Differential Regulation of Immune Responses by Highly and Weakly Virulent Cryptococcus neoformans Isolates (original) (raw)
Abstract
Early inflammatory responses, delayed-type hypersensitivity (DTH) responses, and cytokine profiles were studied in mice infected by the pulmonary route with either a highly virulent isolate (NU-2) or a weakly virulent isolate (184A) of Cryptococcus neoformans. After infection, NU-2 remained in the lungs and the capsule became more pronounced during the first 24 h, whereas 184A induced an immediate inflammatory reaction and was rapidly cleared from the lungs. Cryptococcal antigen (GXM) appeared in sera early after infection with NU-2 and increased over the entire observation period. There was no detectable GXM in sera from 184A-infected mice. Both C. neoformans isolates induced anticryptococcal cell-mediated immune responses, but the responses had different profiles. DTH in NU-2-infected mice appeared at day 15 after infection and waned by day 21, whereas DTH in 184A-infected mice was present by day 5 and continued to increase. T helper 1 (Th1) cytokines (interleukin 2 [IL-2] and gamma interferon) were made by spleen cells early after infection with either isolate. NU-2-infected mice lost their ability to produce these cytokines, but 184A-infected mice retained it. IL-4, a Th2 cytokine, was not detected in infected mice. The regulatory cytokine IL-10 was made by spleen cells early but not later after infection with the highly virulent isolate and was not produced by spleen cells from 184A-infected mice. IL-10-deficient mice survived an NU-2 infection significantly longer than wild-type mice, suggesting that IL-10 is important in down-regulating the protective immune response. The induction of anergy appears to be responsible for the inability of NU-2-infected mice to control a C. neoformans infection.
Cryptococcus neoformans infection is believed to be acquired by the inhalation of blastoconidia found in debris around pigeon roosts and in the soil (26). In the normal host, the infection typically is limited to the lung but disseminates to other tissues in the immunosuppressed patient and in the occasional normal host (25, 31). C. neoformans has a predilection for the brain, where it produces a meningoencephalitis that is fatal if it is not treated with antifungal agents (28). In the AIDS patient population, this treatment must be continued for life (31). Although most cryptococcosis patients have their disease diagnosed at the onset of meningitis, the pathobiology can vary among patients. The differences in pathogenesis could be due to differences in the ways individuals respond to the variation in the genetic makeup of the organism.
Mouse models of cryptococcosis have been used to identify the host responses to cryptococcosis that may provide protection from infection (18). However, very limited information is available regarding host responses to different C. neoformans isolates, which may vary greatly in their levels of virulence. We reported that two such isolates display great differences in capsule synthesis under tissue culture conditions and differ in their pathogenic potential for mice (4). A weakly virulent isolate, 184A, does not significantly increase its capsule size when transferred into tissue culture conditions, i.e., RPMI 1640 in the presence of CO2 at 37°C, while a highly virulent isolate, NU-2, exhibits a significant increase in capsule size under the same conditions (4). In addition, the heavily encapsulated NU-2 cells stimulate macrophages to secrete C3 when they are exposed to cryptococcal blastoconidia in tissue cultures (4). These results prompted us to predict that C. neoformans isolates with the ability to produce large quantities of capsular polysaccharide in vivo may induce very different immune responses from those induced by isolates that produce much less capsule.
This investigation was undertaken to further define differences in host responses to a weakly virulent isolate and a highly virulent isolate of C. neoformans in order to identify host factors which might contribute to the pathogenesis of the highly virulent cryptococcal isolate. Our results showed that while the highly virulent isolate induced a cell-mediated immune (CMI) response as detected by delayed-type hypersensitivity (DTH) reactivity and a T helper 1 (Th1) cytokine profile, these responses appeared early after intratracheal infection and were rapidly down-regulated. On the other hand, infection with the weakly virulent isolate induced a CMI response that was slower to develop but was not down-regulated. The down-regulation of DTH and type 1 cytokine responses in mice infected with the highly virulent isolate could not be attributed to the production of the type 2 cytokine interleukin 4 (IL-4) during the later stages of infection but rather to an unresponsive state of the host’s effector cells. The immunoregulatory cytokine IL-10 was significantly elevated during the early stages of NU-2 infection, and IL-10 knockout mice were more resistant to infection with NU-2 than their normal counterparts, suggesting that IL-10 may contribute to the emergence of the unresponsive state which develops later in infection.
MATERIALS AND METHODS
Animals.
CBA/J, C57BL/6J, and C57BL/6-IL10tm1Cgn (IL-10 knockout) female mice (7 to 8 weeks of age) were purchased from the Jackson Laboratory, Bar Harbor, Maine, and were used in experiments when they were 8 to 16 weeks of age. The mice were housed in the University of Oklahoma Health Sciences Center Animal Facility, which is approved by the American Association for the Accreditation of Laboratory Animal Care.
Reagents.
RPMI 1640, penicillin-streptomycin, l-glutamine, sodium pyruvate, essential vitamins, and nonessential amino acids were purchased from GIBCO BRL (Grand Island, N.Y.). HyClone (Ogden, Utah) was the supplier of fetal bovine serum (FBS). Concanavalin A (ConA) and HEPES were purchased from Sigma Chemical Co. (St. Louis, Mo.). Recombinant mouse IL-2 was purchased from Collaborative Biomedical Products, Bedford, Mass. Tumor necrosis factor alpha and gamma interferon (IFN-γ) were purchased from Genzyme (Cambridge, Mass.). PharMingen (San Diego, Calif.) was the supplier of recombinant mouse IL-10. Recombinant mouse IL-4 was generously provided by Sterling Winthrop (Malvern, Pa.).
Fungal strains.
The isolates of C. neoformans used in this investigation included strain NU-2, originally obtained from the spinal fluid of a patient with cryptococcal meningitis at the University of Nebraska Medical Center, and strain 184A which was obtained from L. Friedman, Tulane University Medical School. Both of these cryptococcal isolates are encapsulated and are serotype A. The organisms were maintained in the laboratory by growth on Sabouraud dextrose agar.
Maintenance of endotoxin-free conditions.
To eliminate the influence of endotoxin on experimental results, all experiments were performed under conditions that minimize endotoxin contamination. This included the use of endotoxin-free plasticware or glassware that was heated for 3 h at 180°C and of reagents that contained less than 8 pg of endotoxin/ml (minimal detectable level) with the Limulus amoebocyte lysate assay (Whittaker Bioproducts, Inc., Walkersville, Md.).
Intratracheal infections.
