Independent Control of Gibberellin Biosynthesis and Flowering Time by the Circadian Clock in Arabidopsis (original) (raw)
Abstract
Flowering of the facultative long-day plant Arabidopsis is controlled by several endogenous and environmental factors, among them gibberellins (GAs) and day length. The promotion of flowering by long days involves an endogenous clock that interacts with light cues provided by the environment. Light, and specifically photoperiod, is also known to regulate the biosynthesis of GAs, but the effects of GAs and photoperiod on flowering are at least partially separable. Here, we have used a short-period mutant, toc1, to investigate the role of the circadian clock in the control of flowering time by GAs and photoperiod. We show that toc1 affects expression of several floral regulators and a GA biosynthetic gene, but that these effects are independent.
Flowering in Arabidopsis is regulated by multiple environmental and endogenous cues, such as light, temperature, nutrient availability, and age (or developmental stage; Simpson et al., 1999). In most Arabidopsis accessions, long days, higher growth temperatures, and transient exposure to winter-like low temperatures (vernalization) promote faster flowering, whereas short days, lower growth temperatures, and absence of vernalization represent noninductive conditions, under which flowering is delayed.
In addition to environmental signals, several hormones are known to take part in the control of flowering time. In Arabidopsis, GAs are essential for flowering under noninductive conditions because mutants impaired in GA biosynthesis are unable to flower under short days, whereas mutants with enhanced GA response or plants exogenously treated with GA flower earlier, particularly under short days. The GA antagonist abscisic acid, on the other hand, appears to have a role in delaying flowering because mutants defective in abscisic acid signaling flower slightly earlier than the wild type (Chandler et al., 2000).
Several findings have suggested that an increase in GA biosynthesis contributes to the promotion of flowering by long photoperiods. First, the active GA species, GA1 and GA4, accumulate when short day-grown Arabidopsis plants are induced to flower by transferring them to long days (Xu et al., 1997; Gocal et al., 2000); and second, exogenous application of GAs to certain monocots or to Arabidopsis mutants can be as efficient as single long-day treatments in the promotion of flowering (Pharis et al., 1987; Gocal et al., 2000). On the other hand, GA-deficient mutants are able to flower under long days, and the severity of the delay caused by the lack of GAs varies with the experimental conditions employed, indicating that there is not a simple relationship between day length and the role of GAs in floral induction (Wilson et al., 1992; Blázquez et al., 1998).
To investigate the relationship between GAs and photoperiod, we have made use of the toc1 mutant, in which photoperiodic promotion of flowering is uncoupled from external inputs by disruption of the circadian clock. TOC1, which encodes a nuclear protein with an atypical response regulator receiver domain, is a critical component of the central oscillator, and mutations in it cause day length-independent early flowering that is exclusively because of shortening of circadian rhythms (Somers et al., 1998; Strayer et al., 2000). Here, we show that alteration of circadian clock function affects the expression of a gene involved in GA biosynthesis as well as that of floral regulators in the long-day pathway. These appear to be parallel effects because the photoperiodic control of flowering is independent of GA signaling.
RESULTS
Expression of a GA Biosynthetic Gene Is Affected by Changes in Circadian Period
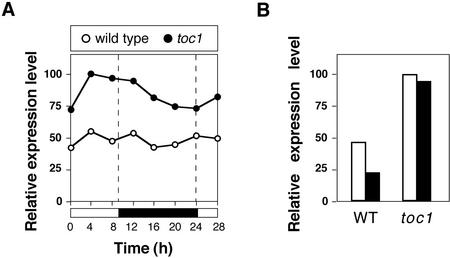
To determine whether changes in circadian clock function affect GA biosynthesis, we analyzed the expression of GA5, which encodes a GA 20-oxidase (Phillips et al., 1995; Xu et al., 1995), in wild type and in toc1 mutants. The expression of GA5 is subject to negative regulation by GAs, and, therefore, can be used to monitor changes in GA concentration (Xu et al., 1995). Plants were entrained in a short-day regime (9 h of light, 15 h of dark), and gene expression was monitored during a 28-h period (Fig. 1A). GA5 expression was elevated 1.5 to 2 times in the toc1 mutant compared with the wild type over the entire period of time analyzed. A weak rhythmic oscillation of GA5 expression was apparent in the toc1 mutant, but not in the wild type. Although this observation suggests that alterations in circadian clock elements may slightly affect GA biosynthesis, it remains unclear whether this is because of circadian regulation of GA5 expression, or because of a clock-independent activity of TOC1. To test whether the effect of toc1 on GA5 expression affects apparent GA concentration, we also compared the response of GA5 expression, which is under end product repression (Xu et al., 1995), with exogenous GA3 in wild type and toc1 mutants (Fig. 1B). Exogenous GA3 was not sufficient to suppress the increase in GA5 caused by the toc1 mutation, suggesting that GA levels are increased in toc1 mutants.
