Hypophosphatemia leads to rickets by impairing caspase-mediated apoptosis of hypertrophic chondrocytes (original) (raw)
Abstract
Rickets is seen in association with vitamin D deficiency and in several genetic disorders associated with abnormal mineral ion homeostasis. Studies in vitamin D receptor (VDR)-null mice have demonstrated that expansion of the late hypertrophic chondrocyte layer, characteristic of rickets, is secondary to impaired apoptosis of these cells. The observation that normalization of mineral ion homeostasis in the VDR-null mice prevents rachitic changes suggests that rickets is secondary to hypocalcemia, hypophosphatemia, or hyperparathyroidism, rather than impaired VDR action. To determine which of these abnormalities is responsible for impaired chondrocyte apoptosis and subsequent rachitic changes, two additional models were examined: diet-induced hypophosphatemia/hypercalcemia and hypophosphatemia secondary to mutations in the Phex gene. The former model is associated with suppressed parathyroid hormone levels as a consequence of hypercalcemia. The latter model demonstrates normal calcium and parathyroid hormone levels, but 1,25-dihydroxyvitamin D levels that are inappropriately low for the degree of hypophosphatemia. Our studies demonstrate that normal phosphorus levels are required for growth plate maturation and implicate a critical role for phosphate-regulated apoptosis of hypertrophic chondrocytes via activation of the caspase-9-mediated mitochondrial pathway.
Keywords: Phex gene, vitamin D receptor
Rickets is a classical feature of vitamin D deficiency in growing animals and humans. Rachitic changes in the growth plate are seen when the actions of 1,25-dihydroxyvitamin D [1,25(OH)2D] are impaired as a consequence of dietary vitamin D deficiency, impaired activation of vitamin D because of mutations in the 25-hydroxyvitamin D 1-α-hydroxylase gene (pseudovitamin D deficiency rickets) (1) or when the nuclear actions of 1,25(OH)2D are impaired because of mutations in the vitamin D receptor (VDR) (hereditary vitamin D-resistant rickets), suggesting that the actions of 1,25(OH)2D are critical for the maintenance of a normal growth plate (2). Rachitic growth plates also are associated with inherited mutations that impair the actions of the Phex gene (X-linked hypophosphatemic rickets) (3, 4) and the metabolism of FGF-23 (autosomal dominant hypophosphatemic rickets) (5). Both of these disorders are associated with reduced 25-hydroxyvitamin D 1-α-hydroxylase activity, leading to a reduction in 1,25(OH)2D synthesis. It was, therefore, unclear whether the pathophysiologic abnormality leading to rachitic growth plates in the hereditary hypophosphatemias is similar to that underlying rickets due to VDR deficiency and whether these rachitic changes are a direct consequence of impaired 1,25(OH)2D action.
Targeted ablation of the VDR in mice is a phenocopy of the human disease hereditary vitamin D resistant rickets (6). These mice are born metabolically normal, but as a consequence of impaired 1,25(OH)2D-dependent intestinal calcium absorption, they develop hypocalcemia and secondary hyperparathyroidism, the latter of which leads to an increase in urinary phosphate excretion resulting in hypophosphatemia. The VDR-null mice develop rickets and osteomalacia by the fourth week of life. Investigations addressing the cellular basis for these rachitic changes demonstrate that the growth-plate abnormality in the VDR-null mice is due to an expansion of the late hypertrophic chondrocyte layer, a consequence of impaired apoptosis of these cells. Chondrocyte proliferation and acquisition of markers of chondrocyte differentiation as proliferative chondrocytes mature are unaffected by VDR ablation, as is signaling for vascular invasion assessed by VEGF mRNA expression (7). Of note, prevention of abnormal mineral ion homeostasis by institution of a diet high in calcium, phosphorus, and lactose by 18 days of age prevents the development of hyperparathyroidism, rickets, and osteomalacia in the VDR-null mice (8). This phenotype suggests that rickets is not a direct consequence of impaired VDR action, but rather, is due to the resultant hypocalcemia, hypophosphatemia, or hyperparathyroidism.