Cryptococci were grown on Sabouraud dextrose agar for 72 h, harvested from the surface of the agar plate with sterile physiological saline solution (SPSS), and washed three times with SPSS. Mice were anesthetized with ketamine (50 mg/kg) and xylazine (5 mg/kg), and the trachea of each mouse was surgically exposed. A 22-gauge angiocatheter was threaded into the trachea until the needle hub was at the level of the mouth of the mouse. Twenty-five microliters of a solution of cryptococci (4 × 106 blastoconidia/ml) was injected into the angiocatheter tube, followed by 50 μl of air to expel all of the inoculum from the catheter into the lung. The incision was closed with wound clips. Normal control mice received a sham operation with the instillation of 25 μl of SPSS into the lung instead of cryptococcal cells.
Determination of cryptococcal CFU in infected lungs.
Groups of five mice were euthanized immediately after infection or 24 h thereafter, respectively, to determine the number of cryptococci deposited into the lungs and to evaluate the clearance of organisms from the lung over the first 24 h of infection. All lobes of the lungs were removed and placed in 5 ml of SPSS in a sterile stomacher bag. Lungs from individual mice were homogenized with a stomacher homogenizer (Stomacher 80 Lab Blender; Techmar). Each lung homogenate was serially diluted in SPSS, and the dilutions were plated in duplicate on Sabouraud dextrose agar plates. CFU were enumerated after 3 days of incubation at room temperature.
Histological examination of infected lungs.
Lungs from experimental animals were removed at various times after infection with C. neoformans. The lungs were fixed with buffered formalin, sectioned, and stained with hematoxylin and eosin or with mucicarmine. The tissues were examined for the type and intensity of cellular infiltrate in the infected lungs, for the presence of cryptococci, and for the amount of capsular polysaccharide associated with the cryptococcal cells. Sections were examined in a blind fashion. The intensity of the inflammatory responses was graded on a scale of 1 (mild) to 3 (severe), where mild represents a definite but slight inflammatory response with cellular infiltrate detectable when compared with lungs of sham-treated controls. The severe lesions were characterized by an intense inflammatory infiltrate composed predominantly of aggregates of polymorphonuclear leukocytes and few lymphocytes. Inflammatory lesions, intermediate between these two categories, were scored as moderate with a numeric score of 2. The criteria for these scores were set after a careful evaluation of the varying severity of inflammation seen in a series of experiments.
Since the inflammatory process is patchy in distribution, the extent of lung involvement was determined by dividing each hematoxylin and eosin-stained lung cross section into five relatively equal regions. The presence of the inflammatory process within these regions was determined and expressed as a percentage of the entire cross section of the lung. Although the lungs were not inflated, by comparing experimental lungs to lungs of sham-treated mice we were able to rule out lung collapse as a factor in our assessment.
Measurement of serum GXM levels.
Titers of the cryptococcal antigen glucuronoxylomannan (GXM) in the sera of experimental animals were determined by using a commercially available latex agglutination assay (Immuno-Mycologics, Inc., Norman, Okla.). Serial twofold dilutions of serum were tested for the ability to agglutinate latex particles coated with anti-GXM according to the manufacturer’s protocol.
Preparation of cryptococcal antigen.
Cryptococcal culture filtrate antigen (CneF) was prepared from C. neoformans 184A as described previously (5). The preparation used in this investigation had a protein content of 243 μg/ml as determined by the bicinchoninic acid assay (Pierce Chemical Co., Rockford, Ill.) and a carbohydrate concentration of 3.2 mg/ml as determined by the phenol-sulfuric acid assay (11). When tested in the Limulus assay, this lot of CneF gave a reaction equivalent to less than 0.1 ng of endotoxin/ml. Because the extract contains a high concentration of GXM, which gives a positive reaction in the Limulus assay due to its content of glucuronic acid (32), this Limulus reactivity is considered to be due to the glucuronic acid rather than to endotoxin contamination.
Elicitation of the anticryptococcal DTH response.
Hind footpads of mice were measured with a gauge micrometer (Mitutoyo, Aurora, Ill.), 30 μl of SPSS was injected in the left footpad, and 30 μl of CneF was injected in the right footpad. The footpads were measured again 24 h later.
The increase in footpad thickness was calculated as the difference in swelling between the 0- and 24-h measurements. Specific DTH reactivity was calculated as the difference between the swelling of the SPSS-injected footpads and the swelling of the CneF-injected footpads.
In vitro stimulation of cytokine synthesis by spleen cells.
Spleen cells were harvested from groups of mice at various times after intratracheal infection with C. neoformans NU-2 or 184A. Single cell suspensions were prepared by pressing the spleens through a sterile 60-mesh wire screen into sterile Hanks’ balanced salt solution–3% FBS. Erythrocytes were lysed with 0.83% ammonium chloride, and the mononuclear cells were washed three times in Hanks’ balanced salt solution-FBS. The cells were then suspended in Bretcher’s medium (RPMI 1640 containing 100 U of penicillin per ml, 100 μg of streptomycin per ml, 25 mM HEPES, 5 × 10−3 M 2-mercaptoethanol, 2 mM l-glutamine, 1 mM sodium pyruvate, 1% essential vitamins, 1% nonessential amino acids, and 10% FBS). The spleen cells at a concentration of 5 × 106/ml were stimulated with cryptococcal CneF at a final dilution of 1:8, with concanavalin A (25 μg/ml) as a positive control, or they were cultured without stimulation to determine the constitutive or background level of cytokine secretion. Cultures were incubated at 37°C in an atmosphere of 5% CO2, and supernatant fluids were collected 24 and 48 h after the initiation of cultures for the assessment of cytokines.
Quantitation of cytokine levels in culture supernatants.
Enzyme-linked immunosorbent assays for the detection of IL-2, IFN-γ, IL-4, and IL-10 in tissue culture supernatants were constructed by using commercially available paired monoclonal antibodies for each cytokine (PharMingen) according to our previously described method (30). The minimal levels of detection for the IL-2, IL-4, IL-10, and IFN-γ assays were 4 pg/ml, 6.25 pg/ml, 200 pg/ml, and 128 pg/ml, respectively.
Determination of survival after infection with C. neoformans.
The virulence of cryptococcal isolate NU-2 was determined in C57BL/6J mice and in the IL-10 knockout strain C57BL/6-IL10tm1Cgn. Nineteen mice of each strain were infected by the intratracheal route with 105 C. neoformans NU-2 organisms. The animals were observed daily for deaths, to determine the mean survival time in the normal and IL-10 knockout mouse strains.
Statistical analysis.
The significant differences between experimental groups were evaluated by Student’s t test. Survival data were analyzed by Kaplan-Meier survival plots followed by log rank tests. Data with a P value of 0.05 or less were considered to be significant.
RESULTS
Clearance of cryptococcal isolates NU-2 and 184A from infected lungs.
