Role of CCL5 (RANTES) in Viral Lung Disease (original) (raw)
Abstract
CCL5/RANTES is a key proinflammatory chemokine produced by virus-infected epithelial cells and present in respiratory secretions of asthmatics. To examine the role of CCL5 in viral lung disease, we measured its production during primary respiratory syncytial virus (RSV) infection and during secondary infection after sensitizing vaccination that induces Th2-mediated eosinophilia. A first peak of CCL5 mRNA and protein production was seen at 18 to 24 h of RSV infection, before significant lymphocyte recruitment occurred. Treatment in vivo with Met-RANTES (a competitive chemokine receptor blocker) throughout primary infection decreased CD4+ and CD8+ cell recruitment and increased viral replication. In RSV-infected, sensitized mice with eosinophilic disease, CCL5 production was further augmented; Met-RANTES treatment again reduced inflammatory cell recruitment and local cytokine production. A second wave of CCL5 production occurred on day 7, attributable to newly recruited T cells. Paradoxically, mice treated with Met-RANTES during primary infection demonstrated increased cellular infiltration during reinfection. We therefore show that RSV induces CCL5 production in the lung and this causes the recruitment of RSV-specific cells, including those making additional CCL5. If this action is blocked with Met-RANTES, inflammation decreases and viral clearance is delayed. However, the exact effects of chemokine modulation depend critically on time of administration, a factor that may potentially complicate the use of chemokine blockers in inflammatory diseases.
Bronchiolitis resulting from respiratory syncytial virus (RSV) infection is the single major cause of infant hospitalization in the developed world (25). It is characterized by excessive cell recruitment to the lung, leading to bronchiolar obstruction and sometimes ventilatory failure (24). RSV bronchiolitis is associated with the recurrent wheezing and asthma diagnosis in later childhood (33).
CCL5 (RANTES) is a potent chemoattractant cytokine that recruits monocytes, T cells, and eosinophils, acting via the receptors CCR1, CCR3, and CCR5 (30). Infection of respiratory epithelial cells with RSV causes upregulation of CCL5 secretion (21) by NF-κB translocation (39) and by increasing the stability of CCL5 mRNA (16), as does stimulation of epithelial cells with the Th1 cytokine gamma interferon (IFN-γ) (37). Children with RSV infections have increased CCL5 protein levels in both the upper and lower airway secretions, and levels of CCL5 in upper airway secretions correlate positively with disease severity (2, 9, 11, 36). In mice, CCL5 induction by RSV infection contributes to subsequent allergic pulmonary inflammation (14). CCL5 is a key chemokine in recruitment of CD8 T cells to the lung (6) and has been implicated in classical IFN-γ dominant Th1 responses, and yet it is also involved in eosinophilic disease driven by Th2 cells (7, 8, 17, 27).
In mice, RSV infection can prime for Th1- or Th2-biased T-cell populations that control infection but also enhance inflammation upon subsequent exposure to RSV, allowing us to examine situations in which polarized cytokine responses can be achieved in the context of identical viral challenge (24). In this system, we have recently shown that the pattern of chemokine release is directly affected by priming with individual RSV proteins (4).
To further explore the timing of CCL5 production, its cellular source, and the associated pattern of pathology, we used the inhibitory CCL5 analogue Met-RANTES (28) to block CCL5 activity in a mouse model of acute viral lung disease. To further investigate the role of CCL5 in Th2-biased immunopathology, we primed mice with RSV-G prior to RSV challenge. In the first 2 days of RSV infection, CCL5 protein was abundant in bronchoalveolar lavage fluid but not in the eluted cells by intracellular staining or by enzyme-linked immunospot (ELISPOT) assay; at 7 days this situation was reversed. Therefore, CCL5 and its coligands have potent time-dependent actions in viral lung disease, both in recruitment of inflammatory cells and in controlling virus infection.
MATERIALS AND METHODS
Virus stocks, mouse infection, and cell recovery.
RSV (strain A2) and recombinant vaccinia virus expressing RSV G protein (rVV-G) or β-galactosidase (rVV-βgal) was grown in HEp-2 cells (ATCC). UV inactivation of virus was performed in a UV Stratalinker (Stratagene) for 10 min.
Eight-week-old female BALB/c mice (Harlan Ltd., Veryan, United Kingdom) were maintained in pathogen-free conditions according to institutional and United Kingdom Home Office guidelines. All studies were reviewed and approved by the local institutional review committee. In some experiments, mice were primed to individual RSV proteins by scarification on the rump using 3 × 106 PFU of vaccinia virus, 2 weeks prior to RSV challenge. Mice were infected with 2 × 106 PFU RSV intranasally (i.n.) in 100 μl.
Bronchoalveolar lavage (BAL) fluids and lung tissues were harvested as described previously (12). Briefly, the lungs of each mouse were inflated six times with 1 ml of 12 mM lidocaine in Eagle’s minimal essential medium and BAL fluid kept on ice; 100 μl was centrifuged onto glass slides and stained with hematoxylin and eosin. The remainder was centrifuged, the supernatant retained at −80°C, and the pellet resuspended at 106 cells/ml. Lungs were homogenized by passage through 100-μm cell strainers (Falcon), red blood cells lysed in ammonium chloride buffer, and the remaining cells resuspended in RPMI medium with 10% fetal calf serum. Viable cell numbers were determined by trypan blue exclusion.
Met-RANTES.
