T-Cell Apoptosis in Inflammatory Brain Lesions: Destruction of T Cells Does Not Depend on Antigen Recognition (original) (raw)
Abstract
Elimination of inflammatory T cells by apoptosis appears to play an important role in the down-regulation of inflammation in the central nervous system. Here we report that apoptosis of T lymphocytes occurs to a similar extent in different models of autoimmune encephalomyelitis. Apoptosis is restricted to cells located in the neuroectodermal parenchyma, thereby leaving T cells present in the brain’s connective tissue compartments unharmed. Death of T cells in the parenchyma does not depend on antigen presentation by resident microglial cells or astrocytes. Adoptive transfer experiments with T lymphocytes carrying a specific genetic marker revealed that in the central nervous system these cells are destroyed regardless of their antigen specificity or state of activation. Although many of both antigen-dependent and -independent mechanisms in the induction of T-cell apoptosis may act simultaneously, our results suggest that the nervous system harbors a specific, currently undefined, mechanism that effectively eliminates infiltrating T lymphocytes.
For many years the central nervous system (CNS) has been considered an “immune-privileged site.” Among others, one reason for this was thought to reside in the presence of a blood-brain barrier that hides antigens from the immune system and impedes traffic of leukocytes into the tissue. This view was challenged when it became clear that the CNS is monitored continuously by surveying lymphocytes. 1,2 Recent data suggest that, like in other “immune-privileged” organs such as the eye, 3 the control of immune reactions in the CNS may rather take place by active local suppression of the inflammatory response than by restriction of immune surveillance or limitation of leukocyte traffic. One such suppressive mechanism recently became evident in experimental autoimmune encephalomyelitis (EAE). This disease, which is one of the best studied models of organ-specific autoimmunity, can be induced in rodents by active immunization with myelin antigens or adoptive transfer of myelin-reactive T cells. 4-6 The abundance of T-cell apoptosis in this model suggests that inflammation in the CNS is controlled by the efficient local destruction of lymphocytes. 7,8
In vitro studies have identified a variety of mechanisms that lead to apoptosis of T cells. Several of these mechanisms may also operate in the CNS in vivo. Apoptosis may ensue in the course of antigen presentation 9 either induced by excessive antigen in the lesions 10 or caused by unbalanced signaling by CNS antigen-presenting cells. 11-13 Alternatively, various antigen-independent mechanisms of apoptosis may be involved. Steroids induce apoptosis of T cells in the thymus, 14 and in the course of EAE, glucocorticoid levels peak at the time of recovery, 15 when T-cell apoptosis is most prominent. 8 Cytokines such as transforming growth factor-β, which can induce T-cell apoptosis in vitro, 16 are locally produced in EAE lesions. 17 Furthermore, activation of the Fas-dependent cell death pathway may be involved. In inflammatory eye lesions, as an example, T cells undergo Fas-FasL induced apoptosis caused by abundant expression of FasL in the corneal epithelium and retina. 18
To comprehend the mechanisms operating in the CNS in vivo, a number of unresolved questions need to be addressed: Is apoptosis unique to certain autoreactive T-cell clones such as myelin basic protein (MBP)-specific T cells, or is it a general feature of T cell-mediated CNS autoimmunity? Is apoptosis restricted to the autoantigen-specific T cells or are all incoming T cells destroyed, regardless of their antigen specificity? Which resident cells in the CNS are essential for apoptosis induction and, more specifically, is apoptosis dependent on major histocompatibility complex-restricted antigen presentation by facultative resident antigen-presenting cells such as astrocytes or microglial cells?
This study addresses these questions in several different models of EAE. A transgenic model used allowed unequivocal identification of transferred autoantigen-specific or secondarily recruited “bystander” T cells in the lesions and also permitted their fate to be discerned during local proliferation and destruction. Our data suggest that the CNS possesses a mechanism of T-cell elimination by apoptosis that is not dependent on the presence of the antigen for which the T cell is specific, nor does it require the activation of the lymphocyte that enter the CNS.
Materials and Methods
Animals
For the induction of EAE and for the production of antigen-specific T-cell lines, 6–12-week-old inbred Lewis rats or TK-tsA transgenic rats were used that were either purchased from Charles River (Sulzfeld, Germany) or provided by the local animal facilities from the Max Planck Institute for Psychiatry (Martinsried, Germany). The production of the TK-tsA transgenic Lewis rats has been described before. 19 These animals harbor 250 to 300 copies of a transgene cassette encompassing the thymidine kinase (TK) promoter of herpes simplex virus, the temperature-sensitive simian virus 40 T antigen tsA58/dl884 (tsA), and simian virus 40 polyadenylation and splice sites. Northern blot analysis for tsA58 mRNA of thymus cells from transgenic animals showed that the transgene is not expressed and, thus, can be used as a stable genomic marker of otherwise normal Lewis rat cells. For the production of bone marrow chimeras, Lewis rats were obtained from Charles River Laboratories (Wilmington, MA), and DA rats were supplied by Bantin and Kingman Breeders (Fremont, CA). The (DA × Lewis) F1 hybrids that served as bone marrow donors were generated in the animal colony at Washington University (St. Louis, MO) from these two parental stocks. Briefly, the bone marrow chimeras were created by lethally irradiating (1,000 rad) 2-month-old DA rats and subsequently infusing 10 8 bone marrow cells from the (DA × Lewis) F1 via the tail vein, as described previously 20 (Table 1) ▶ . All animals were maintained in accordance with United States and German institutional and federal guidelines for the treatment of rodents.
Table 1.
Sources of Animals Used
| Cell line | Number of cells | Antigen specificity | Epitope specificity | Type of EAE | Source |
|---|---|---|---|---|---|
| C1 | 5 × 105 | MBP | 68–88 | Monophasic | Kojima et al 55 |
| xx* | 1 × 107 | MBP | 68–88 | Monophasic | Lassmann et al 31 |
| RM3(OG3) | 5 × 107 | MOG | 35–55 | Monophasic† | Linington et al 56 |
| LS1 | 2 × 107 | S100 | 76–91 | Monophasic | Kojima et al 55 |
| MP1.1 | 5 × 107 | MAG | 20–34 | Monophasic† | Berger et al 25 |
| yy‡ | 1 × 107 | MBP | 68–88 | Monophasic | |
| zz§ | 1 × 107 | OVA | Unknown |
Establishment of T-Cell Lines
The specific establishment of the different T-cell lines is described in detail in the respective publications cited in Table 1 ▶ . In general, 8 to 12-week-old Lewis rats or TK-tsA-transgenic Lewis rats were immunized with 0.05–0.2 mg of various peptides in 0.2 ml complete Freund’s adjuvant. Draining lymph nodes were isolated 9 to 13 days after sensitization, and stable T-cell lines were established as described. 6 Briefly, these cells were propagated by alternating cycles of antigen-driven T-cell activation, using irradiated syngeneic thymus cells as antigen-presenting cells, and interleukin (IL)-2-dependent expansion of activated T cells. Phenotype and antigen specificity of these cells was determined as reported elsewhere. 21
Induction of EAE
Adoptive transfer experiments were performed using freshly activated T-cell blasts specific for MBP, MOG, myelin-associated glycoprotein (MAG), and S100b after 72 hours of stimulation with the appropriate antigen. T-cell blasts, dependent on the cell line varying in numbers, were injected intravenously. EAE in the radiation bone marrow chimeras and respective controls was induced by intravenous injection of 10 7 MBP-specific Lewis T lymphoblasts. In the TK-tsA experiments, EAE was induced in 8 to 12-week-old TK-tsA transgenic rats by injection of normal Lewis MBP-specific T blasts. Alternatively, EAE was induced in 8 to 12-week-old Lewis rats by passive transfer of freshly activated (TK-tsA) MBP-specific T-line blasts. A third experiment comprised Lewis rats that received (TK-tsA) OVA-specific T lymphocytes, either as freshly activated T-cell blasts or as completely resting cells no longer able to respond to IL-2. In some of these animals the Tk-tsA OVA T cells were co-transferred with MBP-reactive Lewis T-cell blasts. The animals were examined daily for clinical signs of EAE, which was scored on the following scale: 0, healthy; 1, complete loss of tail tonus; 2, additional hind limb weakness; 3, hind limb paralysis and incontinence; 4, complete paralyses; and 5, death.
