Contrasting Roles of Endosomal pH and the Cytoskeleton in Infection of Human Glial Cells by JC Virus and Simian Virus 40 (original) (raw)
Abstract
Infection of eukaryotic cells by pathogens requires the efficient use of host cell endocytic and cytoplasmic transport mechanisms. Understanding how these cellular functions are exploited by microorganisms allows us to better define the basic biology of pathogenesis while providing better insight into normal cellular functions. In this report we compare and contrast intracellular transport and trafficking of the human polyomavirus JC virus (JCV) with that of simian virus 40 (SV40). We have previously shown that infection of human glial cells by JCV requires clathrin-dependent endocytosis. In contrast, infection of cells by SV40 proceeds by caveola-dependent endocytosis. We now examine the roles of endosomal pH and the cellular cytoskeleton during infection of glial cells by both viruses. Our results demonstrate that JCV infection is sensitive to disruption of endosomal pH, whereas SV40 infection is pH independent. Infection by JCV is inhibited by treatment of glial cells with cytochalasin D, nocodazole, and acrylamide, whereas SV40 infection is affected only by nocodazole. These data point to critical differences between JCV and SV40 in terms of endocytosis and intracellular trafficking of their DNA genomes to the nucleus. These data also suggest a unique sequential involvement of cytoskeletal elements during infection of glial cells by JCV.
The human polyomavirus JC virus (JCV) is the etiologic agent of the fatal demyelinating disease progressive multifocal leukoencephalopathy (38, 44). JCV infection is prevalent, occurring in 70% to 90% of the human population worldwide (39). Reactivation of JCV in immunosuppressed individuals leads to virus dissemination to the central nervous system, where the primary targets of infection are astrocytes and oligodendrocytes (30).
Progressive multifocal leukoencephalopathy occurs as a consequence of the lytic destruction of oligodendrocytes (30). Although JCV is closely related to the simian polyomavirus simian virus 40 (SV40), we have elucidated critical differences between these viruses that relate to receptor specificity (28), sialic acid dependence (29), and mechanisms of internalization (42). In this paper, we explore the roles of low endosomal pH and the cellular cytoskeleton during infection by both viruses.
We have previously shown that JCV, unlike SV40, enters cells through clathrin-dependent endocytosis (42). Entry into endosomes exposes the virus to an acidic environment, which is known to induce conformational changes in several viral glycoproteins, thereby promoting uncoating of viruses (4, 34, 51, 58, 59, 61). In order to determine whether acidic pH was necessary for uncoating and hence productive infection by JCV, we used two different inhibitors of endosomal acidification. The weak base ammonium chloride (NH4Cl) diffuses into acidic endosomes, where it becomes protonated. Once protonated, it is unable to diffuse out, thereby increasing the pH (37). A second inhibitor, bafilomycin A1, is a potent and specific inhibitor of the vacuolar H+-ATPase, which is the proton pump responsible for the acidification of intracellular compartments in eukaryotic cells (5, 16). SV40 entry is thought to be mediated via a pH-neutral “caveosome,” and infection by SV40 would therefore be pH independent (40). Our studies with these inhibitors indicate that while SV40 infection is pH independent, JCV infection shows sensitivity to the disruption of endosomal pH.
Upon delivery to the interior of the host cell, viruses do not rely on passive diffusion for their trafficking but rather require active cellular transport systems (47). Cytoplasmic transport in eukaryotic cells is dependent on a complex network of three types of filaments: microtubules, microfilaments, and intermediate filaments. We therefore wanted to examine the roles of these cytoskeletal elements during infection by JCV and SV40. Treatment with nocodazole, cytochalasin D, and acrylamide is known to disassemble microtubules, microfilaments, and intermediate filaments, respectively (8, 10, 14, 26, 35). Our results from studies examining the effects of these agents on virus infection demonstrate that SV40 is sensitive solely to nocodazole treatment, while JCV is sensitive to all three types of inhibitors.
As both viruses depend on an intact microtubule network for productive infection, we investigated the role of the most abundant member of the negative-end-directed microtubule-associated motor proteins, dynein 1, during infection by these viruses. Dynein 1 requires a multisubunit activator protein, dynactin, to serve as an adaptor mediating dynein binding to cargo (18, 22). The cargo-binding and dynein-binding domains of dynactin are linked by the protein dynamitin, which, when overexpressed, causes uncoupling of these activities of dynactin (9, 13). Dynamitin overexpression can thus be used to analyze loss of dynein function in various contexts, including viral infection. We found that overexpression of dynamitin in glial cells does not inhibit infection by either JCV or SV40, suggesting that dynein 1 function is not required for the microtubule-mediated transport of these viruses.
MATERIALS AND METHODS
Cells, viruses, and antibodies.
SVG-A cells are a subclone of the original SVG human glial cell line established by transformation of human fetal glial cells by an origin-defective SV40 mutant (31). SVG-A cells were maintained in a humidified 37°C CO2 incubator in Eagle's minimal essential medium (Mediatech, Inc., Herndon, Va.) supplemented with 10% heat-inactivated fetal bovine serum (Mediatech, Inc.). CV1 cells were obtained from the American Type Culture Collection (Rockville, Md.) and were maintained identically to SVG-A cells.
The PAB597 hybridoma produces a monoclonal antibody against the SV40 major capsid protein VP1 and was a generous gift from Ed Harlow. This antibody has been previously shown to cross-react with JCV VP1 (3) and was used to stain both JCV- and SV40-infected cells. The generation of the rabbit anti-JCV antiserum has been described previously (42). Rabbit anti-SV40 antiserum was obtained from Lee Biomolecular Research, Inc. (San Diego, Calif.). The generation and propagation of the Mad-1/SVEΔ strain of JCV have been described previously (27, 28). SV40 strain 776 was propagated in the African green monkey kidney cell line CV1.
Indirect immunofluorescent analysis of JCV and SV40 infection.
SVG-A cells were grown to 70% confluence on coverslips and incubated with 514 HAU of JCV or 4.8 × 106 PFU of SV40 per ml for 4 h at 37°C. This corresponds to a multiplicity of infection of approximately 10 for each virus. At the end of the incubation, cells were rinsed in Eagle's minimal essential medium and incubated with neutralizing concentrations of anti-JCV or anti-SV40 antiserum in Eagle's minimal essential medium for 72 h. Cells were then fixed in ice-cold acetone for 10 min and washed several times in phosphate-buffered saline (PBS) (137 mM NaCl, 2.682 mM KCl, 8.1 mM Na2HPO4, 1.47 mM KH2PO4, pH 7.2). Cells were then incubated with a 1:10 dilution of the PAB597 monoclonal antibody against V antigen for 30 min at 37°C. Cells were then washed three times in PBS and incubated with a 1:50 dilution of goat anti-mouse immunoglobulin antibody conjugated to fluorescein isothiocyanate (Jackson Immunoresearch Labs, Inc., West Grove, Pa.) for 30 min at 37°C. Cells were then rinsed in PBS, followed by 0.02% Evan's blue in PBS (red counterstain). Coverslips were mounted onto slides with fluorescence mounting medium (Vector Labs, Burlingame, Calif.). V antigen-positive cells were visualized on a Nikon epifluorescence microscope (Eclipse E800; Nikon Inc., Melville, N.Y.) and scored by counting. A minimum of 10 fields were counted for each sample.
