Rapid Detection of Clostridium difficile in Feces by Real-Time PCR (original) (raw)
Abstract
Clostridium difficile is the major causative agent of nosocomial antibiotic-associated diarrhea, colitis, and pseudomembranous colitis. The pathogenicity of C. difficile is closely related to the production of toxins A and B. Toxigenic C. difficile detection by a tissue culture cytotoxin assay is often considered the “gold standard.” However, this assay is time consuming, as it implies an incubation period of at least 24 h. We have developed a rapid real-time fluorescence-based multiplex PCR assay targeting the C. difficile toxin genes tcdA and tcdB, with the Smart Cycler. Two molecular beacons bearing different fluorophores were used as internal probes specific for each amplicon type. The analytical sensitivity of the assay was around 10 genome copies for all nine C. difficile strains tested, representing the 6 most common toxinotypes. The specificity was demonstrated by the absence of amplification with DNA purified from bacterial species other than C. difficile (n = 14), including Clostridium sordellii for which the lethal toxin gene sequence is closely related to the toxin genes of C. difficile. Following a rapid (15 min) and simple fecal sample preparation protocol, both tcdA and tcdB were efficiently amplified from 28 of 29 cytotoxin-positive feces samples. There was no amplification observed with all 27 cytotoxin-negative feces samples tested. This is the first real-time PCR assay for the detection of C. difficile. It is rapid, sensitive, and specific and allows detection of C. difficile directly from feces samples.
Clostridium difficile, the main etiological agent of antibiotic-associated diarrhea and pseudomembranous colitis, is also the major recognized cause of nosocomial diarrhea (5, 27). The use of antibiotics such as clindamycin, cephalosporins, and ampicillin disrupts the normal intestinal flora, predisposing patients to colonization by C. difficile, which is encountered mainly in health care centers (13, 21, 28, 34). This organism is carried asymptomatically in about 50% of neonates and 20% of hospitalized patients but only in 2% of healthy adults (20, 26). In fact, asymptomatic carriers usually outnumber symptomatic patients (14). Therefore, the high level of healthy carriers among hospitalized patients coupled with the presence of patients under antibiotic treatment explains the high rate of nosocomial diarrhea associated with C. difficile.
The pathogenicity of this organism is associated with the production of two large toxins, toxin A (TcdA) and toxin B (TcdB), both implicated in mucosal damage (21). Nontoxigenic strains are not pathogenic. Most strains produce both toxins, but pathogenic strains of C. difficile producing toxin B only have been reported (16, 17, 29, 31). Variability in the toxin genes of C. difficile has been investigated by Rupnik et al. (32, 33) by using restriction fragment length polymorphism analysis. The majority of strains studied had the same toxinotype as the reference strain VPI 10463 (toxinotype 0), whereas approximately 8% of the strains were variants distributed in toxinotypes I to XV.
The detection of toxin B by the tissue culture cytotoxicity assay is considered to be the “gold standard” for the detection of C. difficile from fecal samples (9, 20). This assay is efficient but still requires 24 to 48 h for completion. Enzyme immunoassays (EIA) are also commonly used for toxin detection. However, these EIA are not as sensitive as the cytotoxicity assay (8). A simple, rapid (≤1 h), and specific diagnostic test would be helpful to the clinician for the prescription of an efficient treatment at the beginning of the infection to avoid complications. We report here the development of the first real-time PCR assay for the rapid detection of all toxigenic C. difficile strains directly from feces samples. This assay is based on the amplification of the genes encoding toxins A and B, the major virulence factors of this bacterial species.
MATERIALS AND METHODS
Bacterial strains.
Bacterial strains were obtained from the American Type Culture Collection (Manassas, Va.) and from Michel Delmée (Université Catholique de Louvain, Brussels, Belgium). Nine characterized C. difficile strains from various origins were used in this study (Table 1). Four strains are classified as toxinotype 0, whereas the others are representatives of the most frequent variant toxinotypes (I, III, IV, VI, and VIII) (32). The specificity of the PCR-based assay was demonstrated by using 14 non-C. difficile strains, which comprise 13 Clostridium species (Table 1).
TABLE 1.
