Cochaperone immunophilin FKBP52 is critical to uterine receptivity for embryo implantation (original) (raw)
Abstract
Embryo implantation in the uterus is a critical step in mammalian reproduction, requiring preparation of the uterus receptive to blastocyst implantation. Uterine receptivity, also known as the window of implantation, lasts for a limited period, and it is during this period blastocysts normally implant. Ovarian steroid hormones estrogen and progesterone (P4) are the primary regulators of this process. The immunophilin FKBP52 serves as a cochaperone for steroid hormone nuclear receptors to govern appropriate hormone action in target tissues. Here we show a critical role for FKBP52 in mouse implantation. This immunophilin has unique spatiotemporal expression in the uterus during implantation, and females missing the Fkbp52 gene have complete implantation failure due to lack of attainment of uterine receptivity. The overlapping uterine expression of FKBP52 with nuclear progesterone receptor (PR) in wild-type mice together with reduced P4 binding to PR, attenuated PR transcriptional activity and down-regulation of several P4-regulated genes in uteri of _Fkbp52_-/- mice, establishes this cochaperone as a critical regulator of uterine P4 function. Interestingly, ovulation, another P4-mediated event, remains normal. Collectively, the present investigation provides evidence for an in vivo role for this cochaperone in regulating tissue-specific hormone action and its critical role in uterine receptivity for implantation.
Keywords: mouse, uterus, ovulation, blastocyst, progesterone receptor
Progesterone (P4) is essential for implantation and pregnancy maintenance in all mammalian species studied. In mice, P4 priming of the uterus is obligatory for estrogen to prepare the uterus to the receptive state conducive to blastocyst implantation. P4 acting through the nuclear P4 receptor (PR) modulates uterine physiology and expression of various genes that are required for implantation (1, 2). Numerous defects in mice lacking the Pgr gene that encodes PR include failure in ovulation, mammary gland development, and sexual behavior along with uterine hyperplasia and inflammation, reflecting the critical role of P4 in female reproduction (3). Appropriate functioning of nuclear steroid hormone receptors depends on interactions with the molecular chaperone machinery to maintain a functional state competent for hormone binding and subsequent transcriptional activation. Functionally mature steroid receptor complexes consist of a receptor monomer, a 90-kDa heat shock protein (Hsp90) dimer, the cochaperone p23, and one of four cochaperones that contain a tetratricopeptide repeat (TPR) domain. The TPR cochaperones include two members of the FK506 binding family of immunophilins, FKBP52/FKBP4 and FKBP51/FKBP5, a member of the cyclosporin-binding immunophilin cyclophilin 40 (CyP40) or the protein phosphatase PP5. FKBP52 and FKBP51 are similar to other FKBP family members in that both contain an active peptidylprolyl cis/trans isomerase domain that catalyzes conformational changes in protein substrates (4, 5).
Roles for Hsp90 and p23 in initiating and maintaining receptor competency for hormone binding are well established (6), but the contribution of cochaperones in receptor complexes are not clearly understood. Although FKBP51 and FKBP52 share 60% sequence identity, have similar x-ray crystallographic structures (7, 8), and display similar peptidylprolyl cis/trans isomerase and Hsp90-binding activities, there are clear functional distinctions between these cochaperones in steroid receptor complexes. There is evidence based on in vitro cellular assays that, whereas FKBP52 potentiates the function of glucocorticoid receptors (GR) (9), androgen receptors (10), and PR (5), FKBP51 antagonizes GR and PR functions (11, 12). However, physiological roles of FKBP52 and FKBP51 in an in vivo context have not been examined. More specifically, a physiological role for FKBP52 and FKBP51 in P4-dependent processes including ovarian and uterine functions remains unknown. Here we show that female mice missing the Fkbp52 gene have compromised PR functions leading to total failure of the uterus to support blastocyst implantation.
Materials and Methods
Mice. The disruption of the Fkbp52 gene was achieved by homologous recombination as described (10). Tail genomic DNA was used for genotyping by PCR. Mice on C57BL/6/129SvJ were housed and used in the present investigation in accordance with the National Institutes of Health and institutional guidelines on the care and use of laboratory animals.