Met-RANTES was produced as previously described. It was reconstituted from a lyophilized powder to 80 μg/ml in phosphate-buffered saline (PBS) and 200 μl given intravenously on each day of treatment, from days 0 to 7 during primary RSV infection and days 5 to 6 during RSV challenge following vaccinia sensitization.
Analysis of cell types.
Surface and intracellular staining was carried out as previously described (20). Cells (106/ml) were incubated with 50 ng/ml phorbol myristate acetate, 500 ng/ml ionomycin, and 10 μg/ml Brefeldin A for 4 h at 37°C. Cells were blocked with Fc block prior to being stained with fluorescently labeled antibody against surface markers for 30 min on ice and then fixed for 20 min at room temperature with 2% formaldehyde. Samples were permeabilized with 0.5% saponin in PBS (1% bovine serum albumin-0.1% azide) for 10 min. Anticytokine antibodies (anti-IFN-γ:BD and anti-CCL5:R&D) were added for a further 20 min at room temperature, washed with PBS (1% bovine serum albumin-0.1% azide), and analyzed on a Coulter EPICS Elite flow cytometer collecting data on at least 40,000 lymphocytes. All chemicals were from Sigma (Poole, United Kingdom), and all antibodies were from BD-Pharmingen (Oxford, United Kingdom) except where stated.
ELISPOT.
Assays were performed as described previously (23), with minor modifications. Briefly, plates were coated with 1 μg/ml of the appropriate anticytokine antibody in 0.1 M carbonate/bicarbonate buffer, pH 9.6, overnight at 4°C. One hundred microliters of cell suspension in doubling dilutions (from 105 to 1.25 × 104 cells) was added per well. For live RSV stimulation, 2 PFU RSV per cell was added and incubated overnight at 37°C. Controls of equivalent amounts of UV-inactivated RSV were also performed, as was mock stimulation by the addition of lysate from uninfected HEp-2 cells. Each well was then incubated with 100 μl of the appropriate biotinylated antibody: anti-CCL5 overnight at 4°C or anti-IFN-γ/interleukin 5 (IL-5) antibody for 2 to 4 h, followed by 100 μl of streptavidin-alkaline phosphatase at 1:1,000 in PBS for 2 h. Bound antibody was visualized with the alkaline phosphatase substrate 5-bromo-4-chloro-3-indolyl phosphate/Nitro Blue Tetrazolium.
CCL5 mRNA and protein quantification.
RNA was extracted from snap frozen whole lung using RNA-Stat-60 (Tel-Test, Inc.). CCL5 mRNA expression was determined by using the probe kit mCK5 (BD Biosciences). Probes were transcribed using T7 RNA polymerase and α-32P-labeled UTP (Amersham Pharmacia). Assays were carried out using the RNase protection assay III kit (Ambion) with analysis by polyacrylamide gel electrophoresis, and band intensity was assessed electronically (Storm PhosphorImager; Molecular Dynamics). Results were normalized to two housekeeping genes (L32 and glyceraldehyde phosphate-3-dehydrogenase genes). CCL5 enzyme-linked immunosorbent assay was performed with matched antibody pairs (R&D) according to the manufacturer's instructions.
Lung RSV titer.
Clearance of RSV was assessed in lung homogenates 4 days after virus challenge. Lungs were removed and homogenized. After 4 min of centrifugation at 4,000 × g, supernatants were titrated in doubling dilutions on HEp-2 cell monolayers in 96-well flat-bottom plates. Twenty-four hours later, monolayers were washed, fixed with methanol, and incubated with peroxidase-conjugated goat anti-RSV antibody (Biogenesis; Poole, United Kingdom). Infected cells were detected using 3-amino-9-ethylcarbazole and infectious units enumerated by light microscopy.
RESULTS
CCL5 expression during primary RSV infection and Th2 T-cell-mediated immunopathology.
To study the cellular sources and kinetics of CCL5 production during primary and Th2 immune-augmented RSV infection, mice were sensitized to RSV by priming with rVV-G or the control, rVV-βGal, previously demonstrated to not affect primary infection.
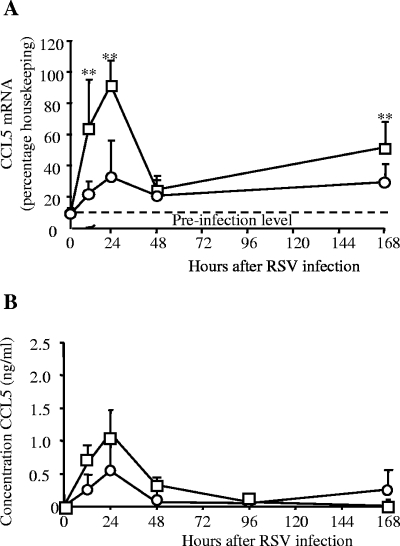
In all groups, lung CCL5 mRNA levels increased 12 h after RSV infection compared to preinfection controls, peaked at 18 to 24 h, and declined by 48 h (Fig. 1A; also not depicted). In rVV-G primed mice, this early response was significantly greater than that with rVV-βGal (P < 0.01). In both groups at all time points, the CCL5 mRNA level was significantly greater than that for the preinfection control (P < 0.01). Seven days after infection, CCL5 mRNA was still expressed at levels significantly above background in all groups (Fig. 1A; also data not depicted). The CCL5 protein was detected in the BAL fluid of RSV-infected mice, peaking 24 h after infection, and then declined rapidly (Fig. 1B) and remained low on days 2 to 4, an effect seen consistently in independent experiments (not shown). The profile of CCL5 production in primary infection was not significantly different from that for rVV-βGal-treated mice (results not depicted).