Bacterial strains used in this study
| Species (strain no.) | Toxinotype | Tcd production | PCR detection |
|---|---|---|---|
| Clostridium difficile (ATCC 9689) | 0 | TcdA and TcdB | tcdA and tcdB |
| Clostridium difficile (VPI 10463)a | 0 | TcdA and TcdB | tcdA and tcdB |
| Clostridium difficile (MD 32275)a | 0 | TcdA and TcdB | tcdA and tcdB |
| Clostridium difficile (MD 56484)a | 0 | TcdA and TcdB | tcdA and tcdB |
| Clostridium difficile (MD EX 623)a | I | TcdA and TcdB | tcdA and tcdB |
| Clostridium difficile (MD SE 847)a | III | TcdA and TcdB | tcdB |
| Clostridium difficile (MD 55767)a | IV | TcdA and TcdB | tcdB |
| Clostridium difficile (MD 51377)a | VI | TcdA and TcdB | tcdB |
| Clostridium difficile (MD 20376)a | VIII | TcdB | tcdA and tcdB |
| Clostridium beijerinckii (ATCC 8260) | NAb | −c | − |
| Clostridium bifermentans (ATCC 638) | NA | − | − |
| Clostridium botulinum (CHUL 20:1.2) | NA | − | − |
| Clostridium histolyticum (ATCC 19401) | NA | − | − |
| Clostridium innocuum (ATCC 14501) | NA | − | − |
| Clostridium novyi (ATCC 19402) | NA | − | − |
| Clostridium perfringens (ATCC 13124) | NA | − | − |
| Clostridium ramosum (ATCC 25582) | NA | − | − |
| Clostridium septicum (ATCC 12464) | NA | − | − |
| Clostridium sordellii (ATCC 9714) | NA | − | − |
| Clostridium sphenoides (ATCC 19403) | NA | − | − |
| Clostridium tertium (ATCC 14573) | NA | − | − |
| Clostridium tetani (ATCC 19406) | NA | − | − |
| Escherichia coli (ATCC 11775) | NA | − | − |
Stool specimens.
A total of 56 consecutive unformed or liquid feces samples were obtained from _C. difficile_-infected and noninfected patients. These samples were submitted by physicians to the microbiology laboratory for C. difficile testing by the cytotoxicity assay. All stool specimens were stored at 4°C and tested within 72 h of receipt.
Cytotoxicity assay.
Detection of C. difficile in fecal samples was made by using the cytotoxicity assay on Vero cells. The presence of C. difficile was detected by the characteristic round-up (asteroid-like) of the cultivated Vero cells due to the cytotoxicity of toxin B in the fecal filtrate. The specificity of the assay was demonstrated by neutralization of this toxin by the appropriate antitoxin (Bartels Inc., Issaquah, Wash.).
DNA extraction and sample preparation.
Genomic DNA was purified from the strains listed in Table 1 by using the G NOME DNA kit (Qbiogene Inc., Carlsbad, Calif.). Two types of fecal samples were prepared. First, in order to evaluate the analytical sensitivity of the assay, two noncytotoxic feces samples were spiked with 10-fold dilutions of C. difficile ATCC 9689 culture in the exponential growth phase, to obtain concentrations of 107 to 103 CFU per g of feces. Next, 75 μl of liquid stool sample, or the same volume of a suspension of unformed stool specimen, was prepared for PCR by using a rapid (15 min) DNA extraction kit [Infectio Diagnostic (I.D.I.) Inc., Sainte-Foy, Québec, Canada] (19).
Oligonucleotides.
The tcdA and tcdB C. difficile toxin gene sequences available from public databases were analyzed with the GCG Wisconsin package (version 10; Accelrys, San Diego, Calif.). Multiple-sequence alignments revealed conserved and specific regions for both genes. Conserved regions were chosen to design two PCR primer pairs and molecular beacon probes (35) with the help of the Oligo primer analysis software version 5.0 (National Biosciences, Plymouth, Minn.) and the DNA fold program (M. Zuker, http://www.bioinfo.rpi.edu/applications/mfold/old/dna/). The stem of each molecular beacon was formed by joining a 5-nucleotide complementary sequence at each end of the probe sequence. To avoid nonspecific fluorescence emission, the stem sequence was designed to ensure that the molecular beacons adopt a hairpin structure at the optimal annealing temperature of the PCR when no target amplicons are present. Oligonucleotide primers were synthesized by using a model 391 DNA synthesizer (Perkin-Elmer Corp., Applied Biosystems Division, Mississauga, Ontario, Canada). Molecular beacons were synthesized by Biosearch Technologies (Novato, Calif.). The PCR primers and fluorescent probes used in this study are listed in Table 2.