Sampling and Embedding
At various time points after T-cell transfer, animals were perfused with 4% paraformaldehyde in phosphate-buffered saline under deep anesthesia. The brain and spinal cord were removed and fixed in the same fixative for an additional 3 hours. This material was then embedded in paraffin. Paraffin sections 3 μm thick from spinal cord were cut and used for general pathological screening, in situ hybridization (ISH) for TK-tsA, in situ tailing (IST) for detection of DNA fragmentation, and immunocytochemistry.
ISH for TK-tsA
ISH was performed as described earlier, 22 with the addition that, before acetylation, the sections were incubated in 10 mmol/L citric acid (pH 6.0) and microwave treated (five times for 5 minutes each, 700 W). Digoxygenin-labeled TK/tsA DNA probe, corresponding to the transgene cassette, was generated by random priming, according to the DIG DNA labeling and detection kit instructions (Boehringer Mannheim, Indianapolis, IN). For use in the ISH, the probe was diluted 1:100.
Immunocytochemistry
Immunocytochemistry was performed with a biotin-avidin technique as described in detail by Vass et al. 23 The following primary antibodies were used: W3/13 (recognizing T cells; DAKO, Glostrup, Denmark) and proliferating cell nuclear antigen (PCNA; recognizing proliferating cells; DAKO). In case of double labeling for PCNA and W3/13, sections were primarily stained for PCNA by using a triple alkaline phosphatase anti-alkaline phosphatase system as described earlier in Breitschopf et al 22 with nitroblue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate as substrate. Next, W3/13 was stained with the same alkaline phosphatase anti-alkaline phosphatase system but visualized with Fast Red. This resulted in PCNA+ nuclei stained black and W3/13+ T cells (cytoplasm and cell membrane) stained red. To analyze nuclear condensation indicative for apoptosis, all sections were counterstained with hematoxylin.
IST
To detect cells with DNA fragmentation, IST was performed as described by Gold et al. 24 Briefly, 3-μm paraffin sections from spinal cord were deparaffinized, treated with chloroform, and air dried. Next, sections were incubated in 50 μl of the reaction mixture (5 μl 10× tailing buffer, 1 μl digoxigenin-labeled nucleotides, and 44 μl transferase). As a secondary step, the sections were incubated with an alkaline phosphatase anti-digoxigenin F(ab′)2 antibody at a dilution of 1:250. Alkaline phosphatase was visualized with nitroblue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate. All materials for the IST were obtained from Boehringer Mannheim. Subsequently, the sections were stained with monoclonal antibody W3/13 as described above.
Quantification of (Apoptotic) T Cells
TK-tsA-positive cells were quantified in spinal cord cross-sections. Per cross-section the numbers of nonapoptotic (one or two distinctive spots; see Results) or apoptotic (diffuse labeling over the entire nucleus; see Results) cells were counted in the various compartments (parenchyma, meninges, and perivascular space of blood vessels). In general, from each animal, three cross-sections representing a total area of 10 mm 2 were studied. In case of low numbers of infiltrating TK-tsA-positive cells (such as by injection of OVA-specific T cells), a total of five cross-sections were investigated. The total numbers of infiltrating T cells were quantified in the various compartments on spinal cord cross-sections. In the case of TK-tsA animals, these W3/13-stained sections were consecutive to the sections used for ISH. Apoptotic W3/13+ T cells were distinguished from nonapoptotic T cells by morphological criteria (nuclear condensation and fragmentation).
Results
Clearance of Inflammation by T-Cell Apoptosis Is a General Feature of CNS Autoimmunity
Apoptotic cells were identified in W3/13-stained sections by their characteristic nuclear changes, ie, the condensation of chromatin and nuclear fragmentation (Figure 1a) ▶ . DNA fragmentation in these cells was confirmed by their positive reaction in IST-stained sections. 8 T-cell apoptosis was found in all models of EAE (induced by MBP-, MOG-, MAG-, and S100-reactive T cells) despite quite pronounced variations in the patterns of inflammation in these different conditions 25 (Figure 2a) ▶ . In general, apoptotic T cells were dispersed throughout the CNS parenchyma, although in larger or confluent lesions where inflammatory cells were densely packed, apoptotic cells occurred in clusters of variable size (Figure 1b) ▶ . Because in vitro apoptosis of T cells frequently follows a transient phase of cell cycle activation and/or proliferation, 26 we studied, by using the cell cycle marker PCNA, whether these clustered T cells also might proliferate and thereafter rapidly undergo apoptosis. Double staining for PCNA and W3/13 showed that on day 4 of EAE, PCNA+ cells were only found in the parenchyma. Considering the size and shape of the nucleus, most of these cells were identified as macrophages (Figure 1c) ▶ . PCNA+ T cells were found to be extremely rare (one to two cells/section) and in some cases appeared to have a condensed nucleus, indicating that these cells were undergoing apoptosis (Figure 1, d and e) ▶ . This sparse proliferation of T cells in the CNS confirms the results from Ohmori et al, 27 who used bromodeoxyuridine labeling to detect proliferating T cells in the CNS. None of the cells in the apoptotic clusters showed labeling for PCNA, opposing the view that such clusters developed from a local expansion of antigen-stimulated T-cell clones.
Figure 1.