FIG. 1.
CCL5 mRNA and protein levels after RSV infection. BALB/c mice were scarified with recombinant vaccinia viruses expressing the G (□) protein of RSV or a control construct (rVV-βgal [○]). Two weeks later, they were infected intranasally with RSV. (A) CCL5 mRNA levels in whole, snap-frozen lung, determined by RNase protection assay. (B) CCL5 protein concentration in the BAL measured by enzyme-linked immunosorbent assay; data points represent means (n > 4) ± standard errors. **, P < 0.01. This graph shows one of three similar independent experiments.
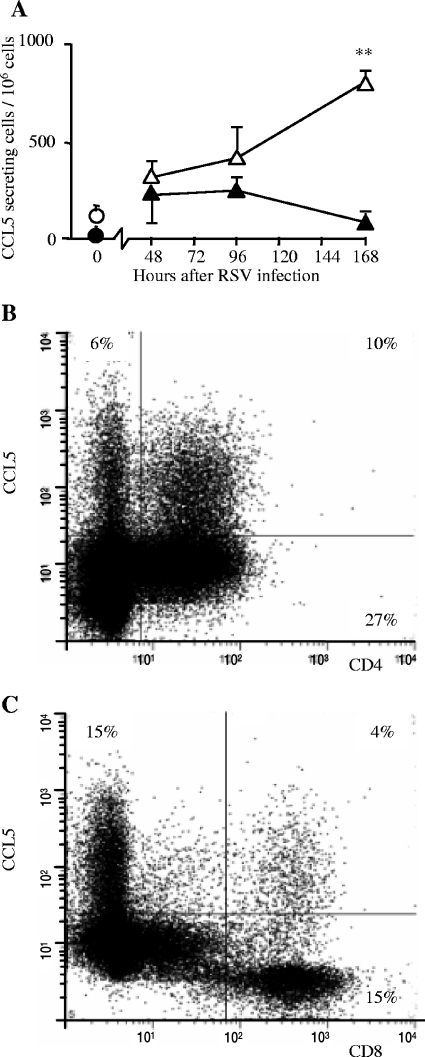
We wished to determine whether CCL5 production was due to resident or infiltrating cells and if this production was RSV specific. Since CCL5 production was highest in rVV-G primed mice, we isolated lung cells from rVV-G-primed, RSV-infected mice on various days after infection and examined ex vivo CCL5 production by ELISPOT. In vitro stimulation of lung cells from mice that were uninfected or early in infection with live RSV resulted in a detectable but low frequency of production of CCL5. However, cells from mice 7 days after RSV infection showed a large increase in CCL5 production after live RSV restimulation, compared to unstimulated cells and compared to cells from uninfected mice (Fig. 2A). This response required replicating virus, since UV-inactivated RSV did not lead to augmented CCL5 production. Levels of protein production in stimulated culture supernatant showed similar effects (not depicted). This directly measured virus-specific local production was not evident in the first 4 days of infection.
FIG. 2.
Cellular CCL5 production during RSV infection. BALB/c mice were scarified with rVV-G. Two weeks later, they were infected intranasally with RSV. (A) CCL5 production after in vitro live RSV stimulation (open symbols) or mock stimulation (HEp-2 cell lysate [filled symbols]) from isolated cells measured by ELISPOT; results are means (n = 5 mice) ± standard errors. *, P < 0.05; circles, uninfected mice; triangles, rVV-G-primed mice. (B and C) Intracellular staining for CCL5 in lung CD4+ (B) or CD8 + (C) lymphocytes isolated on day 7 after infection from rVV-G primed, RSV infected mice and analyzed by flow cytometry, gated on lymphocytes by forward/side scatter.
Since this response was present only in the lungs of infected mice during later stages of infection, we hypothesized that the source of CCL5 was infiltrating cells. Intracellular staining for CCL5 and fluorescence-activated cell sorter (FACS) analysis on lung cells taken from mice scarified with rVV-G and infected with RSV confirmed that CCL5 was produced by both CD4+ (27% of infiltrating CD4+ T cells in the example shown in Fig. 2B) and CD8+ (21% of infiltrating CD8+ T cells; Fig. 2C) T cells. Interestingly, CD8+ cells that were not high CCL5 producers contained no detectable CCL5, whereas all CD4+ cells contained low levels of stainable CCL5. Although few T cells were present in the early stages of infection (days 0 to 4), those that were recovered contained no detectable CCL5. Therefore, we observed two phases of CCL5 production: the first we believe to be from resident cells and the second from an infiltrating population of CD4 or CD8 T cells that secrete CCL5 in a virus-specific manner.
Effect of Met-RANTES on RSV disease.
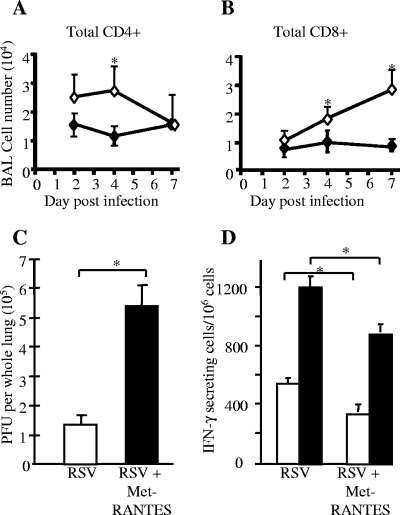
Having seen that RSV influences levels of CCL5 in the lung, we wished to determine whether blocking CCL5 would affect cell recruitment and viral clearance. Met-RANTES is a competitive blocker of the CCL5 receptors CCR1 and -5. Treatment of RSV-infected mice with Met-RANTES reduced the recruitment of CD4+ T cells to the BAL on day 4 of infection and of BAL CD8+ T cells over the last 3 days of infection, as measured by FACS (Fig. 3A and B). There was no difference in activation of these cells as determined by the level of CD45RB expression, and B-cell numbers were not altered by Met-RANTES treatment (not depicted).
