Stimulation of Menaquinone-Dependent Electron Transfer in the Respiratory Chain of Bacillus subtilis by Membrane Energization (original) (raw)
Abstract
At a pH of ≤7, respiration of Bacillus subtilis cells on endogenous substrates shut down almost completely upon addition of an uncoupler (carbonyl cyanide _m_-chlorophenylhydrazone [CCCP]) and a K+-ionophore (valinomycin). The same effect was observed with cell spheroplasts lacking the cell wall. The concentration of CCCP required for 50% inhibition of the endogenous respiration in the presence of K+-valinomycin was below 100 nM. Either CCCP or valinomycin alone was much less efficient than the combination of the two. The inhibitory effect was easily reversible and depended specifically on the H+ and K+ concentrations in the medium. Similar inhibition was observed with respect to the reduction of the artificial electron acceptors 2,6-dichlorophenolindophenol (DCPIP) and N,N,_N_′,_N_′-tetramethyl-_p_-phenylenediamine cation (TMPD+), which intercept reducing equivalents at the level of menaquinol. Oxidation of the reduced DCPIP or TMPD in the bacterial cells was not sensitive to uncoupling. The same loss of the electron transfer activities as induced by the uncoupling was observed upon disruption of the cells during isolation of the membranes; the residual activities were not further inhibited by the uncoupler and ionophores. We conclude that the menaquinone-dependent electron transfer in the B. subtilis respiratory chain is facilitated, thermodynamically or kinetically, by membrane energization. A requirement for an energized state of the membrane is not a specific feature of succinate oxidation, as proposed in the literature, since it was also observed in a mutant of B. subtilis lacking succinate:quinone reductase as well as for substrates other than succinate. Possible mechanisms of the energy-dependent regulation of menaquinone-dependent respiration in B. subtilis are discussed.
There is a classical phenomenon of a so-called respiratory control in mitochondrial oxidative phosphorylation (35; reviewed in reference 18). Electron flow in the mitochondrial respiratory chain becomes slow as the membrane is energized (the state of respiratory control) and can be stimulated under these conditions by addition of ADP, which turns on ATP synthesis, or by the uncouplers of oxidative phosporylation, which dissipate the transmembrane electrochemical proton potential difference (ΔμH+). Although described initially for mitochondria, the effect of respiratory control is often assumed to apply to bacterial coupled respiration as well (see, e.g., reference 43).
It is therefore interesting that respiration of the aerobic bacterium Bacillus subtilis is stimulated rather than suppressed by proton motive force. The fact that the respiration of B. subtilis cells can be inhibited by K+/H+ and Na+/H+ antiporters and protonophores was noticed about 10 years ago (3, 37, 46) and confirmed more recently by Shirawski and Unden (47). A potentially relevant observation is that the succinate oxidase activity of B. subtilis cells decreases drastically during isolation of the membranes (36, 47); this effect could also be a consequence of membrane deenergization.
The mechanism underlying the energy requirement for B. subtilis respiration remains to be established. Acidification of cytoplasm (3) and loss of cytoplasmic K+ (46) were proposed to be involved in the uncoupler-induced inhibition of respiration of bacterial cells. In contrast, Unden and collaborators (37, 47) assigned the energy dependence of respiration specifically to proton motive force-driven transmembrane electron transfer from succinate to menaquinone (MQ) through succinate:MQ reductase as described below.
Succinate:quinone oxidoreductases (SQR) of bacteria and mitochondria (called also complex II of the respiratory chain) catalyze two-electron reduction of membrane-bound quinone (usually ubiquinone [UQ] or MQ) to quinol by succinate. Besides this, many bacteria contain enzymes that are homologous to SQR but are more efficient catalytically in performing the reverse quinol:fumarate reductase (QFR) reaction (see reference 33 for classification). The midpoint redox potential (Em) of the MQ-menaquinol couple (MQ-MQH2) is ca. −80 mV at pH 7, which is some 110 mV more negative than the Em of the succinate-fumarate couple (ca. +30 mV at pH 7 [9, 14, 37]). Therefore, the succinate:quinone reductase activity in MQ-containing bacteria such as B. subtilis (10) poses an obvious thermodynamic problem (36).
The dehydrogenase partial activity of SQR, i.e., electron transfer from succinate to artificial electron acceptors such as _N_-methylphenazinium sulfate (PMS) or ferricyanide, is associated in both mitochondria and bacteria with the water-soluble two-subunit peripheral part of the enzyme, which contains the dicarboxylate binding center, covalently bound FAD, and three different iron-sulfur centers (reviewed in references 14, 20, and 38). The ability to reduce hydrophobic quinones is conferred to the enzyme by a so-called membrane anchor subunit, whose structure and composition can vary significantly in different organisms (14, 38). In B. subtilis, this subunit is a diheme cytochrome b (15), with the two porphyrin groups arranged across the membrane on top of each other, as predicted by mutagenesis studies (14, 16) and confirmed recently by the three-dimensional crystal structure of complex II from Wolinella succinogenes (32, 34). The low-potential heme b (approximately isopotential with MQ-MQH2 [15, 50]) is located close to the outer periplasmic side of the membrane and is believed to be the MQ reducing site (20, 39, 42, 47, 50). The high-potential heme b located near the cytoplasmic side appears to be within fast electron transfer distance from the terminal iron-sulfur center S-3 of the dehydrogenase part of the enzyme.
Such a “vectorial” arrangement of the two b hemes across the membrane implies that the membrane potential and proton motive force can promote reduction of the low-potential heme b by succinate via the high-potential heme b and thus stimulate the thermodynamically unfavorable MQ reductase activity of SQR. According to this model, the reverse QFR reaction catalyzed by SQR or QFR should generate ΔμH+ (31). Although they are consistent with the crystal structure of complex II, these predictions remain to be proved. Recent experiments with whole B. subtilis cells provide some evidence for electrogenicity of fumarate reduction with glucose as an electron donor (48). On the other hand, detailed studies with a structurally similar diheme QFR from W. succinogenes, carried out both with the whole cells and with the purified liposome-reconstituted enzyme, did not reveal either membrane potential or transmembrane pH difference (ΔpH) generation coupled to the QFR reaction (6, 13, 27, 28, 41). No proton pumping could also be demonstrated for homologous but heme-deficient QFR from Escherichia coli (see references 8, 22, and 42 for a discussion of a possibility of vectorial charge transfer in different types of complex II).
In several recent works, the putative energy-linked reduction of MQ by succinate via the low-potential heme b has been invoked to explain the energy-dependent stimulation of electron transfer in the respiratory chain of B. subtilis (39, 42, 47). On the other hand, the energy-dependent activation of SQR may well be a separate phenomenon, not related to the specific mechanism of the endergonic MQ reduction by B. subtilis SQR. For instance, proton motive force activates SQR in plant mitochondria (2), although the enzyme catalyzes exergonic reduction of UQ and its membrane anchor contains only one heme b.