TABLE 2.
Oligonucleotides used in this study
| Primer or probe | Target gene | Oligonucleotide sequence (5′ → 3′)a | Amplicon size (bp) |
|---|---|---|---|
| Primers | |||
| tcdA441 | tcdA | TCT ACC ACT GAA GCA TTA C | 158 |
| tcdA579 | TAG GTA CTG TAG GTT TAT TG | ||
| tcdB2667 | tcdB | ATA TCA GAG ACT GAT GAG | 101 |
| tcdB2746 | TAG CAT ATT CAG AGA ATA TTG T | ||
| Probesb | |||
| tcdAB1FAM | tcdA | CAC GCG GAT TTT GAA TCT CTT CCT CTA GTA GCG CGT G | |
| tcdBB2TET | tcdB | CAC GCC TGG AGA ATC TAT ATT TGT AGA AAC TGG CGT G |
PCR amplification.
Amplification was performed either from purified genomic DNA or from crude DNA extracted from feces samples. PCR conditions and reagent concentrations were optimized to obtain the final parameters described hereafter. Multiplex amplification was carried out by using 0.7 μM (each) primers tcdA442 and tcdA579, 0.45 μM (each) primers tcdB2667 and tcdB2746, 0.3 μM concentrations of each molecular beacon, 8 mM MgCl2, 12.25 μg of bovine serum albumin, 0.2 mM concentrations of each of the four deoxynucleoside triphosphates (Amersham Biosciences, Uppsala, Sweden), 50 mM Tris-HCl, 16 mM (NH4)2SO4, 2.5 U of KlenTaq DNA polymerase (AB Peptides, St. Louis, Mo.) combined with TaqStart antibody (BD Biosciences Clontech, Palo Alto, Calif.), and 1 μl of purified genomic DNA or 1.5 μl of DNA prepared from a fecal sample, all in a final volume of 26.5 μl. The PCR amplification (180 s at 94°C and then 45 cycles of three steps consisting of 10 s at 94°C, 15 s at 57°C, and 7 s at 72°C) was performed with a Smart Cycler thermal cycler (Cepheid, Sunnyvale, Calif.). This rapid PCR cycling protocol required approximately 45 min. The presence of amplified products was confirmed when the fluorescent signal exceeded an automatic noise-based defined threshold.
Sensitivity and specificity tests.
Specificity of the PCR assay was tested by using 1 ng (representing ≈2 × 105 genome copies per PCR) of genomic DNA purified from the 14 non-C. difficile strains. The analytical sensitivity of the assay was determined by using twofold dilutions of genomic DNA purified from the nine C. difficile strains. Detection of the PCR products was made in real-time by measuring the fluorescent signal emitted by the molecular beacon when it hybridizes to its target at the end of each annealing step. A control reaction was used to monitor PCR inhibition by each of the fecal samples tested. Each amplification control reaction was carried out with a separate reaction mixture with 100 copies of a linearized recombinant plasmid that was previously constructed for that purpose (19). This plasmid template was amplified as previously described (19) in the presence of 1.5 μl of fecal sample.
RESULTS
Specificity and analytical sensitivity of the PCR assay.
Sensitivity tests demonstrated that the assay efficiently detected tcdA from all nine C. difficile strains tested (Table 1). The detection limit was around 10 genome copies per PCR for these nine strains. However, for three strains representing variant toxinotypes (i.e., III, IV, and VI), tcdB was not detected by the PCR assay. All 14 non-C. difficile strains tested showed no amplification signal, thereby demonstrating the specificity of the PCR assay. Clostridium sordellii, which carries a closely related lethal toxin gene (22), was not detected.
