Intracellular ceramide synthesis and protein kinase Cζ activation play an essential role in palmitate-induced insulin resistance in rat L6 skeletal muscle cells (original) (raw)
Abstract
Non-esterified fatty acids (NEFAs) have been implicated in the pathogenesis of skeletal muscle insulin resistance that may develop, in part, as a consequence of a direct inhibitory effect on early insulin signalling events. Here we report work investigating the mechanism by which palmitate (a saturated free fatty acid) inhibits insulin action in rat L6 myotubes. Palmitate suppressed the insulin-induced plasma membrane recruitment and phosphorylation of protein kinase B (PKB) and this was associated with a loss in insulin-stimulated glucose transport. The inhibition in PKB was not due to a loss in insulin receptor substrate (IRS)1 tyrosine phosphorylation, IRS-1/p85 (phosphoinositide 3-kinase) association or suppression in phosphatidyl 3,4,5 triphosphate synthesis, but was attributable to an elevated intracellular synthesis of ceramide (6-fold) from palmitate and a concomitant activation of protein kinase PKCζ (5-fold). Inhibitors of serine palmitoyl transferase suppressed the intracellular synthesis of ceramide from palmitate, prevented PKCζ activation, and antagonized the inhibition in PKB recruitment/phosphorylation and the loss in insulin-stimulated glucose transport elicited by the NEFA. Inhibiting the palmitate-induced activation of PKCζ with Ro 31.8220, also prevented the loss in the insulin-dependent phosphorylation of PKB caused by palmitate. These findings indicate that intracellular ceramide synthesis and PKCζ activation are important aspects of the mechanism by which palmitate desensitizes L6 muscle cells to insulin.
Keywords: adipocytes, free fatty acids, muscle, protein kinase B (PKB)/Akt, serine palmitoyl transferase (SPT)
Abbreviations: DAG, _sn_-1,2-diacylglycerol; DMEM, Dulbecco's modified essential medium; FBS, foetal bovine serum; GSK3, glycogen synthase kinase 3; GST, glutathione S-transferase; IRS-1, insulin receptor substrate 1; MEM, minimal essential medium; NEFA, non-esterified fatty acid; PDK, 3-phosphoinositide dependent kinase; PH, pleckstrin homology; PIP3, phosphatidylinositol 3,4,5-triphosphate; PI3-kinase, phosphoinositide 3-kinase; PKB, protein kinase B; PKCζ, protein kinase Cζ; SPT, serine palmitoyl transferase; TCA, trichloroacetic acid
INTRODUCTION
Although the pathogenesis of insulin resistance in skeletal muscle is poorly understood, reduced insulin sensitivity and glucose utilization have been correlated with an increase in circulating non-esterified fatty acids (NEFAs) and intramuscular triglyceride content [1,2]. In addition to storage as triglyceride, fatty acids can also be utilized by muscle for conversion into components of membranes (e.g. phospholipids and sphingolipids), bioactive lipids that can regulate cell signalling events (e.g. diacylglycerol) or be directed for mitochondrial oxidation. The latter is likely to be increased significantly during periods of lipid over supply and this has long been proposed to underlie the impaired utilization of carbohydrate as a muscle fuel. The classic Randle cycle provided a conceptual basis for understanding the reciprocal relationship between carbohydrate and fat metabolism, and how fatty acids may inhibit skeletal muscle insulin action and glucose metabolism (for review see [3]). Randle proposed that the increased availability of free fatty acids to muscle would lead to inhibition of both pyruvate dehydrogenase (PDH) and phosphofructokinase by accumulation of acetyl CoA and citrate respectively [3]. The inhibition of these enzymes would lead to reduced glycolytic flux and accumulation of glucose 6-phosphate, which, in turn, would inhibit hexokinase and thereby decrease glucose uptake. However, the finding that reduced muscle glycogen synthesis precedes any change in intramuscular glucose 6-phosphate following an elevation in circulating NEFAs [4] implies that the ‘glucose–fatty acid cycle’ is likely to be just one of a number of mechanisms by which NEFAs influence insulin action and glucose metabolism in skeletal muscle. Indeed, there is mounting evidence showing that NEFAs can antagonize insulin action by modulating the activity of proteins with a prominent role in proximal insulin signalling [5–7].
One potential mechanism by which a sustained increase in circulating NEFAs may lead to impaired insulin signalling involves the enhanced intracellular synthesis of ceramide from saturated fatty acids, such as palmitate. This proposition is substantiated by studies in murine C2C12 muscle cells showing that palmitate impairs insulin signalling via ceramide generation [8,9], and also by data showing that palmitic fatty acids comprise nearly one third of the total ceramides present in skeletal muscle [10]. Although the molecular basis by which ceramide inhibits insulin signalling is not fully understood, a number of studies have reported that this sphingolipid suppresses the hormonal activation of protein kinase B (PKB, also known as Akt), a serine/threonine kinase implicated strongly in the stimulation of glycogen synthesis and glucose transport by insulin [11,12]. Activation of PKB by insulin relies upon its recruitment to the plasma membrane, a process facilitated by the binding of 3-phosphoinositides to the N-terminal pleckstrin homology (PH) domain of the kinase [13]. This lipid-binding event also serves to induce conformational changes in PKB that allow phosphorylation of its two regulatory sites, one localized in the kinase domain (Thr308 for PKBα) and the other in the C-terminal hydrophobic domain (Ser473 for PKBα) by two distinct upstream 3-phosphoinositide-dependent kinases, termed PDK1 and PDK2 respectively [13,14]. Studies with short chain cell permeant analogues of ceramide have revealed that, depending on cell type, PKB activation can be reduced by either dephosphorylation of its two regulatory sites or alternatively by inhibition in its cell surface recruitment and phosphorylation [15,16]. Activation of a Type 2A-like phosphatase activity has been implicated in the dephosphorylation of PKB in some studies [15,17], whereas others, including work from our own lab, have suggested that activation of atypical protein kinase Cζ (PKCζ) may underlie the suppressive action of ceramide on PKB [18,19].
In an attempt to understand the mechanism by which palmitate dysregulates insulin signalling in skeletal muscle, we have used L6 myotubes, an established rat skeletal muscle cell line that exhibits many of the archetypal features and responses of mammalian skeletal muscle to insulin (such as insulin-stimulated glycogen synthesis and glucose transport, GLUT4 expression and translocation etc [16,20,21]). Here we show that L6 myotubes incubated with palmitate exhibit impaired insulin-stimulated PKB phosphorylation and glucose transport. This dysregulation stems from the de novo synthesis of ceramide and the resulting activation of PKCζ, as suppressing either of these events nullifies the effects of palmitate. We also present comparative data from 3T3-L1 adipocytes showing that, unlike muscle cells, palmitate fails to induce ceramide synthesis in fat cells. Nevertheless, incubation of adipocytes with a cell permeant analogue of ceramide leads to a profound loss in PKB activation by an intracellular mechanism similar to that observed in L6 muscle cells. Collectively, our findings suggest that an increase in intracellular ceramide and the consequent activation of PKCζ are important determinants of insulin sensitivity in these cell types.
EXPERIMENTAL
Materials
α-Minimal essential medium (α-MEM), Dulbeco's modified essential medium (DMEM), foetal bovine serum (FBS), and antibiotic/antimycotic solution were from Life Technologies (Paisley, Scotland, U.K.). All other reagent-grade chemicals, insulin, BSA, palmitate, palmitoleate, histone type III-SS, myriocin and L-cycloserine were obtained from Sigma-Aldrich (Poole, U.K.). Ro 31-8220, GF 109203X, okadaic acid, and di-hydroceramide were purchased from Calbiochem-Novabiochem Ltd. (Nottingham, U.K.) and C2-ceramide was obtained from Tocris (Bristol, U.K.). PKC lipid activator was purchased from Upstate Biotechnology (New York, U.S.A.). Antibodies against PKBα, phospho-PKB308, phospho-PKB473, phospho-glycogen synthase kinase 3α (GSK3α/β(9/21)), PI3K and IRS-1 were from New England Biolabs (Hitchin, Herts, U.K.). Anti-PKCζ was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, U.S.A.). Horseradish peroxidase-conjugated anti-rabbit IgG, anti-mouse IgG, and anti-sheep/goat IgG were obtained from Scottish Antibody Production Unit (Law Hospital, Carluke, Lanarkshire, Scotland, U.K.). Protein A–Sepharose beads, ATP and _sn_-1,2-diacylglycerol (DAG) kinase assay kit was purchased from Amersham Biosciences (Piscataway, NJ, USA). Complete protein phosphatase inhibitor tablets were purchased from Boehringer-Roche Diagnostics (Basel, Switzerland).
Cell culture
L6 muscle cells were cultured to myotubes as described previously [22] in αMEM containing 2% (v/v) FBS and 1% (v/v) antibiotic/antimycotic solution (100 units/ml penicillin, 100 μg/ml streptomycin, 250 ng/ml amphotericin B) at 37 °C with 5% CO2. 3T3-L1 preadipocytes (provided by Dr Howard Green, Department of Cell Biology, Harvard Medical School, Boston, MA, U.S.A.) were cultured in DMEM and differentiated into adipocytes as described previously [23,24].