In this work we have explored the effect of the uncoupling on electron transfer in the respiratory chain of B. subtilis in more detail. Special attention was paid to the proposal that the phenomenon is specifically associated with succinate oxidation (47). Both intact cells and isolated membranes were studied with different respiratory substrates as electron donors and either oxygen or artificial redox dyes as electron acceptors. We find that deenergization of the B. subtilis coupling membrane either by uncoupling agents or as a consequence of breaking the cells during preparation of the membranes severely inhibits respiration with oxygen as well as reduction of 2,6-dichlorophenolindophenol (DCPIP) and oxidized N,N,_N_′,_N_′-tetramethyl-_p_-phenylenediamine (TMPD+) at the level of MQH2. However, contrary to earlier conclusions (37, 47), these effects are observed not only with succinate but also with other physiological electron donors such as NADH or glycerol-3-phosphate (GP) as well as with endogenous substrates in cells grown on succinate, glycerol, or malate. Moreover, results very similar to those observed with wild-type bacteria were obtained with a mutant strain deficient in SQR.
We conclude that the stimulating effect of energization on electron transfer in the respiratory chain of B. subtilis is not associated uniquely with the succinate:quinone reductase complex of the respiratory chain. Accordingly, the widely discussed specific molecular mechanism of the effect based on a unique structure of SQR (47) cannot explain the phenomenon. We propose that electron transfer in the respiratory chain of B. subtilis, and perhaps in other MQ-dependent bacteria as well, may be kinetically controlled (stimulated) by membrane energization at the level of MQ reduction by the dehydrogenases. Possible mechanisms of energy-dependent stimulation of electron flow in the respiratory chain of B. subtilis that may be common for different MQ-dependent pathways are discussed.
MATERIALS AND METHODS
Strains and preparations.
The following B. subtilis strains, kindly provided by Lars Hederstedt and Claes von Wachenfeldt (Lund University), were used: (i) the “wild-type” strain 168 A (trpC2), (ii) the SQR-lacking strain LUH-16 (trpC2ΔsdhCAB::ble), and (iii) a strain with two- to threefold-enhanced expression of SQR and lacking the _bd_-type terminal oxidase, LUW-20/pBSD1400 (trpC2ΔcydABCD::tet, transformed by the low-copy-number pHP13 plasmid carrying the sdhCAB DNA region). The cells were grown in flasks at 37°C and pH 7.0, with high aeration, in medium containing 0.5% yeast extract (Difco), 50 mM potassium phosphate, and 1 mM MgSO4 and supplemented with 50 mM sodium malate or 50 mM sodium succinate with or without 0.5% glycerol. The culture was harvested near the end of the exponential phase of growth, when the optical density of the cell suspension at 600 nm reached ∼1.5. Membranes were isolated essentially as described previously (19). All of the chemicals used were commercial products from conventional sources (Sigma, Serva, Fluka, or Merck).
Measurements.
Oxygen consumption was measured with a Clark-type oxygen electrode in a 1.1-ml cell at 25°C. Reduction of DCPIP (50 μM, with or without 0.4 mM PMS) and TMPD+ (100 μM) by the cells and membranes was monitored in an SLM-Aminco DW-2000 UV-visible spectrophotometer with a 3-nm slit width in 1-cm-optical-pathway spectrophotometric cells thermostatted at 25°C with permanent stirring. Extinction of the oxidized DCPIP was monitored at 609 nm in a split-beam mode (ɛ =16 to 17 mM−1 cm−1 under our conditions). A stock solution of the oxidized form of TMPD (TMPD+; Wurster's Blue) was prepared by mixing TMPD with a mixture of potassium ferrocyanide-ferricyanide at a ratio of 1:0.5:1.75. Extinction of the oxidized TMPD at 612 nm versus the 700-nm reference point was monitored in a dual-wavelength mode using an approximate extinction coefficient of 9 mM−1 cm−1 as determined for these conditions. The cytochrome _aa_3 concentration in the membranes was determined from dithionite (reduced) minus ferricyanide (oxidized) difference spectra of the samples, using Δɛ600 nm − 575 nm = 20 mM−1 cm−1. When the _aa_3 content of the cells was to be determined spectrophotometrically, the cells were suspended in 50 mM MOPS [3-(_N_-morpholino)propanesulfonic acid]-NaOH (pH 7.0) and treated with lysozyme (1 mg/ml, 45 min, 37°C) and 15 mM sodium EDTA (20 min). In order to obtain spheroplasts, the cells were subjected to a similar lysozyme treatment in a hypertonic medium with 0.8 M sucrose and 20 mM MgSO4. To induce osmotic lysis, spheroplasts were placed in the same medium but without sucrose. Formation and lysis of spheroplasts were controlled visually with a phase-contrast microscope.
RESULTS
Energy-dependent respiration of the cells.
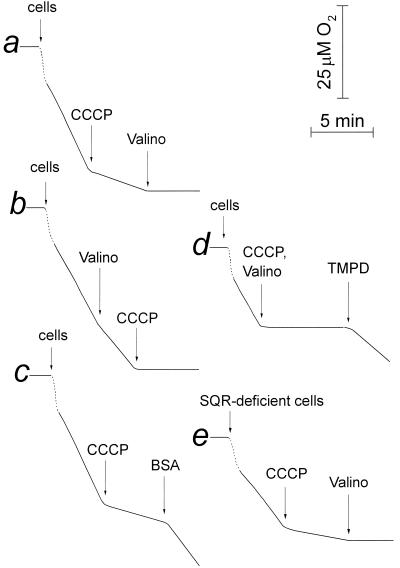
Figure 1 illustrates the effect of a protonophorous uncoupler, carbonyl cyanide _m_-chlorophenylhydrazone (CCCP), and the K+-ionophore valinomycin on the respiration of B. subtilis cells with endogenous substrates as electron donors. The combination of 10 μM CCCP and 5 μM valinomycin brought about an almost full inhibition of oxygen consumption, while when added alone these agents were much less efficient (Fig. 1, traces a and b). We could not reproduce the >90% inhibition of respiration by valinomycin alone reported previously (47), provided that care was taken to wash the reaction vessel thoroughly from the uncoupler used in the previous probes. The inhibitory effect of CCCP could be fully reversed by bovine serum albumin (Fig. 1, trace c) or charcoal (not shown), which adsorb the compound. Addition of the reduced TMPD (trace d) or 50 μM DCPIP (not shown) after the ionophores restored a high respiration rate, indicating that these mediators donate electrons to the respiratory chain below the inhibition site.
FIG. 1.
Inhibition of respiration of B. subtilis cells by CCCP and valinomycin. Oxygen consumption was monitored in 50 mM potassium phosphate-1 mM MgSO4, pH 7.0. The initial downward deflections of the traces (dotted lines) result from addition of oxygen-depleted cell suspension. The results are shown for cells grown on malate. Traces a to d, wild-type (strain 168 A); trace e, SQR-deficient mutant (strain LUH-16). Additions: bacterial cells, up to an optical density at 600 nm of 0.1; CCCP, 10 μM; valinomycin (Valino), 5 μM; bovine serum albumin (BSA), 1 mg/ml; reduced TMPD, 0.5 mM.