Ovulation, Fertilization, Implantation, and Blastocyst Transfer. Mice were examined for ovulation, fertilization, and implantation as described (13). To examine ovulation and fertilization, mice were mated with fertile males. On day 2 of pregnancy (day 1 = vaginal plug), oviducts were flushed with Whitten's medium to recover ovulated eggs and to examine the fertilization rate. Implantation sites on days 5 and 6 of pregnancy were visualized by an i.v. injection (0.1 ml per mouse) of a Chicago Blue B dye solution (1% solution in saline) and the number of implantation sites, as demarcated by distinct blue bands, was recorded. For blastocyst transfer, pseudopregnant recipients were generated by mating females with vasectomized males. Day 4 wild-type blastocysts were transferred into day 4 uteri of wild-type, heterozygous or _Fkbp52_-/- pseudopregnant recipients, and implantation sites were recorded 24 h later (day 5) by the blue dye method. All mice used were between 2 and 4 months of age.
Comparative RT-PCR and Southern Hybridization. Comparative RT-PCR and Southern blotting were performed as described (14).
P4 Binding Assay. Ovariectomized wild-type or _Fkbp52_-/- mice were injected s.c. with estradiol-17β (1 μg per mouse in sesame oil) or vehicle for 14 days. Aliquots of wild-type or _Fkbp52_-/- uterine cytosol (200 μg of total protein) were incubated overnight at 4°C in the presence of indicated concentrations of [3H]P4 (NEN, specific activity = 102.1 Ci/mmol; 1 Ci = 37 GBq), a 100-fold molar excess of cortisol plus or minus a 100-fold molar excess of unlabeled P4. Unbound ligand was removed by incubation with dextran-coated charcoal, and bound radioactivity in the supernatant was measured by liquid scintillation counting (10).
Transfection and PR Transcriptional Activity Assay. Cells (8 × 104 per well) were cotransfected with 20 ng of pCMV-β-galactosidase plasmid (Clontech), 0.3 μg of pCR3.1-hPRB expression plasmid (provided by N. Weigel, Baylor College of Medicine, Houston), 0.3 μg of pMMTV-luc reporter plasmid (provided by J. Scammell, University of South Alabama, Mobile), and 0.3 μg of pCI-neo vector (Promega) lacking or containing human Fkbp51 or Fkbp52 cDNA. Cells were incubated overnight with P4 as indicated. Luciferase and β-galactosidase assays were performed essentially as described (10).
In Situ Hybridization. Sense or antisense 35S-labeled probes were generated by using appropriate polymerases from cDNAs for in situ hybridization as described (15). Sections hybridized with sense probes served as negative controls.
Northern Hybridization. Total RNA (6.0 μg) was denatured, separated by formaldehyde-agarose gel electrophoresis, and transferred onto nylon membranes. Cross-linked blots were prehybridized, hybridized, and washed as described (15). The hybrids were detected by autoradiography.
Isolation and Culture of Embryonic Fibroblasts. Day 13 embryos were isolated and genotyped by PCR. The head, limbs, and liver were excluded from each embryo, and the remaining tissues were minced and digested with 0.25% trypsin at 37°C for 15 min, and plated in 60-mm plastic dishes in DMEM supplemented with 10% FBS plus essential amino acids and penicillin/streptomycin. Fibroblasts were immortalized by the 3T3 protocol of Todaro and Green (16).
Histology and Immunostaining of Ki67. Frozen (10 μm) or formalin-fixed paraffin embedded uterine sections (5 μm) were subjected to hematoxylin and eosin staining or immunostaining by using a Ki67 antigen staining kit, respectively (Novacastra, Newcastle, U.K.). Nuclear brown color indicates positive Ki67 staining.
Results and Discussion
Ovulation Is Normal in _Fkbp52_-/- Mice. To delineate whether FKBP51 or FKBP52 plays a critical role in ovarian and uterine functions, we generated mice with targeted deletion of either Fkbp51 or Fkbp52 genes. We found that, although _Fkbp51_-deficient mice show no overt reproductive failures (unpublished observations), both males and females lacking Fkbp52 are infertile (10), indicating the importance of this immunophilin in reproduction. The infertility status of _Fkbp52_-/- females is shown (Table 3, which is published as supporting information on the PNAS web site). Whereas male infertility is due to compromised androgen receptor function (10), the site and cause of female infertility in _Fkbp52_-/- mice remains unexplored.