To clarify which of these three metabolic abnormalities is the pathophysiologic basis for rickets, studies were performed in two additional murine models: mice with hypophosphatemia secondary to mutations in the Phex gene (Hyp mouse) (3), associated with normal calcium and parathyroid hormone (PTH) levels; and mice with diet-induced hypophosphatemia/hypercalcemia in which PTH levels are suppressed. These studies demonstrate that impaired apoptosis of terminal hypertrophic chondrocytes secondary to hypophosphatemia is the common mediator of rickets in the three models investigated. In vitro analyses in primary murine chondrocytes demonstrate that phosphate mediates hypertrophic chondrocyte apoptosis by activating the caspase-9-dependent mitochondrial pathway. Studies in mice treated with a caspase-9 inhibitor confirm the critical importance of the mitochondrial pathway in growth plate maturation in vivo.
Materials and Methods
Animals. All studies performed were approved by the institutional animal care committee. Mice were maintained in a virus- and parasite-free barrier facility and exposed to a 12-h light/dark cycle. All mice in this study have a C57BL/6J background. The mice were weaned at 18 days of age onto a standard diet containing 1% calcium, 0% lactose, and 0.44% phosphorus. To normalize the blood mineral ion levels of the VDR-knockout mice, the animals were fed a γ-irradiated test diet (TD96348, Teklad, Madison, WI) containing 2% calcium, 1.25% phosphorus, and 20% lactose from 18 days of age. To induce hypophosphatemia/hypercalcemia in wild-type mice, the animals were weaned at 18 days of age onto a diet (TD98103, Teklad) containing 2% calcium, 20% lactose, and 0.02% phosphorus. To evaluate caspase-dependent apoptosis in vivo, caspase-3 inhibitor (Z-DEVD-FMK) or caspase-9 inhibitor (Z-LEHD-FMK) (Calbiochem) was injected daily (4 nmol/g of body weight) i.p. from 18 to 23 days of age into C57BL/6J mice fed a regular diet.
Biochemical Parameters. Serum phosphate levels were measured by using the Phosphorus Liqui-UV kit (Stanbio Laboratory, Boerne, TX). Serum calcium levels were measured by using the Calcium CPC LiquiColor test kit (Stanbio Laboratory). Serum PTH levels were determined by using a mouse intact PTH ELISA kit (Immutopics, San Clemente, CA).
Tissue Histology. Tibiae were fixed and decalcified in 10% formalin–PBS/20% EDTA (pH 8.0) for 24–48 h. Samples were then processed, embedded in paraffin, and sectioned with a Microtome (RM 2025, Leica, Deerfield, IL). To evaluate mineralization, nondecalcified sections were embedded in methylmethacrylate before sectioning.
Evaluation of Apoptosis. Apoptotic cells were identified by using a TUNEL-based in situ cell death detection kit (Roche Diagnostics). After treatment with 10 μg/ml proteinase K for 30 min at 37°C, sections were incubated with the TUNEL reaction mixture for 2 h at 37°C, rinsed, counterstained with 2% Evans blue solution (Sigma), and mounted with Vectashield (Vector Laboratories).
Immunohistochemistry. Immunohistochemistry using an antibody to cleaved caspase-3 (Cell Signaling Technology, Beverly, MA) (9) and the TSA biotin system kit (PerkinElmer) was performed as recommended by the manufacturer. Immunoreactive proteins were visualized by using streptavidin–alkaline phosphatase (PerkinElmer), followed by incubation with Vector red alkaline phosphatase substrate (Vector Laboratories).
Primary Chondrocyte Cultures. The costochondral region of embryonic day 18.5 embryos was dissected and transferred to PBS. Chondrocytes were isolated by collagenase digestion (10) and plated at a density of 3 × 105 cells per well in 0.1% gelatin-coated six-well plates (Becton Dickinson). Cells were cultured in DMEM/10% FBS containing 25 μg/ml l-ascorbic acid. To evaluate activation of apoptotic pathways, cells were treated with phosphate (NaH2PO4) at concentrations varying from 1 mM to 7 mM for 18 h. Control cultures were treated with 7 mM Cl- (NaCl). To block mitochondrial permeability transition, which is involved in the release of caspase-activating molecules and subsequent metabolic failure in the mitochondria (11), cells were treated with 1 μM cyclosporin A (Sigma) (12).
