An EP2 receptor-selective prostaglandin E2 agonist induces bone healing (original) (raw)
Abstract
The morbidity and mortality associated with impaired/delayed fracture healing remain high. Our objective was to identify a small nonpeptidyl molecule with the ability to promote fracture healing and prevent malunions. Prostaglandin E2 (PGE2) causes significant increases in bone mass and bone strength when administered systemically or locally to the skeleton. However, due to side effects, PGE2 is an unacceptable therapeutic option for fracture healing. PGE2 mediates its tissue-specific pharmacological activity via four different G protein-coupled receptor subtypes, EP1, -2, -3, and -4. The anabolic action of PGE2 in bone has been linked to an elevated level of cAMP, thereby implicating the EP2 and/or EP4 receptor subtypes in bone formation. We identified an EP2 selective agonist, CP-533,536, which has the ability to heal canine long bone segmental and fracture model defects without the objectionable side effects of PGE2, suggesting that the EP2 receptor subtype is a major contributor to PGE2's local bone anabolic activity. The potent bone anabolic activity of CP-533,536 offers a therapeutic alternative for the treatment of fractures and bone defects in patients.
The skeleton has the unique ability to repair and heal itself after injury (1, 2). This process is a cascade of synchronized events involving many systemic and local signaling molecules (3). However, in ≈10% of cases, fractured bones heal more slowly (malunion) or fail to heal (nonunion), requiring additional costly medical intervention to repair the fracture (4). These malunions and nonunions cause significant patient morbidity, significantly limiting quality of life and increasing healthcare costs. New therapies that could ensure rapid healing of fractures and bone defects would lessen the need for further medical intervention and greatly reduce the morbidity and loss of independence associated with immobilization.
The discovery of bone morphogenetic proteins has increased our understanding of the cascade of events that takes place during fracture healing. Several clinical studies demonstrate the capability of these proteins to induce and facilitate this process (5–8). However, the cost effectiveness, degree of clinical benefit, and long-term safety of these therapies have not been fully elucidated. These issues prompted us to identify additional mechanisms and pathways involved in bone formation that could be modulated with a nonpeptidyl small molecule. Such a compound could be used as a therapy to promote fracture healing and prevent malunions. Prostaglandin E2 (PGE2) has been shown to have multiple biological effects in many tissues, including bone. PGE2 causes significant increases in bone mass and bone strength when administered systemically or locally to the skeleton (9–11). However, due to side effects, including diarrhea, lethargy, and flushing, PGE2 is an unacceptable therapeutic option for bone healing. PGE2 binds to and elicits its pharmacological activity from four different cell surface receptor subtypes, EP1, -2, -3, and -4 (12–16). These four G proteincoupled receptors are responsible for mediating the tissue-specific actions of PGE2. Of these four receptors, three are involved in modulation of cAMP levels (15). Activation of the EP2 and -4 receptor subtypes results in the elevation of intracellular cAMP levels, whereas activation of the EP3 receptor results in a reduction of intracellular cAMP levels (15). The fourth receptor, EP1, is involved in regulating intracellular calcium levels. In bone, PGE2 has an anabolic action that has been linked to an elevated level of cAMP, thereby implicating the EP2 and/or EP4 receptor subtypes in bone formation. We initiated a discovery effort to identify EP2 and -4 receptor selective agonists.
In this report, we show that CP-533,536, a highly selective and potent functional EP2 receptor agonist, is able to mimic the effects of PGE2 on bone when directly injected into the marrow of rats. When administered locally to bone in canine models such as the critical defect of the ulna or tibial osteotomy, CP-533,536 successfully induced bone repair without the side effects associated with PGE2. Given the high morbidity associated with fracture malunions and the limitations of current procedures for augmenting fracture healing such as the use of autografts and allografts, the development of this alternative treatment for bone healing has the potential to meet a significant medical need (17–21).