FIG. 3.
Met-RANTES treatment during primary RSV infection. Mice were treated with Met-RANTES daily throughout the first 7 days of a primary RSV infection. (A and B) Met-RANTES treatment (⧫) reduced CD4+ and CD8+ lymphocyte accumulation in BAL, compared to results for untreated mice (⋄); data points represent means (n = 5 per group) ± standard errors. (C) RSV PFU were measured in whole homogenized lung taken 4 days after RSV infection; results are means (n = 5) ± standard errors. (D) IFN-γ-secreting lung cells 7 days after i.n. RSV infection measured by ELISPOT; data points represent means (n = 4 mice per group) ± standard errors. *, P < 0.05. (Open bars, mock stimulation; filled bars, live RSV stimulation in vitro.).
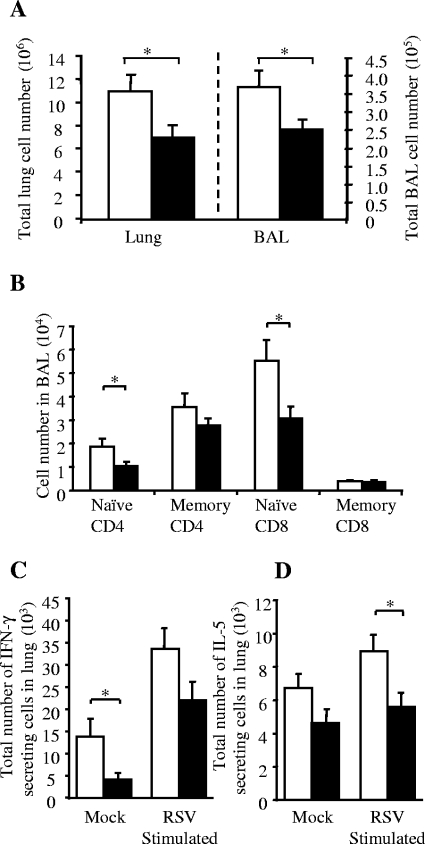
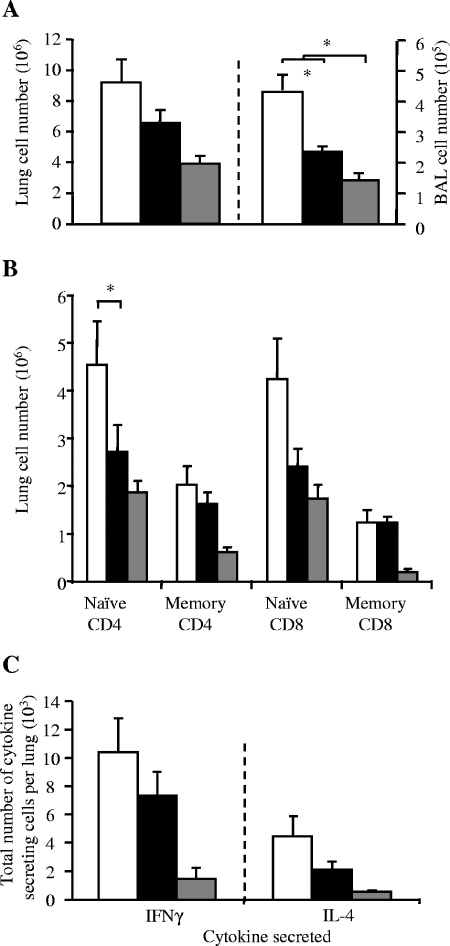
However, there was a large increase in RSV titer recovered from the lung 4 days after infection (Fig. 3C). This was accompanied by a >25% decrease in the proportion of lung cells secreting IFN-γ 7 days after RSV infection (measured by ELISPOT), both spontaneously and after ex vivo RSV stimulation (Fig. 3D). Because Met-RANTES reduced cellular infiltration during primary infection, we wished to test if it would be effective as a treatment for established “exacerbated” pathology. Therefore, rVV-G-primed RSV-infected mice were treated with Met-RANTES on days 5 and 6 days after RSV challenge. Treatment with Met-RANTES reduced cell recruitment in both lung (not shown) and BAL on day 7 (Fig. 4A). Lower numbers of naive CD4+ and CD8+ T cells were found in both the lung and BAL (Fig. 4B), and the total numbers of IFN-γ- and IL-5-secreting cells in the lung, both with and without ex vivo RSV stimulation, were decreased (P < 0.05) (Fig. 4C and D).
FIG. 4.
Met-RANTES treatment during RSV infection following Th2 disease exacerbation. Mice were primed with rVV-G and infected with RSV 2 weeks later. Met-RANTES treatment was given on days 5 and 6 after RSV infection. Mice were harvested on day 7. (A) Total cells isolated from the BAL and lung following Met-RANTES treatment. (B) Effect of Met-RANTES treatment on CD4+ and CD8+ populations in BAL; memory, CD44hi/CD62Llo. Effect of late Met-RANTES treatment on number of IFN-γ-secreting (C) or IL-5-secreting (D) cells in the lung. Data points represent means (n = 5 mice per group) ± standard errors. *, P < 0.05. (Open bars, untreated mice; filled bars, Met-RANTES-treated mice). Memory phenotype cells were defined as CD62Llo/CD44hi.
