Subcellular distribution of Wnt pathway proteins in normal and neoplastic colon (original) (raw)
Abstract
Mutations in the APC tumor suppressor gene are present in approximately 85% of colorectal tumors and are thought to contribute early in the process of tumorigenesis. The truncated protein resulting from most APC mutations can lead to elevated β-catenin levels in colon tumor cells. APC and associated proteins thus form a β-catenin regulatory complex, with axin playing a key role. Although cell culture studies have revealed intriguing aspects of this complex, little characterization has been done in human colonocytes, the target tissue of colon carcinogenesis. The present study of intact human colon crypts, adenomatous polyps, and adenocarcinomas focuses on subcellular localization of some key elements of the complex: β-catenin, APC, axin, and axin2. We examined endogenous protein localization within the framework of three-dimensional tissue architecture by using laser scanning confocal microscopy, and immunofluorescence staining of whole-mount fixed tissue from more than 50 patients. Expression patterns suggest that APC and axin colocalize in the nucleus and at lateral cell borders, and show that axin2 is limited to the nucleus. Altered nuclear expression of axin seen in colon polyps and carcinomas may be a consequence of the loss of full-length APC and the advent of nuclear β-catenin. The observation of nuclear β-catenin in fewer than half of carcinoma images and only rarely in polyps indicates that nuclear translocation of β-catenin may not be an immediate consequence of the loss of APC.
Germ-line mutations in APC are associated with an inherited colorectal cancer syndrome, familial adenomatous polyposis. Somatic mutations are found in a majority of sporadic adenomatous colon polyps and adenocarcinomas. Identification of β-catenin as a binding partner of, and target for proteolytic regulation by APC initially suggested a role for APC in cell adhesion. Later, β-catenin was shown to be present in the nucleus in association with a transcription factor, suggesting a nuclear regulatory role for APC. We now understand APC to be a member of the Wnt signaling pathway, a nuclear shuttling protein, and a participant in chromosome segregation (1).
The role of β-catenin in the Wnt pathway was identified by work done in embryos and in cultured cells. One of the most important findings was the identification of axin as part of a multimeric complex with APC. Axin and APC form a scaffold, mediating the interaction of regulatory proteins such as GSK3β with β-catenin. The sum of a decade of in vitro investigation shaped an influential paradigm and led to a model describing the molecular mechanisms of colon carcinogenesis. This model asserts that as APC/axin-mediated degradation of β-catenin is inhibited by Wnt signaling or mutation, levels of cytoplasmic and nuclear β-catenin become elevated, leading to cell proliferation. As a test of this model for colon carcinogenesis, it is of interest to compare these in vitro predictions with in vivo observations. This study was thus undertaken in an effort to validate current molecular models in the context of human colon tissue.
The human colon is a mucosal membrane adapted for water absorption and mucus production (2). Its inner surface is densely carpeted with tube-shaped invaginations of epithelial cells known as the crypts of Lieberkühn. These crypts are contiguous with the luminal surface of the colon and expand its total surface area to roughly 20 m2 (3). Stem cells located near the base of each crypt give rise to a proliferative compartment that extends midway up the crypt and is responsible for completely regenerating the lining of the colon every 5–7 days (4). Immunofluorescence staining was used to reveal the subcellular localization of APC, β-catenin, and axin proteins in crypts from normal colonic mucosa, adenomatous polyps (benign precursor lesions), and adenocarcinomas.
Based on the model described above, we expected to find β-catenin localized in the nucleus and cytoplasm in proliferative regions of normal crypts. Furthermore, we expected adenomatous polyps and adenocarcinomas to demonstrate uniform nuclear and cytoplasmic β-catenin accompanying the loss of APC throughout. Some of these initial expectations were met completely, some partially, and others, surprisingly, were not met at all.
Materials and Methods
Tissue Acquisition.
Tissues from grossly normal colon and sporadic adenomas and carcinomas were obtained through the Huntsman Cancer Institute Tissue Access Core Facility (University of Utah). Samples were collected from surgical pathology immediately following surgery, and maintained in CO2-Independent Media (Life Technologies) on ice until processing. Tissue was pinned flat and fixed in fresh 2% paraformaldehyde + 0.1% Triton X-100 in PBS for 1 h at 4°C. Following a brief PBS wash, tissue was visualized under a dissecting microscope and individual crypts separated from surrounding tissue with microscalpels. Crypts were then affixed to Teflon printed slides (Electron Microscopy Sciences, Fort Washington, PA) previously coated with Cell Tak Cell and Tissue Adhesive (Collaborative Biomedical Products, Bedford, MA), and stained immediately.
Antibodies.
