Cryo-electron tomography of clathrin coated vesicles – structural implications for coat assembly (original) (raw)
. Author manuscript; available in PMC: 2008 Jan 19.
Published in final edited form as: J Mol Biol. 2006 Oct 14;365(3):892–899. doi: 10.1016/j.jmb.2006.10.036
Abstract
Clathrin-coated vesicles mediate vesicular traffic in cells. Three-dimensional image reconstructions of homogenous populations of in vitro assembled clathrin coats have yielded a molecular model for clathrin and its interactions with some of its partners. The intrinsic averaging required for those calculations has precluded detailed analysis of heterogeneous populations of clathrin-coated vesicles isolated from cells. We have therefore used cryo-electron tomography to study the lattice organization of individual clathrin coated vesicles and the disposition of the captured vesicle with respect to the surrounding coat. We find a wide range of designs for the clathrin lattice, with different patterns of pentagonal, hexagonal, and occasionally heptagonal facets. Many coats, even smaller ones, enclose membrane vesicles, which are generally offset from the center of the clathrin shell. The electron density distribution between the coat and the underlying vesicle is not uniform, and the number of apparent contacts that anchor the clathrin lattice to the vesicle membrane is significantly less than the number of clathrin heavy chains in the assembly. We suggest that the eccentric position of the vesicle reflects the polarity of assembly, from initiation of coat formation to membrane pinching.
Keywords: clathrin, clathrin coated vesicles, endocytosis, cryo-electron tomography, cryo-electron microscopy
Introduction
Clathrin-coated vesicles are specialized organelles that carry endocytic membrane traffic from the plasma membrane to endosomes and secretory traffic between the trans-Golgi network and endosomes (reviewed in 1; 2). They also participate in synaptic vesicle recycling 3. Clathrin, the protein that forms the lattice-like coat of these structures, is a spider-like trimer (a “triskelion”), with three legs radiating from a central hub. Additional “adaptor” proteins link the clathrin scaffold to the enclosed membrane vesicle and to membrane-anchored cargo proteins 1; 4; 5; 6.
Formation of clathrin-coated vesicles follows a sequence of coordinated steps, in which membrane invagination is coupled to growth of the clathrin lattice, leading to lattice closure and vesicle budding 1; 7; 8. Early electron microscopy (EM) studies of negatively stained clathrin coated vesicles purified from pig brain showed coats with a wide variety of designs accommodating vesicles of different sizes and shapes 4. In vitro assembled clathrin lattices 9; 10; 11 can have more uniform and symmetrical designs (e.g., lattices with icosahedral, D6 or tetrahedral symmetry), particularly if assembled with the endocytic adaptor protein complex AP-2. A recent study based on cryo-electron microscopy (cryo-EM) of clathrin/AP-2 coats imaged in vitrified ice produced electron density maps at sub-nanometer resolution, obtained from averaged images of these structures 12. When combined with the atomic structures of individual clathrin domains 13; 14, these maps yielded a molecular model for a clathrin lattice 12. The approach was extended to the analysis of how auxilin, the co-factor required for the Hsp70 and ATP-dependent dissociation of the coat, interacts with clathrin within the lattice 15.
Clathrin coated vesicles purified from tissues contain many protein components in addition to adaptors, mostly in sub-stoichiometric ratios with respect to clathrin, and our understanding of their disposition with respect to the encapsulated, cargo-carrying lipid vesicles remains largely inferential. We describe here reconstructions of individual coated vesicles obtained by cryo-electron tomography. Even the smallest of the many lattices we detect can enclose a vesicle, which has an unexpected eccentric disposition with respect to the surrounding coat.
Results and discussion
Cryo-electron tomography of single clathrin-coated vesicles
Clathrin coated vesicles were purified from bovine brain 16, using a gentle isosmotic procedure to prevent membrane shrinkage 17, and embedded in vitreous ice. Cryo-EM images (Fig. 1a) of these frozen hydrated coated vesicles recorded with a conventional low dose of ~20e−/Å2 show that the individual assemblies differ in size and shape and that many contain membrane vesicles. These preparations contain all the assembly components: clathrin triskelions, adaptor protein complexes, membrane vesicles and possibly cargo proteins trapped within the vesicles (data not shown). Because of their obvious heterogeneity, we could not use single particle averaging (as used to reach sub-nanometer resolution from images of reassembled coats 12) for analyzing these structures, and we have therefore used cryo-electron tomography to obtain 3D reconstructions of individual frozen hydrated coated vesicles at lower resolution.
Figure 1.