Analytical sensitivity with spiked fecal samples.
The PCR assay was then used to detect C. difficile in fecal samples spiked with different concentrations of target bacteria. Following the rapid (15 min) sample preparation protocol, C. difficile was detected in two spiked fecal samples. The detection limit with these two samples was found to be around 5 × 104 CFU per g of feces.
Detection of C. difficile in feces from infected patients.
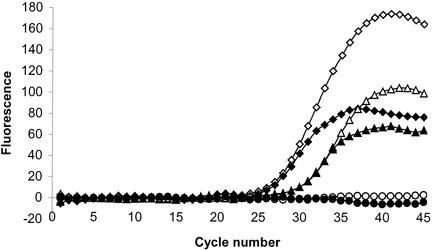
To evaluate the applicability of the assay to detect C. difficile in infected patients, 56 fecal samples collected from hospitalized patients were tested. All of these samples were either unformed or of liquid consistency, as recommended for C. difficile testing (2, 14). Of these 56 feces samples, 29 were positive for C. difficile by the conventional cytotoxicity test. Of these 29 cytotoxic samples, 28 were also positive by PCR. The 27 other samples were negative with both techniques. All PCR-positive samples were positive for the detection of both tcdA and tcdB. The cycle threshold for the PCR-positive samples ranged from around 22 to 35, thereby showing a wide variation in the bacterial load of toxigenic C. difficile in feces from colonized patients (Fig. 1). The only discordant feces sample was of different consistency, not as homogenous as the other samples tested, with distinctive large solid particles. The amplification control of the PCR did not show any significant inhibition by any of the samples tested. Overall, this PCR assay showed (i) a specificity and a positive predictive value of 100% (for specificity, 27 samples negative by both cytotoxicity assay and PCR/27 samples negative by the cytotoxicity assay; for the positive predictive value, 28 samples positive by both cytotoxicity assay and PCR/28 samples positive by PCR), (ii) a sensitivity of 97% (28 samples positive by both cytotoxicity assay and PCR/29 samples positive by the cytotoxicity assay), and (iii) a negative predictive value of 96% (27 samples negative by both cytotoxicity assay and PCR/28 samples negative by PCR) when compared to the standard cytotoxicity assay.
FIG. 1.
Example of real-time detection of C. difficile in feces from patients by multiplex amplification of tcdA (open symbols) and tcdB (filled symbols) genes. Fecal samples 1 (diamonds) and 2 (triangles) are positive for both tcdA and tcdB genes while sample 3 (circles) is negative. Sample 1 has a higher bacterial load of toxigenic C. difficile (cycle threshold, ≈25) than sample 2 (cycle threshold, ≈29).
DISCUSSION
C. difficile is the major cause of antibiotic-associated diarrhea as well as nosocomial diarrhea. The use of the appropriate antibiotic therapy is crucial to prevent the progression of C. difficile pathogenesis. Thus, the rapid diagnosis of this pathogen is decisive in allowing clinicians to prescribe the appropriate therapy. Several diagnostic tools for the detection of this pathogen in clinical microbiology laboratories are available. Although widely used and very rapid, immunoassays lack sensitivity (4, 10). The tissue culture cytotoxicity assay is considered the gold standard, since it is specific and highly sensitive (8, 30). However, this assay is time consuming as it requires an incubation period of 24 to 48 h; moreover, it detects only toxin B.
The most important advantage of PCR in the clinical microbiology field is the rapidity that it offers for pathogen diagnosis. To date, some PCR assays have been developed for the specific detection of C. difficile in feces samples, but they are all coupled with time-consuming post-PCR manipulations for analysis of the amplification products, i.e., agarose gel electrophoresis and Southern hybridization (3, 11, 12, 18, 23, 36). Moreover, most of these assays target only one of the two C. difficile toxin genes, potentially missing strains carrying only one of them. Thus, we developed a real-time PCR assay for the rapid detection of all toxigenic C. difficile strains, relying on the direct detection of tcdA and tcdB gene sequences from fecal samples. By combining a rapid sample preparation protocol and real-time PCR technology, this assay is much more rapid than all _C. difficile_-specific PCR assays previously described. In addition, the analytical sensitivity of the assay is excellent, as the detection limit obtained with spiked fecal samples was 5 × 104 CFU/g of stools. Our assay is thus 100-fold more sensitive than the PCR method described by Guilbault et al. (11).