Neutralization of endosomal pH.
SVG-A cells were treated for 4 h at 37°C with NH4Cl at 5 or 25 mM or with 0.5 μM bafilomycin A1. Following this treatment, cells were infected with JCV or SV40 for 4 h in the continued presence of the drugs. At the end of 4 h, the cells were washed several times in Eagle's minimal essential medium and incubated with Eagle's minimal essential medium containing neutralizing concentrations of either anti-JCV or anti-SV40 antiserum. Cells were fixed and stained 72 h postinfection as described above.
Disassembly of microfilaments, microtubules, and intermediate filaments.
Cytochalasin D (Sigma Aldrich, St. Louis, Mo.) nocodazole (Sigma Aldrich), and acrylamide (Calbiochem-Novabiochem Corp., San Diego, Calif.) were used at 15 μg/ml, 5 μg/ml, and 10 mM, respectively. Cells were treated with cytochalasin D and nocodazole for 1 h at 37°C and with acrylamide for 6 h at 37°C. Infection with JCV or SV40 was then carried out in the continued presence of the drug for 4 h, and infected cells were scored at 72 h postinfection as described above.
To detect microfilaments, cells were washed twice in PBS and fixed in 2% paraformaldehyde. They were then incubated with a 1:50 dilution of rhodamine-conjugated phalloidin (Molecular Probes Inc., Eugene, Oreg.) for 30 min at 37°C. Cells were then washed three times in PBS and mounted on slides with fluorescence mounting medium (Vector Labs). Staining was visualized by laser scanning confocal microscopy at 63× magnification (LSM 410; Zeiss, Inc., Thornwood, N.Y.). Images were analyzed with Adobe Photoshop (version 6 LE).
To detect microtubules, cells were washed twice in PBS and fixed in 2% paraformaldehyde prior to incubation with a 1:50 dilution of rabbit α-tubulin antibody (Sigma Aldrich) for 30 min at 37°C. Cells were then washed three times in PBS and incubated with goat anti-rabbit immunoglobulin conjugated to tetramethylrhodamine (Jackson Immunoresearch Labs) for 30 min at 37°C. Cells were mounted and visualized as described above.
To detect intermediate filaments, cells were washed three times in PBS, fixed in 2% paraformaldehyde, and incubated with a 1:50 dilution of mouse antivimentin antibody (Sigma Aldrich) for 30 min at 37°C. Cells were washed three times in PBS and incubated with a 1:50 dilution of goat anti-mouse immunoglobulin-fluorescein isothiocyanate antibody (Jackson Immunoresearch Labs) for 30 min at 37°C. Cells were mounted and visualized as described above.
Inhibition of dynein function.
The Myc-tagged p50/dynamitin construct was a kind gift of R. Vallee (13, 53). Dynamitin DNA was used at 2 and 10 μg/ml with 20 μg of Lipofectamine (Gibco-BRL Inc., Rockville, Md.) per ml to transfect SVG-A cells. At 24 h posttransfection, cells were infected with JCV or SV40. Cells were fixed and stained for V antigen as described above.
To detect p50/dynamitin expression, fixed cells were washed three times in PBS and incubated with a 1:50 dilution of rabbit anti-Myc antibody (Sigma Aldrich) for 30 min at 37°C. Cells were washed three times in PBS and incubated with a 1:50 dilution of goat anti-rabbit immunoglobulin-fluorescein isothiocyanate (Jackson Immunoresearch Labs Inc.) secondary antibody for 30 min at 37°C. Cells were then washed in PBS and mounted on slides with fluorescence mounting medium (Vector Labs). V antigen- and dynamitin-positive cells were visualized on a Nikon epifluorescence microscope (Nikon Inc.). The Golgi apparatus was stained in untransfected and transfected cells by incubating with 0.5 μM BODIPY-Texas Red-ceramide (Molecular Probes) for 30 min at 37°C. Cells were then washed twice in 1× PBS and fixed in 2% paraformaldehyde. Staining was visualized with laser scanning confocal microscopy under 63× magnification (Zeiss, Inc.), and images were analyzed with Adobe Photoshop (version 6 LE).
RESULTS
Role of low pH in infection of glial cells by JCV and SV40.
The role of low pH during infection by JCV and SV40 was examined by using two different methods. In the first method, we used bafilomycin A1, a specific inhibitor of the vacuolar proton ATPase, to prevent acidification of endosomes in glial cells. SVG-A cells growing on coverslips were treated with 0.5 μM bafilomycin A1 and infected with either JCV or SV40. Control cells were either left untreated and uninfected (Fig. 1, A and D) or infected with JCV or SV40 in the absence of bafilomycin A1 (Fig. 1, B and E). Infected cells were scored at 72 h postinfection by staining for V antigen expression.
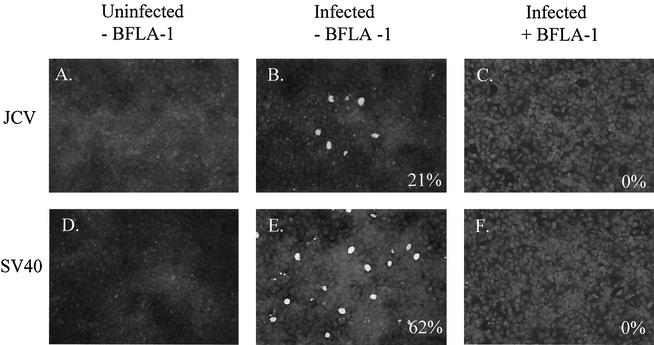
FIG. 1.
Bafilomycin A1 inhibits infection by JCV and SV40. (A and D) Uninfected SVG-A cells. (B and E) SVG-A cells were incubated with JCV or SV40 for 4 h at 37°C, washed, and incubated with medium containing neutralizing JCV or SV40 antiserum. Cells were fixed 72 h postinfection and stained for V antigen. (C and F) SVG-A cells were treated for 1 h at 37°C with 5 × 10−4 mM bafilomycin A1 (BFLA-1), infected with JCV or SV40, fixed, and stained for V antigen. Numbers at the bottom right of each panel refer to the percentage of infected cells per field and represent the mean of at least four different fields of cells from each of three independent experiments.
