res. 1991;15:1145–1152. doi: 10.1016/0145-2126(91)90183-t. [DOI] [PubMed] [Google Scholar]
- 60.Breitman TR, Selonick SE, Collins SJ. Induction of differentiation of the human promyelocytic leukemia cell line (HL-60) by retinoic acid. Proc Natl Acad Sci USA. 1980;77:2936–2940. doi: 10.1073/pnas.77.5.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aglietta M, Piacibello W, Stacchini A, Sanavio F, Gavosto F. In-vitro effect of retinoic acid on normal and chronic myeloid leukemia granulopoiesis. Leuk Res. 1985;9:879–883. doi: 10.1016/0145-2126(85)90309-1. [DOI] [PubMed] [Google Scholar]
- 62.Nagler A, Ricklis I, Gazit E, Tatarsky I, Fabian I. Differentiation of bone marrow cells from myelodysplastic patients in the presence of 1,25 dihydroxyvitamin D3 or 13-cis retinoic acid. Eur J Clin Invest. 1986;16:297–301. doi: 10.1111/j.1365-2362.1986.tb01345.x. [DOI] [PubMed] [Google Scholar]
- 63.Chan S, Fung M, Mak N, Leung K. Involvement of interleukin-1 in the differentiation-inducing activity of tumor necrosis factor-alpha on a murine myeloid leukemia (WEHI-3B JCS) Int J Oncol. 1997;10:821–826. doi: 10.3892/ijo.10.4.821. [DOI] [PubMed] [Google Scholar]
- 64.Samal BB, Stearns GW, Boone TC, Arakawa T. Comparative analysis of the effects of recombinant cytokines on the growth and differentiation of ML-1, a human myelogenous leukemic cell line. Leuk Res. 1993;17:299–304. doi: 10.1016/0145-2126(93)90016-e. [DOI] [PubMed] [Google Scholar]
- 65.Mak NK, Fung MC, Leung KN, Hapel AJ. Monocytic differentiation of a myelomonocytic leukemic cell (WEHI 3B JCS) is induced by tumour necrosis factor-alpha (TNF-alpha) Cell Immunol. 1993;150:1–14. doi: 10.1006/cimm.1993.1173. [DOI] [PubMed] [Google Scholar]
- 66.Leung KN, Mak NK, Fung MC, Hapel AJ. Synergistic effect of IL-4 and TNF-alpha in the induction of monocytic differentiation of a mouse myeloid leukaemic cell line (WEHI-3B JCS) Immunology. 1994;81:65–72. [PMC free article] [PubMed] [Google Scholar]
- 67.Krutzik SR, Hewison M, Liu PT, Robles JA, Stenger S, Adams JS, Modlin RL. IL-15 links TLR2/1-induced macrophage differentiation to the vitamin D-dependent antimicrobial pathway. J Immunol. 2008;181:7115–7120. doi: 10.4049/jimmunol.181.10.7115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tanigawa T, Elwood N, Metcalf D, Cary D, DeLuca E, Nicola NA, Begley CG. The SCL gene product is regulated by and differentially regulates cytokine responses during myeloid leukemic cell differentiation. Proc Natl Acad Sci USA. 1993;90:7864–7868. doi: 10.1073/pnas.90.16.7864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Netea MG, Lewis EC, Azam T, Joosten LA, Jaekal J, Bae SY, Dinarello CA, Kim SH. Interleukin-32 induces the differentiation of monocytes into macrophage-like cells. Proc Natl Acad Sci USA. 2008;105:3515–3520. doi: 10.1073/pnas.0712381105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tamura T, Ozato K. ICSBP/IRF-8: its regulatory roles in the development of myeloid cells. J Interferon Cytokine Res. 2002;22:145–152. doi: 10.1089/107999002753452755. [DOI] [PubMed] [Google Scholar]
- 71.Perussia B, Dayton ET, Fanning V, Thiagarajan P, Hoxie J, Trinchieri G. Immune interferon and leukocyte-conditioned medium induce normal and leukemic myeloid cells to differentiate along the monocytic pathway. J Exp Med. 1983;158:2058–2080. doi: 10.1084/jem.158.6.2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kolb-Maurer A, Weissinger F, Kurzai O, Maurer M, Wilhelm M, Goebel W. Bacterial infection of human hematopoietic stem cells induces monocytic differentiation. FEMS Immunol Med Microbiol. 2004;40:147–153. doi: 10.1016/S0928-8244(03)00305-5. [DOI] [PubMed] [Google Scholar]
- 73.Adams DO. The structure of mononuclear phagocytes differentiating in vivo. II. The effect of Mycobacterium tuberculosis. Am J Pathol. 1975;80:101–116. [PMC free article] [PubMed] [Google Scholar]
- 74.Hall RE, Agarwal S, Kestler DP. Induction of leukemia cell differentiation and apoptosis by recombinant P48, a modulin derived from Mycoplasma fermentans. Biochem Biophys Res Commun. 2000;269:284–289. doi: 10.1006/bbrc.2000.2282. [DOI] [PubMed] [Google Scholar]
- 75.Petit AJ, Terpstra FG, Miedema F. Human immunodeficiency virus infection down-regulates HLA class II expression and induces differentiation in promonocytic U937 cells. J Clin Invest. 1987;79:1883–1889. doi: 10.1172/JCI113032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Roulston A, D’Addario M, Boulerice F, Caplan S, Wainberg MA, Hiscott J. Induction of monocytic differentiation and NF-kappa B-like activities by human immunodeficiency virus 1 infection of myelomonoblastic cells. J Exp Med. 1992;175:751–763. doi: 10.1084/jem.175.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chang ZL. Recent development of the mononuclear phagocyte system: in memory of Metchnikoff and Ehrlich on the 100th Anniversary of the 1908 Nobel Prize in Physiology or Medicine. Biol Cell. 2009;101:709–721. doi: 10.1042/BC20080227. [DOI] [PubMed] [Google Scholar]
- 78.Livneh E, Fishman DD. Linking protein kinase C to cell-cycle control. Eur J Biochem. 1997;248:1–9. doi: 10.1111/j.1432-1033.1997.t01-4-00001.x. [DOI] [PubMed] [Google Scholar]
- 79.Katagiri K, Hattori S, Nakamura S, Yamamoto T, Yoshida T, Katagiri T. Activation of Ras and formation of GAP complex during TPA-induced monocytic differentiation of HL-60 cells. Blood. 1994;84:1780–1789. [PubMed] [Google Scholar]
- 80.Hass R, Prudovsky I, Kruhoffer M. Differential effects of phorbol ester on signaling and gene expression in human leukemia cells. Leuk Res. 1997;21:589–594. doi: 10.1016/s0145-2126(97)00010-6. [DOI] [PubMed] [Google Scholar]
- 81.Schwende H, Fitzke E, Ambs P, Dieter P. Differences in the state of differentiation of THP-1 cells induced by phorbol ester and 1,25-dihydroxyvitamin D3. J Leukoc Biol. 1996;59:555–561. [PubMed] [Google Scholar]
- 82.Kim SJ, Bang OS, Lee YS, Kang SS. Production of inducible nitric oxide is required for monocytic differentiation of U937 cells induced by vitamin E-succinate. J Cell Sci. 1998;111:435–441. doi: 10.1242/jcs.111.4.435. [DOI] [PubMed] [Google Scholar]
- 83.Coccia EM, Del Russo N, Stellacci E, Testa U, Marziali G, Battistini A. STAT1 activation during monocyte to macrophage maturation: role of adhesion molecules. Int Immunol. 1999;11:1075–1083. doi: 10.1093/intimm/11.7.1075. [DOI] [PubMed] [Google Scholar]
- 84.Zhang T, He YM, Wang JS, Shen J, Xing YY, Xi T. Ursolic acid induces HL60 monocytic differentiation and upregulates C/EBPbeta expression by ERK pathway activation. Anticancer Drugs. 2011;22:158–165. doi: 10.1097/CAD.0b013e3283409673. [DOI] [PubMed] [Google Scholar]
- 85.Ji Y, Lee HJ, Goodman C, Uskokovic M, Liby K, Sporn M, Suh N. The synthetic triterpenoid CDDO-imidazolide induces monocytic differentiation by activating the Smad and ERK signaling pathways in HL60 leukemia cells. Mol Cancer Ther. 2006;5:1452–1458. doi: 10.1158/1535-7163.MCT-06-0136. [DOI] [PubMed] [Google Scholar]
- 86.Akashi K, Traver D, Zon LI. The complex cartography of stem cell commitment. Cell. 2005;121:160–162. doi: 10.1016/j.cell.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 87.Gotze KS, Ramirez M, Tabor K, Small D, Matthews W, Civin CI. Flt3high and Flt3low CD34 + progenitor cells isolated from human bone marrow are functionally distinct. Blood. 1998;91:1947–1958. [PubMed] [Google Scholar]
- 88.Rusten LS, Lyman SD, Veiby OP, Jacobsen SE. The FLT3 ligand is a direct and potent stimulator of the growth of primitive and committed human CD34 + bone marrow progenitor cells in vitro. Blood. 1996;87:1317–1325. [PubMed] [Google Scholar]
- 89.Gabbianelli M, Pelosi E, Montesoro E, Valtieri M, Luchetti L, Samoggia P, Vitelli L, Barberi T, Testa U, Lyman S, et al. Multi-level effects of flt3 ligand on human hematopoiesis: expansion of putative stem cells and proliferation of granulomonocytic progenitors/monocytic precursors. Blood. 1995;86:1661–1670. [PubMed] [Google Scholar]
- 90.Kolb-Maurer A, Wilhelm M, Weissinger F, Brocker EB, Goebel W. Interaction of human hematopoietic stem cells with bacterial pathogens. Blood. 2002;100:3703–3709. doi: 10.1182/blood-2002-03-0898. [DOI] [PubMed] [Google Scholar]
- 91.Mackarehtschian K, Hardin JD, Moore KA, Boast S, Goff SP, Lemischka IR. Targeted disruption of the flk2/flt3 gene leads to deficiencies in primitive hematopoietic progenitors. Immunity. 1995;3:147–161. doi: 10.1016/1074-7613(95)90167-1. [DOI] [PubMed] [Google Scholar]
- 92.Haas SC, Huber R, Gutsch R, Kandemir JD, Cappello C, Krauter J, Duyster J, Ganser A, Brand K. ITD- and FL-induced FLT3 signal transduction leads to increased C/EBPbeta-LIP expression and LIP/LAP ratio by different signalling modules. Br J Haematol. 2010;148:777–790. doi: 10.1111/j.1365-2141.2009.08012.x. [DOI] [PubMed] [Google Scholar]
- 93.Mizuki M, Schwable J, Steur C, Choudhary C, Agrawal S, Sargin B, Steffen B, Matsumura I, Kanakura Y, Bohmer FD, Muller-Tidow C, Berdel WE, Serve H. Suppression of myeloid transcription factors and induction of STAT response genes by AML-specific Flt3 mutations. Blood. 2003;101:3164–3173. doi: 10.1182/blood-2002-06-1677. [DOI] [PubMed] [Google Scholar]
- 94.Steelman LS, Abrams SL, Whelan J, Bertrand FE, Ludwig DE, Basecke J, Libra M, Stivala F, Milella M, Tafuri A, Lunghi P, Bonati A, Martelli AM, McCubrey JA. Contributions of the Raf/MEK/ERK, PI3K/PTEN/Akt/mTOR and Jak/STAT pathways to leukemia. Leukemia. 2008;22:686–707. doi: 10.1038/leu.2008.26. [DOI] [PubMed] [Google Scholar]
- 95.Polak R, Buitenhuis M. The PI3K/PKB signaling module as key regulator of hematopoiesis: implications for therapeutic strategies in leukemia. Blood. 2012;119:911–923. doi: 10.1182/blood-2011-07-366203. [DOI] [PubMed] [Google Scholar]
- 96.Zhang Y, Zhang J, Studzinski GP. AKT pathway is activated by 1, 25-dihydroxyvitamin D3 and participates in its anti-apoptotic effect and cell cycle control in differentiating HL60 cells. Cell Cycle. 2006;5:447–451. doi: 10.4161/cc.5.4.2467. [DOI] [PubMed] [Google Scholar]
- 97.Wang Y, Wang H, Piper MG, McMaken S, Mo X, Opalek J, Schmidt AM, Marsh CB. sRAGE induces human monocyte survival and differentiation. J Immunol. 2010;185:1822–1835. doi: 10.4049/jimmunol.0903398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hsu MJ, Lin WW, Tsao WC, Chang YC, Hsu TL, Chiu AW, Chio CC, Hsieh SL. Enhanced adhesion of monocytes via reverse signaling triggered by decoy receptor 3. Exp Cell Res. 2004;292:241–251. doi: 10.1016/j.yexcr.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 99.Gaurnier-Hausser A, Rothman VL, Dimitrov S, Tuszynski GP. The novel angiogenic inhibitor, angiocidin, induces differentiation of monocytes to macrophages. Cancer Res. 2008;68:5905–5914. doi: 10.1158/0008-5472.CAN-07-6179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Maekawa T, Imoto A, Satoh T, Okazaki T, Takahashi S. Induction of beta-catenin by the suppression of signal regulatory protein alpha1 in K562 cells. Int J Mol Med. 2011;27:865–872. doi: 10.3892/ijmm.2011.630. [DOI] [PubMed] [Google Scholar]
