Evidence for the Requirement of ITAM Domains but Not SLP-76/Gads Interaction for Integrin Signaling in Hematopoietic Cells (original) (raw)
Abstract
Syk tyrosine kinase and Src homology 2 (SH2) domain-containing leukocyte-specific phosphoprotein of 76 kDa (SLP-76) are signaling mediators activated downstream of immunoreceptor tyrosine-based activation motif (ITAM)-containing immunoreceptors and integrins. While the signaling cascades descending from integrins are similar to immunoreceptors, the mechanism of Syk activation and SLP-76 recruitment remains unclear. We used an in vivo structure-function approach to study the requirements for the domains of Syk and SLP-76 in immunoreceptor and integrin signaling. We found that both SH2 domains and the kinase domain of Syk are required for immunoreceptor-dependent signaling and cellular response via integrins. While the Gads-binding domain of SLP-76 is needed for immunoreceptor signaling, it appears dispensable for integrin signaling. Syk and SLP-76 also are required for initiating and/or maintaining separation between the blood and lymphatic vasculature. Therefore, we correlated the signaling requirement of the various domains of Syk and SLP-76 to their requirement in regulating vascular separation. Our data suggest ITAMs are required in Syk-dependent integrin signaling, demonstrate the separation of the structural features of SLP-76 to selectively support immunoreceptor versus integrin signaling, and provide evidence that the essential domains of SLP-76 for ITAM signals are those which most efficiently support separation between lymphatic and blood vessels.
Immunoreceptors, including the B-cell receptor, T-cell receptor (TCR), FcRγ, and glycoprotein VI (GPVI) collagen receptor, utilize a common signaling pathway. Upon receptor activation, Src family kinases phosphorylate tyrosine residues within immunoreceptor tyrosine-based activation motifs (ITAMs), which then serve as binding sites for Syk and/or ζ-associated protein of 70 kDa (ZAP-70) kinase. Activated Syk or ZAP-70 can then phosphorylate a number of downstream molecules, including the adaptor protein Src homology 2 (SH2) domain-containing leukocyte-specific phosphoprotein of 76 kDa (SLP-76). This common signal transduction pathway is utilized also by integrin receptors, which do not contain ITAM domains, in various cell types, including neutrophils and platelets. In this study, we examined the differential requirement of the domains of Syk and SLP-76 in immunoreceptor and integrin signaling.
Syk is expressed throughout the hematopoietic compartment and is necessary for development and function in a number of lineages (8, 13, 26, 43, 52). Structurally, it contains two tandem SH2 domains and a C-terminal kinase domain. The SH2 domains selectively bind to the diphosphorylated ITAM within the cytoplasmic tail of immunoreceptors, including the B-cell receptor (44), FcRγ in neutrophils and macrophages (14, 26), FcɛRI in mast cells (41), and the γ chain of the collagen receptor in platelets (19). Binding of Syk to diphosphorylated ITAMs results in kinase activation.
In addition to functioning downstream of immunoreceptors, Syk is activated by integrins in neutrophils (31, 63) and platelets (9). Integrins are heterodimeric proteins consisting of an α and a β chain that mediate interaction with the extracellular matrix and other cell surface receptors (20). Syk is activated by β1 (24, 50) and β3 integrins (9, 18) in platelets and β1, β2, and β3 integrins in neutrophils (31). In contrast to immunoreceptor activation of Syk, integrin activation of Syk has been reported to be phosphotyrosine independent, requiring only the N-terminal SH2 domain and interdomain A region (56, 57). These results indicate that minimal mutations within the C-terminal SH2 domain of Syk that abrogate phosphotyrosine binding should prevent immunoreceptor signaling but not integrin signaling, allowing for dissection of the two pathways.
SLP-76 is expressed in nearly all hematopoietic lineages (11) and is required for thymocyte development (12, 40), platelet activation by collagen (10), neutrophil response to FcRγ stimulation (34), and FcɛRI-dependent mast cell activation (41). SLP-76 is composed of three distinct domains: an N-terminal acidic domain, a central proline-rich domain, and a C-terminal SH2 domain. The acidic domain contains three tyrosine residues that upon phosphorylation bind to Vav (36, 51, 58), Nck (61), and Tec family kinases (6, 49). The central proline-rich region is constitutively bound to the SH3 domain of the adaptor Gads (2, 5, 16, 28, 29) and the enzymes phospholipase C-γ1 (PLCγ1) and PLCγ2 (62). The C-terminal SH2 domain of SLP-76 binds phosphorylated tyrosines in adhesion- and degranulation-promoting adaptor protein (15, 32) and hematopoietic progenitor kinase 1 (46).
During immunoreceptor signaling, receptor engagement results in sequential activation of Src and Syk family kinases. Syk then phosphorylates a number of downstream substrates, including SLP-76 and the transmembrane adaptor linker of activated T cells (LAT) (54, 64). LAT is constitutively targeted to lipid rafts, cholesterol-rich membrane fractions that are enriched in signaling molecules. Gads binds to phosphorylated LAT, recruiting SLP-76 and its binding partners to the membrane rafts to form a multimolecular signaling complex (2). Like Syk, SLP-76 is also required for activation of platelets and neutrophils after engagement of β1, β2, and β3 integrins ex vivo (22, 34, 35). In contrast, LAT, which is needed for immunoreceptor signaling in T cells (65), mast cells (45), and platelets (38), appears to be dispensable for integrin signaling, at least in platelets (7, 23).
In addition to a loss of hematopoietic cell development and/or function, mice deficient in either Syk or SLP-76 display an aberrant vascular pattern, resulting from inappropriate connections between blood and lymphatic vessels (1). The causal cell type remains unidentified, but reconstitution of lethally irradiated wild-type (WT) mice with Syk-deficient fetal liver or SLP-76-deficient bone marrow recapitulates the vascular phenotype (1). This model provides a means for studying the structural requirements of Syk and SLP-76 in vascular separation via retroviral reconstitution of deficient fetal liver cells (FLCs) with various structural mutants of Syk or SLP-76.
Syk and SLP-76 play key roles in the proximal signal cascade activated by both integrins and immunoreceptors. Loss of either protein has profound effects on a number of hematopoietic lineages as well as on vascular development. Therefore, differentiating between Syk and SLP-76 requirements downstream of integrins versus immunoreceptors for a particular cellular or developmental function is difficult in vivo. One approach to this complex question is to investigate differences in Syk and SLP-76 functions downstream of the two receptor classes. In this study, we investigated the in vivo requirements of the various domains of Syk and SLP-76 in immunoreceptor or integrin receptor signaling and correlated these data with their roles in the regulation of vascular separation.
MATERIALS AND METHODS
Mice.
Syk-deficient mice (52) were a gift of V. Tybulewicz (National Institute for Medical Research, Mill Hill, London, England). SLP-76-deficient mice have been previously described (12). All mice were housed under pathogen-free conditions at the University of Pennsylvania in accordance with National Institutes of Health (NIH) guidelines and approved animal protocols.
Constructs.
WT Syk and a kinase-dead (KD) mutant were kindly provided by Reuben P. Siraganian (National Institute of Dental and Craniofacial Research, NIH). The KD mutant contains a lysine-to-arginine mutation at amino acid 395. The N-RK (arginine to lysine at amino acid 41) and C-RK (arginine to lysine at amino acid 194) mutants were generated from WT Syk cDNA in pSVL by use of transformer site-directed mutagenesis (Clontech). WT Syk and the Syk mutants were subcloned into MIGR1 (provided by W. Pear, University of Pennsylvania) (39) in the XhoI and SacII sites. The Y3F mutant of SLP-76 was generated by site-directed mutagenesis of SLP-76 cDNA in pBluescript and was cloned into the BglII site of MIGR1. Generation of an MIGR1 vector containing WT SLP-76, G2 mutants, and MTS have been previously described (47).
Transient transfections.
Chinese hamster ovary (CHO) cells stably expressing αIIbβ3 (CHO-A5) have been previously described (18). All cells were maintained in Dulbecco's modified Eagle's medium (DMEM) with 10% fetal calf serum (FCS), penicillin (1,000 units/ml), streptomycin (1,000 units/ml), and glutamine (20 mM). For transient transfections, 4 × 106 CHO-A5 cells were plated on 150-mm tissue culture plates and 24 h later were transfected with 20 μg of DNA using GeneJammer (Stratagene) following the manufacturer's protocol. Twenty hours posttransfection, the serum concentration in the medium was lowered to 0.5% FCS and the cells were serum starved for an additional 24 h.
Immunoprecipitation and Western blotting.
Forty-eight hours posttransfection, cells were treated with Versene, harvested, and then added to bacterial plates coated with 5 mg/ml bovine serum albumin (BSA) or 100 μM fibrinogen and incubated at 37°C for 60 min. Cells were harvested, washed twice in phosphate-buffered saline (PBS), normalized for the percentage of cells transfected, and lysed in Nonidet P-40 buffer containing a 1:100 dilution of a protease inhibitor mixture (Sigma), 1 mM phenylmethylsulfonyl fluoride, and the following protein phosphatase inhibitors: 400 μM sodium vanadate, 10 μM sodium fluoride, and 10 μM sodium pyrophosphate. For Syk immunoprecipitation, 2 μg of anti-Syk (Santa Cruz Biotechnology) was conjugated to GammaBind Plus-Sepharose beads (Amersham Biosciences) for 12 h; lysates were tumbled with the antibody-conjugated beads for 4 h at 4°C. The immune complexes were washed three times with lysis buffer, resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and transferred to nitrocellulose for immunoblotting with 4G10. To ensure equal protein loading, blots were stripped with Restore Western blot stripping buffer (Pierce Biotechnology, Rockford, IL) and reprobed with anti-Syk monoclonal antibody (kindly provided by A. Weiss, University of California, San Francisco). For SLP-76 studies, cell lysates were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to nitrocellulose, and immunoblotted with anti-SLP-76 antibody (Upstate).