Effect of Met-RANTES treatment on immunity to a subsequent RSV challenge.
Given that Met-RANTES appears to control the recruitment of CD4+ and CD8+ T cells, it was hypothesized that Met-RANTES might affect the formation of RSV-specific memory. To test this, mice were treated with Met-RANTES during primary RSV infection and rechallenged with RSV 4 weeks later. Paradoxically, mice treated with Met-RANTES during the first infection showed an increased cellular response 7 days after a subsequent RSV challenge compared to the response with a standard secondary infection (Fig. 5A and B). This was reflected in increased CD4+ and CD8+ T-cell numbers in the lung, measured by FACS (Fig. 5C), and a higher proportion of cells of the CD62Llo/CD44hi “effector memory” phenotype, although “naive” CD8+ T-cell numbers also increased (Fig. 5C). There were also more cytokine-secreting lung cells than with primary infection, but Met-RANTES treatment did not alter this. There was no difference in RSV-induced illness assessed by weight loss and illness scores, nor was there any difference in the ability of the two groups to clear RSV.
FIG. 5.
Effect of Met-RANTES treatment on cell recruitment during subsequent RSV challenge. Mice were infected i.n. with RSV on day 0 and rechallenged on day 28. During days 0 to 5 of primary RSV infection, some mice were treated with Met-RANTES. (A) Total cells isolated from the BAL and lung following RSV rechallenge. (B) Effect of Met-RANTES treatment during primary infection on CD4 and CD8 memory (CD44hi/CD62Llo) populations after RSV rechallenge. (C) Effect of Met-RANTES treatment on number of IFN-γ or IL-5 secreting cells in the lung; data points represent means (n = 5 mice per group) ± standard errors. *, P < 0.05; **, P < 0.01. (Open bars, untreated mice; filled bars, Met-RANTES-treated mice; gray bars, primary RSV infection).
DISCUSSION
We found that CCL5 expression after RSV infection occurs in two phases: the first, seen during the first 48 h of infection, appears to be an innate response to viral challenge by resident lung cells arising from epithelial cells (10) and lung macrophages (21). Since lymphocyte recruitment is minimal before day 3 (24), this early peak in CCL5 transcription is unlikely to be due to infiltrating lymphocytes. The second peak occurs 7 days after infection. By this time, replicating virus is virtually eliminated, but infiltrating inflammatory cells are numerous (24).
This biphasic pattern resembles that observed previously (21), but the source of the second phase of production has not been demonstrated. From the results of intracellular cytokine staining, we believe that CD4+ and CD8+ T cells are primarily responsible for the second wave of CCL5 production, although resident cells may also increase CCL5 production as a response to virus-induced inflammation.
To further delineate the role of CCL5 in vivo, we treated mice with the CCL5 analogue, Met-RANTES. We found that Met-RANTES inhibited the recruitment of both CD4+ and CD8+ T cells to the bronchial epithelium, as sampled by BAL. It is possible that Met-RANTES blocks and/or downregulates expression of the CCL5 receptors (CCR1 and CCR5) on both CD4+ and CD8+ T cells. Alternatively, recruitment and activation of dendritic cells may be blocked by Met-RANTES treatment, therefore lessening T-cell responses (1, 35).
Met-RANTES treatment caused a striking increase in RSV replication during primary infection in vivo. This may be a direct consequence of the reduced recruitment of antiviral T cells but could also be due to inhibition of innate mechanisms of viral clearance. CCL5 can act as a chemoattractant for NK cells (which are recruited early in this RSV infection model (13) and can also block apoptosis of alveolar macrophages (18), an effect that may be important in viral clearance (10). In addition, pretreatment of HEp-2 cells with CCL5 or Met-RANTES has been shown to reduce virus growth (5). However, we saw an increase in viral titer in vivo, despite the potential of Met-RANTES to block viral replication.
Studies of T-cell responses in asthma often show local helper T cells that preferentially make IL-4, IL-5, IL-9, and IL-13. Such “′Th2” cells are thought to mediate eosinophilia, goblet cell hyperplasia, and bronchial hyperresponsiveness. However, there is growing evidence that Th1 cells may act in concert with Th2 cells to enhance inflammation in asthma or may themselves be immunopathogenic. In humans, it is notable that pulmonary bacterial and viral infections (which normally induce Th1-like immune responses) frequently trigger asthmatic exacerbations. Indeed, upper respiratory viral infections are present in 80 to 85% of asthma exacerbations in school-age children (15). This may be explained by the observation that Th1-polarized T cells can drive the recruitment of Th2 cells into the lung, even in the absence of Th2 antigen (29, 34). Pulmonary viral infections can also drive recruitment of allergen-specific T cells during infection or enhance subsequent sensitization to allergen (19, 22, 31).