Western Blot Analysis. Primary hypertrophic chondrocytes were lysed in Tris-bufferred saline containing 1 mM EDTA, 1 mM DTT, 0.2% Triton X-100, 0.1% SDS, and protease inhibitor mixture (Roche Diagnostics). Ten micrograms of protein was subjected to SDS/PAGE under reducing conditions. Immunodetection was performed with anti-caspase-9 (Cell Signaling Technology). Immunoreactive proteins were visualized by using a chemiluminescence detection kit (NEN) according to the manufacturer's instructions.
Statistical Analysis. Student's t test was used to analyze significance between two groups. P < 0.05 was considered significant.
Results
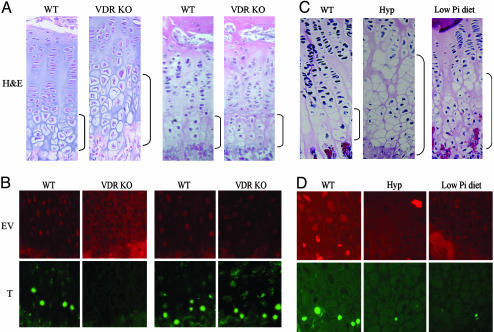
We have demonstrated previously that VDR-null mice develop rickets secondary to impaired apoptosis of late hypertrophic chondrocytes (ref. 7 and Fig. 1_A_, regular diet). However, prevention of abnormal mineral ion homeostasis in VDR-null mice, by institution of a “rescue diet” at 18 days of age, prevents the development of these rachitic changes (ref. 13 and Fig. 1 A, rescue diet). These studies suggest that impaired mineral ion homeostasis, not VDR ablation per se, is the cause of impaired hypertrophic chondrocyte apoptosis in this model. To address this hypothesis, TUNEL assays were performed in VDR-null mice and their wild-type littermates, under both dietary conditions. As reported in ref. 7 and shown in Fig. 1_B_ (regular diet), VDR-null mice with abnormal mineral ion homeostasis have a marked reduction in hypertrophic chondrocyte apoptosis; however, rescue of the growth plate phenotype by normalizing mineral ion homeostasis restores apoptosis (Fig. 1_B_, rescue diet). These data support the hypothesis that rickets associated with VDR ablation is due to the impaired apoptosis of terminally differentiated hypertrophic chondrocytes, which is a direct consequence of impaired mineral ion homeostasis.
Fig. 1.
Histological analysis and evaluation of hypertrophic chondrocyte apoptosis in 24-day-old mice. Shown are hematoxylin/eosin-stained sections (A and C) and TUNEL assay (B and D). (A and B) Wild-type (WT) and VDR knockout (KO) mice fed a regular diet (Left) or a rescue diet (Right) that normalizes mineral ion homeostasis. (C and D) Wild-type mice fed a regular diet, Hyp mice fed a regular diet, and wild-type mice fed a high-calcium/low-phosphate diet from 18 to 24 days of age. Brackets indicate the hypertrophic chondrocyte layer. Data are representative of experiments performed on sections from three or more mice for each condition. EV, Evans blue; T, TUNEL.
Histologic analysis of the growth plate of 24-day-old Hyp mice, an animal model for the human disorder X-linked hypophosphatemia, reveals marked expansion of the hypertrophic chondrocyte layer (Fig. 1_C_). These rachitic changes also were associated with a significant decrease in the number of TUNEL-positive late hypertrophic chondrocytes, compared with those of their wild-type littermates (Fig. 1_D_). Because the Phex gene is expressed in the growth plate, the pathophysiology of these rachitic changes could be multifactorial, thus a second model was examined to identify whether hypophosphatemia per se leads to the development of rickets. Wild-type C57BL/6J mice were weaned onto a phosphorus-restricted/high-calcium diet from 18 to 24 days of age. Expansion of the late hypertrophic chondrocyte layer was observed in these wild-type mice (Fig. 1_C_), associated with marked decrease in the number of TUNEL-positive hypertrophic chondrocytes, compared with wild-type mice fed a regular diet (Fig. 1_D_). These data demonstrate that in all three rachitic models, expansion of the growth plate is associated with impaired apoptosis of late hypertrophic chondrocytes.
