The temporal and spatial distribution of ciliogenesis in the tracheobronchial airways of mice (original) (raw)
. Author manuscript; available in PMC: 2006 Jul 5.
Published in final edited form as: Am J Physiol Lung Cell Mol Physiol. 2005 May 6;289(3):L454–L459. doi: 10.1152/ajplung.00036.2005
Abstract
Little is known about ciliogenesis as it proceeds through the entire airway tree, from the trachea to the terminal bronchioles, especially during the postnatal period. The purpose of this study was to define the spatial and temporal (prenatal and postnatal) pattern of normal cilia development in the mouse. Three airway generations representing the entire airway tree were examined: trachea, lobar bronchi, and terminal bronchiole. Ciliated cells in lung lobe whole mounts were labeled with a fluorescent dye for confocal microscopy, and ciliated cell surface density was measured for each airway generation and age. The same samples were examined by scanning electron microscopy to verify the appearance of ciliated cells among the differentiating epithelium of the airways. Ciliated cells were first detected in the trachea and lobar bronchi at 16 days gestational age (DGA) and in the terminal bronchioles at 18 DGA. Ciliated cell surface density increased with prenatal and postnatal age at all airway levels. However, the ciliated cell surface density of the trachea and lobar bronchi was always greater compared to the terminal bronchiole. In conclusion, the study revealed that in developing tracheobrochial airways of the mouse: 1) Ciliogenesis differs temporally and spatially by airway generation; 2) Ciliated cell surface density increases with age in all airway generations, but density decreases in a proximal to distal direction; and 3) A significant portion of ciliogenesis continues after birth. This study provides a healthy basis for investigations of neonatal pulmonary disease or pollutant toxicity affecting cilia and its functions.
Keywords: gestational, postnatal, cilia, lung
INTRODUCTION
Ciliated cells are found throughout the lung, as well as in other parts of the body. Most studies of ciliogenesis in the lung have been limited to the prenatal period and/or they are based on a single area of the airway tree, such as the trachea (9,10,16,19). These studies have established that ciliogenesis begins prenatally, although at different phases of lung development depending upon the species, and occurs in a proximal to distal direction in the select airway generations that were examined. However, little is known about how ciliogenesis proceeds through the entire airway tree, from the trachea to the terminal bronchioles, especially during the postnatal period of lung development. This information is important to know because ciliated cells are responsible for efficient mucociliary transport, a major defense system of the lung that removes pathogenic microbes and inhaled particles from the respiratory tract. Impairment of mucociiary function is a component of such diseases as cystic fibrosis (15), asthma (2), and primary ciliary dyskinesia (26), which have their beginnings in childhood while the lung is developing. Furthermore, ciliated cells are negatively affected by cigarette smoke (4) as well as air pollutants such as ozone (22), sulfur dioxide (11), and nitrogen dioxide (7). Children are often chronically exposed to these pollutants and epidemiological studies have shown a correlation between pollutant exposure and reduced pediatric lung health (see (21) for review).
The goal of this study was to define the spatial and temporal (prenatal and postnatal) pattern of normal cilia development in three different airway generations representing the entire airway tree: the trachea, the lobar bronchi, and the terminal bronchiole. Mice were chosen because they are commonly used in studies of airway disease. Ciliated cells were labeled with a fluorescent dye for confocal microscopy of lung lobe whole mounts, and ciliated cell surface density was measured for each airway generation and age. The same specimens were examined by scanning electron microscopy (SEM) to correlate ciliated cell surface density with the appearance of ciliated cells among the differentiating epithelium of the airways. SEM was chosen because it provides exact structure and spatial information. We also felt that SEM would enable us to identify cilia that might have a weak fluorescent signal. Using these methods, the following conclusions were made: 1) Ciliogenesis differs temporally and spatially by airway generation; 2) Ciliated cell surface density increases with age in all airway generations, but density decreases in a proximal to distal direction; and 3) A significant portion of ciliogenesis continues after birth.
MATERIALS AND METHODS
Animals and lung fixation
Female time-pregnant (shipped at 6th day of gestation) and male Swiss Webster mice (Charles River Breeding Laboratory, Wilmington, MA) were used. Animals were housed in AAALAC approved facilities on a 12/12 hour light/dark cycle with food and water ad libitum for at least five days prior to use. Male and female animals were selected at 15, 16, 17, 18, and 19 days gestation age (DGA) and at 1, 7, 14, 21 days postnatal age (DPN). Adult animals were 10 to 12 weeks old. Three to five animals were used per age group. All animals were anesthetized with sodium pentobarbital, the trachea cannulated, and the animals killed by exsanguination. Postnatal and adult lungs were inflated and fixed under 30 cm of pressure with 330 mOsm Karnovsky’s fixative (1% glutaraldehyde/0.5% paraformaldehyde in cacodylate buffer, pH 7.4) for 1 hour. Prenatal mouse lungs were inflated and fixed with injection of Karnovsky’s fixative via tracheal cannula holding the pressure constant for 10 minutes.