Figure 1.
Expression of GA5 as determined by semiquantitative reverse transcription (RT)-PCR. Tissue was harvested from 10-d-old seedlings entrained in a 9-h-light/15-h-dark cycle. Expression levels were normalized against UBQ, and expressed as fraction of the maximal expression level. A, Effect of the toc1 mutation on circadian GA5 expression. B, Effect of exogenous GA3 on GA5 expression measured 4 h after dawn. Seedlings were grown for 10 d on Murashige and Skoog plates without exogenous GA3 (white bars) or with 50 μm GA3 (black bars).
Expression of Flowering Time Genes Is Affected by Changes in Circadian Period
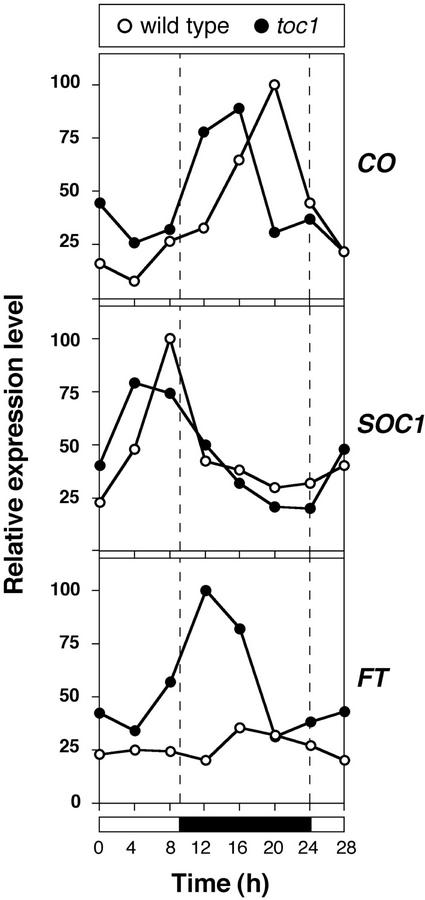
The circadian clock acts upstream of the photoperiod, or long-day, pathway promoting flowering. Circadian regulation of CO (CONSTANS) RNA expression appears to be an integral part of the molecular mechanism that regulates flowering in a day length-dependent manner. Light is apparently required for CO activity, and because peak levels of CO RNA are present during the day in long, but not short days, this coincidence mechanism has been proposed to allow CO to promote flowering only in long days (Suárez-López et al., 2001). If CO mediates between the clock and flowering, CO expression should be affected by changes in the clock independently of external photoperiod. To test this hypothesis, we analyzed the expression of CO in toc1 mutants, which flower early in noninductive short-day conditions (Somers et al., 1998). As previously reported, CO expression was subject to circadian regulation in short day-grown wild-type seedlings (Suárez-López et al., 2001), with a peak of expression in the middle of the dark period (Fig. 2). In the toc1 mutant, CO expression levels had a similar circadian rhythm, but the peak was reached 4 h earlier than in wild type. The maximal level of CO expression was similar to that of wild type.
Figure 2.
Expression of floral regulators under short days as determined by semiquantitative RT-PCR. Tissue was harvested from 10-d-old seedlings entrained in a 9-h-light/15-h-dark cycle. Expression levels were normalized against UBQ, and expressed as fraction of the maximal expression level.