Materials and Methods
Screening for EP2 Selective Agonists. Compounds were categorized for further characterization on the basis of their ability to selectively bind the EP2 receptor. EP2 receptor agonism was defined by the ability of compounds to selectively bind to the EP2 receptor and increase intracellular cAMP levels.
Receptor Binding. Membranes were prepared from stably transfected HEK-293 cells (American Type Culture Collection) expressing PGE2 EP1, -2, -3, or -4 receptor subtypes as well as those for prostaglandin D2, prostaglandin F2α, prostacyclin, and thromboxane. Receptor binding was measured as described by Castleberry et al. (22).
Determination of cAMP. Cellular cAMP levels in the HEK-293/EP2 line were determined after pretreatment of 2 × 105 cells with 1 mM 3-isobutyl-1-methylxanthine (Calbiochem) for 10 min at 37°C followed by treatment with the indicated concentrations of test compounds for 12 min at 37°C. cAMP was quantitated by using an RIA kit according to the manufacturer's instructions (DuPont/NEN).
Rat Experiments. All animal experiments were conducted in accordance with relevant institutional guidelines for animal research. Forty male rats (Taconic Farms) at 6 weeks of age were injected with 10 ×l of either vehicle (5% ethanol in sterile injection water, n = 10) or CP-533,536 at 0.3, 1, and 3 mg/kg (n = 10 per dose) into the marrow cavity of the proximal tibial metaphysis underneath the secondary spongiosa. At 7 days postinjection, the animals were necropsied, and tibial injection sites were analyzed cross-sectionally by using peripheral quantitative computerized tomography (Stratec XCT Research M, Norland Medical Systems, Fort Atkinson, WI), as described by Ke et al. (23). Briefly, a 1-mm-thick cross section of the injection site was analyzed with a voxel size of 0.10 mm. Total bone area, bone mineral content, and total bone mineral density were determined as percent changes compared with vehicle-treated controls.
Canine Experiments
Critical Defect. Experiment 1. Male beagle dogs (Greenhill, Montichiari, Italy) were surgically prepared by creating a 1.5-cm segmental critical defect in the midulna by using a pendular saw according to accepted veterinary surgical practices. The radius and remaining interosseal membrane were left intact, and the soft tissues were closed in layers. Animals were divided into three experimental groups (n = 8 per group) and treated with three (Group A), seven (Group B), or 14 (Group C) daily injections of an aqueous solution of CP-533,536 [100 mg/ml in calcium magnesium-free PBS after surgery and implantation of Helistat (2.5 × 5 cm) precut collagen sponges into the defect area, Colla-Tec, Plainsboro, NJ]. Radiographs of the forelimbs were obtained immediately after surgery and every 2 weeks until termination of the study (week 10).
Experiment 2. In another set of ulnar critical defect experiments, male beagle dogs (n = 28), surgically prepared as above, were divided into four groups and treated with 1.0 ml of poly(D,L-lactide-co-glycolide) (PLGH) matrix (Atrix Laboratories) alone (Group A), 50 mg of CP-533,536 dissolved in 1.0 ml of matrix (Group B), 10 mg of CP-533,536 dissolved in 1.0 ml of matrix (Group C), or 10 mg of CP-533,536 dissolved in 0.2 ml of matrix (Group D). In these experiments, no precut collagen sponge was used in the defect area. The compound was administered into the defect area in a single dose at surgery. Blood (1.0 ml) was collected from animals 30 min, 2 h, 4 h, 24 h, 72 h, and 7 days after surgery. Animals were monitored postsurgically for side effects, and radiographs of the forelimbs were obtained immediately after surgery and every week until the termination of the study (week 24).