The common metabolic parameter that unifies these rachitic models is hypophosphatemia (Fig. 2_A_): the Hyp mice had normal calcium (Fig. 2_B_) and PTH levels (Fig. 2_C_) at day 24, whereas the mice fed the low-phosphorus/high-calcium diet were hypercalcemic with suppressed PTH levels. As previously reported, in the third rachitic model being examined, VDR-null mice fed a regular diet were hypophosphatemic with elevated PTH levels at day 24 (8).
Fig. 2.
Biochemical parameters. Serum phosphate (A), serum calcium (B), and immunoreactive PTH (C) were measured in 24-day-old wild-type mice fed a regular diet (black bars), Hyp mice fed a regular diet (white bars), and wild-type mice fed a high-calcium/low-phosphate diet (gray bars). (D) Serum phosphate levels at 18.5 days postcoitum and at 0.5, 10, and 24 days of age in wild-type (♦) and Hyp (○) mice fed a regular diet. Data represent the mean ± SD of values from five mice for each assay. *, P ≤ 0.05.
Placental transfer of mineral ions in vitamin D-deficient murine models has been shown to favor normal mineral ion homeostasis in the fetus, despite profound maternal hypocalcemia and hypophosphatemia (14). Furthermore, infants with Phex mutations develop hypophosphatemia and rickets only after birth. To determine whether the evolution of the rachitic changes in the Hyp mice parallels the development of hypophosphatemia, serum phosphate levels were evaluated from 18.5 days postcoitum until 24 days of age. At 18.5 days postcoitum, serum phosphate levels of the Hyp mice were indistinguishable from those of their wild-type littermates (Fig. 2_D_). However, by 10 days of age, significant hypophosphatemia was present in the Hyp mice. Correlating with this observation, the growth plates of newborn Hyp mice were histologically indistinguishable from those of their wild-type littermates (Fig. 3_A_), and TUNEL-positive cells were observed in the growth plates of mice of both genotypes at this age (Fig. 3_B_). However, by 10 days of age, correlating with the development of hypophosphatemia, expansion of the late hypertrophic chondrocyte layer is evident in the Hyp mice (Fig. 3_C_) and is associated with a significant decrease in the number of TUNEL-positive cells, compared with their wild-type littermates (Fig. 3_D_). Interestingly, in both the Hyp mice and the mice with dietary-induced hypophosphatemia, impaired apoptosis is observed at a time when there is still significant mineralization of the extracellular matrix surrounding the late hypertrophic chondrocytes (Fig. 4). These data demonstrate that hypophosphatemia is the common factor leading to impaired apoptosis of the late hypertrophic chondrocytes and, consequently, rickets, in these models. Furthermore, the data suggest that the presence of local mineral, as evidenced by von Kossa staining, is insufficient to maintain normal programmed cell death.
Fig. 3.
Histological analysis and evaluation of hypertrophic chondrocyte apoptosis in Hyp mice. Hematoxylin/eosin staining (A and C) and TUNEL assays (B and D) were performed on sections from wild-type (WT) and Hyp mice at 0.5 day (A and B) and 10 days of age (C and D). Brackets indicate the hypertrophic chondrocyte layer. Data are representative of experiments performed on sections from three or more mice for each condition. EV, Evans blue; T, TUNEL.
Fig. 4.
Growth-plate mineralization in 24-day-old mice. von Kossa staining was performed on sections from 24-day-old wild-type mice fed a regular diet, Hyp mice fed a regular diet, and wild-type mice fed a high-calcium/low-phosphate diet from 18 to 24 days of age. Insets show a magnification of the hypertrophic chondrocytes. Brackets indicate the hypertrophic chondrocyte layer. Data are representative of sections from five mice for each condition.