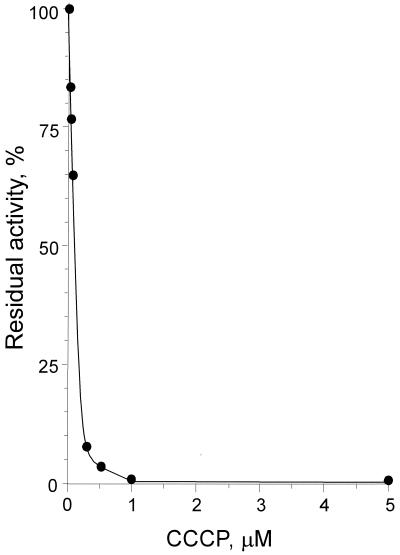
In the presence of 5 μM valinomycin, 50% inhibition of respiration with endogenous substrates was attained at a CCCP concentration of as low as 0.1 μM (Fig. 2) (actually, even less; see the legend to Fig. 2). A similar inhibition pattern was observed with pentachlorophenol, a less potent protonophore uncoupler, taken at about a 10-fold-higher concentration. SCN− (a membrane-penetrating anion) at 50 mM also could substitute for K+-valinomycin. Among other ionophores tested, gramicidin D (5 μM), alamethicin (15 μM), and monensin (10 μM) were found to inhibit respiration significantly (3- to 10-fold).
FIG. 2.
Concentration dependence of the CCCP inhibitory effect on respiration of B. subtilis cells. Endogenous respiration of malate-grown 168A cells was measured in 50 mM Tris-HCl-1 mM MgSO4-5 μM valinomycin, pH 7.0. Other conditions were as described for Fig. 1. Note that at below 0.5 μM, the actual CCCP concentration in the probe is decreased significantly by adsorption of the compound on the surface of the vessel (N. Azarkina, unpublished); therefore, the true concentration at which 50% inhibition is attained is probably even lower than the uncorrected observed value of 0.1 μM quoted in the text.
All of the experiments carried out with the wild-type B. subtilis cells were duplicated with an SQR-deficient strain, LUH-16, without any noticeable difference in the results (e.g., cf. traces a and e in Fig. 1). Therefore, the inhibition of respiration induced by the uncoupling is not associated specifically with the succinate-oxidizing pathway of the respiratory chain as proposed previously (47). Accordingly, respiration of wild-type cells grown on malate was found to be only slightly less sensitive to CCCP and valinomycin than respiration of cells grown on 50 mM succinate. In contrast, when the cells were grown on glucose, about half of the oxygen consumption was not sensitive to CCCP plus valinomycin. However, in this case the uncoupler-resistant part also is not inhibited by cyanide, and its mechanism is not fully clear. It is possible that the uncoupler-insensitive respiration on glucose, as opposed to the uncoupler-sensitive succinate oxidation described previously (47), could originate similarly in the cyanide-resistant electron flow. The results shown below were obtained mainly with malate-grown cells, if not stated otherwise.
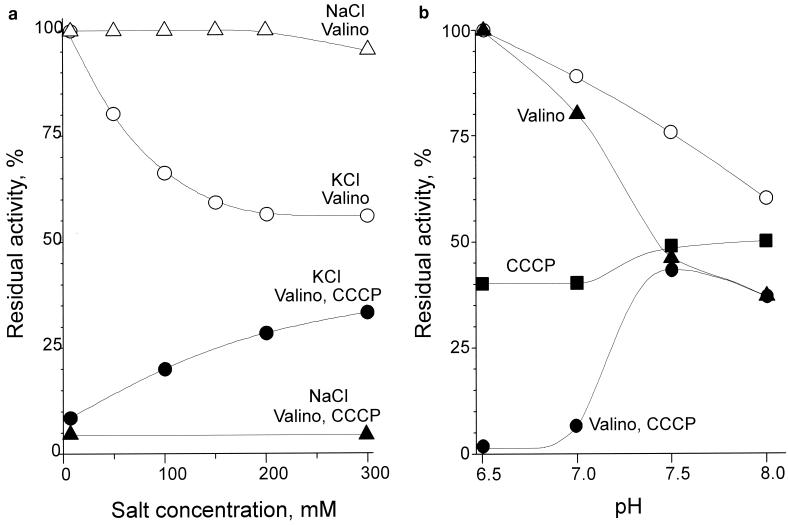
The inhibitory effect of uncoupling agents on respiration may depend on the exact experimental conditions. The nature of the pH buffer used in the experiments (e.g., Tris or phosphate) did not show any noticeable effect on the results. However, the data were quite sensitive to the concentrations of K+ and H+ in the medium (Fig. 3a and b, respectively). At pH 7, KCl (but not NaCl) potentiated the inhibition imposed by valinomycin but markedly counteracted the inhibition induced by valinomycin plus CCCP. The effects of K+ saturated at about 200 mM KCl, which is close to concentration of K+ in the cytoplasm of B. subtilis (40). The inhibition by valinomycin plus CCCP changed steeply from a virtually complete block at a pH of ≤7 to a moderate effect similar to that induced by valinomycin alone at a pH of above 7.5, which is the normal cytoplasmic pH value (30). A very similar pH dependence has been shown for the inhibition imposed by monensin. These observations are in agreement with the data in references 3 and 46. We give these results mainly to characterize more fully the phenomenology of the effect; however, the data are consistent with the role of both the transmembrane electrical potential difference (Δψ) and ΔpH components of the proton motive force in the energy dependence of respiration.
FIG. 3.
K+ and H+ dependence of B. subtilis cell respiration in the presence of uncouplers. (a) Dependence on the concentration of KCl (circles) and NaCl (triangles). Oxygen consumption by the wild-type cells was measured as described for Fig. 1 but in 50 mM Tris-HCl-1 mM MgSO4, pH 7.0. The data obtained in the presence of valinomycin (Valino) and valinomycin-CCCP are indicated by open and filled symbols, respectively. (b) pH dependence measured in 50 mM potassium phosphate-1 mM MgSO4. Data were obtained with no additions (open circles) or in the presence of valinomycin (triangles), CCCP (squares), or valinomycin plus CCCP (filled circles).
Energy-dependent reduction of DCPIP and TMPD+ by the cells.
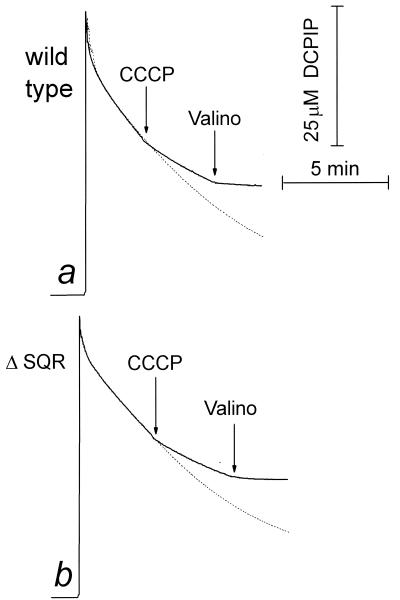
In a second set of experiments, the effect of the uncoupling on the reduction of DCPIP and TMPD+ by endogenous donors in B. subtilis cells was investigated. Studies on both mitochondrial and B. subtilis SQR (15, 51, 52) as well as on bacterial type-II NADH:quinone oxidoreductases (see, e.g., reference 7) indicate that in membrane preparations these artificial electron acceptors receive electrons mostly from the membrane-bound quinols rather than directly from the dehydrogenase metal or flavin redox centers. It can be seen that as in the case of respiration, consecutive addition of CCCP and valinomycin inhibits the reduction of the artificial acceptor (Fig. 4; cf. Fig. 1, traces a and e). The same final level of inhibition was achieved with the inverse order of the additions. Once again, no significant differences between the wild-type and SQR-deficient cells were found. Oxidation of the reduced DCPIP and TMPD by the cells, monitored spectrophotometrically, was resistant to CCCP plus valinomycin (but was fully sensitive to cyanide), which corroborates the data obtained by measurement of oxygen consumption as described in the previous section.