Genetic reconstitution of Syk- and SLP-76-deficient fetal liver.
293T cells were transfected with MIGR1 expressing green fluorescent protein (GFP) alone or GFP and WT Syk, WT SLP-76, or the various mutants using GeneJammer (Stratagene) per the manufacturer's protocol. Viral supernatants were collected 48 and 72 h posttransfection. Embryonic day 14.5 to 17.5 Syk- or SLP-76-deficient embryos were harvested, and single-cell suspensions of fetal liver were generated in DMEM supplemented with 10% FCS. Mononuclear cells were purified by Ficoll gradient and cultured for 3 days in DMEM containing 10% FCS, 20 ng/ml recombinant murine interleukin-3, 10 ng/ml recombinant murine interleukin-6, and 100 ng/ml recombinant murine stem cell factor. All recombinant murine cytokines were obtained from Peprotech. At 24 and 48 h postharvest, fetal liver cells were spin infected. At 72 hours postharvest, cells were collected, washed twice in PBS, and intravenously injected into lethally irradiated (1,100-rad Co) C57BL6 mice. Recipient mice were sacrificed for study 8 to 10 weeks postreconstitution. Severity of vascular phenotype was scored as described in detail below in the figure legends. Differences between the various mutant constructs and WT (Syk or SLP-76) or MIGR1 were compared by chi-square test. A statistically significant difference was defined as a P value of <0.05.
Platelet degranulation and spreading.
Isolation of washed platelets has been previously described (22). Washed platelets (1 × 106) were stimulated with 5 nM convulxin (CVX) or 0.5 mM AYPGKF in the presence of phycoerythrin-conjugated anti-murine P-selectin antibody (BD Pharmingen) for 20 min at 37°C, and P-selectin surface expression was quantified by flow cytometry. Purified CVX from the venom of Crotalus durissus terrificus was from Alexis Biochemicals. AYPGKF peptide was synthesized by the University of Pennsylvania Protein Chemistry Laboratory. For the spreading experiments, 100 μg/ml fibrinogen (Enzyme Research Laboratories) was incubated on a chamber slide (Fischer) overnight at 4°C. After washing twice with PBS, the slide was blocked with 10 mg/ml heat-denatured BSA. Washed platelets (20 × 106 cells/ml) were incubated for 45 min at 37°C. Slides were gently rinsed with PBS, and adhered platelets were fixed in 2% paraformaldehyde, permeabilized, and stained with rhodamine-phalloidin (Molecular Probes). Images were captured at 1,000× magnification. The area of GFP+ platelets was determined as the number of pixels per platelet by IP Labs Scientific Image Processing (Scanalytics, Inc.).
Flow cytometry.
Spleens were harvested from chimeric mice and made into single-cell suspensions, and expression of surface antigens was determined by standard flow cytometric methods using anti-B220-allophycocyanin (BD Pharmingen).
Isolation of bone marrow neutrophils.
Bone marrow-derived neutrophils were prepared as previously described (34). Purity of the neutrophil preparation was assessed using flow cytometry; standard preparations yielded neutrophils of >90% purity as determined by surface expression of Gr-1 (Pharmingen).
Adherent respiratory burst.
Ninety-six-well Immulon 4 HBX plates (Thermo Labsystems) were coated with 150 μg/ml fibrinogen (F-9754; Sigma) in PBS, 15 μg/ml poly(RGD) (F-5022; Sigma) in PBS, or 20 μg/ml anti-CD18 (C71/16) or isotype control antibody (Pharmingen) in carbonate buffer. Antibody-coated wells were blocked with 10% FCS for 30 min. One hundred μl of prewarmed neutrophils suspended at 4 × 106 cells/ml in Hank's balanced salt solution plus 10 mM HEPES, 100 mM ferricytochrome c (Sigma-Aldrich), and 1 mM Ca2+ was added to the wells. Adherent respiratory burst was measured through oxidation-induced changes in ferricytochrome c absorbance as previously described (31). When indicated, 50 ng/ml murine tumor necrosis factor alpha (TNF-α) was added.
Measurement of FcγR-induced calcium flux.
For labeling with the calcium-sensitive fluorochrome Indo-1, neutrophils were resuspended at 107 cells/ml in Hank's balanced salt solution prep containing 3 μg/ml Indo-1 (Molecular Probes) and 4 mM Probencid and were incubated at 37°C for 20 min. Cells were washed twice in PBS and then incubated with 2 μg/ml anti-FcγRII/III (Pharmingen) in PBSg (125 mM sodium chloride, 8 mM sodium phosphate, 2 mM sodium phosphate monobasic, 5 mM potassium chloride, 5 mM glucose) for 15 min at 4°C. Following three washes in cold PBSg, cells were resuspended in ice-cold PBSg at 107 cells/ml until initiation of the assay. Two minutes prior to stimulation, cells were diluted to 0.5 million cells/ml in 37°C KRP buffer (PBSg plus 1 mM calcium chloride and 1.5 mM magnesium chloride). Cell stimulation was initiated by adding 15 to 30 μg/ml of goat anti-rat immunoglobulin G (FcγR; Pharmingen). Calcium levels were analyzed on an LSR flow cytometer (Becton Dickinson Immunocytometry Systems).
Histology and immunohistochemistry.
Formaldehyde-fixed and paraffin-embedded sections were studied as described at the website www.uphs.upenn.edu/mcrc/histology/histologyhome.html using anti-LYVE-1 antibody (3) at a final concentration of 1:1,000.
RESULTS
Phosphotyrosine-binding activity of Syk is dispensable for activation by αIIbβ3 in CHO cells.
Syk activation by immunoreceptors requires binding of the tandem SH2 domains to diphosphorylated tyrosines of the ITAM (44). Studies in CHO cells have shown that Syk can directly bind to and be activated by the cytoplasmic tail of integrin β chains in a phosphotyrosine-independent manner (56, 57). A minimal mutation in the SH2 domains of Syk that abrogated phosphotyrosine binding was predicted, therefore, to interfere with ITAM-dependent signaling without affecting integrin signaling.
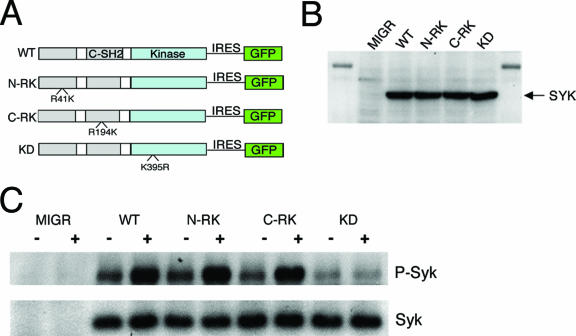
We generated Syk mutants with an arginine-to-lysine mutation at R41 within the amino-terminal SH2 domain (N-RK) or R194 within the carboxy-terminal SH2 domain (C-RK), amino acid changes that have been shown to destroy this phosphotyrosine-binding capacity (53) (Fig. 1A). The KD variant has a point mutation that abrogates kinase activity but retains phosphotyrosine binding of both SH2 domains (37) (Fig. 1A). WT Syk and the N-RK, C-RK, and KD mutants were subcloned into the MIGR1 retrovirus vector and were transfected into CHO cells. Anti-Syk immunoblot analysis showed all constructs were expressed similarly (Fig. 1B).
FIG. 1.
SH2 domains of Syk are dispensable for activation by αIIbβ3 in CHO cells. (A) Schematic of Syk constructs. N-RK and C-RK contain arginine-to-lysine mutations at amino acids 41 and 194, respectively, and KD contains a lysine-to-arginine mutation at amino acid 395. (B) CHO cells were transiently transfected with empty vector, WT Syk, or Syk mutant. Syk expression was assayed by Western blotting after normalizing for the percentage of CHO cells transfected. (C) CHO cells were transiently transfected with the MIGR vector expressing either Syk or a Syk mutant. Cells were plated upon fibrinogen-coated (+) or BSA-coated (−) plates, and lysates from equivalent numbers of transfected cells were collected and immunoprecipitated for Syk, followed by Western blotting for phosphotyrosine. Blots were then stripped and reprobed for Syk.
To investigate if the RK mutations interfere with Syk activation by integrins, we transfected CHO cells stably expressing αIIbβ3 integrin with a vector expressing GFP alone (MIGR1), GFP plus WT Syk, or GFP plus mutant Syk. Cells were plated on fibrinogen, a ligand for αIIbβ3 integrin, and Syk activation was assessed by immunoprecipitation followed by immunoblotting for phosphotyrosine. Upon fibrinogen ligation of αIIbβ3, WT Syk was phosphorylated while the KD mutant remained unphosphorylated (Fig. 1C). Like WT Syk, the N-RK and C-RK mutants were phosphorylated upon fibrinogen binding (Fig. 1C), supporting previous findings of SH2 domain-independent activation of Syk by integrins.
Minimal mutation of Syk SH2 domains results in loss of immunoreceptor and integrin-dependent activation.
