Decreased interferon-gamma response in respiratory syncytial virus compared to other respiratory viral infections in infants (original) (raw)
Abstract
An inappropriate interferon-gamma response has been implicated in the pathogenesis of severe respiratory syncytial virus (RSV) lower respiratory tract illness (LRTI). To assess whether this is unique for RSV primary LRTI compared to a first non-RSV LRTI, intracellular interferon-gamma was determined by flow cytometry in peripheral blood mononuclear cells from 32 infants with a primary RSV infection, 28 with a first non-RSV LRTI due to adenoviral, parainfluenzaviral and rhinoviral infection and 13 healthy infants. Interferon-γ responses were increased significantly during adenoviral, parainfluenzaviral and the majority of the rhinoviral infections, but remained low during RSV and severe rhinoviral infection. Low interferon-γ responses were associated with a more severe clinical course of LRTI. This indicates that depending on the nature of the viral pathogen, respiratory virus infections in infants differ significantly with regard to the quantity of the interferon-γ production and that this may contribute to the clinical course of the disease.
Keywords: respiratory virus infection (RSV), T cells, viruses/ viral immunity
INTRODUCTION
Respiratory syncytial virus (RSV) causes serious respiratory tract illness. Infants below the age of 6 months are especially prone to severe bronchiolitis and pneumonia. It is not known why some infants develop severe illness requiring mechanical ventilation while others display only mild symptoms. There is some evidence, however, to implicate compromised ability to develop sufficient type I-like immune responses during primary RSV infection in the pathogenesis of severe lower respiratory tract illness (LRTI) [1]. Interferon-gamma (IFN-γ) is a type I cytokine which has direct antiviral activity, stimulates antigen presentation through induction of MHC molecule expression and promotes cytotoxic activity of natural killer cells and virus-specific T cells [2]. The strength of the early IFN-γ response may therefore determine to a significant extent the clinical course of RSV disease, and decreased production of IFN-γ may lead to longer periods of infection and concomitant detrimental effects on pulmonary function. We have shown previously that severe RSV bronchiolitis is associated with decreased mRNA IFN-γ expression in peripheral blood [3]. In addition, severe RSV LRTI was shown to be associated with decreased IFN-γ secretion in both the respiratory tract and peripheral blood [4,5].
The strength of IFN-γ responses to viral infection depends on multiple factors, including genetic and developmental variability (e.g. lung/or immunological maturation), age and the infecting viral pathogen. It is still unknown whether a diminished IFN-γ response is unique to RSV primary infection.
We compared IFN-γ responses in peripheral blood mononuclear cells (PBMC) from infants of similar age who had been hospitalized with a primary RSV infection or a first episode of LRTI due to adenoviral, parainfluenzaviral or rhinoviral infection (non-RSV LRTI). IFN-γ responses were also analysed with respect to the severity of the illness.
PATIENTS AND METHODS
Patients
Children who had been hospitalized during the winter epidemics of 2001/2 and 2002/3 with proven viral LRTI (n = 60) were included in the study. The patients included did not have any underlying disease, were less than 32 weeks of age and experiencing a first episode of an acute LRTI. No infants included in the study were born prematurely. RSV disease was confirmed in 32 patients and 28 were hospitalized for LRTI due to rhinovirus (n = 16), adenovirus (n = 7) and parainfluenzavirus (n = 5). Oxygen saturation was monitored by 24 h percutaneous oximetry as part of the routine care. The lowest values of minimal oxygen saturation (MOS) measured with the patient breathing ambient air were used as an objective indication of the severity of illness, as described previously [3,6]. Infants who had ‘hypoxic bronchiolitis’ with MOS <93% for at least 30 min or MOS ≤90% for 15 min were considered severely ill. Heparinized blood was obtained within 24 h of admission from 28 infants hospitalized for non-RSV RTI and from 22 of the 32 RSV-infected infants. Upon discharge (within 7 days of admission to hospital) blood samples were obtained from 16 RSV-infected infants, six of them representing follow-up samples. Heparinized blood samples were obtained from 13 healthy age-matched infants who had been hospitalized for minor surgery.