- 101.Sengupta S, Peterson TR, Sabatini DM. Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol Cell. 2010;40:310–322. doi: 10.1016/j.molcel.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fu CT, Zhu KY, Mi JQ, Liu YF, Murray ST, Fu YF, Ren CG, Dong ZW, Liu YJ, Dong M, Jin Y, Chen Y, Deng M, Zhang W, Chen B, Breslin P, Chen SJ, Chen Z, Becker MW, Zhu J, Zhang JW, Liu TX. An evolutionarily conserved PTEN-C/EBPalpha-CTNNA1 axis controls myeloid development and transformation. Blood. 2010;115:4715–4724. doi: 10.1182/blood-2009-11-255778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Smink JJ, Tunn PU, Leutz A. Rapamycin inhibits osteoclast formation in giant cell tumor of bone through the C/EBPbeta: MafB axis. J Mol Med. 2010;90:25–30. doi: 10.1007/s00109-011-0823-6. [DOI] [PubMed] [Google Scholar]
- 104.Carney L, Pierce A, Rijnen M, Gonzalez Sanchez MB, Hamzah HG, Zhang L, Tamura T, Whetton AD. THOC5 couples M-CSF receptor signaling to transcription factor expression. Cell Signal. 2009;21:309–316. doi: 10.1016/j.cellsig.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 105.Hazzalin CA, Mahadevan LC. MAPK-regulated transcription: a continuously variable gene switch? Nat Rev Mol Cell Biol. 2002;3:30–40. doi: 10.1038/nrm715. [DOI] [PubMed] [Google Scholar]
- 106.Wagner EF, Nebreda AR. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat Rev Cancer. 2009;9:537–549. doi: 10.1038/nrc2694. [DOI] [PubMed] [Google Scholar]
- 107.Chung E, Kondo M. Role of Ras/Raf/MEK/ERK signaling in physiological hematopoiesis and leukemia development. Immunol Res. 2011;49:248–268. doi: 10.1007/s12026-010-8187-5. [DOI] [PubMed] [Google Scholar]
- 108.Coleman ML, Marshall CJ, Olson MF. RAS and RHO GTPases in G1-phase cell-cycle regulation. Nat Rev Mol Cell Biol. 2004;5:355–366. doi: 10.1038/nrm1365. [DOI] [PubMed] [Google Scholar]
- 109.Pylayeva-Gupta Y, Grabocka E, Bar-Sagi D. RAS oncogenes: weaving a tumorigenic web. Nat Rev Cancer. 2011;11:761–774. doi: 10.1038/nrc3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Malumbres M, Perez De Castro I, Hernandez MI, Jimenez M, Corral T, Pellicer A. Cellular response to oncogenic ras involves induction of the Cdk4 and Cdk6 inhibitor p15(INK4b) Mol Cell Biol. 2000;20:2915–2925. doi: 10.1128/mcb.20.8.2915-2925.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Palmero I, Pantoja C, Serrano M. p19ARF links the tumour suppressor p53 to Ras. Nature. 1998;395:125–126. doi: 10.1038/25870. [DOI] [PubMed] [Google Scholar]
- 112.Gartel AL, Tyner AL. Transcriptional regulation of the p21(WAF1/CIP1) gene. Exp Cell Res. 1999;246:280–289. doi: 10.1006/excr.1998.4319. [DOI] [PubMed] [Google Scholar]
- 113.Passioura T, Dolnikov A, Shen S, Symonds G. N-ras-induced growth suppression of myeloid cells is mediated by IRF-1. Cancer Res. 2005;65:797–804. [PubMed] [Google Scholar]
- 114.Jin DI, Jameson SB, Reddy MA, Schenkman D, Ostrowski MC. Alterations in differentiation and behavior of monocytic phagocytes in transgenic mice that express dominant suppressors of ras signaling. Mol Cell Biol. 1995;15:693–703. doi: 10.1128/mcb.15.2.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pearn L, Fisher J, Burnett AK, Darley RL. The role of PKC and PDK1 in monocyte lineage specification by Ras. Blood. 2007;109:4461–4469. doi: 10.1182/blood-2006-09-047217. [DOI] [PubMed] [Google Scholar]
- 116.Hibi S, Lohler J, Friel J, Stocking C, Ostertag W. Induction of monocytic differentiation and tumorigenicity by v-Ha-ras in differentiation arrested hematopoietic cells. Blood. 1993;81:1841–1848. [PubMed] [Google Scholar]
- 117.Fatrai S, van Gosliga D, Han L, Daenen SM, Vellenga E, Schuringa JJ. KRAS(G12V) enhances proliferation and initiates myelomonocytic differentiation in human stem/progenitor cells via intrinsic and extrinsic pathways. J Biol Chem. 2011;286:6061–6070. doi: 10.1074/jbc.M110.201848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mavilio F, Kreider BL, Valtieri M, Naso G, Shirsat N, Venturelli D, Reddy EP, Rovera G. Alteration of growth and differentiation factors response by Kirsten and Harvey sarcoma viruses in the IL-3-dependent murine hematopoietic cell line 32D C13(G) Oncogene. 1989;4:301–308. [PubMed] [Google Scholar]
- 119.Pierce JH, Aaronson SA. Myeloid cell transformation by ras-containing murine sarcoma viruses. Mol Cell Biol. 1985;5:667–674. doi: 10.1128/mcb.5.4.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dunbar CE, Crosier PS, Nienhuis AW. Introduction of an activated RAS oncogene into murine bone marrow lymphoid progenitors via retroviral gene transfer results in thymic lymphomas. Oncogene Res. 1991;6:39–51. [PubMed] [Google Scholar]
- 121.Hawley RG, Fong AZ, Ngan BY, Hawley TS. Hematopoietic transforming potential of activated ras in chimeric mice. Oncogene. 1995;11:1113–1123. [PubMed] [Google Scholar]
- 122.MacKenzie KL, Dolnikov A, Millington M, Shounan Y, Symonds G. Mutant N-ras induces myeloproliferative disorders and apoptosis in bone marrow repopulated mice. Blood. 1999;93:2043–2056. [PubMed] [Google Scholar]
- 123.Gallagher AP, Burnett AK, Bowen DT, Darley RL. Mutant RAS selectively promotes sensitivity of myeloid leukemia cells to apoptosis by a protein kinase C-dependent process. Cancer Res. 1998;58:2029–2035. [PubMed] [Google Scholar]
- 124.Skorski T, Nieborowska-Skorska M, Szczylik C, Kanakaraj P, Perrotti D, Zon G, Gewirtz A, Perussia B, Calabretta B. C-RAF-1 serine/threonine kinase is required in BCR/ABL-dependent and normal hematopoiesis. Cancer Res. 1995;55:2275–2278. [PubMed] [Google Scholar]
- 125.Keller JR, Ruscetti FW, Heidecker G, Linnekin DM, Rapp U, Troppmair J, Gooya J, Muszynski KW. The effect of c-raf antisense oligonucleotides on growth factor-induced proliferation of hematopoietic cells. Curr Top Microbiol Immunol. 1996;211:43–53. doi: 10.1007/978-3-642-85232-9_5. [DOI] [PubMed] [Google Scholar]
- 126.Kharbanda S, Saleem A, Emoto Y, Stone R, Rapp U, Kufe D. Activation of Raf-1 and mitogen-activated protein kinases during monocytic differentiation of human myeloid leukemia cells. J Biol Chem. 1994;269:872–878. [PubMed] [Google Scholar]
- 127.Yen A, Williams M, Platko JD, Der C, Hisaka M. Expression of activated RAF accelerates cell differentiation and RB protein down-regulation but not hypophosphorylation. Eur J Cell Biol. 1994;65:103–113. [PubMed] [Google Scholar]
- 128.Wang X, Studzinski GP. Raf-1 signaling is required for the later stages of 1,25-dihydroxyvitamin D3-induced differentiation of HL60 cells but is not mediated by the MEK/ERK module. J Cell Physiol. 2006;209:253–260. doi: 10.1002/jcp.20731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Miranda MB, Xu H, Torchia JA, Johnson DE. Cytokine-induced myeloid differentiation is dependent on activation of the MEK/ERK pathway. Leuk Res. 2005;29:1293–1306. doi: 10.1016/j.leukres.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 130.Miranda MB, McGuire TF, Johnson DE. Importance of MEK-1/-2 signaling in monocytic and granulocytic differentiation of myeloid cell lines. Leukemia. 2002;16:683–692. doi: 10.1038/sj.leu.2402400. [DOI] [PubMed] [Google Scholar]
- 131.Whelan RD, Kiley SC, Parker PJ. Tetradecanoyl phorbol acetate-induced microtubule reorganization is required for sustained mitogen-activated protein kinase activation and morphological differentiation of U937 cells. Cell Growth Differ. 1999;10:271–277. [PubMed] [Google Scholar]
- 132.Ober-Blobaum JL, Engelhardt G, Hebel S, Rink L, Haase H. Cadmium ions promote monocytic differentiation of human leukemia HL-60 cells treated with 1alpha,25-dihydroxyvitamin D3. Biol Chem. 2010;391:1295–1303. doi: 10.1515/BC.2010.135. [DOI] [PubMed] [Google Scholar]
- 133.Hu X, Moscinski LC, Valkov NI, Fisher AB, Hill BJ, Zuckerman KS. Prolonged activation of the mitogen-activated protein kinase pathway is required for macrophage-like differentiation of a human myeloid leukemic cell line. Cell Growth Differ. 2000;11:191–200. [PubMed] [Google Scholar]
- 134.Saulnier DM, Santos F, Roos S, Mistretta TA, Spinler JK, Molenaar D, Teusink B, Versalovic J. Exploring metabolic pathway reconstruction and genome-wide expression profiling in Lactobacillus reuteri to define functional probiotic features. PLoS ONE. 2012;6:e18783. doi: 10.1371/journal.pone.0018783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Okuma E, Inazawa Y, Saeki K, Yuo A. Potential roles of extracellular signal-regulated kinase but not p38 during myeloid differentiation of U937 cells stimulated by cytokines: augmentation of differentiation via prolonged activation of extracellular signal-regulated kinase. Exp Hematol. 2002;30:571–581. doi: 10.1016/s0301-472x(02)00801-9. [DOI] [PubMed] [Google Scholar]
- 136.Wang X, Studzinski GP. Activation of extracellular signal-regulated kinases (ERKs) defines the first phase of 1,25-dihydroxyvitamin D3-induced differentiation of HL60 cells. J Cell Biochem. 2001;80:471–482. doi: 10.1002/1097-4644(20010315)80:4<471::aid-jcb1001>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 137.Lee JK, Jung JC, Chun JS, Kang SS, Bang OS. Expression of p21WAF1 is dependent on the activation of ERK during vitamin E-succinate-induced monocytic differentiation. Mol Cells. 2002;13:125–129. [PubMed] [Google Scholar]
- 138.Ji Y, Kutner A, Verstuyf A, Verlinden L, Studzinski GP. Derivatives of vitamins D2 and D3 activate three MAPK pathways and upregulate pRb expression in differentiating HL60 cells. Cell Cycle. 2002;1:410–415. doi: 10.4161/cc.1.6.269. [DOI] [PubMed] [Google Scholar]
- 139.Marcinkowska E, Garay E, Gocek E, Chrobak A, Wang X, Studzinski GP. Regulation of C/EBPbeta isoforms by MAPK pathways in HL60 cells induced to differentiate by 1,25-dihydroxyvitamin D3. Exp Cell Res. 2006;312:2054–2065. doi: 10.1016/j.yexcr.2006.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Jack GD, Zhang L, Friedman AD. M-CSF elevates c-Fos and phospho-C/EBPalpha(S21) via ERK whereas G-CSF stimulates SHP2 phosphorylation in marrow progenitors to contribute to myeloid lineage specification. Blood. 2009;114:2172–2180. doi: 10.1182/blood-2008-11-191536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Nakahara T, Moroi Y, Uchi H, Furue M. Differential role of MAPK signaling in human dendritic cell maturation and Th1/Th2 engagement. J Dermatol Sci. 2006;42:1–11. doi: 10.1016/j.jdermsci.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 142.Dreskin SC, Thomas GW, Dale SN, Heasley LE. Isoforms of Jun kinase are differentially expressed and activated in human monocyte/macrophage (THP-1) cells. J Immunol. 2001;166:5646–5653. doi: 10.4049/jimmunol.166.9.5646. [DOI] [PubMed] [Google Scholar]