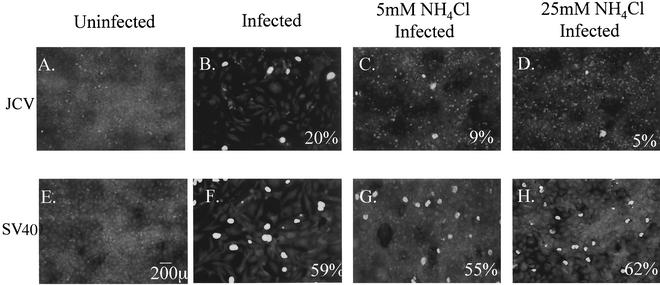
At concentrations of bafilomycin A1 as low as 0.5 μM, infection by both viruses was completely inhibited (Fig. 1, C and F). This suggested that low pH was necessary during infection by both viruses. As bafilomycin A1 has been shown to have additional effects on cells besides neutralization of endosomal pH, we chose to raise the pH within acidic endosomes by accumulation of the weak amine ammonium chloride (NH4Cl). SVG-A cells cultured on coverslips were treated with 5 or 25 mM NH4Cl and infected with either JCV or SV40. Infection was scored at 72 h postinfection by staining for V antigen. Control cells were either left untreated and uninfected (Fig. 2, A and E) or infected with JCV and SV40 in the absence of NH4Cl (Fig. 2, B and F). At concentrations of 5 and 25 mM, NH4Cl treatment inhibited infection by JCV by approximately 60 to 80% compared to untreated controls (Fig. 2, C and D), whereas SV40 infection was unaltered (Fig. 2, G and H). These data, taken together, indicate that infection of glial cells by JCV is sensitive to pH. Our data also confirm that infection of cells by SV40 is pH independent.
FIG. 2.
NH4Cl inhibits infection of glial cells by JCV but not by SV40. (A and E) Uninfected SVG-A cells. (B and F) SVG-A cells were incubated with JCV or SV40 for 4 h at 37°C, washed, and incubated in medium containing neutralizing JCV or SV40 antiserum. Cells were fixed 72 h postinfection and stained for V antigen (green nuclei). (C and G) SVG-A cells were treated for 4 h at 37°C with 5 mM NH4Cl, infected with JCV or SV40, fixed, and stained for V antigen. (D and H) SVG-A cells were treated for 4 h at 37°C with 25 mM NH4Cl, infected with JCV or SV40, fixed, and stained for V antigen. Numbers at the bottom right of each panel refer to the percentage of infected cells per field and represent the mean of at least four different fields of cells from each of three independent experiments.
Role of microfilaments during infection by JCV and SV40.
In order to examine the role of intact microfilaments during infection by these two viruses, we treated SVG-A cells growing on coverslips with the fungal toxin cytochalasin D, which destabilizes microfilaments by binding to the fast-growing end of the filament (35). We first determined whether microfilaments were indeed disassembled by cytochalasin D at concentrations that were not toxic to the cells by using rhodamine-labeled phalloidin, which binds to the interface between actin monomers. We could show that cytochalasin D at 15 μg/ml was able to disassemble microfilaments in SVG-A cells (Fig. 3A).
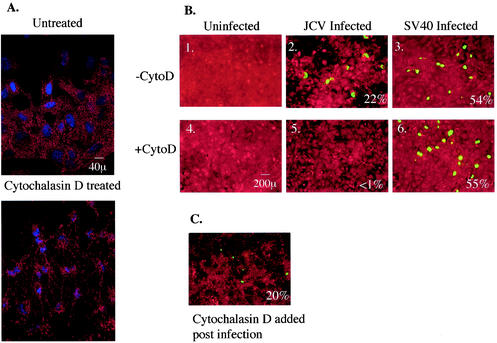
FIG. 3.
Microfilaments are required for infection by JCV but not by SV40. (A) Untreated SVG-A cells or SVG-A cells treated with 15 μg of cytochalasin D per ml for 1 h at 37°C were stained with rhodamine-phalloidin to detect the disassembly of microfilaments upon treatment with cytochalasin D. (B 1 and 4) Uninfected SVG-A cells left untreated or treated with 15 μg cytochalasin D (CytoD) per ml, respectively. (B 2 and 5) SVG-A cells were either left untreated or treated for 1 h at 37°C with 15 μg of cytochalasin D per ml, respectively. They were then infected with JCV, fixed, and stained for V antigen. (B 3 and 6) SVG-A cells were either left untreated or treated for 1 h at 37°C with 15 μg of cytochalasin D per ml, respectively. They were then infected with SV40, fixed, and stained for V antigen. (C) SVG-A cells were infected with JCV and treated for 5 h at 37°C with 15 μg of cytochalasin D per ml at 24 h postinfection. Cells were fixed and stained for V antigen 72 h postinfection. Numbers at the bottom right of each panel refer to the percentage of infected cells per field and represent the mean of at least four different fields of cells from each of three independent experiments.
To examine the effect of microfilament disassembly on early stages of infection by both viruses, we treated cells with 15 μg of cytochalasin D per ml and maintained the cells in the drug throughout the 4-h virus internalization period. Cells were then washed free of the drug and incubated with medium containing neutralizing antiserum against JCV or SV40 for 72 h. Infected cells were then scored by staining for V antigen. Negative control cells were either left untreated and uninfected (Fig. 3B, panel 1) or treated with cytochalasin D and left uninfected (Fig. 3B, panel 4). Positive control cells were infected with JCV or SV40 in the absence of cytochalasin D (Fig. 3B, panels 2 and 3).
Treatment with cytochalasin D severely inhibited infection by JCV (≈94%) but had no effect on infection by SV40 (Fig. 3, panels 5 and 6). To confirm that this inhibition was indeed due to the lack of intact microfilaments during the early stages of infection and not due to nonspecific effects on viral DNA replication or gene expression, cytochalasin D was added 24 h postinfection with JCV. In this case, there was no inhibition of virus infection (Fig. 3C). These data suggest that internalization of JCV requires intact microfilaments, while SV40 internalization into glial cells appears to be microfilament independent.
Role of microtubules during infection by JCV and SV40.