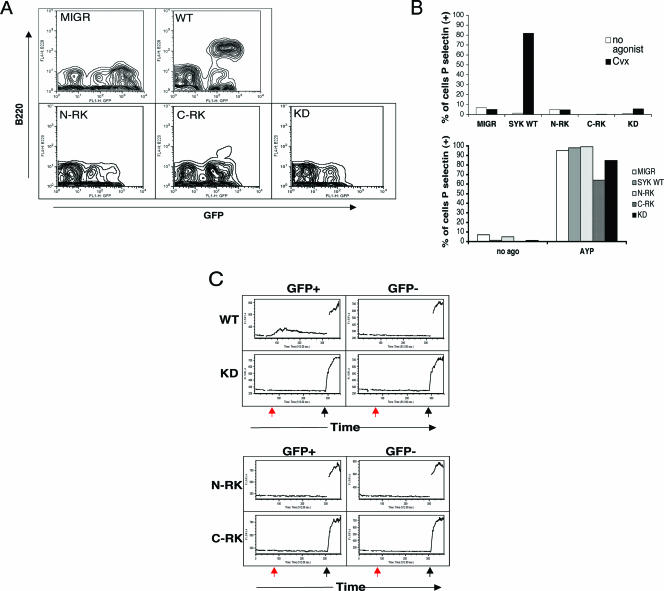
The phosphotyrosine binding of the Syk SH2 domains is required for Syk activation by ITAM-containing receptors but appears dispensable, in the CHO cell system, for activation by integrins. To extend this work to primary cells, we generated chimeric mice expressing WT or mutant Syk within the hematopoietic compartment. Syk-deficient FLCs were transduced with GFP alone or GFP plus WT or mutant Syk and used to reconstitute lethally irradiated mice. GFP expression in T cells, platelets, and neutrophils was ascertained by flow cytometric analysis to determine the percentage of transduced donor cells. Eight to 10 weeks after reconstitution, mice were sacrificed, and immunoreceptor and integrin signaling were studied in B cells, platelets, and neutrophils.
Syk is required for B-cell development beyond the pro-B-cell stage (8, 52). Syk−/− FLCs transduced with GFP alone failed to give rise to B220+ B cells in the spleen, whereas transduction with WT Syk rescued B-cell development (Fig. 2A). As expected, the N-RK, C-RK, and KD mutants of Syk all failed to rescue B-cell development. CVX is an agonist for the platelet collagen receptor GPVI, which signals via the FcγR common chain (42). Activation of platelets by CVX results in degranulation and surface expression of P-selectin, a Syk-dependent response (42). Expression of WT Syk reconstituted the response to CVX, but there was no rescue when the N-RK, C-RK, or KD mutant was expressed, consistent with the requirement for ITAM-bearing receptors in collagen signaling (Fig. 2B, top). All platelets responded to AYPGKF, a PAR4 receptor agonist peptide that activates platelets in a Syk-independent manner (Fig. 2B, bottom).
FIG. 2.
Mutation in either SH2 domain of Syk results in loss of ITAM-dependent signaling in vivo. FLCs from Syk-deficient embryos were transduced with virus expressing GFP alone (MIGR1) or GFP with WT Syk or mutant Syk and were used to reconstitute lethally irradiated C57BL6 mice. Eight to 10 weeks postreconstitution, chimeric mice were sacrificed for study. (A) Splenocytes from reconstituted mice were isolated and stained for B220, followed by flow cytometric analysis to distinguish GFP-positive from GFP-negative cells. Note that B cells (upper quadrants) appear only when WT Syk is successfully transduced. (B) Retrovirally transduced platelets were either left unstimulated or stimulated with 5 ng CVX (top) or 0.5 mM AYPGKF (AYP) peptide (bottom). GFP-positive platelets were then assessed for P-selectin expression. Results are plotted as the percentage of platelets staining positive for P-selectin. (C) Neutrophils were labeled with Indo-1 and incubated with an antibody against FcRγ. Calcium flux was measured by flow cytometry following cross-linking of the receptors with anti-rat immunoglobulin G (red arrow) and ionomycin (black arrow).
Neutrophil stimulation via the FcγR receptor results in Syk activation and mobilization of Ca2+ (30, 55). Syk-deficient neutrophils failed to flux Ca2+ upon activation, while expression of WT Syk restored Ca2+ release (Fig. 2C). As with B-cell development and platelet degranulation, the kinase domain and both SH2 domains of Syk are required for neutrophil Ca2+ flux (Fig. 2C). Ionomycin stimulation, a control, induced a similar Ca2+ rise in all neutrophils.
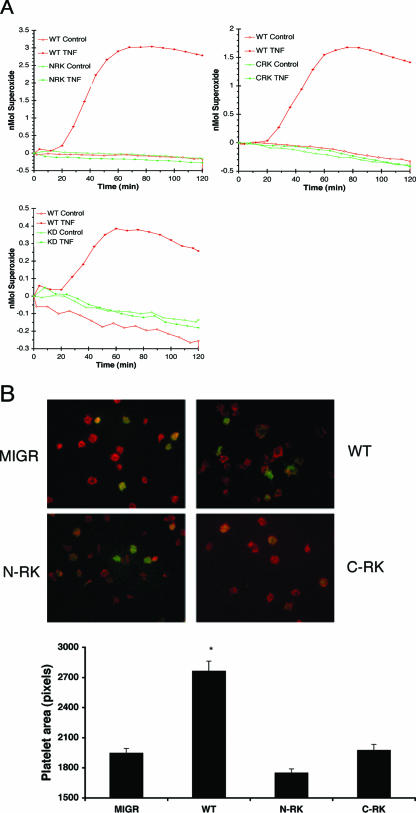
To determine the structural requirements of Syk for integrin signaling, we evaluated neutrophil and platelet responses to fibrinogen. Fibrinogen is a cognate ligand for both β3 integrins in platelets and β2 integrins in neutrophils. Syk-deficient neutrophils failed to generate detectable levels of reactive oxygen intermediates (ROIs) upon plating on fibrinogen in the presence of TNF (Fig. 3A). Neutrophils expressing WT Syk produced ROIs, while neutrophils expressing the KD mutant were unresponsive (Fig. 3A). Surprisingly, transduction with either the C-RK or N-RK mutant failed to rescue ROI production (Fig. 3A). To extend these results beyond neutrophils, we examined platelet spreading on fibrinogen. Syk-deficient platelets failed to spread when plated on a fibrinogen-coated surface, a deficit that was rescued with expression of WT Syk (Fig. 3B). Platelet spreading, as with the neutrophil response to fibrinogen, required both SH2 domains of Syk, inasmuch as neither C-RK Syk-transduced nor N-RK Syk-transduced cells were able to spread (Fig. 3B). Quantification of platelet area showed that platelets expressing either SH2 mutant of Syk spread significantly less than platelets expressing WT Syk. These results argue that the phosphotyrosine-binding capacity of Syk is required for activation by integrins in primary cells.
FIG. 3.
Mutation in either SH2 domain of Syk results in loss of the integrin-dependent cellular response in vivo. (A) Neutrophils from chimeric mice were stimulated with TNF or left unstimulated after plating upon a fibrinogen-coated surface. ROI production was measured by cytochrome c oxidation. Each graph includes neutrophils expressing WT Syk (red line) and one Syk mutant. (B) Platelets were incubated on coverslips coated with fibrinogen, fixed, permeabilized, and stained for F-actin (phalloidin). GFP-positive platelets represent platelets transduced with virus. Original magnification, ×1,000. The average area of spread of GFP+ platelets (n > 100) is shown in pixels per platelet. Error bars represent standard errors. *, significant difference in spreading between MIGR and WT, N-RK, or C-RK (P < 0.001).
The Gads-binding region of SLP-76 is required for ITAM but not integrin signaling.
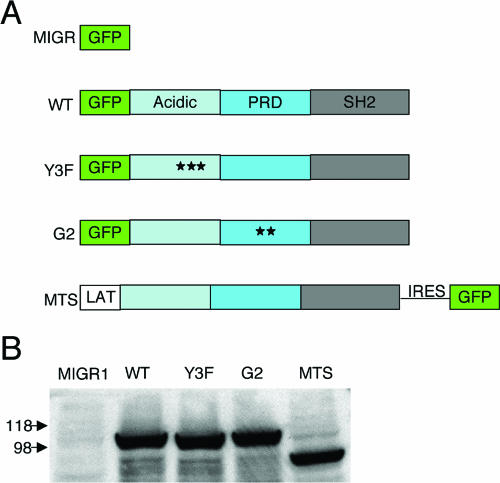
SLP-76 consists of multiple domains that mediate binding to other proteins (60). We generated mutants in which the three tyrosines in the N-terminal domain were altered to phenylalanine (Y3F) or two amino acids within the Gads-binding region were mutated (G2) (Fig. 4A). Given that the G2 mutant has been shown to abrogate SLP-76 recruitment to the membrane (47), we made use of a complementary construct, SLP-76 cDNA fused to cDNA encoding the transmembrane domain of LAT and its two juxtamembrane cysteine residues, to target SLP-76 to the membrane (MTS) (4) (Fig. 4A). WT SLP-76, Y3F, G2, and MTS were cloned into the MIGR1 retrovirus vector and transfected into CHO cells. Anti-SLP-76 immunoblot analysis showed equal protein expression (Fig. 4B).
FIG. 4.
Expression of WT SLP-76, mutant SLP-76, and MTS. (A) Schematic of WT and mutant GFP-tagged SLP and raft-targeted SLP-76 (MTS). PRD represents the proline-rich domain of SLP-76. For the Y3F mutant, stars represent mutations of tyrosine to phenylalanine at amino acids 112, 128, and 145. For the G2 mutant, stars represent amino acid changes from arginine to alanine at amino acid 237 and lysine to alanine at position 240. MTS consists of the first 35 amino acids of LAT fused to full-length SLP-76. MTS is not GFP tagged and hence runs at a lower molecular weight. (B) CHO cells were transiently transfected with empty vector or vector expressing WT SLP-76, mutant SLP-76, or MTS. Cell lysates of equivalent numbers of transfected cells were examined by immunoblot analysis with anti-SLP-76 antibody.