Cell lysis and cellular fractionation
L6 myotubes and 3T3-L1 adipocytes were incubated for the time and with the appropriate amount of effectors described in the figure legends. Plates were washed 3 times with 0.9% (w/v) ice-cold saline, then 200 μl of lysis buffer [50 mM Tris, pH 7.4, 0.27 M sucrose, 1 mM Na-orthovanadate, pH 10, 1 mM EDTA, 1 mM EGTA, 10 mM Na β-glycerophosphate, 50 mM NaF, 5 mM Na pyrophosphate, 1% (w/v) Triton X-100, 0.1% (v/v) 2-mercaptoethanol, 0.1 μM microcystin-LR and protease inhibitors] were added. Whole cell lysate was centrifuged (15000 g, 4 °C for 10 min) and stored at −20 °C. In some experiments, confluent L6 myotubes were sub-fractionated following pre-treatment with NEFAs and/or insulin or enzyme inhibitors to isolate plasma membranes as described previously [22]. Membrane protein concentration was determined by the method of Bradford [25] using BSA as standard.
Fatty acid treatment
L6 cells were maintained in medium containing 2% FBS (v/v), 1% (v/v) antibiotic/antimycotic and 2% BSA (w/v) for 12 h and then serum deprived for the last 4 h. 3T3-L1 adipocytes were cultured in DMEM containing 1% (v/v) antibiotic/antimycotic and 2% BSA (w/v) and maintained in serum-free media for 16 h. Both cell types were exposed to fatty acids which had been conjugated to BSA (fraction V) for times and at concentrations indicated in the figure legends (controls were incubated with vehicle containing BSA but lacking the fatty acid) and incubated with insulin (100 nM) in the penultimate 10 min incubation period.
SDS/PAGE and immunoblotting
Cell lysates (50 μg protein) and plasma membrane fractions from L6 myotubes (20 μg protein) were subjected to SDS/PAGE on 10% resolving gels and transferred on Immobilon-P or Hybond-C membranes (Millipore, Harts, U.K.), as described previously [22]. Membranes were probed with primary antibodies against proteins of interest. Primary antibody detection was performed using either horseradish peroxidase (HRP)-conjugated anti-rabbit IgG, anti-mouse IgG, or anti-sheep/goat IgG and visualized using enhanced chemiluminescence (Pierce-Perbio Biotechnology, Tattenhall, Cheshire, U.K.) on Kodak X-OMAT film (Eastman-Kodak, Rochester, Kent, U.K.).
IRS-1 immunoprecipitation
Following treatment with insulin and/or palmitate, L6 myotubes were lysed in lysis buffer [50 mM Tris/HCl, pH 7.5, 1 mM EDTA, 1 mM EGTA, 1% (v/v) Triton X-100, 1 mM Na3VO4, 10 mM sodium β-glycerophosphate, 50 mM NaF, 5 mM Na4P2O7, 1 μM microcystin-LR, 270 mM sucrose, 1 mM benzamidine, 10 μg/ml leupeptin, complete protein proteinase inhibitor cocktail (one tablet per 50 ml), and 0.1% (v/v) 2-mercaptoethanol]. IRS-1 was immunoprecipitated using an antibody against the C-terminal domain of IRS-1 (Upstate Biotechnology). Immunocomplexes were captured by incubation with protein-A–Sepharose beads and solubilized in Laemmli sample buffer prior to immunoblotting as described above.
Analysis of cellular phosphatidylinositol 3,4,5-triphosphate (PIP3)
The effects of palmitate on insulin-stimulated PIP3 synthesis was assessed using a sensitive time-resolved fluorescence resonance energy transfer (FRET)-based assay that monitors the displacement of glutathione S-transferase (GST)-tagged GRP1 PH domain from a sensor complex consisting of Eu Lance Chelate labelled anti-GST antibody, the GST-tagged GRP1 PH domain, biotinylated-PIP3 and the FRET acceptor, streptavidin allophycocyanin (APC) by non-biotinylated lipid [26]. After the appropriate incubation of cells with palmitate, insulin and/or wortmannin cells were rapidly washed and cellular material precipitated by the immediate addition of 0.5 ml of ice-cold 0.5 M trichloroacetic acid (TCA). After standing the cells on ice for 5 min they were harvested from the plates and the acid precipitate pelleted by centrifugation at 15000 g for 5 min at 22 °C. The pellet was washed twice with 1 ml of 5% TCA/1 mM EDTA. Neutral lipids were extracted from the pellet and the PIP3 content was determined as described previously using an LJL Analyst plate reader [26]. The abundance of PIP3 present was calculated by reference to a standard curve constructed by addition of known amounts of the 3-phosphoinositide to the sensor complex.
Analysis of cellular ceramide content
After the indicated incubations, myotubes were lysed in ice-cold PBS and ceramide, and the DAG content was determined in a 50 μl aliquot using a radiometric DAG kinase assay kit (Amersham Biosciences, N.J, U.S.A). Lipids were extracted from the 50 μl aliquot by the addition of chloroform/methanol (1:2, v/v) and the phases broken by the addition of chloroform and 1 M NaCl. The organic phase was separated, dried down, and used for ceramide and DAG measurements. The abundance of these lipids was evaluated using Escherichia coli DAG kinase. Briefly, the lipids were incubated at 22 °C for 30 min in the presence of 2 mM dithiothreitol and DAG kinase and the reaction initiated by the addition of 2.5 mM ATP (mixed with [γ-32P]ATP, 37 kBq) in a final assay volume of 100 μl. ATP and DAG kinase were both in excess within the reaction assay to ensure that the reaction remained linear within the range of standards used. The reaction was terminated by the addition of 20 μl of 1% (v/v) perchloric acid and 450 μl of chloroform/methanol (1:2, v/v). The organic phase was isolated and washed twice with 1 ml of 1% (v/v) perchloric acid. The samples were dried and reconstituted in chloroform/methanol (95:5, v/v) and spotted onto a paper-lined TLC plate. Reaction products were separated by placing the TLC plate in a chamber containing chloroform/methanol/acetic acid (65:15:5, by vol.) solvent. Radioactive products (phosphatidic acid and ceramide phosphate) were visualized and quantified using a Packard Instant Imager by reference to a C16-ceramide or DAG standard curve. Changes in ceramide and DAG content in palmitate-treated L6 myotubes and 3T3-L1 adipocytes were expressed as a fold change relative to that measured in untreated cells. For example L6 myotubes (ceramide=38±4 pmol/mg protein, DAG=59±8 pmol/mg protein) and 3T3-L1 adipocytes (ceramide=99±25 pmol/mg protein, DAG=76±12 pmol/mg protein), all values are means±S.E.M. from three separate experiments conducted in duplicate.
PKCζ activity
L6 myotubes were pre-treated with 10 μM myriocin for 30 min prior to incubation with 0.75 mM palmitic acid for a further 16 h. As a positive control for PKCζ activation, cells were also incubated with 100 μM C2-ceramide for 2 h. Following the appropriate incubations cells were harvested in lysis buffer. PKCζ was immunoprecipitated from 500 μg of cell lysate using 1 μg of PKCζ antibody complexed to protein-A–Sepharose beads. Following immunoprecipitation, immune complexes were washed twice and incubated for 10 min at 30 °C in a reaction mix containing 0.1 mM EGTA, 50 μM Tris/HCl, pH 7.5, 10 mM MgCl2, 1 mM Na3VO4, 1.25 μM PKC lipid activator, 10 μg/μl histone type III-SS and 100 μM [γ32P]ATP. The reaction was stopped by adding 5× Laemmli sample buffer and incubated for 30 min at 60 °C. The samples were subsequently resolved on SDS gels, transferred to PVDF membranes and the amount of radioactive label incorporated into the histone substrate quantified using a Packard InstantImager.
Statistical analyses
For multiple comparisons, statistical analysis was performed using ANOVA followed by a Newman Keuls post test. Data analysis was performed using GraphPad Prism software and considered statistically significant at values of P<0.05.