Phosphate ions have been shown to induce apoptosis of avian chondrocytes in culture models (15). To demonstrate that caspase activation is involved in late hypertrophic chondrocyte apoptosis in vivo, immunohistochemistry was performed to evaluate caspase-3 activation in rachitic and normal growth plates. The percentage of late hypertrophic chondrocytes exhibiting cleaved caspase-3 immunoreactivity was significantly decreased in both the Hyp mice (10.4 ± 1.1%) and the mice with diet-induced hypophosphatemia (12.5 ± 1.2%) at 24 days of age, compared with that of their wild-type littermates (54.2 ± 3.6%) (Fig. 5). Because activation of caspase-3 is a common terminal marker of the membrane and mitochondrial apoptotic pathways (16), primary chondrocyte cultures were used to dissect which of these pathways mediates the effects of phosphate ions on chondrocyte apoptosis.
Fig. 5.
Cleaved caspase-3 immunohistochemistry. Sections from 24-day-old wild-type (WT) mice fed a regular diet, Hyp mice fed a regular diet, and mice fed a high-calcium/low-phosphate diet from 18 to 24 days of age were immunostained with antibody to cleaved caspase-3. Brackets indicate the hypertrophic chondrocyte layer. Data are representative of experiments performed on sections from three or more mice for each condition.
Previous investigations have demonstrated that primary cultures of mouse costal chondrocytes provide a useful model for the study of chondrocyte differentiation (10, 17). When plated at high density, these cultures express type II collagen mRNA and subsequently undergo hypertrophic differentiation, expressing type X collagen, and form cartilage nodules (10, 17). We therefore isolated and cultured primary mouse costal chondrocytes under differentiating conditions. Differentiation into hypertrophic chondrocytes was evaluated by morphology and confirmed by expression of type X collagen (data not shown). Upon treatment of primary hypertrophic chondrocytes with 7 mM phosphate, caspase-9, a key enzyme in the mitochondrial apoptotic pathway (18), is cleaved to its active form (Fig. 6). In contrast, control cultures treated with 7 mM sodium chloride demonstrated only the full-length inactive form of caspase-9 (Fig. 6). Cyclosporin A is an inhibitor of mitochondrial permeability transition and effectively blocks activation of caspase-9 by this pathway. Treatment of primary cultures of hypertrophic chondrocytes with 1 μM cyclosporin A prevented caspase-9 activation by 7 mM phosphate, demonstrating that phosphate leads to apoptosis of the hypertrophic chondrocytes by activating mitochondrial permeability transition. Interestingly, 7 mM phosphate did not lead to caspase-9 activation in 3T3 cells or in cultures of primary chondrocytes that had not undergone hypertrophic differentiation, demonstrating cell-type and differentiation-dependent specificity of phosphate-induced apoptosis (Fig. 6).
Fig. 6.
Phosphate induces hypertrophic chondrocyte apoptosis in vitro. Hypertrophic chondrocytes were treated with 7 mM NaCl or 7 mM NaH2PO4 for 18 h in the presence or absence of 1 μM cyclosporin A (CsA). Cell lysates were subjected to Western blot analysis using anti-caspase-9. Lysates of 3T3 fibroblasts and proliferating chondrocytes (PC) treated with 7 mM NaH2PO4 were similarly analyzed. Data are representative of those obtained from three independent cell preparations.
Caspase inhibitors have been administered to animals to demonstrate the involvement of the apoptotic pathway in a number of biological processes ranging from development to malignancy (19, 20). In vivo studies were, therefore, undertaken to examine whether inhibition of apoptosis by administration of a caspase-3 inhibitor (Z-DVED-FMK) could recapitulate the growth-plate phenotype observed in the rachitic models studied. As demonstrated in Fig. 7, administration of a caspase-3 inhibitor from 18 to 23 days of age resulted in a 37% (P ≤ 0.001) increase in the number of hypertrophic chondrocytes per column in the growth plates of wild-type mice (6.3 ± 0.4 cells per column), compared with control (DMSO)-treated littermates (4.0 ± 0.2 cells per column). Because caspase-3 activation is a terminal event in both the membrane and mitochondrial apoptotic pathways, analogous studies were performed with a caspase-9 inhibitor (Z-LEHD-FMK) that specifically inhibits the mitochondrial pathway. A 40% (P ≤ 0.001) increase in the number of cells per column in the hypertrophic chondrocyte layer was observed in the caspase-9 inhibitor-treated mice (6.6 ± 0.3 cells per column), demonstrating that inhibition of the mitochondrial apoptotic pathway leads to a rachitic phenotype. Treatment with these caspase inhibitors did not affect serum calcium or phosphorus levels (data not shown).