The hands-on technologist time (approximately 15 to 20 min) is similar in all types of detection assays used for C. difficile (cytotoxicity test, EIA, or real-time PCR) (1). However, on average, the cytotoxicity assay takes 24 to 48 h for completion, whereas EIA and real-time PCR are completed in approximately 1 h. EIA are prone to a lack of sensitivity (ranging from 54 to 76%) when compared to the gold standard cytotoxicity assay (4, 10). Thus, the higher cost of a PCR assay is well justified by the savings realized once a rapid and accurate diagnosis is obtained: procedures such as patient isolation, empirical antibiotic therapy, and longer hospitalization time are all avoided (7, 25).
Moreover, the specificity of this assay in comparison to conventional PCR assays was enhanced by the use of molecular beacons as fluorescent internal probes for real-time detection. The ubiquity (i.e., the ability to detect all toxigenic C. difficile strains) (7) of the assay was demonstrated by efficient amplification of genomic DNA purified from nine C. difficile strains from diverse geographical origins (Table 1). These strains included four strains of the predominant toxinotype 0 and the five most common variant toxinotypes (I, III, IV, VI, and VIII) (32). Even if tcdB was not detected by our assay in toxinotypes III, IV, and VI, the fact that tcdA was detected for these toxinotypes confirmed the identification of all toxinogenic C. difficile strains tested. It should also be noted that variant toxinotypes III, IV, and VI represent less than 3% of the C. difficile toxinotypes (32). One advantage of our PCR assay over conventional clinical testing or the other PCR assays described to date is that both toxin genes are targeted. Some toxin-A-negative, toxin-B-positive strains of C. difficile causing serious disease have been reported (15), and those strains are undetectable in clinical laboratories that use only toxin A immunoassays for C. difficile testing.
DNA sequencing of a portion of the tcdB gene was performed in order to investigate further the genetic variability of toxinotypes III, IV, and VI. A fragment of the tcdB gene was amplified from purified genomic DNA extracted from strains MD 35004, MD 55767, and MD 51377 by using primers B1C and B2N as previously described (32). An additional primer, tcdBseq2438 (5′ TTG AAC TAG AAG AAA AAG TAA TGT TA 3′) was designed to sequence both strands of the region amplified by our PCR assay. Nucleotide polymorphisms were observed at positions corresponding to the last nucleotide at the 3′ end of primers tcdB2667 and tcdB2746 (data not shown). Mismatches with the 3′ end of primers are known to drastically reduce amplification of the desired target. As more tcdA and tcdB sequence information will become available from other C. difficile toxinotypes, minor modifications to the primers used in our detection assay will ensure that all variants are detected. Also, the absence of amplification observed with DNA from non-C. difficile strains demonstrated that the assay is specific for the detection of C. difficile. Furthermore, the assay was sensitive, as it allowed the detection of around 10 genome copies for all nine C. difficile strains tested.
One of the major challenges in optimizing an assay for the detection of C. difficile in feces samples is overcoming the PCR-inhibitory components found in feces. Following a rapid (10 min) sample preparation protocol, we were able to directly detect by PCR C. difficile from fecal samples obtained from infected patients. Thus, from 29 cytotoxicity assay-positive samples, 28 were detected by PCR, whereas none of the 27 cytotoxicity assay-negative samples were detected, demonstrating the sensitivity and specificity of the assay. A possible explanation for the discordant result could be a false-positive result with the cytotoxicity assay, as the antitoxin control can also neutralize the C. sordellii lethal toxin (22). It could also be a DNA extraction failure from this distinctive sample, as it was of a different consistency and not as homogenous as the other samples. Another possibility is that we encountered a strain in which variant toxin genes were present, with mutations and/or deletions in the regions corresponding to our PCR primers and molecular beacons. However, we cannot rule out at this moment a lack of sensitivity of the DNA-based assay compared with the highly sensitive cytotoxicity assay. For example, in a healing patient where the C. difficile cells are present in reduced numbers, toxin levels could still remain at a level detectable only by the cytotoxicity assay. This situation may also be encountered in a patient infected with a C. difficile strain producing high levels of toxin, leading to high toxin concentrations despite a low bacterial load (37). All PCR results were obtained within 1 h from the initiation of sample processing, much more rapidly than the conventional cytotoxicity assay, which takes at least 24 h to complete.