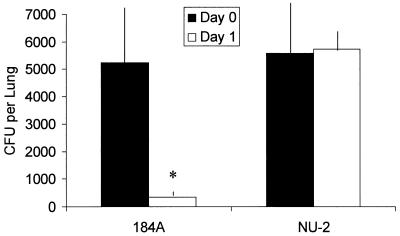
The ability of the host to clear NU-2 or 184A was assayed by the determination of CFU in infected lungs immediately after infection and 24 h after infection. Cryptococcal isolates were grown on Sabouraud dextrose agar, a medium previously shown to allow limited capsule production in both isolates (4). Cryptococci were harvested from these cultures and used to infect the lungs of CBA/J mice. The CFU analysis revealed that isolate 184A was virtually eliminated from the lungs during the first 24 h of infection (Fig. 1). On the other hand, NU-2-infected mice had similar numbers of CFU in their lungs immediately after inoculation (day 0) and at 24 h.
FIG. 1.
CFU cultured from the lungs of mice immediately or 24 h after intratracheal infection with C. neoformans NU-2 or 184A. ∗, statistically significant (P < 0.001) compared to NU-2-infected lungs 24 h after infection. The data presented represent the mean CFU ± standard errors of the means (error bars) for five animals/experimental group.
Histological examination of lungs of mice infected with cryptococcal isolates NU-2 and 184A.
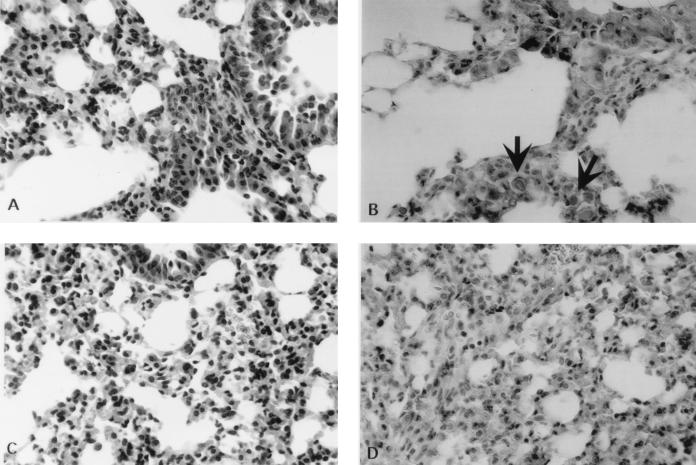
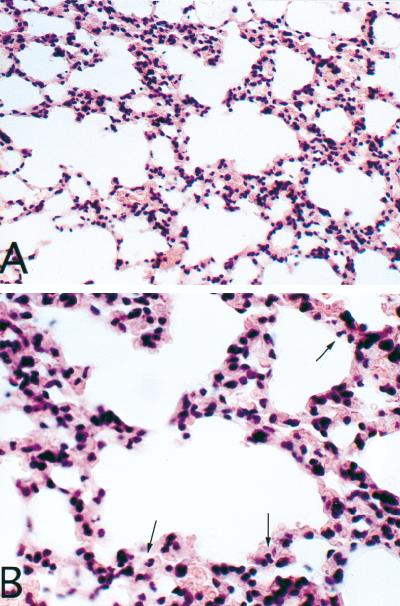
Lung tissue taken from mice infected 24 h earlier with NU-2 displayed cryptococcal cells associated with a mixed inflammatory response consisting of neutrophils and mononuclear cells (Fig. 2A). In the lungs from 184A-infected mice no cryptococci could be seen but mixed inflammatory infiltrates were evident (Fig. 2C). A direct comparison of an area of inflammation adjacent to tissue without inflammatory cells is seen in Fig. 3A. The identification of polymorphonuclear leukocytes at 24 h in NU-2-infected lungs can be seen more clearly at a higher magnification (Fig. 3B). The lungs of experimental mice were examined at several different levels, and in both the NU-2- and 184A-infected groups, patchy inflammatory reactions were distributed throughout the lung in focally cellular areas. Mucicarmine-stained sections of NU-2-infected lungs revealed many cryptococci with large capsules (Fig. 2B), whereas similarly stained sections of 184A-infected lungs showed no evidence of the organism (Fig. 2D). The findings in the histological sections corroborated the observations of disparate cryptococcal CFU numbers isolated from lungs between 0 and 24 h after infection with NU-2 or 184A.
FIG. 2.
Lung sections taken from mice 24 h after infection with C. neoformans NU-2 (A and B) or 184A (C and D). Sections were stained with hematoxylin and eosin (A and C) or mucicarmine (B and D). There were three mice in each experimental group. Photomicrographs are from one representative animal from each group. Encapsulated cryptococcal cells are indicated by the arrows. Magnification, ×200 (A and C) or ×400 (B and D).
FIG. 3.
Lung section taken 24 h after infection with C. neoformans NU-2. Shown in both panels is normal lung tissue (lower left) adjacent to patchy inflammed tissue (central and lower right). Magnification, ×200 (A) or ×400 (B), with polymorphonuclear leukocytes indicated by the arrows.
The hematoxylin and eosin-stained sections of lungs from infected mice taken over the first 24 h of infection were graded in a blind fashion for the intensity of the inflammatory response. In addition, each lung was assessed for the percentage of the lung tissue in which inflammation could be observed. The intensity of the inflammatory response was higher during the first 3 h of infection when the mice were infected with the 184A isolate (Table 1). In addition, the percentage of lung tissue in which inflammation was present was statistically higher in the 184A-infected lungs when assessed after 1 h of infection (P = 0.0079) than in NU-2-infected lungs at 1 h (Table 1). The percent involvement of the inflammatory response in the 184A-infected lungs was significantly lower by 3 h of infection (P = 0.0144) than in 184A-infected lungs at 1 h, while the inflammation of NU-2-infected lungs appeared to increase in intensity with time. When the percentage of lung involvement was compared at 1, 3, 12, and 24 h in the NU-2-infected mice, the differences were not significant from one time period to the next. The inability to obtain statistical differences could be due to the small numbers of mice in each group. However, the percent involvement of NU-2-infected lungs pooled from the early (1 and 3 h) time points (11.8% ± 2.02%) and the percent involvement of NU-2-infected lungs from the later (12 and 24 h) time points (36.2% ± 9.3%) were significantly different (P = 0.0079).
TABLE 1.
Inflammation in mouse lungs after infection with C. neoformans NU-2 or 184A
| Time (h) after infection | Average intensitya for: | % Lung involvementb (SEM) for: | ||
|---|---|---|---|---|
| NU-2 | 184A | NU-2 | 184A | |
| 1 | 1.0 | 1.7 | 14.5 (4.5) | 72.0 (6.5)c |
| 3 | 1.0 | 1.3 | 10.0 (1.5) | 23.7 (9.7)d |
| 12 | 1.3 | 1.6 | 40.7 (17.4) | 21.7 (1.5) |
| 24 | 1.7 | 2.0 | 31.7 (10.7) | 32.7 (8.7) |
Serum GXM levels in mice infected with cryptococcal isolates NU-2 and 184A.