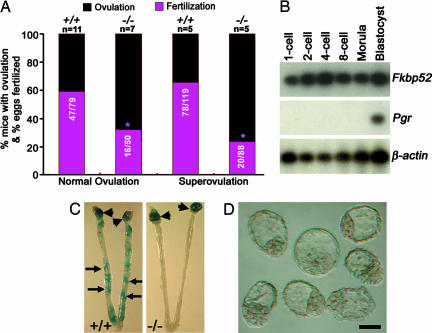
Because Pgr null mice show ovulation failure and uterine refractoriness to implantation (17, 18), and because FKBP52 associates with PR-Hsp90 complexes (5), we first examined whether ovulation is normal in _Fkbp52_-/- females. Because _Fkbp52_-/- males are infertile, normally cyclic or gonadotropin-stimulated _Fkbp52_-/- females were mated with wild-type fertile males. The number of normally ovulated or superovulated eggs in these mutant females is comparable to that of wild-type females (Fig. 1_A_); however, both in vivo and in vitro fertilization rates of eggs arising from mutant females are compromised (Fig. 1_A_). To determine whether oocyte FKBP52 contributes to this reduction, we examined the expression of Fkbp52 and Pgr in preimplantation wild-type embryos from one-cell through blastocyst stages by RT-PCR. We found that, although embryos at all stages of development express Fkbp52, Pgr is expressed only at the blastocyst stage (Fig. 1_B_); our observation of Pgr expression in preimplantation embryos is consistent with a previous report (19). These results suggest that Fkbp52 is maternally derived in the oocyte and may have a role in fertilization and preimplantation development independent of PR. Alternatively, oocyte maturation due to follicular deficiency arising from compromised PR function in the absence of FKBP52 may contribute to the reduced fertilization rate. Indeed, there is evidence that compromised PR function impairs the expansion of cumulus-oocyte complexes (20). Further investigation is needed to address this problem. However, recovery of blastocysts from _Fkbp52_-/- uteri suggests that FKBP52 is not an absolute requirement for fertilization or preimplantation embryo development. Collectively, these results imply that complete infertility observed in mutant females is not due to failure in ovulation as in _Pgr_-/- mice, or total absence of fertilization, but is the result of defective implantation and/or pregnancy failure following implantation.
Fig. 1.
Female reproductive events in _Fkbp52_-/- mice. (A) Normal ovulation and fertilization were examined on day 2 of pregnancy. Superovulated eggs retrieved on day 1 were subjected to in vitro fertilization. Numbers within the bars indicate number of fertilized eggs/total number of ovulated eggs. Normal and superovulation rates are not significantly different between +/+ and -/- mice (unpaired t test); however, normal or in vitro fertilization rates are significantly different between +/+ and -/- mice (χ2 analysis; *, P = 0.01). (B) Fkbp52 and Pgr expression in preimplantation embryos as assessed by RT-PCR. (C) Representative photographs of wild-type uteri with implantation sites (IS) and _Fkbp52_-/- uteri without IS on day 5 of pregnancy. Arrowheads and arrows indicate sites of ovary and implantation, respectively. (D) Representative photomicrograph of blastocysts recovered from _Fkbp52_-/- mice on day 5 of pregnancy. (Bar, 50 μm.)
_Fkbp52_-/- Mice Show Implantation Failure. In mice, blastocyst attachment to the uterine luminal epithelium initiates the implantation process and is accompanied by an increased endometrial vascular permeability at the sites of blastocyst apposition, which can be visualized as distinct blue bands after an i.v. injection of Chicago Blue B dye solution (15). We observed that, although wild-type and heterozygous females had an expected number of implantation sites when examined on day 5 of pregnancy, none of the mutant females showed any sign of implantation (Fig. 1_C_ and Table 1). It is possible that blastocyst implantation is delayed but eventually occurs in _Fkbp52_-/- mice like in _cPLA2_α-/- or _LPA3_-/- mice (13, 21); however, no signs of implantation were evident even on day 6 of pregnancy (Table 1). A small number of unimplanted blastocysts were recovered from mutant females (Fig. 1_D_ and Table 1). Compromised fertilization in _Fkbp52_-/- mice could explain the small number of recovered blastocysts in mutant mice. Therefore, we used blastocyst transfer experiments to confirm that FKBP52 is critical to uterine receptivity for implantation. Day 4 wild-type blastocysts were transferred to wild-type, heterozygous, or homozygous mutant day 4 pseudopregnant recipients. Similar to the results of natural mating, none of the transferred wild-type blastocysts showed implantation in any of the mutant recipients when examined 24 h later, whereas wild-type or heterozygous recipients showed an expected number of implantation sites. Again, a small number of blastocysts were recovered from mutant uteri (Table 2). Reciprocal embryo transfers with homozygous mutant embryos could not be performed because _Fkbp52_-/- males are infertile (10). Collectively, these results show that, whereas P4-mediated ovulation is normal in _Fkbp52_-/- mice, the mutant uterus is completely nonreceptive to blastocyst implantation, implying an essential role for this immunophilin specifically for uterine receptivity. Our next objective was to determine whether these phenotypes resulting from Fkbp52 deficiency are caused by impaired P4 functions in the uterus.