Tibial Osteotomy. For the tibial osteotomy model, male beagle dogs (n = 14) were prepared by making a surgical osteotomy on the distal portion of the dog tibia by using a Gigli saw according to accepted veterinary surgical practices. The proximal and distal bone segments were stabilized by using an AO plate. The remaining interosseal membrane was left intact. The defect site was irrigated with saline to remove bone debris and filled with PLGH matrix alone or matrix containing CP-533,536 in the following manner: Group A, dogs were left untreated (n = 3); Group B, dogs were treated with 0.5 ml of matrix alone (n = 3); Group C, dogs were treated with 5 mg of CP-533,536 dissolved in 0.5 ml of matrix (n = 4); and Group D, dogs were treated with 25 mg of CP-533,536 dissolved in 0.5 ml of matrix (n = 4). Blood (1.0 ml) was collected from all animals at 30 min, 2 h, 4 h, and 24 h after surgery. Radiographs of the forelimbs were obtained immediately after surgery and every week until the termination of the study (week 8).
Radiographs. Radiographs were interpreted by an experienced radiologist blinded to the treatment protocol and postoperative interval. They were graded on a 0–6 scale as described by Cook et al. (ref. 24; see Table 3).
Table 3.
Radiographic healing of ulnar critical defect in dogs treated with CP-533,536 at 24 weeks
Treatment groups
| Control, 1.0 ml of PLGH | 50 mg of CP-533,536 + 1.0 ml of PLGH | 10 mg of CP-533,536 + 1.0 ml of PLGH | 10 mg of CP-533,536 + 0.2 ml of PLGH | ||||
|---|---|---|---|---|---|---|---|
| Animal | Radiographic healing | Animal | Radiographic healing | Animal | Radiographic healing | Animal | Radiographic healing |
| 1 | 0 | 9 | 6 | 13 | 6 | 25 | 3 |
| 2 | 0 | 10 | 6 | 14 | 2 | 26 | 6 |
| 3 | 0 | 11 | 2 | 15 | 3 | 27 | 6 |
| 4 | 0 | 12 | 5 | 16 | 6 | 28 | 6 |
| 5 | 0 | 17 | 6 | ||||
| 6 | 0 | 18 | 6 | ||||
| 7 | 0 | 19 | 6 | ||||
| 8 | 0 | 20 | 4 | ||||
| 21 | 6 | ||||||
| 22 | 6 | ||||||
| 23 | 6 | ||||||
| 24 | 6 |
Histology. On termination, in the ulnar defect or tibial osteotomy model, bones containing the defect site were dissected and muscles removed. The bone defect site with adjacent bone was cut with a saw, fixed in 10% neutral buffered formalin for 7 days, and then embedded in methyl methacrylate. Serial sections were cut through each defect and stained with toluidine blue and safranine O-fast green (23).
Analysis of CP-533,536 in Plasma. Plasma samples were thawed, and 20 ×l of each sample was injected into a PE-Sciex (Thornhill, ON, Canada) API 3000 triple quadrapole mass spectrometer with a turbo ionspray source. A Luna (Phenomenex, Torrance, CA) phenyl-hexyl (4.6 × 50 mm × 3 ×m) column was used for separation. CP-533,536 and the internal standard were determined in negative ion mode by using multireaction monitoring by following the mass transitions of 467.3/303.2 m/z and 388.3/198.1 m/z, respectively. The linear dynamic range of the assay was from 1 to 2,000 ng/ml. The mean accuracy of the assay characterized with quality control standards was 80–116%.
Statistical Analysis. Statistics were calculated by using STATVIEW 4.0 packages (Abacus Concepts, Berkeley, CA). The ANOVA test was used for all group comparisons, and Fisher's protected least significant difference was used to compare the differences between each group.