Scanning laser confocal microscopy and morphometry
The fixed trachea and right cardiac lobe were affixed to a glass coverslip mediastinal side down with cyanoacrylate tissue glue (Nexaband; Veterinary Products, Phoenix, AZ). The lumena, from the trachea to the terminal bronchioles, were exposed by removing the dorsal half of the airway by microdissection while immersed in phosphate-buffered saline (PBS). The microdissected airways were incubated for 20 minutes with 20 μl/ml fluorescein-labeled tomato lectin (lycopersicon esculentum) (Vector, Burlingame, CA). Samples were washed in PBS for ten minutes. A scanning laser confocal microscope (BioRad MRC 1024 ES mounted on an Olympus BX50WI microscope) with a 40X long working distance water-immersion objective and BPS filter (EX 488 nm; EM 520 nm) was used to see ciliated cells labeled with tomato lectin (Figure 1A). We chose tomato lectin to measure the ciliated cell surface density (described below) because it binds to the surface of airway epithelium (1) and has a very high affinity for the surface of ciliated cells. For each animal, five random areas per trachea and lobar bronchi, and one area per five terminal bronchioles (due to their small size) were selected for imaging. An airway generation was defined as the region between the proximal branch point and the distal branch point. A series of images were taken at focal planes that were 10 microns apart and had a depth of 20 microns. Fields were selected based on obtaining areas of low curvature and distortion. The size of the field measured increased in direct proportion to increasing airway surface area resulting in an equal percentage of total airway surface area being measured for each age. The final image was composed of 4 to 10 serial images, which were stacked to produce a three-dimensional composite. NIH Image software (National Technical Information Service, PB 90-500687) was used to measure the luminal surface area occupied by ciliated cells and total luminal surface area of epithelium within the composite. Briefly, a known distance was calibrated based on pixel number. Ciliated cells and the total field in each composite were outlined by freehand, and the ciliated cell surface area per image computed. Total ciliated cell surface density was found for each animal and defined as the percent of airway surface area covered by ciliated cells (ciliated cell surface area / total epithelial surface area). Then, the average ciliated cell surface density of the group of animals was calculated for each age and airway generation.
Figure 1.
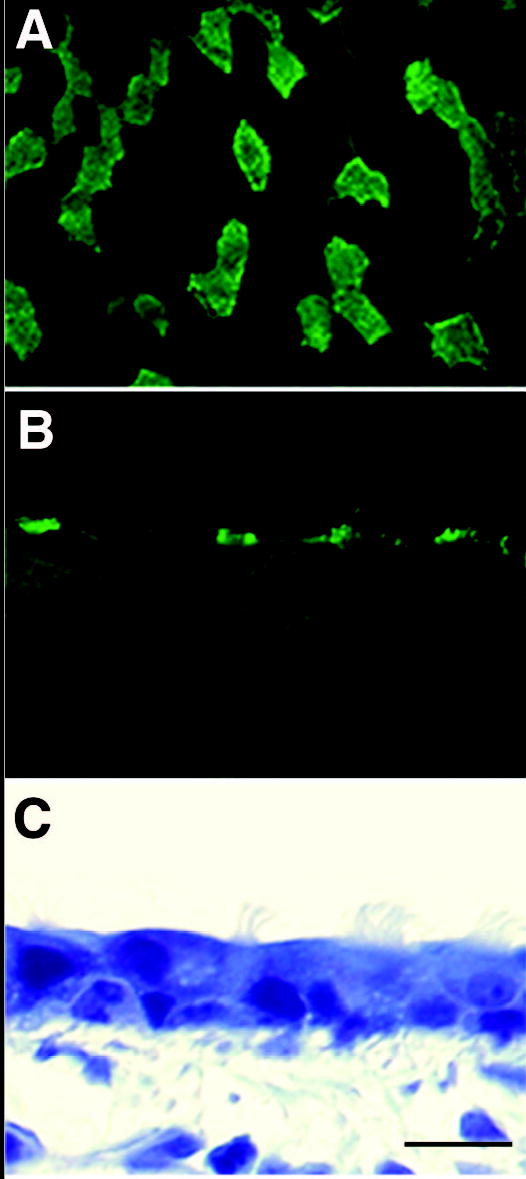
Microscopic images of adult mouse trachea labeled with FITC conjugated tomato lectin taken with (A) scanning confocal microscope (B) epifluorescence (C) transmitted light. Ciliated areas shown with epifluorescence are the same areas observed with transmitted light. Bar = 10 micrometers.