Table 1.
**Fkbp52**−/− female mice show implantation failure
| Day of pregnancy | Genotypes | No. of mice | No. of mice with IS (%) | No. of IS | No. of embryos recovered |
|---|---|---|---|---|---|
| 5 | +/+/+/− | 12 | 9 (75) | 6.5 ± 2.0 | 0* |
| 5 | −/− | 9 | 0 | 0 | 37† |
| 6 | +/+/+/− | 14 | 10 (71) | 7.9 ± 2.0 | 2‡ |
| 6 | −/− | 5 | 0 | 0 | 5§ |
Table 2.
**Wild-type blastocysts fail to implant in Fkbp52**−/− recipients
| Genotype | No. of blastocysts transferred | No. of blastocysts recovered | |||||
|---|---|---|---|---|---|---|---|
| Blastocysts | Recipients | No. of recipients | No. of mice with IS (%) | No. of IS (%) | No. of IS | ||
| +/+ | +/+/+/− | 178 | 10 | 8 (80) | 74 (41) | 7.3 ± 4.0 | 2* |
| +/+ | −/− | 79 | 4 | 0 (0) | 0 | 0 | 14† |
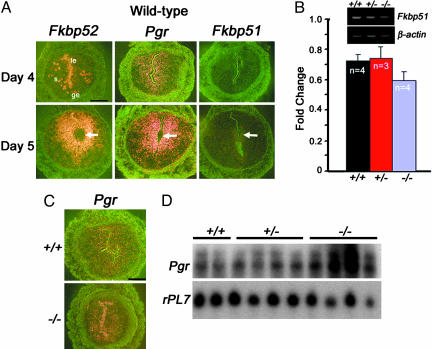
Fkbp52 and Pgr Show Overlapping Uterine Expression During Implantation. We speculated that, if FKBP52 functions as a cochaperone to PR in vivo, cell specific expression of these two genes must overlap. In situ hybridization results show that, on day 4 of pregnancy, Fkbp52 and Pgr expression overlaps in the stroma. However, Fkbp52 is also expressed in the luminal and glandular epithelium. Both Fkbp52 and Pgr become more localized in stromal cells surrounding the blastocyst on day 5 after implantation (Fig. 2_A_). This expression pattern coincides with an increased stromal cell proliferation on day 4 and more intensely localized stromal cell proliferation at the implantation site on day 5. Uterine Fkbp51, although significantly lower compared to Fkbp52, is also expressed on days 4 and 5. The lower expression of Fkbp51 on days 4 and 5 implies a limited role for this immunophilin during implantation and is consistent with normal fertility of _Fkbp51_-/- mice. However, we wanted to determine whether an interaction between FKBP51 and FKBP52 modulates PR transcriptional activity. Using mouse embryonic fibroblasts in cellular assays, we found that FKBP51 is antagonistic to FKBP52 in modulating PR transcriptional activity (Fig. 3). To determine whether Fkbp51 is overexpressed in _Fkbp52_-/- uteri producing the observed phenotypes, we used comparative RT-PCR experiments and found that Fkbp51 expression is not exacerbated in mutant uteri on day 4 of pregnancy (Fig. 2_B_). Collectively, these results indicate that Fkbp52 null phenotypes are specific to Fkbp52 deficiency and not due to overexpression of Fkbp51. Given these results, we then compared Pgr expression in Fkbp52 mutant uteri with wild-type uteri. In situ hybridization results show that Pgr is expressed in both the stroma and epithelium similar to that in wild-type uteri (Fig. 2_C_), and Northern blot results show that Pgr expression is not compromised in _Fkbp52_-/- uteri, but levels are rather increased (Fig. 2_D_). Together, these results show that uterine refractoriness to implantation in _Fkbp52_-/- mice is not due to reduced Pgr expression or overexpression of Fkbp51, but instead could be due to reduced PR function.
Fig. 2.