Results and Discussion
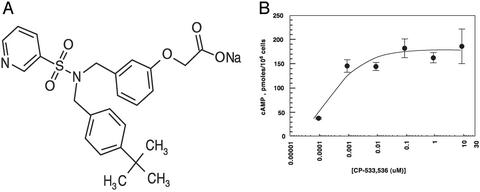
CP-533,536, a Pyridyl Sulfonamide, Is an EP2 Receptor Agonist. An EP2 receptor selective agonist was sought based on PGE2's linkage to bone anabolism (16, 25). A previously published acyclic sulfonamide (26) was determined to be a weak selective EP2 agonist. Chemical modifications of the sulfonamide, amino acid, and bottom chains of the lead molecule resulted in identification of the more potent and highly EP2 selective pyridyl sulfonamide, CP-533,536 (Fig. 1_A_). CP-533,536 bound with high affinity to the rat EP2 receptor (IC50 = 50 nM) and is >50-fold more selective for binding to the EP2 receptor subtype compared with the EP4 receptor subtype (IC50 = >3,200 nM). Similarly, CP-533,536 was selective for EP2 binding when measured against other prostanoid receptors, including those for prostaglandin D2, prostaglandin F2a, prostacyclin, and thromboxane (Table 1). To ascertain whether CP-533,536 was an agonist or antagonist of the EP2 receptor, we studied the effect of CP-533,536 treatment on intracellular cAMP levels. The addition of PGE2 to cells transfected with the EP2 receptor resulted in receptor activation, and the expected increase in intracellular cAMP was observed. Similarly, addition of CP-533,536 resulted in an equivalent increase in intracellular cAMP with an IC50 of 5 nM, suggesting that CP-533,536 was a potent and full EP2 receptor agonist (Fig. 1_B_).
Fig. 1.
(A) Chemical structure of CP-533,536. (B) Analysis of intracellular cAMP levels on treatment of cells with CP-533,536. HEK-293 cells stably transfected with the EP2 receptor were treated with increasing concentrations of CP-533,536, and intracellular cAMP levels were measured.
Table 1. CP-533, 536-binding activity for EP and prostanoid receptors.
| Receptors | EP1 | EP2 | EP3 | EP4 | DP | FP | IP | TP |
|---|---|---|---|---|---|---|---|---|
| IC50, nM | >2,800 | 50 | >2,800 | >3,200 | 820 | >3,200 | >3,200 | >3,200 |
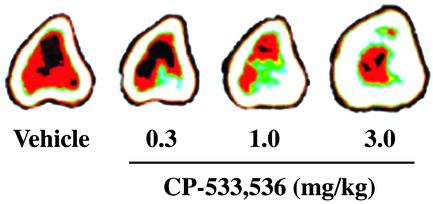
CP-533,536 Injected into Bone Marrow Induces Robust Bone Formation in the Rat. On the basis of the use of receptor subtype selective agonists and a series of in vivo models of bone formation, it was concluded that the EP2 receptor subtype contributed to the local bone anabolic activity of PGE2. Initial lead compounds were identified on the basis of the screening of a large number of selective EP2 agonists in a local rat bone marrow injection model. In this model, it was observed that the EP2 selective agonist CP-533,536 at 0.3, 1.0, or 3.0 mg/kg (n = 10 per group) dose-dependently increased total bone area, total bone mineral content, and total bone mineral density at the injection sites (Fig. 2 and Table 2).
Fig. 2.
Peripheral quantitative computerized tomography images of proximal tibial cross sections from rats injected with vehicle or CP-533,536 directly into the bone marrow. White area, within the bone circumference, represents areas of high bone mass, whereas other colors represent areas of lower-density tissues including bone marrow (red).
Table 2. CP-533,536 induced changes in total bone area (TBA), total bone mineral content (BMC), and total bone mineral density (BMD) in the rat local injection model.