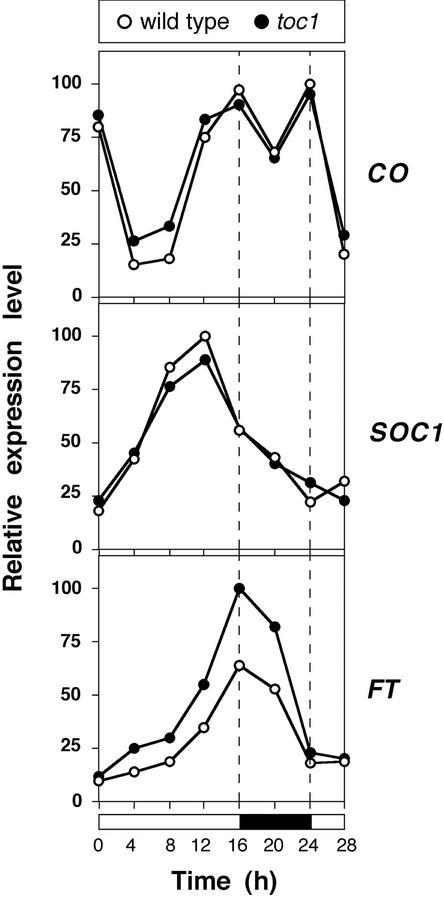
Promotion of flowering by CO is mediated by at least two genes, FT and SOC1, whose expression levels are critical in determining flowering time (Samach et al., 2000). To determine whether the changed CO expression pattern in toc1 mutants affected these downstream genes, we examined their expression as well. As observed for CO, expression of SOC1 in the wild type followed a circadian rhythm under short days, with the peak of expression occurring at dusk (Fig. 2). In the toc1 mutant, the peak of SOC1 expression was reached 3 to 4 h earlier (Fig. 2). The most dramatic effect of the toc1 mutation was observed in the case of FT. Although FT expression levels were very low in wild-type plants grown in short days (Fig. 2; Kardailsky et al., 1999; Kobayashi et al., 1999), maximal levels of FT in the toc1 mutant were at least 4 times higher than in the wild type, and FT expression oscillated with a circadian rhythm, similar to what has been observed in wild-type plants under long days (Suárez-López et al., 2001; Fig. 3), with a peak of expression shortly after dusk. The toc1 mutation had no effect on the expression of CO, SOC1, and FT under long days (Fig. 3), consistent with the similar flowering time of toc1 and wild-type plants under these conditions.
Figure 3.
Expression of floral regulators under long days as determined by semiquantitative RT-PCR. Tissue was harvested from 10-d-old seedlings entrained in a 16-h-light/8-h-dark cycle. Expression levels were normalized against UBQ, and expressed as fraction of the maximal expression level.
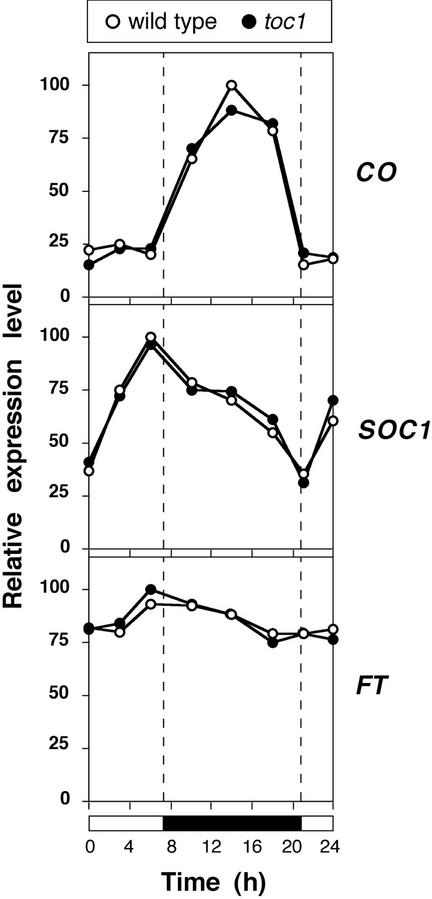
The short-day early flowering phenotype of toc1 mutants is suppressed in 21-h light-dark cycles, which match the endogenous period of the clock in toc1 (Strayer et al., 2000). In agreement with this phenotype, the dramatic up-regulation of FT and the shift in the circadian rhythm of CO and SOC1 seen under short-day cycles that were 24 h long were completely suppressed when the short-day cycles were shortened to 21 h (Strayer et al., 2000; Fig. 4). These results confirm that the activity of the photoperiod pathway is under the control of the circadian clock.