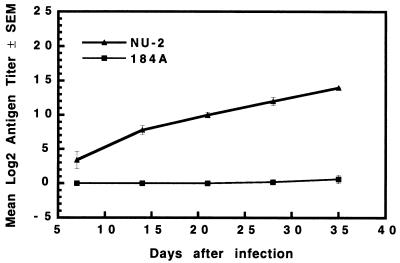
GXM levels in the sera of mice infected with NU-2 or 184A were evaluated weekly over the first 5 weeks of infection. Elevated GXM levels were found in NU-2-infected mice as early as 7 days after infection, and these levels continued to rise over the 5-week observation period (Fig. 4). GXM could not be detected in 184A-infected mice at any time during the 5-week observation period.
FIG. 4.
Serum GXM concentrations in mice infected with NU-2 and 184A. Data presented are means ± standard errors of the means (SEM) (error bars) for three to five individual mice tested at each time period.
DTH responses in mice infected with cryptococcal isolates NU-2 or 184A.
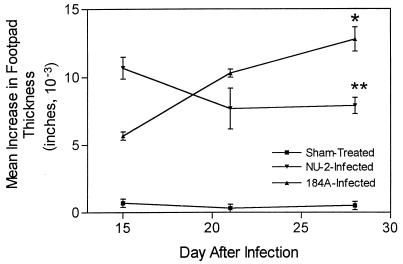
Mice were assayed for their abilities to respond to cryptococcal culture filtrate (CneF) antigen with a DTH response at 15, 21, and 28 or 29 days after infection. The results are shown in Fig. 5. NU-2-infected mice developed a strong DTH reaction early in the infection (day 15), whereas 184A-infected mice showed peak DTH reactivity later (day 28 to 29). By the 21st day of infection with NU-2, the DTH response was waning. On the other hand, DTH responsiveness in the 184A-infected mice increased throughout the entire observation period. In addition, the DTH response of 184A-infected animals at the day 28 to 29 was significantly higher than the DTH response of NU-2-infected mice tested at this same time period (P = 0.0003).
FIG. 5.
DTH footpad reaction to cryptococcal CneF antigen in CBA/J mice infected with C. neoformans NU-2 or 184A. Statistically significant (P = 0.0003) data compared to the DTH response of NU-2-infected mice at 28 to 29 days of infection (∗) and statistically significant (P = 0.02) data compared to the response of NU-2-infected mice at day 15 of infection (∗∗) are indicated. The data shown are combined from two separate experiments and represent the mean increases in paw thickness ± standard errors of the means (error bars) for three to nine animals/time period.
Cytokine responses in mice infected with cryptococcal isolates NU-2 and 184A.
Cytokines associated with Th1 (IL-2 and IFN-γ) cells, Th2 (IL-4) cells, and the regulatory cytokine IL-10 were examined in NU-2- and 184A-infected mice over the first 27 to 30 days of infection. CBA/J mice were infected intratracheally, and their spleen cells were removed at various times after infection. Spleen cells were cultured in tissue culture medium alone or in a medium containing CneF for 24 h (IL-2 analysis) or 48 h (all other cytokines). Supernatant fluids were collected, and the concentration of each cytokine was assessed by an enzyme-linked immunosorbent assay specific for each cytokine. Spleen cells from age-matched, uninfected, sham-treated CBA/J mice were cultured under identical conditions to obtain information regarding the response of normal spleen cells to the cryptococcal antigen CneF. CneF did not induce the production of IL-2, IL-4, or IL-10 by normal spleen cells. However, CneF did stimulate normal spleen cells to produce low levels of IFN-γ. ConA controls were also included (data not shown) which provided evidence that the cells remained viable under our tissue culture conditions and that there were cells present in the spleens that were capable of secreting each of the cytokines studied.
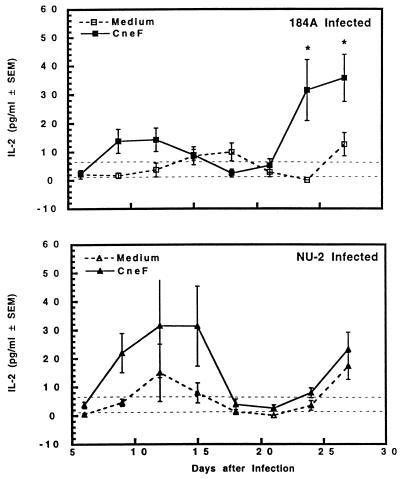
Constitutively produced levels of IL-2 by spleen cells from mice infected with either isolate were not significantly higher than levels made by spleen cells of sham-treated controls. CneF-stimulated spleen cells removed early (day 9 to 15) after infection with NU-2 produced larger amounts of IL-2 than did similarly treated spleen cell cultures from 184A-infected mice (Fig. 6). While the difference between the groups was not statistically significant due to large variation between individual mice, this observation was consistently observed in repeat experiments. Between days 18 and 21, spleen cells from NU-2- or 184A-infected mice did not produce IL-2 when stimulated with CneF. A second cycle of IL-2 production was found with CneF-stimulated spleen cells from 184A-infected mice starting at 24 days of infection. The levels of IL-2 produced by CneF-stimulated spleen cells from 184A-infected mice were significantly (P = 0.04) higher than the levels detected in supernatant fluids from CneF-stimulated spleen cells from NU-2-infected mice at the 24- and 27-day time periods.
FIG. 6.
IL-2 secreted by spleen cells taken from CBA/J mice at various times after infection with C. neoformans NU-2 or 184A. Spleen cells were cultured with or without the addition of cryptococcal antigen CneF, and supernatants were harvested 24 h after the initiation of culture. There was no difference in IL-2 levels between the medium alone and CneF-stimulated cultures when spleen cells were harvested from sham-treated mice; therefore, the data shown as dashed horizontal lines represent the variation (± standard errors of the means [SEM]) of the cytokine response of the sham-treated controls to medium alone and to CneF. ∗, statistically significant (P = 0.04) compared to IL-2 secretion from spleen cells of NU-2-infected mice at day 24 or 27 of infection. Vertical error bars represent the variation (±SEM). There were three to four animals in each experimental group, and the experiment was repeated once.