- 143.Ito Y, Mishra NC, Yoshida K, Kharbanda S, Saxena S, Kufe D. Mitochondrial targeting of JNK/SAPK in the phorbol ester response of myeloid leukemia cells. Cell Death Differ. 2001;8:794–800. doi: 10.1038/sj.cdd.4400886. [DOI] [PubMed] [Google Scholar]
- 144.Wang Q, Wang X, Studzinski GP. Jun N-terminal kinase pathway enhances signaling of monocytic differentiation of human leukemia cells induced by 1,25-dihydroxyvitamin D3. J Cell Biochem. 2003;89:1087–1101. doi: 10.1002/jcb.10595. [DOI] [PubMed] [Google Scholar]
- 145.Yang Z, Kondo T, Voorhorst CS, Nabinger SC, Ndong L, Yin F, Chan EM, Yu M, Wurstlin O, Kratz CP, Niemeyer CM, Flotho C, Hashino E, Chan RJ. Increased c-Jun expression and reduced GATA2 expression promote aberrant monocytic differentiation induced by activating PTPN11 mutants. Mol Cell Biol. 2009;29:4376–4393. doi: 10.1128/MCB.01330-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Himes SR, Sester DP, Ravasi T, Cronau SL, Sasmono T, Hume DA. The JNK are important for development and survival of macrophages. J Immunol. 2006;176:2219–2228. doi: 10.4049/jimmunol.176.4.2219. [DOI] [PubMed] [Google Scholar]
- 147.Arnold R, Frey CR, Muller W, Brenner D, Krammer PH, Kiefer F. Sustained JNK signaling by proteolytically processed HPK1 mediates IL-3 independent survival during monocytic differentiation. Cell Death Differ. 2007;14:568–575. doi: 10.1038/sj.cdd.4402042. [DOI] [PubMed] [Google Scholar]
- 148.Zhang Y, Morgan MJ, Chen K, Choksi S, Liu ZG. Induction of autophagy is essential for monocyte-macrophage differentiation. Blood. 2012;119:2895–2905. doi: 10.1182/blood-2011-08-372383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Shaulian E, Karin M. AP-1 as a regulator of cell life and death. Nat Cell Biol. 2002;4:E131–E136. doi: 10.1038/ncb0502-e131. [DOI] [PubMed] [Google Scholar]
- 150.Mackay HJ, Twelves CJ. Targeting the protein kinase C family: are we there yet? Nat Rev Cancer. 2007;7:554–562. doi: 10.1038/nrc2168. [DOI] [PubMed] [Google Scholar]
- 151.Hass R, Pfannkuche HJ, Kharbanda S, Gunji H, Meyer G, Hartmann A, Hidaka H, Resch K, Kufe D, Goppelt-Strube M. Protein kinase C activation and protooncogene expression in differentiation/retrodifferentiation of human U-937 leukemia cells. Cell Growth Differ. 1991;2:541–548. [PubMed] [Google Scholar]
- 152.Lin YF, Lee HM, Leu SJ, Tsai YH. The essentiality of PKCalpha and PKCbetaI translocation for CD14 + monocyte differentiation towards macrophages and dendritic cells, respectively. J Cell Biochem. 2007;102:429–441. doi: 10.1002/jcb.21305. [DOI] [PubMed] [Google Scholar]
- 153.Lin YF, Leu SJ, Huang HM, Tsai YH. Selective activation of specific PKC isoforms dictating the fate of CD14(+) monocytes towards differentiation or apoptosis. J Cell Physiol. 2010;226:122–131. doi: 10.1002/jcp.22312. [DOI] [PubMed] [Google Scholar]
- 154.Solomon DH, O’Driscoll K, Sosne G, Weinstein IB, Cayre YE. 1 alpha,25-dihydroxyvitamin D3-induced regulation of protein kinase C gene expression during HL-60 cell differentiation. Cell Growth Differ. 1991;2:187–194. [PubMed] [Google Scholar]
- 155.Kang SN, Lee MH, Kim KM, Cho D, Kim TS. Induction of human promyelocytic leukemia HL-60 cell differentiation into monocytes by silibinin: involvement of protein kinase C. Biochem Pharmacol. 2001;61:1487–1495. doi: 10.1016/s0006-2952(01)00626-8. [DOI] [PubMed] [Google Scholar]
- 156.Rosse C, Linch M, Kermorgant S, Cameron AJ, Boeckeler K, Parker PJ. PKC and the control of localized signal dynamics. Nat Rev Mol Cell Biol. 2010;11:103–112. doi: 10.1038/nrm2847. [DOI] [PubMed] [Google Scholar]
- 157.Whetton AD, Heyworth CM, Nicholls SE, Evans CA, Lord JM, Dexter TM, Owen-Lynch PJ. Cytokine-mediated protein kinase C activation is a signal for lineage determination in bipotential granulocyte macrophage colony-forming cells. J Cell Biol. 1994;125:651–659. doi: 10.1083/jcb.125.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Nicholls SE, Heyworth CM, Dexter TM, Lord JM, Johnson GD, Whetton AD. IL-4 promotes macrophage development by rapidly stimulating lineage restriction of bipotent granulocyte-macrophage colony-forming cells. J Immunol. 1995;155:845–853. [PubMed] [Google Scholar]
- 159.Shearman MS, Heyworth CM, Dexter TM, Haefner B, Owen PJ, Whetton AD. Haemopoietic stem cell development to neutrophils is associated with subcellular redistribution and differential expression of protein kinase C subspecies. J Cell Sci. 1993;104:173–180. doi: 10.1242/jcs.104.1.173. [DOI] [PubMed] [Google Scholar]
- 160.Rossi F, McNagny M, Smith G, Frampton J, Graf T. Lineage commitment of transformed haematopoietic progenitors is determined by the level of PKC activity. EMBO J. 1996;15:1894–1901. [PMC free article] [PubMed] [Google Scholar]
- 161.Seales EC, Shaikh FM, Woodard-Grice AV, Aggarwal P, McBrayer AC, Hennessy KM, Bellis SL. A protein kinase C/Ras/ERK signaling pathway activates myeloid fibronectin receptors by altering beta1 integrin sialylation. J Biol Chem. 2005;280:37610–37615. doi: 10.1074/jbc.M508476200. [DOI] [PubMed] [Google Scholar]
- 162.Mahoney CW, Shuman J, McKnight SL, Chen HC, Huang KP. Phosphorylation of CCAAT-enhancer binding protein by protein kinase C attenuates site-selective DNA binding. J Biol Chem. 1992;267:19396–19403. [PubMed] [Google Scholar]
- 163.Ting Y, Medina DJ, Strair RK, Schaar DG. Differentiation-associated miR-22 represses Max expression and inhibits cell cycle progression. Biochem Biophys Res Commun. 2010;394:606–611. doi: 10.1016/j.bbrc.2010.03.030. [DOI] [PubMed] [Google Scholar]
- 164.Pierce A, Heyworth CM, Nicholls SE, Spooncer E, Dexter TM, Lord JM, Owen-Lynch PJ, Wark G, Whetton AD. An activated protein kinase C alpha gives a differentiation signal for hematopoietic progenitor cells and mimicks macrophage colony-stimulating factor-stimulated signaling events. J Cell Biol. 1998;140:1511–1518. doi: 10.1083/jcb.140.6.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Mischak H, Pierce JH, Goodnight J, Kazanietz MG, Blumberg PM, Mushinski JF. Phorbol ester-induced myeloid differentiation is mediated by protein kinase C-alpha and -delta and not by protein kinase C-beta II, -epsilon, -zeta, and -eta. J Biol Chem. 1993;268:20110–20115. [PubMed] [Google Scholar]
- 166.Lopez-Bergami P, Habelhah H, Bhoumik A, Zhang W, Wang LH, Ronai Z. RACK1 mediates activation of JNK by protein kinase C. Mol Cell. 2005;19:309–320. doi: 10.1016/j.molcel.2005.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Pandey P, Nakazawa A, Ito Y, Datta R, Kharbanda S, Kufe D. Requirement for caspase activation in monocytic differentiation of myeloid leukemia cells. Oncogene. 2000;19:3941–3947. doi: 10.1038/sj.onc.1203751. [DOI] [PubMed] [Google Scholar]
- 168.Macfarlane DE, Manzel L. Activation of beta-isozyme of protein kinase C (PKC beta) is necessary and sufficient for phorbol ester-induced differentiation of HL-60 promyelocytes. Studies with PKC beta-defective PET mutant. J Biol Chem. 1994;269:4327–4331. [PubMed] [Google Scholar]
- 169.Slosberg ED, Yao Y, Xing F, Ikui A, Jirousek MR, Weinstein IB. The protein kinase C beta-specific inhibitor LY379196 blocks TPA-induced monocytic differentiation of HL60 cells the protein kinase C beta-specific inhibitor LY379196 blocks TPA-induced monocytic differentiation of HL60 cells. Mol Carcinog. 2000;27:166–176. [PubMed] [Google Scholar]
- 170.Laouar A, Collart FR, Chubb CB, Xie B, Huberman E. Interaction between alpha 5 beta 1 integrin and secreted fibronectin is involved in macrophage differentiation of human HL-60 myeloid leukemia cells. J Immunol. 1999;162:407–414. [PubMed] [Google Scholar]
- 171.Martin GS. The hunting of the Src. Nat Rev Mol Cell Biol. 2001;2:467–475. doi: 10.1038/35073094. [DOI] [PubMed] [Google Scholar]
- 172.Yeatman TJ. A renaissance for SRC. Nat Rev Cancer. 2004;4:470–480. doi: 10.1038/nrc1366. [DOI] [PubMed] [Google Scholar]
- 173.Willman CL, Stewart CC, Longacre TL, Head DR, Habbersett R, Ziegler SF, Perlmutter RM. Expression of the c-fgr and hck protein-tyrosine kinases in acute myeloid leukemic blasts is associated with early commitment and differentiation events in the monocytic and granulocytic lineages. Blood. 1991;77:726–734. [PubMed] [Google Scholar]
- 174.Kazansky AV, Rosen JM. Signal transducers and activators of transcription 5B potentiates v-Src-mediated transformation of NIH-3T3 cells. Cell Growth Differ. 2001;12:1–7. [PubMed] [Google Scholar]
- 175.Kloth MT, Laughlin KK, Biscardi JS, Boerner JL, Parsons SJ, Silva CM. STAT5b, a mediator of synergism between c-Src and the epidermal growth factor receptor. J Biol Chem. 2003;278:1671–1679. doi: 10.1074/jbc.M207289200. [DOI] [PubMed] [Google Scholar]
- 176.Ptasznik A, Traynor-Kaplan A, Bokoch GM. G protein-coupled chemoattractant receptors regulate Lyn tyrosine kinase.Shc adapter protein signaling complexes. J Biol Chem. 1995;270:19969–19973. doi: 10.1074/jbc.270.34.19969. [DOI] [PubMed] [Google Scholar]
- 177.Li Y, Mohammad RM, al-Katib A, Varterasian ML, Chen B. Bryostatin 1 (bryo1)-induced monocytic differentiation in THP-1 human leukemia cells is associated with enhanced c-fyn tyrosine kinase and M-CSF receptors. Leuk Res. 1997;21:391–397. doi: 10.1016/s0145-2126(96)00078-1. [DOI] [PubMed] [Google Scholar]
- 178.Xue ZH, Zhao CQ, Chua GL, Tan SW, Tang XY, Wong SC, Tan SM. Integrin alphaMbeta2 clustering triggers phosphorylation and activation of protein kinase C delta that regulates transcription factor Foxp1 expression in monocytes. J Immunol. 2010;184:3697–3709. doi: 10.4049/jimmunol.0903316. [DOI] [PubMed] [Google Scholar]
- 179.Biscardi JS, Ishizawar RC, Silva CM, Parsons SJ. Tyrosine kinase signalling in breast cancer: epidermal growth factor receptor and c-Src interactions in breast cancer. Breast Cancer Res. 2000;2:203–210. doi: 10.1186/bcr55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Lennartsson J, Wernstedt C, Engstrom U, Hellman U, Ronnstrand L. Identification of Tyr900 in the kinase domain of c-Kit as a Src-dependent phosphorylation site mediating interaction with c-Crk. Exp Cell Res. 2003;288:110–118. doi: 10.1016/s0014-4827(03)00206-4. [DOI] [PubMed] [Google Scholar]
- 181.Gee CE, Griffin J, Sastre L, Miller LJ, Springer TA, Piwnica-Worms H, Roberts TM. Differentiation of myeloid cells is accompanied by increased levels of pp 60c-src protein and kinase activity. Proc Natl Acad Sci USA. 1986;83:5131–5135. doi: 10.1073/pnas.83.14.5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Willman CL, Stewart CC, Griffith JK, Stewart SJ, Tomasi TB. Differential expression and regulation of the c-src and c-fgr protooncogenes in myelomonocytic cells. Proc Natl Acad Sci USA. 1987;84:4480–4484. doi: 10.1073/pnas.84.13.4480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Matikainen S, Hurme M. Comparison of retinoic acid and phorbol myristate acetate as inducers of monocytic differentiation. Int J Cancer. 1994;57:98–103. doi: 10.1002/ijc.2910570118. [DOI] [PubMed] [Google Scholar]