Uterine expression of Fkbp52, Pgr and Fkbp51. (A) In situ hybridization of Fkbp52, Pgr, and Fkbp51 in wild-type uteri on days 4 and 5 of pregnancy. (B) Comparative RT-PCR of uterine Fkbp51 in Fkbp52+/+ (+/+), Fkbp52+/- (+/-), and _Fkbp52_-/- (-/-) mice on day 4 of pregnancy. The data are presented as fold changes (mean ± SEM) of three to four independent RNA samples. Fold changes are not significantly different between +/+, +/-, or -/- mice (unpaired t test). (C) In situ hybridization of uterine Pgr in wild-type (+/+) and _Fkbp52_-/- (-/-) mice on day 4. (D) Northern blot analysis of uterine Pgr in +/+, +/-, and -/- mice on day 4. rPL7 is a housekeeping gene. Arrows in A indicate the location of blastocysts. le, luminal epithelium; ge, glandular epithelium; s, stroma. (Bar, 400 μm.)
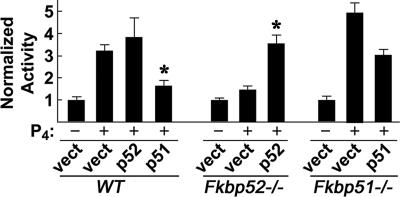
Fig. 3.
Differential modulation of PR transactivation by FKBP52 and FKBP51. Fibroblast cell lines prepared from wild-type (WT), _Fkbp52_-/-, or _Fkbp51_-/- mouse embryos were cotransfected with four plasmids as described in Materials and Methods. Cells were treated with or without 0.5 nM P4 for 16 h. The bars indicate the activity (mean ± SD; n = 3) normalized to uninduced levels of luciferase activity in each cell background. In WT cells expressing endogenous FKBP52 and FKBP51, overexpression of FKBP52 show marginal increases in the reporter activity, whereas overexpression of FKBP51 decreases the reporter activity (*, P = 0.05). In _Fkbp52_-/- cells, P4-induced reporter activity is minimal but restored by exogenous FKBP52 (*, P = 0.05). In _Fkbp51_-/- cells retaining endogenous FKBP52, maximal reporter activity is induced; however, induced expression of FKBP51 decreases the activity. Statistical analysis was performed by using one-way ANOVA followed by paired t test.
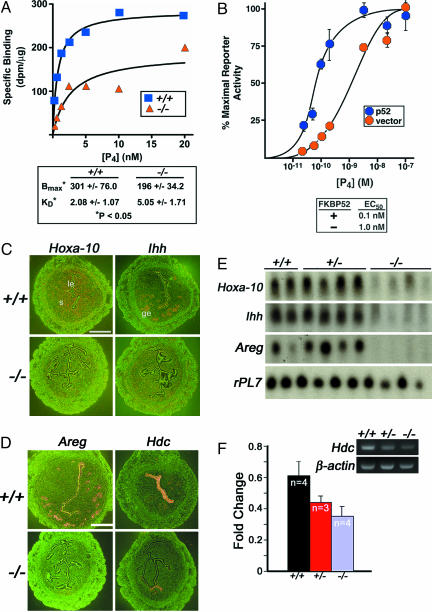
Uterine PR Activity Is Compromised in _Fkbp52_-/- Mice. If FKBP52 is required for optimal PR function, then uterine PR activity would be compromised in the absence of FKBP52. Indeed, binding assays using radiolabeled P4 show that P4 binding to PR is reduced ≈2-fold in _Fkbp52_-/- uterine cytosol compared with that of wild-type mice. There is also a fractional reduction in the number of P4 binding sites (Fig. 4_A_). Reduced PR transcriptional activity in the absence of FKBP52 is also noted in a transfected cell system in vitro. Embryonic fibroblasts isolated from _Fkbp52_-/- mice were cotransfected with human PR-B plasmid, a luciferase reporter plasmid, and either an empty vector or a plasmid containing FKBP52 (p52). P4-dependent reporter activity was measured over a range of P4 concentrations. Cells expressing exogenous FKBP52 have enhanced P4-dependent reporter gene activation as compared to cells lacking FKBP52 (Fig. 4_B_). PR-B results are shown because PR-A normally displays only modest reporter transactivation in cell culture systems (22); however, loss of FKBP52 also reduced the modest activity of PR-A (data not shown). These results provide evidence that FKBP52 is required for optimal uterine PR activity; thus, defects in uterine receptivity for implantation in _Fkbp52_-/- mice are likely caused by compromised uterine P4 function.
Fig. 4.