FIG. 4.
Uncoupling-induced inhibition of DCPIP reduction in wild-type and SQR-deficient B. subtilis cells. The reduction of DCPIP by endogenous substrates in malate-grown cells was monitored in the same buffer as used for Fig. 1. (a) Wild-type strain 168 A; (b) SQR-deficient strain LUH-16. The reaction was started by addition of 50 μM DCPIP, which gives the initial increment of absorption. Dotted lines, reaction in the absence of further additions. Valino, valinomycin.
Interestingly, the DCPIP reduction appears to be inhibited by valinomycin alone much more strongly than is oxygen consumption. This can be explained by some protonophoric activity of DCPIP, which has ionizable groups with pKa values of around 6 and 7 in the oxidized and reduced forms, respectively (9). Indeed, we have observed that, similarly to the uncoupler CCCP, DCPIP inhibits endogenous respiration drastically when added in combination with valinomycin.
Reduction of electron transfer rates upon isolation of membranes.
Table 1 compares the specific rates of respiration as well as of DCPIP and TMPD+ reduction observed in B. subtilis membranes with those in the intact cells. In the membranes, all of the activities are strikingly low (10- to 70-fold lower than in the intact cells) and, notably, fully resistant to further inhibition by the uncouplers (10 μM CCCP plus 5 μM valinomycin). Similar low values are manifested by the uncoupled cells. The inhibition of succinate oxidation agrees with the data in references 36 and 47. However, the same inhibition is observed also with NADH and glycerophosphate as substrates, in contrast to the previous results (37, 47).
TABLE 1.
Electron transfer activities in B. subtilis cells and membranes
| Assay | Addition(s) | Activity (s−1)a | ||||
|---|---|---|---|---|---|---|
| Cellsb | Membranes with substratec | |||||
| Wild type | ΔSQR | NADH | Succinate | Glycerol-3-phosphate | ||
| Respiration | None | 160 | 145 | 1.7 | 1.5 | 4.3 |
| CCCP + valinomycin | 8 | 7 | 1.7 | |||
| DCPIP reduction | None | 121 | 112 | 6.3 | 7.4 | 5.1 |
| CCCP + valinomycin | 14 | 7 | 6.3 | |||
| PMS | 153 | 133 | 246 | |||
| TMPD+ reductiond | None | 313 | 266 | 14 | 30 | 18 |
| CCCP + valinomycin | 0 | 0 | 14 |
The rates of PMS reduction by different substrates (Fig. 5; Table 1) measured with DCPIP as the final electron acceptor (1) were found to be similar in the cells and membranes, in agreement with previous results (47). Therefore, the dehydrogenase activities per se are not likely to be impaired during isolation of membranes. Once again, very similar results were obtained with the wild-type and SQR-deficient strains (Table 1; see the legend to Fig. 5), showing that the effect is not specifically associated with SQR.
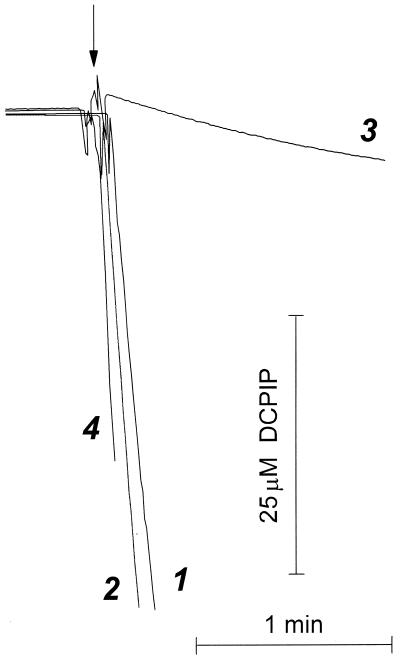
FIG. 5.
Comparison of DCPIP reduction by intact cells and membranes from B. subtilis. DCPIP reduction was measured as described for Fig. 4 with malate-grown 168 A cells (curves 1 and 2) and membranes prepared from these cells (curves 3 and 4). The reaction mixture contained 100 mM MOPS-NaOH buffer (pH 7.0) with 0.2 mM EDTA and, in experiments with the cells (curves 1 and 2), 10 mM malate. PMS at 0.4 mM was added in the case of curves 2 and 4. The reaction was initiated by addition of either cells (curves 1 and 2) or, in the case of membranes, NADH (1 mM in curve 3 and 50 μM in curve 4). Curves 3 and 4 have been corrected for nonenzymatic DCPIP reduction by NADH. The curves obtained with cells and membranes are normalized to the same concentration of cytochrome _aa_3 (0.022 μM).
To determine at which stage(s) of the membrane isolation the respiratory chain becomes inhibited, spheroplasts were first prepared from the cells in the presence of 0.8 M sucrose to prevent osmotic shock (see Materials and Methods). In the course of this procedure, ∼90% of the cells lose their cell wall. Spheroplasts have been found to respire on the endogenous substrates as actively as the untreated cells, and their respiration revealed the same high sensitivity to CCCP plus valinomycin. Notably, added NADH (1 mM) had no effect on the oxygen consumption by spheroplasts, indicating that their membrane is intact. When the medium was replaced with a hypotonic one (without sucrose), the spheroplasts underwent visually detectable lysis, and, concomitantly, a drastic drop in respiration rate was observed (a similar observation for B. subtilis cell homogenates was reported previously [47]). Addition of NADH to osmotically shocked spheroplasts stimulated oxygen consumption but to no more than 15% of the initial rate observed in the cells. Moreover, oxidation of the added NADH was cyanide sensitive but was not inhibited by CCCP plus valinomycin.
DISCUSSION
Electron transport in the respiratory chain of B. subtilis requires ΔμH+.
Our data corroborate earlier observations (3, 37, 46, 47) indicating that electron transfer in the respiratory chain of B. subtilis requires an energized state of the membrane. In this work we aimed to design experiments so as to rule out a number of possible alternative explanations for the inhibitory effect of uncouplers that were not excluded by the conditions of the previous studies.
(i) The effect is not due to direct inhibition of the electron transfer chain by the ionophores.
Uncouplers, such as CCCP, are known to inhibit directly the mitochondrial respiratory chain at relatively high concentration (see, e.g., Fig. 68 of reference 49), presumably acting as UQ antagonists. This is not likely to be the case in our experiments, as evidenced in particular by the very low (submicromolar) concentrations of CCCP required to inhibit respiration in the presence of valinomycin. The widely diverse structures of the ionophores used in this work that exert the inhibitory effect also argue against this possibility. Moreover, an inhibition similar to that induced by the uncoupling agents takes place upon breaking the cell coupling membrane, and the residual electron transfer activities in the membranes are no longer uncoupler sensitive (37).