- 184.Chiaradonna F, Fontana L, Iavarone C, Carriero MV, Scholz G, Barone MV, Stoppelli MP. Urokinase receptor-dependent and -independent p56/59(hck) activation state is a molecular switch between myelomonocytic cell motility and adherence. EMBO J. 1999;18:3013–3023. doi: 10.1093/emboj/18.11.3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Katagiri K, Katagiri T, Koyama Y, Morikawa M, Yamamoto T, Yoshida T. Expression of src family genes during monocytic differentiation of HL-60 cells. J Immunol. 1991;146:701–707. [PubMed] [Google Scholar]
- 186.Katagiri K, Katagiri T, Kajiyama K, Yamamoto T, Yoshida T. Tyrosine-phosphorylation of tubulin during monocytic differentiation of HL-60 cells. J Immunol. 1993;150:585–593. [PubMed] [Google Scholar]
- 187.Yao Y, Zhou Q, Ericson SG. Vanadate stimulates monocytic differentiation activity of IL-6 by enhancing actin filament polymerization in HL-60 cells. J Biomed Sci. 2004;11:940–949. doi: 10.1159/000081841. [DOI] [PubMed] [Google Scholar]
- 188.Mermel CH, McLemore ML, Liu F, Pereira S, Woloszynek J, Lowell CA, Link DC. Src family kinases are important negative regulators of G-CSF-dependent granulopoiesis. Blood. 2006;108:2562–2568. doi: 10.1182/blood-2006-05-024307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Harder KW, Parsons LM, Armes J, Evans N, Kountouri N, Clark R, Quilici C, Grail D, Hodgson GS, Dunn AR, Hibbs ML. Gain- and loss-of-function Lyn mutant mice define a critical inhibitory role for Lyn in the myeloid lineage. Immunity. 2001;15:603–615. doi: 10.1016/s1074-7613(01)00208-4. [DOI] [PubMed] [Google Scholar]
- 190.Napolitani G, Bortoletto N, Racioppi L, Lanzavecchia A, D’Oro U. Activation of src-family tyrosine kinases by LPS regulates cytokine production in dendritic cells by controlling AP-1 formation. Eur J Immunol. 2003;33:2832–2841. doi: 10.1002/eji.200324073. [DOI] [PubMed] [Google Scholar]
- 191.Mirnics ZK, Caudell E, Gao Y, Kuwahara K, Sakaguchi N, Kurosaki T, Burnside J, Mirnics K, Corey SJ. Microarray analysis of Lyn-deficient B cells reveals germinal center-associated nuclear protein and other genes associated with the lymphoid germinal center. J Immunol. 2004;172:4133–4141. doi: 10.4049/jimmunol.172.7.4133. [DOI] [PubMed] [Google Scholar]
- 192.Zhou HR, Jia Q, Pestka JJ. Ribotoxic stress response to the trichothecene deoxynivalenol in the macrophage involves the SRC family kinase Hck. Toxicol Sci. 2005;85:916–926. doi: 10.1093/toxsci/kfi146. [DOI] [PubMed] [Google Scholar]
- 193.Schreiner SJ, Schiavone AP, Smithgall TE. Activation of STAT3 by the Src family kinase Hck requires a functional SH3 domain. J Biol Chem. 2002;277:45680–45687. doi: 10.1074/jbc.M204255200. [DOI] [PubMed] [Google Scholar]
- 194.Carotta S, Wu L, Nutt SL. Surprising new roles for PU.1 in the adaptive immune response. Immunol Rev. 2010;238:63–75. doi: 10.1111/j.1600-065X.2010.00955.x. [DOI] [PubMed] [Google Scholar]
- 195.Rosmarin AG, Yang Z, Resendes KK. Transcriptional regulation in myelopoiesis: Hematopoietic fate choice, myeloid differentiation, and leukemogenesis. Exp Hematol. 2005;33:131–143. doi: 10.1016/j.exphem.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 196.Gangenahalli GU, Gupta P, Saluja D, Verma YK, Kishore V, Chandra R, Sharma RK, Ravindranath T. Stem cell fate specification: role of master regulatory switch transcription factor PU.1 in differential hematopoiesis. Stem Cells Dev. 2005;14:140–152. doi: 10.1089/scd.2005.14.140. [DOI] [PubMed] [Google Scholar]
- 197.Scott EW, Simon MC, Anastasi J, Singh H. Requirement of transcription factor PU.1 in the development of multiple hematopoietic lineages. Science. 1994;265:1573–1577. doi: 10.1126/science.8079170. [DOI] [PubMed] [Google Scholar]
- 198.McKercher SR, Torbett BE, Anderson KL, Henkel GW, Vestal DJ, Baribault H, Klemsz M, Feeney AJ, Wu GE, Paige CJ, Maki RA. Targeted disruption of the PU.1 gene results in multiple hematopoietic abnormalities. EMBO J. 1996;15:5647–5658. [PMC free article] [PubMed] [Google Scholar]
- 199.Kastner P, Chan S. PU.1: a crucial and versatile player in hematopoiesis and leukemia. Int J Biochem Cell Biol. 2008;40:22–27. doi: 10.1016/j.biocel.2007.01.026. [DOI] [PubMed] [Google Scholar]
- 200.Sakhinia E, Byers R, Bashein A, Hoyland J, Buckle AM, Brady G. Gene expression analysis of myeloid and lymphoid lineage markers during mouse haematopoiesis. Br J Haematol. 2006;135:105–116. doi: 10.1111/j.1365-2141.2006.06254.x. [DOI] [PubMed] [Google Scholar]
- 201.Dakic A, Metcalf D, Di Rago L, Mifsud S, Wu L, Nutt SL. PU.1 regulates the commitment of adult hematopoietic progenitors and restricts granulopoiesis. J Exp Med. 2005;201:1487–1502. doi: 10.1084/jem.20050075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Iwasaki H, Somoza C, Shigematsu H, Duprez EA, Iwasaki-Arai J, Mizuno S, Arinobu Y, Geary K, Zhang P, Dayaram T, Fenyus ML, Elf S, Chan S, Kastner P, Huettner CS, Murray R, Tenen DG, Akashi K. Distinctive and indispensable roles of PU.1 in maintenance of hematopoietic stem cells and their differentiation. Blood. 2005;106:1590–1600. doi: 10.1182/blood-2005-03-0860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Zhang P, Zhang X, Iwama A, Yu C, Smith KA, Mueller BU, Narravula S, Torbett BE, Orkin SH, Tenen DG. PU.1 inhibits GATA-1 function and erythroid differentiation by blocking GATA-1 DNA binding. Blood. 2000;96:2641–2648. [PubMed] [Google Scholar]
- 204.Chateauvieux S, Eifes S, Morceau F, Grigorakaki C, Schnekenburger M, Henry E, Dicato M, Diederich M. Valproic acid perturbs hematopoietic homeostasis by inhibition of erythroid differentiation and activation of the myelo-monocytic pathway. Biochem Pharmacol. 2011;81:498–509. doi: 10.1016/j.bcp.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 205.Liew CW, Rand KD, Simpson RJ, Yung WW, Mansfield RE, Crossley M, Proetorius-Ibba M, Nerlov C, Poulsen FM, Mackay JP. Molecular analysis of the interaction between the hematopoietic master transcription factors GATA-1 and PU.1. J Biol Chem. 2006;281:28296–28306. doi: 10.1074/jbc.M602830200. [DOI] [PubMed] [Google Scholar]
- 206.Walsh JC, DeKoter RP, Lee HJ, Smith ED, Lancki DW, Gurish MF, Friend DS, Stevens RL, Anastasi J, Singh H. Cooperative and antagonistic interplay between PU.1 and GATA-2 in the specification of myeloid cell fates. Immunity. 2002;17:665–676. doi: 10.1016/s1074-7613(02)00452-1. [DOI] [PubMed] [Google Scholar]
- 207.Wang D, D’Costa J, Civin CI, Friedman AD. C/EBPalpha directs monocytic commitment of primary myeloid progenitors. Blood. 2006;108:1223–1229. doi: 10.1182/blood-2005-12-008763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.DeKoter RP, Singh H. Regulation of B lymphocyte and macrophage development by graded expression of PU.1. Science. 2000;288:1439–1441. doi: 10.1126/science.288.5470.1439. [DOI] [PubMed] [Google Scholar]
- 209.Li CY, Zhan YQ, Li W, Xu CW, Xu WX, Yu DH, Peng RY, Cui YF, Yang X, Hou N, Li YH, Dong B, Sun HB, Yang XM. Overexpression of a hematopoietic transcriptional regulator EDAG induces myelopoiesis and suppresses lymphopoiesis in transgenic mice. Leukemia. 2007;21:2277–2286. doi: 10.1038/sj.leu.2404901. [DOI] [PubMed] [Google Scholar]
- 210.Chen H, Ray-Gallet D, Zhang P, Hetherington CJ, Gonzalez DA, Zhang DE, Moreau-Gachelin F, Tenen DG. PU.1 (Spi-1) autoregulates its expression in myeloid cells. Oncogene. 1995;11:1549–1560. [PubMed] [Google Scholar]
- 211.Dahl R, Simon MC. The importance of PU.1 concentration in hematopoietic lineage commitment and maturation. Blood Cells Mol Dis. 2003;31:229–233. doi: 10.1016/s1079-9796(03)00152-9. [DOI] [PubMed] [Google Scholar]
- 212.Friedman AD. C/EBPalpha induces PU.1 and interacts with AP-1 and NF-kappaB to regulate myeloid development. Blood Cells Mol Dis. 2007;39:340–343. doi: 10.1016/j.bcmd.2007.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Rosenbauer F, Wagner K, Kutok JL, Iwasaki H, Le Beau MM, Okuno Y, Akashi K, Fiering S, Tenen DG. Acute myeloid leukemia induced by graded reduction of a lineage-specific transcription factor, PU.1. Nat Genet. 2004;36:624–630. doi: 10.1038/ng1361. [DOI] [PubMed] [Google Scholar]
- 214.Yeamans C, Wang D, Paz-Priel I, Torbett BE, Tenen DG, Friedman AD. C/EBPalpha binds and activates the PU.1 distal enhancer to induce monocyte lineage commitment. Blood. 2007;110:3136–3142. doi: 10.1182/blood-2007-03-080291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Houston IB, Kamath MB, Schweitzer BL, Chlon TM, DeKoter RP. Reduction in PU.1 activity results in a block to B-cell development, abnormal myeloid proliferation, and neonatal lethality. Exp Hematol. 2007;35:1056–1068. doi: 10.1016/j.exphem.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.de Bruin AM, Libregts SF, Valkhof M, Boon L, Touw IP, Nolte MA. IFNgamma induces monopoiesis and inhibits neutrophil development during inflammation. Blood. 2012;119:1543–1554. doi: 10.1182/blood-2011-07-367706. [DOI] [PubMed] [Google Scholar]
- 217.Tamura T, Yanai H, Savitsky D, Taniguchi T. The IRF family transcription factors in immunity and oncogenesis. Annu Rev Immunol. 2008;26:535–584. doi: 10.1146/annurev.immunol.26.021607.090400. [DOI] [PubMed] [Google Scholar]
- 218.Laslo P, Spooner CJ, Warmflash A, Lancki DW, Lee HJ, Sciammas R, Gantner BN, Dinner AR, Singh H. Multilineage transcriptional priming and determination of alternate hematopoietic cell fates. Cell. 2006;126:755–766. doi: 10.1016/j.cell.2006.06.052. [DOI] [PubMed] [Google Scholar]
- 219.Xie H, Ye M, Feng R, Graf T. Stepwise reprogramming of B cells into macrophages. Cell. 2004;117:663–676. doi: 10.1016/s0092-8674(04)00419-2. [DOI] [PubMed] [Google Scholar]
- 220.Dionne CJ, Tse KY, Weiss AH, Franco CB, Wiest DL, Anderson MK, Rothenberg EV. Subversion of T lineage commitment by PU.1 in a clonal cell line system. Dev Biol. 2005;280:448–466. doi: 10.1016/j.ydbio.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 221.Laiosa CV, Stadtfeld M, Xie H, de Andres-Aguayo L, Graf T. Reprogramming of committed T cell progenitors to macrophages and dendritic cells by C/EBP alpha and PU.1 transcription factors. Immunity. 2006;25:731–744. doi: 10.1016/j.immuni.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 222.Feng R, Desbordes SC, Xie H, Tillo ES, Pixley F, Stanley ER, Graf T. PU.1 and C/EBPalpha/beta convert fibroblasts into macrophage-like cells. Proc Natl Acad Sci USA. 2008;105:6057–6062. doi: 10.1073/pnas.0711961105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Olson MC, Scott EW, Hack AA, Su GH, Tenen DG, Singh H, Simon MC. PU. 1 is not essential for early myeloid gene expression but is required for terminal myeloid differentiation. Immunity. 1995;3:703–714. doi: 10.1016/1074-7613(95)90060-8. [DOI] [PubMed] [Google Scholar]