Uterine progesterone (P4) binding and PR activity. (A) P4 binding in uterine cytosol of wild-type (+/+) and Fkbp52-/- (-/-) mice. Both +/+ and -/- female mice were ovariectomized and treated with estrogen to increase uterine PR levels. Uterine cytosolic samples from +/+ and -/- mice were used for binding assays for comparison. The data shown are representative of four independent experiments. (B) FKBP52 effects on reporter gene activation in embryonic fibroblasts isolated from _Fkbp52_-/- mice cotransfected with plasmid expressing human PR-B, a luciferase reporter plasmid, and an empty vector or plasmid expressing FKBP52 (p52). Each value represents the mean ± SD for three replicate samples. (C and D) In situ hybridization of Hoxa-10 and Ihh (C) and Areg and Hdc (D) in +/+ and -/- mice on day 4 of pregnancy. (E) Northern blot analysis of uterine Hoxa-10, Ihh, and Areg in +/+, +/-, and -/- mice on day 4. rPL7 is a housekeeping gene. le, luminal epithelium; ge, glandular epithelium; s, stroma. (Bar, 400 μm.) (F) Comparative RT-PCR of uterine Hdc in +/+, +/-, and -/- mice on day 4. The data are presented as fold changes (mean ± SEM) of three to four independent samples. Fold changes in _Fkbp52_-/- mice are significantly different compared to combined fold changes in +/+ and +/- mice (P = 0.05, unpaired t test).
PR Responsive Genes and Functions Are Aberrant in Uteri of _Fkbp52_-/- Mice. We speculated that if PR functions are compromised in _Fkbp52_-/- uteri, uterine P4-dependent genes would be aberrantly expressed. We selected P4-regulated genes that encode Hoxa-10, Indian hedgehog (Ihh), amphiregulin (Areg), and histidine decarboxylase (Hdc) (23-26). Wild-type and _Fkbp52_-/- females were mated with wild-type males to collect samples on day 4 when the uterus is under major P4 influence and expresses these genes. Northern and in situ hybridization results show that expression of Hoxa-10, Ihh, and Areg are drastically down-regulated in _Fkbp52_-/- uteri, indicating compromised P4 function (Fig. 4 _C_-E). There are two isoforms of PR, PR-A and PR-B. Although mice lacking both PR-A and PR-B exhibit multiple defects in ovarian and uterine functions leading to female infertility, mice missing only PR-B display normal ovarian and uterine responses to P4. These findings suggest that normal P4-regulated ovarian and uterine functions are primarily mediated by PR-A (18). Because the mouse uterus expresses both PR-A and PR-B, we sought to examine whether FKBP52 differentially influences these isoforms. Areg and Hdc are known PR-A and PR-B regulated genes in the uterus, respectively (3). Down-regulation of both Areg and Hdc in _Fkbp52_-/- uteri (Fig. 4 _D_-F) provides evidence that FKBP52 influences both PR-A and PR-B functions. Because mice lacking PR-B have normal fertility (18), we believe that compromised uterine PR-A activity in _Fkbp52_-/- mice contributes to implantation failure.
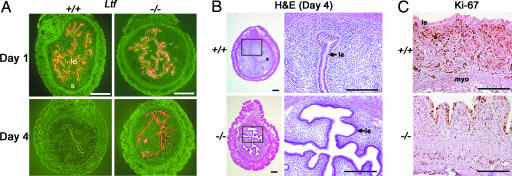
To confirm that the observed changes in uterine gene expression are not due to aberrant responsiveness of the uterus to estrogen, we examined uterine expression of an estrogen-responsive gene lactoferrin (Ltf) on day 1 of pregnancy when the uterus is primarily under the influence of estrogen (Fig. 5_A_). As previously observed in the wild-type uterus (27), normal expression of Ltf on day 1 confirms that _Fkbp52_-/- uteri appropriately respond to estrogen. On day 4 of pregnancy, when the uterus is under major P4 influence, Ltf expression is undetectable in wild-type uteri as expected, but persists in _Fkbp52_-/- uteri, indicating reduced P4 function with exaggerated estrogenic influence in the absence of FKBP52 (Fig. 5_A_). This concept is consistent with a striking difference that we observed between wild-type and _Fkbp52_-/- uteri at the histological and cellular level. Normally, uterine lumens in wild-type mice on day 4 of pregnancy under P4 dominance show luminal closure with a slit-like structure with nonproliferating differentiated cuboidal epithelia. In addition, stromal cell proliferation is intense on this day (2). Surprisingly, uteri of _Fkbp52_-/- mice have compromised luminal closure on day 4, displaying proliferating columnar luminal epithelia and nonproliferating stromal cells, consistent with defects in P4-regulated events (Fig. 5 B and C). However, proliferation in uterine luminal epithelia of _Fkbp52_-/- and wild-type mice on day 1 of pregnancy, when the uterus is under estrogenic influence, remains similar (data not shown). Collectively, these results suggest that responsiveness of _Fkbp52_-/- uteri to coordinated estrogen and progesterone action is skewed to favor estrogen.