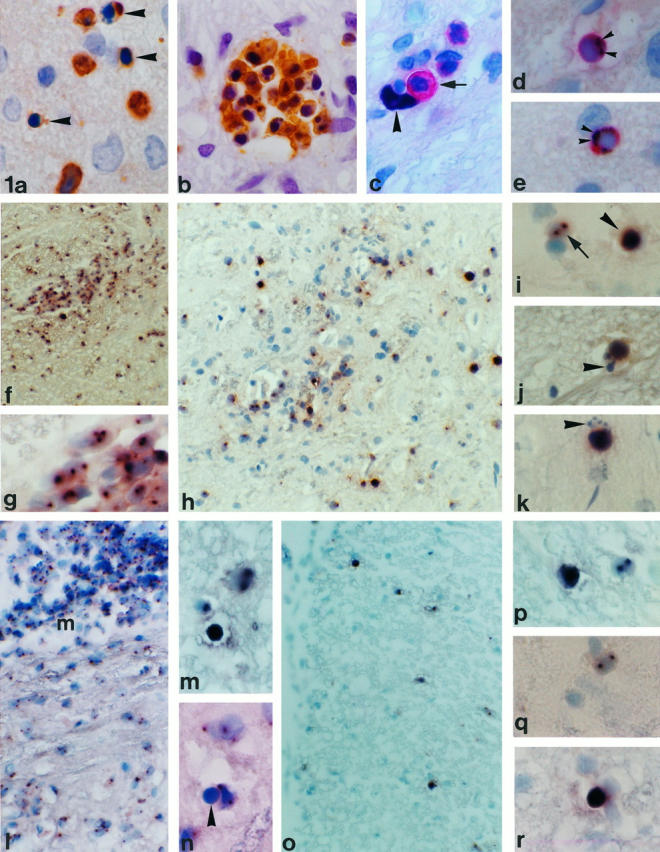
a: Apoptosis in W3/13+ T cells. Apoptotic cells with a condensed nucleus (arrowhead) mostly are found singularly in the spinal cord parenchyma (MBP EAE, day 4; magnification, ×855). b: In some cases, apoptotic T cells are found in clusters of variable size (S100 EAE, day 4; ×855). c to e: Double staining for proliferation marker PCNA and W3/13. c shows a W3/13+ T cell (red, arrow) in close apposition to a PCNA+ nucleus (arrowhead) probably from a macrophage (MBP EAE, day 4; ×855). d and e: PCNA (black, arrowhead) and W3/13 (red) double-stained T cells. The homogenously stained nucleus with PCNA immunoreactivity against the nucleus (arrowheads) shows clear condensation, suggesting that these cells are undergoing apoptosis (MBP EAE, day 4; ×855). f: ISH for TK-tsA showing labeling of cells in the spinal cord of a TK-tsA transgenic rat with EAE (day 6) induced by TK-tsA MBP-specific T cells (×213). g: Higher magnification of f. All cells in the perivascular infiltrate show TK-tsA labeling in one or two distinct (brown) spots on the nucleus (×855). h to k: Transfer of TK-tsA MBP-specific T cells in normal Lewis rats. h: Low magnification of a Lewis rat with EAE induced by TK-tsA+ MBP-specific T cells on day 4 after transfer. High numbers of TK-tsA+ cells can be found around blood vessels and deeply infiltrated in the parenchyma (×336). i: Normal TK-tsA+ MBP cells are labeled with two clear distinct spots on the nucleus (arrow), whereas apoptotic cells (arrowhead) show a strong diffuse labeling over the entire nucleus (×855). j and k: ISH for TK-tsA showing two examples of apoptotic MBP-specific T cells in a Lewis rat EAE lesion that demonstrate the presence of apoptotic bodies (arrowheads) near the main part of the apoptotic cells (×855). l to n: ISH for TK-tsA. Transfer of Lewis MBP-specific T cells in TK-tsA rats (day 6). l: Low magnification of a longitudinal section of the spinal cord. High numbers of TK-tsA-labeled T cells are present in the meninges (m; ×266). m: TK-tsA-positive cells with the morphological appearance (diffuse staining over the entire nucleus) of apoptosis (arrowhead) can be found in between normal TK-tsA-labeled cells with one or two spots on the nucleus (×855). n: In addition to the TK-tsA-labeled apoptotic cells, TK-tsA-negative (MBP-specific) apoptotic cells (arrowhead) can also be found. In this case, the apoptotic cells lie in close apposition to a TK-tsA-labeled macrophage (×855). o and p: Transfer of activated TK-tsA OVA-specific T cells in a Lewis rat with EAE induced by Lewis MBP-specific T cells (day 4 after transfer). o: ISH for TK-tsA reveals the presence of many infiltrated OVA-specific T cells in the spinal cord (×266). p: ISH for TK-tsA in a higher magnification shows that besides normal TK-tsA+ OVA-specific T cells (two dots on the nucleus), apoptotic OVA-specific T cells can also be found (×855). q and r: Transfer of resting TK-tsA OVA-specific T cells in animals with EAE results in migration of low numbers of OVA-specific T cells in the spinal cord parenchyma (day 4 after transfer). Both normal OVA-specific T cells (q; ×855) and OVA-specific T cells with an apoptotic appearance (r; ×855) can be found.
Figure 2.
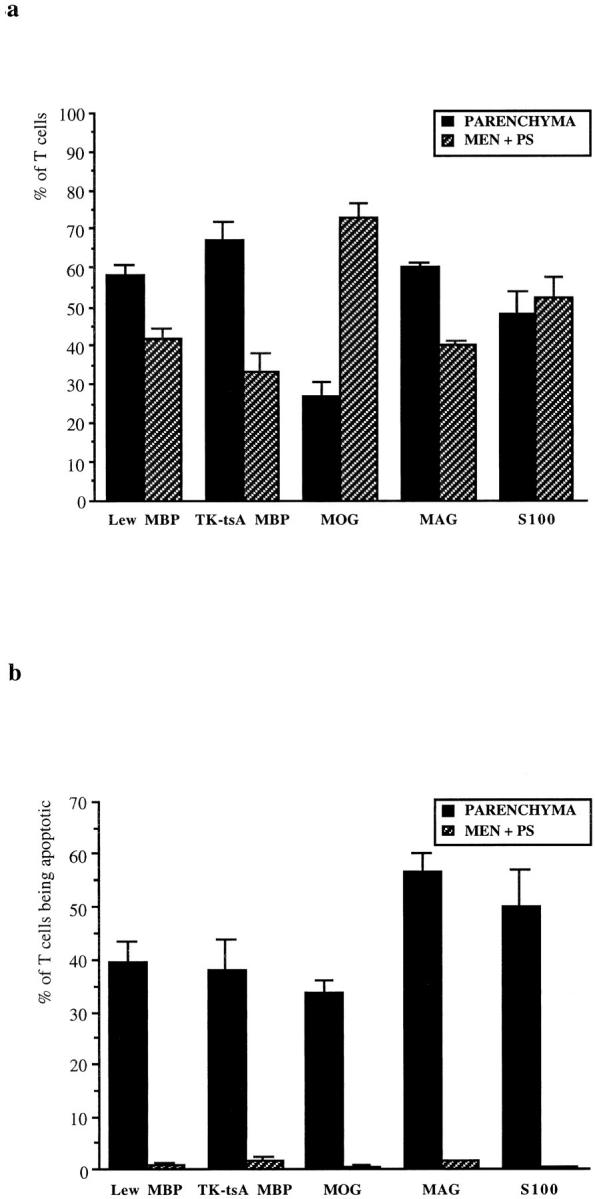
Infiltration and apoptosis of T cells in various models of EAE. a: percentage of T cells (±SEM) in the parenchyma and in meninges/perivascular space in models of EAE induced by antigen-specific T-cell lines against MBP (cell line C1; Lew MBP), MOG, MAG, and S100. In addition, EAE was induced by TK-tsA MBP-specific T cells in Lewis rats (TK-tsA MBP). b: percentages (±SEM) of apoptotic T cells in the parenchyma and in the meninges/perivascular space in these different models of EAE.
