Dissemination of New Methicillin-Resistant Staphylococcus aureus Clones in the Community (original) (raw)
Abstract
Multiple methicillin-resistant Staphylococcus aureus (MRSA) clones carrying type IV staphylococcal cassette chromosome mec were identified in the community-acquired MRSA strains of both the United States and Australia. They multiplied much faster than health-care-associated MRSA and were resistant to fewer non-beta-lactam antibiotics. They seem to have been derived from more diverse S. aureus populations than health-care-associated MRSA strains.
Methicillin-resistant Staphylococcus aureus (MRSA), besides having established itself as a major hospital pathogen, is now beginning to prevail in the wider community as well (1, 3-5). However, we do not know if the subgroup of MRSA designated community-acquired MRSA (C-MRSA) share a common origin of derivation with the other subgroup of MRSA in hospitals, namely the health-care-associated MRSA (H-MRSA). The majority of H-MRSA strains carry one of the three types of staphylococcal cassette chromosome mec (SCC_mec_) as the methicillin resistance determinant on their chromosomes (19, 22). However, members of our group have recently identified a novel SCC_mec_, designated type IV, in the C-MRSA strains isolated at a Chicago children's hospital (23). This raised a possibility that C-MRSA might have an origin of derivation distinct from that of H-MRSA, and type-IV SCC_mec_ could be its unique genetic marker (14). To further test this view, we now analyzed 23 well-characterized C-MRSA strains (2-4, 24-26, 28) whose sources of isolation were not associated with risk factors for H-MRSA infection (e.g., recent hospitalization, recent surgery, residence in a long-term care facility, drug use, etc.) (7, 11) and 12 Australian MRSA strains designated non-multiresistant oxacillin-resistant S. aureus (NORSA) (9) and compared them with the representative H-MRSA strains. NORSA strains, though frequently isolated in hospitals, are considered to be the descendants of C-MRSA strains in Australia (10).
Table 1 shows that the majority of C-MRSA strains were susceptible to most of the non-beta-lactam antibiotics, as NORSA strains are by definition (9). Although the non-multiresistant nature of C-MRSA has been well recognized as a characteristic of C-MRSA (16), this was not without exception: strain 01083 was resistant to four non-beta-lactam antibiotics (Table 1). This indicates that though it is a rare occurrence, C-MRSA strains may also acquire resistance to non-beta-lactam antibiotics, presumably through exposure to the antibiotics.
TABLE 1.
Genotyping and antibiogram of tested MRSA strains
| Categorya | Sourceb | Strain name | Coagulase isotype | MIC (mg/liter) ofc: | SCC_mec_ typed | PFGE pattern | MLST | luk-PVg | Doubling time (min)h | Reference(s) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ERY | KAN | TOB | GEN | TET | NOR | OXA | CZX | IMP | ST, allelic profilee | CCf | |||||||||
| C | MN, US | C1998000370 | 7 | 0.5 | 8 | 0.5 | 0.5 | 32 | 1 | 4 | 512 | 0.25 | IVa | J4 | 1, 1-1-1-1-1-1-1 | 1 | + | 27.89 | 3, 24 |
