Quality control of transmembrane domain assembly in the tetraspanin CD82 (original) (raw)
Abstract
Retention of misfolded proteins in the endoplasmic reticulum (ER) is a primary mechanism of quality control. To discover whether quality control can monitor assembly inside the hydrophobic ER membrane, we characterized the folding and transport of the tetraspanin glycoprotein CD82. Truncated forms of CD82 that are missing one or more transmembrane segments remain in the ER. A construct (TM 2–4) that is missing the first transmembrane segment remains in the ER, even though its extracellular domain, which is facing the ER lumen, has folded to the native structure. Transport to the cell surface is restored by co-expressing the missing segment (TM 1) as a separate polypeptide. Prior to leaving the ER, CD82 transiently associates with the membrane-bound chaperone calnexin but not with its soluble homolog calreticulin. TM 2–4, in contrast, remains in a prolonged interaction with calnexin that is partially reversed by co-expressing TM 1. These findings establish a simple system to study transmembrane domain assembly, show that ER quality control can directly monitor assembly inside the lipid bilayer and suggest that calnexin may play a role in this process.
Keywords: calnexin/chaperone/endoplasmic reticulum/quality control/tetraspanin
Introduction
Protein folding in the endoplasmic reticulum (ER) is aided by chaperones and folding factors, which minimize irreversible aggregation and increase the efficiency of folding. Chaperones such as calnexin, calreticulin, BiP and GRP 94 interact specifically with proteins that have not yet attained their correct three-dimensional structure, forming a quality control apparatus that keeps partially folded or unassembled proteins in the ER and thereby prevents the harmful effects of their deployment (Hurtley and Helenius, 1989; Helenius et al., 1997). The details of how protein folding in the aqueous environment of the ER lumen is monitored are beginning to emerge (for a recent review see Ellgaard et al., 1999), but little is known of how folding inside the lipid bilayer might be scrutinized. The potential adverse consequences of expressing a misfolded ion channel, receptor or signal transduction molecule on the cell surface suggest that the cell must have some means to directly monitor folding within the lipid bilayer.
The tetraspanins are a recently discovered family of proteins that provide a simple model for studies of the quality control of transmembrane domain assembly. The tetraspanin CD82 is a 267-amino-acid glycoprotein found on the surface and in endosomal compartments of B cells (Hammond et al., 1998), macrophages and activated T cells, where it interacts with a large number of proteins including integrins, major histocompatibility complex (MHC) class I and II, co-stimulatory molecules and other tetraspanins (reviewed in Maecker et al., 1997). Like other tetraspanins, CD82 is thought to organize associated proteins into functionally important networks that influence cell growth and motility (Maecker et al., 1997). Compared with more complex polytopic membrane proteins, tetraspanins are structurally quite simple. One domain, composed of four membrane-spanning segments with a number of conserved hydrophilic residues, is buried in the lipid bilayer. The other major domain is a large extracellular loop between transmembrane segments 3 and 4 (Wright and Tomlinson, 1994; Maecker et al., 1997). Short N- and C-terminal regions (9 and 13 amino acids, respectively, for CD82) are found in the cytoplasm.
To determine whether ER quality control can monitor the intramolecular assembly of transmembrane segments, we characterized the folding and transport of truncated forms of CD82 that are missing one or more transmembrane segments. The results indicate that quality control can directly monitor assembly of the transmembrane domain of CD82 and suggest that calnexin may contribute to the retention of proteins with incomplete transmembrane domains.
Results
Characterization of the folding, glycosylation and transport of CD82
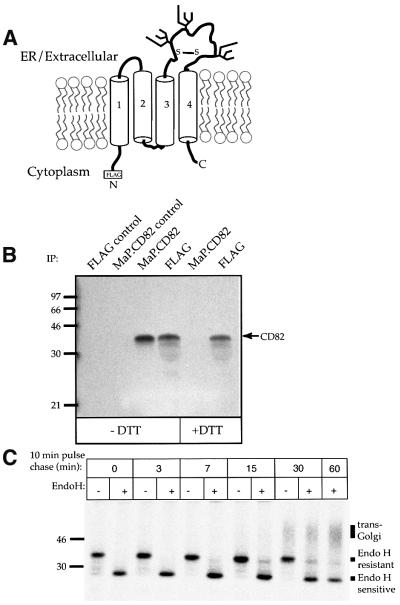
CD82 has six cysteine residues in the large extracellular domain between transmembrane segments 3 and 4 (Figure 1A). To determine whether these cysteines form disulfide bonds, COS-7 cells expressing FLAG-tagged CD82 were radiolabeled with [35S]methionine for 30 min. After solubilization, labeled proteins were immuno precipitated with the monoclonal antibody (mAb) MaP.CD82, M2 anti-FLAG or control mAb. Immune complexes were washed thoroughly and then disrupted by heating (3 min, 70°C) in a low concentration of SDS in the presence or absence of the reducing agent dithiothreitol (DTT). SDS was quenched by dilution into a 10-fold volume of 1% Triton X-100 buffer containing an excess of _N_-ethyl maleimide (NEM) to block free sulfhydryls (Braakman et al., 1991). After 30 min at room temperature, the released proteins were immunoprecipitated with MaP.CD82 or M2.
Fig. 1. Folding and transport of CD82. (A) A model of CD82 showing the four segments (labeled 1–4) of the transmembrane domain and the large extracellular loop with its six cysteine residues and three consensus glycosylation sites. Disulfide bond(s) are represented by a single S–S although their actual number is unknown. (B) MaP.CD82 recognizes only oxidized CD82. COS-7 cells expressing FLAG-tagged CD82 were labeled for 30 min with [35S]methionine. After solubiliz ation in 1% Triton X-100 buffer, labeled proteins were immuno precipitated with bead-conjugated MaP.CD82 or M2 anti-antibodies. Immune complexes were disrupted by warming to 70°C for 3 min in 0.2% SDS in the presence or absence of DTT. Released proteins were diluted 10-fold in TBS buffer containing 1% Triton X-100 and the alkylating agent NEM to quench SDS and block free sulfhydryls, left at room temperature for 30 min to allow refolding, and then immuno precipitated again with M2 or MaP.CD82. Precipitated glycoproteins were separated by 12.5% SDS–PAGE and visualized by fluorography. Controls are MaP.CD82 and M2 immunoprecipitations of lysates from COS-7 cells transfected with vector. (C) Glycosylation can be used to follow ER to Golgi transport of CD82. COS-7 cells expressing FLAG.CD82 were labeled for 10 min with [35S]methionine and chased with unlabeled methionine for 0–60 min. Total solubilized FLAG.CD82 was immunoprecipitated using a mixture of M2 and MaP.CD82 and treated or mock-treated with Endo H. The positions of Endo H-resistant, Endo H-sensitive and complex glycosylated (_trans_-Golgi) CD82 are indicated. Representative results from at least three independent experiments are shown in this and each of the following figures.
The mobility of CD82 on SDS–PAGE (∼35 kDa) is not altered by the reduction of disulfide bonds (Figure 1B). MaP.CD82 precipitated oxidized but not reduced CD82, indicating that disulfide bonds in the extracellular domain of CD82 stabilize the epitope recognized by this mAb (Figure 1B). Thus, recognition of the protein by MaP.CD82 is a useful indication that conformational maturation up to and including the formation of disulfide bonds has occurred.