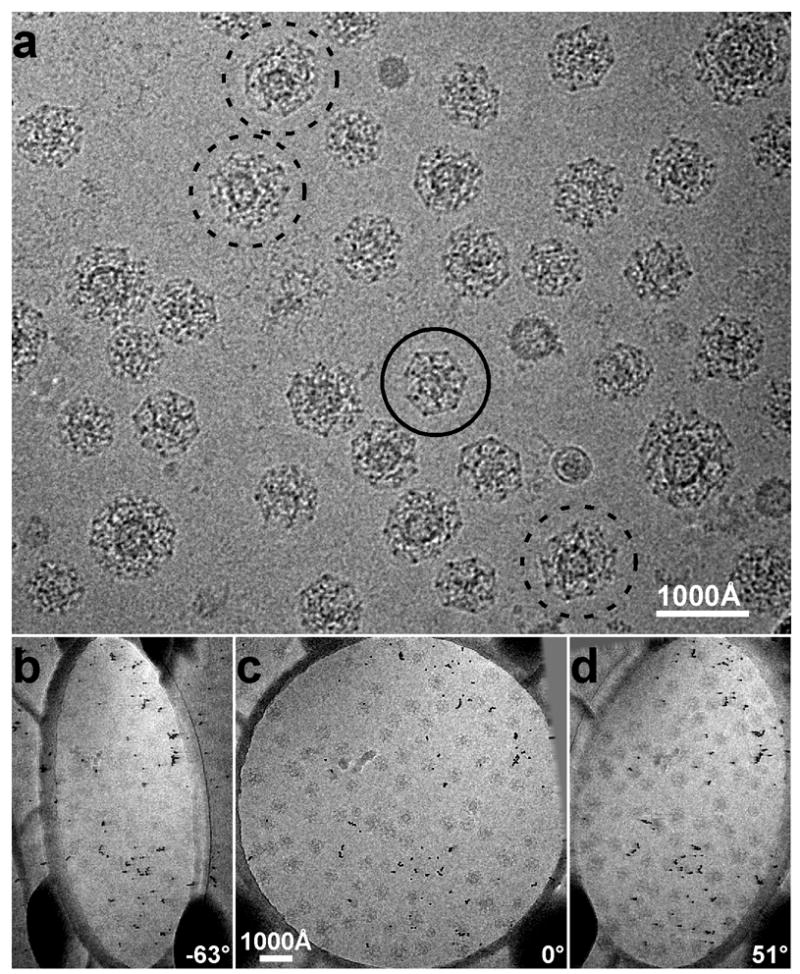
a: Cryo-electron microscopy image of clathrin coated vesicles embedded in vitreous ice. The particle marked by a continuous circle is probably the top view of a D6 barrel. From the projection image alone it is difficult to determine whether the coat contains a vesicle or not. The particles marked by dashed circles are larger and contain vesicles obviously offset from the centers of the coats. b–d: Three representative raw images of a tilt series used to calculate cryo-tomograms of vitrified clathrin-coated vesicles. The tilt axis is approximately along the vertical direction.
Images in a tilt series (Fig. 1b-d), recorded as described in Methods, were aligned with each other to calculate the electron tomograms. Fig. 2a shows a central section from a reconstructed tomogram of a vitreous ice slab with embedded clathrin coated structures. The membrane vesicles are clearly visible within many of the coats (arrow heads). One of them (circled in Fig. 2a), boxed out from the tomogram, is presented as a series of sequential parallel 62 Å thick slices (Fig. 2b). Comparison with similarly sliced views of an empty clathrin coat boxed out from the same field (Fig. 3) makes it clear that the particle shown in Fig. 2b is indeed a vesicle surrounded by a clathrin coat. The continuous membrane of the vesicle and the lattice of the coat (especially its relatively highly contrasted vertices) are all clearly visible. It has been suggested that coats smaller than the icosahedral “soccer ball” might not be able to accommodate a membrane vesicle 18. Without a full reconstruction, it has not been possible to test this proposal, because it is difficult to distinguish between a coated vesicle and a reassembled empty coat with randomly captured protein content. The tomographic reconstructions here show that even the smallest lattices we have isolated often contain membrane vesicles. The vesicle in Fig. 2b is spherical in shape, with a mean diameter of about 340 Å; the clathrin lattice has an outer diameter of about 700 Å.
Figure 2.
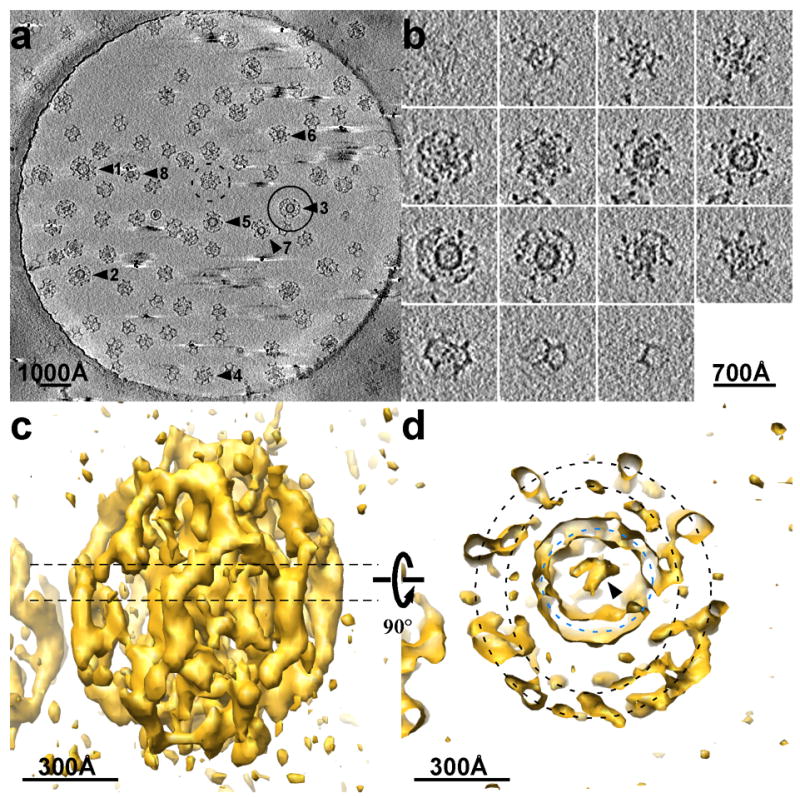
Tomography of clathrin coated vesicles. a: Central section (155 Å thick) of an electron tomogram of coated vesicles embedded in a slab of vitreous ice over a hole in a carbon film. The diameter of the hole is 1 μm. b: Slices through a single coated vesicle (circled in A, and particle 3 in Fig. 4). The slice thickness is 62 Å. c: Isodensity surface for the coated vesicle shown in b viewed from the side. d: Central section of the same density map, corresponding to the dashed line in c, viewed from the top. The black dashed circles mark the locations of two separate layers of density that surround the vesicle; the blue dashed circle marks the membrane of the vesicle itself.