RESULTS
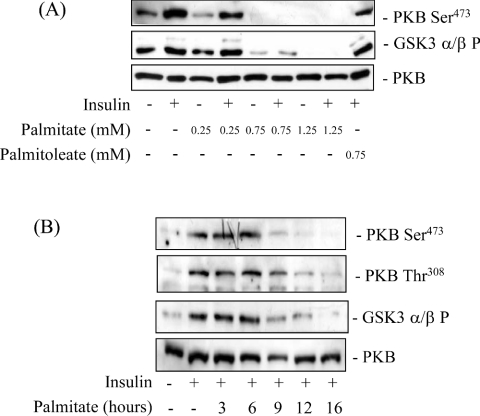
To assess the effective dose at which palmitate induced a loss in PKB-directed insulin signalling we incubated L6 myotubes with three different concentrations of the fatty acid for 16 h prior to an acute 10 min insulin challenge and subsequent analysis of PKB and GSK3 phosphorylation. Figure 1(A) shows that in the absence of palmitate, insulin induced a significant increase in phosphorylation of the Ser473 PKB site, but that this was reduced upon incubation of muscle cells with palmitate in a dose-dependent manner, and was undetectable in cells treated with 0.75 mM palmitate. This loss in PKB phosphorylation could not be attributed to reduced cellular PKB expression, which was unaltered after 16 h of palmitate treatment (Figure 1A). Phosphorylation of GSK3β, a physiological downstream PKB target, mirrored that of its upstream inactivating kinase. The phospho-GSK3 antibody used in this particular experiment showed much greater immunoreactivity for the β than the α isoform of GSK3, but prolonged exposure of autoradiographic film revealed that phosphorylation of GSK3α was also reduced significantly in cells pre-incubated with 0.75 mM palmitate (results not shown). Unlike palmitate, cis-9-hexadecenoic acid also known as palmitoleate, a mono-unsaturated free fatty acid, failed to suppress the insulin-induced phosphorylation of PKB (Figure 1A). This latter finding implies that saturation of the fatty acyl long chain is an important requirement of the mechanism by which palmitate promotes a loss in PKB signalling. It is important to stress that fully differentiated L6 myotubes (and 3T3-L1 adipocytes used later in this study) appear to be relatively resistant to palmitate-induced cell death (based on analysis of cell adherence, trypan blue exclusion and DAPI staining; results not shown), at least at the palmitate concentration and incubation periods used in this study (0.75 mM, 16 h). In contrast, under similar conditions, undifferentiated L6 myoblasts and 3T3-L1 pre-adipocytes exhibit reduced cell viability. The increased loss of L6 myoblasts is likely to be associated with the much greater sensitivity to ceramide generated from palmitate, as we have recently reported that viability of myoblasts, but not myotubes, is reduced significantly by incubation with a cell permeant ceramide analogue [19].
Figure 1. Effects of palmitate and palmitoleate on the insulin-stimulated phosphorylation of PKB and GSK3 in L6 myotubes.
(A) L6 myotubes were pre-incubated with different concentrations of palmitate (between 0.25 mM and 1.25 mM) or palmitoleate (0.75 mM) for 16 h prior to incubation with 100 nM insulin for 10 min. (B) L6 myotubes were pre-incubated with 0.75 mM palmitate (for times indicated), and with insulin (100 nM) during the penultimate 10 min prior to cell lysis and immunoblotting. Cell lysates from (A) and (B) were immunoblotted with phospho-specific antibodies directed against PKB-Ser473, PKB-Thr308, GSK3α/β-Ser21/9 (GSK3α/β P) or antibodies against native PKB. Immunoblots are representative of three separate experiments.
We next investigated the rapidity with which palmitate induced the loss in PKB phosphorylation and downstream signalling. Figure 1(B) shows that palmitate (at maximally effective doses of 0.75 mM) had little inhibitory effect on the insulin-induced phosphorylation of PKB and GSK3 when cells were incubated with palmitate for periods of up to 6 h. However, beyond 6 h, phosphorylation of PKB (on both Ser473 and Thr308) was noticeably reduced and was undetectable following a 16 h incubation of cells with the NEFA. Again, phosphorylation of GSK3β mirrored that of PKB, consistent with it being a target for the latter. In line with the data presented in Figure 1(A) there were no significant changes in the cellular expression of PKB over the 16 h period of cell incubation with palmitate (Figure 1B). Based on the dose- and time-response studies, all subsequent experiments involved incubation of muscle cells with 0.75 mM palmitate for 16 h.
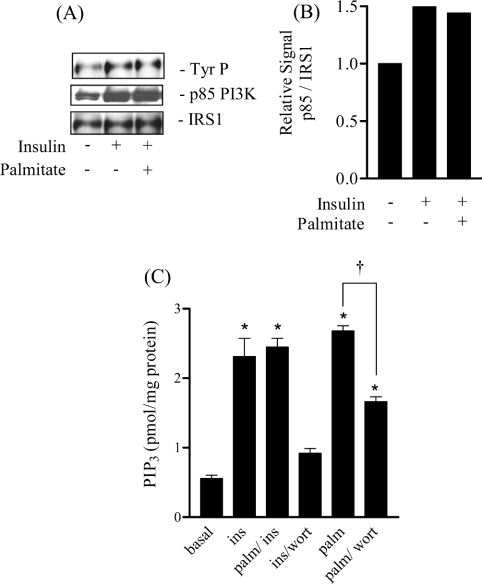
In order to understand how palmitate induces the loss in PKB activation we monitored the effects of the NEFA on upstream signalling events at the level of IRS-1 and PI3-kinase (phosphoinositide 3-kinase). Figure 2(A) shows that neither tyrosine phosphorylation of IRS-1 nor the association of the p85 subunit of PI3-kinase with IRS-1, responses normally induced by insulin, were affected by pre-incubation of muscle cells with palmitate for 16 h. Since PKB activation is crucially dependent on insulin-stimulated PIP3 synthesis it is possible that reduced production of this lipid in palmitate-treated cells underpins the diminished activation of PKB. Figure 2(B) shows that insulin enhanced PIP3 synthesis by 3-fold and that this was abolished by pre-incubating muscle cells with the PI3-kinase inhibitor, wortmannin. In contrast, insulin retained its ability to induce PIP3 synthesis in palmitate-treated cells, suggesting that the reduced hormonal activation of PKB was not as a result of impaired PI3-kinase activation or a suppression of PIP3 synthesis. Intriguingly, exposure of muscle cells to palmitate for 16 h alone, increased cellular PIP3 to a level comparable with that seen in response to an acute insulin challenge. However, despite this increase in PIP3 content, palmitate did not induce an associated increase in the phosphorylation of PKB (Figure 1A). The palmitate-induced increase in PIP3 was reduced significantly by the PI3-kinase inhibitor, wortmannin. The inability of wortmannin to suppress PIP3 levels completely in response to palmitate may, in part, be a reflection of its poor stability in solution over a prolonged (16 h) cell incubation period.
Figure 2. Effects of palmitate and palmitoleate on the insulin-mediated phosphorylation of IRS-1, IRS-1/p85 association and PIP3 production in L6 myotubes.
(A) L6 myotubes were pre-incubated with palmitate (0.75 mM) for 16 h and, during the last 10 min of this period, were treated with insulin (100 nM) prior to lysis. IRS-1 was immunoprecipitated from cell lysates as described in the Experimental section. Immunoprecipitates were resolved by SDS/PAGE and immunoblotted with anti-phosphotyrosine, the p85 subunit of PI3K and IRS-1. (B) IRS-1 and p85-PI3K-immunoreactive bands were quantified and expressed as a ratio, the data represent the mean values from two experiments. (C) L6 myotubes were either incubated with 100 nM insulin (ins) and/or 100 nM wortmannin (wort) for 10 min or cells were incubated with 0.75 mM palmitate (palm) for 16 h and with 100 nM insulin during the penultimate 10 min. Cells were lysed and PIP3 content assessed as described in the Experimental. Values are means±S.E.M. for three separate experiments performed in triplicate. *P<0.05 compared with the untreated control and †P<0.05 between the indicated bars.
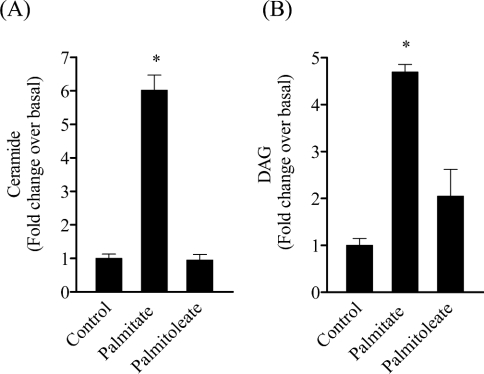
We subsequently explored whether the inhibitory effects of palmitate may have arisen from its intracellular conversion to ceramide, a lipid molecule which inhibits PKB activation in response to insulin in L6 myotubes [16,19]. Figure 3(A) shows that incubation of cells with palmitate, but not palmitoleate, led to a 6-fold increase in intracellular ceramide as judged using a DAG-kinase assay. This assay also allows measurement of DAG, a molecule whose intramuscular accumulation has been implicated in desensitizing insulin-stimulated glucose uptake in response to saturated fatty acids [27]. Figure 3(B) shows that palmitate treatment led to a near 5-fold increase in intramyocellular DAG content. Whilst there was a modest increase in DAG formation in response to cell incubation with palmitoleate, this did not achieve statistical significance (Figure 3B).
Figure 3. Palmitate, but not palmitoleate, stimulates both ceramide and DAG accumulation.
L6 myotubes were incubated with palmitate or palmitoleate (both at 0.75 mM for 16 h). Muscle cells were lysed and intracellular ceramide (A) and DAG (B) content were assayed using the DAG kinase kit, as described in the Experimental section. Bars represent the fold-increase relative to muscle cells incubated under identical conditions, but which were not exposed to these fatty acids. Values are means±S.E.M. for three separate experiments performed in triplicate. *P<0.05 compared with the respective untreated control.