(ii) The effect does not involve transport of substrates.
In the earlier studies, the effect of uncoupling on the respiration of B. subtilis cells was investigated with added substrates, such as glucose, lactate, citrate, or succinate (37, 46, 47). Therefore, the inhibitory effect of the uncouplers could be due to inhibition of the energy-dependent uptake of these compounds (23, 47, 57). This explanation is ruled out by the experiments in this work that were performed with freshly harvested cells oxidizing endogenous substrates.
(iii) The effect is reversible.
The effect of energization could be indirect. One could visualize that a drop in ΔμH+ triggers a cascade of regulatory reactions which eventually shuts down the respiratory chain by some kind of long-term regulatory effect, involving, for instance, proteolysis or phosphorylation. The ease with which the inhibitory effect is reversed by albumin or charcoal, which resorb the uncoupler, makes such a possibility unlikely.
(iv) Local acidification of the periplasmic space is not involved in the effect.
The cytoplasmic coupling membrane of B. subtilis cells does not directly face the ambient water phase but is surrounded by a cell wall. It was reported that in B. subtilis cells, proton pumping across the cytoplasmic membrane results in positive charging of the cell wall, acidification of the periplasmic space, and, accordingly, modification of electrostatic interactions of periplasmic components, including several enzymes, with the membrane (24, 25, 53). All of these effects were abolished by protonophorous uncouplers, and a similar mechanism could be involved in the energy-dependent modulation of respiration. As we have shown, removal of the cell wall has no effect on the respiration and its sensitivity to the uncouplers. Hence, this possibility can also be excluded.
Those control experiments allow us to assume provisionally that it is indeed proton motive force across the coupling membrane that stimulates electron transfer in the respiratory chain of B. subtilis cells. The effects of CCCP, pentachlorophenol, valinomycin, KSCN, alameticin, gramicidin, and monensin, either alone or in combination**,** as well as the K+ and pH dependencies of the phenomenon, are consistent with contributions of both the Δψ and ΔpH constituents of ΔμH+. It is, however, difficult to discriminate ultimately whether it is an acidic shift on the cytoplasmic side of the membrane (3) or dissipation of ΔpH across the membrane that accounts for the H+-dependent component.
Which step in the respiratory chain is blocked by deenergization?
The inhibition of respiration induced by the uncouplers or by disruption of the cell membrane appears to be associated with the dehydrogenase-MQ part of the respiratory chain. This is evidenced by inhibition of reduction of DCPIP and TMPD+, both of which are believed to accept electrons in the membrane-bound respiratory chain at the level of UQ (MQ-7 in case of B. subtilis) (51, 52, 54), and it agrees with the inhibition of the succinate:MQ reductase activity observed previously (36, 47).
It is noteworthy that the succinate:DCPIP reductase activity was emphasized to be insensitive to the uncoupling in reference 47. This apparent discrepancy with our data is due to confusion in terminology. The “succinate-DCPIP-reductase activity” as measured in (47) is actually a classical succinate dehydrogenase assay (see, e.g., references 15 and 37), with 0.4 mM PMS as the immediate electron acceptor for the dehydrogenase and DCPIP serving as the final colored electron acceptor for PMS. In agreement with the previous study (47), both the membranes and the cells reveal quite a high rate of PMS-mediated reduction of DCPIP (Table 1; Fig. 5). However, reduction of DCPIP or TMPD+ by endogenous substrates in the absence of PMS (which was not assayed in the previous study [47]) is strongly inhibited by uncoupling.
At the same time, oxidation of the reduced TMPD and DCPIP through the terminal oxidases is not inhibited by deenergization. Apparently, the inhibition site is located above the point(s) at which those dyes feed electrons in the respiratory chain and, so terminal oxidases are not the targets of the inhibitory effect. In addition, we have screened B. subtilis strains deficient in different terminal oxidases and the _bc_1 complex and have found all of them to show a similar inhibitory effect of the uncoupling on respiration (unpublished work in collaboration with L. Hederstedt). On the basis of these data, specific association of the inhibition with any particular oxidase does not seem to be likely.
The uncoupler-induced inhibition of electron transfer is thus likely to occur at the level of MQ. Either reduction or oxidation of the quinone could be affected. Formally, one cannot fully exclude the possibility that MQ is reduced by dehydrogenases to MQH2 in the deenergized membranes while oxidation of MQH2 by the cytochrome chain is impaired. Alternatively, it can be reduction of MQ by the dehydrogenases to MQH2 or MQ·− that is energy dependent. Monitoring of the redox state of endogenous MQ in the energized and deenergized membranes is required to directly resolve this alternative. However, it is not easy to visualize how the reduced MQ can be protected from direct nonenzymatic oxidation by TMPD+ and DCPIP (this work) or by 2,3-dimethyl-1,4-naphthoquinone (47). Therefore, we assume provisionally that it is MQ reduction rather than oxidation that shuts down upon deenergization of the membrane.
The inhibition is not associated specifically with SQR_._
We confirm the finding of Schirawski and Unden that deenergization impairs succinate-dependent electron transfer to oxygen or artificial acceptors in the respiratory chain of B. subtilis (47). However, our results do not support their conclusion on the effect being specific for succinate oxidation (47). Contrast to the results in references 37 and 47 and in accordance with those in reference 3, the inhibitory effect of the uncoupling was observed with the NADH-dependent substrates as well. Moreover, the same inhibition was obtained in this work with the B. subtilis mutant lacking SQR. Hence, the data used previously to substantiate the conclusion that “menaquinone-dependent succinate dehydrogenase of bacteria catalyzes reversed electron transport driven by the proton potential” (47) may not be relevant to the hypothesis. It is noted that the hypothesis of vectorial electron transfer through SQR is not necessarily compromised by our results. The membrane potential-driven reduction of MQ in B. subtilis SQR is predicted explicitly by the structure of the enzyme and therefore remains an attractive possibility (14, 32, 42), which, however, should not be confused or identified with the effect of energy-dependent stimulation of respiration.
Possible mechanisms of the energy-dependent stimulation of electron transfer in B. subtilis.
According to our observations, membrane energization is required for any of the MQ-dependent electron transfer pathways in the respiratory chain of B. subtilis. It cannot be excluded formally that each of the dehydrogenases is endowed with its own specific mechanism of energy-dependent reduction of MQ. However, such a possibility does not look appealing. Notably, many of the dehydrogenases, such as type II NADH dehydrogenase (4) or GP dehydrogenase (T. Hedin and L. Hederstedt, unpublished data), are not integral membrane proteins and lack anchor subunits or membrane-spanning helices that could sense or mediate the effect of ΔμH+.
