CXCR3 and Its Ligand CXCL10 Are Expressed by Inflammatory Cells Infiltrating Lung Allografts and Mediate Chemotaxis of T Cells at Sites of Rejection (original) (raw)
Abstract
The attraction of T lymphocytes into the pulmonary parenchyma represents an essential step in mechanisms ultimately leading to lung allograft rejection. In this study we evaluated whether IP-10 (CXCL10), a chemokine that is induced by interferon-γ and stimulates the directional migration of activated T cells, plays a role in regulating the trafficking of effector T cells during lung allograft rejection episodes. Immunohistochemical examination showed that areas characterized by acute cellular rejection (grades 1 to 4) and active obliterative bronchiolitis (chronic rejection, Ca) were infiltrated by T cells expressing CXCR3, ie, the specific receptor for CXCL10. In parallel, T cells accumulating in the bronchoalveolar lavage of lung transplant recipients with rejection episodes were CXCR3+ and exhibited a strong in vitro migratory capability in response to CXCL10. In lung biopsies, CXCL10 was abundantly expressed by graft-infiltrating macrophages and occasionally by epithelial cells. Alveolar macrophages expressed and secreted definite levels of CXCL10 capable of inducing chemotaxis of the CXCR3+ T-cell line 300-19; the secretory capability of alveolar macrophages was up-regulated by preincubation with interferon-γ. Interestingly, striking levels of CXCR3 ligands could be demonstrated in the fluid component of the bronchoalveolar lavage in individuals with rejection episodes. These data indicate the role of the CXCR3/CXCL10 interactions in the recruitment of lymphocytes at sites of lung rejection and provide a rationale for the use of agents that block the CXCR3/CXCL10 axis in the treatment of lung allograft rejection.
The bronchiolitis obliterans syndrome (BOS) is a fibroproliferative process sustained by the migration and proliferation of mesenchymal cells in the vascular and airway lumen that manifests clinically as an unexplained chronic graft deterioration. 1-3 Although BOS represents an important cause of morbidity and mortality among lung allograft recipients, 1-3 effector mechanisms triggering the local fibroproliferation are still debated. Because the acute cell-mediated rejection has been identified as an important risk factor for the development of BOS, it has been suggested that BOS is an immune-mediated complication of lung transplantation. 3,4 In particular, as in other pulmonary-immunomediated disorders characterized by a fibroproliferative response, activated macrophages and T cells are likely to play a pathogenetic role in mediating an early hypersensitivity reaction, perhaps triggered by epithelial expression of donor-specific major histocompatibility complex molecules. 2,4 Despite immunosuppressive therapy, the presence of perivascular, interstitial, and intra-alveolar lymphocytic infiltrate may in fact persist in approximately a third of rejecting patients. 1 Hypersensitivity reaction against alloantigens is followed by local injury, uncontrolled fibroproliferative repair processes, and the consequent filling of the airway lumen. 5 Given the above, it is accepted that the severity and persistence of lymphocyte infiltrates represent the limiting factors determining the extension of the tissue involvement by local hypersensitivity reactions and driving the permanent structural alterations of the respiratory tract.
The central event in the pathogenesis of pulmonary T cell responses is the organ-specific traffic of T lymphocytes. 6 Recently, a number of chemoattractants for leukocytes have been described. These molecules belong to the superfamily of chemokines that can be divided into four groups (C, CC, CXC, and CX3C), according to the positioning of the cysteine residues in the mature protein. 7-10 Expression of chemokine receptors on the cell surface of pulmonary immunocompetent cells and interactions with their specific ligands are involved in the mechanisms accounting for the accumulation of inflammatory cells within the lung microenvironment and the establishment of local hypersensitivity reactions. 6 Although the interactions of chemokine receptors are often characterized by considerable promiscuity, a human chemokine receptor that is selectively involved in the recruitment of T cells is CXCR3. 11,12 CXCR3 is selectively expressed by activated T lymphocytes, and, in turn, its ligands interferon (IFN)-γ-inducible protein-10 (IP-10, CXCL10 according to the new nomenclature of chemokine and chemokine receptors, 13 ), monokine induced by IFN-γ (Mig/CXCL9), interferon-inducible T-cell α-chemoattractant (I-TAC/CXCL11) are specifically chemotactic for activated T cells.