We found that _C. difficile_-infected samples presented a broad range of bacterial loads, as shown by cycle thresholds ranging from 22 to 35. These cycle thresholds correspond to a range of approximately 107 to 104 CFU per g, based on testing with spiked fecal samples. This situation contrasts with another assay previously developed in our laboratory for the detection of Shiga toxin-producing Escherichia coli (6) which showed that most feces samples had similar bacterial loads (a cycle threshold of around 25).
The possibility that some of the patients for which the samples were weakly positive by PCR (i.e., a cycle threshold of ≈35 cycles) were having diarrheal symptoms of another etiology cannot be excluded. Indeed, these patients may have been asymptomatic carriers of C. difficile, as the proportion of asymptomatic hospitalized patients is about 20% (28). To further complicate the picture, it appears that the antibody response against toxin A can protect against C. difficile diarrhea (24). The clinical significance of samples weakly positive for C. difficile either by the toxin B cytotoxic assay or by a DNA-based assay is therefore debatable and will require further studies.
In summary, our results demonstrate that this 1-h real-time PCR assay targeting both C. difficile toxin genes tcdA and tcdB is quite promising for direct detection of this pathogen from fecal samples. To our knowledge, this is the first real-time PCR assay for the detection of C. difficile directly from feces. This new diagnostic tool may improve the management of C. difficile infection and may lead to a more rational use of antibiotics, as the clinicians will rapidly obtain the clinical microbiology results. However, a large-scale clinical trial is needed to further validate this assay.
Acknowledgments
This study was supported by Infectio Diagnostic (I.D.I.) Inc. and by grant PA-15586 from the Medical Research Council of Canada. S.D.B. received a studentship from the FCAR-FRSQ Santé Fund.
We thank Louise Côté and Nathalie Boucher from the microbiology laboratory of CHUQ, Pavillon CHUL, for providing the clinical samples. We thank Michel Delmée for providing bacterial strains.
REFERENCES
- 1.Alfa, M. J., B. Swan, P. VanDekerkhove, P. Pang, and G. K. M. Harding. 2002. The diagnosis of _Clostridium difficile_-associated diarrhea: comparison of triage C. difficile panel, EIA for Tox A/B and cytotoxin assays. Diagn. Microbiol. Infect. Dis. 43**:**257-263. [DOI] [PubMed] [Google Scholar]
- 2.Allen, S. D., C. L. Emery, and J. A. Siders. 1999. Clostridium, p. 654-671. In P. R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.), Manual of clinical microbiology, 7th ed. American Society for Microbiology, Washington, D.C.