Figure 4.
Expression of floral regulators under short days in 21-h light/dark cycles as determined by semiquantitative RT-PCR. Tissue was harvested from 10-d-old seedlings entrained in a 7-h-light/14-h-dark cycle. Expression levels were normalized against UBQ, and expressed as fraction of the maximal expression level.
Interaction of GAs and the Circadian Clock in Controlling Flowering Time
In short days, GAs are essential for flowering of Arabidopsis (Wilson et al., 1992). To determine whether the promotion of flowering by the circadian clock required GA, we tested whether the early flowering phenotype of toc1 mutants could be abolished by blocking GA biosynthesis. In long days, the late-flowering phenotype of the ga1 mutant was alleviated by the toc1 mutation (Table I). More dramatically, the inability of ga1 mutants to flower in short days was suppressed by the toc1 mutation, indicating that GAs are no longer limiting in short day-grown toc1 mutants. As with the induction of CO, FT, and SOC1, 21-h light/dark cycles suppressed the ability of toc1 to promote flowering in short day-grown ga1 mutants (Table I), confirming that suppression of the ga1 flowering defect by toc1 is because of its effects on the clock.
Table I.
Flowering time of ga1-3 and toc1-3 lines under different light regimes
| Line | Genotype | Long Days (24 h) | Short Days (24 h) | Short Days (21 h) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| RL | CL | TL | RL | CL | TL | RL | CL | TL | ||
| 150-304 | Wild type | 7.9 ± 0.6 | 2.6 ± 0.5 | 10.5 ± 0.5 | 25.1 ± 1.2 | 7.1 ± 0.9 | 32.3 ± 1.3 | 31.8 ± 2.6 | 7.1 ± 0.9 | 38.9 ± 2.8 |
| 281L4 | toc1-3 | 7.9 ± 0.7 | 2.2 ± 0.4 | 10.1 ± 0.2 | 8.0 ± 0.6 | 2.4 ± 0.5 | 10.4 ± 0.6 | 29.6 ± 1.3 | 6.2 ± 0.7 | 35.8 ± 1.2 |
| 304G1 | ga1-3 | 12.5 ± 0.8 | –a | 12.5 ± 0.8 | –b | –b | >60 | –b | –b | >60 |
| M281 | ga1-3 toc1-3 | 11.2 ± 0.8 | –a | 11.2 ± 0.8 | 12.8 ± 1.2 | –a | 12.8 ± 1.2 | –b | –b | >60 |
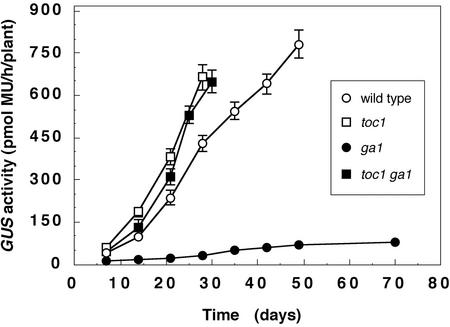
A critical output of floral induction is activation of the floral meristem identity gene LFY (LEAFY), which encodes a transcription factor that activates a flower-specific developmental program (Parcy et al., 1998). The LFY promoter integrates signals from the GA and long-day pathways, and GAs are essential for activation of the LFY promoter when the long-day pathway is inactive (Blázquez and Weigel, 2000). To determine how the circadian clock affects LFY expression, we analyzed LFY promoter activity in toc1 and toc1 ga1 mutants. In wild type, the LFY promoter is up-regulated as development proceeds; when a critical threshold is reached, flowers are initiated (Blázquez et al., 1997). In short day-grown ga1 mutants, the LFY promoter fails to be strongly up-regulated, causing the inability of ga1 mutants to flower under these conditions (Fig. 5). LFY promoter activity was restored in ga1 toc1 mutants grown in short days, to levels similar to those observed in toc1 mutants, which were higher than those in wild-type plants. These results indicate that the LFY promoter is an ultimate target for the control of flowering by the clock, and that the clock regulates LFY expression through a mechanism that is independent of GAs.
Figure 5.