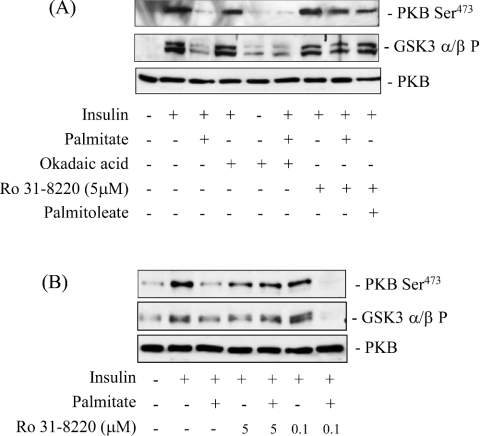
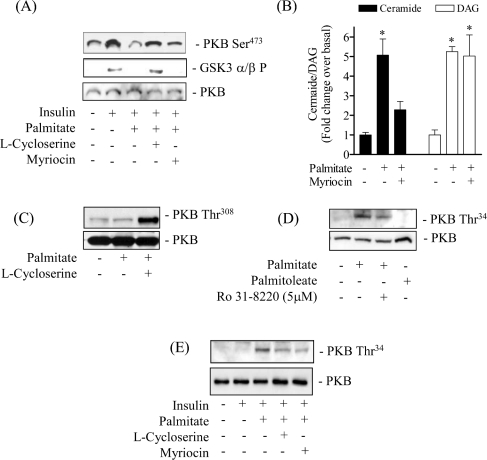
In murine C2C12 muscle cells the enhanced synthesis of ceramide from palmitate has been shown to suppress PKB activation in an okadaic-acid-sensitive manner [9], implicating the involvement of a Type 2A-like phosphatase activity. However, okadaic acid fails to protect against ceramide-mediated inhibition of PKB in L6 muscle cells [16] and in cultured 3T3-L1 adipocytes [28] suggesting that alternative mechanisms to inhibit PKB may be utilized by ceramide in these cell types. Indeed, recent work from our lab and that of others suggests that ceramide invokes activation of atypical PKCζ which results in the negative regulation of PKB [18,19]. To assess whether palmitate inhibition of PKB involves either of these mechanisms, we investigated the effects of okadaic acid and Ro 31.8220, a PKC inhibitor. Consistent with earlier data, Figure 4(A) shows that 16 h of palmitate treatment reduced the activation of PKB by insulin substantially, and that this was not protected by pretreatment of muscle cells with okadaic acid at concentrations that inhibit protein phosphatase 2A. In contrast, pretreatment of cells with 5 μM Ro 31.8220 negated the inhibitory effects of palmitate on both PKB and its downstream target kinase, GSK3. At this concentration, Ro 31.8220 inhibits novel, conventional and atypical PKC isoforms; however, at submicromolar concentrations the compound only inhibits the novel and conventional PKC isoforms [29,30]. Thus the finding that Ro 31.8220 was effective at 5 μM, but not at the lower concentration of 0.1 μM (Figure 4B) implies that atypical PKCs may mediate the palmitate-induced inhibition of PKB in L6 myotubes.
Figure 4. Effects of palmitate, Ro 31.8220 and okadaic acid on the insulin-induced phosphorylation of PKB and GSK3 in L6 myotubes.
L6 myotubes were incubated with palmitate or palmitoleate (both at 0.75 mM for 16 h). In some experiments, cells were pre-treated for 30 min with okadaic acid (100 nM) or Ro 31.8220 (0.1 μM or 5 μM) prior to incubation with fatty acids. Insulin (100 nM) was added in the penultimate 10 min of the 16 h incubation, after which cells were lysed. Cell lysates (50 μg protein) were then immunoblotted with phospho-specific antibodies directed against PKB-Ser473 and GSK3α/β-Ser21/9 (GSK3α/βP) and a PKB antibody.
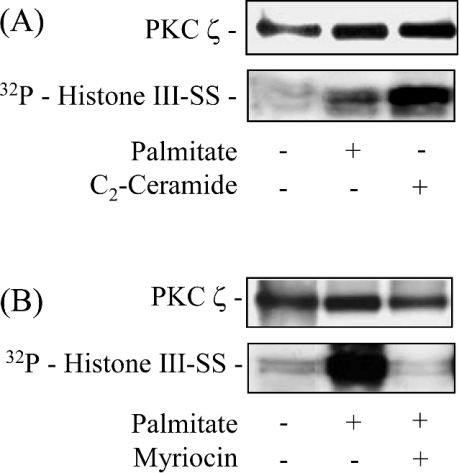
To explore whether PKCζ is involved, we immunoprecipitated the kinase from L6 myotubes following 16 h of pre-incubation with palmitate and assessed its ability to promote phosphorylation of histone III-SS, which was used as an in vitro protein substrate. Figure 5(A) shows that compared with untreated control cells, palmitate stimulated (∼5-fold) PKCζ. The observation that PKCζ can be activated by palmitate is not unprecedented and has been shown to occur also in pancreatic β cells, where it has been implicated in NEFA-induced changes in mitogenesis and insulin secretion [31,32]. Cell treatment with 100 μM C2-ceramide, which potently activates (9-fold) PKCζ [19,33], was used as a positive control in this experiment. The ability of palmitate to activate PKCζ is consistent with the idea that this stems from the increased conversion of palmitate into ceramide. To test this proposition we utilized inhibitors of serine palmitoyl transferase (SPT), a key enzyme that commits palmitate to the de novo synthesis of ceramide. Figure 5(B) shows that compared with untreated cells, PKCζ immunoprecipitated from palmitate-treated cells exhibited a 7-fold greater capacity to phosphorylate histone III-SS, whereas this capability was not apparent in cells treated simultaneously with palmitate and the SPT inhibitor, myriocin.
Figure 5. Effects of palmitate and myriocin on PKCζ activity in L6 myotubes.
(A) L6 myotubes were incubated with 0.75 mM palmitate (for 16 h) or with 100 μM C2-Ceramide (for 2 h). In (B) L6 myotubes were also treated with 0.75 mM palmitate (for 16 h) but in the absence or presence of 10 μM myriocin. Myriocin was added to the extracellular media 30 min prior to exposure of cells to the fatty acid and was retained in the extracellular media for the subsequent 16 h with palmitate. PKCζ was immunoprecipitated from L6 cell lysates (500 μg protein) and activity in immunoprecipitates assayed using histone type III-SS as substrate. Phosphorylated substrate was visualized by resolving it on a 4–12% Novex gradient gel transferred to PVDF membrane followed by autoradiography. The results are representative of three similar experiments.
To investigate further the importance of endogenous ceramide synthesis from palmitate we subsequently investigated how myriocin, and another structurally unrelated SPT inhibitor, L-cycloserine, affected the insulin-dependent phosphorylation of PKB and GSK3 in palmitate-treated cells. Figure 6(A) shows that pre-treatment of L6 muscle cells with either of the two SPT inhibitors protected against the palmitate-induced inhibition of PKB and GSK3. To demonstrate that this protection is likely to be afforded by suppressing intracellular synthesis of ceramide from the NEFA, Figure 6(B) shows that myriocin reduces significantly the ceramide content in palmitate-treated cells; similar results were obtained with L-cycloserine (results not shown). In contrast, myriocin had little impact upon accumulation of DAG whose synthesis from the fatty acid is known not to be affected by SPT inhibition (Figure 6B). In Figure 2(C) we illustrated the surprising finding that exposing muscle cells to palmitate for 16 h led to an increase in cellular PIP3 to a level that was comparable with that seen in response to an acute insulin challenge. Figures 1(A) and 6(C) show that despite this increase in PIP3, palmitate did not induce phosphorylation of PKB. However, given that palmitate also promotes an increase in intracellular ceramide, we hypothesized that this would inhibit PKB phosphorylation despite the attendant increase in PIP3. Consistent with this idea, when intracellular synthesis of ceramide from palmitate was suppressed using L-cycloserine, we observed phosphorylation of PKB on Thr308 in response to palmitate (Figure 6C).
Figure 6. Effects of Insulin, palmitate, Ro 31.8220 and SPT inhibitors on PKB and GSK3 phosphorylation, ceramide and DAG content in L6 myotubes.
L6 myotubes were primed for 30 min with SPT inhibitors, 1 mM L-cycloserine or 10 μM myriocin or 5 μM Ro 31.8220, prior to incubation of muscle cells with 0.75 mM palmitate (for 16 h). In some experiments, 100 nM insulin was added in the penultimate 10 min period of the 16 h incubation. Following this incubation period muscle cells were lysed and immunoblotted with phospho-specific antibodies directed against PKB-Ser473 and GSK3α/β-Ser21/9 (GSK3α/β P) (A, C) or PKB-Thr34 (D, E) and a PKB antibody (A, C, D and E) In other experiments, following the incubation period with myriocin and or palmitate, cells were lysed in PBS and lysates used for analysis of intracellular ceramide and DAG content by the DAG kinase method (see Experimental section). The bar values are the means±S.E.M. of three separate experiments each performed in triplicate. *P<0.05 compared with the untreated control.
We have recently demonstrated that in L6 muscle cells PKCζ interacts physically with PKB and that, when activated by ceramide, it promotes inhibition of PKB by phosphorylation of the PKB-PH domain at Thr34 (in PKBα) [19]. An important consequence of this phosphorylation is that it impairs PIP3 binding to the PKB-PH domain and thereby blocks recruitment of the kinase to the plasma membrane where it is normally activated by its upstream kinases [16,19]. To test whether palmitate-induces inhibition of PKB by this mechanism we used a phospho-specific antibody to monitor PKB phosphorylation of Thr34 in palmitate-treated cells, and the sensitivity of this phosphorylation to Ro 31.8220 and SPT inhibitors. Figure 6(D) shows that palmitate, but not palmitoleate, induced Thr34 phosphorylation in a Ro 31.8220-sensitive manner consistent with the finding that PKCζ was activated by palmitate (Figure 5). The suggestion that PKCζ mediates this phosphorylation in response to enhanced ceramide synthesis from palmitate was strengthened by the demonstration that phosphorylation of PKB at Thr34 in cells treated with the NEFA was reduced noticeably upon pre-treatment of muscle cells with either L-cycloserine or myriocin (Figure 6E).