| CP-533,536, mg/kg | |||
|---|---|---|---|
| Measurements, % changes from vehicle | 0.3 | 1.0 | 3.0 |
| TBA | -4.7 | 4.4 | 20.6* |
| BMC | 2.5 | 26.3* | 53.5* |
| BMD | 7.5* | 20.7* | 27.5* |
CP-533,536 Heals Critical Bone Defects in the Dog. Experiment 1. The findings in the rat bone marrow injection model led us next to test the ability of CP-533,536 to heal critical bone defects and osteotomies in canine models (27). We used the canine ulnar critical defect model and surgically implanted a collagen carrier (Helistat) into the defect site, followed by daily injections of CP-533,536 (100 mg/ml dissolved in PBS) into the collagen sponges for 3, 7, or 14 days. This experiment yielded rebridgement success rates of 20%, 30%, and 60%, respectively, when radiographically analyzed at 10 weeks postsurgery (data not shown). When injected i.v. in a saline solution, CP-533,536 had a half-life of ≈1.7 h, which suggested that sustained exposure of CP-533,536 would lead to improved efficacy in bone healing. Experiment 2. To obtain a sustained exposure, CP-533,536 was formulated with the ATRIGEL Delivery System (Atrix Laboratories) consisting of PLGH dissolved in _N_-methyl-2-pyrrolidone (NMP) (28, 29). After placement at the defect site, NMP diffuses out of the implant, encapsulating the drug in a PLGH matrix.
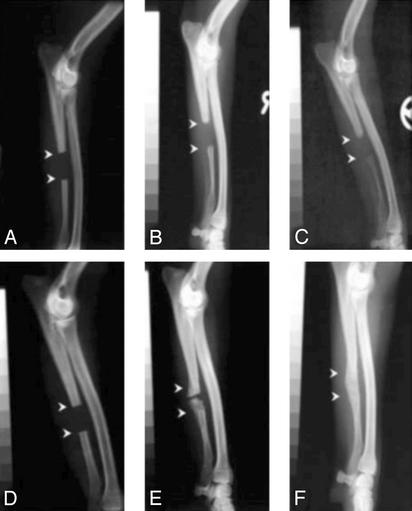
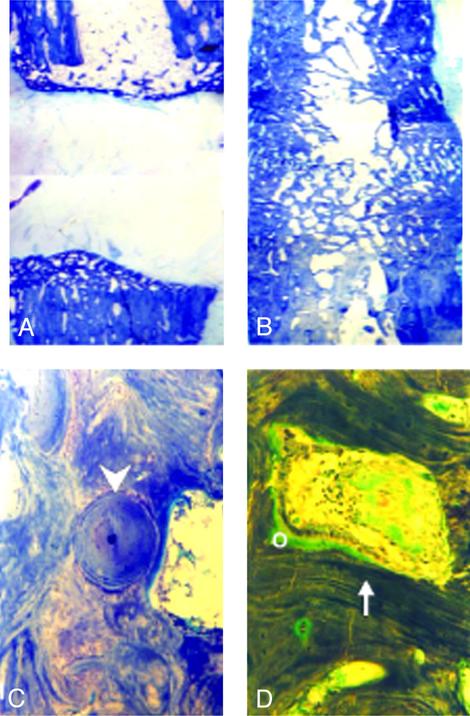
Application of CP-533,536 to the canine ulnar critical defect showed a markedly accelerated rate of bone healing. In these experiments, two different doses (10 and 50 mg per dog) of CP-533,536 were tested. In addition, the 10-mg dose was delivered in either 1 or 0.2 ml of the PLGH formulation. As shown in Fig. 3 A–C and quantitated in Table 3), none of the dogs treated with the PLGH matrix alone (1 ml) exhibited healing of the ulnar critical defect (Fig. 3 A–C, Fig. 4_A_, Table 3). In animals treated with 50 mg of CP-533,536, complete rebridgement of the defect was found in 50% of the dogs, whereas the 10-mg dose in either volume of PLGH matrix showed a time-dependent rebridgement success rate of 75% at 24 weeks after surgery (Fig. 3 D–F and Table 3). Although no healing was observed at 2 weeks (Fig. 3_D_), pronounced new bone formation within the defect was observed in treated animals at 12 weeks postsurgery (Fig. 3_F_). At 24 weeks, complete rebridgement of the defect was revealed by x-rays (Table 3 and Fig. 3_E_) and histology (Fig. 4). The newly formed bone at the rebridgement site was remodeled with cortices and the medullar cavity fully restored (Fig. 4_B_) and was undergoing osteonal remodeling (Fig. 4_C_) with the appearance of osteoblasts and active vascular channels containing hematopoietic marrow (Fig. 4_D_).