Figure 3.
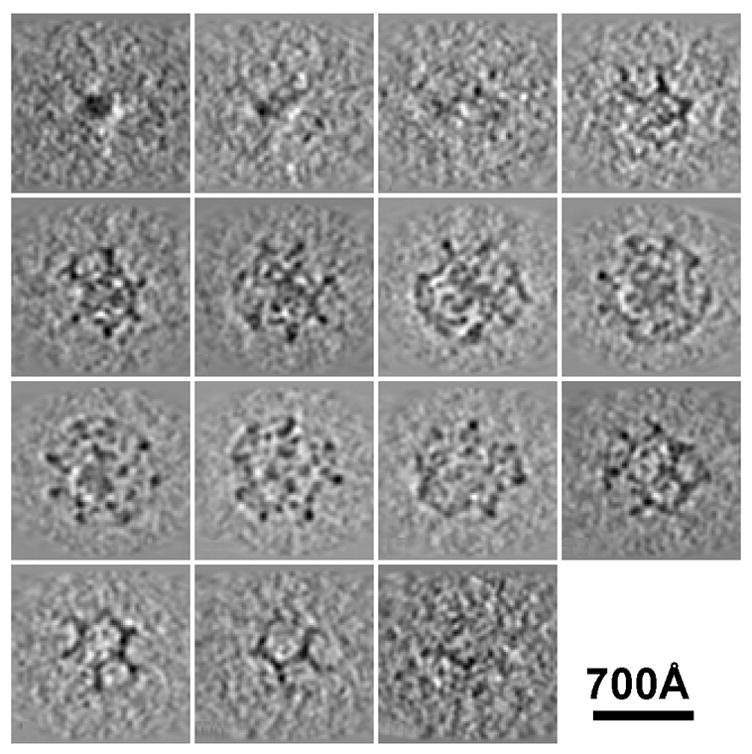
Slices through a single clathrin coat that does not contain a membrane vesicle. Coats like this are probably assembled spontaneously from clathrin triskelions during the purification process.
The 3D density map of this coated vesicle (Fig. 2c), viewed along the ice slab and perpendicular to the tilt axis, shows that the missing wedge effect leads to non-uniformity in the resolution of the tomographic 3D reconstruction 19. The details in the equator of the reconstruction are substantially sharper than at its top and bottom. The representation of the vesicle membrane is continuous in the vicinity of the equator but broken axially, and the reconstructed image elongates perpendicular to the vitreous-ice slab. Nevertheless, the pentagons and hexagons of the clathrin lattice on the surfaces of the assemblies, including the top and bottom of the reconstruction, are all well resolved (Fig. 2b and c). The density surrounding the membrane vesicle divides into two layers (Fig. 2d). The outer layer clearly defines the hexagons and pentagons of the clathrin lattice. The much higher (~ 8 Å) resolution images obtained from single-particle averaging of in vitro assembled clathrin coats show that the hexagonal and pentagonal edges of the polyhedron are bundles (about 70 Å in diameter) of two anti-parallel clathrin heavy-chain proximal segments (each with an associated light chain) and two anti-parallel distal segments 12. Therefore, the inner density layer in the tomographic reconstruction must correspond to the heavy-chain N-terminal domains and linkers; it is weaker than the outer layer and less defined in its features. There appear to be some connections between the inner layer and the vesicle membrane. The membrane vesicle is of uniform curvature with a relatively smooth surface, except where contacts from the clathrin coat impinge. Adjusting the threshold of the 3D density map reveals within the vesicle a feature, perhaps protein cargo, connecting to the membrane opposite a position where inner-layer density from the clathrin coat makes contact. Density inside the vesicle varies from particle to particle, but the inner spaces of these brain-derived coated vesicles are clearly not crowded.