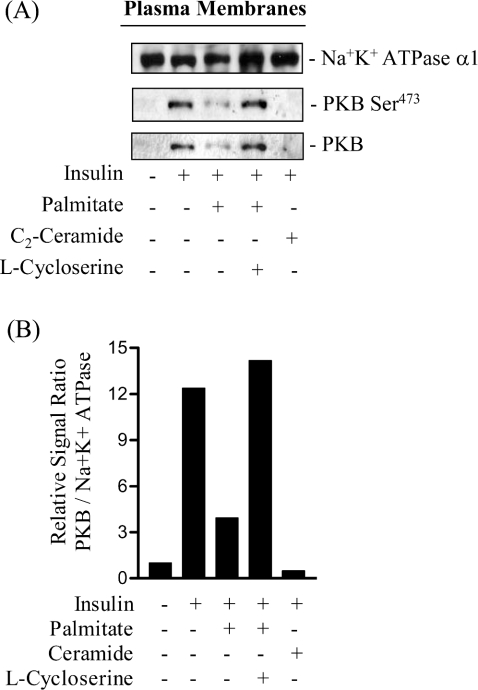
We have shown previously in L6 myotubes that one feature of the mechanism by which ceramide disrupts PKB involves a loss in its translocation to the plasma membrane [16]. To assess whether impaired PKB activation in palmitate-treated cells also involves diminished insulin-stimulated recruitment of the kinase to the cell surface, we assessed the abundance of the kinase in L6 plasma membranes by immunoblotting. Figure 7 shows that insulin induces a near 12-fold increase in the amount of PKB associated with the plasma membrane, as determined using a pan-PKB antibody. Identical results were obtained when we used a phospho-specific antibody against the Ser473 site to trace the localization/abundance of the activated/phosphorylated kinase (Figure 7A). Incubation of muscle cells with palmitate led to a substantial loss in insulin's ability to recruit PKB, which was prevented by prior treatment of cells with the SPT inhibitor, L-cycloserine. As an additional control, and as reported previously [16], incubation of muscle cells with 100 μM C2-ceramide completely abolished insulin-stimulated PKB recruitment to the plasma membrane (Figure 7A). To ensure that the differences in the immunoreactive PKB signals observed did not arise through aberrant loading of protein on SDS gels, membranes immunoblotted with the pan-PKB antibody were subsequently reprobed with a monoclonal antibody directed against the α1-subunit of the Na,K-ATPase, a plasma membrane marker [34].
Figure 7. Effects of palmitate and L-cycloserine on the insulin-dependent recruitment of PKB to the plasma membrane of L6 myotubes.
(A) L6 myotubes were primed with 1 mM L-cycloserine for 30 min prior to incubation with 0.75 mM palmitate (for 16 h). In some experiments muscle cells were incubated with 100 μM C2-ceramide for 2 h. In the penultimate 30 min of incubation with palmitate or ceramide L6 myotubes were challenged with 100 nM insulin. At the end of the insulin treatment, cells were harvested and plasma membranes isolated by subcellular fractionation. Plasma membranes (20 μg of protein) were subjected to SDS/PAGE and immunoblotted with antibodies against the α1 subunit of the Na/K-ATPase (plasma membrane marker) or with antibodies against PKB or phospho-PKB-Ser473 (PKB-Ser473). (B) PKB and α1-immunoreactve bands were quantified and expressed as a ratio (lower panel). The blots are representative of two separate experiments and the histogram depicts the mean ratio values from the two experiments.
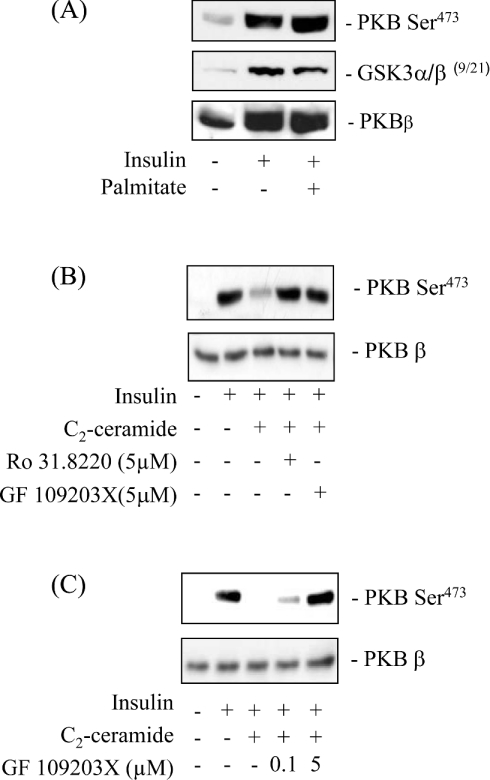
As with L6 muscle cells [16,19], ceramide also promotes a profound loss in the hormonal activation of PKB in 3T3-L1 adipocytes, another well-established insulin-responsive cell line [28]. Moreover, in both cell types, expression of a membrane-targeted form of PKB bypasses the inhibition exerted by ceramide on PKB signalling, suggesting that impaired recruitment of PKB to the plasma membrane is a common feature that underlies the inhibition of the kinase in both cell types [16,28]. To assess whether palmitate also inhibits PKB in cultured 3T3-L1 adipocytes, we immunoblotted fat-cell lysates with phospho-specific antibodies to PKB Ser473 (that cross-react with the Ser474 residue of PKBβ) and GSK3β following 16 h of palmitate incubation. However, unlike L6 myotubes (Figure 1), palmitate did not suppress the insulin-induced phosphorylation of PKB or GSK3 in fully differentiated 3T3-L1 adipocytes (Figure 8A). Nevertheless, when adipocytes were incubated with 100 μM C2-ceramide we observed a profound reduction in the phosphorylation of PKB, which could be prevented by pre-treatment of cells with Ro 31.8220 and GF 109203X (another PKC inhibitor) but only when used at a concentration that inhibits atypical PKCs (Figures 8B and 8C). This latter finding implies a common mechanism of PKB inhibition by ceramide in both cell types.
Figure 8. Effects of palmitate on insulin-stimulated PKB and GSK3 phosphorylation in differentiated 3T3-L1 adipocytes.
Differentiated 3T3-L1 adipocytes were cultured for 16 h in serum-free DMEM supplemented with 0.5% BSA (w/v) in the absence or presence of 0.75 mM palmitate (A). Alternatively, adipocytes were incubated with C2-ceramide (100 μM) for 2 h and were pre-treated with either Ro 31-8220 or GF 109203X for 30 min prior to incubation with ceramide at the concentrations shown and/or with insulin (100 nM) in the penultimate 10 min followed by cell lysis (B and C). Cell lysates (50 μg) were immunoblotted with anti-phosphospecific antibodies against PKB (Ser473 and Thr308), GSK3 α/β (Ser219) and with native PKBβ antibodies. Immunoblots are representative of 5 different experiments.
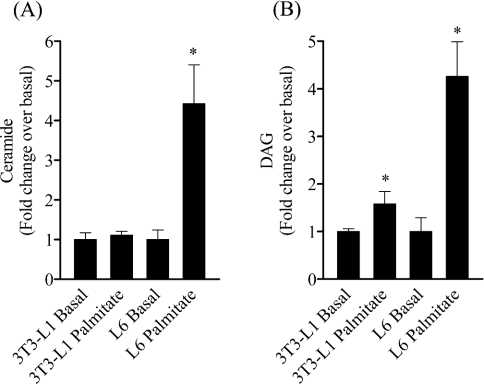
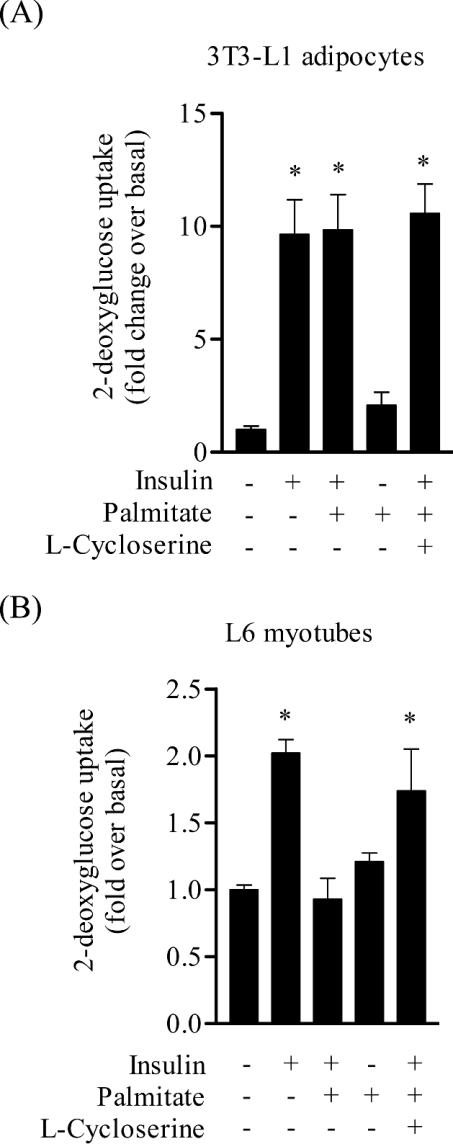
To understand why palmitate failed to elicit any inhibition of PKB in 3T3-L1 adipocytes we subsequently compared ceramide and DAG abundance in muscle and fat cells following the 16 h incubation with palmitate. Figure 9(A) shows that whilst palmitate enhanced intracellular ceramide by over 4-fold in L6 myotubes, it failed to do so in fat cells. Analysis of DAG content in 3T3-L1 adipocytes revealed that palmitate induced a modest, but significant increase (∼50%) in DAG accumulation, but which in relative terms was considerably lower than that observed in muscle cells, which exhibit a 4-fold increase in DAG accumulation (Figure 9B). An important end-point response of insulin in both muscle and fat cells is the stimulation of glucose uptake. Insulin stimulated glucose uptake by over 12-fold in 3T3-L1 adipocytes, and, consistent with the lack of any significant inhibition of PKB, palmitate did not have any discernible effect on insulin-stimulated sugar uptake in these cells (Figure 10A). In contrast, palmitate abolished the 2-fold increase in glucose uptake elicited by insulin in L6 myotubes, but interestingly this inhibition was considerably less in muscle cells that had been pre-treated with the SPT inhibitor, L-cycloserine (Figure 10B).