β-catenin expression was assessed using a mouse monoclonal antibody Clone 14 (1:700; Transduction Laboratories, Lexington, KY) and a rabbit polyclonal antibody (1:100; Sigma). A panel of APC antibodies was used. C-terminal mouse monoclonals: Ab2 (1:10), Ab4 (1:50), and Ab6 (1:10) (Oncogene Research, Cambridge, MA). C-terminal rabbit polyclonals: C20 (1:300; Santa Cruz Biotechnology) and APC 64 (1:100) and APC 34 (middle region; 1:100) (both gifts from Arnold Levine). N-terminal monoclonals: Ab1 (1:50), Ab3 (1:20), Ab5 (1:20), and Ab7 (1:20) (Oncogene Research) and N-terminal polyclonal N15 (1:100; Santa Cruz Biotechnology). Axin expression was evaluated using rabbit polyclonal AX1 (1:30; Zymed) and results were corroborated using two additional axin antibodies made in rabbit (gifts of R. Nusse, Stanford University School of Medicine, Stanford, CA), 288–514 (1:30), and 1 + 3 (1:30) (data not shown). Axin2 expression was evaluated using rabbit polyclonal (1:30; Zymed). GSK3β was assessed using rabbit polyclonal (1:25; Chemicon) and APC2 was determined using goat polyclonals, A-17 and T-17 (1:20; Santa Cruz Biotechnology) (data not shown). The secondary antibodies and reagents used were: Alexa 488 goat anti-mouse IgG, F(ab′)2 (1:1,200), Alexa 568 goat anti-rabbit IgG (1:400), Alexa 568 phalloidin (1:100), and nuclear counterstain To-Pro 3 (1:1,500), all from Molecular Probes.
Immunofluorescence Microscopy.
Slide-mounted crypts were permeabilized using 0.2% Triton X-100 in PBS for 1 h at 20°C. This and all subsequent incubations and washes were carried out on an orbital shaker. Samples were then blocked (1% BSA/5% normal goat serum/0.02% Triton X-100 in TBS) overnight at 4°C. Wash buffer (1% BSA/0.5% normal goat serum/0.02% Triton X-100 in TBS) was used for all subsequent dilutions and washes. Primary antibodies were added to samples for 2–3 days at 4°C. Slides were washed for 4 h at 20°C, secondary antibodies applied for 1 h at 20°C, and the slides washed overnight at 4°C. Actin was stained in some samples by applying Alexa 568 Phalloidin for 20 min, and nuclei were then counterstained using ToPro-3 for 10 min in the last wash. Slides were mounted in ProLong Antifade Mounting Media (Molecular Probes). Images were collected using an Olympus Fluoview 200 (New Hyde Park, NY) laser scanning confocal microscope. Microscopic fields were selected for scanning based on focus and clarity of the various elements. Proteins in the image were scored for nuclear, cytoplasmic, or cell border expression, and the image was scored positive if the field contained one or more positive cells. Nuclear expression was scored positive if the general shape or location of the nucleus could be determined from the protein expression alone.
Cell Fractionation and Immunoblot.
293 cells and HCT 116 cells were fractionated essentially as described (5, 6). Antibodies were used to probe western immunoblots at the following dilutions: Axin (AX1, Zymed) 1:500, 1 + 3 (1:2,000), α-tubulin (ICN, 1:500), lamin B (Calbiochem, 1:200), goat anti-rabbit IgG-HRP conjugate (Bio-Rad, 1:25,000), goat-anti-mouse IgG1-HRP conjugate (Accurate Chemical, 1:33,000).
Results
Dissection of Whole Crypts from Fixed Tissue Preserved Antigen and Allowed Selection of Precise Focal Planes.
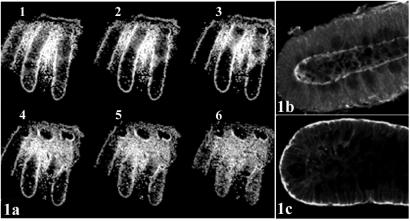
Optical sectioning of whole-mount crypts, using laser scanning confocal microscopy, enabled selection of complete longitudinal sections for analysis (Fig. 1a). Crypts manually dissected intact from fixed tissue (Fig. 1b) ensured preservation of protein localization, which may be altered in the course of chemical or enzymatic isolation of crypts from tissue before fixation (Fig. 1c). Because immunofluorescence is typically performed on monolayer cultured cells or thin tissue sections, standard protocols required modification to optimize fixation and allow for adequate permeabilization and access of reagents to whole-mount tissue. Tissues from more than 50 patients were examined, with careful attention to consistent handling and fixation. To avoid the pitfalls of relying on a single antibody, where possible, a panel of antibodies was investigated for each protein. In all, protein expression patterns revealed using ten different APC antibodies, two β-catenin antibodies, three axin antibodies, and one axin2 antibody were examined. Staining patterns of the individual antibodies were compared with one another and conclusions were based on consensus. Commercial APC monoclonal antibodies Ab1 and Ab4 were tested for specificity by preincubation with an APC peptide containing the epitope (10-fold excess) and commercial polyclonal antibodies were tested similarly, using control peptides provided by the vendors (see Fig. 5, which is published as supporting information on the PNAS web site, www.pnas.org).
Figure 1.