| C | ND, US | C1999000529 | 7 | 0.5 | 4 | 0.5 | 0.25 | 0.5 | 1 | 8 | >512 | 0.5 | IVa | J4 | 1, 1-1-1-1-1-1-1 | 1 | + | 26.38 | 3, 24 |
| C | MN, US | C1999000193 | 7 | 0.5 | 8 | 0.5 | 0.5 | 0.5 | 1 | 4 | >512 | 0.25 | IVa | J3 | 1, 1-1-1-1-1-1-1 | 1 | + | 26.73 | 3, 24 |
| C | ND, US | MW2 | 7 | 0.5 | 4 | 0.25 | 0.25 | 0.5 | 1 | 8 | >512 | 0.5 | IVa | J4 | 1, 1-1-1-1-1-1-1 | 1 | + | 28.67 | 2, 3, 24 |
| C | MN, US | C2001001201 | 7 | >512 | 8 | 0.25 | 0.25 | 0.5 | 1 | 8 | >512 | 0.5 | IVa | J4 | 1, 1-1-1-1-1-1-1 | 1 | + | 27.4 | 3, 24 |
| C | MN, US | C2001000101 | 7 | 0.5 | 8 | 0.5 | 0.5 | 0.5 | 1 | 4 | >512 | 0.25 | IVa | J4 | 1, 1-1-1-1-1-1-1 | 1 | + | 27.25 | 3, 24 |
| C | MN, US | C200100818 | 7 | >512 | 8 | 0.5 | 0.25 | 0.5 | 1 | 16 | >512 | 0.25 | IVa | J5 | 1, 1-1-1-1-1-1-1 | 1 | + | 27.72 | 3, 24 |
| C | Woo, AU | A80 3355 | 4 | 0.5 | 4 | 0.25 | 0.25 | 0.5 | 2 | 16 | >512 | 0.125 | IVa | H1 | 30, 2-2-2-2-6-3-2 | 30 | + | 26.22 | 25 |
| C | Woo, AU | A82 3549 | 4 | 0.5 | 2 | 0.25 | 0.25 | 0.5 | 2 | 16 | >512 | 0.125 | IVa | H3 | 30, 2-2-2-2-6-3-2 | 30 | + | 28.09 | 25 |
| C | Woo, AU | A83 0528 | 4 | 0.5 | 4 | 0.25 | 0.25 | 0.5 | 2 | 16 | >512 | ≤0.063 | IVa | H1 | 30, 2-2-2-2-6-3-2 | 30 | + | 27.08 | 25 |
| C | Woo, AU | B82 6559 | 4 | 0.5 | 2 | 0.25 | 0.25 | 0.5 | 2 | 16 | 512 | ≤0.063 | IVa | H1 | 30, 2-2-2-2-6-3-2 | 30 | + | 27.57 | 25 |
| C | Woo, AU | D82 1552 | 4 | 0.5 | 2 | 0.25 | 0.25 | 0.5 | 2 | 16 | >512 | 0.125 | IVa | H1 | 30, 2-2-2-2-6-3-2 | 30 | + | 61.03 | 25 |
| C | Woo, AU | E80 2537 | 4 | 0.5 | 4 | 0.25 | 0.25 | 0.5 | 2 | 8 | >512 | ≤0.063 | IVa | H2 | 30, 2-2-2-2-6-3-2 | 30 | + | 26.97 | 25 |
| C | Woo, AU | F81 0539 | 4 | 0.5 | 4 | 0.25 | 0.25 | 0.5 | 2 | 16 | >512 | ≤0.063 | IVa | H1 | 30, 2-2-2-2-6-3-2 | 30 | + | 26.44 | 25 |
| C | Woo, AU | 180 2552 | 4 | 0.5 | 2 | 0.25 | 0.25 | 0.5 | 2 | 16 | 512 | 0.125 | IVa | H3 | 30, 2-2-2-2-6-3-2 | 30 | + | 26.86 | 25 |
| C | Per, AUi | M9N | 3 | >512 | 4 | 0.25 | 0.25 | 0.5 | 2 | 16 | 512 | 0.125 | IVa | I1 | 78, 22-1-14-23-12-53-31 | 298 | − | 26.75 | 26 |
| C | Per, AUi | M33T | 3 | >512 | 4 | 0.25 | 0.25 | 0.5 | 2 | 16 | 512 | 0.125 | IVa | I2 | 78, 22-1-14-23-12-53-31 | 298 | − | 26.44 | 26 |
| C | Per, AUi | W1S | 1 | 0.5 | 2 | 0.5 | 0.25 | 0.5 | 0.5 | 2 | 32 | 0.063 | New | E2 | 45, 10-14-8-6-10-3-2 | 45 | − | 27.74 | 26 |
| C | Per, AUi | C7N | 7 | >512 | 4 | 0.5 | 0.5 | 0.5 | 2 | 16 | 512 | 0.25 | IVa | J6 | 1, 1-1-1-1-1-1-1 | 1 | − | 30.26 | 26 |
| C | TN, USj | 00215 | 7 | 0.25 | 4 | 0.5 | 0.25 | 0.5 | 0.5 | 64 | >512 | 2 | IVa | E1 | 45, 10-14-8-6-10-3-2 | 45 | − | 27.71 | 28 |
| C | MS, USk | 01083 | 3 | 64 | >512 | 0.5 | 0.5 | 32 | 16 | 8 | 512 | 0.125 | IVa | A3 | 8, 3-3-1-1-4-4-3 | 8 | + | 27.78 | 4 |
| C | MS, USk | 01093 | 5 | 16 | 512 | 0.5 | 0.5 | 0.5 | 2 | 16 | >512 | 0.125 | IVa | F | 72, 1-4-1-8-4-4-3 | 8 | − | 25.94 | 4 |
| C | MS, USk | 01102 | 3 | 0.25 | 4 | 0.5 | 0.5 | 0.5 | 1 | 8 | >512 | 0.25 | IVb | A2 | 8, 3-3-1-1-4-4-3 | 8 | + | 28.29 | 4 |
| N | Ade, AU | 81 0342 | 3 | 0.25 | 4 | 0.5 | 0.5 | 0.5 | 8 | 2 | 16 | ≤0.063 | New | A1 | 8, 3-3-1-1-4-4-3 | 8 | − | 26.86 | This study |