To examine CD82 transport, cells were pulsed for 10 min with [35S]methionine and chased for 0–60 min with unlabeled methionine. Samples removed at various times of chase were immunoprecipitated with a mixture of MaP.CD82 and M2, washed and digested or mock-digested with the enzyme endoglycosidase H (Endo H). At the end of the pulse period, a prominent band of newly synthesized CD82 as well as several faint bands of lower molecular weight were visible (Figure 1C). These faint bands are due to incomplete utilization of glycosylation sites, a consequence of transient overexpression in COS-7 cells (Phan et al., 2000). Endo H treatment (Figure 1C) caused a decrease in the apparent molecular weight of CD82, consistent with removal of its three N-glycans (Figure 1A). A band of Endo H-resistant CD82 was visible after 15 min of chase (Figure 1C). After 30 min, the Endo H-resistant form of CD82 began to shift to a diffuse band of 40–70 kDa. This broad increase in apparent molecular weight occurs as a result of galactose and sialic acid addition in the _trans_-Golgi compartment (Kornfeld and Kornfeld, 1985; Rabouille et al., 1995; Hammond et al., 1998). A fraction of the total CD82 remained Endo H-resistant even after 60 min of chase in COS-7 cells. Inefficient transport/glycan-processing, like incomplete glycosylation, is due to limitations in the COS-7 expression system and is not seen when CD82 is analyzed in other cell types (Hammond et al., 1998).
A role for the transmembrane segments of CD82 in quality control
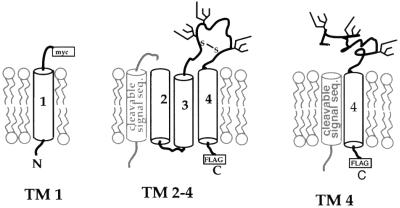
To examine the assembly of the transmembrane domain of CD82, we constructed a pair of deletion constructs that effectively split CD82 between the first and second transmembrane segments (illustrated in Figure 2). The TM 1 construct was created by inserting a myc tag followed by a stop codon just prior to the beginning of the second transmembrane segment of CD82. The TM 2–4 construct was created by fusing a cleavable signal sequence from the mouse MHC class I allele Kb (Huppa and Ploegh, 1997) to the beginning of the second transmembrane segment of CD82. A C-terminal FLAG tag was added to TM 2–4 to facilitate detection.
Fig. 2. Cartoon depicting truncated forms of CD82. TM 1 was generated by inserting a myc epitope tag and a stop codon just prior to the second transmembrane segment. TM 2–4 was made by inserting a cleavable signal sequence in front of the second transmembrane segment. A C-terminal FLAG tag was added to facilitate detection. Note that together TM 1 and TM 2–4 form a ‘whole’ CD82 molecule. TM 4 was generated by replacing the first three transmembrane segments of CD82 with a cleavable signal sequence and contains a C-terminal FLAG tag. In TM 2–4 the extracellular loop is depicted as folded/oxidized while in TM 4 it is not. See Figure 5A for evidence that this is the case.
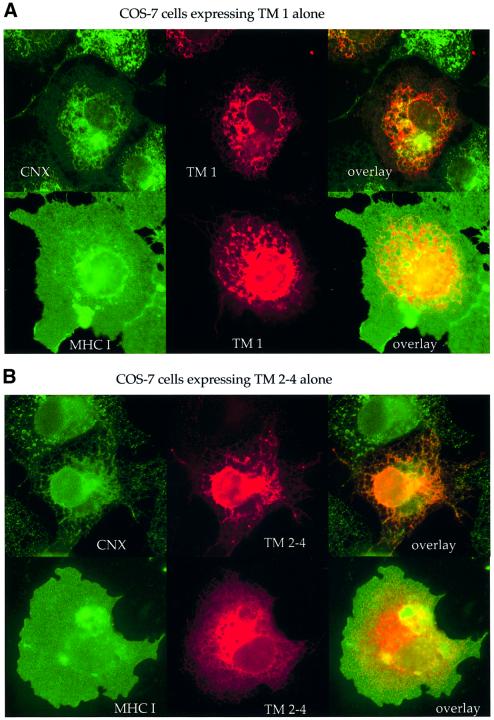
We used indirect immunofluorescence microscopy to identify the location of TM 1 and TM 2–4 in COS-7 cells relative to calnexin, an ER marker, and to MHC class I, which is found primarily on the cell surface. When expressed alone, TM 1 co-localized well with calnexin but poorly with class I (Figure 3A), suggesting that it remains in the ER and is not efficiently transported to the cell surface. Similarly, TM 2–4 was seen in a reticular pattern identical to calnexin but distinct from MHC class I (Figure 3B).
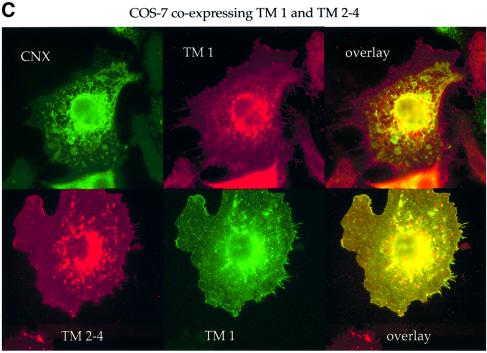
Fig. 3. TM 1 and TM 2–4 remain in the ER when expressed alone but move to the cell surface when co-expressed. COS-7 cells transfected with (A) TM 1, (B) TM 2–4, or (C) TM 1 plus TM 2–4 were fixed and permeabilized. The location of each construct relative to that of calnexin (CNX) and MHC class I, which are found primarily in the ER and on the cell surface, respectively, was determined by indirect immunofluorescence with antibodies against the indicated proteins. Primary antibodies used were rabbit anti-calnexin and anti-myc mAb 9E10 (A, top row); mouse anti-class I mAB HC10 and rabbit anti-myc antibody A14 (A, bottom row); rabbit anti-calnexin and MaP.CD82 (B, top row); HC10 and rabbit anti-whole CD82 (B, bottom row); rabbit anti-calnexin and 9E10 (C, top row); and MaP.CD82 with A14 (C, bottom row); followed by FITC or Texas Red-conjugated goat anti-rabbit or anti-mouse secondary antibodies.
If the constructs were retained because of the missing transmembrane segment(s), we reasoned that co-expressing TM 1 with TM 2–4, which together form a ‘whole’ CD82 molecule, might restore transport. Consistent with this, the staining pattern seen for TM 1 and TM 2–4 in cells co-expressing the two constructs was no longer reticular (Figure 3C, top row). TM 1 and TM 2–4 were now both located on the cell surface and were clearly co-localized, even in small, finger-like projections of the plasma membrane (Figure 3C, bottom row). Flow cytometry verified that co-expression with TM 1 did in fact allow TM 2–4 to be transported to the cell surface at much higher levels than when TM 2–4 was expressed alone (data not shown).
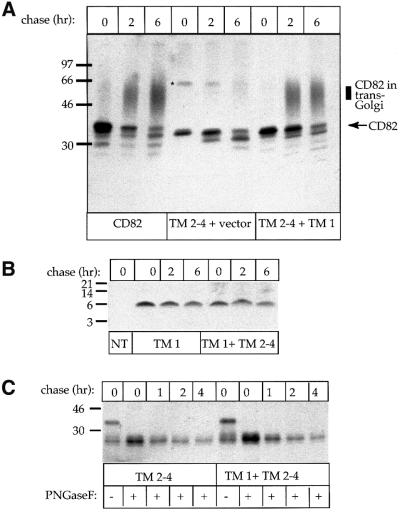
Biochemical confirmation that transport of TM 2–4 along the secretory pathway is restored by adding TM 1
To confirm the immunofluorescence data, we transfected COS-7 cells with CD82 (as a positive control), TM 2–4, or TM 2–4 plus TM 1. After radiolabeling for 30 min and chasing for ≤6 h, proteins were immunoprecipitated with MaP.CD82. Only upon addition of TM 1 did we see a diffuse band of complex glycosylated TM 2–4 (Figure 4A), indicative of transport through the _trans_-Golgi. To determine whether co-expression affected the stability of either TM 1 or TM 2–4, we performed pulse–chase experiments followed by immunoprecipitation on cells expressing TM 1, TM 2–4, or TM 1 plus TM 2–4. The enzyme protein N-glycanase F (PNGase F) was used to remove the N-glycans from TM 2–4 to simplify the comparison. The results of these experiments, shown in Figure 4B and C, demonstrate that the truncated forms of CD82 are held in a pre-_trans_-Golgi compartment but are not rapidly degraded. Co-expression thus leads to a restoration of transport but does not significantly alter the stability of either TM 1 or TM 2–4.