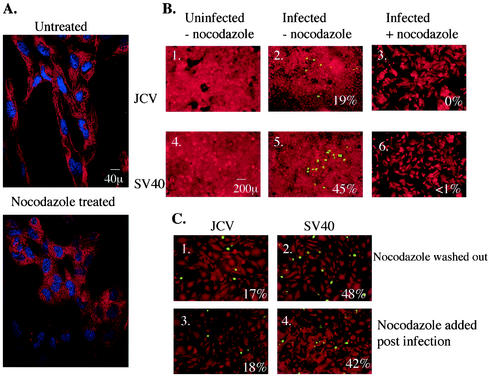
We next examined the role of microtubules in JCV and SV40 intracellular trafficking. Nocodazole is a reversible mitotic inhibitor that binds the fast-growing ends of microtubules and prevents monomer addition. We first determined whether nocodazole could cause depolymerization of microtubules at a concentration that was not toxic to the SVG-A cells. Anti-α-tubulin staining revealed that nocodazole at 20 μg/ml caused microtubule depolymerization without cell death (Fig. 4A). We therefore treated SVG-A cells with 20 μg of nocodazole per ml and assayed for its effect on infection by both viruses. Control cells were either left untreated and uninfected (Fig. 4B, panels 1 and 4) or infected with JCV or SV40 in the absence of nocodazole (Fig. 4B, panels 2 and 5). Nocodazole treatment completely inhibited infection by JCV and SV40 (Fig. 4B, panels 3 and 6), confirming the critical role of microtubules during infection by both polyomaviruses. To further confirm that this inhibition of infection was due to improper trafficking of the viruses as a result of microtubule depolymerization and not due to some nonspecific effects on the host cell or virion structure, we allowed nocodazole-treated and infected cells to recover for 48 h in drug-free medium and then scored for V antigen-positive infected cells. We were now able to restore infection of glial cells by both viruses (Fig. 4C, panels 1 and 2). One possible biological explanation for this is that the viruses are trapped in an intracellular compartment and are unable to deliver their genome to the nucleus of the host cell. Nocodazole added 24 h postinfection had no effect on infection by JCV and SV40 (Fig. 4C, panels 3 and 4).
FIG. 4.
Microtubules are required for infection by JCV and SV40. (A) Untreated SVG-A cells or SVG-A cells treated with 5 μg of nocodazole per ml for 1 h at 37°C were stained with anti-α-tubulin antibody to detect the disassembly of microtubules upon treatment with this inhibitor. (B 1 and 4) Uninfected SVG-A cells. (B 2 and 5) SVG-A cells were incubated with JCV or SV40 for 4 h at 37°C, washed, and incubated with medium containing neutralizing JCV or SV40 antiserum. Cells were fixed at 72 h postinfection and stained for V antigen. (B 3 and 6) SVG-A cells were treated for 1 h at 37°C with 5 μg of nocodazole per ml, infected with JCV or SV40, fixed, and stained for V antigen. (C) SVG-A cells were treated for 1 h at 37°C with 5 μg of nocodazole per ml and infected with JCV or SV40. At 72 h postinfection, cells were washed free of the drug and allowed to recover in drug-free medium for an additional 48 h before fixation and staining for V antigen. Numbers at the bottom right of each panel refer to the percentage of infected cells per field and represent the mean of at least four different fields of cells from each of three independent experiments.
Role of dynein during infection by JCV and SV40.
JCV accumulates in a perinuclear region in the vicinity of the microtubule organizing center during the early stages of infection (our unpublished observations). This is analogous to the perinuclear accumulation of herpes simplex virus type 1 and adenovirus, which require a dynein-dynactin complex to move along microtubules (48, 50). We have shown that infection by JCV and SV40 is microtubule dependent. We therefore examined the role of the minus-end-directed motor protein dynein and its activator, dynactin, during infection by both viruses.
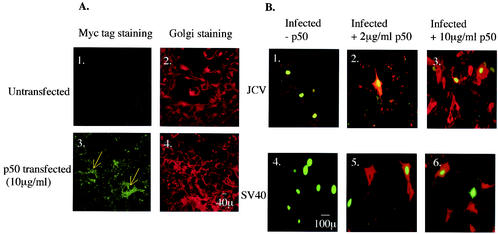
SVG-A cells grown on coverslips were transfected with 10 μg of Myc-tagged p50/dynamitin, which is a dominant-negative inhibitor of dynein function, per ml. SVG-A cells overexpressing p50/dynamitin were identified by staining with an anti-Myc tag antibody (Fig. 5A, panels 1 and 3). At 24 h posttransfection, cells were infected with either JCV or SV40, and infected cells were scored 72 h later by staining for V antigen (Fig. 5B). We were able to detect double-labeled SVG-A cells upon JCV and SV40 infection (Fig. 5B, panels 2 to 6), suggesting that cells overexpressing p50/dynamitin were still susceptible to infection by both viruses. The functional overexpression of p50/dynamitin was confirmed by staining of the Golgi apparatus with BODIPY-Texas Red-ceramide. Transfected cells stained with the anti-Myc tag antibody showed dispersion of the Golgi throughout the cytoplasm, as expected in cells that are disrupted in dynein function (Fig. 5A, panel 4).
FIG. 5.
Negative-end-directed transport by dynein-1 along microtubules is not required for infection by JCV and SV40. (A) Untransfected SVG-A cells or SVG-A cells transfected with 10 μg of p50/dynamitin DNA per ml were stained with an anti-Myc tag antibody to detect cells expressing the dynamitin construct. Blue arrows point to transfected cells. Bar, 10 μm. (B 1 and 4) Untransfected SVG-A cells were incubated with JCV or SV40 for 4 h at 37°C, washed, and incubated with medium containing neutralizing JCV or SV40 antiserum. Cells were fixed at 72 h postinfection and stained for V antigen. (B 2 and 5) SVG-A cells transfected with 2 μg of p50/dynamitin DNA per ml were infected with JCV or SV40, fixed, and stained for V antigen. (B 3 and 6) SVG-A cells transfected with 10 μg of p50/dynamitin DNA per ml were infected with JCV or SV40, fixed, and stained for V antigen. Bar, 100 μm.
Role of intermediate filaments in JCV and SV40 infection.
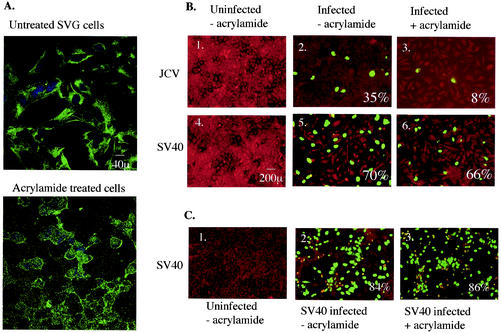
Intermediate filaments are another class of cytoskeletal proteins in animal cells and are specifically disrupted by the neurotoxin acrylamide. We first determined whether acrylamide could cause disassembly of intermediate filaments in glial cells by staining with an antivimentin antibody which detects the presence of the most abundantly expressed type III intermediate filament protein vimentin. Acrylamide at 10 mM caused depolymerization of the vimentin network in glial cells, as shown in Fig. 6A. Infection inhibition assays were performed as described above for cytochalasin D. Control cells were either left untreated and uninfected (Fig. 6B, panels 1 and 4) or infected with either JCV or SV40 in the absence of acrylamide (Fig. 6B, panels 2 and 5). Treatment with 10 mM acrylamide severely inhibited infection by JCV (≈77% reduction) but had no effect on infection by SV40 (Fig. 6B, panels 3 and 6). Addition of acrylamide 24 h postinfection did not inhibit JCV infection of glial cells (data not shown). These data indicate that an intact intermediate filament network is an additional requirement for proper early trafficking of JCV in glial cells, while SV40 does not require this cytoskeletal network for proper trafficking and infection.
FIG. 6.