Figure 9. Effect of palmitate on the accumulation of ceramide and DAG in differentiated 3T3-L1 adipocytes and L6 myotubes.
3T3-L1 adipocytes and L6 myotubes were incubated with palmitate (0.75 mM, 16 h) and extracted from plates in PBS. Intracellular ceramide and DAG concentrations were assessed using the DAG kinase method. Intracellular ceramide (A) and DAG (B) levels are presented as means±S.E.M. of three separate experiments. *P<0.05 signifies a statistically significant change from the appropriate untreated cell type control.
Figure 10. Effect of palmitate on insulin-stimulated 2-deoxyglucose uptake.
3T3-L1 adipocytes and L6 myotubes were incubated in the absence or presence of palmitate (0.75 mM, 16 h) and L-cycloserine (1 mM) as indicated. During the last 30 min of this incubation cells were exposed to 100 nM insulin and then used for assaying 2-deoxyglucose uptake. The bar values represent the fold increase in glucose uptake relative to untreated cells (means±S.E.M.) of three and four experiments in 3T3-L1 adipocytes and L6 myotubes respectively, each performed in triplicate. *P<0.05 compared with the appropriate untreated control.
DISCUSSION
There is increasing acceptance of the idea that sustained elevation of NEFAs may dysregulate early insulin signalling events and that these may act in concert with the ‘Randle mechanism’ [35] to impair glucose utilization in skeletal muscle. In the present study we demonstrate that prolonged exposure of L6 muscle cells to elevated palmitate concentrations induces a profound inhibition in PKB-directed signalling and insulin-stimulated glucose transport. In our experimental system we found no evidence to suggest that palmitate antagonized insulin action upstream of PKB, given that the NEFA did not affect the tyrosine phosphorylation of IRS-1 or the insulin-induced interaction between IRS-1 and the p85 subunit of PI3-kinase. These findings are consistent with similar work reported previously using mouse C2C12 cells [8,9,36] but have been extended here to show also, for the first time, that palmitate has no inhibitory effect on the insulin-stimulated production of PIP3 in vivo. Nevertheless, despite the stimulus-mediated increase in PIP3, we show that palmitate uncouples 3-phosphoinositide synthesis from the activation of PKB leading to a concomitant loss in signalling to important end-point responses, such as glucose transport. Understanding how palmitate dysregulates PKB signalling was therefore a major objective of the current study.
One metabolic fate of the palmitate taken up by the cells is, that following its conversion into palmitoyl CoA, it can be condensed with serine in a reaction catalysed by SPT [37] to generate 3-ketodihydrosphingosine. This latter molecule can be reduced to sphinganine whose acylation produces dihydroceramide, which can be desaturated to yield ceramide [38]. Ceramide has been implicated in the regulation of diverse cellular responses including cell death, differentiation and in the pathogenesis of insulin resistance [39]. Indeed, evidence exists showing that its concentration is elevated in muscle of insulin resistant animals [40], and that sustained muscle exercise, which is known to enhance insulin sensitivity, can promote a reduction in intramuscular ceramide [10]. The suggestion that ceramide may be an intracellular effector molecule mediating palmitate-induced insulin resistance is supported by two separate lines of evidence. First, incubation of muscle cells with palmitate promotes intracellular accumulation of ceramide (Figure 3A and [8,9]), and we have shown previously that a cell permeant analogue of ceramide is capable of suppressing both the hormonal stimulation of PKB and glucose uptake in L6 muscle cells in a fashion similar to that reported for palmitate in the present study [16]. Secondly, data presented here and also reported by others [9] shows that pre-incubation of muscle cells with structurally unrelated SPT inhibitors suppresses ceramide synthesis and antagonizes the inhibitory effect of palmitate on PKB activation. In addition, we also demonstrate for the first time that SPT inhibition safeguards against the loss in insulin-stimulated glucose transport in L6 myotubes elicited by palmitate (Figure 10B). This latter observation provides further, albeit indirect, support for the concept that PKB is an important component of the insulin signalling cascade regulating glucose uptake in skeletal muscle.
Whilst there is general agreement that ceramide does not affect insulin signalling events upstream of PKB, the precise mechanism by which ceramide impairs PKB activation remains somewhat equivocal. In murine C2C12 myotubes, for example, ceramide generated de novo from palmitate invokes the activation of a Type 2A-like phosphatase that dephosphorylates and inactivates PKB [17]. In contrast, okadaic acid (a Type 2A phosphatase inhibitor) fails to alleviate the palmitate- or C2 ceramide-induced inhibition of PKB in rat L6 muscle cells (Figure 4) [16] or indeed in murine 3T3-L1 adipocytes [28] and in a neuroblastoma-motor neuron cell line [41]. These observations suggest that, depending on cell type, ceramide can inhibit PKB by distinct mechanisms. This notion is supported by the finding that, in addition to activating phosphatases, ceramide also activates atypical PKCs {e.g. PKCζ (for review see [39])}, which can directly interact with and inhibit PKB [18,42–44]. The interaction between these two members of the AGC kinase family requires the PH domain of PKB and we have shown recently that, when activated by ceramide, PKCζ phosphorylates Thr34 within the PH domain of PKBα [19]. This phosphorylation reduces the ability of the PKB-PH domain to bind 3-phosphoinositides and underpins the loss in kinase recruitment and activation at the plasma membrane [19]. We propose that such a mechanism also forms the basis by which palmitate inhibits PKB in our cultured muscle cells. This supposition is based on the collective strength of a number of observations that show (i) palmitate promotes intracellular accumulation of ceramide, (ii) palmitate stimulates PKCζ activity and induces phosphorylation of PKB on Thr34, (iii) phosphorylation of PKB Thr34 in palmitate-treated cells was sensitive to Ro 31.8220, a PKC inhibitor, and (iv) inhibitors of SPT not only suppressed ceramide production from palmitate, but also inhibited the activation of PKCζ, the phosphorylation of PKB-Thr34, and, moreover, reinstated PKB recruitment/activation at the plasma membrane in response to insulin.
In both L6 muscle cells and 3T3-L1 adipocytes the ability of C2-ceramide to suppress PKB-directed signalling can be circumvented by the expression of a membrane-targeted form of the kinase [16,28] implying that, in these cell lines, stimuli that induce ceramide synthesis may impair PKB activation by a common mechanism. However, unlike muscle cells, we found that incubation of adipocytes with palmitate did not elicit any detectable inhibition of PKB or insulin-stimulated glucose transport, despite a clear suppressive effect of C2-ceramide on PKB phosphorylation (Figure 8B). The apparent lack of inhibition by palmitate in adipocytes can be explained by the observation that these cells fail to accumulate appreciable amounts of ceramide from the NEFA. This finding is completely consistent with a recent report suggesting that adipocytes preserve insulin-sensitivity upon incubation with NEFAs by effectively converting fatty acyl-CoAs into triglyceride rather than molecules such as ceramide [9]. This proposition is entirely plausible given that triglyceride storage is one of the principal functions of normal fat cells. However, whilst adipocytes resist converting palmitate to ceramide, their responsiveness to insulin (using PKB phosphorylation as an index) can be reduced dramatically by elevating intracellular ceramide using cell-permeant analogues of the sphingolipid. The mechanism by which C2-ceramide impairs the hormonal activation of PKB in adipocytes has remained unclear. A previous study by Summers et al. [28] excluded the involvement of a ceramide-activated phosphatase or of atypical PKCs based on the inability of okadaic acid or Gö 6983 (a broad spectrum PKC inhibitor) to act as antagonists of ceramide action in adipocytes. However, we show here that the ceramide-mediated inhibition of PKB can be prevented using Ro 31.8220 and also the structurally unrelated bisindolemalemide, GF 109203X, when used at concentrations that inhibit atypical PKCs. Collectively, these inhibitor studies are consistent with the idea that atypical PKCs, activated by exogenously supplied ceramide, impair PKB activation as observed in L6 cells. The reason for the apparent discrepancy in the data obtained with these two inhibitors and Gö 6983 remains unclear, but since the latter has also been used at concentrations of between 1 μM and 5 μM to inhibit atypical PKCs in cell-based assays [45], it is plausible that its use at the lower dose (as in the study of Summers et al. [28]) may have been ineffective in fully suppressing PKCζ in 3T3-L1 adipocytes. Indeed, previous work from our lab showing that 1 μM Ro 31.8220 recovers only ∼20% of the insulin-stimulated PKB phosphorylation in ceramide-treated cells, compared with that at the higher inhibitor concentration (5 μM) [19], suggests that such as possibility cannot be excluded for Gö 6983. It should be stressed that whilst we remain mindful that the selectivity of such inhibitors for their targets is not entirely specific, previous work from our lab using a battery of kinase inhibitors revealed that the loss in PKB activation elicited by ceramide could only be antagonized by Ro 31.8220 when used at concentrations in the μM range that target the atypical PKCs [19].