To confirm the phenotype of cells stained with FITC-labeled tomato lectin, tracheas from 18 DGA and adult mice were dehydrated in ethanol and embedded in glycolmethacrylate (Immuno-bed, Polyscience, Warrington, PA), which does not damage lectin, for high resolution microscopy. Blocks containing trachea were sectioned at 1 micrometer on a Zeiss HM340E microtome. Sections containing epithelium were first imaged under fluorescent conditions to visualize the ciliated cells and then under bright-field (phase contrast) conditions to visualize the epithelium. A Dage MTI CCD camera (Michigan City, IN) mounted on a Zeiss Axioskop microscope (Thornwood, NY) was used.
Scanning electron microscopy
Tissue to be imaged by scanning electron microscopy was dehydrated in a graded ethanol series of ethanol: 70%, 85%, 95%, and 100%. The tissue was briefly washed in an agitated bath of 50/50 solution of 100% ethanol and toluene to remove airway secretions from the epithelium. The tissue was taken to 100% toluene and then passed through the solvents in reverse order to 100% ethanol. Samples were immersed in hexamethyldisilizane (Electron Microscopy Sciences, Fort Washington, PA) for five minutes, and air dried overnight at room temperature. Prior to SEM, samples were covered with gold using a Polaron II ES100 sputter-coater (2.5 kV acceleration voltage in argon atmosphere with current 10 mA) for two minutes. The trachea, lobar bronchi, and terminal bronchioles from the cardiac lobe of two mice in each age group were imaged with a Philips SEM 501 microscope (FEI Corporation, Hillsboro, OR).
Statistics
Ciliated cell surface density at each airway level was measured in trachea, lobar bronchi, and terminal bronchioles at all 10 time points, except for the 15 DGA time point because ciliated cells were not observed. Differences in ciliated cell surface density with age were compared at each airway level by ANOVA, and the significance of post hoc comparisons was determined using the Bonferroni-Dunn method (p < 0.05) (6). Differences in ciliated cell surface density among airway levels at each age were also compared by ANOVA, and the significance of post hoc comparisons was determined using the Tukey method (p < 0.5) (6).
RESULTS
Ciliated cell identification using tomato lectin
Ciliated cells populating the airway were identified after surface labeling with FITC conjugated tomato lectin (Figure 1). Lectin binding was especially strong at the base of the cilia.
Prenatal age
Cilia were not detected until 16 days gestational age (DGA) (pseudoglandular stage of lung development) in the trachea and lobar bronchi. In both airways, short cilia were first visible by SEM on single cells surrounded by undifferentiated, round cells lacking the microvilli or apical projections characteristic of mature nonciliated cells (Figure 3A and B). In contrast, the first cilia were found in the terminal bronchioles at 18 DGA, two days later than in more proximal airways. Single ciliated cells appeared that were fairly flat with short, immature cilia, and microvilli adjacent to round, poorly differentiated nonciliated cells (Figure 4A). Measurements of ciliated cell surface density reflected the SEM findings as at 16 DGA ciliated cell surface density was 1.5% in the trachea, 0.6% in the lobar bronchus, and 0% in the terminal bronchioles (Figure 2). At 18 DGA, the trachea and lobar bronchus had significantly increased ciliated cell surface density compared to the terminal bronchiole. At 19 DGA, the cilia were longer compared to 16 DGA in the trachea and lobar bronchus (Figure 3C vs. A). Most ciliated cells appeared alone or in pairs among nonciliated cells (Figure 3D). Compared to 16 DGA, ciliated cell surface density was significantly increased at 19 DGA in the trachea (19%) and lobar bronchus (13.2%) (Figure 2). Not only was ciliated cell surface density significantly smaller in the terminal bronchiole at 19 DGA (only 1.3%) compared to the two more proximal airways, but it was also unchanged compared to ciliated cell surface density measured at 18 DGA (1.2%). By 19DGA, ciliated cell surface density was significantly different among all three airway generations.
Figure 3.