Fig. 7.
In vivo inhibition of caspase-3 or caspase-9 leads to expansion of the hypertrophic chondrocyte layer. Shown are hematoxylin/eosin-stained sections from 24-day-old mice injected at days 18–23 with DMSO, caspase-3 inhibitor (Z-DEVD-FMK), or caspase-9 inhibitor (Z-LEHD-FMK). Brackets indicate the hypertrophic chondrocyte layer.
Discussion
Development of the endochondral skeleton begins with mesenchymal condensation, followed by differentiation of these cells into chondrocytes (21). During development and postnatal growth, proliferative chondrocytes differentiate into hypertrophic chondrocytes, which subsequently undergo programmed cell death and are replaced by bone (21). As demonstrated by our studies, expansion of the late hypertrophic chondrocyte layer is a feature of hypophosphatemic disorders such as hereditary vitamin D-resistant rickets and X-linked hypophosphatemia. The studies in the Hyp mice, a model of X-linked hypophosphatemia, demonstrate that development of hypophosphatemia is associated with a parallel decrease in the number of apoptotic hypertrophic chondrocytes and expansion of the growth plate, revealing a correlation among serum phosphate levels, programmed cell death of hypertrophic chondrocytes, and the development of rickets. Investigations in the calcium-sensing receptor knockout mice support the hypothesis that phosphate is a key regulator of chondrocyte apoptosis. These mice exhibit hyperparathyroidism (due to impaired parathyroid calcium sensing), which leads to hypophosphatemia. Their growth plates reveal classical rachitic changes (22). Rendering these mice hypoparathyroid by making them null for the Gcm2 gene, which is required for parathyroid gland development (23), prevents hypophosphatemia and rachitic changes (22), demonstrating a link between circulating phosphorus levels and the development of rickets.
Conversely, the type II sodium phosphate cotransporter (Npt2a) knockout mice that exhibit hypophosphatemia, an appropriate elevation of 1,25(OH)2D, hypercalcemia, and hypoparathyroidism have not been reported to develop rickets (24, 25). However, almost half of these mice die before weaning, and those that do survive maintain serum phosphate levels that are 65–80% of those of their wild-type littermates, whereas the mouse models in our studies had a 50% decrease in their serum phosphate levels. It is notable that the surviving Npt2a knockout mice have a 2.5-fold increase in Npt2c protein expression in the kidney, suggesting a compensatory mechanism that may maintain circulating phosphate levels closer to the normal range than that observed in the Hyp mice, in whom an 80% decrease in Npt2c protein levels is seen (26). These observations implicate a threshold in the circulating phosphate levels, below which impairment of hypertrophic chondrocyte apoptosis is observed. Although calcium has been shown to play a role in chondrocyte differentiation (27, 28), our investigations demonstrate that wild-type mice rendered hypercalcemic and hypophosphatemic by dietary means develop rickets, demonstrating that hypercalcemia cannot maintain normal growth-plate maturation in the presence of a 50% reduction in circulating phosphate levels. Therefore, our studies eliminate calcium and PTH as modulators of apoptosis. In addition, Fgf-23 also can be ruled out, because the levels are elevated in the Hyp mouse but are suppressed in both the VDR-null mouse and the diet-induced hypophosphatemic mouse (29).
In vitro studies have implicated phosphate as a modulator of chondrocyte apoptosis. Inorganic phosphate induces chondrocyte apoptosis in a dose- and time-dependent fashion (30). Our study extends these observations, demonstrating the effect of phosphate on chondrocyte apoptosis in vivo. Our studies point to circulating phosphate, rather than locally deposited phosphate, as a key determinant of hypertrophic chondrocyte apoptosis, because impaired apoptosis is observed when there is still marked von Kossa staining (indicating mineralization) of the late hypertrophic chondrocyte layer. Studies in the tissue-nonspecific alkaline phosphatase (TNAP) knockout mouse, a model for the human disorder hypophosphatasia, support this hypothesis (31). The absence of alkaline phosphatase impairs mineral deposition, thus despite normal circulating mineral ion levels, the TNAP-null mice have decreased skeletal mineralization. Rather than an expanded growth plate, developmental arrest of chondrocyte differentiation is observed in these mice. There is a significant reduction in the hypertrophic chondrocyte layer, despite virtual absence of mineralization in this region.