- 3.Arzese, A., G. Trani, L. Riul, and G. A. Botta. 1995. Rapid polymerase chain reaction method for specific detection of toxigenic Clostridium difficile. Eur. J. Clin. Microbiol. Infect. Dis. 14**:**716-719. [DOI] [PubMed] [Google Scholar]
- 4.Barbut, F., C. Kajzer, N. Planas, and J.-C. Petit. 1993. Comparison of three enzyme immunoassays, a cytotoxicity assay, and toxigenic culture for diagnosis of _Clostridium difficile_-associated diarrhea. J. Clin. Microbiol. 31**:**963-967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartlett, J. G. 1990. Clostridium difficile: Clinical considerations. Rev. Infect. Dis. 12(Suppl. 2)**:**S243-S251. [DOI] [PubMed]
- 6.Bélanger, S. D., M. Boissinot, C. Ménard, F. J. Picard, and M. G. Bergeron. 2002. Rapid detection of Shiga toxin-producing bacteria in feces by multiplex PCR with molecular beacons on the Smart Cycler. J. Clin. Microbiol. 40**:**1436-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boissinot, M., and M. G. Bergeron. 2002. Toward rapid real-time molecular diagnostic to guide smart use of antimicrobials. Curr. Opin. Microbiol. 5**:**478-482. [DOI] [PubMed] [Google Scholar]
- 8.Cleary, R. K. 1998. _Clostridium difficile_-associated diarrhea and colitis: clinical manifestations, diagnosis, and treatment. Dis. Colon Rectum 41**:**1435-1449. [DOI] [PubMed] [Google Scholar]
- 9.Delmée, M. 2001. Laboratory diagnosis of Clostridium difficile disease. Clin. Microbiol. Infect. 7**:**411-416. [DOI] [PubMed] [Google Scholar]
- 10.Fedorko, D. P., E. D. Engler, E. M. O'Shaughnessy, E. C. Williams, C. J. Reichelderfer, and W. I. Smith, Jr. 1999. Evaluation of two rapid assays for detection of Clostridium difficile toxin A in stool specimens. J. Clin. Microbiol. 37**:**3044-3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guilbault, C., A.-C. Labbé, L. Poirier, L. Busque, C. Béliveau, and M. Laverdière. 2002. Development and evaluation of a PCR method for detection of the Clostridium difficile toxin B gene in stool specimens. J. Clin. Microbiol. 40**:**2288-2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gumerlock, P. H., Y. J. Tang, J. B. Weiss, and J. Silva, Jr. 1993. Specific detection of toxigenic strains of Clostridium difficile in stool specimens. J. Clin. Microbiol. 31**:**507-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson, S., C. R. Clabots, F. V. Linn, M. M. Olson, L. R. Peterson, and D. N. Gerding. 1990. Nosocomial Clostridium difficile colonisation and disease. Lancet 336**:**97-100. [DOI] [PubMed] [Google Scholar]
- 14.Johnson, S., and D. N. Gerding. 1998. _Clostridium difficile_-associated diarrhea. Clin. Infect. Dis. 26**:**1027-1034. [DOI] [PubMed] [Google Scholar]
- 15.Johnson, S., S. A. Kent, K. J. O'Leary, M. M. Merrigan, S. P. Sambol, L. R. Peterson, and D. N. Gerding. 2001. Fatal pseudomembranous colitis associated with a variant Clostridium difficile strain not detected by toxin A immunoassay. Ann. Intern. Med. 135**:**434-438. [DOI] [PubMed] [Google Scholar]
- 16.Kader, H. A., D. A. Piccoli, A. F. Jawad, K. L. McGowan, and E. S. Maller. 1998. Single toxin detection is inadequate to diagnose Clostridium difficile diarrhea in pediatric patients. Gastroenterology 115**:**1329-1334. [DOI] [PubMed] [Google Scholar]
- 17.Kato, H., N. Kato, K. Watanabe, N. Iwai, H. Nakamura, T. Yamamoto, K. Suzuki, S. M. Kim, Y. Chong, and E. B. Wasito. 1998. Identification of toxin A-negative, toxin B-positive Clostridium difficile by PCR. J. Clin. Microbiol. 36**:**2178-2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kato, N., C. Y. Ou, H. Kato, S. L. Bartley, C. C. Luo, G. E. Killgore, and K. Ueno. 1993. Detection of toxigenic Clostridium difficile in stool specimens by the polymerase chain reaction. J. Infect. Dis. 167**:**455-458. [DOI] [PubMed] [Google Scholar]
- 19.Ke, D., C. Ménard, F. J. Picard, M. Boissinot, M. Ouellette, P. H. Roy, and M. G. Bergeron. 2000. Development of conventional and real-time PCR assays for the rapid detection of group B streptococci. Clin. Chem. 46**:**324-331. [PubMed] [Google Scholar]