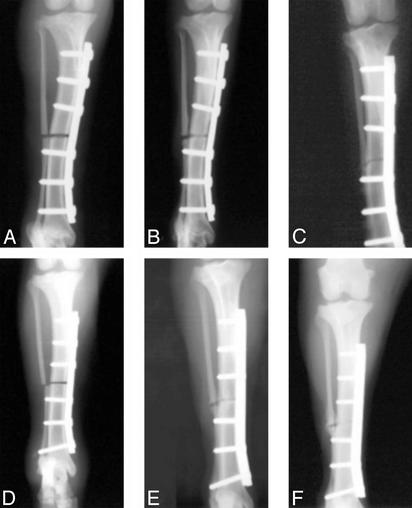
Fig. 3.
X-rays of canine ulnar critical defect treated with 1.0 ml of PLGH matrix show no healing/rebridging sequence at 2 (A), 12 (B), and 24 (C) weeks after surgery. Critical defects treated with 10 mg of CP-533,536 dissolved in 1.0 ml of matrix showed a time-dependent healing/rebridging sequence at 2 (D), 12 (E), and 24 (F) weeks after surgery.
Fig. 4.
A toluidine blue-stained section of the midulnar region from the canine critical defect model after treatment with 1.0 ml of PLGH matrix alone shows no rebridgement at 24 weeks after surgery (A), whereas full rebridgement was observed after treatment with 10 mg of CP-533,536 (B). Intense remodeling of the newly formed cortical bone was observed and consisted of osteons (C, arrow-head), active vascular channels with hematopoietic marrow, rows of osteoblasts, and newly deposited osteoid (O) on the surface of mineralized lamellar bone (D, arrow). Final magnification: A and B = ×25; C and D = ×125.
CP-533,536 Heals Tibial Osteotomy in the Dog. As seen in the ulnar critical defect model, animals with tibial osteotomies either untreated or treated with 0.5 ml of PLGH matrix alone failed to rebridge the defect within 8 weeks of surgery (Fig. 5 A–C). In contrast, animals treated with 5 mg of CP-533,536 in 0.5 ml of PLGH matrix showed a time-dependent rebridging sequence forming new bone of uniform radiodensity, which approximated the density of normal bone during the 8 weeks after surgery (Fig. 5 D–F).
Fig. 5.
X-rays of a canine tibial osteotomy treated with 0.5 ml of PLGH matrix alone show no healing/rebridging sequence at 2 (A), 4 (B), and 8 (C) weeks after surgery. Defects treated with 5 mg of CP-533,536 dissolved in 0.5 ml of matrix showed healing/rebridging sequence at 2 (D), 4 (E), and 8 (F) weeks after surgery.
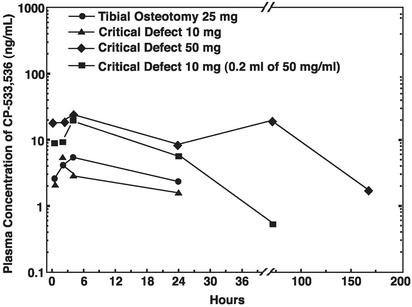
Plasma Levels and Side Effects in Dogs Treated with CP-533,536 and PLGH Matrix. Administration of this CP-533,536 formulation in a PLGH matrix at the site of ulnar critical defect or tibial osteotomy resulted in an extended release of the compound as measured by plasma drug levels over a period beyond 7 days (Fig. 6). At no point in the study did administration of CP-533,536 in PLGH matrix produce diarrhea, lethargy, or flushing, the known systemic side effects observed on PGE2 treatment (9). It should be noted that at the maximum dose used in these studies (50 mg), the peak plasma level of the drug was in the range of only 25 ng/ml or 43 nM (Fig. 6). On the basis of the receptor selectivity (Table 1), only the EP2 receptor is expected to be activated at this concentration of CP-533,536. These data suggest that the EP2 receptor is not associated with the side effects observed after systemic administration of PGE2.