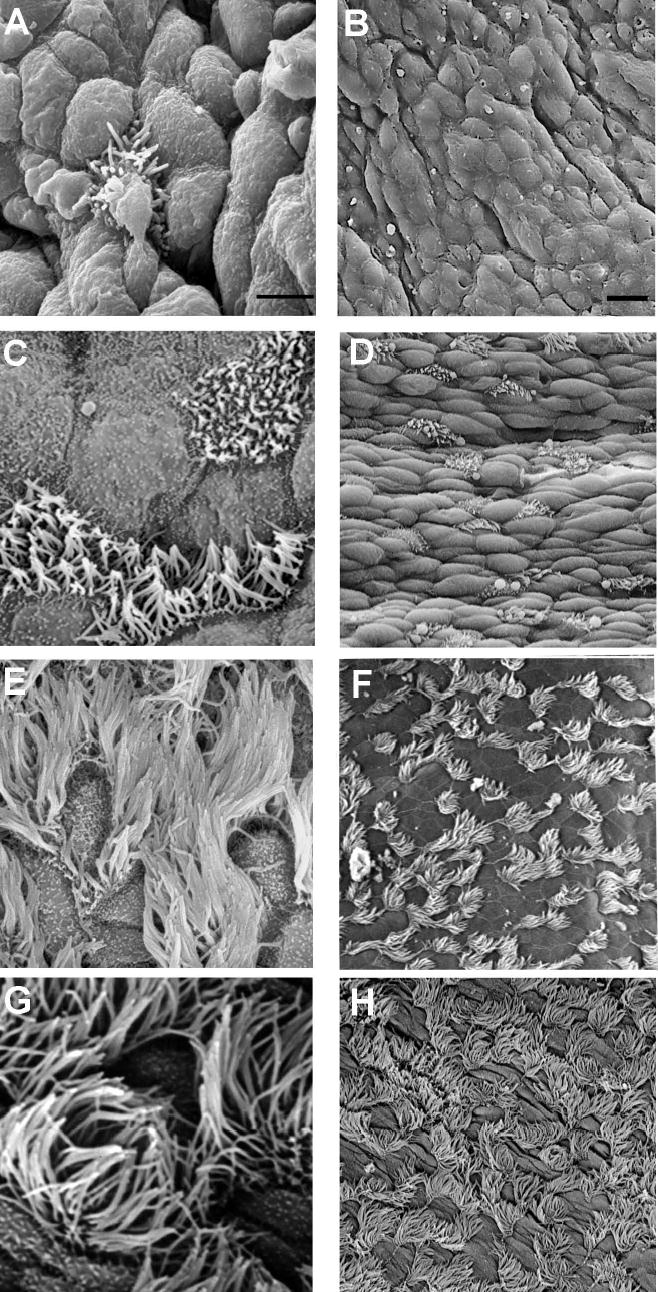
SEM images of the development of cilia in the trachea of mice at different ages: 16 DGA (A, B), 19 DGA (C, D), 14 DPN (E, F), and 21 DPN (G, H). At 16 DGA, sparse, single ciliated cells were visible. The remaining cells were undifferentiated and round, lacking microvilli or apical projections. At 19 DGA, cilia were short and covered the surface of ciliated cells. Cilia cells appeared alone or in pairs. Nonciliated cells were flat and had some microvilli. At 14 DPN, ciliated cells were covered by long cilia and occurred in pairs or in rows of 3–4 cells. At 21 DPN, ciliated cells have long cilia. Bar = 10 micrometers in A, C, E, and G. Bar = 50 micrometers in B, D, F, and H.
Figure 4.
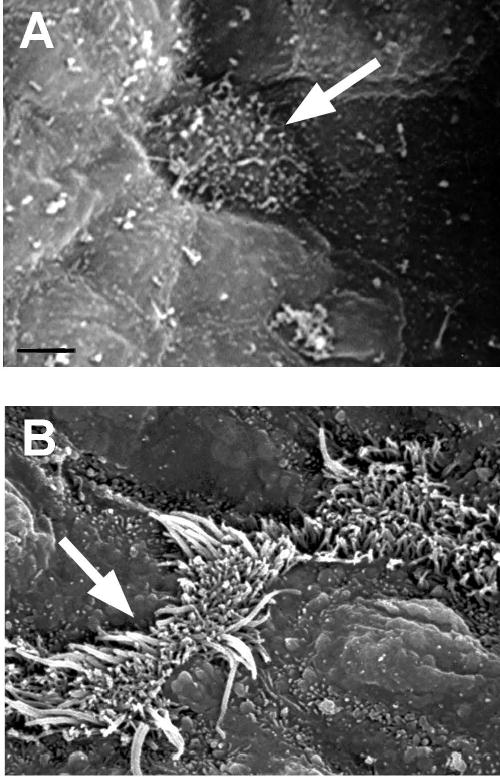
SEM images of the development of cilia in the terminal bronchiole. (A) Ciliated cells were first seen at 18 DGA and were single, fairly flat cells with very short cilia and microvilli. Nonciliated cells surrounding the ciliated cells were undifferentiated and slightly raised. (B) At 14 DPN, ciliated cells had long cilia and were alone or in pairs. Bar = 10 micrometers in A and B. Arrows indicate cilated cells.
Figure 2.