Fig. 5.
Differential expression of an estrogen-responsive gene lactoferrin (Ltf) and cell proliferation. (A) In situ hybridization of Ltf in uteri of +/+ and -/- mice on days 1 and 4 of pregnancy. (Bar, 400 μm.) (B) Histological examination of +/+ and -/- uteri on day 4 of pregnancy. (Bar, 200 μm.) (C) Immunostaining of Ki67, a marker of cell proliferation, in uteri of +/+ and -/- mice on day 4. Note stromal cell proliferation in wild-type uterus versus luminal epithelial proliferation in _Fkbp52_-/- mice. Arrow points to luminal epithelial layer. le, luminal epithelium; s, stroma; myo, myometrium. (Bar, 100 μm.)
The present study identifies FKBP52 as a critical determinant of uterine P4 actions in preparing the uterus receptive for blastocyst implantation. Although genetic and pharmacological evidence has previously established that P4 action via nuclear PR is essential to ovulation, implantation, and pregnancy maintenance, it is remarkable to see that a particular cochaperone for PR is so critical and specific to uterine preparation for implantation. Apparently normal ovulation and a less severe fertilization phenotype as compared to uterine preparation for implantation suggest differential sensitivity of the ovary and uterus to FKBP52-mediated P4 action. This tissue-specific differential sensitivity is not noted in Pgr mutant mice in which both severely compromised ovarian and uterine functions are responsible for female infertility. The physiological significance of PR-associated immunophilins has been underappreciated, and the dramatic infertility of mice lacking Fkbp52 lends credence to the significance of this immunophilin cochaperone in female fertility. This study places FKBP52 as a target for improving female fertility or alternatively developing novel approaches to contraception.
Supplementary Material
Supporting Table
Acknowledgments
This work was supported in parts by the National Institutes of Health and the Mayo Foundation. S. K. Dey is recipient of Method to Extend Research in Time Awards from the National Institute of Child Health and Human Development and the National Institute on Drug Abuse. H.W. is recipient of Solvay/Mortola Research Award from the Society for Gynecologic Investigation. S.T. and M.B.C. are supported by National Institutes of Health Training Grants 5 T 32 DK07563 and 1 F32 DK068983, respectively.
Author contributions: S.T., J.C.-F., T.D., H.W., D.F.S., and S. K. Dey designed research; S.T., J.C.-F., T.D., V.P., M.B.C., H.X., H.W., and S. K. Das performed research; S.T., J.C.-F., T.D., V.P., M.B.C., H.W., S. K. Das, D.F.S., and S. K. Dey analyzed data; and S.T., D.F.S., and S. K. Dey wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: P4, progesterone; PR, P4 receptor.
References
- 1.Paria, B. C., Reese, J., Das, S. K. & Dey, S. K. (2002) Science 296**,** 2185-2188. [DOI] [PubMed] [Google Scholar]
- 2.Dey, S. K., Lim, H., Das, S. K., Reese, J., Paria, B. C., Daikoku, T. & Wang, H. (2004) Endocr. Rev. 25**,** 341-373. [DOI] [PubMed] [Google Scholar]
- 3.Mulac-Jericevic, B., Mullinax, R. A., DeMayo, F. J., Lydon, J. P. & Conneely, O. M. (2000) Science 289**,** 1751-1754. [DOI] [PubMed] [Google Scholar]
- 4.Silverstein, A. M., Galigniana, M. D., Kanelakis, K. C., Radanyi, C., Renoir, J. M. & Pratt, W. B. (1999) J. Biol. Chem. 274**,** 36980-36986. [DOI] [PubMed] [Google Scholar]
- 5.Barent, R. L., Nair, S. C., Carr, D. C., Ruan, Y., Rimerman, R. A., Fulton, J., Zhang, Y. & Smith, D. F. (1998) Mol. Endocrinol. 12**,** 342-354. [DOI] [PubMed] [Google Scholar]