| N | Ade, AU | 91 2572 | 7 | 0.5 | 4 | 0.25 | 0.25 | 0.5 | 1 | 4 | >512 | 0.25 | IVa | J7 | 1, 1-1-1-1-1-1-1 | 1 | − | 27.15 | This study |
| N | Ade, AU | 91 2619 | 7 | 0.5 | 8 | 0.5 | 0.5 | 0.5 | 2 | 64 | >512 | 2 | IVa | J7 | 76, 1-63-1-1-1-1-1 | 1 | − | 27.47 | This study |
| N | Ade, AU | WCH379 | 7 | 0.5 | 2 | 0.25 | 0.5 | 0.5 | 1 | 16 | >512 | 4 | IVa | J8 | 1, 1-1-1-1-1-1-1 | 1 | − | 33.31 | This study |
| N | Ade, AU | 91 2574 | 7 | 0.25 | 1 | 0.25 | 0.25 | 0.5 | 128 | 8 | 256 | ≤0.063 | New | G | 22, 7-6-1-5-8-8-6 | 22 | − | 29.52 | This study |
| N | Ade, AU | SAP260 | 2 | >512 | 4 | 0.5 | 0.25 | 0.5 | 0.5 | 4 | >512 | 0.25 | IVa | B | 73, 1-4-27-4-12-1-10 | 5 | − | 26.64 | This study |
| N | Per, AU | 81 0937 | 7 | 0.5 | 2 | 0.25 | 0.5 | 0.5 | 1 | 4 | >512 | 0.5 | IVa | J1 | 1, 1-1-1-1-1-1-1 | 1 | − | 25.29 | This study |
| N | Per, AU | 91 2125 | 7 | 0.5 | 4 | 0.25 | 0.5 | 0.5 | 0.5 | 16 | >512 | 0.5 | IVa | J2 | 1, 1-1-1-1-1-1-1 | 1 | − | 30.25 | This study |
| N | Per, AU | 91 1703 | 3 | 0.25 | 2 | 0.125 | 0.25 | 8 | 1 | 16 | 512 | 0.125 | IVa | A2 | 8, 3-3-1-1-4-4-3 | 8 | − | 32.0 | This study |
| N | Bri, AU | 81 1238 | 7 | 0.5 | 2 | 0.25 | 0.5 | 0.5 | 1 | 8 | >512 | 0.5 | IVa | J1 | 1, 1-1-1-1-1-1-1 | 1 | − | 26.06 | This study |
| N | Bri, AU | 91 2666 | 7 | 0.5 | 2 | 0.5 | 0.25 | 0.5 | 0.5 | 2 | 512 | 0.125 | IVa | J2 | 1, 1-1-1-1-1-1-1 | 1 | − | 26.65 | This study |
| N | Dar, AU | SAP411 | 6 | ≤0.125 | 4 | 0.25 | 0.5 | 32 | 1 | 4 | 128 | 0.125 | IVa | N | 75, 36-3-43-34-39-52-49 | S | − | 27.68 | This study |
| H | Ade, AU | 81 0508 | 4 | >512 | 256 | 256 | 0.5 | 0.25 | 512 | 512 | >512 | 128 | II | M2 | 36, 2-2-2-2-3-3-2 | 30 | − | 32.22 | This study |
| H | Ade, AU | 91 2231 | 4 | >512 | 2 | 0.25 | 0.5 | 32 | 32 | 128 | >512 | 16 | III | K4 | 239, 2-3-1-1-4-4-3 | 8 | − | 35.8 | This study |
| H | Bri, AU | 91 1573 | 4 | >512 | 128 | 64 | 8 | 16 | 1 | 64 | >512 | 4 | III | K2 | 239, 2-3-1-1-4-4-3 | 8 | − | 41.82 | This study |
| H | Bri, AU | 91 1575 | 4 | >512 | 512 | 16 | 32 | 1 | 32 | 256 | >512 | 32 | III | K6 | 239, 2-3-1-1-4-4-3 | 8 | − | 50.46 | This study |
| H | Bri, AU | 91 2145 | 4 | >512 | 512 | 16 | 32 | 32 | 32 | 64 | >512 | 16 | III | K1 | 239, 2-3-1-1-4-4-3 | 8 | − | 50.64 | This study |
| H | Dar, AU | SAP344 | 4 | >512 | 4 | 0.25 | 0.5 | 32 | 32 | 256 | >512 | 32 | III | K3 | 239, 2-3-1-1-4-4-3 | 8 | − | 34.51 | This study |
| H | Per, AU | 91 2118 | 4 | >512 | 512 | 16 | 32 | 32 | 256 | 512 | >512 | 64 | III | L | 239, 2-3-1-1-4-4-3 | 8 | − | 38.45 | This study |
| H | UK | NCTC10442 | 3 | 0.125 | 1 | 0.125 | 0.5 | 128 | 1 | 256 | >512 | 16 | I | D | 250, 3-3-1-1-4-4-16 | 8 | − | 36.44 | 19 |
| H | JP | N315 | 2 | >512 | >512 | 512 | 0.5 | 0.125 | 2 | 16 | 16 | 1 | II | C | 5, 1-4-1-4-12-1-10 | 5 | − | 34.28 | 19 |
| H | NZ | 85/2082 | 4 | 512 | >512 | 8 | 64 | 128 | 2 | 32 | >512 | 0.5 | III | K5 | 239, 2-3-1-1-4-4-3 | 8 | − | 43.53 | 19 |
| H | UK | Strain 252l | 4 | >512 | 128 | 128 | 0.25 | 0.5 | >512 | 512 | >512 | 64 | II | M1 | 36, 2-2-2-2-3-3-2 | 30 | − | 29.79 | 20, —m |