Fig. 4. Co-expression of TM 1 and TM 2–4 improves transport but does not alter the stability of either protein. (A) COS-7 cells expressing CD82, TM 2–4, or TM 2–4 and TM 1 were pulsed for 3 min and chased for 0–6 h. After solubilization, labeled proteins were precipitated with MaP.CD82. (B) Non-transfected COS-7 cells (NT) or COS-7 cells transfected with TM 1 plus vector or TM 1 plus TM 2–4 were pulsed for 30 min and chased for 0–6 h. After solubilization, labeled proteins were precipitated with M2. (C) COS-7 cells transfected with TM 2–4 or TM 2–4 and TM 1 were pulsed for 30 min and chased for 0–4 h. After solubilization, labeled proteins were precipitated with M2 and exposed to the enzyme PNGase F to remove N-glycans and allow a direct comparison of the amount of labeled protein at each time point. Note, the ∼66 kDa band marked ‘*’ on (A) is a post-lysis oxidation artifact, most likely a dimer. It is also occasionally seen with full-length CD82.
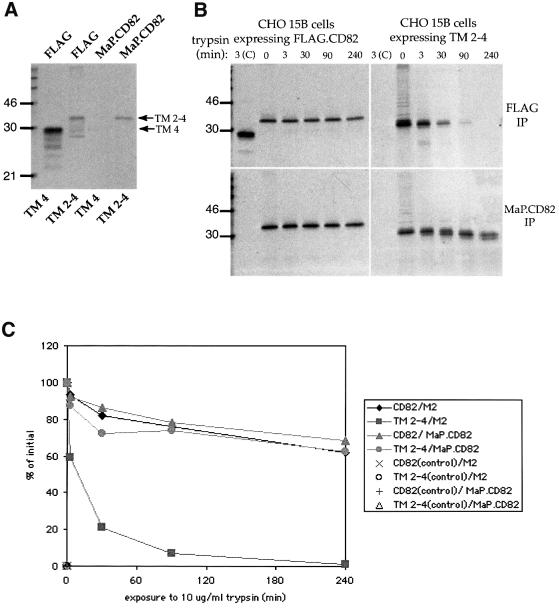
The extracellular domain of TM 2–4 is oxidized and protease resistant
Although we examined a number of CD82 truncations, we decided to focus on TM 2–4 because it is well recognized by the mAb MaP.CD82, which suggests that the extracellular domain is in the native, oxidized state. To illustrate this point, cells expressing TM 2–4 or TM 4, a construct that contains the extracellular loop but is missing the first three transmembrane segments (shown in Figure 2), were labeled with [35S]methionine for 2 h. MaP.CD82 precipitation after detergent extraction revealed that the epitope was formed in TM 2–4 but not in TM 4 (Figure 5A). No _trans_-Golgi processed forms of either protein were visible (Figure 5A).
Fig. 5. The extracellular domain of TM 2–4 is oxidized and protease resistant. (A) COS-7 cells expressing TM 2–4 or TM 4 were radiolabeled for 2 h. Labeled proteins were precipitated with MaP.CD82 to measure folding/oxidation of the large extracellular loop or with M2 to detect labeled protein regardless of its folding state. TM 4 is included as a negative control for folding/oxidation of the large extracellular loop. To emphasize the absence of even very low levels of oxidized TM 4, ∼5× more cell equivalents/lane were loaded compared with TM 2–4 lanes. (B) FLAG.CD82 or TM 2–4 was expressed in the complex–glycosylation-deficient cell line CHO 15B to allow labeled glycoproteins to be visualized as a single discrete band. Cells were pulsed for 2 h with [35S]methionine and chased for 2 h with an excess of unlabled methionine. After lysis at 4°C in buffer containing 1% Triton X-100, post-nuclear supernatants were exposed to 10 µg/ml trypsin for 0–240 min as indicated. Trypsin was inactivated and remaining labeled proteins were isolated by immunoprecipitation with MaP.CD82 or M2. In lanes labeled ‘3(C)’, reduced/alkylated protein exposed to 10 µg/ml trypsin for 3 min is shown as a positive control for proteolysis. (C) Bands of MaP.CD82 or M2-precipitable FLAG.CD82 and TM 2–4 from (B) were quantified by densitometry and graphed as a percentage of the amount precipitated at time 0.
As a further test for folding of the large extracellular domain, we compared the protease sensitivity of TM 2–4 with that of full-length CD82 by exposing the labeled proteins to trypsin for varying lengths of time and immunoprecipitating with either MaP.CD82 or M2 (Figure 5B). Quantitation of MaP.CD82 precipitable protein at each time point revealed that the large extracellular domain is as resistant to proteolysis in the context of TM 2–4 as in FLAG.CD82 (Figure 5C). At least two visible cleavage events were seen for TM 2–4 (Figure 5B). Based on shifts in mobility on SDS–PAGE, the first cleavage appears to remove ∼20 amino acids, including the FLAG tag, while the second removes an additional ∼20 amino acids. Positive controls in which reduced/denatured FLAG.CD82 or TM 2–4 was exposed to trypsin for 3 min demonstrated that both were rapidly degraded [Figure 5B, lanes marked 3(C)]. Taken together these data indicate that even though TM regions of TM 2–4 are incompletely assembled, the extracellular domain is in a native, oxidized conformation.
CD82 associates with calnexin but not calreticulin
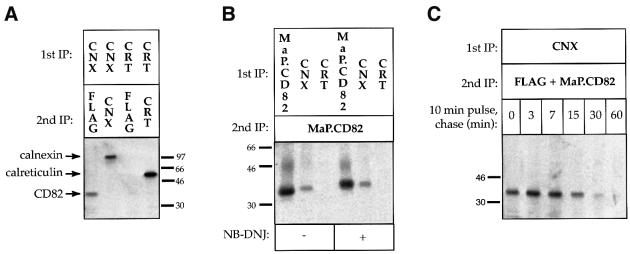
ER chaperones and folding factors facilitate structural maturation and serve as a quality control apparatus that prevents incompletely folded/assembled proteins from leaving the ER. Because CD82 is a glycoprotein, we examined its interaction with the lectin chaperones calnexin and calreticulin. These two chaperones are highly homologous, except that calreticulin lacks the C-terminal region that anchors calnexin to the ER membrane. Surprisingly, although co-immunoprecipitation revealed a clear interaction between CD82 and calnexin, we were unable to demonstrate an association with calreticulin (Figure 6A and B).
Fig. 6. CD82 interacts transiently with calnexin in a glycan-independent manner but does not interact with calreticulin. (A) CD82 interacts with calnexin (CNX) but not calreticulin (CRT). COS-7 cells expressing FLAG.CD82 were radiolabeled for 30 min. Labeled proteins were solubilized in buffer containing 2% CHAPS and immunoprecipitated with the indicated antibodies (1st IP). Immune complexes were disrupted as described in Figure 1 (–DTT) and released proteins were immunoprecipitated a second time (2nd IP). (B) The interaction between CD82 and calnexin is glycan independent. ER glucosidase inhibitor NB-DNJ or solvent was added 30 min prior to and throughout the labeling period. Cells were otherwise labeled and processed as in (A). (C) The interaction with calnexin is transient. COS-7 cells expressing FLAG.CD82 were radiolabeled for 10 min, chased for 0–60 min and processed as in (A), except that the 2nd IP was performed with a mixture of M2 anti-FLAG and MaP.CD82.