Intermediate filaments are required for infection by JCV but not by SV40. (A) Untreated SVG-A cells or SVG-A cells treated with 10 mM acrylamide for 6 h at 37°C were stained with antivimentin antibody to detect the disassembly of intermediate filaments. Bar, 40 μm. (B 1 and 4) Uninfected SVG-A cells. (B 2 and 5) SVG-A cells were incubated with JCV or SV40 for 4 h at 37°C, washed, and incubated with medium containing neutralizing JCV or SV40 antiserum. Cells were fixed at 72 h postinfection and stained for V antigen. (B 3 and 6) SVG-A cells were treated for 6 h at 37°C with 10 mM acrylamide, infected with JCV or SV40, fixed, and stained for V antigen. Bar, 200 μm. (C 1) Uninfected CV1 cells. (C 2) Untreated CV1 cells infected with SV40. (C 3) CV1 cells treated for 6 h with 10 mM acrylamide, infected with SV40, fixed, and stained for V antigen. Numbers at the bottom right of each panel refer to the percentage of infected cells per field and represent the mean of at least four different fields of cells from each of three independent experiments.
Unlike microtubules and microfilaments, intermediate filaments vary greatly in protein composition in different cell types. Since simian-derived cells are the normal host of SV40, we examined the effect of depolymerizing intermediate filaments in CV1 cells on infection by this virus. Figure 6C shows that acrylamide treatment of CV1 cells had no effect on SV40 infection, which is consistent with our results in SVG-A cells. This suggests that cell type-specific intermediate filaments do not play a role in infection of cells by SV40.
DISCUSSION
Productive infection by many viruses requires the efficient use of host cell endocytic and cytoplasmic transport mechanisms. Understanding the ways in which viruses exploit these mechanisms allows us to understand the biology of these viruses while providing us with better insight into basic cellular functions. Here, we have begun to investigate the cellular transport and trafficking mechanisms exploited by the human polyomavirus JCV during infection of permissive glial cells. The related simian polyomavirus SV40 was used as a control in our experiments and served as an important tool to reveal the mechanisms utilized by polyomaviruses in general.
JCV, like several other animal viruses (20, 32), is internalized by clathrin-dependent endocytosis (42) upon binding to an N-linked glycoprotein with α-2,6-linked sialic acid on glial cells (29). Events downstream of endocytosis that lead to delivery of the viral genome to the nucleus of the host cell have not yet been characterized. SV40, on the other hand, is internalized via caveolae (1, 24) upon binding to the major histocompatibility complex class I antigens on the cell surface (2, 6, 49). It has been shown to then enter a pH-neutral “caveosome,” from which it is sorted into caveolin-free vesicles that travel along microtubules to the smooth endoplasmic reticulum (40). In order to understand the trafficking of JCV within glial cells, we used various inhibitors of endosomal pH as well as the cytoskeletal network and assayed their effects on productive infection.
In our first set of experiments, we examined the effect of the acidification inhibitor bafilomycin A1 on infection by JCV and SV40. There was virtually complete inhibition of infection by both viruses when treated with bafilomycin A1. We also examined the effects of ammonium chloride (NH4Cl) on infection by JCV and SV40. Our results show that while JCV infection was decreased by approximately 60 to 80% upon neutralization of endosomal pH, there was no effect on infection by SV40. Other groups have previously shown that an acidic pH is not required for infection by SV40 with similar concentrations of NH4Cl and a different inhibitor, chloroquine (46, 52). These data could be the result of two possibilities, the first being that SV40 infection is in fact pH dependent and that higher concentrations of NH4Cl than that used here are required to observe this inhibition, and the second being that bafilomycin A1 may have additional indirect effects on glial cells besides neutralization of endosomal pH and that the targets of this indirect inhibition are required for productive infection by both viruses. We were unable to rule out the first possibility, as higher concentrations of NH4Cl were toxic to glial cells (data not shown). However, there is considerable support for the second possibility. Several groups have shown that bafilomycin A1 affects transport through the endosomal system as a result of the neutral endosomal pH. The exact point of this inhibition is a topic of considerable controversy and may depend on the specific cell type. Bayer et. al. (4) and Clague et al. (11) suggested that the formation of an intermediate compartment between early and late endosomes, known as the endosomal carrier vesicle, is inhibited by bafilomycin A1. However, Van Deurs et al. (54, 55) and Van Weert et al. (56) suggested that bafilomycin A1 interferes with a downstream fusion step between late endosomes and lysosomes, while Yoshimori et al. (60) attributed decreased lysosomal degradation to bafilomycin A1 treatment. Without further detailed studies, we are unable to define the exact point of inhibition of endocytosis by bafilomycin A1 in glial cells, but we must attribute our findings to inhibition of endosomal transport rather than increased endosomal pH.
Next, we examined the role of microfilaments during infection by JCV and SV40. Our data clearly indicate that disruption of microfilaments with cytochalasin D inhibits infection of glial cells by JCV but not by SV40. The inhibition of infection was due to early events controlling virus trafficking in the cell, as cytochalasin D did not inhibit JCV infection if added at 24 h postinfection. Our data with SV40 are consistent with an earlier report demonstrating that intact microfilaments are not required for SV40 infection (46). Recently, however, it was reported that microfilaments, while not being important for early entry steps of SV40, are required at a step following virus entry into caveolae (41). Our data are consistent with these studies in that microfilaments do not play a role early in infection of cells by SV40. We were unable to ascertain whether disruption of microfilaments plays a role at later time points because cytochalasin D was toxic if left on the cells for longer periods. A role for the microfilament network in entry and internalization of other viruses has been documented (15, 23, 36). In addition, several studies indicate that actin polymerization facilitates clathrin-dependent endocytosis. This is not surprising, as the plasma membrane is intricately linked to the underlying actin cytoskeleton and events such as vesicle budding would require significant rearrangement of this actin cortex. Durrbach et al. (12) have suggested that microfilaments facilitate two steps in endocytosis: first, uptake of ligands, and second, delivery of ligands to degradative compartments. Brown and Song (7) found that microfilaments are involved in the same two steps while studying the trafficking of the B-cell antigen receptor. Lamaze et al. (25) have shown that actin-binding proteins and drugs that sequester actin monomers specifically inhibit clathrin-coated vesicle formation in mammalian cells. The same group later reported that endocytosis in different cell types or even in the same cell type under different conditions differs widely in sensitivity to microfilament-disrupting agents (17). Their conclusions most accurately summarize the literature in saying that microfilaments play a variable but not obligatory role in clathrin-dependent endocytosis in mammalian cells. It is not yet known what role, if any, microfilaments may play during endocytosis in glial cells. It is therefore equally probable that inhibition of JCV infection is due to a direct involvement of actin filaments during entry of the virus or that the effects observed are due to inhibition of clathrin-dependent endocytosis in glial cells.