Clathrin lattices
We have analyzed in detail the tomographic 3D reconstructions of eight individual coated vesicles (Fig. 4a and b). The lattices, reconstructed without ambiguity, show different designs, ranging from 36 to 60 triskelions (Table 1). The smallest (36 triskelions) has the so-called “tennis-ball” structure 4, with D2 symmetry (particle 8 in Fig. 4b). The number of triskelions is the same as in the D6 barrel, frequently found in clathrin coats assembled in vitro, but the twelve pentagons form a continuous chain, like the seam of a tennis ball, rather than two distinct rings of six. The vesicle inside this tennis-ball coat has a diameter of about 310 Å. The largest lattice (particle 1 in Fig. 4b) has the same number of triskelions as does an icosahedral coat. The coat of particle 1, like that of particle 4 in Fig. 4b, contains a heptagon and 13 pentagons. In a lattice composed entirely of hexagons and pentagons, exactly 12 pentagons are needed to form a closed shell. Inclusion of one or more heptagons requires a corresponding number of additional pentagons. Heptagons have been observed in clathrin lattices, especially near edges of the flat clathrin arrays that form where fibroblasts in culture make contact with the substrate on which they are growing 20. Of the two heptagons in the eight structures described here, one is surrounded by 6 pentagons and 1 hexagon and the other, by 4 pentagons and 3 hexagons. In both lattices, the heptagon creates a larger opening as well as a larger radius of curvature in the coat.
Figure 4.
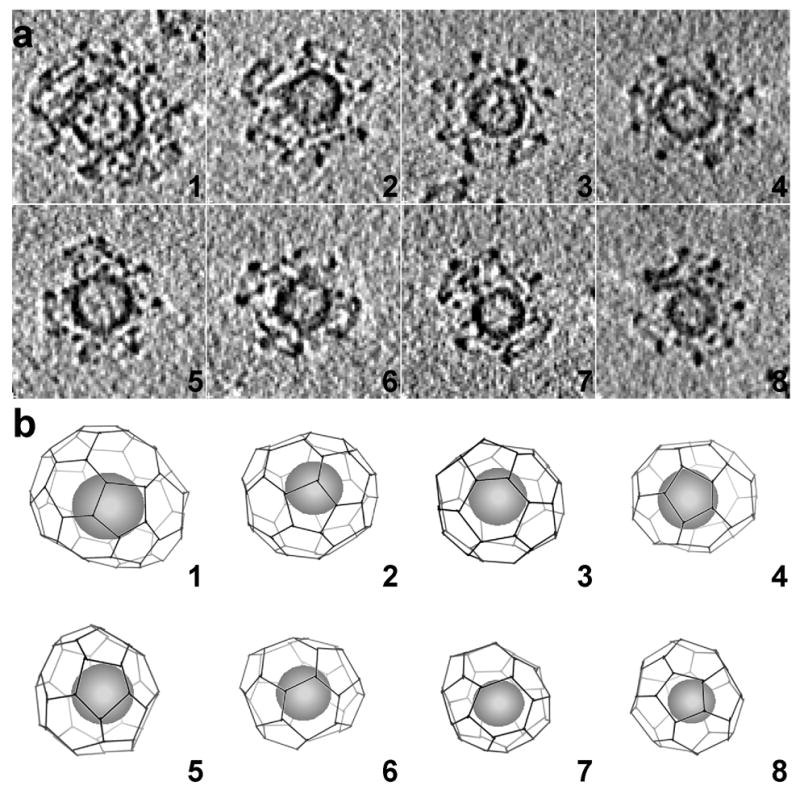
Outline of clathrin lattices in individual coated vesicles. a: The particles correspond to the clathrin-coated vesicles indicated in the tomogram shown in Fig. 2a. The images correspond to central sections (62 Å thick) viewed from the top. b: Clathrin lattices were determined by manual tracing along the electron density of the individual tomograms. The spheres inside the lattices represent the sizes and locations of the membrane vesicles contained within each coat. The views are presented in the same orientation as the central sections of the coated vesicles in A.
Table 1.
Reconstructed clathrin lattices from the cryo-electron tomogram of coated vesicles.
| number of edges | number of triskelions | number of pentagons | number of hexagons | number of heptagons | vesicle diameter (Å) | |
|---|---|---|---|---|---|---|
| 1: | 90 | 60 | 13 | 18 | 1 | 500 |
| 2: | 75 | 50 | 12 | 16 | 0 | 390 |
| 3: | 66 | 44 | 12 | 12 | 0 | 370 |
| 4: | 60 | 40 | 13 | 8 | 1 | 400 |
| 5: | 60 | 40 | 12 | 10 | 0 | 370 |
| 6: * | 57 | 38 | 12 | 9 | 0 | 360 |
| 7: * | 57 | 38 | 12 | 9 | 0 | 340 |
| 8: | 54 | 36 | 12 | 8 | 0 | 310 |
Contrary to our expectations, we found that most of the lattices in Fig. 4b lack overall symmetry (the tennis ball with D2 symmetry is an exception), and they deviate in different degrees from spherical shape. The deviations of the coats from a circular outline come not from the inevitable distortion of a tomographic reconstruction with a missing wedge, but rather reflect the designs of the clathrin lattices themselves. Surface tension or other physical forces are unlikely to have produced the departure from an overall spherical shape, as the distortions appear in all orientations even though the illustrations in Fig. 4b are oriented so that the direction of view is always perpendicular to the surface of the ice.
Interactions between coats and vesicle membrane
The density of the inner layer of the coat is not as well defined as that of the lattice-like outer layer. We can nonetheless identify, especially near the equator where missing-wedge effects are least, connections between inner-layer features and the membrane vesicle within (see asterisk, Fig. 5a). These are presumably positions at which the N-terminal domains of clathrin and AP-2 adaptor proteins (or other proteins) come together in the vicinity of the lipid bilayer. It is difficult to determine how many contacts of this kind are present in any one of the coated vesicles studied here, because of the relatively noisy electron density at the top and bottom of the 3D reconstructions. Nevertheless, these contacts are clearly resolved around the equators (Fig. 6), and in some cases they contain a sufficiently large volume to accommodate a heterotetrameric adaptor complex (Fig. 7).