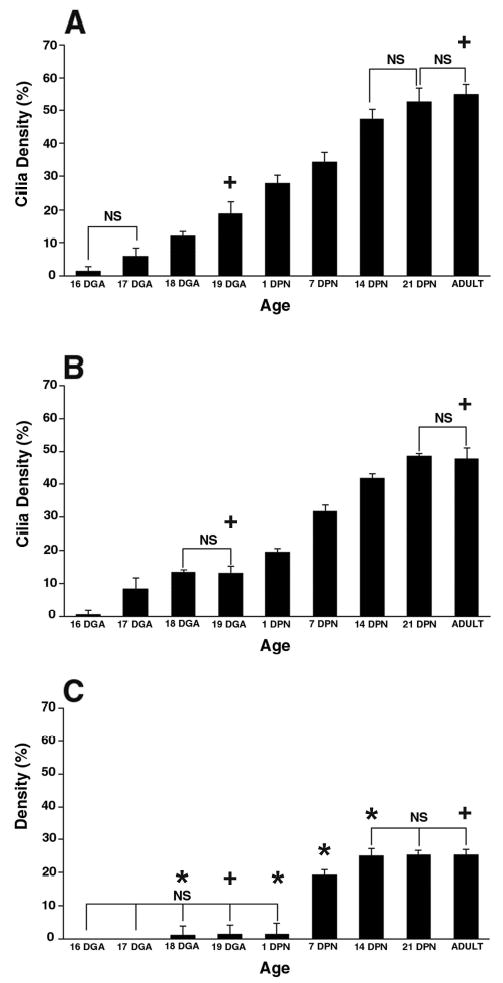
Comparison of age-related ciliated cell surface density in three airways of mouse lung: (A) trachea (B) lobar bronchi (C) terminal bronchioles. Time points include 16, 17, 18, and 19 days gestation (DGA); 1, 7, 14, and 21 days postnatal (DPN); and adult. Ciliated cell surface density is expressed as percentage of airway surface area. NS = No significant differences within each airway level between time points in brackets. Otherwise, the significance within each airway level between time points is p < 0.05. * = significantly different (p < 0.05) from the trachea and lobar bronchi at the same time point. + = significantly different (p < 0.05) from the other two airway generations at the same time point.
Postnatal age
At 1 DPN, ciliated cells appeared alone or in pairs with long cilia surrounded by rounded Clara cells in the trachea and lobar bronchus. The terminal bronchioles still had only single ciliated cells with short cilia. By 1 DPN, ciliated cell surface density had increased dramatically in the trachea and lobar bronchus and was 28.9% and 19.4%, respectively (Figure 2). There was no difference in ciliated cell surface density from 18 DGA to 1 DPN in the terminal bronchioles. By 14 DPN, ciliated cells were in rows of 3–4 cells among nonciliated cells in the trachea and lobar bronchus (Figure 2E). In the terminal bronchiole, ciliated cells appeared alone or in pairs surrounded by many round nonciliated cells (Figure 4B). By 14 DPN, ciliated cell surface density had increased to 47.2% in the trachea and 41.8% in the lobar bronchus (Figure 2). Ciliated cell surface density in the terminal bronchiole did not increase significantly until 7 DPN (19.3%) and, at 14 DPN, was only 25.0%, much less than the two more proximal airways. In fact, ciliated cell surface density was significantly greater in the trachea and lobar bronchus compared to the terminal bronchiole from 1 to 14 DPN. By 21 DPN, ciliated cell surface density in all three airways had reached adult levels (Figure 2). Differences observed in ciliated cell surface density among the different airway levels during development were maintained into adulthood; adult ciliated cell surface density was 54.8% in the trachea, 47.7% in the lobar bronchus, and 25.5% in the terminal bronchioles. At this time, ciliated cell surface density was significantly different among all three airway generations. The disparity in ciliated cell surface density is reflected in the SEM images of adult trachea, lobar bronchus, and terminal bronchioles (Figure 5). Adult ciliated cells were in rows of 3–5 cells in the trachea and lobar bronchus, while they were in pairs or alone, surrounded by Clara cells in the terminal bronchioles. Cilia length also appeared to still be shorter in the terminal bronchioles compared to the more proximal airways (Figure 5C vs. 5A, B). Throughout the study, the findings with confocal microscopy and SEM were comparable.
Figure 5.