In this study, using cells recovered from the bronchoalveolar lavage (BAL) and related biopsy specimens, we have evaluated the role of local CXCR3/CXCL10 receptor interactions in the immunological events ultimately leading to lung allograft rejection. We have shown that T cells accumulating in the lower respiratory tract of patients with acute lung allograft rejection, as well as in individuals suffering from BOS, express a functional CXCR3 because they are able to migrate in response to CXCL10. Furthermore, the specific CXCR ligand CXCL10 was abundantly expressed by graft-infiltrating macrophages of patients showing T cell infiltrate.
Materials and Methods
Study Populations
According to our protocol, 56 consecutive transbronchial biopsies and BALs were performed during the follow-up of 24 lung allograft recipients (14 men and 10 women) who underwent surveillance bronchoscopy for clinical suspicion of infection or rejection. All patients underwent immunosuppression with rabbit anti-thymocyte globulin postoperatively, followed by a triple immunosuppression regimen of cyclosporin A or tacrolimus, azathioprine or RAD, and prednisone.
Transbronchial biopsies (average, 6.1; range, 5.0 to 11.0) were taken from each lobe and serial sections were used for histological diagnosis and immunohistochemical analysis. Biopsy samples were evaluated according to the revised International Society for Heart and Lung Transplantation working formulation. 14 Overall, 47 of 58 biopsy samples showed no histological signs of rejection and eight showed acute rejection ISHLT grade A1 (n = 3), A2 (n = 3), and A3 (n = 2). Three patients were classified as suffering from BOS.
BAL was performed according to the technical recommendations and guidelines for the standardization of BAL procedures. 15 Briefly, a total of 200 ml of saline solution was injected in 25-ml aliquots via fiberoptic bronchoscopy, with immediate vacuum aspiration after each aliquot. Immediately after the BAL, the fluid was filtered through gauze and the volume measured. A volume of 100 to 200 ml of BAL recovery and a sample of 50% of the instilled volume with a minimum of 50 ml was considered acceptable. The percentage of BAL recovery was 53.7 ± 4.2% and 55.1 ± 3.7% of the injected fluid in allograft recipients and control patients, respectively. Cells recovered from the BAL were washed three times with phosphate-buffered saline (PBS), resuspended in endotoxin tested RPMI 1640 (Sigma Chemical Co., St. Louis, MO) supplemented with 20 mmol/L HEPES and l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, and 10% fetal calf serum (ICN Flow, Costa Mesa, CA), and then counted.
A standard morphological and immunological analysis of BAL cellular components was performed and included cell recovery; differential count of macrophages, lymphocytes, neutrophils, and eosinophils; and flow cytometry analysis of CD4/CD8 BAL T cell ratio.
Purification of Alveolar Macrophages (AMs) and T Cells
AMs and T cells were enriched from the BAL cell suspensions by rosetting with neuraminidase-treated sheep red blood cells followed by F/H gradient separations and removing residual CD3+ lymphocytes using high-gradient magnetic separation columns (Mini MACS; Miltenyi Biotec, Germany). 16 After this multistep selection procedure >95% of the above cells were viable, as judged by the trypan blue exclusion test. Staining with mAb showed that >99% of purified lymphocytes were CD3+ T cells.
Monoclonal Antibodies and Cytokines
The commercially available conjugated or unconjugated mAbs used belonged to the Becton Dickinson and PharMingen series and included: CD3, CD4, CD8, CD45R0, CD45RA, and isotype-matched controls. Anti-IL-4 and anti-IFN-γ mAbs were purchased from PharMingen (San Diego, CA). Purified rabbit anti-human CXCL10 polyclonal antibody (R&D Systems Inc., Minneapolis, MN) and anti-hCXCR3 mAb (R&D Systems Inc.) were also used.
Immunohistochemical Analysis of CXCR3+ Cells and CXCL10-Producing Cells
Expression of CXCR3 and CXCL10 was measured by permanent section immunohistochemistry with anti-CXCR3 and anti-IP10 antibodies. Four-μm thick paraffin-embedded sections were used for immunostaining using the standard avidin-biotin complex method (Vectastain ABC Kit; Vector Laboratories, Burlingame, CA). The reliability of both antibodies in paraffin sections was compared with cryostatic (frozen) lung sections of two patients who died from persistent acute airway rejection (A3) and active obliterative bronchiolitis (Ca), respectively. Sections were deparaffinized in xylene (5 minutes, three times) and rehydrated through graded ethanol (twice for 5 minutes in 100% ethanol, 3 minutes in 95% ethanol, 3 minutes in 70% ethanol, and 5 minutes in distilled H20). For the microwave antigen retrieval procedure, slides were placed in a 2-L glass beaker containing 0.01 mol/L citrate buffer, pH 5.9, and microwaved at full power (800 W for 5 minutes, three times) before cooling and equilibration in PBS.