The study was approved by the Ethics Committee of the St Anna Kinderspital, Vienna, and informed consent was obtained from the parents and guardians of all children participating.
Virus detection in nasopharyngeal secretions
Nasopharyngeal aspirates from infants were tested for the presence of RSV, adenoviruses, influenza and parainfluenza viruses 1–3 by enzyme-linked immunosorbent assays, tissue culture virus isolation and seminested reverse-transcribed polymerase chain reaction (RT-PCR), as described previously [3,7,8].
Intracellular cytokine analysis
Sample preparation was performed following the protocol from the FastImmune Intracellular Cytokine Detection Kit (BD Biosciences, Heidelberg, Germany) [9].
Briefly, samples of heparinized blood (300 _µ_l) were incubated in 15 ml polypropylene tubes (BD Biosciences) with PMA (10 ng/ml) (Sigma) and ionomycin (1 _µ_g/ml) (Sigma Aldrich, Vienna, Austria), in addition to 1 _µ_g/ml of co-stimulatory MoAbs against CD28 and CD49d (BD) for 6 h at 37°C. For the final 4 h, 10 _µ_g/ml of brefeldin A (Sigma) was added to the whole blood samples. Cells were incubated at room temperature with 50 _µ_l of a 20 mm EDTA solution in phosphate buffered saline (PBS) (pH 7·3) for 15 min and subsequently in 5 ml FACS lysing solution for 10 min. Cells were then stored frozen at − 80°C. After that, cell preparations were thawed rapidly in a 37°C water bath, washed in 7 ml FACS wash buffer containing 0·5% bovine serum albumin (BSA) and 0·1% NaN3 in sterile PBS and resuspended in 300 _µ_l FACS wash buffer. One hundred _µ_l cell suspensions were then added to 500 _µ_l FACS permeabilizing solution and incubated for 10 min at room temperature. Fixed and permeabilized cells were washed and stained with fluorescein isothiocyanate (FITC)-conjugated anti-IFN-γ (25723·11), cyanine peridinin chlorophyll protein (PerCP–Cy5·5) conjugated anti-CD3 (SK7) and the corresponding isotype controls, IgG1 (X40) or IgG2a (X39), all obtained from BD Biosciences, for 30 min. Cells were washed, resuspended in 1% paraformaldehyde in PBS and stored in a dark place at 4°C until FACS analysis. Stimulated PBMC obtained from 32 infants (nine RSV-infected, 18 non-RSV-infected, five healthy controls) were additionally dual-stained with FITC-conjugated anti-IFN-γ and PerCP–Cy5·5-conjugated anti-CD3. From 28 infants (nine RSV-infected, 14 non-RSV-infected, five healthy controls) enough blood was available for determining absolute counts of CD3+ T lymphocytes; cells were stained with TriTest (CD3FITC/CD16+ CD56PE/CD45PerCP) according to the manufacturer's instructions (BD Biosciences).
Cells were analysed on a FACSCalibur and at least 15 000 events acquired for each preparation. Lymphocytes were gated according to their scatter properties and data analysed with paint-a-gateplus software (BD Biosciences).
Statistical analysis
The Mann–Whitney _U_-test was used for comparisons between groups. Spearman's correlation coefficients were computed to determine the degree of correlation between percentages of IFN-_γ_-producing lymphocytes and patient age.