Two distinct modes of interaction between calnexin and substrates have been described—one that depends on the presence and processing of N-glycans and another that does not (Cannon et al., 1996). Competitive inhibitors of glucose-trimming, such as _N_-butyl deoxynojirimycin (NB-DNJ), prevent glycan-dependent binding of substrates to calnexin. Consistent with an inhibition of glucose trimming, the apparent molecular weight of CD82 increased after a 30 min incubation in the presence of NB-DNJ (Figure 6B, compare – and + NB-DNJ lanes). However, NB-DNJ did not decrease the amount of calnexin-associated CD82 (Figure 6B). In a pulse–chase experiment, the level of calnexin-associated CD82 peaked at 3 min of chase, decreased steadily, and was no longer detectable after 60 min (Figure 6C). These results demonstrate that the observed interaction with calnexin is transient and glycan independent.
TM 2–4 remains associated with calnexin; addition of TM 1 leads to release from calnexin and transport
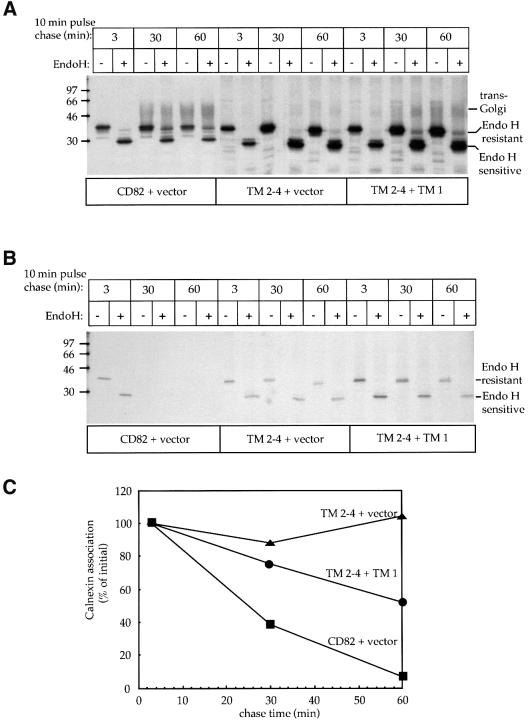
A pulse–chase of TM 2–4 combined with Endo H digestion confirmed that TM 2–4 remains in the ER when expressed alone (Figure 7A, TM 2–4 + vector). Addition of TM 1 leads to the appearance of Endo H-resistant forms of the protein (Figure 7A, TM 2–4 + TM 1). Because CD82 interacts with calnexin and dissociates from the chaperone before it exits the ER, we wondered whether calnexin might play a role in the retention of truncated forms of the molecule. In support of this idea, pulse–chase experiments revealed that unlike full-length CD82, the level of calnexin-associated TM 2–4 did not decrease over time (Figure 7B and C). Co-expressing TM 2–4 with TM 1 led to a decrease in the amount of calnexin-associated TM 2–4 (Figure 7B and C).
Fig. 7. TM 2–4 shows a prolonged interaction with calnexin but is released in the presence of TM 1. (A) COS-7 cells expressing CD82, TM 2–4 or co-expressing TM 1 and TM 2–4 were labeled for 10 min with [35S]methionine and chased for 3, 30 or 60 min. Solubilized proteins were immunoprecipitated with MaP.CD82 and treated or mock treated with Endo H. The positions of Endo H-resistant, Endo H-sensitive and complex glycosylated (_trans_-Golgi) protein are indicated. (B) Samples removed in parallel were immunoprecipitated with an antibody to calnexin. Antibody-bound protein complexes were dissociated, re-immunoprecipitated with MaP.CD82, and treated with Endo H. (C) Bands of calnexin-associated CD82 from (B) were quantified by densitometry and graphed as a percentage of the amount of calnexin-associated protein at 3 min.
Discussion
The ER quality control apparatus prevents incompletely folded or assembled proteins from leaving the ER for other locations in the cell. It has not been clear whether retention results purely from the misfolding of domains that are in the ER lumen or whether quality control can directly oversee the folding and assembly of transmembrane domains. In this study, we characterized the folding and transport of the tetraspanin CD82 and of truncated forms of CD82 that lack one or more transmembrane segments. Unlike the full-length molecule, these truncated forms of CD82 accumulate in the ER, do not have their N-glycans processed by Golgi enzymes and are not transported to the cell surface. Notably, the TM 2–4 construct, which is missing a single transmembrane segment, remains in the ER even though it has no detectable folding defect in regions of the molecule that are exposed to the ER lumen. Retention in the ER thus does not necessarily depend on the misfolding of lumenal domains. Unlike full-length CD82, TM 2–4 remains associated with the ER chaperone calnexin for an extended period of time. Export is restored by co-expressing TM 2–4 with TM 1, a construct that contains the transmembrane segment that is missing from TM 2–4. Co-expression with TM 1 causes TM 2–4 to be released from calnexin, exit the ER, and move through the Golgi to the cell surface. These findings demonstrate that quality control can monitor the intramolecular assembly of transmembrane domains and suggest that calnexin may participate in this process.
Folding of the extracellular domain of CD82
Members of the tetraspanin family feature an extracellular domain formed by the large loop between their third and fourth transmembrane segments. Many anti-tetraspanin mAbs recognize an epitope in this region (Maecker et al., 1997), and like MaP.CD82, the conformation-specific antibody used in this study, they fail to recognize tetraspanins that have been treated with reducing agents (Figure 1B) (Maecker et al., 1997). Recognition by MaP.CD82 indicates that the extracellular domain folds in TM 2–4. Further removal of the second and third transmembrane segments in the TM 4 construct prevented the large loop from folding (Figure 5A), suggesting that membrane proximal regions of the loop may need to be brought together by transmembrane segments 3 and 4 before proper folding can occur. This is not generally true for all tetraspanins, as soluble constructs of CD81 are able to fold in the absence of membrane attachment (Maecker et al., 1998).
The extracellular domain is as protease resistant in the TM 2–4 construct as in wild-type CD82 (Figure 5B and C), indicating that missing portions of the molecule are not necessary for its proper folding. Among the amino acids that form this region of CD82 are six lysines and five arginines, the favored cleavage site for trypsin. This, combined with the observation that when reduced and alkylated, both CD82 and TM 2–4 were rapidly cleaved by trypsin [Figure 5B, control 3(C) lanes], suggests that the extracellular loop is not inherently protease resistant. The degradation product seen at ∼29 kDa for FLAG.CD82, where the epitope tag is N-terminal, but not in the control for TM 2–4, where the FLAG tag is C-terminal, indicates that an initial cleavage of the reduced and alkylated proteins occurs at a residue in the extracellular domain 50–70 amino acids from the N-terminus of the protein.
Interactions between transmembrane segments
Popot and Engleman (1990) have proposed a two stage model for the folding and assembly of integral membrane proteins: hydrophobic transmembrane α-helices, stabi lized by hydrogen bonding of the main chain and association with lipids, fold immediately upon insertion into the bilayer. The α-helical segments then combine without significant rearrangement of their secondary structure to form the protein’s tertiary structure. If helix–helix interactions are strong enough, they form in the absence of covalent linkages (Lemmon et al., 1994). This model predicts that the transmembrane segments of CD82 first fold individually as they are translated and inserted into the ER membrane and later assemble with one another. Our results demonstrate that the transmembrane segments of CD82 need to associate before the protein can be efficiently transported out of the ER. The ability of TM 1 and TM 2–4 to escape the ER when co-expressed suggests that they assemble to form a ‘reconstituted’ CD82 molecule. Thus the interaction between these segments must be quite strong.