We next examined the role of the microtubule network during infection by both viruses. Due to the nuclear proximity of the microtubule organizing center, microtubules are attractive cytoskeletal elements for viruses that replicate within the nucleus of the host cell. Several groups have previously shown that SV40 infection is dependent on an intact microtubule network (40, 46). We used nocodazole to disassemble microtubules in glial cells and found that JCV infection was severely inhibited in these cells. SV40 infection was similarly inhibited, in agreement with previous findings. Microtubules have been shown to be required for infection by several other viruses (33, 48, 57). In the case of SV40, it is thought that microtubules transport SV40 in caveolin-free vesicles from the caveosome to the smooth endoplasmic reticulum. It is possible that JCV requires microtubules for a similar transport step from the cell periphery to a juxtanuclear compartment. We therefore hypothesized that JCV may require a negative-end-directed motor protein for this directional transport along microtubules. Dynein is thought to be nonfunctional for transport unless associated with the activator protein dynactin (18, 22). Overexpression of the p50/dynamitin component of dynactin has been shown to disassemble the dynein-dynactin complex (9, 13). We have shown that overexpression of p50/dynamitin and hence perturbation of dynein function leads to no significant inhibition of JCV or SV40 infection. The role of motor proteins during infection by SV40 has not been examined previously. These observations therefore suggest either that JCV and SV40 require a different member of the dynein family whose function is independent of dynactin or that the viral proteins can interact directly with microtubules for transport. Pelkmans et al. observed the trafficking of SV40 virions along microtubules, which therefore rules out an indirect involvement of the microtubule network during infection by SV40 (40). We are currently exploring whether the microtubule network plays an indirect role in JCV infection.
The third cytoskeletal network examined was intermediate filaments. Very little is known about the involvement of this network in viral infection despite their abundance and important contribution to cellular architecture. There is a large and growing family of intermediate filament-associated proteins, and these proteins vary in different cell types. Vimentin is the predominant intermediate filament-associated protein in astrocytic cell types, and we therefore stained for vimentin networks in SVG-A cells. Acrylamide treatment of cells has been shown to specifically disassemble the intermediate filament network, and we used this inhibitor to analyze the effects of disassembly on both JCV and SV40 infection. Our results imply that an intact intermediate filament network is necessary for productive infection by JCV but not by SV40. Similar results were obtained when SV40 infection was examined in CV1 cells after acrylamide treatment, ruling out the possibility that there might be a cell type-specific intermediate filament-associated protein requirement for SV40 infection.
Recently, vaccinia virus assembly in virus factories has been shown to require intact intermediate filaments (45). Intermediate filaments are emerging as an important cytoskeletal component involved in virus infection. Several groups have suggested that the assembly of intermediate filaments in vivo requires microtubules and microtubule-associated fast-growing-end-directed motor proteins such as kinesin (19, 21, 43). It is therefore possible to hypothesize that JCV infection is dependent on intermediate filaments for proper cytoplasmic transport and that microtubules play an indirect role due to their role in the proper assembly of intermediate filaments.
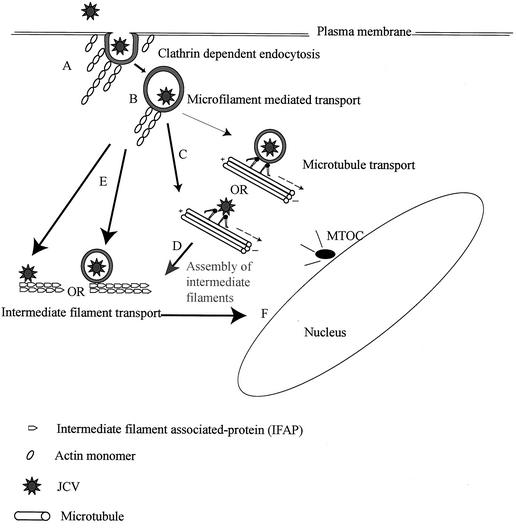
In summary, we suggest the following model for cytoplasmic transport for JCV (Fig. 7), in which actin filaments play an early role during internalization of JCV, due either to a direct interaction with the virus-containing vesicles or to an indirect involvement in clathrin-dependent endocytosis. We then propose a central role for intermediate filaments in the cytoplasmic transport of JCV. Microtubules may be involved either indirectly, due to their role in intermediate filament assembly, or due to a direct involvement that may involve a dynein family member which is dynactin independent. Our current work is aimed at testing this model and describing events downstream of early cytoplasmic transport that culminate in viral gene expression in the nucleus of the host cell.
FIG. 7.
Model for cytoplasmic transport of JCV. (A) The actin cytoskeleton is required for clathrin-dependent endocytosis in glial cells and is therefore required for JCV entry. (B) Microfilaments are required for transport of virus-containing vesicles very early during JCV infection. (C) Microtubules may be involved due to a direct interaction with either viral proteins or dynein family members other than dynein 1 or (D) microtubules may be required for transport of intermediate filament-associated proteins and hence proper assembly of intermediate filaments. (E) Intermediate filaments may therefore play an important role in the cytoplasmic transport of JCV either through a direct interaction with the virions or through interactions with virion-containing vesicles. (F) Further downstream trafficking events that lead to JCV DNA accumulation in the nucleus of the host cell are yet to be characterized. Thin arrows represent possibilities ruled out by our experiments, while dark arrows represent possibilities favored by our data. MTOC, microtubule organizing center.
Acknowledgments
We thank all members of the Atwood laboratory for critical discussion during the course of this work. We thank Eugene Major for the Mad-1 SVEΔ strain of JCV and for the original SVG cell line. We thank Ed Harlow for the PAB597 hybridoma and Richard Vallee for generously providing the Myc-tagged p50 construct.
Work in our laboratory was supported by a grant from the National Cancer Institute, R01 CA71878, and by grants from the National Institutes of Health, R01 NS43097, P20 RR15578, and P30-AI42853.