To neutralize endogenous peroxidase activity, slides were pretreated with 3% hydrogen peroxide for 5 minutes. Primary antibodies were applied at the following concentrations: anti-hCXCR3 mAb 1:100 and anti-hIP-10/CXCL10 polyclonal 1:100 for 1 hour in a humidified chamber at 37°C. Immunoreactivity was detected using biotinylated secondary antibodies (1:50 rabbit anti-goat and 1:1,000 dilution goat anti-rabbit in PBS-bovine serum albumin buffer) incubated for 45 minutes followed by a 30-min incubation with avidin-peroxidase (1:200) and visualized by a 7-minute incubation with the use of 0.1% 3,3′-diaminobenzidene tetrahydrochloride as the chromogen. The intensity of antibody staining was classified in three groups: strong, weak, and negative. Parallel control slides were prepared either lacking primary antibody or lacking primary and secondary antibodies, or stained with normal sera to control for background reactivity.
Phenotypic Evaluation of BAL Cells
The frequency of BAL cells positive for the above reagents was determined by overlaying the flow cytometry histograms of the samples stained with the different reagents as previously reported. 16 Cells were scored using a FACScan analyzer (Becton Dickinson, Mountain View, CA), and data were processed using the Macintosh CELLQuest software program (Becton Dickinson). The expression of cytoplasmic cytokine was evaluated after permeabilization of cell membranes using 1:2 diluted PermeaFix (Ortho, Raritan, NJ) for 40 minutes. After permeabilization procedures, anti-IL-4, anti-IFN-γ, and anti-CXCL10 antibodies were added. Because pulmonary cells bore cytoplasmic cytokines in a unimodal expression pattern, indicating that the entire cell population exhibits relatively homogeneous fluorescence, the percentage of positive cells does not represent the most accurate way of enumerating positive cells. The mean fluorescence intensity was used to compare the positivity of these specific antigens on different cell populations. To evaluate whether the shift of the positive cell peak was statistically significant, the Kolmogorov-Smirnov test for analysis of histograms was used according to the Macintosh CELLQuest software user’s guide (Becton Dickinson).
For immunofluorescence analysis, control IgG1 and IgG2a and IgG2b were obtained from Becton-Dickinson; control rat antiserum consisted of ascites containing an irrelevant rat IgG2b; control rabbit antiserum consisted of rabbit IgG (purified protein) purchased from Serotec (Serotec, UK); goat-anti-rabbit IgG and goat F(ab′)2 anti-rat IgG were obtained from Immunotech (Marseille, France).
Generation of Macrophage Supernatants
To verify the ability of AMs to release CXCL10, AMs (1 × 106/ml) were isolated from the BALs of allograft recipients, resuspended in RPMI medium, and cultured for 24 hours in 24-well plates at 37° in 5% CO2. In separate experiments AMs were stimulated with IFN-γ (100 U/ml), phorbol myrisate acetate (10 ng/ml), and lipopolysaccharide (10 μg/ml; Difco Labs., Detroit, MI). After the incubation period, supernatants were harvested, filtered through a 0.45-μm Millipore filter, and immediately stored at −80°C. At the end of the culture time AM viability was always >95%. Chemotactic activity of supernatants was determined as reported below.
Migration Activity of Pulmonary T Cells in Response to CXCR Chemokines
T-cell migration was measured in a 48-well modified Boyden chamber (AC48 Neuro Probe Inc.). The chamber is made of two sections: different chemotactic stimuli were loaded in the bottom section while cells were added in the top compartment. Polyvinylpyrrolidone-free polycarbonate membranes with 3- to 5-μm pores (for lung T cells obtained from allograft patients and the CXCR3+ and CXCR− T cell lines, respectively; Osmonics, Livermore, CA) and coated with fibronectin were placed between the two chamber parts. Only the bottom face of filters was pretreated with fibronectin; the fibronectin pretreatment maximizes attachment of migrating cells to filters, avoiding the possibility that they may not adhere. Using this procedure in preliminary experiments we demonstrated that only a trivial number of cells may be recovered in the bottoms of the wells. To avoid the shedding of fibronectin, fibronectin-treated filters were extensively washed. In preliminary experiments, fibronectin-treated filters did not induce spontaneous chemotaxis in absence of chemokines.