Quality control of multi-membrane-spanning proteins
Mutants of the cystic fibrosis transmembrane conductance regulator (CFTR) with alterations in their transmembrane segments also fail to leave the ER, and remain in association with multiple chaperones (Skach, 2000). CFTR has two distinct hydrophobic domains each formed by six transmembrane segments and three separate cytosolic domains. This complex structure has made it difficult to determine whether the mutated transmembrane segments themselves or some other more global change causes the protein to be recognized as misfolded (Skach, 2000). Multiple cytosolic and ER chaperones associate with mutant CFTR, making it difficult to determine the contribution of any given chaperone. Thus, it has not been possible to learn how chaperones recognize mutant CFTR as misfolded. CD82 provides a simpler model for the study of transmembrane domain assembly because folding of the extracellular domain does not depend on the assembly of segments that form the transmembrane domain. The truncated forms of CD82 characterized in this study will be useful in future studies to determine precisely how alterations in transmembrane segments cause retention in the ER.
Possible mechanisms for retention of unassembled transmembrane domains
What keeps TM 2–4 and TM 1 in the ER when they are expressed alone? Transmembrane segments with exposed charged or hydrophilic residues may integrate poorly into the membrane and have an increased propensity to aggregate. Truncated versions of CD82 do not appear to form irreversible aggregates, as co-expression of TM 1 with TM 2–4 allows their transport to the cell surface. Molecules that enter transient aggregates can go on to fold properly, a process that may be aided by the presence of calnexin in the aggregate (Kim et al., 1992; de Silva et al., 1993; Ihara et al., 1999). It is possible that truncated versions of CD82 and perhaps even the full-length molecule temporarily enter such aggregates. However, preliminary experiments employing sucrose gradients suggest that CD82-containing complexes are approximately the same size as those that contain TM 2–4 (our unpublished observations).
Calnexin remains bound to TM 2–4 but not to full-length CD82, suggesting that it plays a role in retention. It is possible that cytoplasmic or ER chaperones in addition to calnexin contribute to retention, but we have been unable to demonstrate an interaction between CD82 or truncated forms of CD82 and any other chaperone (our unpublished observations). We have thus far been unable to conclusively demonstrate a specific interaction between TM 1 and any chaperone including calnexin. Further experiments to address the mechanism of its retention are in progress.
Differences in the specificity of calnexin and calreticulin
Calnexin and calreticulin are components of a unique glycan-based system that monitors glycoprotein folding in the ER. These two homologous chaperones are lectins that bind specifically to glycoproteins via the monoglucosylated N-glycans that are exposed when glucosidases I and II trim the outer two glucose residues from core oligosaccharides (Hammond et al., 1994). Glycoproteins are released from the chaperones when glucosidase II removes the third and final glucose residue (Hebert et al., 1995; Cannon and Helenius, 1999). A glucosyl transferase folding sensor in the ER lumen then adds a single glucose residue only to misfolded glycoproteins, causing them to re-bind calnexin and/or calreticulin (Sousa et al., 1992; Hebert et al., 1995; Cannon and Helenius, 1999; Trombetta and Helenius, 2000). In this way misfolded glycoproteins are held in the ER while correctly folded molecules are exported.
Despite the identical lectin properties of calnexin and calreticulin (Vassilakos et al., 1998), there are differences in the spectrum of associated proteins (Peterson et al., 1995; Sadasivan et al., 1996; Van Leeuwen and Kearse, 1996; Halaban et al., 1997; Hebert et al., 1997; Pipe et al., 1998; Danilczyk et al., 2000). In addition to CD82, differences in binding to calnexin versus calreticulin have been observed for vesicular stomatitis virus (VSV) G protein, MHC class I, type I inositol triphosphate receptor and subunits of the T-cell receptor (Peterson et al., 1995; Sadasivan et al., 1996; Van Leeuwen and Kearse, 1996; Joseph et al., 1999). Variations in the number and location of lectin sites may contribute to these differences (Cannon et al., 1996; Rodan et al., 1996; Hebert et al., 1997). The apparent glycan independence of the observed interaction between CD82 and calnexin, however, argues against a lectin-based difference in this case.
There are other possible explanations for the observed difference in association with calnexin versus calreticulin. They may, for example, have different distributions in the ER. Calnexin has a cytoplasmic dibasic retention motif and may be excluded from ER exit sites (Cannon and Helenius, 1999), while calreticulin has a C-terminal KDEL sequence and is recycled from the _cis_-Golgi back to the ER (Peter et al., 1992; Sonnichsen et al., 1994). The observation that calreticulin binds VSV G when the purified proteins are mixed in vitro but not in living cells (Peterson et al., 1995; Peterson and Helenius, 1999) supports the idea of a location-based difference.
The transmembrane anchor of calnexin does more than just fasten the chaperone to the ER membrane. It changes the pattern of proteins that associate with calnexin (Peterson et al., 1995; Hebert et al., 1997; Ho et al., 1999; Danilczyk et al., 2000), perhaps by enhancing access to the translocon or to membrane-proximal glycans (Chen et al., 1995; Hebert et al., 1997; Ho et al., 1999), or perhaps even by allowing calnexin to interact directly with transmembrane domains of substrate proteins (Margolese et al., 1993; Loo and Clarke, 1995).
Several glycan-independent interactions of calnexin with transmembrane proteins have been observed (Rajagopalan et al., 1994; Arunachalam and Cresswell, 1995; Loo and Clarke, 1995; Cannon et al., 1996). Calnexin is known to interact with multi-membrane-spanning proteins such as the inositol triphosphate receptor (Joseph et al., 1999), P glycoprotein (Loo and Clarke, 1995) and of course CFTR (Pind et al., 1994). Although its existence is speculative, having a special site in or near the translocon where multi-membrane-spanning proteins would remain until they are fully assembled could conceptually simplify both the folding of transmembrane domains and, if necessary, their eventual degradation.
As was seen with CD82, complementary fragments of the integral membrane protein rhodopsin, the human erythrocyte anion exchanger 1 (Band 3) and P glycoprotein can assemble and, in some cases, form functional molecules (Groves and Tanner, 1995; Loo and Clarke, 1995; Ridge et al., 1995). Fragments of Band 3 failed to reach the cell surface but the reason for this transport failure was not determined (Groves and Tanner, 1995). Our findings provide a plausible explanation by showing that truncated forms of multi-membrane-spanning proteins can be retained in the ER in an assembly-competent conformation.
The tetraspanin constructs employed in this study will be useful to unravel the mechanism by which the transmembrane region of calnexin gives this versatile chaperone the ability to monitor folding/assembly events that occur inside the membrane and to help prevent multi-membrane-spanning proteins from leaving the ER until they are fully assembled.
Materials and methods
Reagents, cell lines and antibodies
COS-7 (American Type Culture Collection, Manassas, VA) and CHO 15B, a derivative of CHO that is deficient in _N_-acetylglucosaminyl transferase I activity (Gottlieb et al., 1975; Schlesinger et al., 1975), were grown in Iscove’s modified Dulbecco’s medium (IMDM) containing 10% bovine calf serum in an atmosphere of 5% C02 at 37°C. Previously described antibodies were rabbit polyclonal anti-calnexin (Hammond and Helenius, 1994), mouse mAb to CD82 (MaP.CD82) (Hammond et al., 1998) and MHC class I (HC10) (Stam et al., 1986). The anti-FLAG mAb M2 was obtained from Sigma (St Louis, MO). Rabbit polyclonal (A14) and mouse monoclonal (9E10) anti-myc antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). The rabbit polyclonal anti-calreticulin antibody was from Affinity Bioreagents (Golden, CO). Rabbit polyclonal anti-CD82 serum was raised against immunoaffinity-purified CD82. Secondary antibodies used were: fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit F(ab′)2 from Zymed (South San Francisco, CA); and Texas Red X-conjugated goat anti-mouse from Molecular Probes (Eugene, OR). Protein G and concanavalin A Sepharose 4 Fast Flow were from Amersham Pharmacia Biotech AB (Uppsala, Sweden). Endo H was from Roche Diagnostics Corp. (Indianapolis, IN). All other chemicals were obtained from Sigma (St Louis, MO).