We found that CCL5 was induced both in primary (Th1-driven) and in Th2-mediated immunopathology. It is possible that CCL5 production is enhanced by the Th2 cytokine IL-13 (38), which could explain the higher CCL5 mRNA we observed in the Th2-dominated responses seen in rVV-G primed mice. Holtzman's “epithelial-viral-allergic paradigm” asserts that CCL5 produced by virus-infected epithelial cells drives recruitment of both Th1 and Th2 proinflammatory cells in asthma (10). Our model of rVV-G priming followed by RSV infection allowed us the opportunity to use Met-RANTES treatment in an exacerbated inflammatory response, where both antiviral responses and Th2 immunopathology occur. When CCL5 signaling was blocked later during infection of rVV-G-primed animals, T-cell recruitment to the lung was attenuated and both IFN-γ- and IL-5-secreting cells were equally reduced in number. It has been shown that CD8+ cells contain CCL5 bound to proteoglycans within intracellular granules, which may be released upon antigen-specific stimulation (26). This may explain why we found elevated CCL5 mRNA with only low levels of the CCL5 protein in the BAL fluid on day 7 and release of CCL5 on antigen-specific restimulation. An alternative possibility is that the IFN-γ produced by the CD8+ cells indirectly stimulates the lung epithelial cells to produce CCL5 at this later time point.
Others have shown that CCL5 can reduce IL-12 production in primary RSV infection (38). However, in our model of rVV-G priming (in which IL-12 administration can inhibit eosinophilia [12]), Met-RANTES had no effect on eosinophilia or weight loss but did reduce lung inflammation. Interestingly, clinical studies of asthma exacerbation show that CCL5 and T-cell recruitment to the lung correlates with clinical symptoms but not with eosinophilia (3). We have recently shown that CCL11 plays a major role in governing eosinophilia and Th2 cytokine production in this model (20). Therefore, CCL5 production does not selectively bias Th1 or Th2 immunopathology but recruits multiple subsets of lymphocytes to the lung.
Surprisingly, we found that mice treated with Met-RANTES during primary RSV infection showed significantly increased cell recruitment during secondary RSV rechallenge. This paradoxical effect was evident in recruitment of both CD4+ and CD8+ T cells to the lung; the proportion of cells bearing the effector memory (CD62Llo/CD44hi) phenotype increased, but the numbers of naive cells were also raised by Met-RANTES treatment during prior infection. This could result from altered T-cell memory in treated mice, perhaps as a consequence of delayed viral clearance. Alternatively, Met-RANTES treatment during primary infection could create an altered lung environment, perhaps as a consequence of viral persistence (32), that results in a more proinflammatory response during secondary challenge.
By contrast, John et al., observed that anti-CCL5 antibody treatment during RSV infection diminished the extent of subsequent allergic inflammation in the lungs, although interestingly they reported no differenced in viral clearance for antibody-treated mice (14). The difference in tissue penetration of the high-molecular-weight immunoglobulin and low-molecular-weight Met-RANTES may have important differential effects on the effects of chemokines. Met-RANTES is an attractive alternative to depletion of CCL5 with antibodies because of its ability to penetrate tissues: immunoglobulin is larger than synthetic chemokine and is therefore unlikely to reach less-accessible local sites, particularly in noninflamed tissues.
In conclusion, our results suggest that infection of epithelial cells and macrophages results in production of CCL5 by resident cells during the first 48 h of infection. This CCL5 recruits CD4+ and CD8+ T cells and plays an important role in controlling viral replication. Later, CCL5 from resident cells disappears to be replaced by virally induced CCL5 from recruited T cells. Blocking CCL5 with Met-RANTES at this time reduces immunopathology but does not influence the Th1/Th2 balance. This second action is important both during primary infection and during augmented immunopathological responses. Our data suggest that CCL5 plays a central role in driving inflammation in RSV lung disease and that blocking its effect may prove beneficial in bronchiolitis. To this we should also add a note of caution: chemokine blockade might be beneficial in primary exposure but paradoxically enhance disease during reinfection with the same pathogen. Such effects might be difficult to anticipate or detect in clinical studies.
Acknowledgments
This work was supported by Wellcome Trust program grants to P.J.M.O. (54797/Z/98/Z and 071381/Z/03/Z) and by a Wellcome Trust prize studentship (055303/Z/98/Z).
REFERENCES
- 1.Aliberti, J., C. Reis e Sousa, M. Schito, S. Hieny, T. Wells, G. B. Huffnagle, and A. Sher. 2000. CCR5 provides a signal for microbial induced production of IL-12 by CD8 alpha+ dendritic cells. Nat. Immunol. 1**:**83-87. [DOI] [PubMed] [Google Scholar]