| H | UK | MSSA476 | 7 | 0.5 | 4 | 0.5 | 0.5 | 1 | 1 | 0.5 | 8 | ≤0.063 | J1 | 1, 1-1-1-1-1-1-1 | 1 | − | 27.66 | —m |
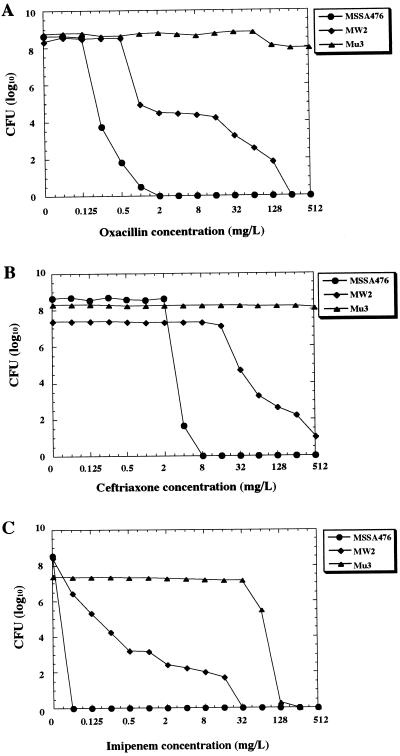
Table 1 also shows that C-MRSA/NORSA strains had relatively lower levels of oxacillin and imipenem resistance than H-MRSA strains (with the exceptions of N315 and 85/2082) (20). This indicated that they had the heterogeneous methicillin resistance phenotype, which was confirmed by population analysis (Fig. 1). MW2, a representative C-MRSA strain (2), possessed typical heterogeneous subpopulations of resistant cells, whereas the “truly” (i.e., _mecA_-negative) methicillin-susceptible strain 476, the putative progenitor strain of MW2 (see below), did not have the resistant subpopulations. Mu3, a typical H-MRSA strain, on the other hand, had a distinct pattern of resistance called homogeneous methicillin resistance. This implied that unlike H-MRSA strains, C-MRSA strains were not selected out by the exposure to these potent beta-lactam antibiotics used in the hospital, testifying further to their predominant propagation occurring in the community.
FIG. 1.
C-MRSA strain shows heterogeneous phenotypic expression of methicillin resistance. Analysis of resistant subpopulations of the C-MRSA strain MW2, the related MSSA strain 476, and strain Mu3, with heterogeneous resistance to vancomycin, was performed with oxacillin (A), ceftriaxone (B), and imipenem (C) as described previously (13). Ceftriaxone was the antibiotic used in vain to treat the patient infected with MW2 (3). MW2 is an American Midwest MRSA strain representing the major C-MRSA (see the text). Strain 476 is an MSSA strain sharing its MLST allotype with MW2 (see Table 1). Mu3 is a representative H-MRSA strain with heterogeneous resistance to vancomycin (13). It is noted that MW2 contains subpopulations resistant to each of the three beta-lactam antibiotics.
C-MRSA/NORSA strains grew significantly faster than H-MRSA strains: the mean doubling times (8) of the former group of strains were 28.79 ± 7.09 and 28.24 ± 2.48 min, respectively, whereas that for the latter was 38.81 ± 7.01 min (see Table 1). The difference was statistically significant (P value of <0.0001 by t test). This high growth rate may be a prerequisite in the absence of antibiotics for C-MRSA to achieve successful colonization of humans by outcompeting the numerous bacterial species in the environment.
The MRSA genotype is the sum of the SCC_mec_ type and the type of its recipient chromosome (12). First, by using multiple locus sequence typing (MLST), we identified the chromosome genotype of the test strains. Enright et al. reported that 356 of 359 MRSA strains from 20 countries were classified into only five clonal complexes (CCs), CC5, CC8, CC22, CC30, and CC45, with the rest, three strains, possessing sequence types (STs) of rare occurrence (6). All the 11 H-MRSA strains used in this study were reasonably classified into three of these five CCs (Table 1). However, 35 C-MRSA/NORSA strains possessed 10 different STs that constituted one singleton (ST75) and seven CCs that, surprisingly, included all five H-MRSA CCs described above (Table 1).