(a) Serial optical sections of mouse colon crypts labeled with the nuclear counterstain To-Pro 3. Sequential images along the _Z_-axis were collected using laser scanning confocal microscopy. Optical section 2 gives the clearest longitudinal section of the center crypt. To compare tissue preparation methods, colon tissue from a single patient was either fixed and the crypts manually microdissected (b), or treated with chelating agents to release the crypts, which were then fixed (c). Crypts were immunostained for APC and the protein localization compared. Crypts manipulated before fixation (c) exhibited altered protein localization.
Full-Length APC Is Expressed in the Nucleus and at Lateral Cell Borders in Normal Crypts.
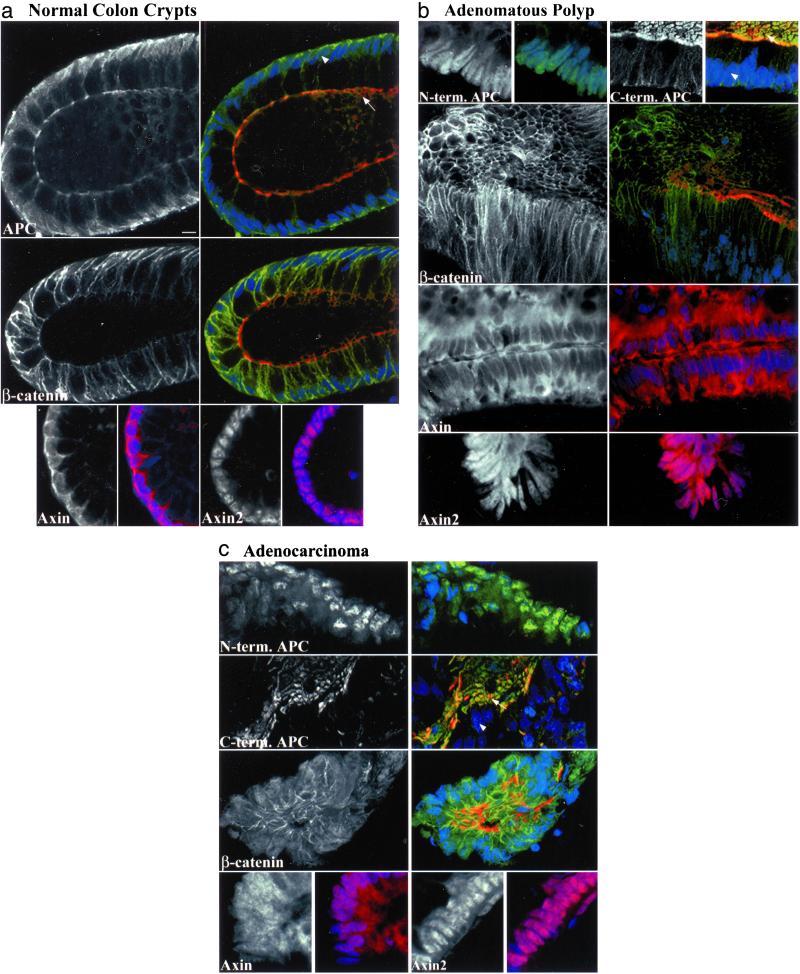
APC protein has been observed in cultured cells at cell–cell junctions, associated with microtubules at the leading edge of migrating cells, in the cytoplasm, in the nucleus, and at the apical membrane (1, 7). It was of interest to determine in colonocytes whether APC and its binding partner β-catenin reside at sites analogous to those seen in cell culture systems. As expected, normal crypts showed strong expression of β-catenin at lateral cell junctions (Fig. 2a and Table 1). However, nuclear and cytoplasmic β-catenin were rarely seen and then only faintly above background.
Figure 2.
Expression of APC, β-catenin, axin, and axin2 in optical sections of whole-mount human colon tissue. The basic units of normal colonic mucosa, the crypts, are vase-shaped structures comprised of rectangular epithelial cells, stacked lengthwise. These cells have an apical side that faces the crypt lumen and a basal side that houses the nucleus. A grayscale image shows the primary antibody alone. Three-color merged images of APC or β-catenin show primary antibody in green, a nuclear counterstain in blue, and phalloidin-labeled actin in red, delineating a ring of adhesion proteins just below the apical cell membrane. The two-color merged images show axin or axin2 in red with a nuclear counter stain in blue. Arrows indicate apical surfaces and arrowheads mark nuclei. In normal crypts (a), APC and axin are found diffusely in the nucleus and near the borders between cells. APC is especially prominent near the apical junctions. β-catenin is found at lateral cell junctions and axin2 is nuclear. Adenomatous polyps (b) show strong nuclear N-terminal (truncated) APC, whereas C-terminal (full-length) APC and axin are missing or reduced in the nucleus. C-terminal APC is variably retained at cell–cell borders, and near the face of the apical membrane. Axin is strongly cytoplasmic. The β-catenin pattern is similar to that in normal crypts and axin2 remains nuclear. In carcinoma (c), expression patterns of the four proteins are similar to those in polyps except that β-catenin and axin are found in the nucleus. (Scale bar, 10 μm.)
Table 1.