- 6.Pratt, W. B. & Toft, D. O. (1997) Endocr. Rev. 18**,** 306-360. [DOI] [PubMed] [Google Scholar]
- 7.Ratajczak, T., Ward, B. K. & Minchin, R. F. (2003) Curr. Top. Med. Chem. 3**,** 1348-1357. [DOI] [PubMed] [Google Scholar]
- 8.Nair, S. C., Rimerman, R. A., Toran, E. J., Chen, S., Prapapanich, V., Butts, R. N. & Smith, D. F. (1997) Mol. Cell. Biol. 17**,** 594-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davies, T. H. & Sanchez, E. R. (2005) Int. J. Biochem. Cell. Biol. 37**,** 42-47. [DOI] [PubMed] [Google Scholar]
- 10.Cheung-Flynn, J., Prapapanich, V., Cox, M. B., Riggs, D. L., Suarez-Quian, C. & Smith, D. F. (2005) Mol. Endocrinol. 19**,** 1654-1666. [DOI] [PubMed] [Google Scholar]
- 11.Denny, W. B., Prapapanich, V., Smith, D. F. & Scammell, J. G. (2005) Endocrinology 146**,** 3194-3201. [DOI] [PubMed] [Google Scholar]
- 12.Hubler, T. R., Denny, W. B., Valentine, D. L., Cheung-Flynn, J., Smith, D. F. & Scammell, J. G. (2003) Endocrinology 144**,** 2380-2387. [DOI] [PubMed] [Google Scholar]
- 13.Song, H., Lim, H., Paria, B. C., Matsumoto, H., Swift, L. L., Morrow, J., Bonventre, J. V. & Dey, S. K. (2002) Development (Cambridge, U.K.) 129**,** 2879-2889. [DOI] [PubMed] [Google Scholar]
- 14.Daikoku, T., Tranguch, S., Friedman, D. B., Das, S. K., Smith, D. F. & Dey, S. K. (2005) Mol. Endocrinol. 19**,** 683-697. [DOI] [PubMed] [Google Scholar]
- 15.Das, S. K., Wang, X. N., Paria, B. C., Damm, D., Abraham, J. A., Klagsbrun, M., Andrews, G. K. & Dey, S. K. (1994) Development (Cambridge, U.K.) 120**,** 1071-1083. [DOI] [PubMed] [Google Scholar]
- 16.Todaro, G. J. & Green, H. (1963) J. Cell. Biol. 17**,** 299-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lydon, J. P., DeMayo, F. J., Funk, C. R., Mani, S. K., Hughes, A. R., Montgomery, C. A., Jr., Shyamala, G., Conneely, O. M. & O'Malley, B. W. (1995) Genes. Dev. 9**,** 2266-2278. [DOI] [PubMed] [Google Scholar]
- 18.Mulac-Jericevic, B., Lydon, J. P., DeMayo, F. J. & Conneely, O. M. (2003) Proc. Natl. Acad. Sci. USA 100**,** 9744-9749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hou, Q. & Gorski, J. (1993) Proc. Natl. Acad. Sci. USA 90**,** 9460-9464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shimada, M., Nishibori, M., Yamashita, Y., Ito, J., Mori, T. & Richards, J. S. (2004) Endocrinology 145**,** 4603-4614. [DOI] [PubMed] [Google Scholar]
- 21.Ye, X., Hama, K., Contos, J. J., Anliker, B., Inoue, A., Skinner, M. K., Suzuki, H., Amano, T., Kennedy, G., Arai, H., et al. (2005) Nature 435**,** 104-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Daikoku, T., Matsumoto, H., Gupta, R. A., Das, S. K., Gassmann, M., DuBois, R. N. & Dey, S. K. (2003) J. Biol. Chem. 278**,** 7683-7691. [DOI] [PubMed] [Google Scholar]
- 23.Das, S. K., Chakraborty, I., Paria, B. C., Wang, X. N., Plowman, G. & Dey, S. K. (1995) Mol. Endocrinol. 9**,** 691-705. [DOI] [PubMed] [Google Scholar]
- 24.Matsumoto, H., Zhao, X., Das, S. K., Hogan, B. L. & Dey, S. K. (2002) Dev. Biol. 245**,** 280-290. [DOI] [PubMed] [Google Scholar]
- 25.Lim, H., Ma, L., Ma, W. G., Maas, R. L. & Dey, S. K. (1999) Mol. Endocrinol. 13**,** 1005-1017. [DOI] [PubMed] [Google Scholar]
- 26.Paria, B. C., Das, N., Das, S. K., Zhao, X., Dileepan, K. N. & Dey, S. K. (1998) Endocrinology 139**,** 3958-3966. [DOI] [PubMed] [Google Scholar]
- 27.McMaster, M. T., Teng, C. T., Dey, S. K. & Andrews, G. K. (1992) Mol. Endocrinol. 6**,** 101-111. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Table