RESULTS
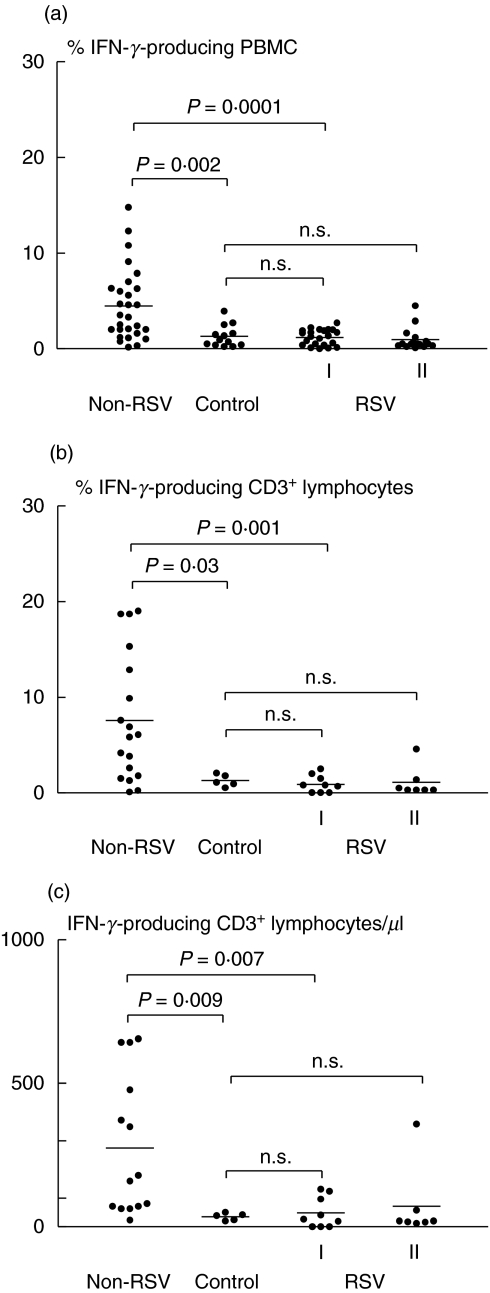
In the acute phase of illness, the percentages of IFN-_γ_-producing PBMC from the RSV-infected infants were similar to those of the healthy, age-matched controls (Fig. 1a). In contrast, infants with non-RSV-related LRTI showed significantly higher percentages of IFN-_γ_-producing PBMC than the healthy control (P = 0·002) and RSV-infected (P = 0·0001) groups (Fig. 1a). No correlation was seen between patient age and percentages of IFN-_γ_-producing PBMC (r = 0·12, P = 0·4; Spearman's correlation co-efficient). The percentages of IFN-_γ_-producing PBMC remained low in follow-up blood analyses obtained from 16 of the RSV-infected infants within 7 days of the onset of illness (Fig. 1a).
Fig. 1.
Percentages of IFN-_γ_-producing (a) peripheral blood mononuclear cells (PBMC), (b) CD3+ T lymphocytes and (c) total numbers of CD3+ T lymphocytes/_µ_l from infants with acute non-RSV-related lower respiratory tract illness (non-RSV), acute RSV infection (RSV, I) assayed again within 7 days (RSV, II) and from healthy, age-matched controls (control). _P_-values were calculated with the Mann–Whitney _U_-test; n.s., not significant.
Significant differences in IFN-γ production between infants with RSV infection and those with non-RSV LRTI were confirmed further by analysis of the T lymphocyte (CD3+) subset. Again, percentages and absolute numbers of IFN-_γ_-producing CD3+ T lymphocytes were significantly higher in non-RSV-infected infants than in those who were RSV-infected (% IFN-γ + CD3+ T cells, P = 0·001; IFN-γ + CD3+ T cell count/_µ_l, P = 0·007) and in the healthy controls (% IFN-γ + CD3+ T cells, P = 0·03; IFN-γ + CD3+ T cell count/_µ_l, P = 0·009) (Figs 1b,c and 2).
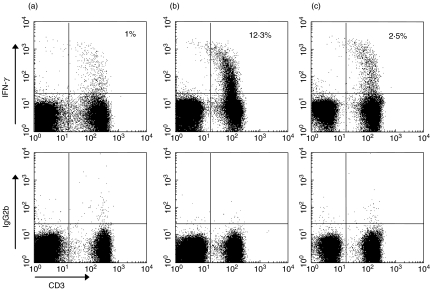
Fig. 2.