Figure 5.
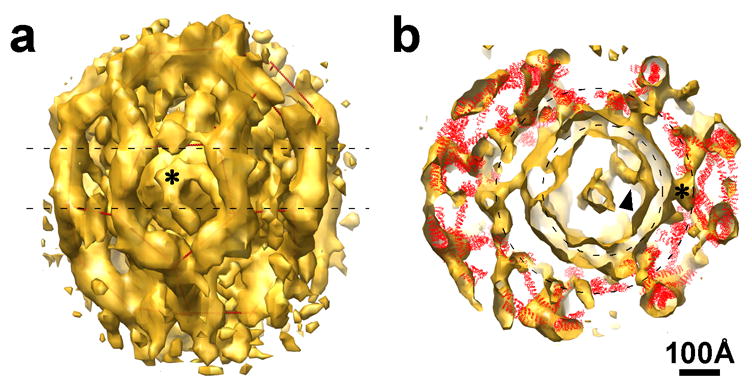
Details of coated vesicle 4 from Fig. 4. a: Surface rendered density map; the asterisk marks a feature anchored in the vesicle membrane. Red lines within the density outline the lattice. b: Central section of the same density map, with the ribbon diagrams of vertices of a clathrin lattice positioned within it. The two dashed circles represent the vesicle membrane (smaller circle) and the radial location of the N-terminal domains of the clathrin heavy chains (larger circle).
Figure 6.
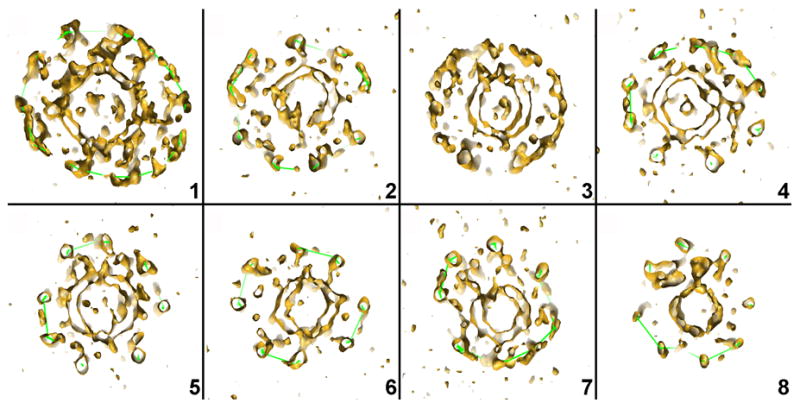
Central sections of the tomographic density maps of each of the 8 coated vesicles shown in Fig. 4, illustrating the eccentric positions of the membrane vesicles and the positions of the N-terminal domains of the clathrin heavy chains with respect to the vesicles.
Figure 7.
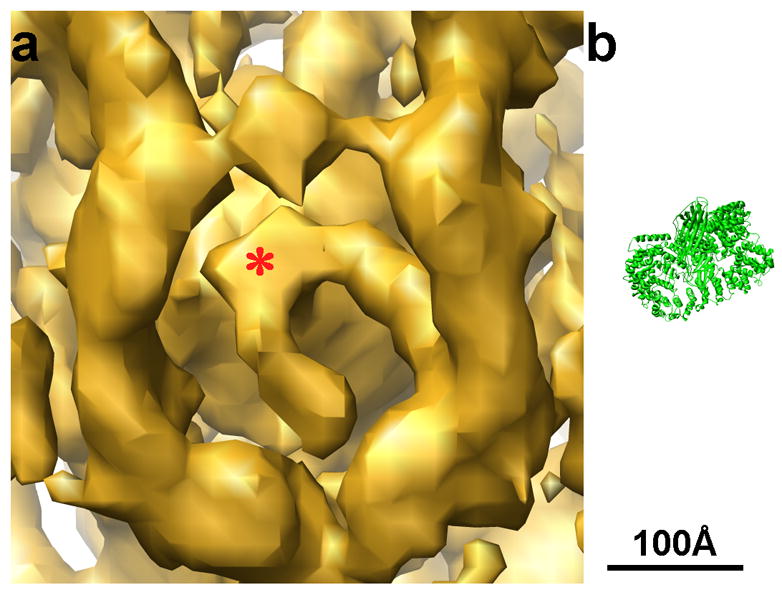
a: Enlarged view of Fig. 5a, showing a feature anchored on the vesicle membrane. b: Ribbon diagram of the AP-1 core structure (pdb code: 1W63, 29) at the same scale as the density map. The size of the AP-1 core matches the size of the density anchored on the membrane in a.