Apoptosis of Inflammatory T Cells Is Restricted to the CNS Parenchyma
The distribution of W3/13+ T cells in spinal cord cross-sections was quantified by counting the numbers of T cells in parenchyma, meninges, and perivascular space. As shown in Figure 2a ▶ , the distribution of T cells on day 6 of EAE induced by MBP-, MAG- or S100-specific T cells in the different compartments of the CNS are similar. In these models, most T cells are located in the parenchyma. In the MOG EAE model, infiltration in the CNS parenchyma for as yet unknown reasons is impeded, and thus, lymphocytes to a large extent are retained in the meninges and perivascular space. From these W3/13+ T cells in the various models, we quantified the proportion of apoptotic W3/13+ cells in the different compartments of the CNS. The results (Figure 2b) ▶ show that apoptotic T cells are present in the CNS neuroectodermal parenchyma, whereas connective tissue compartments such as meninges and the perivascular space are almost completely devoid of apoptotic T cells. This finding strongly suggests that T-cell apoptosis relies on cellular factors or contacts with cells exclusively present in the parenchyma.
Antigen Presentation by Parenchymal Glial Cells Is Not a Prerequisite for T Cell Apoptosis
Evidence that apoptosis of T cells depends on a parenchymal component is supported by in vitro data showing that antigen presentation by astrocytes or microglia but not by thymocytes 12,28,29 triggers programmed cell death of encephalitogenic T cells. To investigate whether such a role of resident microglia or astrocytes in vivo might be demonstrated, we quantified the number of (apoptotic) T cells in the spinal cord of bone marrow chimeras and control Lewis rats on day 6 after transfer of Lewis MBP-specific T cells. In these chimeric animals, antigen presentation to the injected Lewis MBP-specific T cells by resident CNS cells is precluded because of the mismatch between the major histocompatibility complex expressed on the resident cells and the major histocompatibility complex restriction requirements of the infiltrating T cells. 20,30 The results (Table 2) ▶ revealed that in both chimeric and control animals, high numbers of T lymphocytes infiltrated the spinal cord. As in all other MBP T-cell models investigated, in chimeric animals the majority of T cells were present in the parenchyma with lower numbers of T cells in meninges and perivascular space. Quantification of apoptotic T cells (Table 2) ▶ in the parenchyma revealed a similar percentage of T cells undergoing apoptosis in both chimeric and control animals. Earlier studies in bone marrow chimeras have shown that, despite the absence of antigen presentation by CNS-resident glial cells, the induction, progression, and regression of EAE is very similar to that in animals with major histocompatibility complex-matched CNS-resident antigen-presenting cells. 20,31 Our observation that apoptosis of lymphocytes is retained in chimeric animals further delineates that antigen-induced apoptosis by resident astrocytes or microglial cells cannot be held responsible for down-regulation of inflammation in the CNS.
Table 2.
Apoptosis in Bone Marrow Chimeras and Control Lewis Rats
| Group | Parenchyma | Meninges and perivascular space | ||||
|---|---|---|---|---|---|---|
| Number of T cells | Apoptotic T cells | % apoptotic T cells | Number of T cells | Apoptotic T cells | % apoptotic T cells | |
| Lewis (n = 3) | 1504 ± 161 | 205 ± 28 | 12 ± 0.6 | 658 ± 137 | 2.7 ± 0.3 | 0.4 ± 0.06 |
| Chimeras (n = 3) | 746 ± 239 | 78 ± 32 | 8.9 ± 0.9 | 711 ± 550 | 1.3 ± 0.9 | 0.1 ± 0.07 |
Both MBP-Specific and CNS-Irrelevant OVA T Cells Are Eliminated by Apoptosis in CNS Lesions
The definition of the type of T cells undergoing apoptosis in the CNS during EAE is essential to unravel the mechanisms responsible for destruction of these lymphocytes. To address this question, we used a transgenic Lewis rat with a high number of copies of foreign DNA in its genome, which is not transcribed into mRNA but allows reliable identification of transferred T cells even when they undergo several cycles of proliferation or are destroyed by apoptosis. ISH with the TK-tsA DNA probe performed on the spinal cord of such TK-tsA transgenic animals revealed labeling as one or two distinctive spots over the nucleus of each individual cell (Figure 1, f and g) ▶ . In the various ISH reactions performed on the CNS of TK-tsA transgenic animals, a labeling efficiency of 80 to 91% was reached. ISH on nontransgenic Lewis rats showed a background labeling of less than 0.5% of cells. In EAE animals, apoptotic T cells instead of the distinctive spots showed a strong diffuse labeling over the entire nucleus (Figure 1i) ▶ . In some cases, pinched-off apoptotic bodies were seen close to these condensed nuclei (Figure 1, j and k) ▶ . Obviously, the diffuse labeling of TK-tsA+-degenerating T cells is a result of the DNA cleavage in apoptotic cells, thereby spreading the multiple copies of TK-tsA genome (which in hemizygous animals lie in a tandem repeat on one chromosome) over the entire nucleus.
MBP-Specific T Cells Are Abundant at Early Stages of EAE and Are Destroyed by Apoptosis
MBP-specific T cells during the course of EAE were quantified by counting the number of TK-tsA-positive nuclei in the spinal cord. Two hours and 24 hours after injection of TK-tsA MBP-specific T cells, these cells were found in the CNS only in very low numbers (Table 3) ▶ . The number of MBP-specific T cells dramatically increased on day 4 after cell transfer. At this time point, high numbers of MBP-specific T cells were present in the meninges, perivascular space, and the CNS parenchyma (Figure 1h) ▶ . Quantification revealed that at this time point, high numbers of MBP-specific T cells in the parenchyma were undergoing apoptosis (Table 4) ▶ , whereas in the meninges and perivascular space no apoptotic MBP-specific T cells were present.
Table 3.
Migration of MBP-Specific T Cells into the CNS
| Time point | Parenchyma | Meninges and perivascular space | ||||
|---|---|---|---|---|---|---|
| Average MBP T cells | Total T cells | % of total T cells | Average MBP T cells | Total T cells | % of total T cells | |
| 2 hours (n = 3) | 1.3 ± 0.9 | 1.0 ± 0.6 | ND | 0 | 0 | ND |
| Day 1 (n = 6) | 0.5 ± 0.5 | 3.0 ± 0.7 | ND | 0 | 0 | ND |
| Day 4 (n = 4) | 233 ± 64 | 475 ± 117 | 48 ± 9.3 | 92 ± 21 | 415 ± 58 | 25 ± 9.1 |
| Day 6 (n = 8) | 116 ± 32 | 471 ± 57 | 23 ± 5.7 | 16 ± 7.8 | 227 ± 36 | 11 ± 3.3 |
| Day 9 (n = 5) | 14 ± 4.6 | 84 ± 30 | 24 ± 7.8 | 1.2 ± 0.37 | 59 ± 10.1 | 1.9 ± 0.5 |
Table 4.