Tissue staining summary
| No. of images | % Images positive | |||
|---|---|---|---|---|
| Nucleus | Cytoplasm | Border | ||
| β catenin | ||||
| Normal | 58 | 3 | 2 | 100 |
| Polyp | 94 | 9 | 1 | 98 |
| Carcinoma | 119 | 45 | 18 | 95 |
| N-terminal APC | ||||
| Normal | 122 | 99 | 4 | 94 |
| Polyp | 98 | 90 | 49 | 33 |
| Carcinoma | 197 | 97 | 60 | 40 |
| C-terminal APC | ||||
| Normal | 156 | 90 | 7 | 96 |
| Polyp | 78 | 14 | 10 | 77 |
| Carcinoma | 148 | 11 | 24 | 88 |
| Axin | ||||
| Normal | 54 | 91 | 46 | 96 |
| Polyp | 65 | 35 | 80 | 92 |
| Carcinoma | 109 | 65 | 74 | 68 |
| Axin2 | ||||
| Normal | 29 | 100 | 0 | 0 |
| Polyp | 40 | 100 | 18 | 8 |
| Carcinoma | 81 | 99 | 28 | 2 |
APC expression was determined using a panel of ten antibodies raised against the middle, N terminus, or C terminus of APC. Overall staining patterns were similar among the ten antibodies tested (see Table 2, which is published as supporting information on the PNAS web site). Normal crypt cells showed an APC localization pattern characterized by lateral cell border and diffuse nuclear expression (Fig. 2a and Table 1). This pattern was consistent in intensity and localization, throughout the crypt. APC was also especially pronounced at cell junctions near the apical adhesion complex, particularly with the C-terminal antibodies. Thus, APC could be found near presumed sites of β-catenin function, at cell junctions, and in the nucleus.
Axin and Axin2 Localize with Full-Length APC in Distinct Compartments in Normal Colon.
The closely related proteins axin and axin2 bind and interact with APC, β-catenin, GSK3β, and one another. As shown in Fig. 2a, axin appeared, as did APC, diffusely in the nucleus and along lateral cell membranes, near β-catenin. In addition, axin was often found in the cytoplasm, also a site of GSK3β expression (data not shown). Fractionation of cells from two human epithelial lines, HCT116 and 293, confirmed the axin localization pattern seen in tissue. Axin was found primarily in the nucleus of HCT 116 cells and in both the nucleus and cytoplasm of 293 cells (Fig. 6, which is published as supporting information on the PNAS web site, and data not shown). Surprising in light of their similarities, axin and axin2 had distinct expression patterns. Axin2 was found strongly and exclusively in the nuclei of normal crypt cells, whereas axin was found near each of the other proteins: APC, β-catenin, and axin2 (Fig. 2a, Table 1).
Nuclear Expression of Full-Length APC Is Lost in Polyp and Carcinoma.
To determine whether truncated APC protein is aberrantly localized relative to full-length APC, a series of sporadic polyp and carcinoma samples was stained. Mutations of APC typically result in a protein lacking C-terminal regions. Antibodies directed against N- and C-terminal portions of APC gave staining patterns distinct from one another in polyp and carcinoma, and were therefore used to distinguish between truncated and full-length protein, respectively. N-terminal (truncated) APC staining was almost always present in the nucleus, was frequently seen in the cytoplasm, and was diminished at cell junctions of polyp and carcinoma, whereas C-terminal (full-length) APC staining was largely absent from cell nuclei (Fig. 2 b and c and Table 1). Although missing from nuclei, weak variable C-terminal APC expression was retained at some cell margins and especially at the surface of remnants of the apical membranes in areas of both polyp and carcinoma. Although not detected with N-terminal antibodies, this bright apical pattern was identical when using multiple C-terminal antibodies, was abrogated by preincubation of antibody with appropriate peptide, and was inconsistent with the expression pattern of APC homologue APC2 in these lesions (data not shown).
Nuclear Expression of β-Catenin Is Found in Carcinoma, but Not in Polyp.
Elevated nuclear and cytoplasmic β-catenin is thought to be a consequence of the inability of mutant APC to target it for proteasome-mediated proteolysis. We therefore examined the localization of β-catenin in presumed APC-mutant lesions. Similar to the pattern seen in normal tissue, β-catenin was localized at cell–cell junctions in both polyp and carcinoma, but surprisingly was almost never seen in the nucleus or cytoplasm in polyp. In carcinomas, cytoplasmic β-catenin was only occasionally seen and nuclear β-catenin was found in only about half of the images (Fig. 2 b and c and Table 1).
Nuclear Axin Expression Is Reduced in Polyp but Reappears in Carcinoma.
Disappearance of full-length APC from the nucleus in polyps was coincident with a decrease in nuclear axin, and an increase in cytoplasmic axin relative to normal (Fig. 2b and Table 1). In carcinoma, however, nuclear axin was again present in a majority of images (Fig. 2c and Table 1). As in normal tissue, axin2 expression was strongly nuclear in both polyp and carcinoma (Fig. 2 b and c, Table 1), but was seen occasionally in the cytoplasm as well.