Fig. 5b shows an equatorial section of the 3D reconstruction in Fig. 5a, together with the atomic structures of clathrin triskelions (in red) placed into that reconstruction. The fit confirms our assignment of the inner density layer to the N-terminal domains of the clathrin heavy chains. The eccentric position of the vesicle brings these terminal domains into contact with densities anchored on the membrane. On the left-hand side of the image, there is a gap of 50–100 Å between the vesicle membrane and the terminal domains, and the vesicle surface is rather smooth on that side, except for one extended connection. A similar gap between the vesicle membrane and the N-terminal domains of clathrin heavy chains can also be detected in others of our tomographically reconstructed coated vesicles (Fig. 6). As seen in Figs. 4a and b and Table 1, the diameter of the membrane vesicle is generally proportional to the number of the triskelions in the assembly, but the relationship is not a strict one. The vesicle inside lattice 4 is slightly larger than the vesicles inside lattices 2 and 3, which have more triskelions (Table 1). As a result, the vesicles in 2 and 3 are more loosely encased, with more obvious gaps between coat and membrane.
In the smallest structure analyzed here (8 in Fig. 4), with 36 clathrin triskelions and hence 108 heavy chains, the membrane vesicle has a diameter of about 310 Å. If tightly covered, its surface could accommodate a total of about 30 to 40 AP-2 complexes, but the reconstruction clearly shows that such a dense coating is not present. We can conclude that the total number of AP-2 mediated contacts between the clathrin coat and the vesicle membrane is substantially less than the total number of clathrin heavy chains in the lattice.
Most of the coated vesicles in our tomogram probably correspond to precursors of synaptic vesicles 21. The eccentric locations of the vesicles inside the coats are more obvious than in larger coated vesicles observed by EM of thin-sectioned tissues 22, in part because the difference between the maximum and minimum vesicle-to-coat spacing is easier to detect in these highly curved structures. To confirm that this eccentricity is not a property of vesicles that have uncoated and then recoated during isolation, we prepared coated vesicles from bovine brain by essentially our standard procedure (see Methods), but isolated and purified at somewhat higher ionic strength and pH [25 mM Na MES pH 7.0, 100 mM NaCl, 1 mM EGTA, 0.5 mM MgCl2], to minimize even more the possibility of reassembly and artificial recoating of synaptic vesicles 21. We recorded untilted cryo-EM images of these particles and examined a number of fields and many particles are still seen to have vesicle off centered within the coats (Fig. 8a and b).
Figure 8.
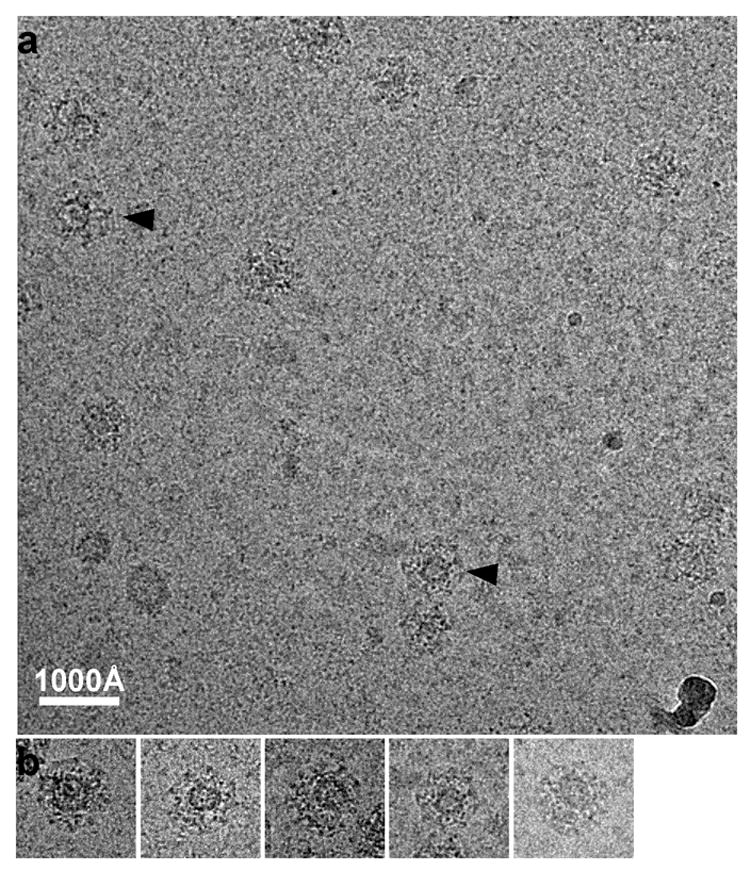
a: Cryo-EM image of clathrin coated vesicles embedded in vitreous ice. The coated vesicles were purified with high ionic strength and pH to prevent recoating during purification. Vesicles within two of the coats (arrow head) are obviously eccentric. b: A gallery of selected clathrin coated vesicles boxed out from various areas of the sample.
We suggest that the eccentric position of the vesicle within the coat comes from the polarity of initiation and completion of the lattice. The positions of close contacts of lattice and vesicle may correspond to preferential locations of adaptors and other proteins that mark the region within a coat at which assembly began, as concluded from recent live-cell imaging studies (S. Saffarian and T. Kirchhausen, in preparation). Unlike initiation, completion of a clathrin lattice, which occurs in vitro even when no vesicle is present, does not require membrane contact by clathrin.