- 224.Henkel GW, McKercher SR, Leenen PJ, Maki RA. Commitment to the monocytic lineage occurs in the absence of the transcription factor PU.1. Blood. 1999;93:2849–2858. [PubMed] [Google Scholar]
- 225.Friedman AD. Transcriptional regulation of granulocyte and monocyte development. Oncogene. 2002;21:3377–3390. doi: 10.1038/sj.onc.1205324. [DOI] [PubMed] [Google Scholar]
- 226.Starck J, Weiss-Gayet M, Gonnet C, Guyot B, Vicat JM, Morle F. Inducible Fli-1 gene deletion in adult mice modifies several myeloid lineage commitment decisions and accelerates proliferation arrest and terminal erythrocytic differentiation. Blood. 2010;116:4795–4805. doi: 10.1182/blood-2010-02-270405. [DOI] [PubMed] [Google Scholar]
- 227.Yang ZF, Drumea K, Cormier J, Wang J, Zhu X, Rosmarin AG. GABP transcription factor is required for myeloid differentiation, in part, through its control of Gfi-1 expression. Blood. 2011;118:2243–2253. doi: 10.1182/blood-2010-07-298802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Feinberg MW, Wara AK, Cao Z, Lebedeva MA, Rosenbauer F, Iwasaki H, Hirai H, Katz JP, Haspel RL, Gray S, Akashi K, Segre J, Kaestner KH, Tenen DG, Jain MK. The Kruppel-like factor KLF4 is a critical regulator of monocyte differentiation. EMBO J. 2007;26:4138–4148. doi: 10.1038/sj.emboj.7601824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Steidl U, Rosenbauer F, Verhaak RG, Gu X, Ebralidze A, Otu HH, Klippel S, Steidl C, Bruns I, Costa DB, Wagner K, Aivado M, Kobbe G, Valk PJ, Passegue E, Libermann TA, Delwel R, Tenen DG. Essential role of Jun family transcription factors in PU.1 knockdown-induced leukemic stem cells. Nat Genet. 2006;38:1269–1277. doi: 10.1038/ng1898. [DOI] [PubMed] [Google Scholar]
- 230.Ghani S, Riemke P, Schonheit J, Lenze D, Stumm J, Hoogenkamp M, Lagendijk A, Heinz S, Bonifer C, Bakkers J, Abdelilah-Seyfried S, Hummel M, Rosenbauer F. Macrophage development from HSCs requires PU.1-coordinated microRNA expression. Blood. 2011;118:2275–2284. doi: 10.1182/blood-2011-02-335141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Kong KY, Owens KS, Rogers JH, Mullenix J, Velu CS, Grimes HL, Dahl R. MIR-23A microRNA cluster inhibits B-cell development. Exp Hematol. 2010;38(629–640):e621. doi: 10.1016/j.exphem.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Ramji DP, Foka P. CCAAT/enhancer-binding proteins: structure, function and regulation. Biochem J. 2002;365:561–575. doi: 10.1042/BJ20020508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Johnson PF. Molecular stop signs: regulation of cell-cycle arrest by C/EBP transcription factors. J Cell Sci. 2005;118:2545–2555. doi: 10.1242/jcs.02459. [DOI] [PubMed] [Google Scholar]
- 234.Wang X, Scott E, Sawyers CL, Friedman AD. C/EBPalpha bypasses granulocyte colony-stimulating factor signals to rapidly induce PU.1 gene expression, stimulate granulocytic differentiation, and limit proliferation in 32D cl3 myeloblasts. Blood. 1999;94:560–571. [PubMed] [Google Scholar]
- 235.Johansen LM, Iwama A, Lodie TA, Sasaki K, Felsher DW, Golub TR, Tenen DG. c-Myc is a critical target for c/EBPalpha in granulopoiesis. Mol Cell Biol. 2001;21:3789–3806. doi: 10.1128/MCB.21.11.3789-3806.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Timchenko NA, Wilde M, Nakanishi M, Smith JR, Darlington GJ. CCAAT/enhancer-binding protein alpha (C/EBP alpha) inhibits cell proliferation through the p21 (WAF-1/CIP-1/SDI-1) protein. Genes Dev. 1996;10:804–815. doi: 10.1101/gad.10.7.804. [DOI] [PubMed] [Google Scholar]
- 237.Friedman AD, Keefer JR, Kummalue T, Liu H, Wang QF, Cleaves R. Regulation of granulocyte and monocyte differentiation by CCAAT/enhancer binding protein alpha. Blood Cells Mol Dis. 2003;31:338–341. doi: 10.1016/s1079-9796(03)00135-9. [DOI] [PubMed] [Google Scholar]
- 238.Kirstetter P, Schuster MB, Bereshchenko O, Moore S, Dvinge H, Kurz E, Theilgaard-Monch K, Mansson R, Pedersen TA, Pabst T, Schrock E, Porse BT, Jacobsen SE, Bertone P, Tenen DG, Nerlov C. Modeling of C/EBPalpha mutant acute myeloid leukemia reveals a common expression signature of committed myeloid leukemia-initiating cells. Cancer Cell. 2008;13:299–310. doi: 10.1016/j.ccr.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 239.Chen Y, Costa RM, Love NR, Soto X, Roth M, Paredes R, Amaya E. C/EBPalpha initiates primitive myelopoiesis in pluripotent embryonic cells. Blood. 2009;114:40–48. doi: 10.1182/blood-2008-11-189159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Suh HC, Gooya J, Renn K, Friedman AD, Johnson PF, Keller JR. C/EBPalpha determines hematopoietic cell fate in multipotential progenitor cells by inhibiting erythroid differentiation and inducing myeloid differentiation. Blood. 2006;107:4308–4316. doi: 10.1182/blood-2005-06-2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Fukuchi Y, Shibata F, Ito M, Goto-Koshino Y, Sotomaru Y, Ito M, Kitamura T, Nakajima H. Comprehensive analysis of myeloid lineage conversion using mice expressing an inducible form of C/EBP alpha. EMBO J. 2006;25:3398–3410. doi: 10.1038/sj.emboj.7601199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Hanna J, Markoulaki S, Schorderet P, Carey BW, Beard C, Wernig M, Creyghton MP, Steine EJ, Cassady JP, Foreman R, Lengner CJ, Dausman JA, Jaenisch R. Direct reprogramming of terminally differentiated mature B lymphocytes to pluripotency. Cell. 2008;133:250–264. doi: 10.1016/j.cell.2008.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Hsu CL, King-Fleischman AG, Lai AY, Matsumoto Y, Weissman IL, Kondo M. Antagonistic effect of CCAAT enhancer-binding protein-alpha and Pax5 in myeloid or lymphoid lineage choice in common lymphoid progenitors. Proc Natl Acad Sci USA. 2006;103:672–677. doi: 10.1073/pnas.0510304103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Heavey B, Charalambous C, Cobaleda C, Busslinger M. Myeloid lineage switch of Pax5 mutant but not wild-type B cell progenitors by C/EBPalpha and GATA factors. EMBO J. 2003;22:3887–3897. doi: 10.1093/emboj/cdg380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Zhang DE, Zhang P, Wang ND, Hetherington CJ, Darlington GJ, Tenen DG. Absence of granulocyte colony-stimulating factor signaling and neutrophil development in CCAAT enhancer binding protein alpha-deficient mice. Proc Natl Acad Sci USA. 1997;94:569–574. doi: 10.1073/pnas.94.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Zhang P, Iwasaki-Arai J, Iwasaki H, Fenyus ML, Dayaram T, Owens BM, Shigematsu H, Levantini E, Huettner CS, Lekstrom-Himes JA, Akashi K, Tenen DG. Enhancement of hematopoietic stem cell repopulating capacity and self-renewal in the absence of the transcription factor C/EBP alpha. Immunity. 2004;21:853–863. doi: 10.1016/j.immuni.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 247.Heath V, Suh HC, Holman M, Renn K, Gooya JM, Parkin S, Klarmann KD, Ortiz M, Johnson P, Keller J. C/EBPalpha deficiency results in hyperproliferation of hematopoietic progenitor cells and disrupts macrophage development in vitro and in vivo. Blood. 2004;104:1639–1647. doi: 10.1182/blood-2003-11-3963. [DOI] [PubMed] [Google Scholar]
- 248.Wang QF, Friedman AD. CCAAT/enhancer-binding proteins are required for granulopoiesis independent of their induction of the granulocyte colony-stimulating factor receptor. Blood. 2002;99:2776–2785. doi: 10.1182/blood.v99.8.2776. [DOI] [PubMed] [Google Scholar]
- 249.Kato N, Kitaura J, Doki N, Komeno Y, Watanabe-Okochi N, Togami K, Nakahara F, Oki T, Enomoto Y, Fukuchi Y, Nakajima H, Harada Y, Harada H, Kitamura T. Two types of C/EBPalpha mutations play distinct but collaborative roles in leukemogenesis: lessons from clinical data and BMT models. Blood. 2010;117:221–233. doi: 10.1182/blood-2010-02-270181. [DOI] [PubMed] [Google Scholar]
- 250.Schwieger M, Lohler J, Fischer M, Herwig U, Tenen DG, Stocking C. A dominant-negative mutant of C/EBPalpha, associated with acute myeloid leukemias, inhibits differentiation of myeloid and erythroid progenitors of man but not mouse. Blood. 2004;103:2744–2752. doi: 10.1182/blood-2003-07-2280. [DOI] [PubMed] [Google Scholar]
- 251.Niebuhr B, Iwanski GB, Schwieger M, Roscher S, Stocking C, Cammenga J. Investigation of C/EBPalpha function in human (versus murine) myelopoiesis provides novel insight into the impact of CEBPA mutations in acute myelogenous leukemia (AML) Leukemia. 2009;23:978–983. doi: 10.1038/leu.2008.332. [DOI] [PubMed] [Google Scholar]
- 252.Matsushita H, Nakajima H, Nakamura Y, Tsukamoto H, Tanaka Y, Jin G, Yabe M, Asai S, Ono R, Nosaka T, Sugita K, Morimoto A, Hayashi Y, Hotta T, Ando K, Miyachi H. C/EBPalpha and C/EBPvarepsilon induce the monocytic differentiation of myelomonocytic cells with the MLL-chimeric fusion gene. Oncogene. 2008;27:6749–6760. doi: 10.1038/onc.2008.285. [DOI] [PubMed] [Google Scholar]
- 253.Schepers H, Wierenga AT, van Gosliga D, Eggen BJ, Vellenga E, Schuringa JJ. Reintroduction of C/EBPalpha in leukemic CD34 + stem/progenitor cells impairs self-renewal and partially restores myelopoiesis. Blood. 2007;110:1317–1325. doi: 10.1182/blood-2006-10-052175. [DOI] [PubMed] [Google Scholar]
- 254.Huber R, Pietsch D, Panterodt T, Brand K. Regulation of C/EBPbeta and resulting functions in cells of the monocytic lineage. Cell Signal. 2012;24:1287–1296. doi: 10.1016/j.cellsig.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 255.Gutsch R, Kandemir JD, Pietsch D, Cappello C, Meyer J, Simanowski K, Huber R, Brand K. CCAAT/enhancer-binding protein beta inhibits proliferation in monocytic cells by affecting the retinoblastoma protein/E2F/cyclin E pathway but is not directly required for macrophage morphology. J Biol Chem. 2011;286:22716–22729. doi: 10.1074/jbc.M110.152538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Hirai H, Zhang P, Dayaram T, Hetherington CJ, Mizuno S, Imanishi J, Akashi K, Tenen DG. C/EBPbeta is required for ‘emergency’ granulopoiesis. Nat Immunol. 2006;7:732–739. doi: 10.1038/ni1354. [DOI] [PubMed] [Google Scholar]
- 257.Popernack PM, Truong LT, Kamphuis M, Henderson AJ. Ectopic expression of CCAAT/enhancer binding protein beta (C/EBPbeta) in long-term bone marrow cultures induces granulopoiesis and alters stromal cell function. J Hematother Stem Cell Res. 2001;10:631–642. doi: 10.1089/152581601753193841. [DOI] [PubMed] [Google Scholar]