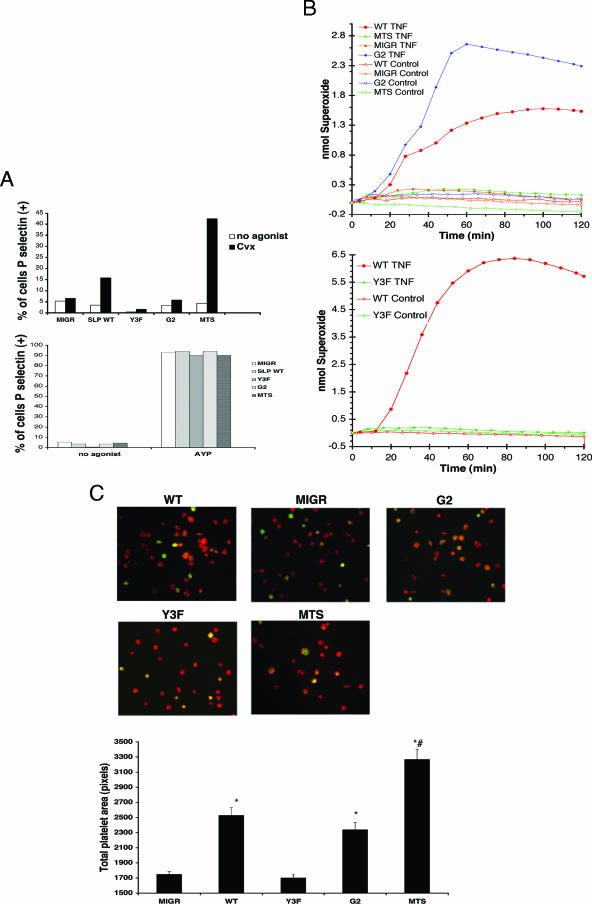
To determine the importance of the domains of SLP-76 in immunoreceptor or integrin signaling, SLP-76-deficient FLCs were transduced with retrovirus expressing GFP and WT SLP-76 or mutant SLP-76 and injected into lethally irradiated mice. Eight to 10 weeks later, neutrophils and platelets were collected from the chimeric mice. Previous studies have shown that the tyrosines and Gads-binding region of SLP-76 are required for immunoreceptor signaling (25, 27, 33, 59). To confirm these results, we evaluated the ability of the SLP-76 mutants to rescue GPVI-dependent platelet degranulation. SLP-76-deficient platelets were unresponsive to CVX, and the expression of WT SLP-76 rescued this defect (Fig. 5A). Both the N-terminal tyrosine residues and the Gads-binding region are required for GPVI signaling, as expression of Y3F SLP-76 or G2 SLP-76 failed to rescue degranulation (Fig. 5A). Previously, we have shown that the MTS supports immunoreceptor signaling in Jurkat T cells and is predominantly targeted to lipid rafts (4). As shown in Fig. 5A, platelets expressing the MTS up-regulated P-selectin after CVX stimulation. All platelets responded to AYPGKF (Fig. 5A, bottom).
FIG. 5.
Membrane localization of SLP-76 is required for immunoreceptor-dependent signaling but is dispensable for an integrin-dependent response. FLCs or bone marrow cells from SLP-76-deficient embryos were transduced with virus expressing empty vector or WT SLP-76, mutant SLP-76, or MTS and were used to reconstitute lethally irradiated C57BL6 mice. Eight to 10 weeks postreconstitution, chimeric mice were sacrificed for study. (A) Retrovirally transduced platelets were either left unstimulated or stimulated with 5 nM CVX (top) or 0.5 mM AYPGKF (AYP) (bottom) for 20 min in the presence of anti-P-selectin antibody. P-selectin expression was assessed by flow cytometry with gates set to distinguish GFP-positive cells from GFP-negative cells. Results are plotted as the percentage of GFP-positive platelets staining positive for P-selectin. (B) Neutrophils from chimeric mice were stimulated with TNF or left unstimulated after plating upon a fibrinogen-coated surface. ROI production was measured by cytochrome c oxidation. (C) Platelets were incubated on coverslips coated with fibrinogen, fixed, permeabilized, and stained for F-actin (phalloidin). Original magnification, ×1,000. The average area of spread of GFP+ platelets (n > 100) is shown in pixels per platelet. Error bars represent standard errors.  , significant difference in spreading between MIGR or Y3F and WT or G2 (P < 0.001); #, significant difference in spreading between MTS and WT (P < 0.001).
, significant difference in spreading between MIGR or Y3F and WT or G2 (P < 0.001); #, significant difference in spreading between MTS and WT (P < 0.001).
To assess integrin-dependent signaling, we assayed neutrophil ROI production and platelet spreading on fibrinogen. SLP-76-deficient neutrophils, like Syk-deficient neutrophils, failed to produce ROI when plated on fibrinogen in the presence of TNF (Fig. 5B). Transduction with virus expressing WT SLP-76 rescued ROI production, while neutrophils expressing the Y3F mutant failed to respond (Fig. 5B). LAT and Gads are dispensable for integrin signaling and, concordantly, the G2 mutant rescued integrin-dependent ROI production (Fig. 5B). In contrast, constitutive targeting of SLP-76 to membrane using the MTS failed to rescue integrin-dependent ROI production (Fig. 5B), presumably because of selective expression of the MTS in lipid rafts.
To extend these findings beyond neutrophils, we examined platelet spreading on fibrinogen via αIIbβ3. Like Syk, SLP-76 has been implicated as a signaling intermediate downstream of αIIbβ3 (22). Platelets deficient in SLP-76 failed to spread on a fibrinogen surface, a response rescued by expression of WT SLP-76 but not the Y3F mutant (Fig. 5C). The inability to recruit SLP-76 to membrane rafts appears to be dispensable for integrin signaling in platelets, as expression of the G2 mutant restored spreading upon fibrinogen (Fig. 5C). Interestingly, however, although it fails to rescue neutrophil ROI production, the MTS rescued platelet spreading on fibrinogen (Fig. 5C). Quantification of platelet area showed that platelets expressing the Y3F mutant of SLP-76 spread significantly less than platelets expressing WT SLP-76, while platelets expressing the G2 mutant spread equivalently to WT SLP-76. Surprisingly, platelets expressing the MTS spread to a greater extent than WT SLP-76-expressing platelets. These studies suggest a differential requirement for SLP-76 membrane localization in platelets versus neutrophils for integrin-mediated responses.
The SH2 domains of Syk and SLP-76 recruitment to the plasma membrane are required for vascular separation.
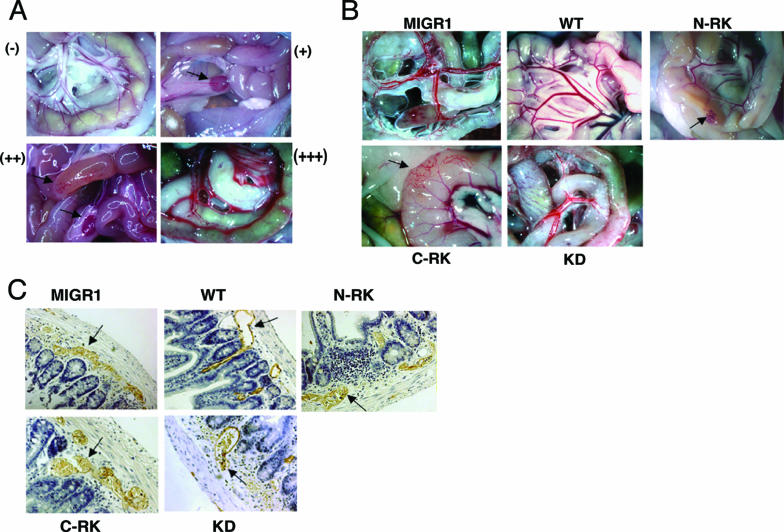
As we have reported previously (1), SLP-76 or Syk deficiency results in failed separation of lymphatic and blood vasculature. High-dose radiation followed by FLC transfer from SLP-76- or Syk-deficient donors recapitulates this phenotype, most notably in the gut. Since WT SLP-76 or Syk completely rescues this defect, we also determined vascular integrity to assess the structural features of SLP-76 or Syk for optimal function. In assessing this vascular defect, we scored each animal as having no phenotype (−), mild phenotype (+), moderate phenotype (++), or severe phenotype (+++) (Fig. 6A). In the most severely affected mice (+++), there was a complete loss of vascular architecture in the gut wall. Blood was clearly visible in mesenteric lymphatic vessels, and there was a bloody chylous effusion in the peritoneum. In the mildly affected mice, the only evidence of abnormality was grossly visible blood in the mesenteric lymph nodes, with no lesions visible in the gut (Fig. 6A). Mice that showed areas of affected gut while other adjacent areas appeared grossly normal were scored as moderately affected.
FIG. 6.
SH2 domains and kinase activity of Syk are required for blood-lymphatic separation. (A) Range of the gross morphological appearances of vascular phenotypes seen in the small intestines of chimeric mice: −, unaffected; +, blood visible in mesenteric lymph nodes; ++, focal loss of normal vascular architecture of gut wall; +++, complete loss of vascular architecture with appearance of chylous effusion within peritoneum. (B) Chimeric mice were sacrificed and examined for appearance of grossly visible vascular lesions in the small intestine and mesenteric lymph nodes. Black arrows identify affected regions. (C) Tissue from the small intestine of chimeric mice was fixed, paraffin embedded, sectioned, and stained for Lyve-1 with horseradish peroxidase-tagged secondary antibody to identify lymphatic vessels. Arrows identify lymphatic vessels. Original magnification: (A and B) ×1, except for C-RK which was at ×2; (C) ×200.