Among the seven C-MRSA CCs, especially notable was CC1, which contained the internationally dominant C-MRSA strains; eight U.S. strains represented by MW2 and six Australian strains belonged to this clonal complex. Remarkably, no H-MRSA strains belonged to this complex (6). Curiously, a highly virulent methicillin-susceptible S. aureus (MSSA) strain, 476, whose whole genome sequence has been determined, belongs to this complex (http://www.mlst.net/new/index.htm). MSSA 476 and two NORSA strains belonging to CC1 even shared an identical pulsed-field gel electrophoresis (PFGE) pattern (Table 1). Detailed comparison revealed that the only significant difference between the two chromosomes was the presence of type IV SCC_mec_ in MW2, which indicated that strain 476 represented the progenitor MSSA strain from which MW2 was generated by acquiring type IV SCC_mec_.
The pattern of clonal distribution of the 35 C-MRSA/NORSA strains was statistically distinct from that of 359 MRSA strains analyzed in a previous study plus 11 H-MRSA strains used in this study (P value of <0.000001 by Fisher's exact test). This clearly indicated that distinct clonal populations were successfully propagated as C-MRSA/NORSA and H-MRSA, presumably through different selective pressures exerted on them, e.g., fast-growing S. aureus or S. epidermidis strains for the former and exposure to multiple antibiotics for the latter.
The MLST data, despite the small number of tested strains, indicated that C-MRSA/NORSA strains were generated from S. aureus clones of much more diverse genetic backgrounds than expected. This was also supported by PFGE analysis (Table 1), which showed that the C-MRSA/NORSA strains were classified into nine unrelated groups according to the criteria described by Tenover et al. (27). Moreover, these strains consisted of producers of as many as seven coagulase isotypes (Table 1). Since only eight coagulase isotypes are known among S. aureus strains isolated from various sources (18), this also supported the view that C-MRSA/NORSA represents diverse S. aureus genomes as the origin of derivation.
Next, we determined SCC_mec_ types by PCR typing of the mec gene complex and ccr gene complex as described previously (19, 21). Table 2 and Fig. 2 show the nucleotide sequences and locations of the primers used (15, 19, 21). In contrast to the heterogeneity of C-MRSA/NORSA chromosomes demonstrated above, all except for three strains harbored type IV SCC_mec_, and the remaining three harbored a novel SCC_mec_ carrying the class C2 mec gene complex (21) (Fig. 2). None of the C-MRSA/NORSA strains possessed any of the three types of SCC_mec_ which the majority of epidemic H-MRSA strains possess (19).
TABLE 2.
Primer sets used for identifying SCC_mec_
| Primer (previous name) for detection of: | Nucleotide sequence | Expected size of product (gene[s] reactive to the primer) | Reference |
|---|---|---|---|
| ccr gene complexa | |||
| βc (β2) | 5′-ATTGCCTTGATAATAGCCITCT-3′ | (all types of ccrB) | 19 |
| αc | 5′-ATCTATTTCAAAAATGAACCA-3′ | 560 bp, βc (all types of ccrA) | This study |
| α1 (α2) | 5′-AACCTATATCATCAATCAGTACGT-3′ | 700 bp, βc (type 1 ccrA) | 19 |
| α2 (α3) | 5′-TAAAGGCATCAATGCACAAACACT-3′ | 1 kbp, βc (type 2 ccrA) | 19 |
| α3 (α4) | 5′-AGCTCAAAAGCAAGCAATAGAAT-3′ | 1.6 kbp, βc (type 3 ccrA) | 19 |
| mec gene complex (all types) | |||
| mecR1 (MS domain) | |||
| mcR4 | 5′-GTCGTTCATTAAGATATGACG-3′ | (_mecR1_—MS domain) | This study |
| mcR3 | 5′-GTCTCCACGTTAATTCCATT-3′ | 310 bp, mcR4 (_mecR1_—MS domain) | 21 |
| mecA | |||
| mA1 | 5′-TGCTATCCACCCTCAAACAGG-3′ | (mecA) | 15 |
| mA2 | 5′-AACGTTGTAACCACCCCAAGA-3′ | 200 bp, mA1 (mecA) | 15 |
| mecA -IS_431mec_ | |||
| IS2 (iS-2) | 5′-TGAGGTTATTCAGATATTTCGATGT-3′ | 4 kbp, mA1 (IS_431mec_) | 21 |
| Class C mec gene complex | |||
| mA2 | 5′-AACGTTGTAACCACCCCAAGA-3′ | <3 kbp, IS_2_ (mecA) | 21 |
| Class B mec gene complex | |||
| IS5 | 5′-AACGCCACTCATAACATATGGAA-3′ | (IS_1272_) | This study |
| mA6 | 5′-TATACCAAACCCGACAAC-3′ | 2 kbp, IS_5_ (mecA) | 21 |
| Class A mec gene complex | |||
| mecI | |||
| mI4 | 5′-CAAGTGAATTGAAACCGCCT-3′ | (mecI) | This study |
| mI3 | 5′-CAAAAGGACTGGACTGGAGTCCAAA-3′ | 180 bp, mI4 (mecI) | This study |
| mecR1 (PB domain) | |||
| mcR2 | 5′-CGCTCAGAAATTTGTTGTGC-3′ | (_mecR1_—PB domain) | 21 |
| mcR5 | 5′-CAGGGAATGAAAATTATTGGA-3′ | 320 bp, mcR2 (_mecR1_—PB domain) | This study |
| SCC_mec_ subtype IVa | |||
| 4a1 | 5′-TTTGAATGCCCTCCATGAATAAAAT-3′ | (J1 region of IVa) | This study |
| 4a2 | 5′-AGAAAAGATAGAAGTTCGAAAGA-3′ | 450 bp, 4a1 (J1 region of IVa) | This study |
| SCC_mec_ subtype IVb | |||
| 4b1 | 5′-AGTACATTTTATCTTTGCGTA-3′ | (J1 region of IVb) | This study |
| 4b2 | 5′-AGTCATCTTCAATATCGAGAAAGTA-3′ | 1 kbp, 4b1 (J1 region of IVb) | This study |
FIG. 2.