Representative plots of IFN-γ production in CD3+ T cells upon 6-h whole-blood activation with phorbol 12-myristate 13-acetate (PMA) and ionomycin within the lymphocyte population from an infant with RSV (a), non-RSV (adenovirus) infection (b) and from a healthy control infant (c).
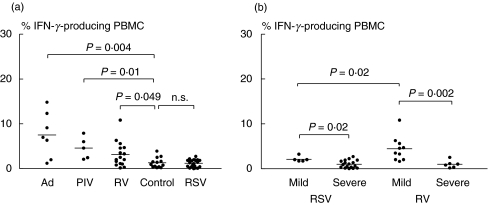
Patients were then grouped according to type of virus identified and IFN-γ responses to adenovirus, parainfluenzavirus and rhinovirus compared (Fig. 3a). A significant increase in IFN-_γ_-producing PBMC over those from healthy controls was observed during adenoviral (P = 0·004), parainfluenzaviral (P = 0·01) and rhinoviral (P = 0·049) infection (Fig. 3a). In addition, remarkable differences between RSV- and non-RSV-infected groups were found in the number of infants who developed a severe LRTI. Severe bronchiolitis was diagnosed in 17 of the 22 RSV-infected patients, in six of the 16 infants with a rhinovirus infection, but not in infants with an adenoviral or parainfluenzaviral infection.
Fig. 3.
Percentages of IFN-_γ_-producing PBMC from (a) infants infected with RSV, adenovirus (Ad), parainfluenzavirus (PIV) and rhinovirus (RV) and (b) infants with mild and severe RSV and RV illness. _P_-values were calculated with the Mann–Whitney _U_-test; n.s., not significant.
The IFN-γ responses in RSV- and in rhinovirus-infected infants were then analysed with regard to the severity of their illness. Infants with mild illness had significantly higher percentages of IFN-_γ_-producing PBMC than those with severe disease, both in RSV-infected (P = 0·02) and rhinovirus-infected groups (P = 0·002) (Fig. 3b). Infants with mild or severe bronchiolitis did not differ with respect to age (median age (range) in weeks: 18 (7–24), RSV-mild; eight (2–30), RSV-severe illness (P = 0·29); 12 (2·5–28), RV-mild; nine (5–28), RV-severe illness (P = 0·95)).
When the IFN-γ response was compared in infants with mild illness, RSV infection was associated with significantly lower percentages of IFN-_γ_-producing PBMC than rhinovirus infection (P = 0·02) (Fig. 3b).
DISCUSSION
The results of this study provide evidence that early production of IFN-γ is either weak or absent in infants experiencing a first RSV infection. In contrast, a strong IFN-γ response was found during non-RSV LRTI, indicating that an infant is able to respond with competent IFN-γ production to a first respiratory viral infection in the first months of life. This was not observed during primary RSV infection. The finding may be explained in several ways.
In comparison to other viruses, RSV may be less immunogenic and may not efficiently prime T cell immunity at an early age. This could be due to a combination of factors, including differences in antigenic motifs or different properties of the virus for inducing innate immune responses. Unlike influenzavirus, for example, RSV stimulates innate immune mechanisms through the toll-like receptor 4 (TLR-4), which is a primary regulator of innate immunity. TLR-4 is required for RSV-induced cytokine responses by macrophages in vitro as well as for virus elimination in a mouse model system [10]. Genetic and developmental variability in receptor expression, especially at an age when immune response mechanisms are still maturing, may therefore exert a greater influence on the infant immune response to RSV than on immune responses to other respiratory viruses. In addition, data obtained from bovine RSV model studies and from humans identified RSV non-structural proteins as potent antagonists of type I IFN-mediated host cell responses, suggesting a role for RSV in counteracting innate host defences [11,12].