Taken together, the models used in the current investigations support the hypothesis that circulating phosphate is a key determinant of hypertrophic chondrocyte apoptosis in vivo. Parallel studies in an in vitro culture model suggest that phosphate acts directly on hypertrophic chondrocytes rather that regulating the production of paracrine and/or endocrine factors that, in turn, promote apoptosis of these cells. Identification of a specific phosphate sensor or transporter expressed in hypertrophic chondrocytes that is responsible for these effects is a critical next step. Characterization of this transporter will identify the basis for the postulated threshold in circulating phosphate, below which impaired apoptosis is observed, as well as play a critical role in identifying potential therapeutic agents for the treatment for rachitic disorders.
Acknowledgments
We thank Paola Divieti for her insightful comments. This work was supported by National Institutes of Health Grant DK46974 (to M.B.D.) and the Yale Core Center for Musculoskeletal Diseases. Y.S. is a recipient of a fellowship from the Canadian Institutes of Health Research.
Author contributions: Y.S. and M.B.D. designed research; Y.S., T.O.C., and M.B.D. performed research; T.O.C. and M.B.D. contributed new reagents/analytic tools; Y.S. and M.B.D. analyzed data; and Y.S., T.O.C., and M.B.D. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: 1,25(OH)2D, 1,25-dihydroxyvitamin D; VDR, vitamin D receptor; PTH, parathyroid hormone.
References
- 1.St.-Arnaud, R., Messerlian, S., Moir, J. M., Omdahl, J. L. & Glorieux, F. H. (1997) J. Bone Miner. Res. 12**,** 1552-1559. [DOI] [PubMed] [Google Scholar]
- 2.Hughes, M. R., Malloy, P. J., Kieback, D. G., Kesterson, R. A., Pike, J. W., Feldman, D. & O'Malley, B. W. (1988) Science 242**,** 1702-1705. [DOI] [PubMed] [Google Scholar]
- 3.Eicher, E. M., Southard, J. L., Scriver, C. R. & Glorieux, F. H. (1976) Proc. Natl. Acad. Sci. USA 73**,** 4667-4671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sabbagh, Y., Jones, A. O. & Tenenhouse, H. S. (2000) Hum. Mutat. 16**,** 1-6. [DOI] [PubMed] [Google Scholar]
- 5.The Autosomal Dominant Hypophosphataemic Rickets Consortium (2000) Nat. Genet. 26**,** 345-348. [DOI] [PubMed] [Google Scholar]
- 6.Li, Y. C., Pirro, A. E., Amling, M., Delling, G., Baron, R., Bronson, R. & Demay, M. B. (1997) Proc. Natl. Acad. Sci. USA 94**,** 9831-9835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donohue, M. M. & Demay, M. B. (2002) Endocrinology 143**,** 3691-3694. [DOI] [PubMed] [Google Scholar]
- 8.Li, Y. C., Amling, M., Pirro, A. E., Priemel, M., Meuse, J., Baron, R., Delling, G. & Demay, M. B. (1998) Endocrinology 139**,** 4391-4396. [DOI] [PubMed] [Google Scholar]
- 9.Nicholson, D. W., Ali, A., Thornberry, N. A., Vaillancourt, J. P., Ding, C. K., Gallant, M., Gareau, Y., Griffin, P. R., Labelle, M., Lazebnik, Y. A., et al. (1995) Nature 376**,** 37-43. [DOI] [PubMed] [Google Scholar]