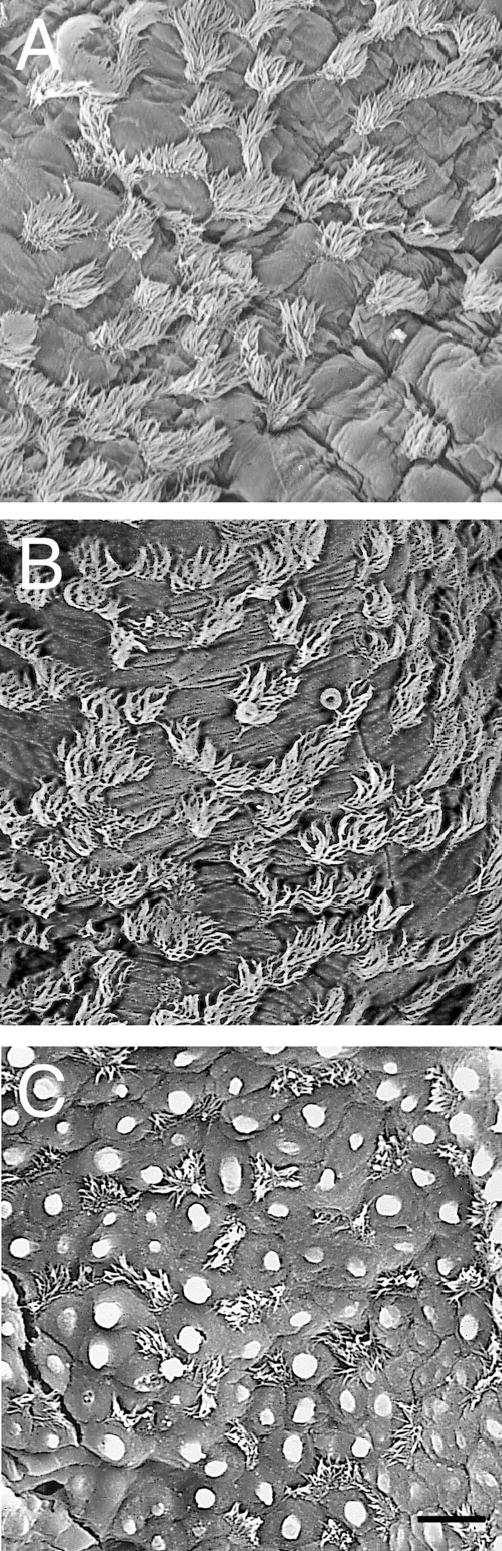
SEM images of (A) trachea (B) lobar bronchi and (C) terminal bronchiole in adult mice. Cilia are long and cover the entire surface of the cell and extend over the cell margins in the trachea and lobar bronchi. Ciliated cells appeared in rows of 4–6 cells. In the terminal bronchioles, cilia are shorter and cells appear in single or in pairs. Bar = 10 micrometers.
DISCUSSION
The purpose of this study was to define the spatial and temporal pattern of ciliogenesis in three airway levels representing the entire conducting airway tree: trachea, lobar bronchi, and terminal bronchiole. We found that in the mouse, ciliogenesis varies by airway generation and age. Initially, ciliated cells appear as single cells in all three airway generations. However, with increasing age, the more proximal airways (trachea and lobar bronchi) develop ciliated cells in rows of 3–4 cells while ciliated cells in the terminal bronchioles remain as single or paired cells. Therefore, ciliated cell surface density is always greater in the trachea and lobar bronchi compared to the terminal bronchiole. Ciliogenesis moves in a proximal to distal direction along the tracheobronchial airways; ciliated cells are first observed in the trachea and lobar bronchi at 16 days gestational age (DGA) and two days later in the terminal bronchioles. Ciliogenesis continues well into the postnatal period as ciliated cell surface density significantly increases from birth to three weeks of age in all airway generations.
It is well established that cilia are vital for mucociliary transport of mucus, macrophages, and environmentally derived particles. Ciliated cell damage and disrupted mucociliary clearance are key components of airway diseases such as asthma (2), cystic fibrosis (15), chronic sinusitis (5,24) and bronchitis (17). Presently, it is not clear what percent of the epithelial surface needs to be covered by ciliated cells in order for mucociliary clearance to be efficient and what rate of mucociliary transport is essential for maintaining respiratory health. As shown by this study, ciliated cells are not uniformly distributed over the epithelial surface and are not always contiguous with each other. Differences in ciliated cell distribution have also been noted in adult rat trachea, where areas covering tracheal ligaments were found to have more ciliated cell density than areas covering cartilage rings (18). We did not see this alternating density pattern in the developing postnatal or adult mouse trachea. However, it is known that ciliary beat frequency, a predictor of the efficiency of mucociliary transport, correlates with ciliated cell density and the number and length of cilia per cell (13,23). In this study, mouse ciliated cell surface density did not reach adult levels until 21 DPN, the age equivalent of human adolescence. It is unknown whether this same pattern of postnatal cilia maturation is present in humans, but if it is similar, then children may be more at risk to compromised mucociliary transport by such factors as pathogens or air pollution than adults.
We feel that the strength of using a three dimensional whole mount approach to studying ciliogenesis is the ability to evaluate the whole airway tree. Studies in the past have been limited to certain airway regions, especially larger airways. When we compare our results with past studies of ciliogenesis in larger airways, the results are similar. For example, using TEM, Kawamata and Fujita (10) found the first signs of differentiating ciliated cells in the trachea of mice at 15 DGA. At 16 DGA, they reported seeing many ciliated cells. This is the same time point that we first observed cilia in the trachea and lobar bronchi. Declining ciliated cell surface density with declining airway size has been reported for adult humans when large bronchi, segmental bronchi, and bronchioles were compared (14). Pack et al. found that adult mice had a ciliated cell density of 39% in the trachea (19) and 36% in the lobar bronchi (19). The greater ciliated cell surface density (i.e. trachea 54.8%) found in this study compared to Pack et al. is probably due to strain differences. As far as we can tell, this is the first study to measure ciliated cell surface density in the terminal bronchioles of mice and the first to show that ciliogenesis continues well into the postnatal period.