MQ is the only common link for the different electron transfer routes in B. subtilis, and it is tempting to propose that MQ itself can be the component whose reduction-oxidation is controlled by energization. Such a control could be imposed via special MQ-binding adapter proteins located in the membrane. Protein-imposed control over redox reactions of MQ could also be meaningful for a more general reason. B. subtilis is a relatively rare example of a strictly aerobic bacterium with MQ as the only quinone species. Due to its low Em (ca. −80 mV, versus ca. +90 mV in the case of, e.g., UQ [42]), MQH2 is much more autoxidizable than ubiquinol (UQH2). In animal mitochondria, reactions of UQ in the respiratory chain are believed to be described by a simple mass action law (the so-called Q-pool model [29]). This model assumes the existence of homogenous pools of mobile free UQ and UQH2 dissolved in the phospholipid milieu of the membrane that shuttle between the dehydrogenases and cytochrome _bc_1 complexes. The UQ pool concept was also used to describe respiration through the branched electron transfer chains of Paracoccus denitrificans, although more complex behavior of the system was noticed in this case (43) and in plant mitochondria (26). The pool behavior may be problematic in B. subtilis, however, since free reduced MQH2 is prone to rapid autoxidation. Therefore, a more specific control over MQ reduction-oxidation would be functionally significant.
First, the time of MQH2 free diffusion in the membrane between the dehydrogenases and quinol-oxidizing enzymes should be minimized (e.g., by virtue of formation of functionally active supercomplexes between different dehydrogenases and quinol oxidases [5, 21, 45]). Second, involvement of the special MQ-binding proteins that stabilize the reduced form(s) of MQ may be envisaged. According to this hypothesis, there may be protein-bound, rather than free, forms of MQ and MQH2 that serve as substrates for the dehydrogenases and quinol oxidases in B. subtilis. The stabilization could be either kinetic, e.g., sequestration of the quinol ring of bound MQH2 inside the protein protecting it from collisions with oxygen dissolved in the membrane, or thermodynamic, raising the effective Em of the bound MQ-MQH2 (or MQ-MQ·−) couple.
Conceivably, the MQ-binding adapter proteins could also mediate the energy-driven stimulation of MQ-dependent electron transfer. First, the energy of ΔμH+ can be utilized for thermodynamic promotion of MQ reduction to MQH2, as discussed, for instance, for SQR (42). In general, such an effect implies that upon reduction, MQ receives electrons from the dehydrogenases at the inner side of the membrane while being protonated from the outer aqueous phase (e.g., via a proton channel in the adapter protein). Second, membrane energization could simply lower the kinetic barrier of the reaction and thus be part of a regulation mechanism. For instance, in plant mitochondria, SQR is activated by proton motive force despite the absence of a thermodynamic barrier between the enzyme and UQ (2). It must be emphasized that in B. subtilis, there is no explicit thermodynamic problem in the overall reduction of MQ to MQH2 by dehydrogenases of substrates other than succinate, as their _Em_s are typically below that of the MQ-MQH2 couple. Therefore, we would favor the more ergonomic kinetic regulation mechanism over the ΔμH+-consuming thermodynamically driven quinone reduction.
Reduction of MQ to the unstable semiquinone radical upon addition of the first electron is likely to be the limiting step of the overall process due to the very low Em of the MQ/MQ·− transition. The reaction can be facilitated by a specific tight binding of the semiquinone intermediate, as is typical of the 2_e_−→1_e_− junctions in the respiratory and photosynthetic electron transfer chains (42). It can be speculated that in B. subtilis, the major peripheral dehydrogenases such as type II NADH dehydrogenase or GP dehydrogenase cannot stabilize MQ·− themselves and that this function requires MQ-binding adapter proteins which are in turn regulated by proton motive force.
First, energization could modulate conformation of the adapter protein, opening the binding crevice for MQ. Second, energization could increase affinity of the adapter protein for MQ·−. The latter effect can be induced, in particular, by membrane potential-driven protonation of a residue in vicinity of the MQ·−-binding site from periplasm via a proton channel. Energy-dependent proton delivery from the periplasmic side will change electric charge near the binding site, e.g., by virtue of protonation of ionized carboxylate. For instance, stabilization of ubisemiquinone anion in E. coli fumarate reductase has been observed upon mutation of a nearby anionic Glu-29 to neutral Leu (17). Accordingly, binding of the semiquinone anion will be greatly enhanced and the Em of the MQ-MQ·− transition will be raised relative to those for the deenergized membrane.
UQ has been shown recently to modulate the structure of the so-called uncoupling proteins (UCP 1, 2, and 3) in the mitochondrial membrane (11, 12), with the effect being specific for the oxidized (UQ) form (12). These findings provide a precedent of a linkage between the redox state of bound quinone and the structure of a nonredox proton-conducting integral membrane protein sensitive to ΔμH+ and thus may be relevant to the hypothesis of the quinone adapter proteins. Interestingly, a fraction of UQH2 that is resistant to aerobic oxidation through the respiratory chain due to interaction with a yet-unidentified protein (A. M. Wagner, unpublished data) has been found in plant mitochondria (44, 56).
Finally, the energy-dependent stimulation of respiration may not be a unique feature of B. subtilis. Relevant effects were described earlier for Micrococcus luteus (M. lysodeikticus) (55) and for two more bacteria (47). In recent work, we have observed the uncoupler-induced inhibition of respiration in more than 20 other bacteria, most of which were gram positive and contained MQ (N. Azarkina, E. Strom, and A. A. Konstantinov, unpublished data). Evidence for membrane potential-dependent modulation of UQ pool behavior in P. denitrificans has been obtained (43), and an energy requirement for active respiration has been observed recently for some animal mitochondria (A. I. Shestopalov and B. S. Kristal, unpublished data). Hence, the ΔμH+-dependent regulation of electron flow through the respiratory chain at the level of coenzyme Q may be a phenomenon of wide physiological significance.
Acknowledgments
The work was supported by grants from the Russian Fund for Basic Research (00-04-48251, 99-04-48095, 01-04-49330, and 01-04-06274), Howard Hughes Medical Institute International Research Scholar Award 55000320, and the INTAS Young Scientist fellowship program (YSF 00-140).