LFY promoter activity in short day-grown plants. Values are expressed as mean ± 2 se of the mean. Time represents days after sowing. Error bars that are not visible are as small or smaller than the graph symbol.
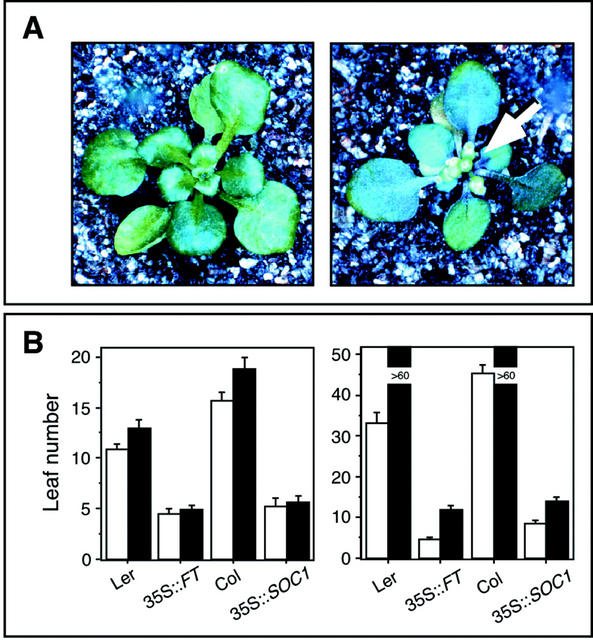
Constitutive expression of LFY has been shown to allow flowering of ga1 mutants under noninductive conditions (Blázquez et al., 1998). If photoperiodic control of flowering time by the circadian clock does not require GA signaling, activation of the long-day pathway under short days should cause flowering even in the absence of GA biosynthesis. To test this idea, we overexpressed two genes, FT and SOC1, which act downstream of CO, in the ga1 mutant background, and in plants grown in the presence of the GA biosynthesis inhibitor paclobutrazol. Constitutive expression of FT restored the ability of ga1 mutants to flower under short days (Fig. 6A). Similarly, treatment of 35S::FT and 35S::SOC1 plants with paclobutrazol did not prevent early flowering either under long or short days, although flowering under short days was delayed by a few leaves compared with the untreated controls (Fig. 6B). That elimination of GA biosynthesis in short days had an effect on the early flowering of 35S::FT and 35S::SOC1 plants (Fig. 6B), but not on toc1 (Table I), suggests that the full effect of the toc1 mutation requires both FT and SOC1.
Figure 6.
A, ga1-3 (left) and ga1-3 35S::FT (right) plants grown in short days. Arrow indicates flowers. B, Flowering time expressed as leaf number on the main shoot. Left, Long days; right, short days. White bars show untreated plants, and black bars show plants treated with paclobutrazol.
DISCUSSION
Modulation of GA biosynthesis and signaling is a common theme in plant development. For instance, the promotion of seed germination by light requires the accumulation of active GA species, achieved by the induction of GA biosynthetic enzymes (Kamiya and Garcia-Martinez, 1999). In other processes, light affects sensitivity to GAs, as deduced from the observation that hypocotyl elongation of plants with a defect in the PHYB photoreceptor is hyperresponsive to GAs (Reed et al., 1996). Both GAs and PHYTOCHROME B (PHYB) also affect flowering, but in this case, they appear to act in parallel because GAs are not required for the early flowering phenotype of phyB mutants, and the expression of the floral meristem identity gene LFY does not become more sensitive to GAs in a phyB mutant background (Blázquez and Weigel, 1999).
A similarly complex relationship is seen between light and flowering. Light provides seasonal information through variation in day length, but it also affects flowering through variation in light quality or intensity. For example, far-red light promotes flowering, whereas red light inhibits flowering. In addition, high irradiance accelerates flowering (Bagnall, 1992; Corbesier et al., 1996).