Construction and expression of CD82 truncations
The cDNA for CD82 in the cloning vector pCR2.1 (Invitrogen, Carlsbad, CA) was as previously described (Hammond et al., 1998). FLAG.CD82, an N-terminally FLAG-tagged version of wild-type CD82, was constructed by Quikchange mutagenesis (Stratagene, La Jolla, CA) with primer 7 (5′-GGTACCGCCACCATGGACTACAAGGATGAC GATGACAAGGGCTCAGCCTG–3′) and its reverse complement primer 8 (5′-CAGGCTGAGCCCTTGTCATCGTCATCCTTGTAGT CCATGGTGGCGGTACC-3′).
TM 1, a C-terminal truncation after amino acid 54, was made by replacing the nucleotides encoding amino acid 55 (GGG) in FLAG.CD82 with a stop codon (TGA) using Quikchange mutagenesis with primer 9 (5′-GCTCGCTTAGGATGTGAGCCTATGTCTTCATCGGC-3′) and its reverse complement primer 10 (5′-GCCGATGAAGACATAGGCTCACATCCTAAGCGAGC-3′).
An alternative TM 1 construct was made by inserting a myc tag followed by a stop codon after amino acid 54 using PCR with primer 19 (5′-GGTACCGCCACCATGGGCTCAGCC-3′) and primer 20 (5′-TCTAGATCACAGATCCTCTTCTGAGATGAGTTTTTGTTCCATCCTAAGCGAGCTGGAGGAGGTTTGC-3′).
TM 2–4, which consists of the 22-amino-acid signal sequence of mouse class I H2-Kb fused to amino acids 55–267 of CD82, was constructed using PCR. In the first reaction, the region coding for the signal sequence of H2-Kb was amplified with an overhang of nucleotides 113–133 from CD82 using primer 1 (5′-GGTACCGCCACCATGGTACCGTGCACGCTGCTCCTGC-3′) and primer 2 (5′-CGCCGATGAAGACATAGGCCCCCGCGCGGGTCTGAGTCGGAGCC-3′). In a second reaction, the region coding for CD82 amino acids 55–267 was amplified with a 5′ overhang of nucleotides 46–66 from mouse class I H2-Kb and a 3′ region coding for C-terminal FLAG-tag using primer 3 (5′-GGCTCCGACTCAGACCCGCGCGGGGGCCTATGTCTTCAT CGGCG-3′) and primer 4 (5′-ATCGATTCACTTGTCATCGTCATCCTTGTAGTCGTACTTGGGGACCTTGCTGTAGTCTTCGG-3′). Finally, the region coding for the entire fusion protein was amplified using the products of the first two reactions as a template and primers 1 and 4.
TM 4, which consists of the 22-amino-acid signal sequence of mouse class I H2-Kb fused to amino acids 109–267 of CD82, was constructed using PCR. In the first reaction, the region coding for the signal sequence of H2-Kb was amplified with an overhang of 25 nucleotides from CD82 using primers 1 (described above) and 5 (5′-GCTTCAGCTTGCCCATGTTCCCCGCGCGGGTCTGAGTCGGAGCCAGGGCG- 3′). In a second reaction, the region coding for CD82 amino acids 109–267 was amplified with a 5′ overhang of 20 nucleotides from mouse class I H2-Kb and a 3′ region coding for C-terminal FLAG-tag using primer 6 (5′-CGCCCTGGCTCCGACTCAGACCCGCGCGGGGAA CATGGGCAAGCTGAAGC-3′) and primer 4 (described above). In a final reaction, the entire cDNA was amplified using the products of the first two reactions as a template and primers 1 and 4.
PCR products were cloned into pCR2.1 and sequenced using an ABI DNA sequencer (Applied Biosystems, Foster City, CA). Constructs were placed downstream of the T7 RNA polymerase initiation site in the mammalian expression vector pcDNA3.1 (–) PAC. pcDNA3.1 (–) PAC was made from pcDNA3.1 (–) Neo (Invitrogen, Carlsbad, CA) by replacing the neomycin resistance gene with the puromycin resistance gene.
Transient expression in COS-7 cells using CellFectin (Life Technologies, Grand Island, NY) was carried out according to the manufacturer’s instructions. All experiments were begun 24 h post-transfection.
For stable expression, CHO 15B cells were transfected using CellFectin according to the manufacturer’s instructions. Because CHO 15B cells are inherently puromycin resistant, FLAG.CD82 and TM 2–4 [in pcDNA3.1 (–) PAC] were co-transfected with a hygromycin-resistance vector. Transfectants were selected by growth in medium containing 500 µg/ml hygromycin (Boehringer Mannheim, IN). Expression was confirmed by metabolic labeling and immunoprecipitation.
Metabolic labeling
Cells were grown to 70% confluence, released with trypsin, washed once in growth medium and once in phosphate-buffered saline, and suspended at 3 × 106 cells/ml in labeling medium (Dulbecco’s modified Eagle’s medium lacking cysteine and methionine plus 3% dialyzed fetal calf serum and 2 mM glutamine). After a 30 min starvation, cells were labeled with [35S]methionine (0.3 µCi/ml). Chase was in normal growth medium supplemented with 5 mM each unlabeled cysteine and methionine. At the end of the chase period, samples were diluted 5-fold on ice for 10 min with chilled TBS (10 mM Tris pH 7.4, 150 mM NaCl) containing 20 mM NEM. Cells were centrifuged, resuspended at <3 × 106 cells/ml in TBS containing 1% Triton X-100 or 2% CHAPS (for co-immunoprecipitations), solubilized for 30 min on ice and centrifuged at 12 000 g for 5 min to remove nuclei.
Immunoprecipitation
After solubilization, labeled glycoproteins in the post-nuclear supernatant were immunoprecipitated with MaP.CD82–agarose, M2–agarose, or 30 µl of protein A–Sepharose plus anti-calnexin or anti-calreticulin polyclonal antiserum. Antibody:protein levels were carefully adjusted to maximize immunoprecipitation. After rotating for 1 h at 4°C, immune complexes were pelleted by centrifugation at 2000 g at 4°C for 1 min, and washed three times with TBS containing 0.1% Triton X-100 or 0.5% CHAPS. To allow unambiguous identification, proteins co-precipitated with anti-calnexin or anti-calreticulin antibodies were released by incubating at 70°C for 3 min in 100 µl of 0.2% SDS/TBS. Supernatants were transferred to fresh tubes containing 900 µl of 1% Triton X-100/TBS and left to stand at room temperature for 30 min to allow quenching of SDS and refolding of partially denatured proteins. After a second round of immunoprecipitation at 4°C, protein–bead complexes were washed three times in TBS/detergent. In Figure 1A, a final round of precipitation with concanavalin A–Sepharose beads was performed to remove non-glycosylated protein. For analysis by SDS–PAGE, immune complexes were disrupted in 40 µl of SDS loading buffer (50 mM Tris–HCl pH 6.8, 2% SDS, 5% glycerol, 0.004% bromophenol blue, 1 mM EDTA), heated to 70°C for 3 min, and centrifuged at 2000 g for 2 min to pellet the beads. Samples were transferred to a new tube with β-mercaptoethanol to 5% (v/v) and heated to 100°C for 1 min prior to loading for separation by 12.5% Laemmli Tris–Glycine or 16.5% Tris–Tricine (Schagger and von Jagow, 1987) PAGE and fluorography.