Irradiated mice reconstituted with Syk-deficient FLCs transduced with virus expressing GFP alone uniformly developed vascular lesions with varying severity (Fig. 6B; Table 1). Immunostaining of histological sections from the small intestine with antibodies against LYVE-1 revealed blood-filled lymphatics (Fig. 6C). Retroviral expression of WT Syk within the hematopoietic compartment uniformly rescued vascular separation (Fig. 6B and C; Table 1). The KD mutant failed to rescue normal vascular development, and, in fact, chimeric mice expressing KD Syk demonstrated a more severe phenotype (Fig. 6B and C; Table 1). Expression of Syk with either SH2 domain mutated partially rescued the vascular phenotype (Fig. 6B and C). However, 7 of 10 mice expressing the N-RK mutant developed vascular lesions, as did 4 of 8 expressing the C-RK mutant (Table 1) (both results were significantly different [P < 0.05] compared to WT), although none of these mice displayed the most severe phenotype. From these studies we conclude a requirement for phosphotyrosine binding by Syk in the signaling pathway to optimally regulate vascular separation.
TABLE 1.
Requirements of domains of Syk for vascular separation
| Retroviral construct (n) | No. with vascular phenotypea | Cellular response to stimulationb | ||||
|---|---|---|---|---|---|---|
| None (−) | Mild (+) | Moderate (++) | Severe (+++) | Immunoreceptor | Integrin | |
| WT (9) | 9 | 0 | 0 | 0 | + | + |
| N-RKc,d (10) | 3 | 5 | 2 | 0 | − | − |
| C-RKc,d (8) | 4 | 2 | 2 | 0 | − | − |
| KDc (4) | 0 | 0 | 1 | 3 | − | − |
| MIGR1c (13) | 0 | 3 | 6 | 4 | − | − |
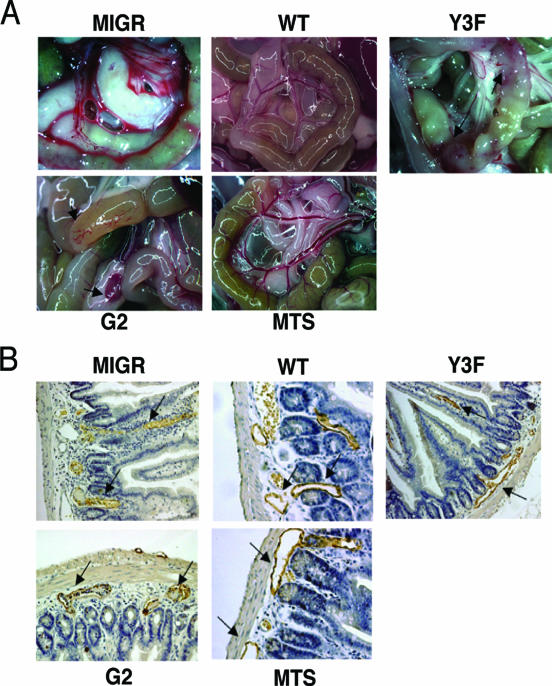
All mice reconstituted with SLP-76-deficient cells expressing GFP alone developed the vascular abnormality with varying degrees of severity (Fig. 7A; Table 2). Expression of WT SLP-76 uniformly rescued the vascular abnormality (Fig. 7A; Table 2). Ten of 12 chimeric mice expressing the Y3F mutant within their hematopoietic compartment displayed evidence of the vascular phenotype (Fig. 7A; Table 2) (P < 0.0002 compared to WT). Mice reconstituted with G2-expressing cells displayed vascular lesions in 11 of 14 recipients although, unlike with the Y3F mutant, none showed the most severe phenotype (Fig. 7A; Table 2) (P < 0.0002 compared to WT). Histological examination of the vascular lesions in the gut wall of affected chimeric mice revealed blood-filled lymphatics (Fig. 7B). As with WT SLP-76, expression of MTS uniformly prevents the development of any vascular lesions (Fig. 7A and B; Table 2).
FIG. 7.
Membrane localization of SLP-76 is required for blood-lymphatic vascular separation. (A) Chimeric mice were sacrificed and examined for the appearance of grossly visible vascular lesions in the small intestine and mesenteric lymph nodes. Original magnification, ×1. (B) Tissue from small intestines of chimeric mice was fixed, paraffin embedded, sectioned, and stained for Lyve-1 with horseradish peroxidase-tagged secondary antibody to identify lymphatic vessels. Arrows identify lymphatic vessels. Original magnification, ×200.
TABLE 2.
Requirement of SLP-76 domains for vascular separation
| Retroviral construct (n) | No. with vascular phenotypea | Cellular response to stimulationb | ||||
|---|---|---|---|---|---|---|
| None (−) | Mild (+) | Moderate (++) | Severe (+++) | Immunoreceptor | Integrin | |
| WT (16) | 16 | 0 | 0 | 0 | + | + |
| Y3Fd (12) | 2 | 4 | 4 | 2 | − | − |
| G2d,e (15) | 4 | 5 | 6 | 0 | − | + |
| MTSe (15) | 15 | 0 | 0 | 0 | + | −/+c |
| MIGR1d (14) | 0 | 3 | 5 | 6 | − | − |
DISCUSSION
Syk and SLP-76 play important roles in the proximal signal cascades initiated by both immunoreceptors and integrins. The functions of Syk and SLP-76 in immunoreceptor signaling have been extensively studied, but less is known about their roles in integrin-dependent cellular activation. In this investigation, we showed that the phosphotyrosine-binding capacity of both SH2 domains of Syk is required for activation by integrins as well as immunoreceptors, in contrast to the CHO cell model system, in which the requirement for Syk activation appears less stringent. These results suggest that, as with immunoreceptor signaling, an ITAM-containing molecule may be required for integrin signaling, at least in platelets and neutrophils. In distinction from our studies of Syk, our results do show a significant difference in the binding partner requirements of SLP-76 for function downstream of integrins versus immunoreceptors, in that the Gads-binding region of SLP-76 is essential for immunoreceptor but not integrin signaling. Using mutant SLP-76 constructs which selectively support immunoreceptor or integrin pathways, we showed that functional integrin-dependent cellular activation does not correlate with rescue of the vascular separation defect observed in deficient mice.
The requirement for phosphotyrosine binding of Syk in ITAM-dependent signaling was expected, but, surprisingly, it was also required for integrin signaling. Previous studies have shown Syk binding directly to the β chain of integrins in a phosphotyrosine-independent manner that requires only the N-terminal SH2 domain and interdomain A (56, 57). Supporting an SH2 domain-independent function of Syk, we found that the RK mutation in either SH2 domain of Syk does not affect Syk activation as assessed by tyrosine phosphorylation following engagement of αIIbβ3 in CHO cells. However, these mutations resulted in complete loss of Syk-dependent integrin responses in both neutrophils and platelets. There are several possible explanations for this difference. First, minimal mutations in the SH2 domains may result in defective interaction with the integrin β chain, which is overcome when Syk and αIIbβ3 are overexpressed in CHO cells. Second, overexpression of αIIbβ3 and Syk in CHO cells could result in nonspecific biochemical interactions, which do not predict function in primary cells. Third, integrins in neutrophils and platelets may utilize ITAM-containing molecules to activate the Syk kinase-dependent signaling pathways. Finally, Syk may directly bind to and be activated by the cytoplasmic tail of the integrin β chain in vivo as in vitro, but to initiate the requisite signaling pathway, a secondary interaction with an ITAM-containing protein is required. Given the specificity of the arginine-to-lysine mutation in the SH2 domain of Syk, our results argue strongly for an ITAM requirement for integrin signaling in platelets and neutrophils. In this regard, while ITAM-based Syk-dependent signaling has been extensively studied in the context of immunoreceptors, recent reports have highlighted the importance of this signaling pathway in a number of nonimmunoreceptor systems. The PSGL-1 adhesion receptor, for example, has been shown to utilize the ITAM-containing cytoplasmic Ezrin/Radixin/Moesin proteins to activate Syk and, therefore, gene transcription (17). These results raise the possibility that integrins may utilize similar ITAM-containing proteins, cytoplasmic or transmembrane, to activate Syk. Further investigation is required to understand the requirement for phosphotyrosine binding by Syk in integrin signaling.
It is possible that the absence of an integrin-dependent response in cells expressing the SH2 domain-mutated Syk reflects a quantitative, rather than a qualitative, signaling deficiency and that high concentrations of ligand would rescue the defect. However, in our experiments stimulating surfaces were covered with saturating concentrations of fibrinogen and therefore addition of more agonist would be unlikely to have any additional effects. It is also important to note that when the percentage of cells expressing WT Syk or SLP-76 was significant (>70%), ROI production (measured as nanomoles of ROI per million cells) was equivalent to that seen when 100% wild-type neutrophils were examined (31, 34).
Another possible caveat for interpretations of our data is that the differences in the cellular response in our various assays could be partially explained by varied levels of expression of the mutated constructs. This is of more significant concern for the Syk constructs, as they are expressed using a bicistronic virus and hence not directly coupled to GFP. However, with the exception of mice reconstituted with stem cells transduced with virus expressing GFP alone (MIGR), there is significant overlap in both the expression level and the percentage of cells transduced for the various constructs in all cell lineages analyzed (including splenic T cells, platelets, neutrophils, and B cells [data not shown]). Further, in CHO cells, when normalized for the percentage of GFP+ cells and mean fluorescence intensity (MFI), Western blotting showed equivalent protein levels with the various constructs (Fig. 1B). Finally, studies by others have shown that expression of GFP even from an internal ribosome entry site correlates with expression of the protein of interest (39).
We recognize that our experimental approach necessarily results in some degree of variability in both number of cells transduced and in the level of protein expressed. However, we do not believe that these variables impact our conclusions. Within the range of transduction efficiency in our studies, the qualitative results of each of our functional assays were independent of the percentage of cells transduced (GFP+) or the MFI. For example, in the neutrophil studies examining the cellular response to integrin stimulation, ROI production was significantly rescued by WT Syk (when compared to the negative control cells [MIGR]) even when as few as 19% of neutrophils expressed the wild-type construct (date not shown). In contrast, C-RK and N-RK Syk were incapable of supporting ROI production even with >60% of neutrophils transduced. All other assays we report here were done as single-cell assays and, again, expression of the SH2 mutants of Syk did not support any cellular response, no matter the MFI (utilizing GFP expression as a surrogate for Syk level). Given the uniform positive response seen in all assays (albeit to various degrees depending on GFP expression) with cells expressing WT Syk, the complete absence of response by cells expressing the Syk mutants is unlikely to be due to low levels of protein expression.