Fig. 6.
Plasma concentrations of CP-533,536 in dogs after administration in a PLGH matrix formulation to the site of a tibial osteotomy (•, 25 mg) or ulnar critical defect [▴, 10mg;♦,50mg;▪, 10 mg (0.2 ml of 50 mg/ml)]. Each point represents drug blood values from 4–10 dogs.
Conclusion
We have presented data that the EP2 receptor subtype is important in skeletal healing. Further, a selective and potent functional EP2 receptor agonist, CP-533,536, induced healing of critical defects in the canine ulna and dramatically accelerated healing in the tibial osteotomy model. A single injection of CP-533,536, administered in a PLGH sustained release matrix at the time the bone defect was created, was efficacious in accelerating healing. Thus, given the high unmet medical need for fracture healing therapy and the current limitations of therapeutic procedures such as autographs and allographs, the potent bone anabolic capacity of CP-533,536 offers a promising therapeutic alternative for the enhancement of bone healing and treatment of bone defects and fractures in patients.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: PGE2, prostaglandin E2; PLGH, poly(D,L-lactide-co-glycolide).
References
- 1.Lieberman, J. R., Daluiski, A. & Einhorn, T. A. (2002) J. Bone Joint Surg. 84-A**,** 1032-1044. [DOI] [PubMed] [Google Scholar]
- 2.Bostrom, M. P. G., Yang, X. & Koutras, I. (2000) Curr. Opin. Orthop. 11**,** 403-412. [Google Scholar]
- 3.Barnes, G. L., Kostenuik, P. J., Gerstenfeld, L. C. & Einhorn, T. A. (1999) J. Bone Miner. Res. 14**,** 1805-1815. [DOI] [PubMed] [Google Scholar]
- 4.Einhorn, T. A. (1995) J. Bone Joint Surg. 77-A**,** 940-956. [DOI] [PubMed] [Google Scholar]
- 5.Rueger, D. C. (2002) in Bone Morphogenetic Proteins: From Laboratory to Clinical Practice, eds. Vukicevic, S. & Sampath, K. T. (Birkhauser, Basel), pp. 1-19.
- 6.Friedlaender, G. E., Perry, C. R., Cole, J. D., Cook, S. D., Cierny, G., Muschler, G. F., Zych, G. A., Calhoun, J. H., LaForte, A. J. & Yin, S. (2001) J. Bone Joint Surg. 83-A**,** S151-S158. [PMC free article] [PubMed] [Google Scholar]
- 7.Govender, S., Csimma, C., Genant, H. K., Valentin-Opran, A., Amit, Y., Arbel, R., Aro, H., Atar, D., Bishay, M., Borner, M. G., et al. (2002) J. Bone Joint Surg. 84-A**,** 2123-2134. [DOI] [PubMed] [Google Scholar]
- 8.Paralkar, V. M. & Owen, T. A. (2002) in Wiley Encyclopedia of Molecular Medicine, ed. Creighton, T. E. (Wiley, New York), Vol. 5.