Apoptosis of MBP-Specific and Host-Derived T Cells in the CNS Parenchyma
| TK-tsA+ MBP T cells → Lewis rats Apoptosis MBP-specific T cells | Lewis MBP T cells → TK-tsA rats Apoptosis host-derived T cells | |||
|---|---|---|---|---|
| Average apoptotic TK-tsA+ cells | % of total apoptotic T cells | Average apoptotic TK-tsA+ cells | % of total apoptotic T cells | |
| Day 4 | 53 ± 17 | 44.0 ± 4.9 | 30 ± 1.5 | 23.7 ± 10.2 |
| Day 6 | 59 ± 17 | 27.3 ± 4.1 | 42 ± 6.4 | 36.0 ± 3.1 |
| Day 9 | 5.2 ± 1.5 | 21.0 ± 3.2 | 65 ± 6.7 | 56.3 ± 8.7 |
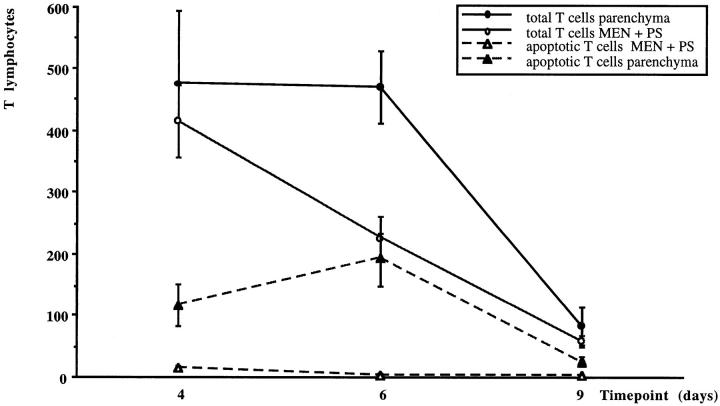
On day 6 after T-cell transfer, the percentage of MBP-specific T cells decreased in the lesions (Table 3) ▶ . However, given that the total numbers of T cells remained the same (Table 3) ▶ , at this time point large numbers of host-derived, secondarily recruited T cells apparently infiltrated the CNS. Also in the meninges and perivascular space, the percentage of MBP-specific T cells decreased (Table 3) ▶ , showing an 83% reduction in numbers compared with a 46% reduction of total T cells. Similarly, in comparison with day 4, the percentage of apoptotic MBP-reactive T cells declined (Table 4) ▶ .
At day 9 of EAE, both the numbers of MBP-specific T cells and the total T-cell population were drastically reduced (Figure 3) ▶ . In the parenchyma, the MBP-specific T cells still comprised 24% of all T cells, whereas, in the meninges and perivascular space, only sporadically were MBP-specific T cells found (Table 3) ▶ . The numbers of apoptotic T cells were also decreased at this late stage of EAE (Figure 3) ▶ . At this period, the vast majority of apoptotic T cells were unlabeled, the MBP-specific T cells contributing only 21% of all apoptotic T lymphocytes (Table 4) ▶ .
Figure 3.
Numbers of (apoptotic) T lymphocytes in parenchyma and meninges/perivascular space during the course of EAE. The average number (with SEM) of total T cells and apoptotic T cells was quantified in lumbar spinal cord cross sections on days 4, 6 and 9 of animals with MBP-T cell-induced EAE.
Secondarily Recruited (Host-Derived) T Lymphocytes Dominate Late Stages of EAE and Are Also Eliminated by Apoptosis
The results described above implied that the numbers of MBP-specific apoptotic T cells could not account for all of the apoptotic W3/13+ T cells in the spinal cord at the various time points of EAE. To demonstrate apoptosis of host-derived T cells directly, we therefore induced EAE in TK-tsA rats by injection of wild-type Lewis MBP-specific T cells. In this experimental paradigm, all TK-tsA-labeled T cells are host-derived. On day 4, the first host-derived inflammatory cells were detected in the meninges, perivascular space, and parenchyma. At this time point, most apoptotic T cells were unlabeled, with only few TK-tsA+ apoptotic cells in the parenchyma (Figure 1, l to n) ▶ . During the course of EAE, the percentage of these host-derived apoptotic T cells gradually increased until a maximum of 56% was reached on day 9 (Table 4) ▶ . Although this experiment established that secondarily recruited host-derived T lymphocytes undergo apoptosis in inflammatory CNS lesions, it does not answer the question whether these host-derived cells are autoreactive or of irrelevant antigen specificity.
Both Activated and Resting OVA-Reactive T Cells Are Recruited into EAE Lesions and Locally Undergo Apoptosis
To determine whether apoptosis of lymphocytes in the nervous system is confined to autoreactive T cells or whether it affects T cells of any specificity, we transferred TK-tsA-labeled, OVA-specific T cells in the presence or absence of Lewis MBP-specific T cells (Table 5) ▶ . In the absence of EAE induced by MBP-specific T cells, 4 days after transfer, OVA-specific T cells were not found in the CNS. In contrast, in animals with transfer EAE (MBP-reactive T cells and OVA-specific T cells injected on day 0), OVA-specific T cells had been recruited to the CNS lesions on day 4 of EAE (Figure 1o) ▶ . In these animals, almost 10% of all T cells in the CNS were derived from the OVA-specific T-cell line. Resting OVA-specific T cells transferred to EAE rats also appeared in the CNS lesions, although to a much lower extent than their activated counterparts (Figure 1q) ▶ . In both the resting and activated OVA-specific T-cell populations that found their way into the CNS parenchyma after adoptive transfer, the presence of apoptotic cells could be documented (Figure 1, p and r) ▶ . In fact, the percentage of apoptotic cells was even higher among the resting than among the activated OVA-specific T lymphocytes, even though the overall number of resting cells entering the CNS was lower than the activated type (Table 5) ▶ .
Table 5.
Migration and Apoptosis of OVA-Specific T Cells in the CNS
| Injection day MBP T cells | Injection day OVA T cells | Day of sampling | Average number of labeled cells in spinal cord | Average % of apoptotic cells | |
|---|---|---|---|---|---|
| Resting | Active | Normal | Apoptotic | ||
| − (n = 3) | 0 | 4 | 0 | 0 | 0 |
| − (n = 3) | 0 | 4 | 0 | 0 | 0 |
| 0 (n = 3) | 0 | 4 | 2.9 ± 1.4 | 2.3 ± 1.2 | ND* |
| 0 (n = 3) | 0 | 4 | 97 ± 45 | 14 ± 5.7 | 13 ± 1.1 |
Discussion
The results presented here demonstrate that apoptosis of T cells in the neuroectodermal parenchyma is a feature common to diverse models of autoimmune CNS inflammation. Apoptosis not only affects autoreactive effector T-cell populations but also secondarily recruited lymphocytes. Moreover, even T cells reactive against non-CNS antigens such as ovalbumin are destroyed after their migration into the CNS parenchyma. Thus, the data presented here indicate that the CNS protects itself against inflammation by destroying infiltrating lymphocytes in a nonselective manner.
The experiments using TK-tsA transgene-labeled, MBP-specific T cells demonstrate that in the early stage of EAE (day 4), almost 50% of all T cells found in the target organ are derived from the encephalitogenic, CNS antigen-specific population. Although homing and/or the selective retention of antigen-specific T cells is not the main subject of the current study, this observation is potentially important, because it challenges the longstanding dogma that antigen-specific T cells only constitute a very small percentage (less than 1%) of all lymphocytes in lesions in preclinical/clinical stages of EAE. 32-34 Our data, however, are in line with the results from others who in early phases of monophasic EAE found high numbers 30,35 of antigen-specific T cells in CNS lesions. A possibility is that parenchymal infiltration of T cells differs extensively between chronic EAE in mice 32-34 and the acute monophasic EAE in rats as presented here. Alternatively, the finding of low numbers of antigen-specific T cells may result from tracing methods (autoradiography) that largely underestimated the actual number of antigen-specific T cells in the CNS parenchyma.