- 2.Bonville, C. A., H. F. Rosenberg, and J. B. Domachowske. 1999. Macrophage inflammatory protein-1alpha and RANTES are present in nasal secretions during ongoing upper respiratory tract infection. Pediatr. Allergy Immunol. 10**:**39-44. [DOI] [PubMed] [Google Scholar]
- 3.Castro, M., S. R. Bloch, M. V. Jenkerson, S. DeMartino, D. L. Hamilos, R. B. Cochran, X. E. Zhang, H. Wang, J. P. Bradley, K. B. Schechtman, and M. J. Holtzman. 2004. Asthma exacerbations after glucocorticoid withdrawal reflects T cell recruitment to the airway. Am. J. Respir. Crit. Care Med. 169**:**842-849. [DOI] [PubMed] [Google Scholar]
- 4.Culley, F. J., A. M. Pennycook, J. S. Tregoning, T. Hussell, and P. J. Openshaw. 2006. Differential chemokine expression following respiratory virus infection reflects Th1- or Th2-biased immunopathology. J. Virol. 80**:**4521-4527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elliott, M. B., P. W. Tebbey, K. S. Pryharski, C. A. Scheuer, T. S. Laughlin, and G. E. Hancock. 2004. Inhibition of respiratory syncytial virus infection with the CC chemokine RANTES (CCL5). J. Med. Virol. 73**:**300-308. [DOI] [PubMed] [Google Scholar]
- 6.Galkina, E., J. Thatte, V. Dabak, M. B. Williams, K. Ley, and T. J. Braciale. 2005. Preferential migration of effector CD8+ T cells into the interstitium of the normal lung. J. Clin. Investig. 115**:**3473-3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gonzalo, J. A., C. M. Lloyd, A. Peled, T. Delaney, A. J. Coyle, and J. C. Gutierrez-Ramos. 2000. Critical involvement of the chemotactic axis CXCR4/stromal cell-derived factor-1 alpha in the inflammatory component of allergic airway disease. J. Immunol. 165**:**499-508. [DOI] [PubMed] [Google Scholar]
- 8.Grone, H. J., C. Weber, K. S. Weber, E. F. Grone, T. Rabelink, C. M. Klier, T. N. Wells, A. E. Proudfoot, D. Schlondorff, and P. J. Nelson. 1999. Met-RANTES reduces vascular and tubular damage during acute renal transplant rejection: blocking monocyte arrest and recruitment. FASEB J. 13**:**1371-1383. [PubMed] [Google Scholar]
- 9.Harrison, A. M., C. A. Bonville, H. F. Rosenberg, and J. B. Domachowske. 1999. Respiratory syncytial virus-induced chemokine expression in the lower airways. eosinophil recruitment and degranulation. Am. J. Respir. Crit. Care Med. 159**:**1918-1924. [DOI] [PubMed] [Google Scholar]
- 10.Holtzman, M. J., J. D. Morton, L. P. Shornick, J. W. Tyner, M. P. O'Sullivan, A. Antao, M. Lo, M. Castro, and M. J. Walter. 2002. Immunity, inflammation, and remodeling in the airway epithelial barrier: epithelial-viral-allergic paradigm. Physiol. Rev. 82**:**19-46. [DOI] [PubMed] [Google Scholar]
- 11.Hornsleth, A., L. Loland, and L. B. Larsen. 2001. Cytokines and chemokines in respiratory secretion and severity of disease in infants with respiratory syncytial virus (RSV) infection. J. Clin. Virol. 21**:**163-170. [DOI] [PubMed] [Google Scholar]
- 12.Hussell, T., U. Khan, and P. J. M. Openshaw. 1997. IL-12 treatment attenuates Th2 and B cell responses but does not improve vaccine-enhanced lung illness. J. Immunol. 159**:**328-334. [PubMed] [Google Scholar]
- 13.Hussell, T., and P. J. M. Openshaw. 1998. Intracellular interferon-gamma expression in natural killer cells precedes lung CD8+ T cell recruitment during respiratory syncytial virus infection. J. Gen. Virol. 79**:**2593-2601. [DOI] [PubMed] [Google Scholar]
- 14.John, A. E., A. A. Berlin, and N. W. Lukacs. 2003. Respiratory syncytial virus-induced CCL5/RANTES contributes to exacerbation of allergic airway inflammation. Eur. J. Immunol. 33**:**1677-1685. [DOI] [PubMed] [Google Scholar]
- 15.Johnston, S. L., P. K. Pattemore, G. Sanderson, S. Smith, F. Lampe, L. Josephs, P. Symington, S. O'Toole, S. H. Myint, D. A. J. Tyrrell, and S. T. Holgate. 1995. Community study of role of viral infections in exacerbations of asthma in 9-11 year old children. Br. Med. J. 310**:**1225-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koga, T., E. Sardina, R. M. Tidwell, M. Pelletier, D. C. Look, and M. J. Holtzman. 1999. Virus-inducible expression of a host chemokine gene relies on replication-linked mRNA stabilization. Proc. Natl. Acad. Sci. USA 96**:**5680-5685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lloyd, C. M., A. W. Minto, M. E. Dorf, A. Proudfoot, T. N. Wells, D. J. Salant, and J. C. Gutierrez-Ramos. 1997. RANTES and monocyte chemoattractant protein-1 (MCP-1) play an important role in the inflammatory phase of crescentic nephritis, but only MCP-1 is involved in crescent formation and interstitial fibrosis. J. Exp. Med. 185**:**1371-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maghazachi, A. A., A. al-Aoukaty, and T. J. Schall. 1994. C-C chemokines induce the chemotaxis of NK and IL-2-activated NK cells. Role for G proteins. J. Immunol. 153**:**4969-4977. [PubMed] [Google Scholar]
- 19.Marsland, B. J., C. B. Scanga, M. Kopf, and G. Le Gros. 2004. Allergic airway inflammation is exacerbated during acute influenza infection and correlates with increased allergen presentation and recruitment of allergen-specific T-helper type 2 cells. Clin. Exp. Allergy 34**:**1299-1306. [DOI] [PubMed] [Google Scholar]