Endo H treatment
Immune complexes isolated as described above were suspended in 20 µl of 100 mM sodium acetate pH 5.2 + 0.2% SDS and heated to 70°C for 3 min. After cooling, an additional 20 µl of 100 mM sodium acetate pH 5.2 with or without 0.002 units of Endo H were added followed by incubation at 37°C for 16 h. At the end of the incubation period, 5 µl of 10× SDS loading buffer were added and samples were separated/analyzed as described above.
Trypsin sensitivity assay
CHO 15B cells expressing TM 2–4 and FLAG.CD82 were labeled for 2 h with [35S]methionine, chased for 2 h with cold methionine, and washed with ice cold TBS. After lysis in 1% Triton X-100 buffer, 10 µg/ml trypsin was added and lysates were incubated for 0–240 min at 37°C. As a positive control, denatured, reduced and alkylated CD82 or TM 2–4 were incubated under identical conditions for 3 min. At the end of the incubation period, 2 mM phenylmethylsulfonyl fluoride was added to inactivate the protease. Labeled proteins in the lysate were then immunoprecipitated with MaP.CD82 or M2 and processed as described.
Indirect immunofluorescence microscopy
COS-7 cells were plated on alcian blue-treated glass cover slips (12 mm) and allowed to settle for 24 h before transfection. At 24 h post-transfection, cells were fixed and prepared for indirect immunofluorescence as previously described (Hammond et al., 1998). Cells were viewed with a Zeiss axiophot 2 fluorescence microscope (Thornwood, NY). Digital images were captured with a Princeton instruments (Trenton, NJ) CCD camera using Improvision (Lexington, MA) OpenLab software and processed with Adobe Photoshop.
Acknowledgments
Acknowledgements
We thank Drs Wendy S.Garrett, E.Sergio Trombetta and Pamela Wearsch for stimulating discussions and proofreading; Craig Hammond, Naveen Bangia and Uyen Phan for ideas and experimental assistance; and Nancy Dometios for preparation of the manuscript. This work was supported by National Institutes of Health Grant AI23081 and by the Howard Hughes Medical Institute.
References
- Arunachalam B. and Cresswell,P. (1995) Molecular requirements for the interaction of class II major histocompatibility complex molecules and invariant chain with calnexin. J. Biol. Chem., 270, 2784–2790. [DOI] [PubMed] [Google Scholar]
- Braakman I., Hoover-Litty,H., Wagner,K.R. and Helenius,A. (1991) Folding of influenza hemagglutinin in the endoplasmic reticulum. J. Cell Biol., 114, 401–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon K.S. and Helenius,A. (1999) Trimming and readdition of glucose to N-linked oligosaccharides determines calnexin association of a substrate glycoprotein in living cells. J. Biol. Chem., 274, 7537–7544. [DOI] [PubMed] [Google Scholar]
- Cannon K.S., Hebert,D.N. and Helenius,A. (1996) Glycan-dependent and -independent association of vesicular stomatitis virus G protein with calnexin. J. Biol. Chem., 271, 14280–14284. [DOI] [PubMed] [Google Scholar]
- Chen W., Helenius,J., Braakman,I. and Helenius,A. (1995) Cotranslational folding and calnexin binding of influenza hemagglutinin in the endoplasmic reticulum. Proc. Natl Acad. Sci. USA, 92, 6229–6233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danilczyk U.G., Cohen-Doyle,M.F. and Williams,D.B. (2000) Functional relationship between calreticulin, calnexin and the endoplasmic reticulum luminal domain of calnexin. J. Biol. Chem., 275, 13089–13097. [DOI] [PubMed] [Google Scholar]
- de Silva A., Braakman,I. and Helenius,A. (1993) Post-translational folding of VSV G protein in the endoplasmic reticulum: involvement of noncovalent and covalent complexes. J. Cell Biol., 120, 647–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellgaard L., Molinari,M. and Helenius,A. (1999) Setting the standards: quality control in the secretory pathway. Science, 286, 1882–1888. [DOI] [PubMed] [Google Scholar]
- Gottlieb C., Baenziger,J. and Kornfeld,S. (1975) Deficient uridine diphosphate-_N_-acetylglucosamine:glycoprotein _N_-acetylglucos- aminyltransferase activity in a clone of Chinese hamster ovary cells with altered surface glycoproteins. J. Biol. Chem., 250, 3303–3309. [PubMed] [Google Scholar]
- Groves J.D. and Tanner,M.J. (1995) Co-expressed complementary fragments of the human red cell anion exchanger (band 3, AE1) generate stilbene disulfonate-sensitive anion transport. J. Biol. Chem., 270, 9097–9105. [DOI] [PubMed] [Google Scholar]
- Halaban R., Cheng,E., Zhang,Y., Moellmann,G., Hanlon,D., Michalak,M., Setaluri,V. and Hebert,D.N. (1997) Aberrant retention of tyrosinase in the endoplasmic reticulum mediates accelerated degradation of the enzyme and contributes to the dedifferentiated phenotype of amelanotic melanoma cells. Proc. Natl Acad. Sci. USA, 94, 6210–6215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond C. and Helenius,A. (1994) Quality control in the secretory pathway: retention of a misfolded viral membrane glycoprotein involves cycling between the ER, intermediate compartment and Golgi apparatus. J. Cell Biol., 126, 41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond C., Braakman,I. and Helenius,A. (1994) Role of N-linked oligosaccharides, glucose trimming and calnexin during glycoprotein folding in the endoplasmic reticulum. Proc. Natl Acad. Sci. USA, 91, 913–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond C., Denzin,L.K., Pan,M., Griffith,J.M., Geuze,H.J. and Cresswell,P. (1998) The tetraspan protein CD82 is a resident of MHC class II compartments where it associates with HLA-DR, -DM and -DO molecules. J. Immunol., 161, 3282–3391. [PubMed] [Google Scholar]
- Hebert D.N., Foellmer,B. and Helenius,A. (1995) Glucose trimming and reglucosylation determines glycoprotein association with calnexin. Cell, 81, 425–433. [DOI] [PubMed] [Google Scholar]
- Hebert D., Foellmer,B. and Helenius,A. (1997) Number and location of N-linked oligosaccharides determines how calnexin and calreticulin associate with a glycoprotein substrate. J. Cell Biol., 139, 613–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helenius A., Trombetta,E.S., Hebert,D.N. and Simons,J.F. (1997) Calnexin, calreticulin and the folding of glycoproteins. Trends Cell Biol., 7, 193–200. [DOI] [PubMed] [Google Scholar]
- Ho S.C., Rajagopalan,S., Chaudhuri,S., Shieh,C.C., Brenner,M.B. and Pillai,S. (1999) Membrane anchoring of calnexin facilitates its interaction with its targets. Mol. Immunol., 36, 1–12. [DOI] [PubMed] [Google Scholar]
- Huppa J.B. and Ploegh,H.L. (1997) In vitro translation and assembly of a complete T cell receptor–CD3 complex. J. Exp. Med., 186, 393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtley S.M. and Helenius,A. (1989) Protein oligomerization in the endoplasmic reticulum. Annu. Rev. Cell Biol., 5, 277–307. [DOI] [PubMed] [Google Scholar]