These studies using SLP-76 mutants also extend our understanding of the importance of the various domains of this adaptor in receptor signaling. In one model of SLP-76 function, immunoreceptor signaling is dependent upon SLP-76 localization to membrane rafts, while its function in integrin signaling is raft independent. One of the key substrates of activated Syk is LAT, a transmembrane adaptor, which is constitutively targeted to lipid rafts (64) and contains a binding site for Gads (48). Binding of Gads results in the recruitment of SLP-76 to the membrane, along with other SLP-76-associated proteins (21). While the Syk/LAT/Gads/SLP-76 pathway appears vital for signaling in immunoreceptor pathways, LAT and Gads are dispensable for integrin signaling, at least in platelets (7, 23).
One prediction from this model is that mutations in the Gads-binding region of SLP-76 should interfere with immunoreceptor but not integrin signaling. In fact, the G2 mutant is unable to rescue GPVI signaling in platelets (as well as FcɛRI in mast cells [25, 59] and TCR signaling in Jurkat cells [47]) but appears to function normally in platelet as well as neutrophil responses to integrin stimulation. These results, obtained in primary cells, suggest either SLP-76 recruitment to the plasma membrane is not required for optimal integrin signaling or a Gads/LAT-independent mechanism exists for SLP-76 membrane localization.
The complementary experiment of constitutively targeting SLP-76 to the membrane (using the MTS) rescued GPVI signaling in platelets but failed to rescue integrin-dependent ROI production in neutrophils. Interestingly, however, it rescued αIIbβ3-dependent spreading of platelets on fibrinogen. One explanation may be that platelet spreading may require a lower threshold of signaling than neutrophil ROI production. Although MTS is predominantly targeted to membrane rafts, it is also present to a limited extent outside of rafts (4), and this expression may support sufficient signaling for spreading of platelets. Alternatively, raft versus nonraft differentiation of immunoreceptor and integrin signaling may be cell type or receptor type specific. Finally, lack of ROI production in neutrophils expressing the MTS may be unrelated to rafts and may result from membrane targeting of SLP-76. Depending on the cell type and signaling pathway, the capacity for SLP-76 to leave the membrane may be as important as localization to the membrane.
Reconstitution of lethally irradiated WT mice with Syk- or SLP-76-deficient cells results in abnormal vascular architecture in the gut, bloody chylous peritoneum, and blood-filled lymphatic vessels (1). This phenotype recapitulates the phenotype of SLP-76-deficient adult mice and, to the extent that it involves blood-filled lymphatics, resembles the phenotype of Syk- and SLP-76-deficient embryos. The development of the vascular phenotype in radiation chimeras provides a tractable model to dissect the molecular pathway in which Syk and SLP-76 function to regulate vascular separation.
Our studies revealed that the kinase domain as well as the phosphotyrosine-binding function of both SH2 domains of Syk are needed for optimal vascular separation. The kinase domain of Syk is absolutely required to prevent mixing between blood and lymphatic vasculature. In fact, chimeric mice expressing the KD Syk mutant in their hematopoietic compartment were more severely affected than mice containing Syk-deficient cells, possibly as a result of a dominant negative effect of KD Syk. Perhaps low-level expression of the related protein tyrosine kinase ZAP-70 ameliorates to some extent the vascular phenotype, and this effect is blocked by the KD Syk. The expression of Syk with a mutation in one SH2 domain conferred vascular mixing in 11 out of 18 mice. This result is consistent with an ITAM-dependent signaling pathway, as both SH2 domains of Syk are required for binding to and activation by phosphorylated ITAMs.
While 11 mice expressing an SH2 domain-mutated Syk displayed a vascular phenotype, 7 mice did not. Two points suggest, however, that the presence of vascular mixing in a subset of mice is more significant than the absence of a phenotype in the remainder. First, expression of WT Syk (and also WT SLP-76 and MTS) uniformly prevents blood-lymphatic mixing, arguing that if signaling is fully restored then vascular mixing never develops. Secondly, even in irradiated mice restored with an unmanipulated Syk-deficient (or SLP-76-deficient) hematopoietic compartment, there is a range of phenotypes, with some mice only mildly affected. While it is unclear why such a range of phenotypes would develop, it is possible that even inefficient signaling may be sufficient to prevent vascular mixing in the subset of mice that would otherwise develop mild phenotypes.
Expression of WT SLP-76 in the hematopoietic compartment prevented development of the vascular phenotype in irradiated recipient mice. The three N-terminal residues of SLP-76 are required for prevention of blood-lymphatic misconnections in the vast majority of experimental mice. These three residues mediate binding to Vav, Nck, and Tec family kinases. Our data imply that one or more of these proteins may play an important role in the signaling pathway regulating lymphatic endothelial cell-blood endothelial cell separation. In fact, Tec/Btk-deficient mice display an in utero phenotype strongly reminiscent of Syk- and SLP-76-deficient mice (W. Ellmeir and M. L. Kahn, unpublished observations). Study of SLP-76 with single tyrosine mutations should identify which binding partner(s) is most critical in maintaining the lymphatic-blood separation.
The Gads-binding region of SLP-76 is also needed for blood-lymphatic separation, although not as absolutely as the N-terminal tyrosines. Structure-function studies in T cells (27, 33) and mast cells (25, 59) also showed a somewhat less severe phenotype with loss of the Gads-binding region compared to loss of the N-terminal tyrosines. Because of the presence of vascular mixing in 11 of 15 mice expressing the G2 mutant of SLP-76, it is reasonable to conclude that optimal signaling in the pathway regulating the separation of blood and lymphatic vasculature requires a functional Gads-binding domain. This finding suggests that localization of SLP-76 to the membrane is required for the signaling pathways regulating vascular separation. Furthermore, in the context of a WT-like response of G2 mutant-expressing neutrophils and platelets to integrin stimulation, the inability of the G2 mutant to rescue vascular separation argues that the vascular misconnections do not result from defects in an integrin pathway.
Given the loss of integrin-dependent as well as immunoreceptor-dependent cellular responses with mutation of either SH2 domain of Syk, it seems likely that integrins utilize ITAM-containing proteins to promote signaling by Syk/SLP-76. The G2 mutant of SLP-76 reveals that the Gads/SLP-76 interaction is likely not required for integrin signaling. Given the importance of SLP-76 membrane recruitment and signaling downstream of the TCR and FcɛR, it would seem likely that an alternative to Gads binding exists for SLP-76 membrane translocation in the context of integrin signaling. Further studies, ideally in the setting of a stable genetic system, are necessary to elucidate such a mechanism. Our report suggests that integrin and immunoreceptor signaling in hematopoietic cells may be more similar in some ways than predicted, in that both may require an ITAM-containing molecule. In contrast, we have also identified a key difference, showing that SLP-76 membrane relocalization is either unnecessary or occurs in a Gads-independent manner. These results suggest the possibility of different requirements for SLP-76 in immunoreceptor versus integrin signaling for various in vivo cellular functions.
Acknowledgments
We thank Reuban Sirgananian and Victor Tybulewicz for critical reagents and Gael Ménasché and Michael Silverman for critical reading of the manuscript.
This work was supported by NIH training grant HL07971 (F.A.), RO1 HL072798 (M.L.K.), Howard Hughes Medical Institute Predoctoral Fellowship (N.B.), and the American Heart Association (E.S.).