Illustrative representation of various types of SCC_mec._ SCC_mec_ type is defined by the combination of the type of ccr gene complex and the class of mec gene complex. Type I SCC_mec_ is defined by the combination of type 1 ccr and class B mec (IS_1272-ΔmecR1-mecA_); type II is defined by type 2 ccr and class A mec (mecI-mecR1-mecA); type III is defined by type 3 ccr and class A mec; and type IV is defined by type 2 ccr and class B mec. Type IV SCC_mec_ is further classified into subtypes (type IVa and type IVb) based on the sequence difference in the J1 region of SCC_mec_ (J stands for “junkyard”). Positions of primers used in this study to identify and type SCC_mec_ are shown (see Table 2 for the nucleotide sequence of each primer). The allelic class of mec gene complex is determined by PCR detection of the presence or absence of IS_1272_, mecI, and mecR1 in two domains (PB, penicillin-binding domain; and MS, membrane-spanning domain), mecA, and IS_431mec_. PCR amplification was performed using 2.5 U of Ex Taq (Takara Shuzo Co., Ltd., Kyoto, Japan) in 50 μl of reaction mixture. Thermal cycling was set at 30 cycles (30 s for denaturation at 94°C, 1 min for annealing at 50°C, and 2 min for elongation at 72°C) using the Gene Amp PCR system 9600 (Perkin-Elmer, Wellesley, Mass.). For the detection of mecA_-IS_431mec, a long-range PCR method was used, set at 10 cycles (15 s for denaturation at 94°C, 30 s for annealing at 50°C, and 8 min for elongation at 68°C) followed by 20 cycles (15 s for denaturation at 94°C, 30 s for annealing at 50°C, and 12 min for elongation at 68°C). Note that this study identified a new type of SCC_mec_ for three C-MRSA strains that carried the class C2 mec gene complex (21). The sequencing of the entire SCC_mec_ is now ongoing.
It is not clear at this moment why type IV SCC_mec_ is prevalent in C-MRSA/NORSA strains. However, type IV SCC_mec_ is short (21 to 25 kb) compared to the three SCC_mec_s prevalent in H-MRSA strains (34 to 67 kb) and lacks any antibiotic resistance genes other than mecA (23) (Fig. 2). This evidently corresponds to the non-multiresistant nature of C-MRSA/NORSA and may alleviate the fitness cost paid by H-MRSA strains carrying big SCC_mec_s with multiple-drug resistance determinants.
Although we need to explore further the reason why type IV SCC_mec_ is prevalent in C-MRSA strains, it seems clear that we are witnessing the emergence and expansion of new MRSA clones in the community. These clones are different from any of the major H-MRSA clones in the world that we have identified by using SCC_mec_ typing and ribotyping combinations (12, 17). In this study we realized that the antibiogram is not completely reliable in discriminating C-MRSA from H-MRSA, nor is the phenotypic expression of methicillin resistance. Even epidemiological information is not sufficient, since, for example, many C-MRSA strains have been carried in Australian hospitals (29). Therefore, no reliable judgment can be made as to whether the strain isolated in the hospital is H-MRSA or C-MRSA even based on the timing of isolation of the strains after admission to hospital. In this regard, SCC_mec_ and MLST typing will become more important in the years to come for discrimina-tion of numerous C-MRSA strains prevailing in both com-munity and hospitals by reference to their ancestral MRSA clones (12).