In summary, cryo-electron tomography has allowed us to relate the structural properties of authentic coated vesicles to molecular details derived from higher-resolution methods. The reconstructed images shows that coats containing as few as 36 triskelions (e.g., the tennis-ball structure and presumably the D6 barrel) can engulf a membrane vesicle of just over 350 Å diameter, similar to the observed diameter of neurotransmitter-loaded synaptic vesicles 23. The variety of lattices in a single preparation is consistent with assembly by sequential incorporation of individual clathrin triskelions, as postulated from live-cell imaging data on the kinetics of coat formation 8. A defined region of close association between the membrane bilayer and the protein shell may mark the position at which assembly was initiated, and further analysis may therefore provide evidence for how coat formation starts.
Methods
Sample preparation and data collection
Clathrin-coated vesicles were isolated from bovine brain 16 and subjected to a final purification step by equilibrium centrifugation for 20 hr of the crude coated vesicles applied to the top of a linear gradient ranging from 2% Ficoll/9% D2O to 20% Ficoll/90% D2O in isolation buffer 17. The fractions enriched in coated vesicles were pooled, diluted 10 fold with isolation buffer, and centrifuged for 10 min at 10,000 rpm; the supernatant containing coated vesicles was used for subsequent analysis within a day of purification. Frozen hydrated samples of clathrin coated vesicles were prepared by applying a 2.5 μl drop of sample (about 1 mg/ml protein concentration) to a glow discharged 200 mesh Quantifoil holey carbon grid (Quantifoil Micro Tools GmbH, Germany). Before plunging the grid into liquid ethane at −180°C, 1 μl of a 10 nM solution of colloidal gold particles (BBInternational, UK) was added to the sample, followed by blotting with filter paper. A tilt series of 62 images from −55° to +67° with 2° increments was collected using a Tecnai F20 field-emission microscope (FEI company, USA) operated at an acceleration voltage of 200 kV. Following strict low-dose procedures, images of the tilt series were recorded manually at a nominal magnification of 29,000X on a 2K×2K CCD camera with the defocus set to −7 μm. The electron dose used for collecting each image of the tilt series was 1.5 e−/Å2, and the total electron dose for the series was about 100 e−/Å2; the total dose used for tracking during the data collection was only about 0.5 e−/Å2. Even in the last image of the tilt series (Fig. 1d) the particles were clearly visible, and there was no evident radiation-induced bubbling. All the images were binned 2×2 before further processing, corresponding to a final calibrated pixel size of 15.5 Å/pixel.
Image processing
The program package IMOD 24 was used to process the images of the tomographic tilt series. After initial coarse alignment using cross correlation between images from different tilt angles, 44 colloidal gold particles were selected as fiducial markers to align all the images of the tilt series. The thickness of the calculated tomogram was 100 pixels (~1550 Å), corresponding to the thickness of the layer of vitreous ice. No segmentation, either manual or automated, was performed to interpret the cryo-electron tomogram. Individual assemblies were boxed out from the 3D reconstructed tomogram using the program label.exe in the MRC package 25. Noise in the boxed-out 3D density maps was reduced by a 3×3×3 median filter 26.
Structure analysis
The program O 27 was used to analyze the individual coats. An artificial multi-Ala α-helix was used as a fiducial to mark the edges of the clathrin lattice and ultimately to draw its outlines as in Figure 4b. Views of the 3D density maps were prepared for illustration using the program Chimera 28.
Supplementary Material
01
02
03
Acknowledgments
This work was supported by National Institutes of Health Grant GM62580 (to David DeRosier) and GM36548 (to T.K.). The molecular EM facility at Harvard Medical School was established by a generous donation from the Giovanni Armenise Harvard Center for Structural Biology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kirchhausen T. Clathrin. Annu Rev Biochem. 2000;69:699–727. doi: 10.1146/annurev.biochem.69.1.699. [DOI] [PubMed] [Google Scholar]
- 2.Brodsky FM, Chen CY, Knuehl C, Towler MC, Wakeham DE. Biological basket weaving: formation and function of clathrin-coated vesicles. Annu Rev Cell Dev Biol. 2001;17:517–568. doi: 10.1146/annurev.cellbio.17.1.517. [DOI] [PubMed] [Google Scholar]
- 3.Sudhof TC. The synaptic vesicle cycle. Annu Rev Neurosci. 2004;27:509–547. doi: 10.1146/annurev.neuro.26.041002.131412. [DOI] [PubMed] [Google Scholar]
- 4.Crowther RA, Finch JT, Pearse BM. On the structure of coated vesicles. J Mol Biol. 1976;103:785–798. doi: 10.1016/0022-2836(76)90209-6. [DOI] [PubMed] [Google Scholar]
- 5.Kirchhausen T, Harrison SC. Protein organization in clathrin trimers. Cell. 1981;23:755–761. doi: 10.1016/0092-8674(81)90439-6. [DOI] [PubMed] [Google Scholar]
- 6.Ungewickell E, Branton D. Assembly units of clathrin coats. Nature. 1981;289:420–422. doi: 10.1038/289420a0. [DOI] [PubMed] [Google Scholar]