Several observations indicate that antigen-induced apoptosis may contribute to the elimination of antigen-specific T cells in the CNS. Critchfield et al 10 showed that intravenous treatment with soluble MBP improved the clinical course of EAE, possibly by high-dose antigen-induced apoptosis of MBP-specific T cells. Similarly, in experimental autoimmune neuritis, intravenous injection of recombinant P2 protein prevented clinical experimental autoimmune neuritis and led to a profound increase of apoptotic cells in the sciatic nerve. 36 The elimination of TK-tsA+ MBP-specific T cells confirms the results from Tabi et al, 37 who described elimination of Vβ8.2+ antigen-specific T cells. Although in our system antigen-specific cells appear to die by apoptosis, our observations do not support a mechanism by which only CNS antigen-specific cells are eliminated. In fact, nonspecifically recruited, host-derived T cells also died in the CNS, a process especially prominent in the late stage of the disease. Because host-derived cells could theoretically contain a large pool of CNS-reactive T lymphocytes, 38 OVA-specific T cells were transferred to animals destined to develop EAE, and they likewise were found to undergo apoptosis in the CNS in high proportions. This finding is in contrast to that of Tabi et al, 37 who provided indirect evidence for selective survival of OVA-reactive T cells and, thus, concluded that apoptosis in EAE is restricted to the antigen-specific T cells. In their model, these authors could not differentiate the injected OVA-specific T cells from the co-transferred MBP-specific T cells. Indirect evidence showing that T cells isolated from the CNS proliferated strongly after stimulation with OVA, whereas no proliferation after MBP stimulation was achieved, was therefore used as an argument that only antigen-specific T cells were selectively eliminated. It may be clear from their work, as well as from that of others, 11,12,28,35 that antigen-specific T cells in the CNS become anergic. However, our results show that apoptosis in the CNS also proceeds independently of anergy.
Recent work in human brain focused on Fas-induced apoptosis as a mechanism to eliminate inflammatory T cells. Fas+ T cells and FasL+ microglial cells and macrophages have been demonstrated in the brain from multiple sclerosis patients, 39,40 suggesting that Fas-induced apoptosis of T cells by microglial cells may occur. In addition, apoptosis of activated T cells may also be induced by Fas-FasL interactions between T cells themselves, 41 expression of both Fas and FasL on apoptotic T cells in rat EAE lesions has been described. 42 Arguing against a role for Fas-FasL-induced apoptosis in EAE are data showing that T-cell apoptosis is not abolished in EAE lesions of lpr and gld mutant mice, although they lack functional Fas or FasL. 43
Another mechanism that at least in vitro can induce death of T cells involves corticosteroids. High-dose corticosteroid treatment augments apoptosis in inflammatory lesions of EAE 44 as well as experimental autoimmune neuritis. 45 The contribution of endogenous corticosteroids to T-cell apoptosis in the natural course of EAE is less clear. Lymphocytes undergo apoptosis in the CNS parenchyma but not in the meninges, the perivascular space, or at the inoculation site after active immunization. 8 Equal, if not greater, levels of apoptosis would be expected at such locations if systemic corticosteroids were responsible. Moreover, the induction of EAE in adrenalectomized rats revealed only a minor reduction in the level of apoptotic cells in the inflammatory CNS lesions in comparison. 46
Thus, the question arises whether the CNS hosts a mechanism of T-cell elimination unrelated to the described Fas-dependent, antigen-specific or corticosteroid-induced mechanisms. 47 One possible group of apoptosis-inducing factors in the CNS might be galectins. Galectins are β-galactoside-binding proteins that are present on hepatic sinusoidal endothelial cells and thymic epithelial cells and have been shown to induce apoptosis in various populations of thymocytes as well as in mature T lymphocytes. 48 Another mechanism involved in the elimination of T cells could be the down-regulation of IL-2 and inhibition of lymphocyte proliferation by sialic acid-containing glycosphingolipids (gangliosides). 49 Such moieties are widely distributed in the CNS. 50 Gangliosides may do so by binding to the IL-2 receptor of T lymphocytes. 51 This down-regulation of IL-2 alone can make T cells susceptible to several mechanisms of apoptosis including the corticosteroid-induced type. 9,16
As shown in this study and former studies 8,37 the rate of T cell apoptosis in the nervous system during acute EAE is exceptionally high and differs strongly from T cell apoptosis during inflammatory diseases in the skin (dermatomyositis) or muscle (myositis) where it is nearly absent. 47,52 Whether in multiple sclerosis (MS) brain T cell apoptosis is operating efficiently is not clear. Apoptosis of CD3+ T cells can be observed in MS brain but the numbers are low and not in any way comparable to the incidence of apoptosis in acute monophasic EAE. 53 However, we have recently studied T cell apoptosis in a case of acute disseminated encephalitis (ADEM), a disease more closely comparable to acute EAE. 54 Apoptosis rates in this case reached about 30% of all T cells, which equals the incidence of apoptosis in acute EAE (Bauer J, Stadelmann C, Bancher C, Jellinger K, and Lassmann H, manuscript in preparation). This suggests that in principle, human and rodent brains have similar mechanisms for elimination of T cells. Whether the low incidence of apoptotic T cells in MS is a consequence of the disease’s chronicity or whether this reflects a disturbed mechanism of T cell elimination has to be determined in future studies.
In conclusion, our study shows that the neuroectodermal CNS parenchyma provides a very efficient instrument of T-cell destruction by apoptosis. A new and important finding is that the mechanism(s) active in the CNS parenchyma affects not only the T cells specific for a CNS antigen, but eliminates all T-cell populations that arrive in inflammatory brain lesions regardless of specificity or activation state. This fundamental program for T-cell deletion in the CNS can then be further assured by a variety of antigen- or activation-dependent T-lymphocyte deletion processes. Overall, the existence of multiple, potentially synergistic T-cell depletion mechanisms in the neural parenchyma makes the CNS an extremely hostile environment for these cellular elements of the immune system.
Acknowledgments
We thank Elisabeth Gurnhofer, Ingeborg Haarmann, Angela Kury, and Marianne Leisser for expert technical assistance.
Footnotes
Address reprint requests to Dr. Hans Lassmann, Institute of Neurology, University of Vienna, Schwarzspanierstrasse 17, 1090 Vienna, Austria. E-mail: hans.lassmann@univie.ac.at.
Supported by the Deutsche Forschungsgemeinschaft Sonderforschungsbereich 217 (Projekt C12), by the European Community Biotechnology programme BIO-960174, by Austrian Science Foundation grant P10608-Med, and by grant NS27321 (to WFH).