Acknowledgments
We thank T. Naimi for providing us C-MRSA strains and for fruitful discussion. We also thank M. C. Enright for kind instruction on MLST and the BURST program and Susan Johnson and David Boxrud in Minnesota and Suwanna Trakulsomboon, Mantana Jamklang, and Fumihiko Takeuchi in Japan for their excellent technical assistance. We also thank Yuh Morimoto for her help in preparing the manuscript.
This work was supported by a Grant-in-Aid for Scientific Research on Priority Areas (13226114) from The Ministry of Education, Science, Sports, Culture and Technology of Japan and the Core University System Exchange Programme under the Japan Society for the Promotion of Science, coordinated by the University of Tokyo Graduate School of Medicine and Mahidol University. The work was also supported by a Grant for International Health Cooperation Research (11C-4) from the Ministry of Health and Welfare, by the Research for the Future Program of the Japan Society for the Promotion of Science, and by the National Health and Medical Research Foundation of Australia and the Health Department of Western Australia.
REFERENCES
- 1.Alghaithy, A. A., N. E. Bilal, M. Gedebou, and A. H. Weily. 2000. Nasal carriage and antibiotic resistance of Staphylococcus aureus isolates from hospital and non-hospital personnel in Abha, Saudi Arabia. Trans. R. Soc. Trop. Med. Hyg. 94**:**504-507. [DOI] [PubMed] [Google Scholar]
- 2.Baba, T., F. Takeuchi, M. Kuroda, H. Yuzawa, K. Aoki, A. Oguchi, Y. Nagai, N. Iwama, K. Asano, T. Naimi, H. Kuroda, L. Cui, K. Yamamoto, and K. Hiramatsu. 2002. Genome and virulence determinants of high virulence community-acquired MRSA. Lancet 359**:**1819-1827. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. 1999. Four pediatric deaths from community-acquired methicillin-resistant _Staphylococcus aureus_—Minnesota and North Dakota, 1997-1999. Morb. Mortal. Wkly. Rep. 48**:**707-710. [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. 2001. Methicillin-resistant Staphylococcus aureus skin or soft tissue infections in a state prison—Mississippi, 2000. Morb. Mortal. Wkly. Rep. 50**:**919-922. [PubMed] [Google Scholar]
- 5.Chambers, H. 2001. The changing epidemiology of Staphylococcus aureus? Emerg. Infect. Dis. 7**:**178-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Enright, M. C., D. Robinson, G. Randle, E. J. Feil, H. Grundmann, and B. G. Spratt. 2002. The evolutionary history of methicillin-resistant Staphylococcus aureus (MRSA). Proc. Natl. Acad. Sci. USA 99**:**7687-7692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garner, J. S., W. R. Jarvis, T. G. Emori, T. C. Horan, and J. M. Hughes. 1988. CDC definitions for nosocomial infections, 1998. Am. J. Infect. Control 16**:**128-140. [DOI] [PubMed] [Google Scholar]
- 8.Gerhardt, P., R. G. E. Murray, W. A. Wood, and N. R. Krieg. 1994. Methods for general and molecular bacteriology, p. 270-271. American Society for Microbiology, Washington, D.C.
- 9.Gosbell, I. B., J. Mercer, S. A. Neville, K. G. Chant, and R. Munro. 2001. Community-acquired, non-multiresistant oxacillin-resistant Staphylococcus aureus (NORSA) in South Western Sydney. Pathology 33**:**206-210. [PubMed] [Google Scholar]
- 10.Gosbell, I. B., J. Mercer, S. A. Neville, S. A. Crone, K. G. Chant, B. B. Jalaludin, and R. Munro. 2001. Non-multiresistant and multiresistant methicillin-resistant Staphylococcus aureus in community-acquired infections. Med. J. Aust. 174**:**627-630. [DOI] [PubMed] [Google Scholar]
- 11.Herold, B. C., L. C. Immergluck, M. C. Maranan, D. S. Lauderdale, R. E. Gaskin, S. Boyle-Vavra, C. D. Leitch, and R. S. Daum. 1998. Community-acquired methicillin-resistant Staphylococcus aureus in children with no identified predisposing risk. JAMA 279**:**593-598. [DOI] [PubMed] [Google Scholar]
- 12.Hiramatsu, K. 1995. Molecular evolution of MRSA. Microbiol. Immunol. 39**:**531-543. [DOI] [PubMed] [Google Scholar]
- 13.Hiramatsu, K., N. Aritaka, H. Hanaki, S. Kawasaki, Y. Hosoda, S. Hori, Y. Fukuchi, and I. Kobayashi. 1997. Dissemination in Japanese hospitals of strains of Staphylococcus aureus heterogeneously resistant to vancomycin. Lancet 350**:**1670-1673. [DOI] [PubMed] [Google Scholar]
- 14.Hiramatsu, K., L. Cui, M. Kuroda, and T. Ito. 2001. The emergence and evolution of methicillin-resistant Staphylococcus aureus. Trends Microbiol. 9**:**486-493. [DOI] [PubMed] [Google Scholar]
- 15.Hiramatsu, K., H. Kihara, and T. Yokota. 1992. Analysis of borderline-resistant strains of methicillin-resistant Staphylococcus aureus using polymerase chain reaction. Microbiol. Immunol. 36**:**445-453. [DOI] [PubMed] [Google Scholar]
- 16.Hiramatsu, K., K. Okuma, X. X. Ma, M. Yamamoto, S. Hori, and M. Kapi. 2002. New trends in Staphylococcus aureus infections: glycopeptide resistance in hospital and methicillin resistance in the community. Curr. Opin. Infect. Dis. 15**:**407-413. [DOI] [PubMed] [Google Scholar]
- 17.Hiramatsu, K., T. Ito, and H. Hanaki. 1999. Mechanisms of methicillin and vancomycin resistance in Staphylococcus aureus, p. 221-242. In R. G. Finch and R. J. Williams (ed.), Bailliere's clinical infectious diseases, vol. 5. Bailliere Tindall, London, United Kingdom.