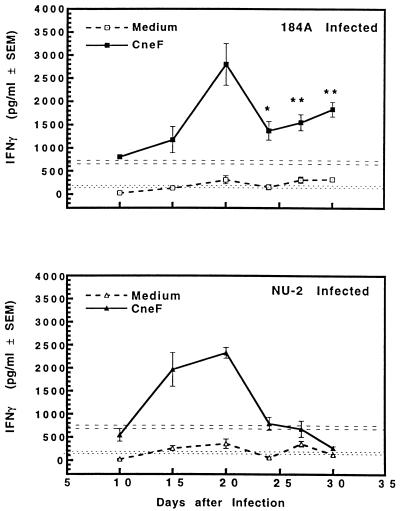
CneF-induced IFN-γ responses of spleen cells from animals infected with either cryptococcal isolate were at a relatively high level by the 15th or 20th day of infection (Fig. 7). At day 15, CneF-stimulated spleen cells from NU-2-infected mice repeatedly produced more IFN-γ than similarly stimulated spleen cells from the 184A-infected animals, but the difference was not statistically significant. The ability of spleen cells to produce IFN-γ after stimulation with CneF continued to increase in both groups of infected mice until day 20. IFN-γ production decreased in both groups at day 24 of infection. Despite this diminution, the levels of IFN-γ in supernatant fluids from CneF-stimulated spleen cells from 184A-infected animals at day 24 and thereafter were still quite substantial in magnitude and were significantly greater than those detected in supernatant fluids from CneF-stimulated spleen cells from mice infected with NU-2 (Fig. 7 [P = 0.049 at day 24; P = 0.0002 at days 27 and 30).
FIG. 7.
IFN-γ secreted by spleen cells taken from CBA/J mice at various times after infection with C. neoformans NU-2 or 184A. Spleen cells were cultured with or without the addition of cryptococcal antigen CneF, and supernatants were harvested 48 h after the initiation of culture. There was a difference in the IFN-γ level between the medium alone and CneF-stimulated cultures when spleen cells were harvested from sham-treated mice; therefore, the data shown for the medium response (± standard errors of the means [SEM]) (error bars) are presented as the horizontal dotted lines, and the response to CneF (±SEM) is represented as horizontal dashed lines. Statistically significant (P = 0.049) data compared to IFN-γ secretion by spleen cells from NU-2-infected mice at day 24 after infection (∗) and statistically significant (P < 0.0002) data compared to the secretion of IFN-γ by spleen cells of NU-2-infected mice at day 27 or 30 after infection (∗∗) are indicated. Vertical error bars represent the variation (±SEM). There were five to six animals/experimental group, and the experiment was repeated once.
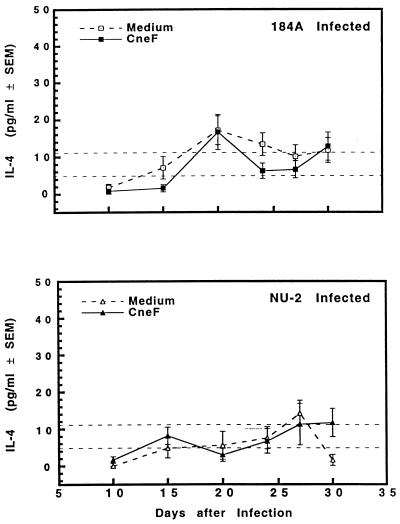
IL-4 responses of spleen cells taken from mice which were infected with C. neoformans 184A or NU-2 were not statistically different from responses of spleen cells from sham-treated mice either after no stimulation or in response to cryptococcal CneF antigen (Fig. 8). For this reason we cannot establish a role for IL-4 as a factor in the immunosuppressive consequences of infection with cryptococcal isolate NU-2.
FIG. 8.
IL-4 secreted by spleen cells taken from CBA/J mice at various times after infection with C. neoformans NU-2 or 184A. Spleens were cultured with or without the addition of cryptococcal antigen CneF, and supernatants were harvested 48 h after the initiation of culture. There was no difference in IL-4 levels between the medium alone and CneF-stimulated cultures when spleen cells were harvested from sham-treated mice; therefore, the data shown as dashed horizontal lines represent the variation (± standard errors of the means [SEM]) of the cytokine response of the sham-treated controls to medium alone and to CneF. Vertical error bars represent the variation (±SEM). There were five to six animals/experimental group, and the experiment was repeated once.
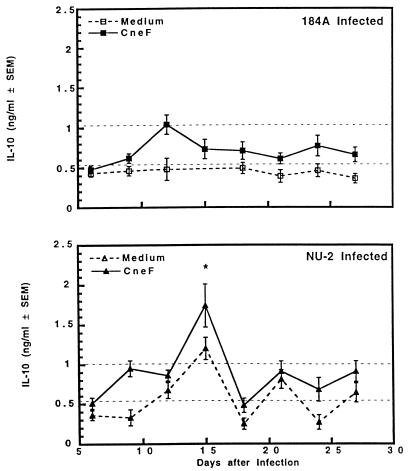
IL-10 production at levels above those from sham-treated mouse spleen cells was observed only in supernatant fluids from CneF-stimulated spleen cells from NU-2-infected mice (Fig. 9). The CneF-stimulated spleen cells from NU-2-infected animals exhibited significantly elevated (P = 0.04) levels of IL-10 early (day 15) in infection compared to those from 184A-infected mice. After the 15th day of NU-2 infection, IL-10 was not produced after the stimulation of spleen cells with CneF and was not present in significantly higher quantities than could be detected in cultures of spleen cells of sham-treated mice. The level of constitutive secretion of IL-10 by spleen cells of 184A-infected mice was no higher than constitutive levels of IL-10 found in culture supernatants of spleen cells from sham-treated mice. In addition, CneF did not stimulate IL-10 secretion from spleen cells from 184A-infected mice (Fig. 9).
FIG. 9.
IL-10 secreted by spleen cells taken from CBA/J mice at various times after infection with C. neoformans NU-2 or 184A. Spleen cells were cultured with or without the addition of cryptococcal antigen CneF, and supernatants were harvested 48 h after the initiation of culture. There was no difference in IL-10 levels between the medium alone and CneF-stimulated cultures when spleen cells were harvested from sham-treated mice; therefore, the data shown as dashed horizontal lines represent the variation (± standard errors of the means [SEM]) of the cytokine response of the sham-treated controls to medium alone and to CneF. Statistically significant (P = 0.04) data compared to IL-10 secretion from spleen cells of 184A-infected mice at day 15 after infection (∗) are indicated. Vertical error bars represent the variation (±SEM). There were three to four animals in each experimental group, and the experiment was repeated once.
Survival of normal and IL-10 knockout mice infected with C. neoformans NU-2.