- 20.Kelly, C. P., and J. T. LaMont. 1998. Clostridium difficile infection. Annu. Rev. Med. 49**:**375-390. [DOI] [PubMed] [Google Scholar]
- 21.Kelly, C. P., C. Pothoulakis, and J. T. LaMont. 1994. Clostridium difficile colitis. N. Engl. J. Med. 330**:**257-262. [DOI] [PubMed] [Google Scholar]
- 22.Knoop, F. C., M. Owens, and I. C. Crocker. 1993. Clostridium difficile: clinical disease and diagnosis. Clin. Microbiol. Rev. 6**:**251-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuhl, S. J., Y. J. Tang, L. Navarro, P. H. Gumerlock, and J. Silva, Jr. 1993. Diagnosis and monitoring of Clostridium difficile infections with the polymerase chain reaction. Clin. Infect. Dis. 16(Suppl. 4)**:**S234-S238. [DOI] [PubMed]
- 24.Kyne, L., M. Warny, A. Qamar, and C. P. Kelly. 2001. Association between antibody response to toxin A and protection against recurrent Clostridium difficile diarrhoea. Lancet 357**:**189-193. [DOI] [PubMed] [Google Scholar]
- 25.Landry, M. L., J. Topal, D. Ferguson, D. Giudetti, and Y. Tang. 2001. Evaluation of biosite triage Clostridium difficile panel for rapid detection of Clostridium difficile in stool samples. J. Clin. Microbiol. 39**:**1855-1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Larson, H. E., F. E. Barclay, P. Honour, and I. D. Hill. 1982. Epidemiology of Clostridium difficile in infants. J. Infect. Dis. 146**:**727-733. [DOI] [PubMed] [Google Scholar]
- 27.Lyerly, D. M., H. C. Krivan, and T. D. Wilkins. 1988. Clostridium difficile: its disease and toxins. Clin. Microbiol. Rev. 1**:**1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McFarland, L. V., M. E. Mulligan, R. Y. Kwok, and W. E. Stamm. 1989. Nosocomial acquisition of Clostridium difficile infection. N. Engl. J. Med. 320**:**204-210. [DOI] [PubMed] [Google Scholar]
- 29.Moncrief, J. S., L. Zheng, L. M. Neville, and D. M. Lyerly. 2000. Genetic characterization of toxin A-negative, toxin B-positive Clostridium difficile isolates by PCR. J. Clin. Microbiol. 38**:**3072-3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Connor, D., P. Hynes, M. Cormican, E. Collins, G. Corbett-Feeney, and M. Cassidy. 2001. Evaluation of methods for detection of toxins in specimens of feces submitted for diagnosis of _Clostridium difficile_-associated diarrhea. J. Clin. Microbiol. 39**:**2846-2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rupnik, M. 2001. How to detect Clostridium difficile variant strains in a routine laboratory. Clin. Microbiol. Infect. 7**:**417-420. [DOI] [PubMed] [Google Scholar]
- 32.Rupnik, M., V. Avesani, M. Janc, C. von Eichel-Streiber, and M. Delmée. 1998. A novel toxinotyping scheme and correlation of toxinotypes with serogroups of Clostridium difficile isolates. J. Clin. Microbiol. 36**:**2240-2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rupnik, M., J. S. Brazier, B. I. Duerden, M. Grabnar, and S. L. J. Stubbs. 2001. Comparison of toxinotyping and PCR ribotyping of Clostridium difficile strains and description of novel toxinotypes. Microbiology 147**:**439-447. [DOI] [PubMed] [Google Scholar]
- 34.Spencer, R. C. 1998. The role of antimicrobial agents in the aetiology of _Clostridium difficile_-associated disease. J. Antimicrob. Chemother. 41(Suppl. C)**:**21-27. [DOI] [PubMed] [Google Scholar]
- 35.Tyagi, S., and F. R. Kramer. 1996. Molecular beacons: Probes that fluoresce upon hybridization. Nat. Biotechnol. 14**:**303-308. [DOI] [PubMed] [Google Scholar]
- 36.Wolfhagen, M. J., A. C. Fluit, R. Torensma, M. J. Poppelier, and J. Verhoef. 1994. Rapid detection of toxigenic Clostridium difficile in fecal samples by magnetic immuno PCR assay. J. Clin. Microbiol. 32**:**1629-1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wren, B., S. R. Heard, and S. Tabaqchali. 1987. Association between production of toxins A and B and types of Clostridium difficile. J. Clin. Pathol. 40**:**1397-1401. [DOI] [PMC free article] [PubMed] [Google Scholar]