- 258.Park MH, Park SY, Kim Y. Induction of proline-rich tyrosine kinase2 (Pyk2) through C/EBPbeta is involved in PMA-induced monocyte differentiation. FEBS Lett. 2008;582:415–422. doi: 10.1016/j.febslet.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 259.Screpanti I, Romani L, Musiani P, Modesti A, Fattori E, Lazzaro D, Sellitto C, Scarpa S, Bellavia D, Lattanzio G, et al. Lymphoproliferative disorder and imbalanced T-helper response in C/EBP beta-deficient mice. EMBO J. 1995;14:1932–1941. doi: 10.1002/j.1460-2075.1995.tb07185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Gorgoni B, Maritano D, Marthyn P, Righi M, Poli V. C/EBP beta gene inactivation causes both impaired and enhanced gene expression and inverse regulation of IL-12 p40 and p35 mRNAs in macrophages. J Immunol. 2002;168:4055–4062. doi: 10.4049/jimmunol.168.8.4055. [DOI] [PubMed] [Google Scholar]
- 261.Tanaka T, Akira S, Yoshida K, Umemoto M, Yoneda Y, Shirafuji N, Fujiwara H, Suematsu S, Yoshida N, Kishimoto T. Targeted disruption of the NF-IL6 gene discloses its essential role in bacteria killing and tumor cytotoxicity by macrophages. Cell. 1995;80:353–361. doi: 10.1016/0092-8674(95)90418-2. [DOI] [PubMed] [Google Scholar]
- 262.Antonson P, Stellan B, Yamanaka R, Xanthopoulos KG. A novel human CCAAT/enhancer binding protein gene, C/EBPepsilon, is expressed in cells of lymphoid and myeloid lineages and is localized on chromosome 14q11.2 close to the T-cell receptor alpha/delta locus. Genomics. 1996;35:30–38. doi: 10.1006/geno.1996.0319. [DOI] [PubMed] [Google Scholar]
- 263.Yoshida H, Ichikawa H, Tagata Y, Katsumoto T, Ohnishi K, Akao Y, Naoe T, Pandolfi PP, Kitabayashi I. PML-retinoic acid receptor alpha inhibits PML IV enhancement of PU.1-induced C/EBPepsilon expression in myeloid differentiation. Mol Cell Biol. 2007;27:5819–5834. doi: 10.1128/MCB.02422-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Nakajima H, Watanabe N, Shibata F, Kitamura T, Ikeda Y, Handa M. N-terminal region of CCAAT/enhancer-binding protein epsilon is critical for cell cycle arrest, apoptosis, and functional maturation during myeloid differentiation. J Biol Chem. 2006;281:14494–14502. doi: 10.1074/jbc.M600575200. [DOI] [PubMed] [Google Scholar]
- 265.Dimberg A, Bahram F, Karlberg I, Larsson LG, Nilsson K, Oberg F. Retinoic acid-induced cell cycle arrest of human myeloid cell lines is associated with sequential down-regulation of c-Myc and cyclin E and posttranscriptional up-regulation of p27(Kip1) Blood. 2002;99:2199–2206. doi: 10.1182/blood.v99.6.2199. [DOI] [PubMed] [Google Scholar]
- 266.Verbeek W, Gombart AF, Chumakov AM, Muller C, Friedman AD, Koeffler HP. C/EBPepsilon directly interacts with the DNA binding domain of c-myb and cooperatively activates transcription of myeloid promoters. Blood. 1999;93:3327–3337. [PubMed] [Google Scholar]
- 267.Bedi R, Du J, Sharma AK, Gomes I, Ackerman SJ. Human C/EBP-epsilon activator and repressor isoforms differentially reprogram myeloid lineage commitment and differentiation. Blood. 2009;113:317–327. doi: 10.1182/blood-2008-02-139741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Halene S, Gaines P, Sun H, Zibello T, Lin S, Khanna-Gupta A, Williams SC, Perkins A, Krause D, Berliner N. C/EBPepsilon directs granulocytic-vs-monocytic lineage determination and confers chemotactic function via Hlx. Exp Hematol. 2010;38:90–103. doi: 10.1016/j.exphem.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Verbeek W, Wachter M, Lekstrom-Himes J, Koeffler HP. C/EBPepsilon -/- mice: increased rate of myeloid proliferation and apoptosis. Leukemia. 2001;15:103–111. doi: 10.1038/sj.leu.2401995. [DOI] [PubMed] [Google Scholar]
- 270.Tavor S, Vuong PT, Park DJ, Gombart AF, Cohen AH, Koeffler HP. Macrophage functional maturation and cytokine production are impaired in C/EBP epsilon-deficient mice. Blood. 2002;99:1794–1801. doi: 10.1182/blood.v99.5.1794. [DOI] [PubMed] [Google Scholar]
- 271.Gombart AF, Krug U, O’Kelly J, An E, Vegesna V, Koeffler HP. Aberrant expression of neutrophil and macrophage-related genes in a murine model for human neutrophil-specific granule deficiency. J Leukoc Biol. 2005;78:1153–1165. doi: 10.1189/jlb.0504286. [DOI] [PubMed] [Google Scholar]
- 272.Shiohara M, Gombart AF, Sekiguchi Y, Hidaka E, Ito S, Yamazaki T, Koeffler HP, Komiyama A. Phenotypic and functional alterations of peripheral blood monocytes in neutrophil-specific granule deficiency. J Leukoc Biol. 2004;75:190–197. doi: 10.1189/jlb.0203063. [DOI] [PubMed] [Google Scholar]
- 273.Chih DY, Park DJ, Gross M, Idos G, Vuong PT, Hirama T, Chumakov AM, Said J, Koeffler HP. Protein partners of C/EBPepsilon. Exp Hematol. 2004;32:1173–1181. doi: 10.1016/j.exphem.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 274.Kreutz M, Andreesen R, Krause SW, Szabo A, Ritz E, Reichel H. 1,25-dihydroxyvitamin D3 production and vitamin D3 receptor expression are developmentally regulated during differentiation of human monocytes into macrophages. Blood. 1993;82:1300–1307. [PubMed] [Google Scholar]
- 275.Chute JP, Ross JR, McDonnell DP. Minireview: nuclear receptors, hematopoiesis, and stem cells. Mol Endocrinol. 2010;24:1–10. doi: 10.1210/me.2009-0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Campbell FC, Xu H, El-Tanani M, Crowe P, Bingham V. The yin and yang of vitamin D receptor (VDR) signaling in neoplastic progression: operational networks and tissue-specific growth control. Biochem Pharmacol. 2010;79:1–9. doi: 10.1016/j.bcp.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Carlberg C, Seuter S, Heikkinen S. The first genome-wide view of vitamin D receptor locations and their mechanistic implications. Anticancer Res. 2012;32:271–282. [PubMed] [Google Scholar]
- 278.Ohta M, Okabe T, Ozawa K, Urabe A, Takaku F. 1 alpha,25-Dihydroxyvitamin D3 (calcitriol) stimulates proliferation of human circulating monocytes in vitro. FEBS Lett. 1985;185:9–13. doi: 10.1016/0014-5793(85)80730-4. [DOI] [PubMed] [Google Scholar]
- 279.Brown G, Choudhry MA, Durham J, Drayson MT, Michell RH. Monocytically differentiating HL60 cells proliferate rapidly before they mature. Exp Cell Res. 1999;253:511–518. doi: 10.1006/excr.1999.4660. [DOI] [PubMed] [Google Scholar]
- 280.Yang W, Freedman LP. 20-Epi analogues of 1,25-dihydroxyvitamin D3 are highly potent inducers of DRIP coactivator complex binding to the vitamin D3 receptor. J Biol Chem. 1999;274:16838–16845. doi: 10.1074/jbc.274.24.16838. [DOI] [PubMed] [Google Scholar]
- 281.Kumagai T, O’Kelly J, Said JW, Koeffler HP. Vitamin D2 analog 19-nor-1,25-dihydroxyvitamin D2: antitumor activity against leukemia, myeloma, and colon cancer cells. J Natl Cancer Inst. 2003;95:896–905. doi: 10.1093/jnci/95.12.896. [DOI] [PubMed] [Google Scholar]
- 282.Hewison M, Dabrowski M, Vadher S, Faulkner L, Cockerill FJ, Brickell PM, O’Riordan JL, Katz DR. Antisense inhibition of vitamin D receptor expression induces apoptosis in monoblastoid U937 cells. J Immunol. 1996;156:4391–4400. [PubMed] [Google Scholar]
- 283.Taoka T, Collins ED, Irino S, Norman AW. 1,25(OH)2-vitamin D3 mediated changes in mRNA for c-myc and 1,25(OH)2D3 receptor in HL-60 cells and related subclones. Mol Cell Endocrinol. 1993;95:51–57. doi: 10.1016/0303-7207(93)90028-i. [DOI] [PubMed] [Google Scholar]
- 284.Liu M, Lee MH, Cohen M, Bommakanti M, Freedman LP. Transcriptional activation of the Cdk inhibitor p21 by vitamin D3 leads to the induced differentiation of the myelomonocytic cell line U937. Genes Dev. 1996;10:142–153. doi: 10.1101/gad.10.2.142. [DOI] [PubMed] [Google Scholar]
- 285.Carlberg C, Molnar F. Current status of vitamin D signaling and its therapeutic applications. Curr Top Med Chem. 2012;12:528–547. doi: 10.2174/156802612799436623. [DOI] [PubMed] [Google Scholar]
- 286.James SY, Williams MA, Kelsey SM, Newland AC, Colston KW. Interaction of vitamin D derivatives and granulocyte-macrophage colony-stimulating factor in leukaemic cell differentiation. Leukemia. 1997;11:1017–1025. doi: 10.1038/sj.leu.2400676. [DOI] [PubMed] [Google Scholar]
- 287.Garay E, Jankowski P, Lizano P, Marczak S, Maehr H, Adorini L, Uskokovic MR, Studzinski GP. Calcitriol derivatives with two different side-chains at C-20. Part 4: further chain modifications that alter VDR-dependent monocytic differentiation potency in human leukemia cells. Bioorg Med Chem. 2007;15:4444–4455. doi: 10.1016/j.bmc.2007.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Choudhuri U, Adams JA, Byrom N, McCarthy DM, Barrett J. 1,25-Dihydroxyvitamin D3 induces normal mononuclear blood cells to differentiate in the direction of monocyte-macrophages. Haematologia (Budap) 1990;23:9–19. [PubMed] [Google Scholar]
- 289.Kreutz M, Andreesen R. Induction of human monocyte to macrophage maturation in vitro by 1,25-dihydroxyvitamin D3. Blood. 1990;76:2457–2461. [PubMed] [Google Scholar]
- 290.Brown G, Bunce CM, Rowlands DC, Williams GR. All-trans retinoic acid and 1 alpha,25-dihydroxyvitamin D3 co-operate to promote differentiation of the human promyeloid leukemia cell line HL60 to monocytes. Leukemia. 1994;8:806–815. [PubMed] [Google Scholar]
- 291.Nakajima H, Kizaki M, Ueno H, Muto A, Takayama N, Matsushita H, Sonoda A, Ikeda Y. All-trans and 9-cis retinoic acid enhance 1,25-dihydroxyvitamin D3-induced monocytic differentiation of U937 cells. Leuk Res. 1996;20:665–676. doi: 10.1016/0145-2126(96)00020-3. [DOI] [PubMed] [Google Scholar]
- 292.Bastie JN, Balitrand N, Guidez F, Guillemot I, Larghero J, Calabresse C, Chomienne C, Delva L. 1 alpha,25-dihydroxyvitamin D3 transrepresses retinoic acid transcriptional activity via vitamin D receptor in myeloid cells. Mol Endocrinol. 2004;18:2685–2699. doi: 10.1210/me.2003-0412. [DOI] [PubMed] [Google Scholar]
- 293.Nakamaki T, Kawakami K, Sato S, Hino K, Tomoyasu S, Tsuruoka N, Honma Y, Hozumi M. The role of granulocyte-macrophage colony-stimulating factor in induction of monocytic differentiation of HL-60 cells: synergistic interaction with 1 alpha, 25-dihydroxyvitamin D3 and interferon-gamma in inducing interleukin-1 beta. Anticancer Res. 1992;12:1331–1337. [PubMed] [Google Scholar]
- 294.Botling J, Oberg F, Torma H, Tuohimaa P, Blauer M, Nilsson K. Vitamin D3- and retinoic acid-induced monocytic differentiation: interactions between the endogenous vitamin D3 receptor, retinoic acid receptors, and retinoid X receptors in U-937 cells. Cell Growth Differ. 1996;7:1239–1249. [PubMed] [Google Scholar]
- 295.Piemonti L, Monti P, Sironi M, Fraticelli P, Leone BE, Dal Cin E, Allavena P, Di Carlo V. Vitamin D3 affects differentiation, maturation, and function of human monocyte-derived dendritic cells. J Immunol. 2000;164:4443–4451. doi: 10.4049/jimmunol.164.9.4443. [DOI] [PubMed] [Google Scholar]