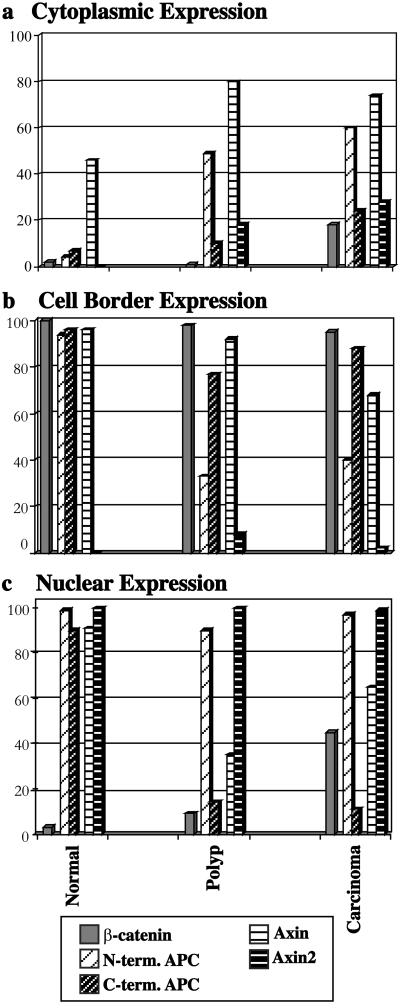
Using data from Table 1, localization patterns of the four proteins studied were grouped according to site of expression (Fig. 3). Presenting the data in this manner illustrates the dynamic nature of the proteins and suggests possible protein interactions. Cytoplasmic expression of all four proteins increased generally as tissue progressed from normal to malignant (Fig. 3a). Cell border expression of N-terminal-stained APC in polyp and carcinoma was diminished (Fig. 3b), suggesting that C-terminal domains of APC may be involved in its lateral distribution.
Figure 3.
Graphic representation of data from Table 1, grouped by site of expression, cytoplasmic, cell border, or nuclear. In a, cytoplasmic expression of axin is seen in normal cells, but is found with greater frequency in polyp and carcinoma, as are cytoplasmic N-terminal (truncated) APC and axin2. Incidence of cytoplasmic β-catenin also rises slightly in carcinoma. In b, cell border expression of N-terminal APC is diminished in polyp and carcinoma. In c, nuclear expression of APC, axin, and axin2 is seen in normal cells. Nuclear C-terminal APC and axin are diminished in polyp, but nuclear axin is found again in carcinoma in conjunction with the expression of nuclear β-catenin.
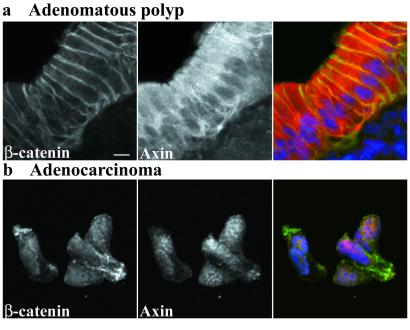
Nuclear β-Catenin and Nuclear Axin Colocalize in Carcinoma Cells.
The correlation of nuclear axin and nuclear β-catenin expression in carcinomas (Fig. 3c) suggested that the proteins might be associated in the nucleus. Dual staining of axin and β-catenin in polyp and carcinoma samples revealed that both proteins were absent from nuclei in the polyp sample (Fig. 4a), but appeared in close association in nuclei of the carcinoma cells (Fig. 4b).
Figure 4.
Double staining of β-catenin and axin in adenomatous polyp and adenocarcinoma. The polyp image (a) shows β-catenin and axin near cell–cell junctions, and axin strongly in the cytoplasm. Neither protein is obvious in the nucleus. The carcinoma image (b) shows both β-catenin and axin in the nucleus. Primary antibodies in each double-stained image are presented singly in grayscale, and in a three-color merged image showing β-catenin in green, axin in red, and nuclei in blue. (Scale bar, 10 μm.)
Discussion
Our current understanding indicates that axin provides a spatial template for the association of APC, β-catenin, and axin2 in a β-catenin degradation complex and our model predicts that Wnt-induced inhibition of this degradation leads to elevated levels of β-catenin in the cytoplasm and nucleus, and transcriptional activation of target genes (1).
In this study, APC was observed in normal crypt cells near sites of β-catenin function: at lateral cell margins, especially near the apical adhesion belt, and diffusely in the nucleus (Fig. 2a). APC expression at lateral cell borders and near apical membranes in mouse and human tissue has been reported (7–10). Nuclear APC was first characterized in tissue culture cells (6), but has also been reported in mouse and human colon tissue (11, 12). The subcellular localization of APC thus reflects its function as a regulator of β-catenin.