References
- 1.Wekerle H, Linington C, Lassmann H, Meyermann R: Cellular immune reactivity within the CNS. Trends Neurosci 1986, 9:271-277 [Google Scholar]
- 2.Hickey H, Hsu BL, Kimura H: T-cell entry into the rat central nervous system. J Neurosci Res 1991, 28:254-260 [DOI] [PubMed] [Google Scholar]
- 3.Griffith TS, Brunner T, Fletcher SM, Green DR, Ferguson TA: Fas ligand-induced apoptosis as a mechanism of immune privilege. Science 1995, 270:1189-1192 [DOI] [PubMed] [Google Scholar]
- 4.Paterson PY: Passive transfer of allergic encephalomyelitis in rats by means of lymph node cells. J Exp Med 1960, 111:119-136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stone SH: Transfer of allergic encephalomyelitis by lymph node cells in inbred guinea pigs. Science 1961, 134:619-620 [DOI] [PubMed] [Google Scholar]
- 6.Ben-Nun A, Wekerle H, Cohen IR: The rapid isolation of clonable antigen-specific T lymphocyte lines capable of mediating autoimmune encephalomyelitis. Eur J Immunol 1981, 11:195-199 [DOI] [PubMed] [Google Scholar]
- 7.Pender MP, Nguyen KB, McCombe PA, Kerr JFR: Apoptosis in the nervous system in experimental allergic encephalomyelitis. J Neurol Sci 1991, 104:81-87 [DOI] [PubMed] [Google Scholar]
- 8.Schmied M, Breitschopf H, Gold R, Zischler H, Rothe G, Wekerle H, Lassmann H: Apoptosis of T lymphocytes in experimental autoimmune encephalomyelitis: evidence for programmed cell death as a mechanism to control inflammation in the brain. Am J Pathol 1993, 143:446-452 [PMC free article] [PubMed] [Google Scholar]
- 9.Boehme SA, Lenardo MJ: Propriocidal apoptosis of mature T lymphocytes occurs at S phase of the cell cycle. Eur J Immunol 1993, 23:1552-1560 [DOI] [PubMed] [Google Scholar]
- 10.Critchfield JM, Racke MK, Zúñiga-Pflücker JC, Cannella B, Raine CS, Goverman J, Lenardo MJ: T cell deletion in high antigen dose therapy of autoimmune encephalomyelitis. Science 1994, 263:1139-1143 [DOI] [PubMed] [Google Scholar]
- 11.Ford AL, Foulcher E, Lemckert FA, Sedgwick J: Microglia induce CD4 T-lymphocyte final effector function and death. J Exp Med 1996, 184:1737-1745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gold R, Schmied M, Tontsch U, Hartung H-P, Wekerle H, Toyka KV, Lassmann H: Antigen presentation by astrocytes primes rat T lymphocytes for apoptotic cell death: a model for T cell apoptosis in vivo. Brain 1996, 119:651-659 [DOI] [PubMed] [Google Scholar]
- 13.Neumann H, Schmidt H, Cavalié A, Jenne D, Wekerle H: Major histocompatibility complex (MHC) class I gene expression in single neurons of the central nervous system: differential regulation by interferon (IFN)-γ and tumor necrosis factor (TNF)-α. J Exp Med 1997, 20:305-316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wyllie AH: Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature 1980, 284:555-556 [DOI] [PubMed] [Google Scholar]
- 15.MacPhee IA, Antoni FA, Mason DW: Spontaneous recovery of rats from experimental allergic encephalomyelitis is dependent on regulation of the immune system by endogenous adrenal corticosteroids. J Exp Med 1989, 169:431-445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weller M, Constam DB, Malipiero U, Fontana A: Transforming growth factor-β2 induces apoptosis of murine T cell clones without down-regulating bcl-2 mRNA expression. Eur J Immunol 1994, 24:1293-1300 [DOI] [PubMed] [Google Scholar]
- 17.Khoury SJ, Hancock WW, Weiner HL: Oral tolerance to myelin basic protein, natural recovery from experimental autoimmune encephalomyelitis are associated with downregulation of inflammatory cytokines, differential upregulation of transforming growth factor β, interleukin 4 and prostaglandin E expression in the brain. J Exp Med 1992, 176:1355-1364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Griffith TS, Ferguson TA: The role of FasL-induced apoptosis in immune privilege. Immunol Today 1997, 18:240-244 [DOI] [PubMed] [Google Scholar]
- 19.Kääb G, Brandl G, Marx A, Wekerle H, Bradl M: The myelin basic protein-specific T cell repertoire in (transgenic) Lewis rat/SCID mouse chimeras: preferential Vβ8.2 T cell receptor usage depends on an intact Lewis thymic microenvironment. Eur J Immunol 1996, 26:981-988 [DOI] [PubMed] [Google Scholar]
- 20.Hickey WF, Kimura H: Perivascular microglial cells of the CNS are bone-marrow derived and present antigen in vivo. Science 1988, 239:290-292 [DOI] [PubMed] [Google Scholar]
- 21.Schluesener HJ, Wekerle H: Autoaggressive T lymphocyte lines recognizing the encephalitogenic region of myelin basic protein: in vitro selection from unprimed rat T lymphocyte populations. J Immunol 1985, 135:3128-3133 [PubMed] [Google Scholar]
- 22.Breitschopf H, Suchanek G, Gould RM, Coleman DR, Lassmann H: In situ hybridisation with digoxigenin-labelled probes: sensitive and reliable detection method applied to myelinating rat brain. Acta Neuropathol 1992, 84:581-587 [DOI] [PubMed] [Google Scholar]
- 23.Vass K, Lassmann H, Wekerle H, Wisniewski HM: The distribution of Ia antigens in the lesions of rat experimental allergic encephalomyelitis. Acta Neuropathol 1986, 70:149-160 [DOI] [PubMed] [Google Scholar]
- 24.Gold R, Schmied M, Giegerich G, Breitschopf H, Hartung H-P, Toyka KV, Lassmann H: Differentiation between cellular apoptosis and necrosis by the combined use of in situ tailing and nick translation techniques. Lab Invest 1994, 71:219-225 [PubMed] [Google Scholar]
- 25.Berger T, Weerth S, Kojima K, Linington C, Wekerle H, Lassmann H: Experimental autoimmune encephalomyelitis: the antigen specificity of T lymphocytes determines the topography of lesions in the central and peripheral nervous system. Lab Invest 1997, 76:355-364 [PubMed] [Google Scholar]
- 26.Lenardo MJ: Interleukin-2 programs mouse αβ T lymphocytes for apoptosis. Nature 1991, 353:858-861 [DOI] [PubMed] [Google Scholar]
- 27.Ohmori K, Hong Y, Fujiwara M, Matsumoto Y: In situ demonstration of proliferating cells in the rat central nervous system during autoimmune encephalomyelitis: evidence suggesting that most infiltrating T cells do not proliferate in the target organ. Lab Invest 1992, 66:54-62 [PubMed] [Google Scholar]
- 28.Matsumoto Y, Hanawa H, Tsuchida M, Abo T: In situ inactivation of infiltrating T cells in the central nervous system with autoimmune encephalomyelitis: the role of astrocytes. Immunology 1993, 79:381-390 [PMC free article] [PubMed] [Google Scholar]
- 29.Ford AL, Goodsall AL, Hickey WF, Sedgwick JD: Normal adult ramified microglia separated from other central nervous system macrophages by flow cytometric sorting: phenotypic differences defined and direct ex vivo antigen presentation to myelin basic protein-reactive CD4+ T cell compared. J Immunol 1995, 154:4309-4321 [PubMed] [Google Scholar]
- 30.Matsumoto Y, Fujiwara M: Adoptively transferred experimental allergic encephalomyelitis in chimeric rats: identification of transferred cells in the lesions of the central nervous system. Immunology 1988, 65:23-29 [PMC free article] [PubMed] [Google Scholar]