REFERENCES
- 1.Ackrell, B. A., E. B. Kearney, and T. P. Singer. 1978. Mammalian succinate dehydrogenase. Methods Enzymol. 53**:**466-483. [DOI] [PubMed] [Google Scholar]
- 2.Affourtit, C., K. Krab, G. R. Leach, D. G. Whitehouse, and A. L. Moore. 2001. New insights into the regulation of plant succinate dehydrogenase. J. Biol. Chem. 276**:**32567-32574. [DOI] [PubMed] [Google Scholar]
- 3.Barsky, E. L., A. V. Nazarenko, V. D. Samuilov, and S. A. Khakimov. 1989. Inhibition of respiration of Bacillus subtilis cells by acidification of cytoplasm. Biol. Membr. (Moscow) 6**:**720-724. [Google Scholar]
- 4.Bergsma, J., M. B. M. van Dongen, and W. N. Konings. 1982. Purification and characterization of NADH dehydrogenase from Bacillus subtilis. Eur. J. Biochem. 128**:**151-157. [DOI] [PubMed] [Google Scholar]
- 5.Berry, E. A., and B. L. Trumpower. 1985. Isolation of ubiquinol oxidase from Paracoccus denitrificans and resolution into cytochrome bc1 and cytochrome c-aa3 complexes. J. Biol. Chem. 260**:**2458-2467. [PubMed] [Google Scholar]
- 6.Biel, S., J. Simon, R. Gross, T. Ruiz, M. Ruitenberg, and A. Kroger. 2002. Reconstitution of coupled fumarate respiration in liposomes by incorporating the electron transport enzymes isolated from Wolinella succinogenes. Eur. J. Biochem. 269**:**1974-1983. [DOI] [PubMed] [Google Scholar]
- 7.Bjorklof, K., V. Zickermann, and M. Finel. 2000. Purification of the 45 kDa, membrane bound NADH dehydrogenase of Escherichia coli (NDH-2) and analysis of its interaction with ubiquinone analogues. FEBS Lett. 467**:**105-110. [DOI] [PubMed] [Google Scholar]
- 8.Cecchini, G., I. Schroder, R. P. Gunsalus, and E. Maklashina. 2002. Succinate dehydrogenase and fumarate reductase from Escherichia coli. Biochim. Biophys. Acta 1553**:**140-157. [DOI] [PubMed] [Google Scholar]
- 9.Clark, W. M. 1960. Oxidation-reduction potentials of organic systems. Williams and Wilkins, Baltimore, Md.
- 10.Collins, M. D., and D. Jones. 1981. Distribution of isoprenoid quinone structural types in bacteria and their taxonomic implications. Microbiol. Rev. 45**:**316-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Echtay, K. S., E. Winkler, K. Frischmuth, and M. Klingenberg. 2001. Uncoupling proteins 2 and 3 are highly active H+ transporters and highly nucleotide sensitive when activated by coenzyme Q (ubiquinone). Proc. Natl. Acad. Sci. USA 98**:**1416-1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Echtay, K. S., E. Winkler, and M. Klingenberg. 2000. Coenzyme Q is an obligatory cofactor for uncoupling protein function. Nature 408**:**609-613. [DOI] [PubMed] [Google Scholar]
- 13.Geisler, V., R. Ullmann, and A. Kroger. 1994. The direction of the proton exchange associated with the redox reactions of menaquinone during electron transport in Wolinella succinogenes. Biochim. Biophys. Acta 1184**:**219-226. [Google Scholar]
- 14.Hagerhall, C. 1997. Succinate: quinone oxidoreductases. Variations on a conserved theme. Biochim. Biophys. Acta 1320**:**107-141. [DOI] [PubMed] [Google Scholar]
- 15.Hagerhall, C., R. Aasa, C. von Wachenfeldt, and L. Hederstedt. 1992. Two hemes in Bacillus subtilis succinate:menaquinone oxidoreductase (complex II). Biochemistry 31**:**7411-7421. [DOI] [PubMed] [Google Scholar]
- 16.Hagerhall, C., and L. Hederstedt. 1996. A structural model for the membrane-integral domain of succinate:quinone oxidoreductases. FEBS Lett. 389**:**25-31. [DOI] [PubMed] [Google Scholar]
- 17.Hagerhall, C., S. Magnitsky, V. Sled, I. Schroder, R. P. Gunsalus, G. Cecchini, and T. Ohnishi. 1999. An Escherichia coli mutant quinol:fumarate reductase contains an EPR-detectable semiquinone stabilized at the proximal quinone-binding site. J. Biol. Chem. 274**:**26157-26164. [DOI] [PubMed] [Google Scholar]
- 18.Hatefi, Y. 1985. The mitochondrial electron transport and oxidative phosphorylation system. Annu. Rev. Biochem. 54**:**1015-1069. [DOI] [PubMed] [Google Scholar]
- 19.Hederstedt, L. 1986. Molecular properties, genetics, and biosynthesis of Bacillus subtilis succinate dehydrogenase complex. Methods Enzymol. 126**:**399-414. [DOI] [PubMed] [Google Scholar]
- 20.Hederstedt, L. 2002. Succinate:quinone oxidoreductase in the bacteria Paracoccus denitrificans and Bacillus subtilis. Biochim. Biophys. Acta 1553**:**74-83. [DOI] [PubMed] [Google Scholar]
- 21.Heron, C., C. I. Ragan, and B. L. Trumpower. 1978. The interaction between mitochondrial NADH-ubiquinone oxidoreductase and ubiquinol-cytochrome c oxidoreductase. Restoration of ubiquinone-pool behaviour. Biochem. J. 174**:**791-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iverson, T. M., C. Luna-Chavez, G. Ceccini, and D. C. Rees. 1999. Structure of the Escherichia coli fumarate reductase respiratory complex. Science 284**:**1961-1966. [DOI] [PubMed] [Google Scholar]
- 23.Janausch, I. G., E. Zientz, Q. H. Tran, A. Kroger, and G. Unden. 2002. C4-dicarboxylate carriers and sensors in bacteria. Biochim. Biophys. Acta 1553**:**39-56. [DOI] [PubMed] [Google Scholar]
- 24.Jolliffe, L. K., R. J. Doyle, and U. N. Streips. 1981. The energized membrane and cellular autolysis in Bacillus subtilis. Cell 25**:**753-763. [DOI] [PubMed] [Google Scholar]
- 25.Kemper, M. A., M. M. Urrutia, T. J. Beveridge, A. L. Koch, and R. J. Doyle. 1993. Proton motive force may regulate cell wall-associated enzymes of Bacillus subtilis. J. Bacteriol. 175**:**5690-5696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krab, K. 1995. Kinetic and regulatory aspect of the alternative oxidase in plant respiration. J. Bioenerg. Biomembr. 27**:**387-396. [DOI] [PubMed] [Google Scholar]
- 27.Kroger, A., S. Biel, J. Simon, R. Gross, G. Unden, and C. R. D. Lancaster. 2002. Fumarate respiration of Wolinella succinogenes: enzymology, energetics and coupling mechanism. Biochim. Biophys. Acta 1553**:**23-38. [DOI] [PubMed] [Google Scholar]
- 28.Kroger, A., V. Geisler, E. Lemma, F. Theis, and R. Lenger. 1992. Bacterial fumarate respiration. Arch. Microbiol. 158**:**311-314. [Google Scholar]
- 29.Kroger, A., and M. Klingenberg. 1973. The kinetics of the redox reactions of ubiquinone related to the electron-transport activity in the respiratory chain. Eur. J. Biochem. 34**:**358-368. [DOI] [PubMed] [Google Scholar]
- 30.Krulwich, T. A., A. A. Guffanti, and I. Masahiro. 1999. pH tolerance in Bacillus: alkaliphiles versus non-alkaliphiles. Novartis Foundation Symposium 221. John Wiley, Chichester, United Kingdom. [DOI] [PubMed]