- 9.Jee, W. S. S. & Ma, Y. F. (1997) Bone 21**,** 297-304. [DOI] [PubMed] [Google Scholar]
- 10.Ke, H. Z., Shen, V. W., Qi, H., Crawford, D. T., Wu, D. D., Liang, X. G., Chidsey-Frink, K. L., Pirie, C. M., Simmons, H. A. & Thompson, D. D. (1998) Bone 23**,** 249-255. [DOI] [PubMed] [Google Scholar]
- 11.Marks, S. M. & Miller, S. C. (1993) Endocr. J. 1**,** 337-344. [Google Scholar]
- 12.Boie, Y., Stocco, R., Sawyer, N., Slipetz, D. M., Ungrin, M. D., Neuschafer, F., Puschel, G. P., Metters, K. M. & Abramovitz, M. (1997) Eur. J. Pharmacol. 340**,** 227-241. [DOI] [PubMed] [Google Scholar]
- 13.Fedyk, E. R. & Phipps, R. P. (1996) Proc. Natl. Acad. Sci. USA 93**,** 10978-10983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schaefer, M., Hofmann, T., Schultz, G. & Gundermann, T. (1998) Proc. Natl. Acad. Sci. USA 95**,** 3008-3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Breyer, R. M., Bagdassarian, C. K., Myers, S. A. & Breyer, M. D. (2001) Annu. Rev. Pharmacol. Toxicol. 41**,** 661-690. [DOI] [PubMed] [Google Scholar]
- 16.Narumiya, S., Sugimoto, Y. & Ushikubi, F. (1999) Physiol. Rev. 79**,** 1193-1226. [DOI] [PubMed] [Google Scholar]
- 17.Hannouche, D., Petite, H. & Sedel, L. (2001) J. Bone Joint Surg. 83-B**,** 157-164. [DOI] [PubMed] [Google Scholar]
- 18.Perry, C. R. (1999) Clin. Orthop. 360**,** 71-86. [DOI] [PubMed] [Google Scholar]
- 19.Lane, J. M. (1998) Clin. Orthop. 355S**,** S359-S360. [PubMed] [Google Scholar]
- 20.Hollinger, J. & Wong, M. E. K. (1996) Oral Surg. Oral Med. Oral Pathol. 82**,** 594-606. [DOI] [PubMed] [Google Scholar]
- 21.Johnson, E. E., Urist, M. R. & Finerman, G. A. M. (1990) Clin. Orthop. 250**,** 234-240. [PubMed] [Google Scholar]
- 22.Castleberry, T., Lu, B., Smock, S. & Owen, T. (2001) Prostaglandins Other Lipid Mediat. 65**,** 167-187. [DOI] [PubMed] [Google Scholar]
- 23.Ke, H. Z., Qi, H., Chidsey-Frink, K. L., Crawford, D. T. & Thompson, D. D. (2001) J. Bone Miner. Res. 16**,** 765-773. [DOI] [PubMed] [Google Scholar]
- 24.Cook, S. D., Baffles, G. C., Wolfe, M. W., Sampath, T. K. & Rueger, D. C. (1994) Clin. Orthop. 301**,** 302-312. [PubMed] [Google Scholar]
- 25.Woodiel, F. N., Fall, P. M. & Raisz, L. G. (1996) J. Bone Miner. Res. 11**,** 1249-1255. [DOI] [PubMed] [Google Scholar]
- 26.Jones, J. H., Holtz, W. J., Bicking, J. B., Cragoe, E. J., Jr., Mandel, L. R. & Kuehl, F. A. (1977) J. Med. Chem. 20**,** 1299-1304. [DOI] [PubMed] [Google Scholar]
- 27.Wolfe, M. W. & Cook, S. D. (1994) Med. Prog. Tech. 20**,** 155-168. [PubMed] [Google Scholar]
- 28.Coonts, B. A., Whitman, S. L., O'Donnell, M., Polson, A. M., Bogle, G., Garrett, S., Swanborn, D. D., Fulfs, J. C., Rodgers, P. W., Southard, G. L., et al. (1998) J. Biomed. Mater. Res. 42**,** 303-311. [DOI] [PubMed] [Google Scholar]
- 29.Royals, M. A., Fujita, S., Yewey, G. L., Rodriguez, J., Schultheiss, P. C. & Dunn, R. L. (1999) J. Biomed. Mater. Res. 45**,** 231-239. [DOI] [PubMed] [Google Scholar]