To evaluate the migratory properties of pulmonary T lymphocytes rhIP-10/CXCL10 (200 ng/ml) were used. The CXCR− and CXCR3+ cell lines (300-19, kindly provided by Dr. B. Moser, Theodor-Kocher Institute, University of Bern, Switzerland) were used as negative and positive controls. Thirty μl of chemokines or control medium were added to the bottom wells, and 50 μl of 5.0 × 10 6 cells/ml T cells or CXCR−/+ cells resuspended in RPMI 1640 were added to the top wells. The chamber was incubated at 37°C with 5% CO2 for 2 hours. The membranes were then removed, washed with PBS on the upper side, fixed, and stained with DiffQuik (Dade AG, Düdingen, Switzerland). Cells were counted in three fields per well at ×800 magnification. All assays were performed in triplicate. In blocking experiments, cell suspensions were preincubated before chemotaxis assay for 30 minutes at 4°C with anti-human CXCR3 mAb at the concentration of 20 μg/ml.
Chemotactic Activity of the Fluid Component of BAL and Macrophage Supernatants
The CXCR− and CXCR3+ cell lines were also used to evaluate both the chemotactic activities of macrophage supernatants and the fluid component of BAL samples. Supernatants from cell cultures and the fluid components of BALs were obtained as reported above and used undiluted; different concentrations of CXCL10 were used as a positive control. Chemotactic assays were performed as reported above. In blocking experiments, anti-CXCL10 was added to the cell supernatants before chemotaxis assay at the concentration of 20 μg/ml.
Statistical Analysis
Data were analyzed with the assistance of the Statistical Analysis System. Data are expressed as mean ± SD. Mean values were compared using the analysis of variance test. A P value <0.05 was considered as significant.
Results
The BAL cell recovery (normal range, 115,500 to 195,500 cells/ml of BAL) was higher in lung transplant recipients with allograft rejection than in recipients with no histological signs of rejection (269,274 ± 207,050 versus 212,907 ± 107,049 cells/ml of BAL, respectively; P = NS). With regard to the differential count of BAL cells, the absolute number of lymphocytes (normal range, 6,500 to 9,500 lymphocytes/ml of BAL) was higher in two patients with A3 acute rejection (77,460 ± 22,946 lymphocytes/ml) than in the six patients with lower grade rejection (three A1 patients: 17,373 ± 4,459 lymphocytes/ml; three A2 patients: 18,111 ± 6,428 lymphocytes/ml) and in the three patients with chronic rejection (15,230 ± 2,600 lymphocytes/ml). Because of the limited number of patients, the differences were not significant. As a consequence of the decrease in the CD4 T cell population and the increase in CD8 T cells, the BAL CD4/CD8 ratio (normal range, 1.2 to 2.3) was decreased both in transplant recipients with signs of rejection (0.35 ± 0.30) and in patients with no histological signs of rejection (0.41 ± 0.35).
Immunohistochemical and Flow Cytometry Analysis of the Expression of CXCR3 by Pulmonary Immunoinflammatory Cells during Lung Allograft Rejection Episodes
Immunohistochemical analysis was used to investigate the pattern of expression of this chemokine receptor. Grafts with histological evidence of rejection (presence of perivascular lymphocytic infiltrates, which in more severe cases, spilled over into the interstitium and alveolar air spaces) showed an infiltrate characterized by T cells expressing CXCR3 (Figure 1, a and b) ▶ . Positively marked lymphocytes showed more intense staining in patients with a higher grade of acute rejection than in patients with lower grade rejection (A3>A2>A1). Strongly stained infiltrates of CXCR3+ lymphocytes were also seen in active obliterative bronchiolitis (Figure 1, c and d) ▶ . Frozen lung samples showed staining intensity and distribution similar to the formalin-fixed lung biopsies used in the study.
Figure 1.