During seed germination, light appears to have a direct effect on GA biosynthesis (Yamaguchi et al., 1998; Kamiya and Garcia-Martinez, 1999). Our finding that expression of GA5, a GA biosynthetic gene that is under end product repression (Xu et al., 1995), is affected in the short-period mutant toc1 independently of changes in the external photoperiod indicates that during adult development, light may affect GA biosynthesis through an interaction with the circadian clock. Similarly, we have found that the toc1 mutation affects expression of the floral regulatory gene CO and its downstream targets FT and SOC1, which are normally regulated by external photoperiod. One possibility is that the advance in the peak of CO expression caused by toc1, or an increase in baseline expression, allows the coincidence of higher CO levels with the presence of light. Considering that CO is required for FT induction, it is likely that the increase in FT expression in toc1 is a consequence of the observed changes in CO expression, although this regulation may require the participation of additional circadian-regulated elements. These results provide experimental evidence that interaction of light with the circadian clock constitutes at least one of the mechanisms for the promotion of flowering by long photoperiods. However, we cannot rule out that light, in addition, has a direct effect on the expression of the floral regulators downstream of CO.
We have described previously a regulatory element in the LFY promoter that is essential for the effects of GA on LFY promoter activity. Mutation of this element does not abolish responsiveness to long days, although the overall activity of the promoter is reduced (Blázquez and Weigel, 2000). Furthermore, the LFY promoter is induced by long days in ga1 mutants (Blázquez et al., 1998), but the magnitude of induction is reduced. These observations point to both GA-dependent and -independent effects in the photoperiodic control of Arabidopsis flowering. It has been shown previously that the concentration of active GAs increases in plants that are transferred from short to long days, coinciding with bolting (Xu et al., 1997). As shown in this work, GA contribution is not quantitatively important in the determination of flowering time by the photoperiod pathway. Therefore, the increase in GA concentration induced by long days might be relevant for cell expansion required during stem elongation, rather than the determination of flowering time.
MATERIALS AND METHODS
Plant Material
The wild type used was Arabidopsis strain Landsberg erecta. ga1-3 (Sun and Kamiya, 1994), 35S::FT (Kardailsky et al., 1999), and 35S::SOC1 (Lee et al., 2000) have been described. The semidominant toc1-3 mutation, originally called fog2-1 (flowering of ga1-3), was isolated because of its ability to suppress the nonflowering phenotype of ga1-3 mutants grown in short days. The mutant was mapped to the TOC1 locus (Strayer et al., 2000), and sequencing identified a C to T mutation at position 2,082 of TOC1 (GenBank accession no. AF272039), causing an Ala-562 to Val change, which is the same change as in the toc1-1 allele (Strayer et al., 2000). The LFY::GUS line (DW150-304) in the Landsberg erecta background has been described previously (Blázquez et al., 1997, 1998).
Growth Conditions
For experiments on soil, seeds were stratified for 2 to 3 d at 4°C before sowing. Plants were grown at 23°C in long (16 h of light and 8 h of dark) or short days (9 h of light and 15 h of dark), under a mixture of 3:1 cool-white and Gro-Lux fluorescent lights (Osram Sylvania, Danvers, MA). For 21-h light/dark cycles, short days were 7 h of light and 14 h of dark.
ga1-3 mutants do not germinate without GAs (Koornneef and van der Veen, 1980) and were incubated with 50 μm GA3 (Sigma, St. Louis) during stratification. Seeds were rinsed thoroughly with water before sowing.
Paclobutrazol (Zeneca Ag Products, Wilmington, DE) was applied by watering with a 37 mg L−1 solution.
β-Glucuronidase Activity Measurements
Quantitative measurements of β-glucuronidase activity in dissected shoot apices using 4-methylumbelliferyl-β-d-glucopyranoside were done as described by Blázquez et al. (1997).
RNA Extraction and Analysis
Total RNA was extracted with TRIzol reagent (Gibco BRL, Grand Island, NY). RT-PCR was performed with 1 μg of total RNA, using a Reverse Transcription Kit (Promega, Madison, WI). CO, FT, and UBQ primers have been described (Blázquez and Weigel, 1999). Primers for amplification of SOC1 were JH1145 (GGA TCG AGT CAG CAC CAA ACC) and JH1146 (CCC AAT GAA CAA TTG CGT CTC); primers for GA5 were MB85 (CCA AGC TTC CAT GGA AGG AG) and MB86 (ACA TGG TCT TGG TGA AGG AT). Signal intensities were determined with a Molecular Dynamics (Sunnyvale, CA) PhosphorImager, and values in the exponential range of amplification were compared. Expression analyses were carried out twice with independent samples, and the results of one of the experiments are shown.