- 20.Matthews, S. P., J. S. Tregoning, A. J. Coyle, T. Hussell, and P. J. Openshaw. 2005. Role of CCL11 in eosinophilic lung disease during respiratory syncytial virus infection. J. Virol. 79**:**2050-2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller, A. L., T. L. Bowlin, and N. W. Lukacs. 2004. Respiratory syncytial virus-induced chemokine production: linking viral replication to chemokine production in vitro and in vivo. J. Infect. Dis. 189**:**1419-1430. [DOI] [PubMed] [Google Scholar]
- 22.O'Donnell, D. R., and P. J. M. Openshaw. 1998. Anaphylactic sensitisation to aeroantigen during respiratory virus infection. Clin. Exp. Allergy 28**:**1501-1508. [DOI] [PubMed] [Google Scholar]
- 23.Olszewska, W., Y. Suezer, G. Sutter, and P. J. M. Openshaw. 2004. Protective and disease-enhancing immune responses induced by recombinant modified vaccinia Ankara (MVA) expressing respiratory syncytial virus proteins. Vaccine 23**:**215-221. [DOI] [PubMed] [Google Scholar]
- 24.Openshaw, P. J., and J. S. Tregoning. 2005. Immune responses and disease enhancement during respiratory syncytial virus infection. Clin. Microbiol. Rev. 18**:**541-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Openshaw, P. J., Y. Yamaguchi, and J. S. Tregoning. 2004. Childhood infections, the developing immune system, and the origins of asthma. J. Allergy Clin. Immunol. 114**:**1275-1277. [DOI] [PubMed] [Google Scholar]
- 26.Ortiz, B. D., P. J. Nelson, and A. M. Krensky. 1997. Switching gears during T-cell maturation: RANTES and late transcription. Immunol. Today 18**:**468-471. [DOI] [PubMed] [Google Scholar]
- 27.Plater-Zyberk, C., A. J. Hoogewerf, A. E. Proudfoot, C. A. Power, and T. N. Wells. 1997. Effect of a CC chemokine receptor antagonist on collagen induced arthritis in DBA/1 mice. Immunol. Lett. 57**:**117-120. [DOI] [PubMed] [Google Scholar]
- 28.Proudfoot, A. E., C. A. Power, A. J. Hoogewerf, M. O. Montjovent, F. Borlat, R. E. Offord, and T. N. Wells. 1996. Extension of recombinant human RANTES by the retention of the initiating methionine produces a potent antagonist. J. Biol. Chem. 271**:**2599-2603. [DOI] [PubMed] [Google Scholar]
- 29.Randolph, D. A., R. Stephens, C. J. Carruthers, and D. D. Chaplin. 1999. Cooperation between Th1 and Th2 cells in a murine model of eosinophilic airway inflammation. J. Clin. Investig. 104**:**1021-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schall, T. J., K. Bacon, K. J. Toy, and D. V. Goeddel. 1990. Selective attraction of monocytes and T lymphocytes of the memory phenotype by cytokine RANTES. Nature (London) 347**:**669-671. [DOI] [PubMed] [Google Scholar]
- 31.Schwarze, J., E. Hamelmann, K. L. Bradley, K. Takeda, and E. W. Gelfand. 1997. Respiratory syncytial virus infection results in airway hyperresponsiveness and enhanced airway sensitization to allergen. J. Clin. Investig. 100**:**226-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwarze, J., D. R. O'Donnell, A. Rohwedder, and P. J. Openshaw. 2004. Latency and persistence of respiratory syncytial virus despite T cell immunity. Am. J. Respir. Crit. Care Med. 169**:**801-805. [DOI] [PubMed] [Google Scholar]
- 33.Sigurs, N., P. M. Gustafsson, R. Bjarnason, F. Lundberg, S. Schmidt, F. Sigurbergsson, and B. Kjellman. 2005. Severe respiratory syncytial virus bronchiolitis in infancy and asthma and allergy at age 13. Am. J. Respir. Crit. Care Med. 171**:**137-141. [DOI] [PubMed] [Google Scholar]
- 34.Stephens, R., D. A. Randolph, G. Huang, M. J. Holtzman, and D. D. Chaplin. 2002. Antigen-nonspecific recruitment of Th2 cells to the lung as a mechanism for viral infection-induced allergic asthma. J. Immunol. 169**:**5458-5467. [DOI] [PubMed] [Google Scholar]
- 35.Stumbles, P. A., D. H. Strickland, C. L. Pimm, S. F. Proksch, A. M. Marsh, A. S. McWilliam, A. Bosco, I. Tobagus, J. A. Thomas, S. Napoli, A. E. Proudfoot, T. N. Wells, and P. G. Holt. 2001. Regulation of dendritic cell recruitment into resting and inflamed airway epithelium: use of alternative chemokine receptors as a function of inducing stimulus. J. Immunol. 167**:**228-234. [DOI] [PubMed] [Google Scholar]
- 36.Sung, R. Y., S. H. Hui, C. K. Wong, C. W. Lam, and J. Yin. 2001. A comparison of cytokine responses in respiratory syncytial virus and influenza A infections in infants. Eur. J. Pediatr. 160**:**117-122. [DOI] [PubMed] [Google Scholar]
- 37.Taguchi, M., D. Sampath, T. Koga, M. Castro, D. C. Look, S. Nakajima, and M. J. Holtzman. 1998. Patterns for RANTES secretion and intercellular adhesion molecule 1 expression mediate transepithelial T cell traffic based on analyses in vitro and in vivo. J. Exp. Med. 187**:**1927-1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tekkanat, K. K., H. Maassab, A. Miller, A. A. Berlin, S. L. Kunkel, and N. W. Lukacs. 2002. RANTES (CCL5) production during primary respiratory syncytial virus infection exacerbates airway disease. Eur. J. Immunol. 32**:**3276-3284. [DOI] [PubMed] [Google Scholar]
- 39.Thomas, L. H., J. S. Friedland, M. Sharland, and S. Becker. 1998. Respiratory syncytial virus-induced RANTES production from human bronchial epithelial cells is dependent on nuclear factor-kappa B nuclear binding and is inhibited by adenovirus-mediated expression of inhibitor of kappa B alpha. J. Immunol. 161**:**1007-1016. [PubMed] [Google Scholar]