REFERENCES
- 1.Anderson, H. A., Y. Chen, and L. C. Norkin. 1996. Bound simian virus 40 translocates to caveolin enriched membrane domains, and its entry is inhibited by drugs that selectively disrupt caveolae. Mol. Biol. Cell 7**:**1825-1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atwood, W. J., and L. C. Norkin. 1989. Class I major histocompatibility proteins as cell surface receptors for simian virus 40. J. Virol. 63**:**4474-4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atwood, W. J., L. Wang, L. C. Durham, K. Amemiya, R. G. Traub, and E. O. Major. 1995. Evaluation of the role of cytokine activation in the multiplication of JC virus (JCV) in human fetal glial cells. J. Neurovirol. 1**:**40-49. [DOI] [PubMed] [Google Scholar]
- 4.Bayer, N., D. Schober, E. Prchla, R. F. Murphy, D. Blaas, and R. Fuchs. 1998. Effect of bafilomycin A1 and nocodazole on endocytic transport in HeLa cells: implications for viral uncoating and infection. J. Virol. 72**:**9645-9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bowman, E. J., A. Siebers, and K. Altendorf. 1988. Bafilomycins: a class of inhibitors of membrane ATPases from microorganisms, animal cells, and plant cells. Proc. Natl. Acad. Sci. USA 85**:**7972-7976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Breau, W. C., W. J. Atwood, and L. C. Norkin. 1992. Class I major histocompatibility proteins are an essential component of the simian virus 40 receptor. J. Virol. 66**:**2037-2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown, B. K., and W. Song. 2001. The actin cytoskeleton is required for the trafficking of the B cell antigen receptor to the late endosomes. Traffic 2**:**414-427. [DOI] [PubMed] [Google Scholar]
- 8.Brown, S. S., and J. A. Spudich. 1981. Mechanism of action of cytochalasin: evidence that it binds to actin filament ends. J. Cell Biol. 88**:**487-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burkhardt, J. K., C. J. Echeverri, T. Nilsson, and R. B. Vallee. 1997. Overexpression of the dynamitin (p50) subunit of the dynactin complex disrupts dynein-dependent maintenance of membrane organelle distribution. J. Cell Biol. 139**:**469-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheung, H. T., and D. S. Terry. 1980. Effects of nocodazole, a new synthetic microtubule inhibitor, on movement and spreading of mouse peritoneal macrophages. Cell Biol. Int. Rep. 4**:**1125-1129. [DOI] [PubMed] [Google Scholar]
- 11.Clague, M. J., S. Urbe, F. Aniento, and J. Gruenberg. 1994. Vacuolar ATPase activity is required for endosomal carrier vesicle formation. J. Biol. Chem. 269**:**21-24. [PubMed] [Google Scholar]
- 12.Durrbach, A., D. Louvard, and E. Coudrier. 1996. Actin filaments facilitate two steps of endocytosis. J. Cell Sci. 109**:**457-465. [DOI] [PubMed] [Google Scholar]
- 13.Echeverri, C. J., B. M. Paschal, K. T. Vaughan, and R. B. Vallee. 1996. Molecular characterization of the 50-kD subunit of dynactin reveals function for the complex in chromosome alignment and spindle organization during mitosis. J. Cell Biol. 132**:**617-633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eckert, B. S. 1985. Alteration of intermediate filament distribution in PtK1 cells by acrylamide. Eur. J. Cell Biol. 37**:**169-174. [PubMed] [Google Scholar]
- 15.Fan, D. P., and B. M. Sefton. 1978. The entry into host cells of Sindbis virus, vesicular stomatitis virus and Sendai virus. Cell 15**:**985-992. [DOI] [PubMed] [Google Scholar]
- 16.Forgac, M. 1998. Structure, function and regulation of the vacuolar H+-ATPases. FEBS Lett. 440**:**258-263. [DOI] [PubMed] [Google Scholar]
- 17.Fujimoto, L. M., R. Roth, J. E. Heuser, and S. L. Schmid. 2000. Actin assembly plays a variable, but not obligatory role in receptor-mediated endocytosis in mammalian cells. Traffic 1**:**161-171. [DOI] [PubMed] [Google Scholar]
- 18.Gill, S. R., T. A. Schroer, I. Szilak, E. R. Steuer, M. P. Sheetz, and D. W. Cleveland. 1991. Dynactin, a conserved, ubiquitously expressed component of an activator of vesicle motility mediated by cytoplasmic dynein. J. Cell Biol. 115**:**1639-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gyoeva, F. K., and V. I. Gelfand. 1991. Coalignment of vimentin intermediate filaments with microtubules depends on kinesin. Nature 353**:**445-448. [DOI] [PubMed] [Google Scholar]
- 20.Helenius, A., J. Kartenbeck, K. Simons, and E. Fries. 1980. On the entry of Semliki forest virus into BHK-21 cells. J. Cell Biol. 84**:**404-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ho, C. L., J. L. Martys, A. Mikhailov, G. G. Gundersen, and R. K. Liem. 1998. Novel features of intermediate filament dynamics revealed by green fluorescent protein chimeras. J. Cell Sci. 111**:**1767-1778. [DOI] [PubMed] [Google Scholar]
- 22.Holleran, E. A., S. Karki, and E. L. Holzbaur. 1998. The role of the dynactin complex in intracellular motility. Int. Rev. Cytol. 182**:**69-109. [DOI] [PubMed] [Google Scholar]
- 23.Hollinshead, M., G. Rodger, H. Van Eijl, M. Law, R. Hollinshead, D. J. Vaux, and G. L. Smith. 2001. Vaccinia virus utilizes microtubules for movement to the cell surface. J. Cell Biol. 154**:**389-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kartenbeck, W., H. Stukenbrok, and A. Helenius. 1989. Endocytosis of simian virus in the endoplasmic reticulum. J. Cell Biol. 109**:**2722-2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lamaze, C., L. M. Fujimoto, H. L. Yin, and S. L. Schmid. 1997. The actin cytoskeleton is required for receptor-mediated endocytosis in mammalian cells. J. Biol. Chem. 272**:**20332-20335. [DOI] [PubMed] [Google Scholar]
- 26.Lee, J. C., D. J. Field, and L. L. Lee. 1980. Effects of nocodazole on structures of calf brain tubulin. Biochemistry 19**:**6209-6215. [DOI] [PubMed] [Google Scholar]
- 27.Liu, C. K., and W. J. Atwood. 2001. Propagation and assay of the JC virus. Methods Mol. Biol. 165**:**9-17. [DOI] [PubMed] [Google Scholar]
- 28.Liu, C. K., A. P. Hope, and W. J. Atwood. 1998. The human polyomavirus JC virus does not share receptor specificity with SV40 on human glial cells. J. Neurovirol. 4**:**49-58. [DOI] [PubMed] [Google Scholar]