Recently, transcription factors and regulatory proteins involved in the ciliogenesis of mouse lung have been identified (3,8,27). The primary intent of this study was to establish the temporal and spatial pattern by which ciliated cells differentiate in the airways, not the specific intra and extracellular steps by which a particular cell matures. However, the three-dimensional whole mount approach used in this study lends itself to site specific studies of protein expression and structural changes during airway development and cell differentiation. For example, it is not clear what role epithelial neural components or extracellular signaling molecules such as neurotransmitters play in the coordination of ciliary motion and the rate of ciliary beat frequency, especially during ciliated cell differentiation. The approach could also be applicable to studying factors dictating ciliated and noncilated cell distribution during airway development. We have successfully used the three-dimensional whole mount approach to evaluate the nerve density and distribution within the epithelial compartment of airways in infant rhesus monkeys exposed to oxidant air pollution and allergen (12). An added benefit of the three dimension whole mount approach is that the same preparations can be sliced and embedded for high resolution two-dimensional histopathology (20,25). Thus, basal lateral areas can be evaluated.
We feel that this study provides a healthy basis for investigations of neonatal pulmonary disease or pollutant toxicity affecting cilia and its functions. Future studies using the same whole mount technique could investigate the effect of viral/bacterial infection, inhaled pollutants, or particles on ciliogenesis. In conclusion, the study found that in the developing tracheobrochial airways of the mouse: 1) Ciliogenesis differs temporally and spatially by airway generation; 2) Ciliated cell surface density increases with age in all airway generations, but density decreases in a proximal to distal direction; and 3) A significant portion of ciliogenesis continues after birth.
Acknowledgments
This work was supported by NIEHS grants ES00628, ES06700, and ES05707. The authors would like to thank Dr. Michelle V. Fanucchi for providing mouse tissue and Dr. Laura S. Van Winkle for providing technical assistance.
References
- 1.Bankston PW, Porter GA, Milici AJ, Palade GE. Differential and specific labeling of epithelial and vascular endothelial cells of the rat lung by Lycopersicon esculentum and Griffonia simplicifolia I lectins. Eur J Cell Biol. 1991;54:187–195. [PubMed] [Google Scholar]
- 2.Bateman JR, Pavia D, Sheahan NF, Agnew JE, Clarke SW. Impaired tracheobronchial clearance in patients with mild stable asthma. Thorax. 1983;38:463–467. doi: 10.1136/thx.38.6.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blatt EN, Yan XH, Wuerffel MK, Hamilos DL, Brody SL. Forkhead transcription factor HFH-4 expression is temporally related to ciliogenesis. Am J Respir Cell Mol Biol. 1999;21:168–176. doi: 10.1165/ajrcmb.21.2.3691. [DOI] [PubMed] [Google Scholar]
- 4.Camner P, Mossberg B, Philipson K. Tracheobronchial clearance and chronic obstructive lung disease. Scand J Respir Dis. 1973;54:272–281. [PubMed] [Google Scholar]
- 5.Ferguson JL, McCaffrey TV, Kern EB, Martin WJ., 2nd The effects of sinus bacteria on human ciliated nasal epithelium in vitro. Otolaryngol Head Neck Surg. 1988;98:299–304. doi: 10.1177/019459988809800405. [DOI] [PubMed] [Google Scholar]
- 6.Glantz, S Primer of Biostatistics New York: McGraw-Hill, Inc., 1997, p. 1–473.