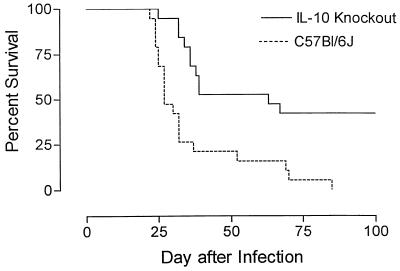
Because significant elevations in IL-10 secretion levels by CneF-stimulated spleen cells from NU-2-infected mice were observed, we assessed how IL-10 may relate to virulence. Survival studies were done in NU-2-infected normal mice (C57BL/6J) and C57BL/6J mice containing a targeted disruption of the IL-10 gene (IL-10 knockout mice). The survival curves are shown in Fig. 10. The mean survival time of C57BL/6 mice was 36.4 ± 4.24 days, whereas the IL-10 knockout mice survived significantly (P = 0.0002) longer with a mean survival time of greater than 65.3 ± 7.1 days. All of the C57BL/6 mice had expired by the 85th day of infection, and the experiment was terminated at the 100th day with 8 of the 19 NU-2-infected IL-10 knockout mice still surviving.
FIG. 10.
Survival of C57BL/6J or IL-10 knockout mice infected by the intratracheal route with 105 C. neoformans NU-2 organisms. The survival rate of IL-10 knockout mice was significantly higher (P = 0.0002) than that of C57BL/6J mice. Data represent the survival rates for 19 animals/mouse strain.
DISCUSSION
Previous reports from our laboratories showed that cryptococcal isolates which vary in their virulence for mice can be differentiated by their abilities to up-regulate the amount of capsule produced when grown under tissue culture conditions (4). In addition, the increased expression of capsule on the highly virulent isolate is responsible, in part, for the induction of increased secretion of the third component of complement by normal peritoneal macrophages (4). Differences in survival rates for NU-2-infected mice and 184A-infected mice and the induction of secretion of this inflammatory mediator by NU-2-stimulated macrophages suggest that the amount of capsule expressed in vivo by the cryptococcal isolates might contribute to differences in the early inflammatory responses elicited and subsequently to the type of immune response that develops during infection.
Analysis of the clearance of cryptococcal cells from the lungs of mice infected with highly and weakly virulent isolates revealed that the weakly virulent isolate was cleared rapidly, whereas the highly virulent isolate remained in the lungs in stable numbers over the first 24 h after infection. At the time of infection, the capsules of NU-2 and 184A were similar in size (data not shown). This observation is in agreement with our previous findings that capsule size is similar for these two isolates when they are grown on Sabouraud medium (4). Histological analysis indicated that NU-2 up-regulated capsule synthesis within 24 h of infection. This suggestion was corroborated by our finding that soluble capsular polysaccharide was found in the sera from NU-2-infected mice as early as 7 days after infection. The poor clearance of NU-2 from the lungs then may be due to the fact that NU-2 develops a large capsule that is protective against phagocytosis (6, 24).
The weakly virulent isolate, 184A, remained in the lungs less than 24 h after infection, which was such a short period, and the numbers of organisms were so low, that we were unable to assess the capsule size in lung tissue. However, it might be assumed from data derived from in vitro tissue culture studies and the lack of serum cryptococcal antigen titers in 184A-infected mice that 184A did not up-regulate its capsule synthesis to a significant degree in the lungs. Our inability to find 184A cells in the lungs beyond 24 h is in accord with an earlier report (27), in which 184A could not be cultured from the lungs of mice infected intranasally. In that earlier report, 95% of the mice developed DTH responses and viable cryptococci were cultured from extrapulmonary tissues of at least 50% of the mice at some time after infection. In the present study, 100% of the 184A-infected mice had positive anticryptococcal DTH reactions by day 21 or 28 of infection. The combined data demonstrate that 184A-infected animals were indeed infected and that 184A is not completely cleared from the body after infection via the lungs even though the lungs appear sterile.
Histological analysis of infected lungs also provided important information regarding the early inflammatory response to these two cryptococcal isolates. While an acute inflammatory response was elicited after infection with both cryptococcal strains, the weakly virulent isolate (184A) elicited a response that tended to be more intense during the first 3 h of infection. In addition, more of the lung was involved early after 184A was introduced into the lungs than in lungs from NU-2-infected mice. At 12 and 24 h after infection, the inflammatory response was diminished in mice infected with 184A. On the other hand, the intensity of the inflammatory response and the percent lung involvement increased with time over the first 12 h when mice were infected with the highly virulent isolate NU-2.
Associations of protection against C. neoformans with an anticryptococcal CMI response and a Th1 cell response, i.e., the production of IL-2 and IFN-γ, have been made by several groups of investigators (1, 9, 15, 19, 21–23, 27, 37). To determine how the level of CMI responsiveness related to the progression of cryptococcosis, we followed the anticryptococcal CMI responsiveness as determined by the level of anticryptococcal DTH reactivity and the cytokine profiles in response to cryptococcal antigen. Results in both DTH and Th1 cytokine production assays indicated that mice infected with the highly virulent or weakly virulent isolate developed anticryptococcal CMI responsiveness. After the initial anticryptococcal immune responsiveness (DTH and Th1 cytokine production) was observed, mice infected with the highly virulent isolate were unable to sustain during the later stages of disease either the anticryptococcal DTH response or the ability to produce Th1 cytokines. In contrast, mice infected with the weakly virulent isolate were delayed in the development of an anticryptococcal DTH response but retained their ability to display the anticryptococcal DTH responsiveness, and their spleen cells retained the ability to react to cryptococcal antigen with the production of IFN-γ. IFN-γ has been shown to be protective against C. neoformans (14, 20, 29), so it is reasonable to predict that the inability of the NU-2-infected mice to produce IFN-γ would leave the animals vulnerable to progressive infection with C. neoformans.
A cryptococcus-specific Th2 response did not appear to be induced, because the level of the Th2 cell-associated lymphokine IL-4 was not significantly elevated above background levels at any time after infection with either the highly virulent or the weakly virulent isolate. From these findings one might conclude that Th2 cells, if induced, are induced at low or undetectable levels. Consequently, one would not expect Th2 cells and their cytokines to be responsible for the observed inability of spleen cells from NU-2-infected mice to make Th1 cytokines when stimulated with cryptococcal antigen. Our conclusion that Th2 cells or their cytokines are not responsible for the diminution of Th1 cell-associated cytokines and the lack of protection in the NU-2-infected mice is in agreement with that of Hoag and coworkers (16), who reported that the susceptibility of C57BL/6 mice to C. neoformans infection cannot be clearly attributed to the induction of Th2 cells.
Because of the inability of spleen cells from NU-2-infected mice to produce any cytokines in response to cryptococcal antigen during the later part of the disease, we postulate that the NU-2-infected mice developed anergy. Functionally impaired CD4+ T cells have been shown to be induced and to persist for prolonged periods in vivo in mice made tolerant to ovalbumin by injecting the antigen intravenously (33). In the ovalbumin model, there is an early transient responsiveness of T cells followed by the lack of an ability to make cytokines, and this unresponsiveness is independent of Th2 cells (33). The immune response in the NU-2-infected mice follows a pattern similar to that seen in the ovalbumin model, in which tolerance is induced.