ACKNOWLEDGMENTS
We thank Nuria González and Thuy Nguyen for their technical assistance; David Somers and Carl Strayer for helpful discussions and material; and David Alabadí, José L. García-Martínez, and Francisco Madueño for useful comments on the manuscript. We also acknowledge Juan Carbonell's generosity and support by providing space to carry out part of the experimental work.
Footnotes
1
This work was supported by the Spanish Ministry of Science (grant no. BIO2001–1558 to M.A.B.), by the National Science Foundation (grant no. MCB–0078277 to D.W.), by the U.S. Department of Agriculture (grant no. 99–35301–8047 to D.W.), by the Human Frontiers Science Program Organization (grant no. RGP0235/2001–M to D.W. and fellowship to M.A.B.), and by the Spanish Ministry of Education (fellowship to M.A.B.). D.W. is a Director of the Max Planck Institute.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.007625.
LITERATURE CITED
- Bagnall DJ. Control of flowering in Arabidopsis thaliana by light, vernalisation and gibberellins. Aust J Plant Physiol. 1992;19:401–409. [Google Scholar]
- Blázquez MA, Green R, Nilsson O, Sussman MR, Weigel D. Gibberellins promote flowering of Arabidopsis by activating the LEAFY promoter. Plant Cell. 1998;10:791–800. doi: 10.1105/tpc.10.5.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blázquez MA, Soowal L, Lee I, Weigel D. LEAFY expression and flower initiation in Arabidopsis. Development. 1997;124:3835–3844. doi: 10.1242/dev.124.19.3835. [DOI] [PubMed] [Google Scholar]
- Blázquez MA, Weigel D. Independent regulation of flowering by phytochrome B and gibberellins in Arabidopsis. Plant Physiol. 1999;120:1025–1032. doi: 10.1104/pp.120.4.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blázquez MA, Weigel D. Integration of floral inductive signals in Arabidopsis. Nature. 2000;404:889–892. doi: 10.1038/35009125. [DOI] [PubMed] [Google Scholar]
- Chandler J, Martinez-Zapater JM, Dean C. Mutations causing defects in the biosynthesis and response to gibberellins, abscisic acid and phytochrome B do not inhibit vernalization in Arabidopsis fca-1. Planta. 2000;210:677–682. doi: 10.1007/s004250050059. [DOI] [PubMed] [Google Scholar]
- Corbesier L, Gadisseur I, Silvestre G, Jacqmard A, Bernier G. Design in Arabidopsis thaliana of a synchronous system of floral induction by one long day. Plant J. 1996;9:947–952. doi: 10.1046/j.1365-313x.1996.9060947.x. [DOI] [PubMed] [Google Scholar]
- Gocal GFW, Sheldon CC, Gubler F, Moritz T, Bagnall D, Li SF, Parish RW, Dennis ES, Weigel D, King RW. GAMYB-like genes, flowering, and gibberellin signaling in Arabidopsis. Plant Physiol. 2000;127:1682–1693. [PMC free article] [PubMed] [Google Scholar]
- Kamiya Y, Garcia-Martinez JL. Regulation of gibberellin biosynthesis by light. Curr Opin Plant Biol. 1999;2:398–403. doi: 10.1016/s1369-5266(99)00012-6. [DOI] [PubMed] [Google Scholar]
- Kardailsky I, Shukla V, Ahn JH, Dagenais N, Christensen SK, Nguyen JT, Chory J, Harrison MJ, Weigel D. Activation tagging of the floral inducer FT. Science. 1999;286:1962–1965. doi: 10.1126/science.286.5446.1962. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Kaya H, Goto K, Iwabuchi M, Araki T. A pair of related genes with antagonistic roles in mediating flowering signals. Science. 1999;286:1960–1962. doi: 10.1126/science.286.5446.1960. [DOI] [PubMed] [Google Scholar]
- Koornneef M, van der Veen JH. Induction and analysis of gibberellin sensitive mutants in Arabidopsis thaliana. Int Z Theor Angew Genet. 1980;58:257–263. doi: 10.1007/BF00265176. [DOI] [PubMed] [Google Scholar]