- 31.Lassmann H, Schmied M, Vass K, Hickey WF: Bone marrow derived elements and resident microglia in brain inflammation. Glia 1993, 7:19-24 [DOI] [PubMed] [Google Scholar]
- 32.Stohl W, Gonatas NK: Chronic permeability of the central nervous system to mononuclear cells in experimental allergic encephalomyelitis in the Lewis rat. J Immunol 1978, 121:844-850 [PubMed] [Google Scholar]
- 33.Cross AH, Cannella B, Brosnan CF, Raine CS: Homing to central nervous system vasculature by antigen-specific lymphocytes. I. Localization of 14C-labeled cells during acute chronic and relapsing experimental allergic encephalomyelitis. Lab Invest 1990, 162:162-170 [PubMed] [Google Scholar]
- 34.Steinman L: A few autoreactive cells in an autoimmune infiltrate control a vast population of nonspecific cells: a tale of smart bombs and the infantry. Proc Natl Acad Sci USA 1996, 93:2253-2256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Körner H, Goodsall AL, Lemckert FA, Scallon BJ, Ghrayeb J, Ford AL, Sedgwick J: Unimpaired autoreactive T-cell traffic within the central nervous system during tumor necrosis factor receptor-mediated inhibition of experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA 1995, 92:11066-11070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weishaupt A, Gold R, Gaupp S, Giegerich G, Hartung H-P, Toyka KV: Antigen therapy eliminates T cell inflammation by apoptosis: effective treatment of experimental autoimmune neuritis with recombinant myelin protein P2. Proc Natl Acad Sci USA 1997, 94:1338-1343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tabi Z, McCombe PA, Pender MP: Apoptotic elimination of Vβ8.2+ cells from the central nervous system during recovery from experimental autoimmune encephalomyelitis induced by passive transfer of Vβ8.2+ encephalitogenic T cells. Eur J Immunol 1994, 24:2609-2617 [DOI] [PubMed] [Google Scholar]
- 38.van Noort JM, van Sechel AC, Bajramovic J, EI Ouagmiri M, Polman CH, Lassmann H, Ravid R: A novel candidate autoantigen in multiple sclerosis: αB-crystallin, a small heat shock protein. Nature 1995, 375:798-801 [DOI] [PubMed] [Google Scholar]
- 39.D’Souza SD, Bonetti B, Balasingam V, Cashman NR, Barker PA, Troutt AB, Raine CS, Antel JP: Multiple sclerosis: Fas signaling in oligodendrocyte cell death. J Exp Med 1996, 184:2361-2370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dowling P, Shang G, Raval S, Menonna J, Cook S, Husar W: Involvement of the CD95 (APO-1/Fas) receptor/ligand system in multiple sclerosis brain. J Exp Med 1996, 184:1513-1518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hargreaves RG, Borthwick NJ, Montani MSG, Piccolella E, Carmichael P, Lechler RI, Akbar AN, Lombardi G: Dissociation of T cell anergy from apoptosis by blockade of Fas/Apo-1 (CD95) signaling. J Immunol 1997, 158:3099-3107 [PubMed] [Google Scholar]
- 42.White CA, McCombe PA, Pender MP: The roles of Fas, Fas ligand and Bcl-2 in T cell apoptosis in the central nervous system in experimental autoimmune encephalomyelitis. J Neuroimmunol 1998, 82:47-55 [DOI] [PubMed] [Google Scholar]
- 43.Malipiero U, Frei K, Spanaus K-S, Agresti C, Lassmann H, Hahne M, Tschopp J, Eugster H-P, Fontana A: Myelin oligodendrocyte glycoprotein-induced autoimmune encephalomyelitis is chronic/relapsing in perforin knockout mice, but monophasic in Fas- and Fas ligand-deficient lpr and gld mice. Eur J Immunol 1997, 27:3151-3160 [DOI] [PubMed] [Google Scholar]
- 44.McCombe PA, Nickson I, Tabi Z, Pender MP: Corticosteroid treatment of experimental autoimmune encephalomyelitis in the Lewis rat results in loss of Vβ82+ and myelin basic protein-reactive cells from the spinal cord, with increased total T cell apoptosis but reduced apoptosis of Vβ8.2+ cells. J Neuroimmunol 1996, 70:93-101 [DOI] [PubMed] [Google Scholar]
- 45.Zettl UK, Gold R, Toyka KV, Hartung H-P: Intravenous glucocorticosteroid treatment augments apoptosis of inflammatory T cells in experimental autoimmune neuritis (EAN) of the Lewis rat. J Neuropathol Exp Neurol 1995, 54:540-547 [DOI] [PubMed] [Google Scholar]
- 46.Smith T, Schmied M, Hewson AK, Lassmann H, Cuzner ML: Apoptosis of T cells, macrophages in the central nervous system of intact, and adrenalectomized Lewis rats during experimental allergic encephalomyelitis. J Autoimmun 1996, 9:167-174 [DOI] [PubMed] [Google Scholar]
- 47.Gold R, Hartung H-P, Lassmann H: T-cell apoptosis in autoimmune diseases: termination of inflammation in the nervous system and other sites with specialized immune-defense mechanisms. Trends Neurosci 1997, 20:399-404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Perillo NL, Uittenbogaart CH, Nguyen JT, Baum LG: Galectin-1, an endogenous lectin produced by thymic epithelial cells, induces apoptosis of human thymocytes. J Exp Med 1997, 185:1851-1558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Irani DN, Lin K-I, Griffin DE: Regulation of brain-derived T cells during acute central nervous system inflammation. J Immunol 1997, 158:2318-2326 [PubMed] [Google Scholar]
- 50.Byrne MC, Farooq M, Sbaschnig-Agler M, Norton WT, Ledeen RW: Ganglioside content of astroglia, and neurons isolated from maturing rat brain: consideration of the source of astroglial gangliosides. Brain Res 1988, 461:87-93 [DOI] [PubMed] [Google Scholar]
- 51.Chu JWK, Sharom FJ: Gangliosides inhibit T-lymphocyte proliferation by preventing the interaction of interleukin-2 with its cell surface receptor. Immunology 1993, 79:10-17 [PMC free article] [PubMed] [Google Scholar]
- 52.Schneider C, Gold R, Dalakas MC, Schmied M, Lassmann H, Toyka KV, Hartung H-P: MHC class I-mediated cytotoxicity does not induce apoptosis in muscle fibers nor in inflammatory T cells: studies in patients with polymyositis, dermatomyositis and inclusion body myositis. J Neuropathol Exp Neurol 1996, 55:1205-1209 [DOI] [PubMed] [Google Scholar]
- 53.Ozawa K, Suchanek G, Breitschopf H, Brück W, Budka H, Jellinger K, Lassmann H: Patterns of oligodendroglia in multiple sclerosis. Brain 1994, 117:1311-1322 [DOI] [PubMed] [Google Scholar]
- 54.Alford EC: Acute disseminated encephalomyelitis and allergic neuro-encephalopathies. Vinken PJ Bruyn GW eds. Handbook of Clinical Neurology. 1970, vol 9.:pp 500-571 North-Holland Publishing Co. Amsterdam [Google Scholar]
- 55.Kojima K, Berger T, Lassmann H, Hinze-Selch D, Zhang Y, Gehrmann J, Reske K, Wekerle H, Linington C: Experimental autoimmune panencephalitis and uveoretinitis transferred to the Lewis rat by T lymphocytes specific for the S100β molecule, a calcium binding protein of astroglia. J Exp Med 1994, 180:817-829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Linington C, Berger T, Perry L, Weerth S, Hinze-Selch D, Zhang Y, Lu H, Lassmann H, Wekerle H: T cells specific for the myelin oligodendrocyte glycoprotein mediate an unusual autoimmune inflammatory response in the central nervous system. Eur J Immunol 1993, 23:1364-1372 [DOI] [PubMed] [Google Scholar]