- 31.Lancaster, C. R., R. Gross, A. Haas, M. Ritter, W. Mantele, J. Simon, and A. Kroger. 2000. Essential role of Glu-C66 for menaquinol oxidation indicates transmembrane electrochemical potential generation by Wolinella succinogenes fumarate reductase. Proc. Natl. Acad. Sci. USA 97**:**13051-13056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lancaster, C. R., and A. Kroger. 2000. Succinate:quinone oxidoreductases: new insights from X-ray crystal structures. Biochim. Biophys. Acta 1459**:**422-431. [DOI] [PubMed] [Google Scholar]
- 33.Lancaster, C. R. D. 2002. Succinate:quinone oxidoreductases: an overview. Biochim. Biophys. Acta 1553**:**1-6. [DOI] [PubMed] [Google Scholar]
- 34.Lancaster, C. R. D., A. Kroger, M. Auer, and H. Michel. 1999. Structure of fumarate reductase from Wolinella succinogenes at 2.2 A resolution. Nature 402**:**377-385. [DOI] [PubMed] [Google Scholar]
- 35.Lardy, H. A., and H. Wellman. 1952. Oxidative phosphorylations: role of inorganic phosphate and acceptor systems in control of metabolic rates. J. Biol. Chem. 195**:**215-224. [PubMed] [Google Scholar]
- 36.Lemma, E., C. Hagerhall, V. Geisler, U. Brandt, G. von Jagow, and A. Kroger. 1991. Reactivity of the Bacillus subtilis succinate dehydrogenase complex with quinones. Biochim. Biophys. Acta 1059**:**281-285. [DOI] [PubMed] [Google Scholar]
- 37.Lemma, E., G. Unden, and A. Kroger. 1990. Menaquinone is an obligatory component of the chain catalyzing succinate respiration in Bacillus subtilis. Arch. Microbiol. 155**:**62-67. [DOI] [PubMed] [Google Scholar]
- 38.Lemos, R. S., A. S. Fernandes, M. M. Pereira, C. M. Gomes, and M. Teixeira. 2002. Quinol:fumarate oxidoreductases and succinate:quinone oxidoreductases: phylogenetic relationships, metal centres and membrane attachment. Biochim. Biophys. Acta 1553**:**158-170. [DOI] [PubMed] [Google Scholar]
- 39.Matsson, M., D. Tolstoy, R. Aasa, and L. Hederstedt. 2000. The distal heme center in Bacillus subtilis succinate:quinone reductase is crucial for electron transfer to menaquinone. Biochemistry 39**:**8617-8624. [DOI] [PubMed] [Google Scholar]
- 40.Measures, J. C. 1975. Role of amino acids in osmoregulation of non-halophilic bacteria. Nature 257**:**398-400. [DOI] [PubMed] [Google Scholar]
- 41.Mell, H., C. Wellnitz, and A. Kroger. 1986. The electrochemical proton potential and the proton/electron ratio of the electron transport with fumarate in Wolinella succinogenes. Biochim. Biophys. Acta 852**:**212-221. [Google Scholar]
- 42.Ohnishi, T., C. C. Moser, C. C. Page, P. L. Dutton, and T. Yano. 2000. Simple redox-linked proton-transfer design: new insights from structures of quinol-fumarate reductase. Structure **8:**R23-R32. [DOI] [PubMed]
- 43.Otten, M. F., W. N. M. Reijnders, J. J. M. Bedaux, H. V. Westerhoff, K. Krab, and R. J. M. Van Spanning. 1999. The reduction state of the Q-pool regulates the electron flux through the branched respiratory network of Paracoccus denitrificans. Eur. J. Biochem. 261**:**767-774. [DOI] [PubMed] [Google Scholar]
- 44.Popov, V. N., A. C. Purvis, V. P. Skulachev, and A. M. Wagner. 2001. Stress-induced changes in ubiquinone concentration and alternative oxidase in plant mitochondria. Biosci. Rep. 21**:**369-379. [DOI] [PubMed] [Google Scholar]
- 45.Ragan, C. I., and C. Heron. 1978. The interaction between mitochondrial NADH-ubiquinone oxidoreductase and ubiquinol-cytochrome c oxidoreductase. Evidence for stoichiometric association. Biochem. J. 174**:**783-790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Samuilov, V. D., and S. A. Khakimov. 1991. Dependence of respiration of Bacillus subtilis cells on monovalent cations. Biochemistry (Moscow) 56**:**1209-1214. [PubMed] [Google Scholar]
- 47.Schirawski, J., and G. Unden. 1998. Menaquinone-dependent succinate dehydrogenase of bacteria catalyzes reversed electron transport driven by the proton potential. Eur. J. Biochem. 257**:**210-215. [DOI] [PubMed] [Google Scholar]
- 48.Schnorpfeil, M., I. G. Janausch, S. Biel, A. Kroger, and G. Unden. 2001. Generation of a proton potential by succinate dehydrogenase of Bacillus subtilis functioning as a fumarate reductase. Eur. J. Biochem. 268**:**3069-3074. [DOI] [PubMed] [Google Scholar]
- 49.Skulachev, V. P. 1969. Energy accumulation in the cell. Nauka, Moscow, Russia. (In Russian.)
- 50.Smirnova, I. A., C. Hagerhall, A. A. Konstantinov, and L. Hederstedt. 1995. HOQNO interaction with cytochrome b in succinate:menaquinone oxidoreductase from Bacillus subtilis. FEBS Lett. 359**:**23-26. [DOI] [PubMed] [Google Scholar]
- 51.Tkachenko, T. A., V. S. Kunts, and A. A. Konstantinov. 1983. The site of interaction of the oxidized N,N,N′,N′-tetramethyl-p-phenilenediamine (Wurster Blue) with the bc1 respiratory chain complex. Biochemistry (Moscow) **273:**242-245. (In Russian.) [PubMed]
- 52.Tushurashvili, P. R., E. V. Gavrikova, A. N. Ledenev, and A. D. Vinogradov. 1985. Studies on the succinate dehydrogenating system. Isolation and properties of the mitochondrial succinate-ubiquinone reductase. Biochim. Biophys. Acta 809**:**145-159. [DOI] [PubMed] [Google Scholar]
- 53.Urrutia, M. M., M. Kemper, R. Doyle, and T. J. Beveridge. 1992. The membrane-induced proton motive force influences the metal binding ability of Bacillus subtilis cell walls. Appl. Environ. Microbiol. 58**:**3837-3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vinogradov, A. D. 1986. Succinate-ubiquinone reductase of the respiratory chain. Biochemistry (Moscow) 51**:**1944-1973. [PubMed] [Google Scholar]
- 55.Votyakova, T. V., V. Y. Artzatbanov, G. V. Mukamolova, and A. S. Kaprelyants. 1994. On the relationship between bacterial cell integrity and respiratory chain activity: a fluorescence anisotropy study. Arch. Biochem. Biophys. 314**:**280-283. [DOI] [PubMed] [Google Scholar]
- 56.Wagner, A. M., and A. C. Purvis. 1998. Production of reactive oxygen species in plant mitochondria. A dual role for ubiquinone?, p. 537-541. In I. M. Muller, P. Gardestrom, K. Glimelius, and E. Glaser (ed.), Plant mitochondria: form gene to function. Backhuys Publishers, Leiden, The Netherlands.
- 57.Willecke, K., and R. Lange. 1974. C4-dicarboxylate transport in Bacillus subtilis studied with 3-fluoro-l-erythro-malate as a substrate. J. Bacteriol. 117**:**373-378. [DOI] [PMC free article] [PubMed] [Google Scholar]