Evidence for immunosuppressive activity of the virus itself is also provided by in vitro studies [13–15]. RSV may inhibit lymphocyte responses either directly or through the induction of cytokines with antiproliferative or immunosuppressive activity such as interleukin (IL)-10 [14]. Virus-specific suppression of IFN-γ synthesis is also suggested by the finding that RSV-infected PBMC from schoolchildren produce less IFN-γ following PHA stimulation than non-infected controls [16].
A growing set of data show that RSV with its two distinct viral envelope glycoproteins is capable of inducing type I and type II cytokine responses in mice [17]. The F (fusion) protein induces predominantly type I cytokine (i.e. IFN-γ) production, whereas the G (attachment) protein elicits a predominantly type II response, including IL-4, IL-5 and IL-10. The polarized production of type II cytokines was associated with enhanced immunopathology following subsequent RSV infection [17,18]. Both type I and type II cytokine production can also be induced upon RSV infection in humans [19], and similar immune mechanisms with deficient type I and/or excess type II cytokine responses have been suggested for RSV bronchiolitis in infants [3,4,20–22].
A deficient type I immune response may also result from the immaturity of an infant's immune system. It is known that the competence to generate IFN-γ responses in infancy is regulated developmentally, and that the maturation process of IFN-γ production is heterogeneous in the normal population [23,24]. The importance of age at first RSV infection in determining the subsequent pattern of T cell responses during re-infection was shown recently in the murine model [17]. Neonatal RSV infection produced more severe disease and strong type II cytokine responses during re-infection, whereas delayed RSV infection led to enhanced IFN-γ production and less severe disease during re-infection [17]. These findings would suggest that early neonatal RSV infection may induce a long-lasting bias toward type II immune responses to rechallenge, thus emphasizing the importance of early life infections in determining subsequent patterns of the disease.
There is also evidence that both genetic and environmental factors determine the type of immune response to acute RSV infection [25]. In this study, we observed differences in the strength of IFN-γ responses to viral infection within the group of rhinovirus-infected infants as well. A few of the infants with a rhinovirus infection had a significantly reduced IFN-γ response and a severe course of LRTI, whereas the majority of the rhinovirus-infected infants showed strong IFN-γ responses and a relatively mild illness. However, the number of infants in individual subgroups, especially of those with severe rhinovirus infection, is small, and further studies are required to confirm these findings. In contrast, an adverse scenario was observed during RSV primary infection, where (a) the majority of infants had severe LRTI and (b) all infants, including those with mild illness, had weak IFN-γ responses. All the infants investigated were hospitalized with a first LRTI, were of similar age, had no family history of atopy, and were therefore comparable. Within these groups, significantly reduced IFN-γ production was found not only in RSV-infected infants with severe LRTI but, in contrast to non-RSV infected infants, also in those with relatively mild illness. This suggests that in addition to a probable intrinsic disposition, virus-specific factors may affect the ongoing immune response. This is also supported by a recent study on IFN-γ responses from infants with upper respiratory tract infection [26]. The authors found that acute RSV infection was associated with reduced levels of IFN_-γ_ in nasopharyngeal aspirates compared to rhinovirus or other respiratory viral infections. However, the temporal sequence of events (low IFN-γ predisposing for RSV infection and/or RSV infection deteriorating ongoing immune responses) in the individual infant can be determined only by a prospective study that, due to the low incidence of severe LRTI in infants, would have to include a substantial number of newborns for the results to be sufficiently meaningful.
A strong stimulus for IFN-γ production may also be important for the maturation of cellular immune functions and for the promotion of Th 1 cell development in the first months of life. The infection history of an infant rather than age may therefore exert a significant influence on the clinical development of respiratory tract disease, especially with regard to the so-called sensitization period during infancy.
In summary, the results of this study suggest that depending on the nature of the viral pathogen, respiratory virus infections in infants differ significantly with regard to the induced immune response, corresponding to differences in outcome and long-term prognosis.
Acknowledgments
The authors would like to thank Ursula Sinzinger for her excellent technical assistance and Annemarie Witzelsberger for collection of specimens.