REFERENCES
- 1.Abtahian, F., A. Guerriero, E. Sebzda, M. M. Lu, R. Zhou, A. Mocsai, E. E. Myers, B. Huang, D. G. Jackson, V. A. Ferrari, V. Tybulewicz, C. A. Lowell, J. J. Lepore, G. A. Koretzky, and M. L. Kahn. 2003. Regulation of blood and lymphatic vascular separation by signaling proteins SLP-76 and Syk. Science 299**:**247-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asada, H., N. Ishii, Y. Sasaki, K. Endo, H. Kasai, N. Tanaka, T. Takeshita, S. Tsuchiya, T. Konno, and K. Sugamura. 1999. Grf40, a novel Grb2 family member, is involved in T cell signaling through interaction with SLP-76 and LAT. J. Exp. Med. 189**:**1383-1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banerji, S., J. Ni, S. X. Wang, S. Clasper, J. Su, R. Tammi, M. Jones, and D. G. Jackson. 1999. LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J. Cell Biol. 144**:**789-801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boerth, N. J., J. J. Sadler, D. E. Bauer, J. L. Clements, S. M. Gheith, and G. A. Koretzky. 2000. Recruitment of SLP-76 to the membrane and glycolipid-enriched membrane microdomains replaces the requirement for linker for activation of T cells in T cell receptor signaling. J. Exp. Med. 192**:**1047-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bourette, R. P., S. Arnaud, G. M. Myles, J. P. Blanchet, L. R. Rohrschneider, and G. Mouchiroud. 1998. Mona, a novel hematopoietic-specific adaptor interacting with the macrophage colony-stimulating factor receptor, is implicated in monocyte/macrophage development. EMBO J. 17**:**7273-7281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bunnell, S. C., M. Diehn, M. B. Yaffe, P. R. Findell, L. C. Cantley, and L. J. Berg. 2000. Biochemical interactions integrating Itk with the T cell receptor-initiated signaling cascade. J. Biol. Chem. 275**:**2219-2230. [DOI] [PubMed] [Google Scholar]
- 7.Chen, H., and M. L. Kahn. 2003. Reciprocal signaling by integrin and nonintegrin receptors during collagen activation of platelets. Mol. Cell. Biol. 23**:**4764-4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng, A. M., B. Rowley, W. Pao, A. Hayday, J. B. Bolen, and T. Pawson. 1995. Syk tyrosine kinase required for mouse viability and B-cell development. Nature 378**:**303-306. [DOI] [PubMed] [Google Scholar]
- 9.Clark, E. A., S. J. Shattil, M. H. Ginsberg, J. Bolen, and J. S. Brugge. 1994. Regulation of the protein tyrosine kinase pp72syk by platelet agonists and the integrin alpha IIb beta 3. J. Biol. Chem. 269**:**28859-28864. [PubMed] [Google Scholar]
- 10.Clements, J. L., J. R. Lee, B. Gross, B. Yang, J. D. Olson, A. Sandra, S. P. Watson, S. R. Lentz, and G. A. Koretzky. 1999. Fetal hemorrhage and platelet dysfunction in SLP-76-deficient mice. J. Clin. Investig. 103**:**19-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clements, J. L., S. E. Ross-Barta, L. T. Tygrett, T. J. Waldschmidt, and G. A. Koretzky. 1998. SLP-76 expression is restricted to hemopoietic cells of monocyte, granulocyte, and T lymphocyte lineage and is regulated during T cell maturation and activation. J. Immunol. 161**:**3880-3889. [PubMed] [Google Scholar]
- 12.Clements, J. L., B. Yang, S. E. Ross-Barta, S. L. Eliason, R. F. Hrstka, R. A. Williamson, and G. A. Koretzky. 1998. Requirement for the leukocyte-specific adapter protein SLP-76 for normal T cell development. Science 281**:**416-419. [DOI] [PubMed] [Google Scholar]
- 13.Costello, P. S., M. Turner, A. E. Walters, C. N. Cunningham, P. H. Bauer, J. Downward, and V. L. Tybulewicz. 1996. Critical role for the tyrosine kinase Syk in signalling through the high affinity IgE receptor of mast cells. Oncogene 13**:**2595-2605. [PubMed] [Google Scholar]
- 14.Crowley, M. T., P. S. Costello, C. J. Fitzer-Attas, M. Turner, F. Meng, C. Lowell, V. L. Tybulewicz, and A. L. DeFranco. 1997. A critical role for Syk in signal transduction and phagocytosis mediated by Fcγ receptors on macrophages. J. Exp. Med. 186**:**1027-1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.da Silva, A. J., Z. Li, C. de Vera, E. Canto, P. Findell, and C. E. Rudd. 1997. Cloning of a novel T-cell protein FYB that binds FYN and SH2-domain-containing leukocyte protein 76 and modulates interleukin 2 production. Proc. Natl. Acad. Sci. USA 94**:**7493-7498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ellis, J. H., C. Ashman, M. N. Burden, K. E. Kilpatrick, M. A. Morse, and P. A. Hamblin. 2000. GRID: a novel Grb-2-related adapter protein that interacts with the activated T cell costimulatory receptor CD28. J. Immunol. 164**:**5805-5814. [DOI] [PubMed] [Google Scholar]
- 17.Fodor, S., Z. Jakus, and A. Mocsai. 2006. ITAM-based signaling beyond the adaptive immune response. Immunol. Lett. 104**:**29-37. [DOI] [PubMed] [Google Scholar]
- 18.Gao, J., K. E. Zoller, M. H. Ginsberg, J. S. Brugge, and S. J. Shattil. 1997. Regulation of the pp72syk protein tyrosine kinase by platelet integrin alpha IIb beta 3. EMBO J. 16**:**6414-6425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gibbins, J., J. Asselin, R. Farndale, M. Barnes, C. L. Law, and S. P. Watson. 1996. Tyrosine phosphorylation of the Fc receptor gamma-chain in collagen-stimulated platelets. J. Biol. Chem. 271**:**18095-18099. [DOI] [PubMed] [Google Scholar]
- 20.Hynes, R. O. 2002. Integrins: bidirectional, allosteric signaling machines. Cell 110**:**673-687. [DOI] [PubMed] [Google Scholar]
- 21.Jordan, M. S., A. L. Singer, and G. A. Koretzky. 2003. Adaptors as central mediators of signal transduction in immune cells. Nat. Immunol. 4**:**110-116. [DOI] [PubMed] [Google Scholar]
- 22.Judd, B. A., P. S. Myung, L. Leng, A. Obergfell, W. S. Pear, S. J. Shattil, and G. A. Koretzky. 2000. Hematopoietic reconstitution of SLP-76 corrects hemostasis and platelet signaling through alpha IIb beta 3 and collagen receptors. Proc. Natl. Acad. Sci. USA 97**:**12056-12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Judd, B. A., P. S. Myung, A. Obergfell, E. E. Myers, A. M. Cheng, S. P. Watson, W. S. Pear, D. Allman, S. J. Shattil, and G. A. Koretzky. 2002. Differential requirement for LAT and SLP-76 in GPVI versus T cell receptor signaling. J. Exp. Med. 195**:**705-717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keely, P. J., and L. V. Parise. 1996. The α2β1 integrin is a necessary co-receptor for collagen-induced activation of Syk and the subsequent phosphorylation of phospholipase Cγ2 in platelets. J. Biol. Chem. 271**:**26668-26676. [PubMed] [Google Scholar]
- 25.Kettner, A., V. Pivniouk, L. Kumar, H. Falet, J. S. Lee, R. Mulligan, and R. S. Geha. 2003. Structural requirements of SLP-76 in signaling via the high-affinity immunoglobulin E receptor (Fc epsilon RI) in mast cells. Mol. Cell. Biol. 23**:**2395-2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kiefer, F., J. Brumell, N. Al-Alawi, S. Latour, A. Cheng, A. Veillette, S. Grinstein, and T. Pawson. 1998. The Syk protein tyrosine kinase is essential for Fcγ receptor signaling in macrophages and neutrophils. Mol. Cell. Biol. 18**:**4209-4220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumar, L., V. Pivniouk, M. A. de la Fuente, D. Laouini, and R. S. Geha. 2002. Differential role of SLP-76 domains in T cell development and function. Proc. Natl. Acad. Sci. USA 99**:**884-889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Law, C. L., M. K. Ewings, P. M. Chaudhary, S. A. Solow, T. J. Yun, A. J. Marshall, L. Hood, and E. A. Clark. 1999. GrpL, a Grb2-related adaptor protein, interacts with SLP-76 to regulate nuclear factor of activated T cell activation. J. Exp. Med. 189**:**1243-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu, S. K., N. Fang, G. A. Koretzky, and C. J. McGlade. 1999. The hematopoietic-specific adaptor protein gads functions in T-cell signaling via interactions with the SLP-76 and LAT adaptors. Curr. Biol. 9**:**67-75. [DOI] [PubMed] [Google Scholar]
- 30.Lofgren, R., L. Serrander, M. Forsberg, A. Wilsson, A. Wasteson, and O. Stendahl. 1999. CR3, FcγRIIA and FcγRIIIB induce activation of the respiratory burst in human neutrophils: the role of intracellular Ca2+, phospholipase D and tyrosine phosphorylation. Biochim. Biophys. Acta 1452**:**46-59. [DOI] [PubMed] [Google Scholar]
- 31.Mocsai, A., M. Zhou, F. Meng, V. L. Tybulewicz, and C. A. Lowell. 2002. Syk is required for integrin signaling in neutrophils. Immunity 16**:**547-558. [DOI] [PubMed] [Google Scholar]
- 32.Musci, M. A., L. R. Hendricks-Taylor, D. G. Motto, M. Paskind, J. Kamens, C. W. Turck, and G. A. Koretzky. 1997. Molecular cloning of SLAP-130, an SLP-76-associated substrate of the T cell antigen receptor-stimulated protein tyrosine kinases. J. Biol. Chem. 272**:**11674-11677. [DOI] [PubMed] [Google Scholar]
- 33.Myung, P. S., G. S. Derimanov, M. S. Jordan, J. A. Punt, Q. H. Liu, B. A. Judd, E. E. Meyers, C. D. Sigmund, B. D. Freedman, and G. A. Koretzky. 2001. Differential requirement for SLP-76 domains in T cell development and function. Immunity 15**:**1011-1026. [DOI] [PubMed] [Google Scholar]