IL-10 is a cytokine that is made primarily by macrophages and B-lymphocytes (17) and is known to down-regulate the Th1 response (13). IL-10 was transiently produced by spleen cells from NU-2-infected mice but not 184A-infected mice when stimulated with cryptococcal antigen, so it is possible that IL-10 either alone or in conjunction with other cytokines is responsible for down-regulating the Th1 response in NU-2-infected mice. IL-10 is known to contribute to immunosuppression in other infectious diseases (3), and the expression of IL-10 in human immunodeficiency virus-positive individuals correlates with the early loss of Th function (7), so there is some precedent for this speculation. It is possible that IL-10 does contribute to the unresponsiveness that subsequently is detected in NU-2-infected mice due to its ability to deactivate macrophages and other antigen-presenting cells (APC) (10). IL-10 regulates the production or action of IL-12 (8), IL-18 (35), and tumor necrosis factor alpha (34) by macrophages, thereby inhibiting the production of cytokines that are known to be important for the induction of protective immunity in cryptococcosis (19). If APC function is eliminated by this mechanism, then subsequent T-cell activation by cryptococcal antigen may be negated, thereby preventing the further production of IL-2 and IFN-γ by activated T cells. The effects on APC function may favor the induction of unresponsiveness in both the Th1 cells and Th2 cells that are specific for cryptococcal antigen. IL-10 has been reported to be involved in the induction of anergy of T cells during specific immunotherapy for allergic disease (2) and to enhance the induction of tolerance to the contact allergen 2,4,6-trinitrochlorobenzene (12).
We have not yet studied the cellular subpopulation that may be responsible for secreting IL-10 during cryptococcal infection. Because it is known that the cryptococcal capsular polysaccharide can cause human monocytes to secrete IL-10 (36), it is possible that macrophages and not lymphocytes are responsible for the production of IL-10 in NU-2-infected mice. Enhanced IL-10 secretion in response to NU-2 may then reflect the increase in capsule production that is detected on the NU-2 cell. While the GXM antigen that is in our CneF preparation would also be expected to stimulate the secretion of IL-10 from macrophages of normal mice and 184A-infected mice, we cannot exclude the possibility that the macrophages in the spleens of NU-2-infected mice secrete more IL-10 in response to GXM, due to a difference in their states of activation.
By introducing an infection with NU-2 via the pulmonary route in mice that were unable to produce IL-10 we were able to determine whether IL-10 displayed a negative regulatory activity on the protective anticryptococcal immune response. IL-10-deficient mice survived significantly longer than wild-type mice, demonstrating that mice without IL-10 were better able to control the C. neoformans infection with the highly virulent isolate than mice with the potential to produce IL-10 in response to C. neoformans infection. This observation, combined with the observation that IL-10 was made by spleen cells in response to cryptococcal antigen at a time in infection prior to the loss of the animals’ ability to mount an anticryptococcal DTH reaction and make IFN-γ, supports the concept that IL-10 plays some role in the anergy observed and the reduced ability of the mice to control the C. neoformans infection.
ACKNOWLEDGMENTS
We thank Fredda Schafer and Anny Alsup for their excellent technical assistance.
This work was supported by Public Health Service grants AI-15716 (J.W.M.), AI18895 (J.W.M.), HL-59852 (J.W.M.), and AI43325 (R.B.) and by the Presbyterian Health Foundation (R.B.). K.L.B. is a Burroughs Wellcome Fund New Investigator in Molecular Pathogenic Mycology.
REFERENCES
- 1.Aguirre K, Havell D A, Gibson G W, Johnson L L. Role of tumor necrosis factor and gamma interferon in acquired resistance to Cryptococcus neoformans in the central nervous system of mice. Infect Immun. 1995;63:1725–1731. doi: 10.1128/iai.63.5.1725-1731.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akdis C A B T, Akdis M, Wuthrich B, Blaser K. Role of interleukin 10 in specific immunotherapy. J Clin Investig. 1998;102:98–106. doi: 10.1172/JCI2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bermudez L E, Champsi J. Infection with Mycobacterium avium induces production of interleukin-10 (IL-10), and administration of anti-IL-10 antibody is associated with enhanced resistance to infection in mice. Infect Immun. 1993;61:3093–3097. doi: 10.1128/iai.61.7.3093-3097.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blackstock R, Murphy J W. Secretion of the C3 component of complement by peritoneal cells cultured with encapsulated Cryptococcus neoformans. Infect Immun. 1997;65:4114–4121. doi: 10.1128/iai.65.10.4114-4121.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buchanan K L, Murphy J W. Characterization of cellular infiltrates and cytokine production during the expression phase of the anticryptococcal delayed-type hypersensitivity response. Infect Immun. 1993;61:2854–2865. doi: 10.1128/iai.61.7.2854-2865.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bulmer G S, Sans M D. Cryptococcus neoformans. III. Inhibition of phagocytosis. J Bacteriol. 1968;95:5–8. doi: 10.1128/jb.95.1.5-8.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clerici M, Wynn T A, Berzofsky J A, Blatt S P, Hendrix C W, Sher A, Coffman R L, Shearer G M. Role of interleukin-10 in T helper cell dysfunction in asymptomatic individuals infected with human immunodeficiency virus. J Clin Investig. 1994;93:768–774. doi: 10.1172/JCI117031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dandrea A, Asteamezaga M, Valiante N M, Ma X J, Kubin M, Trinchieri G. Interleukin-10 (IL-10) inhibits human lymphocyte interferon gamma-production by suppressing natural killer cell stimulatory factor/IL-12 synthesis in accessory cells. J Exp Med. 1993;178:1041–1048. doi: 10.1084/jem.178.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Decken K, Köhler G, Palmer-Lehmann K, Wunderlin A, Mattner F, Magram J, Gately M K, Alber G. Interleukin-12 is essential for a protective Th1 response in mice infected with Cryptococcus neoformans. Infect Immun. 1998;66:4994–5000. doi: 10.1128/iai.66.10.4994-5000.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ding L, Linsley P S, Huang L Y, Germain R N, Shevach E M. IL-10 inhibits macrophage costimulatory activity by selectively inhibiting the up-regulation of B7 expression. J Immunol. 1993;151:1224–1234. [PubMed] [Google Scholar]