- 10.Cormier, S. A., Mello, M. A. & Kappen, C. (2003) BMC Dev. Biol. 3**,** 4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Green, D. R. & Kroemer, G. (2004) Science 305**,** 626-629. [DOI] [PubMed] [Google Scholar]
- 12.Halestrap, A. (2005) Nature 434**,** 578-579. [DOI] [PubMed] [Google Scholar]
- 13.Amling, M., Priemel, M., Holzmann, T., Chapin, K., Rueger, J. M., Baron, R. & Demay, M. B. (1999) Endocrinology 140**,** 4982-4987. [DOI] [PubMed] [Google Scholar]
- 14.Brommage, R. & DeLuca, H. F. (1984) Am. J. Physiol. 246**,** F526-F529. [DOI] [PubMed] [Google Scholar]
- 15.Mansfield, K., Rajpurohit, R. & Shapiro, I. M. (1999) J. Cell. Physiol. 179**,** 276-286. [DOI] [PubMed] [Google Scholar]
- 16.Cohen, G. M. (1997) Biochem. J. 326**,** 1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lefebvre, V., Garofalo, S., Zhou, G., Metsaranta, M., Vuorio, E. & De Crombrugghe, B. (1994) Matrix Biol. 14**,** 329-335. [DOI] [PubMed] [Google Scholar]
- 18.Bossy-Wetzel, E. & Green, D. R. (1999) Mutat. Res. 434**,** 243-251. [DOI] [PubMed] [Google Scholar]
- 19.Philchenkov, A. (2004) J. Cell. Mol. Med. 8**,** 432-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Onteniente, B. (2004) Curr. Drug Targets CNS Neurol. Disord. 3**,** 333-340. [DOI] [PubMed] [Google Scholar]
- 21.Olsen, B. R., Reginato, A. M. & Wang, W. (2000) Annu. Rev. Cell Dev. Biol. 16**,** 191-220. [DOI] [PubMed] [Google Scholar]
- 22.Tu, Q., Pi, M., Karsenty, G., Simpson, L., Liu, S. & Quarles, L. D. (2003) J. Clin. Invest. 111**,** 1029-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gunther, T., Chen, Z. F., Kim, J., Priemel, M., Rueger, J. M., Amling, M., Moseley, J. M., Martin, T. J., Anderson, D. J. & Karsenty, G. (2000) Nature 406**,** 199-203. [DOI] [PubMed] [Google Scholar]
- 24.Beck, L., Karaplis, A. C., Amizuka, N., Hewson, A. S., Ozawa, H. & Tenenhouse, H. S. (1998) Proc. Natl. Acad. Sci. USA 95**,** 5372-5377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gupta, A., Tenenhouse, H. S., Hoag, H. M., Wang, D., Khadeer, M. A., Namba, N., Feng, X. & Hruska, K. A. (2001) Bone 29**,** 467-476. [DOI] [PubMed] [Google Scholar]
- 26.Tenenhouse, H. S., Martel, J., Gauthier, C., Segawa, H. & Miyamoto, K. (2003) Am. J. Physiol. 285**,** F1271-F1278. [DOI] [PubMed] [Google Scholar]
- 27.Wang, D., Canaff, L., Davidson, D., Corluka, A., Liu, H., Hendy, G. N. & Henderson, J. E. (2001) J. Biol. Chem. 276**,** 33995-34005. [DOI] [PubMed] [Google Scholar]
- 28.Chang, W., Tu, C., Pratt, S., Chen, T. H. & Shoback, D. (2002) Endocrinology 143**,** 1467-1474. [DOI] [PubMed] [Google Scholar]
- 29.Yu, X., Sabbagh, Y., Davis, S.I., Demay, M. B., White, K.E. (2005) Bone 36**,** 971-977. [DOI] [PubMed] [Google Scholar]
- 30.Mansfield, K., Teixeira, C. C., Adams, C. S. & Shapiro, I. M. (2001) Bone 28**,** 1-8. [DOI] [PubMed] [Google Scholar]
- 31.Fedde, K. N., Blair, L., Silverstein, J., Coburn, S. P., Ryan, L. M., Weinstein, R. S., Waymire, K., Narisawa, S., Millan, J. L., MacGregor, G. R. & Whyte, M. P. (1999) J. Bone Miner. Res. 14**,** 2015-2026. [DOI] [PMC free article] [PubMed] [Google Scholar]