Axin has been interpreted as a scaffold protein for the β-catenin degradation complex (13), and APC's ability to bind axin is required for its mediation of β-catenin degradation (14). In this study, we found that normal cells displayed axin at sites where other members of the complex were expressed. Axin was found at lateral cell margins with APC and β-catenin, diffusely in the nucleus with APC and axin2, and occasionally in the cytoplasm where GSK3β was also seen (Fig. 2a and data not shown). Ectopically expressed axin in Xenopus has also been seen at the plasma membrane (15). Unexpectedly, we found axin2, the human homologue of mouse conductin and close relative of axin, to be strongly, uniformly, and exclusively nuclear in normal crypt cells. Despite the similarities between the two proteins, the location and intensity of their expression patterns were distinct from one another. The fact that members of the degradation complex were seen singly and in combination in different cellular compartments (Fig. 3) suggests a dynamic equilibrium rather than a static complex.
Elements of the β-catenin regulation complex were thus found at relevant locations as expected, but there were no detectable amounts of the β-catenin protein itself in normal crypt cells except at cell junctions, even in regions expected to be mitotically active. Although β-catenin was not obvious in the nucleus or cytoplasm, it may have been present there at levels too low or too transient to be readily detected by staining. The role of APC, axin, and axin2 in the nuclei of normal crypt cells thus may be to ensure that β-catenin is maintained at only very low levels, if at all.
Mutation of APC is the earliest known step in the transition from normal crypt to adenocarcinoma. Our data showed a loss of C-terminal APC from the nucleus in sporadic adenomatous polyps and adenocarcinomas, whereas truncated protein retained a strong nuclear presence (Fig. 3). Nuclear expression of truncated APC protein has been reported in cultured mammalian cells (16, 17). Although truncated APC typically lacks the two known nuclear localization signals (5), it is reported to be capable of nuclear translocation and usually retains at least one known nuclear export signal (16, 18). The presence of truncated APC in both nucleus and cytoplasm in this study lends support to the idea that it continues to shuttle between the two (16, 18). That truncated APC is diminished at cell junctions suggests the protein may have lost domains crucial to its localization there. Surprisingly, although full-length APC protein was missing from nuclei in cells of the lesions we studied, it could be found weakly expressed at some lateral cell borders and strongly expressed at the surface of vestiges of the apical membranes (Fig. 2 b and c). This apical surface expression was seen with multiple C-terminal antibodies and was eliminated by blocking with peptide. It was never found in normal tissue and was distinct from the apical junction staining typical of normal crypts. Neither was it detected using N-terminal antibodies. One explanation for the presence of C-terminal antibody-reactive APC protein in locations unique to colon tumors is that it may represent the product of a mutation-induced splice form. APC splice forms specific to particular tissues or states of differentiation have been reported (reviewed in ref. 19). In fact, in both cells and tissue, smaller APC isoforms shown by immunoblot were recognized by antibodies directed against both N- and C-terminal portions of the protein (12, 20). In addition, several known splice forms do eliminate exon 1 (21), which might explain the failure of N-terminal antibodies to detect expression in this instance.
We found that altered expression of APC and β-catenin in polyps and carcinomas was accompanied by altered distribution of axin (Fig. 3). In polyps, axin was diminished in the nucleus and increased in the cytoplasm relative to normal. Because both APC and β-catenin are capable of entering the nucleus independently (5, 22), it is possible that entry of axin into the nucleus depends on one or both. Alternatively, axin might be capable of independent nuclear import and export, but be stabilized in the nucleus through binding to APC or β-catenin. The most common mutations of APC result in a truncated protein lacking known binding sites for axin. The truncated APC in polyps, although still able to localize in the nucleus, may have been unable to transport or stabilize axin there, resulting in diminished nuclear and increased cytoplasmic axin. In our study, nuclear axin was increased in carcinoma relative to polyp (Fig. 3c). This increase was coincident with the appearance of nuclear β-catenin, suggesting that perhaps β-catenin and APC are both able to facilitate the nuclear localization of axin.
The role of axin2 in degradation of β-catenin and in the events leading to colon carcinogenesis is unknown. In this study, axin2 distribution was strongly and uniformly nuclear in all instances (Fig. 3). The only easily discernable difference in axin2 expression between normal crypt and APC-mutant lesion was modest cytoplasmic expression in a subset of tumors. The few facts known about axin2 are intriguing: interaction with β-catenin, APC, axin, and GSK3β, overexpression in colorectal cancer, and the observation that its mutant form is linked to colorectal tumor formation (23, 24). It has been suggested, based on a study of axin mutations in hepatocellular cancer (25), that because axin2 fails to compensate for the mutant axin in those tumors, there may be differences between the two proteins in function and/or expression (26). Liu et al. (24) identified a set of axin2 mutations in colorectal tumors. All had lost the DIX domain, a domain common to both axin and axin2, and known to be necessary for the homo-oligomerization of axin (27). If the DIX domain were also a site of interaction between axin and axin2, our data indicate that this interaction would likely occur in the nucleus, suggesting a new set of associations.