- Ihara Y., Cohen-Doyle,M.F., Saito,Y. and Williams,D.B. (1999) Calnexin discriminates between protein conformational states and functions as a molecular chaperone in vitro. Mol. Cell, 4, 331–341. [DOI] [PubMed] [Google Scholar]
- Joseph S.K., Boehning,D., Bokkala,S., Watkins,R. and Widjaja,J. (1999) Biosynthesis of inositol trisphosphate receptors: selective association with the molecular chaperone calnexin. Biochem. J., 342, 153–161. [PMC free article] [PubMed] [Google Scholar]
- Kim P.S., Bole,D. and Arvan,P. (1992) Transient aggregation of nascent thyroglobulin in the endoplasmic reticulum: relationship to the molecular chaperone BiP. J. Cell Biol., 118, 541–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld R. and Kornfeld,S. (1985) Assembly of asparagine-linked oligosaccharides. Annu. Rev. Biochem., 54, 631–664. [DOI] [PubMed] [Google Scholar]
- Lemmon M.A., Treutlein,H.R., Adams,P.D., Brunger,A.T. and Engelman,D.M. (1994) A dimerization motif for transmembrane α-helices. Nature Struct. Biol., 1, 157–163. [DOI] [PubMed] [Google Scholar]
- Loo T.W. and Clarke,D.M. (1995) P-glycoprotein. Associations between domains and between domains and molecular chaperones. J. Biol. Chem., 270, 21839–21844. [DOI] [PubMed] [Google Scholar]
- Maecker H.T., Todd,S.C. and Levy,S. (1997) The tetraspanin superfamily: molecular facilitators. FASEB J., 11, 428–442. [PubMed] [Google Scholar]
- Maecker H.T., Do,M.S. and Levy,S. (1998) CD81 on B cells promotes interleukin 4 secretion and antibody production during T helper type 2 immune responses. Proc. Natl Acad. Sci. USA, 95, 2458–2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolese L., Waneck,G.L., Suzuki,C.K., Degen,E., Flavell,R.A. and Williams,D.B. (1993) Identification of the region on the class I histocompatibility molecule that interacts with the molecular chaperone, p88 (calnexin, IP90). J. Biol. Chem., 268, 17959–17966. [PubMed] [Google Scholar]
- Peter F., Nguyen Van,P. and Söling,H.-D. (1992) Different sorting of Lys-Asp-Glu-Leu proteins in rat liver. J. Biol. Chem., 267, 10631–10637. [PubMed] [Google Scholar]
- Peterson J.R. and Helenius,A. (1999) In vitro reconstitution of calreticulin–substrate interactions. J. Cell Sci., 112, 2775–2784. [DOI] [PubMed] [Google Scholar]
- Peterson J.R., Ora,A., Nguyen Van,P. and Helenius,A. (1995) Transient, lectin-like association of calreticulin with folding intermediates of cellular and viral glycoproteins. Mol. Biol. Cell, 6, 1173–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan U.T., Arunachalam,B. and Cresswell,P. (2000) γ-interferon-inducible lysosomal thiol reductase (GILT). Maturation, activity and mechanism of action. J. Biol. Chem., 275, 25907–25914. [DOI] [PubMed] [Google Scholar]
- Pind S., Riordan,J.R. and Williams,D.B. (1994) Participation of the endoplasmic reticulum chaperone calnexin (p88, IP90) in the biogenesis of the cystic fibrosis transmembrane conductance regulator. J. Biol. Chem., 269, 12784–12788. [PubMed] [Google Scholar]
- Pipe S.W., Morris,J.A., Shah,J. and Kaufman,R.J. (1998) Differential interaction of coagulation factor VIII and factor V with protein chaperones calnexin and calreticulin. J. Biol. Chem., 273, 8537–8544. [DOI] [PubMed] [Google Scholar]
- Popot J.L. and Engelman,D.M. (1990) Membrane protein folding and oligomerization: the two-stage model. Biochemistry, 29, 4031–4037. [DOI] [PubMed] [Google Scholar]
- Rabouille C., Hui,N., Hunte,F., Kieckbusch,R., Berger,E.G., Warren,G. and Nilsson,T. (1995) Mapping the distribution of Golgi enzymes involved in the construction of complex oligosaccharides. J. Cell Sci., 108, 1617–1627. [DOI] [PubMed] [Google Scholar]
- Rajagopalan S., Xu,Y. and Brenner,M.B. (1994) Retention of unassembled components of integral membrane proteins by calnexin. Science, 263, 387–390. [DOI] [PubMed] [Google Scholar]
- Ridge K.D., Lee,S.S. and Yao,L.L. (1995) In vivo assembly of rhodopsin from expressed polypeptide fragments. Proc. Natl Acad. Sci. USA, 92, 3204–3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodan A.R., Simons,J.F., Trombetta,E.S. and Helenius,A. (1996) N-linked oligosaccharides are necessary and sufficient for association of RNase B with calnexin and calreticulin. EMBO J., 15, 6921–6930. [PMC free article] [PubMed] [Google Scholar]
- Sadasivan B., Lehner,P.J., Ortmann,B., Spies,T. and Cresswell,P. (1996) Roles for calreticulin and a novel glycoprotein, tapasin, in the interaction of MHC class I molecules with TAP. Immunity, 5, 103–114. [DOI] [PubMed] [Google Scholar]
- Schagger H. and von Jagow,G. (1987) Tricine–sodium dodecyl sulfate–polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem., 166, 368–379. [DOI] [PubMed] [Google Scholar]
- Schlesinger S., Gottlieb,C., Feil,P., Gelb,N. and Kornfeld,S. (1975) Growth of enveloped RNA viruses in a line of chinese hamster ovary cells with deficient _N_-acetylglucosaminyltransferase activity. J. Virol., 17, 239–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skach W.R. (2000) Defects in processing and trafficking of the cystic fibrosis transmembrane conductance regulator. Kidney Int., 57, 825–831. [DOI] [PubMed] [Google Scholar]
- Sonnichsen B., Fullekrug,J., Nguyen Van,P., Diekmann,W., Robinson,D.G. and Mieskes,G. (1994) Retention and retrieval: both mechanisms cooperate to maintain calreticulin in the endoplasmic reticulum. J. Cell Sci., 107, 2705–2717. [DOI] [PubMed] [Google Scholar]
- Sousa M.C., Ferrero-Garcia,M.A. and Parodi,A.J. (1992) Recognition of the oligosaccharide and protein moities of glycoproteins by the UDP-Glc:glycoprotein glucosyltransferase. Biochemistry, 31, 97–105. [DOI] [PubMed] [Google Scholar]
- Stam N.J., Spits,H. and Ploegh,H.L. (1986) Monoclonal antibodies raised against denatured HLA-B locus heavy chains permit biochemical characterization of certain HLA-C locus products. J. Immunol., 137, 2299–2306. [PubMed] [Google Scholar]
- Trombetta E.S. and Helenius,A. (2000) Conformational requirements for glycoprotein reglucosylation in the endoplasmic reticulum. J. Cell Biol., 148, 1123–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Leeuwen J.E.M. and Kearse,K.P. (1996) The related molecular chaperones calnexin and calreticulin differentially associate with nascent T cell antigen receptor proteins within the endoplasmic reticulum. J. Biol. Chem., 271, 25345–25349. [DOI] [PubMed] [Google Scholar]
- Vassilakos A., Michalak,M., Lehrman,M.A. and Williams,D.B. (1998) Oligosaccharide binding characteristics of the molecular chaperones calnexin and calreticulin. Biochemistry, 37, 3480–3490. [DOI] [PubMed] [Google Scholar]
- Wright M.D. and Tomlinson,M.G. (1994) The ins and outs of the transmembrane 4 superfamily. Immunol. Today, 15, 588–594. [DOI] [PubMed] [Google Scholar]