- 34.Newbrough, S. A., A. Mocsai, R. A. Clemens, J. N. Wu, M. A. Silverman, A. L. Singer, C. A. Lowell, and G. A. Koretzky. 2003. SLP-76 regulates Fcγ receptor and integrin signaling in neutrophils. Immunity 19**:**761-769. [DOI] [PubMed] [Google Scholar]
- 35.Obergfell, A., B. A. Judd, M. A. del Pozo, M. A. Schwartz, G. A. Koretzky, and S. J. Shattil. 2001. The molecular adapter SLP-76 relays signals from platelet integrin αIIbβ3 to the actin cytoskeleton. J. Biol. Chem. 276**:**5916-5923. [DOI] [PubMed] [Google Scholar]
- 36.Onodera, H., D. G. Motto, G. A. Koretzky, and D. M. Rothstein. 1996. Differential regulation of activation-induced tyrosine phosphorylation and recruitment of SLP-76 to Vav by distinct isoforms of the CD45 protein-tyrosine phosphatase. J. Biol. Chem. 271**:**22225-22230. [DOI] [PubMed] [Google Scholar]
- 37.Paolini, R., R. Molfetta, L. O. Beitz, J. Zhang, A. M. Scharenberg, M. Piccoli, L. Frati, R. Siraganian, and A. Santoni. 2002. Activation of Syk tyrosine kinase is required for c-Cbl-mediated ubiquitination of FcɛRI and Syk in RBL cells. J. Biol. Chem. 277**:**36940-36947. [DOI] [PubMed] [Google Scholar]
- 38.Pasquet, J. M., B. Gross, L. Quek, N. Asazuma, W. Zhang, C. L. Sommers, E. Schweighoffer, V. Tybulewicz, B. Judd, J. R. Lee, G. Koretzky, P. E. Love, L. E. Samelson, and S. P. Watson. 1999. LAT is required for tyrosine phosphorylation of phospholipase Cγ2 and platelet activation by the collagen receptor GPVI. Mol. Cell. Biol. 19**:**8326-8334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pear, W. S., J. P. Miller, L. Xu, J. C. Pui, B. Soffer, R. C. Quackenbush, A. M. Pendergast, R. Bronson, J. C. Aster, M. L. Scott, and D. Baltimore. 1998. Efficient and rapid induction of a chronic myelogenous leukemia-like myeloproliferative disease in mice receiving P210 bcr/abl-transduced bone marrow. Blood 92**:**3780-3792. [PubMed] [Google Scholar]
- 40.Pivniouk, V., E. Tsitsikov, P. Swinton, G. Rathbun, F. W. Alt, and R. S. Geha. 1998. Impaired viability and profound block in thymocyte development in mice lacking the adaptor protein SLP-76. Cell 94**:**229-238. [DOI] [PubMed] [Google Scholar]
- 41.Pivniouk, V. I., T. R. Martin, J. M. Lu-Kuo, H. R. Katz, H. C. Oettgen, and R. S. Geha. 1999. SLP-76 deficiency impairs signaling via the high-affinity IgE receptor in mast cells. J. Clin. Investig. 103**:**1737-1743. [PMC free article] [PubMed] [Google Scholar]
- 42.Polgar, J., J. M. Clemetson, B. E. Kehrel, M. Wiedemann, E. M. Magnenat, T. N. Wells, and K. J. Clemetson. 1997. Platelet activation and signal transduction by convulxin, a C-type lectin from Crotalus durissus terrificus (tropical rattlesnake) venom via the p62/GPVI collagen receptor. J. Biol. Chem. 272**:**13576-13583. [DOI] [PubMed] [Google Scholar]
- 43.Poole, A., J. M. Gibbins, M. Turner, M. J. van Vugt, J. G. van de Winkel, T. Saito, V. L. Tybulewicz, and S. P. Watson. 1997. The Fc receptor gamma-chain and the tyrosine kinase Syk are essential for activation of mouse platelets by collagen. EMBO J. 16**:**2333-2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rowley, R. B., A. L. Burkhardt, H. G. Chao, G. R. Matsueda, and J. B. Bolen. 1995. Syk protein-tyrosine kinase is regulated by tyrosine-phosphorylated Ig alpha/Ig beta immunoreceptor tyrosine activation motif binding and autophosphorylation. J. Biol. Chem. 270**:**11590-11594. [DOI] [PubMed] [Google Scholar]
- 45.Saitoh, S., R. Arudchandran, T. S. Manetz, W. Zhang, C. L. Sommers, P. E. Love, J. Rivera, and L. E. Samelson. 2000. LAT is essential for FcɛRI-mediated mast cell activation. Immunity 12**:**525-535. [DOI] [PubMed] [Google Scholar]
- 46.Sauer, K., J. Liou, S. B. Singh, D. Yablonski, A. Weiss, and R. M. Perlmutter. 2001. Hematopoietic progenitor kinase 1 associates physically and functionally with the adaptor proteins B cell linker protein and SLP-76 in lymphocytes. J. Biol. Chem. 276**:**45207-45216. [DOI] [PubMed] [Google Scholar]
- 47.Singer, A. L., S. C. Bunnell, A. E. Obstfeld, M. S. Jordan, J. N. Wu, P. S. Myung, L. E. Samelson, and G. A. Koretzky. 2004. Roles of the proline-rich domain in SLP-76 subcellular localization and T cell function. J. Biol. Chem. 279**:**15481-15490. [DOI] [PubMed] [Google Scholar]
- 48.Sommers, C. L., L. E. Samelson, and P. E. Love. 2004. LAT: a T lymphocyte adapter protein that couples the antigen receptor to downstream signaling pathways. Bioessays 26**:**61-67. [DOI] [PubMed] [Google Scholar]
- 49.Su, Y. W., Y. Zhang, J. Schweikert, G. A. Koretzky, M. Reth, and J. Wienands. 1999. Interaction of SLP adaptors with the SH2 domain of Tec family kinases. Eur. J. Immunol. 29**:**3702-3711. [DOI] [PubMed] [Google Scholar]
- 50.Suzuki-Inoue, K., O. Inoue, J. Frampton, and S. P. Watson. 2003. Murine GPVI stimulates weak integrin activation in PLCγ2−/− platelets: involvement of PLCγ1 and PI3-kinase. Blood 102**:**1367-1373. [DOI] [PubMed] [Google Scholar]
- 51.Tuosto, L., F. Michel, and O. Acuto. 1996. p95vav associates with tyrosine-phosphorylated SLP-76 in antigen-stimulated T cells. J. Exp. Med. 184**:**1161-1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Turner, M., P. J. Mee, P. S. Costello, O. Williams, A. A. Price, L. P. Duddy, M. T. Furlong, R. L. Geahlen, and V. L. Tybulewicz. 1995. Perinatal lethality and blocked B-cell development in mice lacking the tyrosine kinase Syk. Nature 378**:**298-302. [DOI] [PubMed] [Google Scholar]
- 53.Waksman, G., S. E. Shoelson, N. Pant, D. Cowburn, and J. Kuriyan. 1993. Binding of a high affinity phosphotyrosyl peptide to the Src SH2 domain: crystal structures of the complexed and peptide-free forms. Cell 72**:**779-790. [DOI] [PubMed] [Google Scholar]
- 54.Wardenburg, J. B., C. Fu, J. K. Jackman, H. Flotow, S. E. Wilkinson, D. H. Williams, R. Johnson, G. Kong, A. C. Chan, and P. R. Findell. 1996. Phosphorylation of SLP-76 by the ZAP-70 protein-tyrosine kinase is required for T-cell receptor function. J. Biol. Chem. 271**:**19641-19644. [DOI] [PubMed] [Google Scholar]
- 55.Watson, F., L. Gasmi, and S. W. Edwards. 1997. Stimulation of intracellular Ca2+ levels in human neutrophils by soluble immune complexes. Functional activation of FcγRIIIb during priming. J. Biol. Chem. 272**:**17944-17951. [DOI] [PubMed] [Google Scholar]
- 56.Woodside, D. G., A. Obergfell, L. Leng, J. L. Wilsbacher, C. K. Miranti, J. S. Brugge, S. J. Shattil, and M. H. Ginsberg. 2001. Activation of Syk protein tyrosine kinase through interaction with integrin beta cytoplasmic domains. Curr. Biol. 11**:**1799-1804. [DOI] [PubMed] [Google Scholar]
- 57.Woodside, D. G., A. Obergfell, A. Talapatra, D. A. Calderwood, S. J. Shattil, and M. H. Ginsberg. 2002. The N-terminal SH2 domains of Syk and ZAP-70 mediate phosphotyrosine-independent binding to integrin beta cytoplasmic domains. J. Biol. Chem. 277**:**39401-39408. [DOI] [PubMed] [Google Scholar]
- 58.Wu, J., D. G. Motto, G. A. Koretzky, and A. Weiss. 1996. Vav and SLP-76 interact and functionally cooperate in IL-2 gene activation. Immunity 4**:**593-602. [DOI] [PubMed] [Google Scholar]
- 59.Wu, J. N., M. S. Jordan, M. A. Silverman, E. J. Peterson, and G. A. Koretzky. 2004. Differential requirement for adapter proteins Src homology 2 domain-containing leukocyte phosphoprotein of 76 kDa and adhesion- and degranulation-promoting adapter protein in FɛRI signaling and mast cell function. J. Immunol. 172**:**6768-6774. [DOI] [PubMed] [Google Scholar]
- 60.Wu, J. N., and G. A. Koretzky. 2004. The SLP-76 family of adapter proteins. Semin. Immunol. 16**:**379-393. [DOI] [PubMed] [Google Scholar]
- 61.Wunderlich, L., A. Farago, J. Downward, and L. Buday. 1999. Association of Nck with tyrosine-phosphorylated SLP-76 in activated T lymphocytes. Eur. J. Immunol. 29**:**1068-1075. [DOI] [PubMed] [Google Scholar]
- 62.Yablonski, D., T. Kadlecek, and A. Weiss. 2001. Identification of a phospholipase C-γ1 (PLC-γ1) SH3 domain-binding site in SLP-76 required for T-cell receptor-mediated activation of PLC-gamma1 and NFAT. Mol. Cell. Biol. 21**:**4208-4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yan, S. R., M. Huang, and G. Berton. 1997. Signaling by adhesion in human neutrophils: activation of the p72syk tyrosine kinase and formation of protein complexes containing p72syk and Src family kinases in neutrophils spreading over fibrinogen. J. Immunol. 158**:**1902-1910. [PubMed] [Google Scholar]
- 64.Zhang, W., J. Sloan-Lancaster, J. Kitchen, R. P. Trible, and L. E. Samelson. 1998. LAT: the ZAP-70 tyrosine kinase substrate that links T cell receptor to cellular activation. Cell 92**:**83-92. [DOI] [PubMed] [Google Scholar]
- 65.Zhang, W., C. L. Sommers, D. N. Burshtyn, C. C. Stebbins, J. B. DeJarnette, R. P. Trible, A. Grinberg, H. C. Tsay, H. M. Jacobs, C. M. Kessler, E. O. Long, P. E. Love, and L. E. Samelson. 1999. Essential role of LAT in T cell development. Immunity 10**:**323-332. [DOI] [PubMed] [Google Scholar]