Nuclear expression of β-catenin was not seen in adenomatous polyps in this study, but was found in carcinomas in 45% of images (Fig. 3c). Our data fail to demonstrate a direct correlation between mutation of APC and elevated nuclear and cytoplasmic β-catenin. Although a consensus is lacking with respect to β-catenin localization in human colon tumors, nuclear β-catenin has been described as an infrequent occurrence rather than a homogeneous feature (28–31). One such study found nuclear β-catenin absent in adenomatous polyps and present in only 42% of carcinomas (28). Another group reported nuclear β-catenin primarily in cells at the invasion front and not homogeneously throughout tumors (29), suggesting that APC mutation alone was not sufficient to cause its induction. These studies suggest tumor architecture or stromal influences as modifying factors.
Mutation of APC initiates a sequence of events that can culminate in colon carcinogenesis. Our data suggest the initial consequence of APC loss may not be nuclear accumulation of β-catenin, but aberration of crypt architecture instead. Work done in the small-intestine polyps of mice heterozygous for APC mutations characterized alterations in crypt form and cell migration rates (32, 33). If this work proves relevant to the large-intestine polyps that occur in humans, it implicates crypt structural mechanics as the first casualty of APC mutation. It has been shown that tight junctions in adenomatous polyps and carcinomas are more permeable than those in normal colonic epithelia (34). This might allow colonocytes in these lesions access to stromal factors and other agents normally excluded from the crypt. Recent work using human breast cancer cells showed that exposure of the cells to growth factor triggered the release of β-catenin from cell junctions and its subsequent translocation to the nucleus (35). Adenomatous tissues in the colon, influenced by abnormal external stimuli, might also respond by releasing cell-junctional β-catenin, which could then enter the nucleus, contributing to an invasive phenotype. The observation of nuclear β-catenin only at the invasion front and not in the interior of colon carcinomas (29) is consistent with this scenario. The benign lesions that arise following APC mutation may, because of increased permeability and longer cell transit times, be left vulnerable to an environment not normally encountered, to further mutation, and to the acquisition of a malignant phenotype.
Supplementary Material
Supporting Information
Acknowledgments
We gratefully acknowledge the efforts of Leona Conn, in whose memory this work is dedicated; Kerri Harris and Ryan Jones for collection of tissue; Mary Scriven for graphics expertise; Dr. Wade Samowitz for helpful discussions; and Dr. David Virshup for critical review. This work was supported by National Cancer Institute Grant 5P01 CA73992-02 and the Huntsman Cancer Institute.
References
- 1.Fearnhead N S, Britton M P, Bodmer W F. Hum Mol Genet. 2001;10:721–733. doi: 10.1093/hmg/10.7.721. [DOI] [PubMed] [Google Scholar]
- 2.Junqueira L C, Carneiro J, Kelley R O. Basic Histology. Norwalk, CT: Appleton & Lange; 1992. p. 308. [Google Scholar]
- 3.Hall P A, Coates P J, Ansari B, Hopwood D. J Cell Sci. 1994;107:3569–3577. doi: 10.1242/jcs.107.12.3569. [DOI] [PubMed] [Google Scholar]
- 4.Marshman E, Booth C, Potten C S. BioEssays. 2002;24:91–98. doi: 10.1002/bies.10028. [DOI] [PubMed] [Google Scholar]
- 5.Zhang F, White R, Neufeld K. Proc Natl Acad Sci USA. 2000;97:12577–12582. doi: 10.1073/pnas.230435597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neufeld K L, White R L. Proc Natl Acad Sci USA. 1997;94:3034–3039. doi: 10.1073/pnas.94.7.3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reinacher-Schick A, Gumbiner B M. J Cell Biol. 2001;152:491–502. doi: 10.1083/jcb.152.3.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miyashiro I, Senda T, Matsumine A, Baeg G H, Kuroda T, Shimano T, Miura S, Noda T, Kobayashi S, Monden M, et al. Oncogene. 1995;11:89–96. [PubMed] [Google Scholar]
- 9.Senda T, Miyashiro I, Matsumine A, Baeg G H, Monden T, Kobayashil S, Monden M, Toyoshima K, Akiyama T. Biochem Biophys Res Commun. 1996;223:329–334. doi: 10.1006/bbrc.1996.0894. [DOI] [PubMed] [Google Scholar]