- 11.Dubois M, Gilles K A, Hamilton J K, Rebers P A, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28:350–356. [Google Scholar]
- 12.Enk A H, Saloga J, Becker D, Mohamadzadeh M, Knop J. Induction of hapten-specific tolerance by interleukin-10 in vivo. J Exp Med. 1994;179:1397–1402. doi: 10.1084/jem.179.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fiorentino D F, Bond M W, Mosemann T R. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989;170:2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flesch I E A, Schwamberger G, Kaufmann S H E. Fungicidal activity of IFN-gamma-activated macrophages—extracellular killing of Cryptococcus neoformans. J Immunol. 1989;142:3219–3224. [PubMed] [Google Scholar]
- 15.Hoag K A, Lipscomb M F, Izzo A A, Street N E. IL-12 and IFN-γ are required for initiating the protective Th1 response to pulmonary cryptococcosis in resistant C.B-17 mice. Am J Respir Cell Mol Biol. 1997;17:733–739. doi: 10.1165/ajrcmb.17.6.2879. [DOI] [PubMed] [Google Scholar]
- 16.Hoag K A, Street N E, Huffnagle G B, Lipscomb M F. Early cytokine production in pulmonary Cryptococcus neoformans infections distinguishes susceptible and resistant mice. Am J Respir Cell Mol Biol. 1995;13:487–495. doi: 10.1165/ajrcmb.13.4.7546779. [DOI] [PubMed] [Google Scholar]
- 17.Howard M, Ogarra A. Biological properties of interleukin-10. Immunol Today. 1992;13:198–200. doi: 10.1016/0167-5699(92)90153-X. [DOI] [PubMed] [Google Scholar]
- 18.Huffnagle G B, Lipscomb M F, Lovchik J A, Hoag K A, Street N E. The role of CD4(+) and CD8(+) T cells in the protective inflammatory response to a pulmonary cryptococcal infection. J Leukoc Biol. 1994;55:35–42. doi: 10.1002/jlb.55.1.35. [DOI] [PubMed] [Google Scholar]
- 19.Huffnagle G B, Toews G B, Burdick M D, Boyd M B, McAllister K S, McDonald R A, Kunkle S A, Strieter R M. Afferent phase production of TNF-α is required for the development of protective T cell immunity to Cryptococcus neoformans. J Immunol. 1996;157:4529–4536. [PubMed] [Google Scholar]
- 20.Kawakami K, Kohno S, Kaddota J-I, Tohyama M, Teruya K, Kudeken N, Saito A, Hara K. T cell-dependent activation of macrophages and enhancement of their phagocytic activity in the lungs of mice inoculated with heat-killed Cryptococcus neoformans: involvement of IFN-γ and its protective effect against cryptococcal infection. Microbiol Immunol. 1995;39:135–143. doi: 10.1111/j.1348-0421.1995.tb02180.x. [DOI] [PubMed] [Google Scholar]
- 21.Kawakami K, Tohyama M, Qifeng X, Saito A. Expression of cytokines and inducible nitric oxide synthase mRNA in the lungs of mice infected with Cryptococcus neoformans: effects of interleukin-12. Infect Immun. 1997;65:1307–1312. doi: 10.1128/iai.65.4.1307-1312.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawakami K, Tohyama M, Zie O, Saito A. IL-12 protects mice against pulmonary and disseminated infection caused by Cryptococcus neoformans. Clin Exp Immunol. 1996;104:208–214. doi: 10.1046/j.1365-2249.1996.14723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kawakami K, Wifeng X, Tohyama M, Qureshi M H, Saito A. Contribution of tumour necrosis factor-alpha (TNF-α) in host defense mechanism against Cryptococcus neoformans. Clin Exp Immunol. 1996;106:468–474. doi: 10.1046/j.1365-2249.1996.d01-870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kozel T R, Gotschlich E C. The capsule of Cryptococcus neoformans passively inhibits phagocytosis of the yeast by macrophages. J Immunol. 1982;129:1675–1680. [PubMed] [Google Scholar]
- 25.Kwon-Chung K J, Bennett J E. Medical mycology. Philadelphia, Pa: Lea & Febiger; 1992. Appendix B; pp. 821–822. [Google Scholar]
- 26.Kwon-Chung K J, Bennett J E. Medical mycology. Philadelphia, Pa: Lea & Febiger; 1992. Cryptococcosis; pp. 398–399. [Google Scholar]
- 27.Lim T S, Murphy J W, Cauley L K. Host-etiological agent interactions in intranasally and intraperitoneally induced cryptococcosis in mice. Infect Immun. 1980;29:633–641. doi: 10.1128/iai.29.2.633-641.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mitchell T G, Perfect J R. Cryptococcosis in the era of AIDS—100 years after the discovery of Cryptococcus neoformans. Clin Microbiol Rev. 1995;8:515–548. doi: 10.1128/cmr.8.4.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mody C H, Tyler C L, Sitrin R G, Jackson C, Toews G B. Interferon-gamma activates rat alveolar macrophages for anticryptococcal activity. Am J Respir Cell Mol Biol. 1991;5:19–26. doi: 10.1165/ajrcmb/5.1.19. [DOI] [PubMed] [Google Scholar]
- 30.Murphy J W. Cytokine profiles associated with induction of the anticryptococcal cell-mediated immune response. Infect Immun. 1993;61:4750–4759. doi: 10.1128/iai.61.11.4750-4759.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murphy J W. Cell-mediated immunity. In: Miller J D, Howard D H, editors. The mycota. VII. Animal and human relationships. New York, N.Y: Springer-Verlag; 1996. pp. 67–97. [Google Scholar]
- 32.Nowak T P, Barondes S H. Agglutinin from Limulus polyphemus. Purification with formalinized horse erythrocytes as the affinity adsorbent. Biochim Biophys Acta. 1975;393:115–123. [PubMed] [Google Scholar]
- 33.Pape K A, Merica R, Mondino A, Khoruts A, Jenkins M K. Direct evidence that functionally impaired CD4+ T cells persist in vivo following induction of peripheral tolerance. J Immunol. 1998;160:4719–4729. [PubMed] [Google Scholar]
- 34.Parry S L, Sebbag M, Feldmann M, Brennan F M. Contact with T cells modulates monocyte IL-10 production. Role of T cell membrane TNF-α. J Immunol. 1997;158:3673–3681. [PubMed] [Google Scholar]
- 35.Puren A J, Fantuzzi G, Gu Y, Su M S, Ginarello C A. Interleukin-18 (IFNγ-inducing factor) induces IL-8 and IL-1β via TNFα production from non-CD14+ human blood mononuclear cells. J Clin Investig. 1998;101:711–721. doi: 10.1172/JCI1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vecchiarelli A, Retini C, Monari C, Tascini C, Bistoni F, Kozel T R. Purified capsular polysaccharide of Cryptococcus neoformans induces interleukin-10 secretion by human monocytes. Infect Immun. 1996;64:2846–2849. doi: 10.1128/iai.64.7.2846-2849.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yuan R R, Casadevall A, Oh J, Scharff M D. T cells cooperate with passive antibody to modify Cryptococcus neoformans infection in mice. Proc Natl Acad Sci USA. 1997;94:2483–2488. doi: 10.1073/pnas.94.6.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]