- 7.Heller RF, Gordon RE. Chronic effects of nitrogen dioxide on cilia in hamster bronchioles. Exp Lung Res. 1986;10:137–152. doi: 10.3109/01902148609061489. [DOI] [PubMed] [Google Scholar]
- 8.Huang T, You Y, Spoor MS, Richer EJ, Kudva VV, Paige RC, Seiler MP, Liebler JM, Zabner J, Plopper CG, Brody SL. Foxj1 is required for apical localization of ezrin in airway epithelial cells. J Cell Sci. 2003;116:4935–4945. doi: 10.1242/jcs.00830. [DOI] [PubMed] [Google Scholar]
- 9.Kanda T, Hilding D. Development of respiratory tract cilia in fetal rabbits. Electron microscopic investigation. Acta Otolaryngol. 1968;65:611–624. doi: 10.3109/00016486809119295. [DOI] [PubMed] [Google Scholar]
- 10.Kawamata S, Fujita H. Fine structural aspects of the development and aging of the tracheal epithelium of mice. Arch Histol Jpn. 1983;46:355–372. doi: 10.1679/aohc.46.355. [DOI] [PubMed] [Google Scholar]
- 11.Kienast K, Knorst M, Riechelmann H, Schellenberg J, Muller-Quernheim J, Ferlinz R. [In vitro studies of the beat frequency of ciliary cell cultures after short-term exposure to SO2 and NO2] Med Klin (Munich) 1993;88:520–524. [PubMed] [Google Scholar]
- 12.Larson SD, Schelegle ES, Walby WF, Gershwin LJ, Fanuccihi MV, Evans MJ, Joad JP, Tarkington BK, Hyde DM, Plopper CG. Postnatal remodeling of the neural components of the epithelial-mesenchymal trophic unit in the proximal airways of infant rhesus monkeys exposed to ozone and allergen. Toxicol Appl Pharmacol. 2004;194:211–220. doi: 10.1016/j.taap.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 13.Lucas A, Douglas L. Principles underlying ciliary activity in the respiratory tract. Acta Otolaryngol. 1934;20:518. [Google Scholar]
- 14.Mercer RR, Russell ML, Roggli VL, Crapo JD. Cell number and distribution in human and rat airways. Am J Respir Cell Mol Biol. 1994;10:613–624. doi: 10.1165/ajrcmb.10.6.8003339. [DOI] [PubMed] [Google Scholar]
- 15.Miller WF. Mucociliary transport in cystic fibrosis. N Engl J Med. 1973;288:1243. [PubMed] [Google Scholar]
- 16.Moscoso GJ, Driver M, Codd J, Whimster WF. The morphology of ciliogenesis in the developing fetal human respiratory epithelium. Pathol Res Pract. 1988;183:403–411. doi: 10.1016/S0344-0338(88)80086-4. [DOI] [PubMed] [Google Scholar]
- 17.Muller KM, Schmitz I. Chronic bronchitis--alterations of the bronchial mucosa. Wiad Lek. 1997;50:252–266. [PubMed] [Google Scholar]
- 18.Oliveira MJ, Pereira AS, Guimaraes L, Grande NR, de Sa CM, Aguas AP. Zonation of ciliated cells on the epithelium of the rat trachea. Lung. 2003;181:275–282. doi: 10.1007/s00408-003-1030-1. [DOI] [PubMed] [Google Scholar]
- 19.Pack RJ, Al-Ugaily LH, Morris G, Widdicombe JG. The distribution and structure of cells in the tracheal epithelium of the mouse. Cell Tissue Res. 1980;208:65–84. doi: 10.1007/BF00234174. [DOI] [PubMed] [Google Scholar]
- 20.Postlethwait EM, Joad JP, Hyde DM, Schelegle ES, Bric JM, Weir AJ, Putney LF, Wong VJ, Velsor LW, Plopper CG. Three-dimensional mapping of ozone-induced acute cytotoxicity in tracheobronchial airways of isolated perfused rat lung. Am J Respir Cell Mol Biol. 2000;22:191–199. doi: 10.1165/ajrcmb.22.2.3674. [DOI] [PubMed] [Google Scholar]
- 21.Schwartz J. Air pollution and children's health. Pediatrics. 2004;113:1037–1043. [PubMed] [Google Scholar]
- 22.Schwartz LW, Dungworth DL, Mustafa MG, Tarkington BK, Tyler WS. Pulmonary responses of rats to ambient levels of ozone: effects of 7-day intermittent or continuous exposure. Lab Invest. 1976;34:565–578. [PubMed] [Google Scholar]
- 23.Toskala E, Nuutinen J, Rautiainen M, Torkkeli T. The correlation of mucociliary transport and scanning electron microscopy of nasal mucosa. Acta Otolaryngol. 1995;115:61–65. doi: 10.3109/00016489509133348. [DOI] [PubMed] [Google Scholar]
- 24.Toskala E, Rautiainen M. Electron microscopy assessment of the recovery of sinus mucosa after sinus surgery. Acta Otolaryngol. 2003;123:954–959. doi: 10.1080/00016480310005110. [DOI] [PubMed] [Google Scholar]
- 25.Van Winkle LS, Johnson ZA, Nishio SJ, Brown CD, Plopper CG. Early events in naphthalene-induced acute Clara cell toxicity: comparison of membrane permeability and ultrastructure. Am J Respir Cell Mol Biol. 1999;21:44–53. doi: 10.1165/ajrcmb.21.1.3630. [DOI] [PubMed] [Google Scholar]
- 26.Veerman AJ, van der Baan S, Den Hollander W. [Disorders in mucociliary transport. Primary ciliary dyskinesia] Tijdschr Kindergeneeskd. 1983;51:185–192. [PubMed] [Google Scholar]
- 27.You Y, Huang T, Richer EJ, Schmidt JE, Zabner J, Borok Z, Brody SL. Role of f-box factor foxj1 in differentiation of ciliated airway epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2004;286:L650–657. doi: 10.1152/ajplung.00170.2003. [DOI] [PubMed] [Google Scholar]