- 10.Midgley C A, White S, Howitt R, Save V, Dunlop M G, Hall P A, Lane D P, Wyllie A H, Bubb V J. J Pathol. 1997;181:426–433. doi: 10.1002/(SICI)1096-9896(199704)181:4<426::AID-PATH768>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 11.Wong M H, Hermiston M L, Syder A J, Gordon J I. Proc Natl Acad Sci USA. 1996;93:9588–9593. doi: 10.1073/pnas.93.18.9588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deka J, Herter P, Sprenger-Haussels M, Koosch S, Franz D, Muller K M, Kuhnen C, Hoffmann I, Muller O. Oncogene. 1999;18:5654–5661. doi: 10.1038/sj.onc.1202944. [DOI] [PubMed] [Google Scholar]
- 13.Hart M J, de los Santos R, Albert I N, Rubinfeld B, Polakis P. Curr Biol. 1998;8:573–581. doi: 10.1016/s0960-9822(98)70226-x. [DOI] [PubMed] [Google Scholar]
- 14.Kawahara K, Morishita T, Nakamura T, Hamada F, Toyoshima K, Akiyama T. J Biol Chem. 2000;275:8369–8374. doi: 10.1074/jbc.275.12.8369. [DOI] [PubMed] [Google Scholar]
- 15.Zeng L, Fagotto F, Zhang T, Hsu W, Vasicek T J, Perry W L, III, Lee J J, Tilghman S M, Gumbiner B M, Costantini F. Cell. 1997;90:181–192. doi: 10.1016/s0092-8674(00)80324-4. [DOI] [PubMed] [Google Scholar]
- 16.Henderson B. Nat Cell Biol. 2000;2:653–660. doi: 10.1038/35023605. [DOI] [PubMed] [Google Scholar]
- 17.Smits R, Kielman M F, Breukel C, Zurcher C, Neufeld K, Jagmohan-Changur S, Hofland N, van Dijk J, White R, Edelmann W, et al. Genes Dev. 1999;13:1309–1321. doi: 10.1101/gad.13.10.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neufeld K L, Nix D A, Bogerd H, Kang Y, Beckerle M C, Cullen B R, White R L. Proc Natl Acad Sci USA. 2000;97:12085–12090. doi: 10.1073/pnas.220401797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goss K H, Groden J. J Clin Oncol. 2000;18:1967–1979. doi: 10.1200/JCO.2000.18.9.1967. [DOI] [PubMed] [Google Scholar]
- 20.Pyles R B, Santoro I M, Groden J, Parysek L M. Oncogene. 1998;16:77–82. doi: 10.1038/sj.onc.1201505. [DOI] [PubMed] [Google Scholar]
- 21.Santoro I M, Groden J. Cancer Res. 1997;57:488–494. [PubMed] [Google Scholar]
- 22.Fagotto F, Gluck U, Gumbiner B M. Curr Biol. 1998;8:181–190. doi: 10.1016/s0960-9822(98)70082-x. [DOI] [PubMed] [Google Scholar]
- 23.Behrens J, Jerchow B A, Wurtele M, Grimm J, Asbrand C, Wirtz R, Kuhl M, Wedlich D, Birchmeier W. Science. 1998;280:596–599. doi: 10.1126/science.280.5363.596. [DOI] [PubMed] [Google Scholar]
- 24.Liu W, Dong X, Mai M, Seelan R S, Taniguchi K, Krishnadath K K, Halling K C, Cunningham J M, Boardman L A, Qian C, et al. Nat Genet. 2000;26:146–147. doi: 10.1038/79859. [DOI] [PubMed] [Google Scholar]
- 25.Satoh S, Daigo Y, Furukawa Y, Kato T, Miwa N, Nishiwaki T, Kawasoe T, Ishiguro H, Fujita M, Tokino T, et al. Nat Genet. 2000;24:245–250. doi: 10.1038/73448. [DOI] [PubMed] [Google Scholar]
- 26.Polakis P. Genes Dev. 2000;14:1837–1851. [PubMed] [Google Scholar]
- 27.Kishida S, Yamamoto H, Hino S, Ikeda S, Kishida M, Kikuchi A. Mol Cell Biol. 1999;19:4414–4422. doi: 10.1128/mcb.19.6.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kobayashi M, Honma T, Matsuda Y, Suzuki Y, Narisawa R, Ajioka Y, Asakura H. Br J Cancer. 2000;82:1689–1693. doi: 10.1054/bjoc.1999.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brabletz T, Jung A, Hermann K, Gunther K, Hohenberger W, Kirchner T. Pathol Res Pract. 1998;194:701–704. doi: 10.1016/s0344-0338(98)80129-5. [DOI] [PubMed] [Google Scholar]
- 30.Inomata M, Ochiai A, Akimoto S, Kitano S, Hirohashi S. Cancer Res. 1996;56:2213–2217. [PubMed] [Google Scholar]
- 31.Chung G G, Provost E, Kielhorn E P, Charette L A, Smith B L, Rimm D L. Clin Cancer Res. 2001;7:4013–4020. [PubMed] [Google Scholar]
- 32.Mahmoud N N, Boolbol S K, Bilinski R T, Martucci C, Chadburn A, Bertagnolli M M. Cancer Res. 1997;57:5045–5050. [PubMed] [Google Scholar]
- 33.Oshima H, Oshima M, Kobayashi M, Tsutsumi M, Taketo M M. Cancer Res. 1997;57:1644–1649. [PubMed] [Google Scholar]
- 34.Soler A P, Miller R D, Laughlin K V, Carp N Z, Klurfeld D M, Mullin J M. Carcinogenesis. 1999;20:1425–1431. doi: 10.1093/carcin/20.8.1425. [DOI] [PubMed] [Google Scholar]
- 35.Adam L, Vadlamudi R K, McCrea P, Kumar R. J Biol Chem. 2001;276:28443–28450. doi: 10.1074/jbc.M009769200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information