- Lee H, Suh SS, Park E, Cho E, Ahn JH, Kim SG, Lee JS, Kwon YM, Lee I. The AGAMOUS-LIKE 20 MADS domain protein integrates floral inductive pathways in Arabidopsis. Genes Dev. 2000;14:2366–2376. doi: 10.1101/gad.813600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parcy F, Nilsson O, Busch MA, Lee I, Weigel D. A genetic framework for floral patterning. Nature. 1998;395:561–566. doi: 10.1038/26903. [DOI] [PubMed] [Google Scholar]
- Pharis RP, Evans LT, King RW, Mander LN. Gibberellins, endogenous and applied, in relation to flower induction in the long-day plant Lolium temulentum. Plant Physiol. 1987;84:1132–1138. doi: 10.1104/pp.84.4.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips AL, Ward DA, Uknes S, Appleford NE, Lange T, Huttly AK, Gaskin P, Graebe JE, Hedden P. Isolation and expression of three gibberellin 20-oxidase cDNA clones from Arabidopsis. Plant Physiol. 1995;108:1049–1057. doi: 10.1104/pp.108.3.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed JW, Foster KR, Morgan PW, Chory J. Phytochrome B affects responsiveness to gibberellins in Arabidopsis. Plant Physiol. 1996;112:337–342. doi: 10.1104/pp.112.1.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samach A, Onouchi H, Gold SE, Ditta GS, Schwarz-Sommer Z, Yanofsky MF, Coupland G. Distinct roles of CONSTANS target genes in reproductive development of Arabidopsis. Science. 2000;288:1613–1616. doi: 10.1126/science.288.5471.1613. [DOI] [PubMed] [Google Scholar]
- Simpson GG, Gendall AR, Dean C. When to switch to flowering. Annu Rev Cell Dev Biol. 1999;15:519–550. doi: 10.1146/annurev.cellbio.15.1.519. [DOI] [PubMed] [Google Scholar]
- Somers DE, Webb AA, Pearson M, Kay SA. The short-period mutant, toc1-1, alters circadian clock regulation of multiple outputs throughout development in Arabidopsis thaliana. Development. 1998;125:485–494. doi: 10.1242/dev.125.3.485. [DOI] [PubMed] [Google Scholar]
- Strayer C, Oyama T, Schultz TF, Raman R, Somers DE, Mas P, Panda S, Kreps JA, Kay SA. Cloning of the Arabidopsis clock gene TOC1, an autoregulatory response regulator homolog. Science. 2000;289:768–771. doi: 10.1126/science.289.5480.768. [DOI] [PubMed] [Google Scholar]
- Suárez-López P, Wheatley K, Robson F, Onouchi H, Valverde F, Coupland G. CONSTANS mediates between the circadian clock and the control of flowering in Arabidopsis. Nature. 2001;410:1116–1120. doi: 10.1038/35074138. [DOI] [PubMed] [Google Scholar]
- Sun T-p, Kamiya Y. The Arabidopsis GA1 locus encodes the cyclase ent-kaurene synthetase A of gibberellin biosynthesis. Plant Cell. 1994;6:1509–1518. doi: 10.1105/tpc.6.10.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RN, Heckman JW, Somerville CR. Gibberellin is required for flowering in Arabidopsis thaliana under short days. Plant Physiol. 1992;100:403–408. doi: 10.1104/pp.100.1.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu YL, Gage DA, Zeevaart JA. Gibberellins and stem growth in Arabidopsis thaliana. Effects of photoperiod on expression of the GA4 and GA5 loci. Plant Physiol. 1997;114:1471–1476. doi: 10.1104/pp.114.4.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu YL, Li L, Wu K, Peeters AJ, Gage DA, Zeevaart JA. The GA5 locus of Arabidopsis thaliana encodes a multifunctional gibberellin 20-oxidase: molecular cloning and functional expression. Proc Natl Acad Sci USA. 1995;92:6640–6644. doi: 10.1073/pnas.92.14.6640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi S, Smith MW, Brown RG, Kamiya Y, Sun T. Phytochrome regulation and differential expression of gibberellin 3β-hydroxylase genes in germinating Arabidopsis seeds. Plant Cell. 1998;10:2115–2126. doi: 10.1105/tpc.10.12.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]