- 296.Griffin MD, Lutz W, Phan VA, Bachman LA, McKean DJ, Kumar R. Dendritic cell modulation by 1alpha,25 dihydroxyvitamin D3 and its analogs: a vitamin D receptor-dependent pathway that promotes a persistent state of immaturity in vitro and in vivo. Proc Natl Acad Sci USA. 2001;98:6800–6805. doi: 10.1073/pnas.121172198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Sochorova K, Budinsky V, Rozkova D, Tobiasova Z, Dusilova-Sulkova S, Spisek R, Bartunkova J. Paricalcitol (19-nor-1,25-dihydroxyvitamin D2) and calcitriol (1,25-dihydroxyvitamin D3) exert potent immunomodulatory effects on dendritic cells and inhibit induction of antigen-specific T cells. Clin Immunol. 2009;133:69–77. doi: 10.1016/j.clim.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 298.Gobel F, Taschner S, Jurkin J, Konradi S, Vaculik C, Richter S, Kneidinger D, Muhlbacher C, Bieglmayer C, Elbe-Burger A, Strobl H. Reciprocal role of GATA-1 and vitamin D receptor in human myeloid dendritic cell differentiation. Blood. 2009;114:3813–3821. doi: 10.1182/blood-2009-03-210484. [DOI] [PubMed] [Google Scholar]
- 299.Bhatia M, Kirkland JB, Meckling-Gill KA. Monocytic differentiation of acute promyelocytic leukemia cells in response to 1,25-dihydroxyvitamin D3 is independent of nuclear receptor binding. J Biol Chem. 1995;270:15962–15965. doi: 10.1074/jbc.270.27.15962. [DOI] [PubMed] [Google Scholar]
- 300.O’Kelly J, Hisatake J, Hisatake Y, Bishop J, Norman A, Koeffler HP. Normal myelopoiesis but abnormal T lymphocyte responses in vitamin D receptor knockout mice. J Clin Invest. 2002;109:1091–1099. doi: 10.1172/JCI12392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Chen W, Royer WE., Jr Structural insights into interferon regulatory factor activation. Cell Signal. 2010;22:883–887. doi: 10.1016/j.cellsig.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Manzella L, Conte E, Cocchiaro G, Guarniera E, Sciacca B, Bonaiuto C, Stagno F, Messina A. Role of interferon regulatory factor 1 in monocyte/macrophage differentiation. Eur J Immunol. 1999;29:3009–3016. doi: 10.1002/(SICI)1521-4141(199909)29:09<3009::AID-IMMU3009>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 303.Matikainen S, Ronni T, Hurme M, Pine R, Julkunen I. Retinoic acid activates interferon regulatory factor-1 gene expression in myeloid cells. Blood. 1996;88:114–123. [PubMed] [Google Scholar]
- 304.Lehtonen A, Veckman V, Nikula T, Lahesmaa R, Kinnunen L, Matikainen S, Julkunen I. Differential expression of IFN regulatory factor 4 gene in human monocyte-derived dendritic cells and macrophages. J Immunol. 2005;175:6570–6579. doi: 10.4049/jimmunol.175.10.6570. [DOI] [PubMed] [Google Scholar]
- 305.Ju XS, Ruau D, Jantti P, Sere K, Becker C, Wiercinska E, Bartz C, Erdmann B, Dooley S, Zenke M. Transforming growth factor beta1 up-regulates interferon regulatory factor 8 during dendritic cell development. Eur J Immunol. 2007;37:1174–1183. doi: 10.1002/eji.200636504. [DOI] [PubMed] [Google Scholar]
- 306.Tanaka N, Ishihara M, Lamphier MS, Nozawa H, Matsuyama T, Mak TW, Aizawa S, Tokino T, Oren M, Taniguchi T. Cooperation of the tumour suppressors IRF-1 and p53 in response to DNA damage. Nature. 1996;382:816–818. doi: 10.1038/382816a0. [DOI] [PubMed] [Google Scholar]
- 307.Pamment J, Ramsay E, Kelleher M, Dornan D, Ball KL. Regulation of the IRF-1 tumour modifier during the response to genotoxic stress involves an ATM-dependent signalling pathway. Oncogene. 2002;21:7776–7785. doi: 10.1038/sj.onc.1205981. [DOI] [PubMed] [Google Scholar]
- 308.Kano A, Haruyama T, Akaike T, Watanabe Y. IRF-1 is an essential mediator in IFN-gamma-induced cell cycle arrest and apoptosis of primary cultured hepatocytes. Biochem Biophys Res Commun. 1999;257:672–677. doi: 10.1006/bbrc.1999.0276. [DOI] [PubMed] [Google Scholar]
- 309.Yamamoto M, Kato T, Hotta C, Nishiyama A, Kurotaki D, Yoshinari M, Takami M, Ichino M, Nakazawa M, Matsuyama T, Kamijo R, Kitagawa S, Ozato K, Tamura T. Shared and distinct functions of the transcription factors IRF4 and IRF8 in myeloid cell development. PLoS ONE. 2011;6:e25812. doi: 10.1371/journal.pone.0025812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Tamura T, Nagamura-Inoue T, Shmeltzer Z, Kuwata T, Ozato K. ICSBP directs bipotential myeloid progenitor cells to differentiate into mature macrophages. Immunity. 2000;13:155–165. doi: 10.1016/s1074-7613(00)00016-9. [DOI] [PubMed] [Google Scholar]
- 311.Lu R, Pitha PM. Monocyte differentiation to macrophage requires interferon regulatory factor 7. J Biol Chem. 2001;276:45491–45496. doi: 10.1074/jbc.C100421200. [DOI] [PubMed] [Google Scholar]
- 312.Schmidt M, Bies J, Tamura T, Ozato K, Wolff L. The interferon regulatory factor ICSBP/IRF-8 in combination with PU.1 up-regulates expression of tumor suppressor p15(Ink4b) in murine myeloid cells. Blood. 2004;103:4142–4149. doi: 10.1182/blood-2003-01-0285. [DOI] [PubMed] [Google Scholar]
- 313.Tamura T, Kong HJ, Tunyaplin C, Tsujimura H, Calame K, Ozato K. ICSBP/IRF-8 inhibits mitogenic activity of p210 Bcr/Abl in differentiating myeloid progenitor cells. Blood. 2003;102:4547–4554. doi: 10.1182/blood-2003-01-0291. [DOI] [PubMed] [Google Scholar]
- 314.Zhu C, Saberwal G, Lu Y, Platanias LC, Eklund EA. The interferon consensus sequence-binding protein activates transcription of the gene encoding neurofibromin 1. J Biol Chem. 2004;279:50874–50885. doi: 10.1074/jbc.M405736200. [DOI] [PubMed] [Google Scholar]
- 315.Holtschke T, Lohler J, Kanno Y, Fehr T, Giese N, Rosenbauer F, Lou J, Knobeloch KP, Gabriele L, Waring JF, Bachmann MF, Zinkernagel RM, Morse HC, 3rd, Ozato K, Horak I. Immunodeficiency and chronic myelogenous leukemia-like syndrome in mice with a targeted mutation of the ICSBP gene. Cell. 1996;87:307–317. doi: 10.1016/s0092-8674(00)81348-3. [DOI] [PubMed] [Google Scholar]
- 316.Abdollahi A, Lord KA, Hoffman-Liebermann B, Liebermann DA. Interferon regulatory factor 1 is a myeloid differentiation primary response gene induced by interleukin 6 and leukemia inhibitory factor: role in growth inhibition. Cell Growth Differ. 1991;2:401–407. [PubMed] [Google Scholar]
- 317.Kamijo R, Harada H, Matsuyama T, Bosland M, Gerecitano J, Shapiro D, Le J, Koh SI, Kimura T, Green SJ, et al. Requirement for transcription factor IRF-1 in NO synthase induction in macrophages. Science. 1994;263:1612–1615. doi: 10.1126/science.7510419. [DOI] [PubMed] [Google Scholar]
- 318.Blanco JC, Contursi C, Salkowski CA, DeWitt DL, Ozato K, Vogel SN. Interferon regulatory factor (IRF)-1 and IRF-2 regulate interferon gamma-dependent cyclooxygenase 2 expression. J Exp Med. 2000;191:2131–2144. doi: 10.1084/jem.191.12.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Hobart M, Ramassar V, Goes N, Urmson J, Halloran PF. IFN regulatory factor-1 plays a central role in the regulation of the expression of class I and II MHC genes in vivo. J Immunol. 1997;158:4260–4269. [PubMed] [Google Scholar]
- 320.Testa U, Stellacci E, Pelosi E, Sestili P, Venditti M, Orsatti R, Fragale A, Petrucci E, Pasquini L, Belardelli F, Gabriele L, Battistini A. Impaired myelopoiesis in mice devoid of interferon regulatory factor 1. Leukemia. 2004;18:1864–1871. doi: 10.1038/sj.leu.2403472. [DOI] [PubMed] [Google Scholar]
- 321.Tsujimura H, Nagamura-Inoue T, Tamura T, Ozato K. IFN consensus sequence binding protein/IFN regulatory factor-8 guides bone marrow progenitor cells toward the macrophage lineage. J Immunol. 2002;169:1261–1269. doi: 10.4049/jimmunol.169.3.1261. [DOI] [PubMed] [Google Scholar]
- 322.Becker AM, Michael DG, Satpathy AT, Sciammas R, Singh H, Bhattacharya D. IRF-8 extinguishes neutrophil production and promotes dendritic cell lineage commitment in both myeloid and lymphoid mouse progenitors. Blood. 2012;119:2003–2012. doi: 10.1182/blood-2011-06-364976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Li L, Jin H, Xu J, Shi Y, Wen Z. Irf8 regulates macrophage versus neutrophil fate during zebrafish primitive myelopoiesis. Blood. 2011;117:1359–1369. doi: 10.1182/blood-2010-06-290700. [DOI] [PubMed] [Google Scholar]
- 324.Kinder M, Thompson JE, Wei C, Shelat SG, Blair IA, Carroll M, Pure E. Interferon regulatory factor-8-driven myeloid differentiation is regulated by 12/15-lipoxygenase-mediated redox signaling. Exp Hematol. 2010;38:1036–1046. doi: 10.1016/j.exphem.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Khalfin-Rabinovich Y, Weinstein A, Levi BZ. PML is a key component for the differentiation of myeloid progenitor cells to macrophages. Int Immunol. 2011;23:287–296. doi: 10.1093/intimm/dxr004. [DOI] [PubMed] [Google Scholar]
- 326.Scheller M, Foerster J, Heyworth CM, Waring JF, Lohler J, Gilmore GL, Shadduck RK, Dexter TM, Horak I. Altered development and cytokine responses of myeloid progenitors in the absence of transcription factor, interferon consensus sequence binding protein. Blood. 1999;94:3764–3771. [PubMed] [Google Scholar]
- 327.Konieczna I, Horvath E, Wang H, Lindsey S, Saberwal G, Bei L, Huang W, Platanias L, Eklund EA. Constitutive activation of SHP2 in mice cooperates with ICSBP deficiency to accelerate progression to acute myeloid leukemia. J Clin Invest. 2008;118:853–867. doi: 10.1172/JCI33742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Saberwal G, Horvath E, Hu L, Zhu C, Hjort E, Eklund EA. The interferon consensus sequence binding protein (ICSBP/IRF8) activates transcription of the FANCF gene during myeloid differentiation. J Biol Chem. 2009;284:33242–33254. doi: 10.1074/jbc.M109.010231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Linnekin D, Mou S, Deberry CS, Weiler SR, Keller JR, Ruscetti FW, Longo DL. Stem cell factor, the JAK-STAT pathway and signal transduction. Leuk Lymphoma. 1997;27:439–444. doi: 10.3109/10428199709058310. [DOI] [PubMed] [Google Scholar]
- 330.Boneberg EM, Hartung T. Molecular aspects of anti-inflammatory action of G-CSF. Inflamm Res. 2002;51:119–128. doi: 10.1007/pl00000283. [DOI] [PubMed] [Google Scholar]
- 331.Gomez-Lechon MJ. Oncostatin M: signal transduction and biological activity. Life Sci. 1999;65:2019–2030. doi: 10.1016/s0024-3205(99)00296-9. [DOI] [PubMed] [Google Scholar]
- 332.Dubourdeau M, Chene G, Coste A, Bernad J, Lepert JC, Orfila C, Pipy B, Rousseau D. Opposite roles of STAT and PPARgamma in the induction of p21WAF1 expression by IL-13 in human peripheral blood monocytes. Eur Cytokine Netw. 2008;19:156–165. doi: 10.1684/ecn.2008.0134. [DOI] [PubMed] [Google Scholar]
- 333.Silvennoinen O, Witthuhn BA, Quelle FW, Cleveland JL, Yi T, Ihle JN. Structure of the murine Jak2 protein-tyrosine kinase and its role in interleukin 3 signal transduction. Proc Natl Acad Sci USA. 1993;90:8429–8433. doi: 10.1073/pnas.90.18.8429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.O’Shea JJ, Pesu M, Borie DC, Changelian PS. A new modality for immunosuppression: targeting the JAK/STAT pathway. Nat Rev Drug Discov. 2004;3:555–564. doi: 10.1038/nrd1441. [DOI] [PubMed] [Google Scholar]