In addition to promoting ceramide synthesis, DAG was also a key metabolite that accumulated in muscle cells following incubation with palmitate. The accrual of DAG has been implicated strongly in the development of insulin resistance in peripheral tissues through its ability to activate DAG-sensitive PKC isoforms, which can potentially serine phosphorylate and down-regulate insulin receptor and IRS signalling [46,47]. However, our data suggests that DAG accumulated in muscle and fat cells from palmitate was not a major contributor to the loss in PKB signalling, at least over the 16 h incubation period used in the present study. The ability of SPT inhibitors to antagonize palmitate inhibition of PKB without having any significant impact on DAG accumulation would strongly support this idea.
In summary, the present study has shown that prolonged exposure of L6 skeletal muscle cells to increased concentrations of palmitate reduces insulin-stimulated PKB activity with a concomitant loss in downstream signalling to important end-point responses such as glucose transport. We demonstrate that the inhibition in PKB signalling is derived from the elevated synthesis of ceramide from palmitate, and that this promotes the activation of atypical PKCζ, which negatively regulates PKB by suppressing its cell-surface recruitment and phosphorylation. Inhibiting the de novo synthesis of ceramide from palmitate or the palmitate-induced activation of PKCζ not only prevented the loss in PKB activation, but importantly also retained insulin-stimulated glucose transport. Our results suggest that enhanced intracellular production of ceramide may represent a critical feature of the pathogenic process by which NEFAs induce insulin resistance in skeletal muscle and, moreover, raise the intriguing possibility that suppressing the synthesis and/or accumulation of ceramide may be of therapeutic value in promoting skeletal muscle sensitivity to insulin.
Acknowledgments
This work was supported by the Diabetes Research and Wellness Foundation and by Diabetes U.K. E.H. was supported by a grant from la Fondation pour la Recherche Médicale.
References
- 1.Wang L., Folsom A. R., Zheng Z. J., Pankow J. S., Eckfeldt J. H. Plasma fatty acid composition and incidence of diabetes in middle-aged adults: the Atherosclerosis Risk in Communities (ARIC) study. Am. J. Clin. Nutr. 2003;78:91–98. doi: 10.1093/ajcn/78.1.91. [DOI] [PubMed] [Google Scholar]
- 2.Mcgarry J. D. Banting lecture 2001: dysregulation of fatty acid metabolism in the etiology of Type 2 diabetes. Diabetes. 2002;51:7–18. doi: 10.2337/diabetes.51.1.7. [DOI] [PubMed] [Google Scholar]
- 3.Randle P. J. Regulatory interactions between lipids and carbohydrates: the glucose fatty acid cycle after 35 years. Diabetes Metab. Rev. 1998;14:263–283. doi: 10.1002/(sici)1099-0895(199812)14:4<263::aid-dmr233>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 4.Roden M., Price T. B., Perseghin G., Petersen K. F., Rothman D. L., Cline G. W., Shulman G. I. Mechanism of free fatty acid-induced insulin resistance in humans. J. Clin. Invest. 1996;97:2859–2865. doi: 10.1172/JCI118742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Storz P., Doppler H., Wernig A., Pfizenmaier K., Muller G. Cross-talk mechanisms in the development of insulin resistance of skeletal muscle cells palmitate rather than tumour necrosis factor inhibits insulin-dependent protein kinase B (PKB)/Akt stimulation and glucose uptake. Eur. J. Biochem. 1999;266:17–25. doi: 10.1046/j.1432-1327.1999.00809.x. [DOI] [PubMed] [Google Scholar]
- 6.Reynoso R., Salgado L. M., Calderon V. High levels of palmitic acid lead to insulin resistance due to changes in the level of phosphorylation of the insulin receptor and insulin receptor substrate-1. Mol. Cell Biochem. 2003;246:155–162. [PubMed] [Google Scholar]
- 7.Yu C., Chen Y., Cline G. W., Zhang D., Zong H., Wang Y., Bergeron R., Kim J. K., Cushman S. W., Cooney G. J., et al. Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J. Biol. Chem. 2002;277:50230–50236. doi: 10.1074/jbc.M200958200. [DOI] [PubMed] [Google Scholar]
- 8.Schmitz-Peiffer C., Craig D. L., Biden T. J. Ceramide generation is sufficient to account for the inhibition of the insulin-stimulated PKB pathway in C2C12 skeletal muscle cells pretreated with palmitate. J. Biol. Chem. 1999;274:24202–24210. doi: 10.1074/jbc.274.34.24202. [DOI] [PubMed] [Google Scholar]
- 9.Chavez J. A., Summers S. A. Characterizing the effects of saturated fatty acids on insulin signalling and ceramide and diacylglycerol accumulation in 3T3-L1 adipocytes and C2C12 myotubes. Arch. Biochem. Biophys. 2003;419:101–109. doi: 10.1016/j.abb.2003.08.020. [DOI] [PubMed] [Google Scholar]
- 10.Dobrzyn A., Gorski J. Ceramides and sphingomyelins in skeletal muscles of the rat: content and composition. Effect of prolonged exercise. Am. J. Physiol. Endocrinol. Metab. 2002;282:E277–E285. doi: 10.1152/ajpendo.00151.2001. [DOI] [PubMed] [Google Scholar]
- 11.Whiteman E. L., Cho H., Birnbaum M. J. Role of Akt/protein kinase B in metabolism. Trends Endocrinol. Metab. 2002;13:444–451. doi: 10.1016/s1043-2760(02)00662-8. [DOI] [PubMed] [Google Scholar]
- 12.Hajduch E., Litherland G. J., Hundal H. S. Protein kinase B: a key regulator of glucose transport? FEBS Lett. 2001;492:199–203. doi: 10.1016/s0014-5793(01)02242-6. [DOI] [PubMed] [Google Scholar]
- 13.Vanhaesebroeck B., Alessi D. R. The PI3K-PDK1 connection: more than just a road to PKB. Biochem. J. 2000;346:561–576. [PMC free article] [PubMed] [Google Scholar]
- 14.Milburn C. C., Deak M., Kelly S. M., Price N. C., Alessi D. R., van Aalten D. M. Binding of phosphatidylinositol 3,4,5-trisphosphate to the pleckstrin homology domain of protein kinase B induces a conformational change. Biochem. J. 2003;375:531–538. doi: 10.1042/BJ20031229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salinas M., Lopez-Valdaliso R., Martin D., Alvarez A., Cuadrado A. Inhibition of PKB/Akt1 by C2-ceramide involves activation of ceramide-activated protein phosphatase in PC12 cells. Mol. Cell. Neurosci. 2000;15:156–169. doi: 10.1006/mcne.1999.0813. [DOI] [PubMed] [Google Scholar]
- 16.Hajduch E., Balendran A., Batty I. H., Litherland G. J., Blair A. S., Downes C. P., Hundal H. S. Ceramide impairs the insulin-dependent membrane recruitment of protein kinase B leading to a loss in downstream signalling in L6 skeletal muscle cells. Diabetologia. 2001;44:173–183. doi: 10.1007/s001250051596. [DOI] [PubMed] [Google Scholar]
- 17.Cazzolli R., Carpenter L., Biden T. J., Schmitz-Peiffer C. A role for protein phosphatase 2A-like activity, but not atypical protein kinase Czeta, in the inhibition of protein kinase B/Akt and glycogen synthesis by palmitate. Diabetes. 2001;50:2210–2218. doi: 10.2337/diabetes.50.10.2210. [DOI] [PubMed] [Google Scholar]
- 18.Bourbon N. A., Sandirasegarane L., Kester M. Ceramide-induced inhibition of Akt is mediated through protein kinase Czeta: implications for growth arrest. J. Biol. Chem. 2002;277:3286–3292. doi: 10.1074/jbc.M110541200. [DOI] [PubMed] [Google Scholar]