- 29.Liu, C. K., G. Wei, and W. J. Atwood. 1998. Infection of glial cells by the human polyomavirus JC is mediated by an N-linked glycoprotein containing terminal α2-6 linked sialic acids. J. Virol. 72**:**4643-4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Major, E. O., K. Amemiya, C. S. Tornatore, S. A. Houff, and J. R. Berger. 1992. Pathogenesis and molecular biology of progressive multifocal leukoencephalopathy, the JC virus-induced demyelinating disease of the human brain. Clin. Microbiol. Rev. 5**:**49-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Major, E. O., A. E. Miller, P. Mourrain, R. G. Traub, E. de Widt, and J. Sever. 1985. Establishment of a line of human fetal glial cells that supports JC virus multiplication. Proc. Natl. Acad. Sci. USA 82**:**1257-1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marsh, M., and A. Helenius. 1989. Virus entry into animal cells. Adv. Virus Res. 36**:**107-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mas, P., and R. N. Beachy. 2000. Role of microtubules in the intracellular distribution of tobacco mosaic virus movement protein. Proc. Natl. Acad. Sci. USA 97**:**12345-12349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mellman, I., R. Fuchs, and A. Helenius. 1986. Acidification of the endocytic and exocytic pathways. Annu. Rev. Biochem. 55**:**663-700. [DOI] [PubMed] [Google Scholar]
- 35.Miranda, A. F., G. C. Godman, and S. W. Tanenbaum. 1974. Action of cytochalasin D on cells of established lines. II. Cortex and microfilaments. J. Cell Biol. 62**:**406-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nauwynck, H. J., X. Duan, H. W. Favoreel, P. Van Oostveldt, and M. B. Pensaert. 1999. Entry of porcine reproductive and respiratory syndrome virus into porcine alveolar macrophages via receptor-mediated endocytosis. J. Gen. Virol. 80**:**297-305. [DOI] [PubMed] [Google Scholar]
- 37.Ohkuma, S., and B. Poole. 1978. Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents. Proc. Natl. Acad. Sci. USA 75**:**3327-3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Padgett, B., G. ZuRhein, D. Walker, R. Echroade, and B. Dessel. 1971. Cultivation of papova-like virus from human brain with progressive multifocal leukoencephalopathy. Lancet **i:**1257-1260. [DOI] [PubMed]
- 39.Padgett, B. L., and D. L. Walker. 1973. Prevalence of antibodies in human sera against JC virus, an isolate from a case of progressive multifocal leukoencephalopathy. J. Infect. Dis. 127**:**467-470. [DOI] [PubMed] [Google Scholar]
- 40.Pelkmans, L., J. Kartenbeck, and A. Helenius. 2001. Caveolar endocytosis of simian virus 40 reveals a new two-step vesicular-transport pathway to the endoplasmic reticulum. Nat. Cell Biol. 3**:**473-483. [DOI] [PubMed] [Google Scholar]
- 41.Pelkmans, L., D. Puntener, and A. Helenius. 2002. Local actin polymerization and dynamin recruitment in SV40-induced internalization of caveolae. Science 296**:**535-539. [DOI] [PubMed] [Google Scholar]
- 42.Pho, M. T., A. Ashok, and W. J. Atwood. 2000. JC virus enters human glial cells by clathrin-dependent receptor-mediated endocytosis. J. Virol. 74**:**2288-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prahlad, V., M. Yoon, R. D. Moir, R. D. Vale, and R. D. Goldman. 1998. Rapid movements of vimentin on microtubule tracks: kinesin-dependent assembly of intermediate filament networks. J. Cell Biol. 143**:**159-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Richardson, E. P., Jr. 1961. Progressive multifocal leukoencephalopathy. N. Engl. J. Med. 265**:**815-823. [DOI] [PubMed] [Google Scholar]
- 45.Risco, C., J. R. Rodriguez, C. Lopez-Iglesias, J. L. Carrascosa, M. Esteban, and D. Rodriguez. 2002. Endoplasmic reticulum-Golgi intermediate compartment membranes and vimentin filaments participate in vaccinia virus assembly. J. Virol. 76**:**1839-1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shimura, H., Y. Umeno, and G. Kimura. 1987. Effects of inhibitors of the cytoplasmic structures and functions on the early phase of infection of cultured cells with simian virus 40. Virology 158**:**34-43. [DOI] [PubMed] [Google Scholar]
- 47.Sodeik, B. 2000. Mechanisms of viral transport in the cytoplasm. Trends Microbiol. 8**:**465-472. [DOI] [PubMed] [Google Scholar]
- 48.Sodeik, B., M. W. Ebersold, and A. Helenius. 1997. Microtubule-mediated transport of incoming herpes simplex virus 1 capsids to the nucleus. J. Cell Biol. 136**:**1007-1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stang, E., J. Kartenbeck, and R. G. Parton. 1997. Major histocompatibility complex class I molecules mediate association of SV40 with caveolae. Mol. Biol. Cell 8**:**47-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Suomalainen, M., M. Y. Nakano, S. Keller, K. Boucke, R. P. Stidwill, and U. F. Greber. 1999. Microtubule-dependent plus and minus end-directed motilities are competing processes for nuclear targeting of adenovirus. J. Cell Biol. 144**:**657-672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Suzuki, T., M. Yamaya, K. Sekizawa, M. Hosoda, N. Yamada, S. Ishizuka, K. Nakayama, M. Yanai, Y. Numazaki, and H. Sasaki. 2001. Bafilomycin A(1) inhibits rhinovirus infection in human airway epithelium: effects on endosome and ICAM-1. Am. J. Physiol. Lung Cell. Mol. Physiol. 280**:**L1115-L1127. [DOI] [PubMed] [Google Scholar]
- 52.Upcroft, P. 1987. Simian virus 40 infection is not mediated by lysosomal activation. J. Gen. Virol. 68**:**2477-2480. [DOI] [PubMed] [Google Scholar]
- 53.Vallee, R. B., and M. P. Sheetz. 1996. Targeting of motor proteins. Science 271**:**1539-1544. [DOI] [PubMed] [Google Scholar]
- 54.van Deurs, B., P. K. Holm, L. Kayser, K. Sandvig, and S. H. Hansen. 1993. Multivesicular bodies in HEp-2 cells are maturing endosomes. Eur. J. Cell Biol. 61**:**208-224. [PubMed] [Google Scholar]
- 55.van Deurs, B., P. K. Holm, and K. Sandvig. 1996. Inhibition of the vacuolar H(+)-ATPase with bafilomycin reduces delivery of internalized molecules from mature multivesicular endosomes to lysosomes in HEp-2 cells. Eur. J. Cell Biol. 69**:**343-350. [PubMed] [Google Scholar]
- 56.van Weert, A. W., K. W. Dunn, H. J. Gueze, F. R. Maxfield, and W. Stoorvogel. 1995. Transport from late endosomes to lysosomes, but not sorting of integral membrane proteins in endosomes, depends on the vacuolar proton pump. J. Cell Biol. 130**:**821-834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vihinen-Ranta, M., W. Yuan, and C. R. Parrish. 2000. Cytoplasmic trafficking of the canine parvovirus capsid and its role in infection and nuclear transport. J. Virol. 74**:**4853-4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.White, J., J. Kartenbeck, and A. Helenius. 1982. Membrane fusion activity of influenza virus. EMBO J. 1**:**217-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.White, J., K. Matlin, and A. Helenius. 1981. Cell fusion by Semliki Forest, influenza, and vesicular stomatitis viruses. J. Cell Biol. 89**:**674-679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yoshimori, T., A. Yamamoto, Y. Moriyama, M. Futai, and Y. Tashiro. 1991. Bafilomycin A1, a specific inhibitor of vacuolar-type H+-ATPase, inhibits acidification and protein degradation in lysosomes of cultured cells. J. Biol. Chem. 266**:**17707-17712. [PubMed] [Google Scholar]
- 61.Yu, Y. G., D. S. King, and Y. K. Shin. 1994. Insertion of a coiled-coil peptide from influenza virus hemagglutinin into membranes. Science 266**:**274-276. [DOI] [PubMed] [Google Scholar]