- 335.Tanuma N, Nakamura K, Shima H, Kikuchi K. Protein-tyrosine phosphatase PTPepsilon C inhibits Jak-STAT signaling and differentiation induced by interleukin-6 and leukemia inhibitory factor in M1 leukemia cells. J Biol Chem. 2000;275:28216–28221. doi: 10.1074/jbc.M003661200. [DOI] [PubMed] [Google Scholar]
- 336.Si J, Collins SJ. IL-3-induced enhancement of retinoic acid receptor activity is mediated through Stat5, which physically associates with retinoic acid receptors in an IL-3-dependent manner. Blood. 2002;100:4401–4409. doi: 10.1182/blood-2001-12-0374. [DOI] [PubMed] [Google Scholar]
- 337.Mangan JK, Rane SG, Kang AD, Amanullah A, Wong BC, Reddy EP. Mechanisms associated with IL-6-induced up-regulation of Jak3 and its role in monocytic differentiation. Blood. 2004;103:4093–4101. doi: 10.1182/blood-2003-06-2165. [DOI] [PubMed] [Google Scholar]
- 338.Grossman WJ, Verbsky JW, Yang L, Berg LJ, Fields LE, Chaplin DD, Ratner L. Dysregulated myelopoiesis in mice lacking Jak3. Blood. 1999;94:932–939. [PubMed] [Google Scholar]
- 339.Eilers A, Seegert D, Schindler C, Baccarini M, Decker T. The response of gamma interferon activation factor is under developmental control in cells of the macrophage lineage. Mol Cell Biol. 1993;13:3245–3254. doi: 10.1128/mcb.13.6.3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Eilers A, Kanda K, Klose B, Krolewski J, Decker T. Constitutive STAT1 tyrosine phosphorylation in U937 monocytes overexpressing the TYK2 protein tyrosine kinase does not induce gene transcription. Cell Growth Differ. 1996;7:833–840. [PubMed] [Google Scholar]
- 341.Dimberg A, Nilsson K, Oberg F. Phosphorylation-deficient Stat1 inhibits retinoic acid-induced differentiation and cell cycle arrest in U-937 monoblasts. Blood. 2000;96:2870–2878. [PubMed] [Google Scholar]
- 342.Jerke U, Tkachuk S, Kiyan J, Stepanova V, Kusch A, Hinz M, Dietz R, Haller H, Fuhrman B, Dumler I. Stat1 nuclear translocation by nucleolin upon monocyte differentiation. PLoS ONE. 2009;4:e8302. doi: 10.1371/journal.pone.0008302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Watanabe M, Kashiwakura Y, Huang P, Ochiai K, Futami J, Li SA, Takaoka M, Nasu Y, Sakaguchi M, Huh NH, Kumon H. Immunological aspects of REIC/Dkk-3 in monocyte differentiation and tumor regression. Int J Oncol. 2009;34:657–663. doi: 10.3892/ijo_00000191. [DOI] [PubMed] [Google Scholar]
- 344.Ribechini E, Leenen PJ, Lutz MB. Gr-1 antibody induces STAT signaling, macrophage marker expression and abrogation of myeloid-derived suppressor cell activity in BM cells. Eur J Immunol. 2009;39:3538–3551. doi: 10.1002/eji.200939530. [DOI] [PubMed] [Google Scholar]
- 345.de Koning JP, Soede-Bobok AA, Ward AC, Schelen AM, Antonissen C, van Leeuwen D, Lowenberg B, Touw IP. STAT3-mediated differentiation and survival and of myeloid cells in response to granulocyte colony-stimulating factor: role for the cyclin-dependent kinase inhibitor p27(Kip1) Oncogene. 2000;19:3290–3298. doi: 10.1038/sj.onc.1203627. [DOI] [PubMed] [Google Scholar]
- 346.Yoshida T, Iwamoto T, Adachi K, Yokota T, Miyake Y, Hamaguchi M. Functional analysis of the effect of forced activation of STAT3 on M1 mouse leukemia cells. Int J Mol Med. 2005;15:269–275. [PubMed] [Google Scholar]
- 347.Minami M, Inoue M, Wei S, Takeda K, Matsumoto M, Kishimoto T, Akira S. STAT3 activation is a critical step in gp130-mediated terminal differentiation and growth arrest of a myeloid cell line. Proc Natl Acad Sci USA. 1996;93:3963–3966. doi: 10.1073/pnas.93.9.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Nakajima K, Yamanaka Y, Nakae K, Kojima H, Ichiba M, Kiuchi N, Kitaoka T, Fukada T, Hibi M, Hirano T. A central role for Stat3 in IL-6-induced regulation of growth and differentiation in M1 leukemia cells. EMBO J. 1996;15:3651–3658. [PMC free article] [PubMed] [Google Scholar]
- 349.Shiozawa Y, Jung Y, Ziegler AM, Pedersen EA, Wang J, Wang Z, Song J, Wang J, Lee CH, Sud S, Pienta KJ, Krebsbach PH, Taichman RS. Erythropoietin couples hematopoiesis with bone formation. PLoS ONE. 2010;5:e10853. doi: 10.1371/journal.pone.0010853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Lee CK, Raz R, Gimeno R, Gertner R, Wistinghausen B, Takeshita K, DePinho RA, Levy DE. STAT3 is a negative regulator of granulopoiesis but is not required for G-CSF-dependent differentiation. Immunity. 2002;17:63–72. doi: 10.1016/s1074-7613(02)00336-9. [DOI] [PubMed] [Google Scholar]
- 351.Panopoulos AD, Zhang L, Snow JW, Jones DM, Smith AM, El Kasmi KC, Liu F, Goldsmith MA, Link DC, Murray PJ, Watowich SS. STAT3 governs distinct pathways in emergency granulopoiesis and mature neutrophils. Blood. 2006;108:3682–3690. doi: 10.1182/blood-2006-02-003012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Takeda K, Clausen BE, Kaisho T, Tsujimura T, Terada N, Forster I, Akira S. Enhanced Th1 activity and development of chronic enterocolitis in mice devoid of Stat3 in macrophages and neutrophils. Immunity. 1999;10:39–49. doi: 10.1016/s1074-7613(00)80005-9. [DOI] [PubMed] [Google Scholar]
- 353.Numata A, Shimoda K, Kamezaki K, Haro T, Kakumitsu H, Shide K, Kato K, Miyamoto T, Yamashita Y, Oshima Y, Nakajima H, Iwama A, Aoki K, Takase K, Gondo H, Mano H, Harada M. Signal transducers and activators of transcription 3 augments the transcriptional activity of CCAAT/enhancer-binding protein alpha in granulocyte colony-stimulating factor signaling pathway. J Biol Chem. 2005;280:12621–12629. doi: 10.1074/jbc.M408442200. [DOI] [PubMed] [Google Scholar]
- 354.Karin M, Liu Z, Zandi E. AP-1 function and regulation. Curr Opin Cell Biol. 1997;9:240–246. doi: 10.1016/s0955-0674(97)80068-3. [DOI] [PubMed] [Google Scholar]
- 355.Eferl R, Wagner EF. AP-1: a double-edged sword in tumorigenesis. Nat Rev Cancer. 2003;3:859–868. doi: 10.1038/nrc1209. [DOI] [PubMed] [Google Scholar]
- 356.Shaulian E, Karin M. AP-1 in cell proliferation and survival. Oncogene. 2001;20:2390–2400. doi: 10.1038/sj.onc.1204383. [DOI] [PubMed] [Google Scholar]
- 357.Szabo E, Preis LH, Birrer MJ. Constitutive cJun expression induces partial macrophage differentiation in U-937 cells. Cell Growth Differ. 1994;5:439–446. [PubMed] [Google Scholar]
- 358.Bryja V, Sedlacek J, Zahradnickova E, Sevcikova S, Pachernik J, Soucek K, Hofmanova J, Kozubik A, Smarda J. Lipoxygenase inhibitors enhance tumor suppressive effects of jun proteins on v-myb-transformed monoblasts BM2. Prostaglandins Other Lipid Mediat. 2003;72:131–145. doi: 10.1016/s1098-8823(03)00052-2. [DOI] [PubMed] [Google Scholar]
- 359.Liu MY, Wu MC. Induction of human monocyte cell line U937 differentiation and CSF-1 production by phorbol ester. Exp Hematol. 1992;20:974–979. [PubMed] [Google Scholar]
- 360.Liao HF, Shyu SY, Kuo YH, Yang YC, Chen YJ. Compound 278E, structurally modified from tanshinone, induces monocytic differentiation and regulates proto-oncogene expression in human leukemic HL-60 cells. Anticancer Drugs. 2005;16:175–183. doi: 10.1097/00001813-200502000-00009. [DOI] [PubMed] [Google Scholar]
- 361.Momiyama N, Shimada H, Mitsuhashi M. Suppression of c-jun by antisense oligonucleotides inhibits cell adhesion but not respiratory burst during phorbol ester-induced differentiation of U937 human monoblastic cells. Cell Growth Differ. 1996;7:1005–1012. [PubMed] [Google Scholar]
- 362.Zada AA, Singh SM, Reddy VA, Elsasser A, Meisel A, Haferlach T, Tenen DG, Hiddemann W, Behre G. Downregulation of c-Jun expression and cell cycle regulatory molecules in acute myeloid leukemia cells upon CD44 ligation. Oncogene. 2003;22:2296–2308. doi: 10.1038/sj.onc.1206393. [DOI] [PubMed] [Google Scholar]
- 363.Datta R, Sherman ML, Stone RM, Kufe D. Expression of the jun-B gene during induction of monocytic differentiation. Cell Growth Differ. 1991;2:43–49. [PubMed] [Google Scholar]
- 364.Lord KA, Abdollahi A, Hoffman-Liebermann B, Liebermann DA. Proto-oncogenes of the fos/jun family of transcription factors are positive regulators of myeloid differentiation. Mol Cell Biol. 1993;13:841–851. doi: 10.1128/mcb.13.2.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Passegue E, Jochum W, Schorpp-Kistner M, Mohle-Steinlein U, Wagner EF. Chronic myeloid leukemia with increased granulocyte progenitors in mice lacking junB expression in the myeloid lineage. Cell. 2001;104:21–32. doi: 10.1016/s0092-8674(01)00188-x. [DOI] [PubMed] [Google Scholar]
- 366.Santaguida M, Schepers K, King B, Sabnis AJ, Forsberg EC, Attema JL, Braun BS, Passegue E. JunB protects against myeloid malignancies by limiting hematopoietic stem cell proliferation and differentiation without affecting self-renewal. Cancer Cell. 2009;15:341–352. doi: 10.1016/j.ccr.2009.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Sasaki T, Wada T, Kishimoto H, Irie-Sasaki J, Matsumoto G, Goto T, Yao Z, Wakeham A, Mak TW, Suzuki A, Cho SK, Zuniga-Pflucker JC, Oliveira-dos-Santos AJ, Katada T, Nishina H, Penninger JM. The stress kinase mitogen-activated protein kinase kinase (MKK)7 is a negative regulator of antigen receptor and growth factor receptor-induced proliferation in hematopoietic cells. J Exp Med. 2001;194:757–768. doi: 10.1084/jem.194.6.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Surdziel E, Cabanski M, Dallmann I, Lyszkiewicz M, Krueger A, Ganser A, Scherr M, Eder M. Enforced expression of miR-125b affects myelopoiesis by targeting multiple signaling pathways. Blood. 2011;117:4338–4348. doi: 10.1182/blood-2010-06-289058. [DOI] [PubMed] [Google Scholar]
- 369.Hass R, Brach M, Kharbanda S, Giese G, Traub P, Kufe D. Inhibition of phorbol ester-induced monocytic differentiation by dexamethasone is associated with down-regulation of c-fos and c-jun (AP-1) J Cell Physiol. 1991;149:125–131. doi: 10.1002/jcp.1041490116. [DOI] [PubMed] [Google Scholar]
- 370.Perez C, Vilaboa NE, Aller P. Etoposide-induced differentiation of U937 promonocytic cells: AP-1-dependent gene expression and protein kinase C activation. Cell Growth Differ. 1994;5:949–955. [PubMed] [Google Scholar]
- 371.Li J, King I, Sartorelli AC. Differentiation of WEHI-3B D + myelomonocytic leukemia cells induced by ectopic expression of the protooncogene c-jun. Cell Growth Differ. 1994;5:743–751. [PubMed] [Google Scholar]
- 372.Schuler A, Schwieger M, Engelmann A, Weber K, Horn S, Muller U, Arnold MA, Olson EN, Stocking C. The MADS transcription factor Mef2c is a pivotal modulator of myeloid cell fate. Blood. 2008;111:4532–4541. doi: 10.1182/blood-2007-10-116343. [DOI] [PubMed] [Google Scholar]
- 373.Kobayashi S, Kimura F, Ikeda T, Osawa Y, Torikai H, Kobayashi A, Sato K, Motoyoshi K. BCR-ABL promotes neutrophil differentiation in the chronic phase of chronic myeloid leukemia by downregulating c-Jun expression. Leukemia. 2009;23:1622–1627. doi: 10.1038/leu.2009.74. [DOI] [PubMed] [Google Scholar]