REFERENCES
- 1.Kimpen JLL. Respiratory syncytial virus immunology. Pediatr Allergy Immunol. 1996;7(Suppl. 9):86–90. doi: 10.1111/j.1399-3038.1996.tb00403.x. [DOI] [PubMed] [Google Scholar]
- 2.Boehm U, Klamp T, Groot M, Howard JC. Cellular responses to IFN-γ. Annu Rev Immunol. 1997;15:749–95. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- 3.Aberle JH, Aberle SW, Dworzak MN, et al. Reduced interferon-γ expression in peripheral blood mononuclear cells of infants with severe respiratory syncytial virus disease. Am J Respir Crit Care Med. 1999;160:1263–8. doi: 10.1164/ajrccm.160.4.9812025. [DOI] [PubMed] [Google Scholar]
- 4.Bont L, Heijnen CJ, Kavelaars A, et al. Local interferon-γ levels during respiratory syncytial virus lower respiratory tract infection are associated with disease severity. J Infect Dis. 2001;184:355–8. doi: 10.1086/322035. [DOI] [PubMed] [Google Scholar]
- 5.Bont L, Hejnen CJ, Kavelaars A, et al. Peripheral blood cytokine responses and disease severity in respiratory syncytial virus bronchiolitis. Eur Respir J. 1999;14:144–9. doi: 10.1034/j.1399-3003.1999.14a24.x. [DOI] [PubMed] [Google Scholar]
- 6.Garofalo RP, Patti J, Hintz KA, Hill V, Ogra PL, Welliver RC. Macrophage inflammatory protein-1α (not T helper type 2 cytokines) is associated with severe forms of respiratory syncytial virus bronchiolitis. J Infect Dis. 2001;184:393–9. doi: 10.1086/322788. [DOI] [PubMed] [Google Scholar]
- 7.Aberle SW, Aberle JH, Steininger C, Matthes-Martin S, Pracher E, Popow-Kraupp T. Adenovirus DNA in serum of children hospitalized due to an acute respiratory adenovirus infection. J Infect Dis. 2003;187:311–4. doi: 10.1086/367808. [DOI] [PubMed] [Google Scholar]
- 8.Steininger C, Aberle SW, Popow-Kraupp T. Early detection of acute rhinovirus infections by a rapid reverse transcription-PCR assay. J Clin Microbiol. 2001;39:129–33. doi: 10.1128/JCM.39.1.129-133.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Asanuma H, Sharp M, Maecker HT, Maino VC, Arvin AM. Frequencies of memory T cells specific for varizella-zoster virus, herpes simplex virus, and cytomegalovirus determined by intracellular detection of cytokine expression. J Infect Dis. 2000;181:859–66. doi: 10.1086/315347. [DOI] [PubMed] [Google Scholar]
- 10.Kurt-Jones EA, Popowa L, Kwinn L, et al. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat Immunol. 2000;1:398–401. doi: 10.1038/80833. [DOI] [PubMed] [Google Scholar]
- 11.Schlender J, Bossert B, Buchholz U, Conzelmann KK. Bovine respiratory syncytial virus non-structural proteins NS1 and NS2 cooperatively antagonize alpha/beta interferon-induced antiviral response. J Virol. 2000;74:8234–42. doi: 10.1128/jvi.74.18.8234-8242.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Atreya PL, Kulkarni S. Respiratory syncytial virus strain A2 is resistant to the antiviral effects of type 1 interferons and human MxA. Virology. 1999;261:227–41. doi: 10.1006/viro.1999.9835. [DOI] [PubMed] [Google Scholar]
- 13.Preston FM, Beier PL, Pope JH. Infectious respiratory syncytial virus (RSV) effectively inhibits the proliferative T cell response to inactivated RSV in vitro. J Infect Dis. 1992;165:819–25. doi: 10.1093/infdis/165.5.819. [DOI] [PubMed] [Google Scholar]