- 19.Powell D. J., Hajduch E., Kular G., Hundal H. S. Ceramide disables 3-phosphoinositide binding to the Pleckstrin homology domain of protein kinase B (PKB)/Akt by a PKCzeta-dependent mechanism. Mol. Cell. Biol. 2003;23:7794–7808. doi: 10.1128/MCB.23.21.7794-7808.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsakiridis T., McDowell H. E., Walker T., Downes C. P., Hundal H. S., Vranic M., Klip A. Multiple roles of phosphatidylinositol 3-kinase in regulation of glucose-transport, amino-acid-transport, and glucose transporters in L6 skeletal-muscle cells. Endocrinology. 1995;136:4315–4322. doi: 10.1210/endo.136.10.7664650. [DOI] [PubMed] [Google Scholar]
- 21.Mitsumoto Y., Klip A. Developmental regulation of subcellular distribution and glycosylation of GLUT1 and GLUT4 glucose transporters during myogenesis of L6 muscle cells. J. Biol. Chem. 1992;267:4947–4962. [PubMed] [Google Scholar]
- 22.Hajduch E., Alessi D. R., Hemmings B. A., Hundal H. S. Constitutive activation of protein kinase Bα (PKBα) by membrane targeting promotes glucose and system A amino acid transport, protein synthesis and GSK3 inactivation in L6 muscle cells. Diabetes. 1998;47:1006–1013. doi: 10.2337/diabetes.47.7.1006. [DOI] [PubMed] [Google Scholar]
- 23.Frost S. C., Lane M. D. Evidence for the involvement of vicinal sulfhydryl groups in insulin- activated hexose transport by 3T3-L1 adipocytes. J. Biol. Chem. 1985;260:2646–2652. [PubMed] [Google Scholar]
- 24.Green H., Kehinde O. An established preadipose cell line and its differentiation in culture. II. Factors affecting the adipose conversion. Cell (Washington, D.C.) 1975;5:19–27. doi: 10.1016/0092-8674(75)90087-2. [DOI] [PubMed] [Google Scholar]
- 25.Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 26.Gray A., Olsson H., Batty I. H., Priganica L., Peter D. C. Nonradioactive methods for the assay of phosphoinositide 3-kinases and phosphoinositide phosphatases and selective detection of signaling lipids in cell and tissue extracts. Anal. Biochem. 2003;313:234–245. doi: 10.1016/s0003-2697(02)00607-3. [DOI] [PubMed] [Google Scholar]
- 27.Montell E., Turini M., Marotta M., Roberts M., Noe V., Ciudad C. J., Mace K., Gomez-Foix A. M. DAG accumulation from saturated fatty acids desensitizes insulin stimulation of glucose uptake in muscle cells. Am. J. Physiol. Endocrinol. Metab. 2001;280:E229–E237. doi: 10.1152/ajpendo.2001.280.2.E229. [DOI] [PubMed] [Google Scholar]
- 28.Summers S. A., Garza L. A., Zhou H., Birnbaum M. J. Regulation of insulin-stimulated glucose transporter GLUT4 translocation and Akt kinase activity by ceramide. Mol. Cell. Biol. 1998;18:5457–5464. doi: 10.1128/mcb.18.9.5457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Standaert M. L., Galloway L., Karnam P., Bandyopadhyay G., Moscat J., Farese R. V. Protein kinase Cζ as a downstream effector of phosphatidylinositol 3-kinase during insulin stimulation in rat adipocytes – potential role in glucose transport. J. Biol. Chem. 1997;272:30075–30082. doi: 10.1074/jbc.272.48.30075. [DOI] [PubMed] [Google Scholar]
- 30.Alessi D. R. The protein kinase c inhibitors RO318220 and GF109203X are equally potent inhibitors of MAPKAP kinase-1 beta (Rsk-2) and p70 s6 kinase. FEBS Lett. 1997;402:121–123. doi: 10.1016/s0014-5793(96)01510-4. [DOI] [PubMed] [Google Scholar]
- 31.Cousin S. P., Hugl S. R., Wrede C. E., Kajio H., Myers M. G., Jr, Rhodes C. J. Free fatty acid-induced inhibition of glucose and insulin-like growth factor I-induced deoxyribonucleic acid synthesis in the pancreatic beta-cell line INS-1. Endocrinology. 2001;142:229–240. doi: 10.1210/endo.142.1.7863. [DOI] [PubMed] [Google Scholar]
- 32.Yaney G. C., Korchak H. M., Corkey B. E. Long-chain acyl CoA regulation of protein kinase C and fatty acid potentiation of glucose-stimulated insulin secretion in clonal β-cells. Endocrinology. 2000;141:1989–1998. doi: 10.1210/endo.141.6.7493. [DOI] [PubMed] [Google Scholar]
- 33.Bourbon N. A., Yun J., Kester M. Ceramide directly activates protein kinase C zeta to regulate a stress-activated protein kinase signaling complex. J. Biol. Chem. 2000;275:35617–35623. doi: 10.1074/jbc.M007346200. [DOI] [PubMed] [Google Scholar]
- 34.Hundal H. S., Marette A., Mitsumoto Y., Ramlal T., Blostein R., Klip A. Insulin induces translocation of the α2 and β1 subunits of the Na/K-ATPase from intracellular compartments to the plasma membrane in mammalian skeletal muscle. J. Biol. Chem. 1992;267:5040–5043. [PubMed] [Google Scholar]
- 35.Randle P. J., Newsholme E. A., Garland P. B. Effects of fatty acids, ketone bodies and pyruvate and of alloxan diabetes and starvation on the uptake and metabolic fate of glucose in rat heart and diaphragm muscles. Biochem. J. 1964;93:652–665. doi: 10.1042/bj0930652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chavez J. A., Knotts T. A., Wang L. P., Li G., Dobrowsky R. T., Florant G. L., Summers S. A. A role for ceramide, but not diacylglycerol, in the antagonism of insulin signal transduction by saturated fatty acids. J. Biol. Chem. 2003;278:10297–10303. doi: 10.1074/jbc.M212307200. [DOI] [PubMed] [Google Scholar]
- 37.Hanada K. Serine palmitoyltransferase, a key enzyme of sphingolipid metabolism. Biochim. Biophys. Acta. 2003;1632:16–30. doi: 10.1016/s1388-1981(03)00059-3. [DOI] [PubMed] [Google Scholar]
- 38.Merrill A. H., Jr De novo sphingolipid biosynthesis: a necessary, but dangerous, pathway. J. Biol. Chem. 2002;277:25843–25846. doi: 10.1074/jbc.R200009200. [DOI] [PubMed] [Google Scholar]
- 39.Mathias S., Pena L. A., Kolesnick R. N. Signal transduction of stress via ceramide. Biochem. J. 1998;335:465–480. doi: 10.1042/bj3350465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Turinsky J., O'sullivan D. M., Bayly B. P. 1,2-Diacylglycerol and ceramide levels in insulin-resistant tissues of the rat in vivo. J. Biol. Chem. 1990;265:16880–16885. [PubMed] [Google Scholar]
- 41.Zhou H., Summers S. A., Birnbaum M. J., Pittman R. N. Inhibition of Akt kinase by cell-permeable ceramide and its implications for ceramide-induced apoptosis. J. Biol. Chem. 1998;273:16568–16575. doi: 10.1074/jbc.273.26.16568. [DOI] [PubMed] [Google Scholar]
- 42.Doornbos R. P., Theelen M., van der Hoeven P. C., van Blitterswijk W. J., Verkleij A. J., van Bergen en Henegouwen P. M. Protein kinase Cζ is a negative regulator of protein kinase B activity. J. Biol. Chem. 1999;274:8589–8596. doi: 10.1074/jbc.274.13.8589. [DOI] [PubMed] [Google Scholar]
- 43.Konishi H., Kuroda S., Kikkawa U. The pleckstrin homology domain of RAC protein kinase associates with the regulatory domain of protein kinase Cζ. Biochem. Biophys. Res. Commun. 1994;205:1770–1775. doi: 10.1006/bbrc.1994.2874. [DOI] [PubMed] [Google Scholar]
- 44.Mao M., Fang X., Lu Y., Lapushin R., Bast J. R., Mills G. B. Inhibition of growth-factor-induced phosphorylation and activation of protein kinase B/Akt by atypical protein kinase C in breast cancer cells. Biochem. J. 2000;352:475–482. [PMC free article] [PubMed] [Google Scholar]
- 45.Cussac D., Newman-Tancredi A., Pasteau V., Millan M. J. Human dopamine D(3) receptors mediate mitogen-activated protein kinase activation via a phosphatidylinositol 3-kinase and an atypical protein kinase C-dependent mechanism. Mol. Pharmacol. 1999;56:1025–1030. doi: 10.1124/mol.56.5.1025. [DOI] [PubMed] [Google Scholar]
- 46.Idris I., Gray S., Donnelly R. Protein kinase C activation: isozyme-specific effects on metabolism and cardiovascular complications in diabetes. Diabetologia. 2001;44:659–673. doi: 10.1007/s001250051675. [DOI] [PubMed] [Google Scholar]
- 47.White M. F. IRS proteins and the common path to diabetes. Am. J. Physiol. Endocrinol. Metab. 2002;283:E413–E422. doi: 10.1152/ajpendo.00514.2001. [DOI] [PubMed] [Google Scholar]