- 7.Conner SD, Schmid SL. Regulated portals of entry into the cell. Nature. 2003;422:37–44. doi: 10.1038/nature01451. [DOI] [PubMed] [Google Scholar]
- 8.Ehrlich M, Boll W, Van Oijen A, Hariharan R, Chandran K, Nibert ML, Kirchhausen T. Endocytosis by random initiation and stabilization of clathrin-coated pits. Cell. 2004;118:591–605. doi: 10.1016/j.cell.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 9.Vigers GP, Crowther RA, Pearse BM. Three-dimensional structure of clathrin cages in ice. Embo J. 1986;5:529–34. doi: 10.1002/j.1460-2075.1986.tb04242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith CJ, Grigorieff N, Pearse BM. Clathrin coats at 21 A resolution: a cellular assembly designed to recycle multiple membrane receptors. Embo J. 1998;17:4943–4953. doi: 10.1093/emboj/17.17.4943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Musacchio A, Smith CJ, Roseman AM, Harrison SC, Kirchhausen T, Pearse BM. Functional organization of clathrin in coats: combining electron cryomicroscopy and X-ray crystallography. Mol Cell. 1999;3:761–770. doi: 10.1016/s1097-2765(01)80008-3. [DOI] [PubMed] [Google Scholar]
- 12.Fotin A, Cheng Y, Sliz P, Grigorieff N, Harrison SC, Kirchhausen T, Walz T. Molecular model for a complete clathrin lattice from electron cryomicroscopy. Nature. 2004;432:573–579. doi: 10.1038/nature03079. [DOI] [PubMed] [Google Scholar]
- 13.ter Haar E, Musacchio A, Harrison SC, Kirchhausen T. Atomic structure of clathrin: a beta propeller terminal domain joins an alpha zigzag linker. Cell. 1998;95:563–573. doi: 10.1016/s0092-8674(00)81623-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ybe JA, Brodsky FM, Hofmann K, Lin K, Liu SH, Chen L, Earnest TN, Fletterick RJ, Hwang PK. Clathrin self-assembly is mediated by a tandemly repeated superhelix. Nature. 1999;399:371–375. doi: 10.1038/20708. [DOI] [PubMed] [Google Scholar]
- 15.Fotin A, Cheng Y, Grigorieff N, Walz T, Harrison SC, Kirchhausen T. Structure of an auxilin-bound clathrin coat and its implications for the mechanism of uncoating. Nature. 2004;432:649–53. doi: 10.1038/nature03078. [DOI] [PubMed] [Google Scholar]
- 16.Matsui W, Kirchhausen T. Stabilization of clathrin coats by the core of the clathrin-associated protein complex AP-2. Biochemistry. 1990;29:10791–10798. doi: 10.1021/bi00500a011. [DOI] [PubMed] [Google Scholar]
- 17.Woodman PG, Warren G. Isolation of functional, coated, endocytic vesicles. J Cell Biol. 1991;112:1133–41. doi: 10.1083/jcb.112.6.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pearse BM, Crowther RA. Structure and assembly of coated vesicles. Annu Rev Biophys Biophys Chem. 1987;16:49–68. doi: 10.1146/annurev.bb.16.060187.000405. [DOI] [PubMed] [Google Scholar]
- 19.Baumeister W. From proteomic inventory to architecture. FEBS Lett. 2005;579:933–937. doi: 10.1016/j.febslet.2004.10.102. [DOI] [PubMed] [Google Scholar]
- 20.Heuser J. Three-dimensional visualization of coated vesicle formation in fibroblasts. J Cell Biol. 1980;84:560–583. doi: 10.1083/jcb.84.3.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maycox PR, Link E, Reetz A, Morris SA, Jahn R. Clathrin-coated vesicles in nervous tissue are involved primarily in synaptic vesicle recycling. J Cell Biol. 1992;118:1379–1388. doi: 10.1083/jcb.118.6.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perry MM, Gilbert AB. Yolk transport in the ovarian follicle of the hen (Gallus domesticus): lipoprotein-like particles at the periphery of the oocyte in the rapid growth phase. J Cell Sci. 1979;39:257–272. doi: 10.1242/jcs.39.1.257. [DOI] [PubMed] [Google Scholar]
- 23.Schikorski T, Stevens CF. Quantitative ultrastructural analysis of hippocampal excitatory synapses. J Neurosci. 1997;17:5858–67. doi: 10.1523/JNEUROSCI.17-15-05858.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kremer JR, Mastronarde DN, McIntosh JR. Computer visualization of three-dimensional image data using IMOD. J Struct Biol. 1996;116:71–76. doi: 10.1006/jsbi.1996.0013. [DOI] [PubMed] [Google Scholar]
- 25.Crowther RA, Henderson R, Smith JM. MRC image processing programs. J Struct Biol. 1996;116:9–16. doi: 10.1006/jsbi.1996.0003. [DOI] [PubMed] [Google Scholar]
- 26.Winkler H, Taylor KA. Accurate marker-free alignment with simultaneous geometry determination and reconstruction of tilt series in electron tomography. Ultramicroscopy. 2006;106:240–54. doi: 10.1016/j.ultramic.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 27.Jones TA, Zou JY, Cowan SW, Kjeldgaard Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr A. 1991;47( Pt 2):110–119. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]
- 28.Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. UCSF Chimera - a visualization system for exploratory research and analysis. J Comput Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 29.Heldwein EE, Macia E, Wang J, Yin HL, Kirchhausen T, Harrison SC. Crystal structure of the clathrin adaptor protein 1 core. Proc Natl Acad Sci U S A. 2004;101:14108–13. doi: 10.1073/pnas.0406102101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
01
02
03