- 14.Panuska JR, Merolla R, Rebert NA. Respiratory syncytial virus induces interleukin-10 by human alveolar macrophages. J Clin Invest. 1995;96:2445–53. doi: 10.1172/JCI118302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bartz H, Türkel Ö, Hoffjan S, Rothoeft T, Gonschorek A, Schauer U. Respiratory syncytial virus decreases the capacity of myeloid dendritic cells to induce interferon-γ in naive T cells. Immunol. 2003;109:49–57. doi: 10.1046/j.1365-2567.2003.01629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diaz PV, Calhoun WJ, Hinton KL, et al. Differential effects of respiratory syncytial virus and adenovirus on mononuclear cell cytokine responses. Am J Respir Crit Care Med. 1999;160:1157–64. doi: 10.1164/ajrccm.160.4.9804075. [DOI] [PubMed] [Google Scholar]
- 17.Culley FJ, Pollott J, Openshaw PJM. Age at first viral infection determines the pattern of T cell-mediated disease during reinfection in adulthood. J Exp Med. 2002;196:1381–6. doi: 10.1084/jem.20020943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alwan WH, Kozlowska WJ, Openshaw PJM. Distinct types of lung disease caused by functional subsets of antiviral T cells. J Exp Med. 1994;179:81–9. doi: 10.1084/jem.179.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tripp RA, Moore D, Barskey IVA, et al. Peripheral blood mononuclear cells from infants hospitalized because of respiratory syncytial virus infection express T helper-1 and T helper-2 cytokines and CC chemokine messenger RNA. J Infect Dis. 2002;185:1388–94. doi: 10.1086/340505. [DOI] [PubMed] [Google Scholar]
- 20.Legg JP, Hussai IR, Warner JA, Johnston SL, Warner JO. Type 1 and type 2 cytokine imbalance in acute respiratory syncytial virus bronchiolitis. Am J Respir Crit Care Med. 2003;168:633–9. doi: 10.1164/rccm.200210-1148OC. [DOI] [PubMed] [Google Scholar]
- 21.Roman M, Calhoun WJ, Hinton KL, et al. Respiratory syncytial virus infection in infants is associated with predominant Th-2-like responses. Am J Respir Crit Care Med. 1997;156:190–5. doi: 10.1164/ajrccm.156.1.9611050. [DOI] [PubMed] [Google Scholar]
- 22.Bendelja K, Gagro A, Bace A, et al. Predominant type-2 response in infants with respiratory syncytial virus (RSV) infection demonstrated by cytokine flow cytometry. Clin Exp Immunol. 2000;121:332–8. doi: 10.1046/j.1365-2249.2000.01297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holt PG, Clough JB, Holt BJ, et al. Genetic ‘risk’ for atopy is associated with delayed postnatal maturation of T-cell competence. Clin Exp Allergy. 1992;22:1093–9. doi: 10.1111/j.1365-2222.1992.tb00135.x. [DOI] [PubMed] [Google Scholar]
- 24.Rowe J, Macaubas C, Monger T, et al. Heterogeneity in diphtheria–tetanus–acellular pertussis vaccine-specific cellular immunity during infancy: relationship to variations in the kinetics of postnatal maturation of systemic Th1 function. J Infect Dis. 2001;184:80–8. doi: 10.1086/320996. [DOI] [PubMed] [Google Scholar]
- 25.Martinez FD. Respiratory syncytial virus bronchiolitis and the pathogenesis of childhood asthma. Pediatr Infect Dis J. 2003;22:S76–82. doi: 10.1097/01.inf.0000053889.39392.a7. [DOI] [PubMed] [Google Scholar]
- 26.Joshi P, Shaw A, Kakakios A, Isaacs D. Interferon-gamma levels in nasopharyngeal secretions of infants with respiratory virus and other respiratory viral infections. Clin Exp Immunol. 2003;131:143–7. doi: 10.1046/j.1365-2249.2003.02039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]