- 18.Igarashi, H. 1993. Producibility of staphylococcal enterotoxins and toxic shock syndrome toxin-1 on methicillin-resistant Staphylococcus aureus. Igakunoayumi 166**:**274-278. (In Japanese.) [Google Scholar]
- 19.Ito, T., Y. Katayama, K. Asada, N. Mori, K. Tsutsumimoto, C. Tiesasitorn, and K. Hiramatsu. 2001. Structural comparison of three types of staphylococcal cassette chromosome mec integrated in the chromosome in methicillin-resistant _Staphylococcus aureu_s. Antimicrob. Agents Chemother. 45**:**1323-1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson, A. P., H. M. Aucken, S. Cavendish, M. Ganner, M. C. Wale, M. Warner, D. M. Livermore, B. D. Cookson, and the UK EARSS Participants. 2001. Dominance of EMRSA-15 and -16 among MRSA causing nosocomial bacteraemia in the UK: analysis of isolates from the European Antimicrobial Resistance Surveillance System (EARSS). J. Antimicrob. Chemother. 48**:**143-144. [DOI] [PubMed] [Google Scholar]
- 21.Katayama, Y., T. Ito, and K. Hiramatsu. 2001. Genetic organization of the chromosome region surrounding mecA in clinical staphylococcal strains: role of IS_431_-mediated mecI deletion in expression of resistance in _mecA_-carrying, low-level methicillin-resistant Staphylococcus haemolyticus. Antimicrob. Agents Chemother. 45**:**1955-1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katayama, Y., T. Ito, and K. Hiramatsu. 2000. A new class of genetic element, staphylococcus cassette chromosome mec, encodes methicillin resistance in Staphylococcus aureus. Antimicrob. Agents Chemother. 44**:**1549-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma, X. X., T. Ito, C. Tiensasitorn, M. Jamklang, P. Chongtrakool, S. Boyle-Vavra, R. S. Daum, and K. Hiramatsu. 2002. Novel type of staphylococcal cassette chromosome mec identified in community-acquired methicillin-resistant Staphylococcus aureus strains. Antimicrob. Agents Chemother. 46**:**1147-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Naimi, T. S., K. H. LeDell, D. J. Boxrud, A. V. Groom, C. D. Steward, S. K. Johnson, J. M. Besser, C. O'Boyle, R. N. Danila, J. E. Cheek, M. T. Osterholm, K. A. Moore, and K. E. Smith. 2001. Epidemiology and clonality of community-acquired methicillin-resistant Staphylococcus aureus in Minnesota, 1996-1998. Clin. Infect. Dis. 33**:**990-996. [DOI] [PubMed] [Google Scholar]
- 25.Nimmo, G. R., J. Schooneveldt, G. O'Kane, B. McCall, and A. Vickery. 2000. Community acquisition of gentamicin-sensitive methicillin-resistant Staphylococcus aureus in southeast Queensland, Australia. J. Clin. Microbiol. 38**:**3926-3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Brien, F. G., J. Pearman, M. Gracey, T. V. Riley, and W. B. Grubb. 1999. Community strain of methicillin-resistant Staphylococcus aureus involved in a hospital outbreak. J. Clin. Microbiol. 37**:**2858-2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tenover, F. C., R. D. Arbeit, R. V. Goering, P. A. Mickelsen, P. A. Mickelsen, B. E. Murry, D. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33**:**2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Timothy, F. J., E. K. Molly, S. S. Porter, B. Michael, and S. William. 2002. An outbreak of community-acquired foodborne illness caused by methicillin-resistant _Staphylococcus aure_us. Emerg. Infect. Dis. 8**:**82-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Turnidge, J. D., and J. Bell. 2000. Methicillin-resistant Staphylococcal aureus evolution in Australia over 35 years. Microb. Drug Resist. 6**:**223-229. [DOI] [PubMed] [Google Scholar]