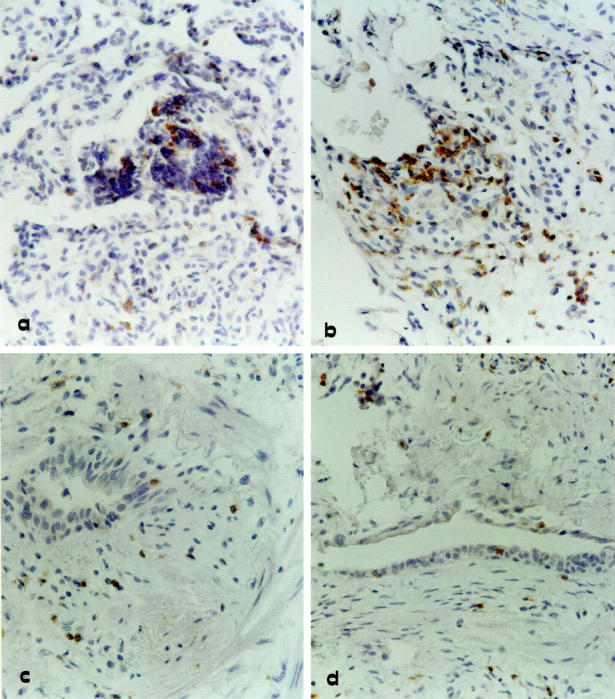
Immunohistochemistry for CXCR3 expression by lung T cells infiltrating transbronchial biopsy of four representative allograft recipients. a and b: Two allograft recipients with a rejection episode classified as A2 and A3 grades, respectively. c and d: Two representative lung allograft recipients with active bronchiolitis obliterans. Strongly stained CXCR3+ lymphocytes can be demonstrated not only in the perivascular space but also in the alveolar septa and air spaces of patients with acute cellular rejection (a and b). In patients with active obliterative bronchiolitis (c and d) subepithelial fibrosis can be observed in the bronchiole and is associated with a moderate infiltrate of CXCR3 stained lymphocytes. Original magnification, ×400.
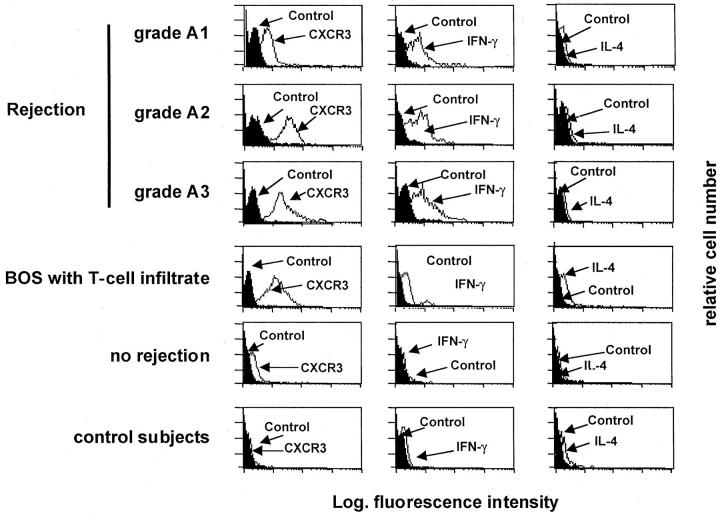
Flow cytometry analysis of BAL T lymphocytes obtained from allograft recipients who showed histological signs of rejection showed that T cells accumulating into alveolar air spaces were CXRC3+ (Figure 2) ▶ and bore IFN-γ but not interleukin (IL)-4, a pattern that is characteristic of Tc1/Th1 cells. BAL T cells of patients with BOS expressed CXCR3 at high density as well as cytoplasmic IFN-γ (Figure 2) ▶ . CXCR3 was found to be expressed at higher intensity in BAL T cells of patients with higher grade acute rejection than in patients with lower grade rejection, the differences in the mean fluorescence intensity of CXCR3+ peaks being significant in the Kolmogorov-Smirnov analysis (P < 0.01). Although CXCR3 was highly expressed by patients with T cell alveolitis, normal BAL T cells and T cells from patients with normal cellular recovery expressed low or insignificant levels of CXCR3 at the Kolmogorov-Smirnov analysis.
Figure 2.
The flow cytometry profile of BAL T cells recovered from three representative patients with A1-A3 rejection episodes, a patient with active BOS, a patient showing no rejection signs, and a control patient. The expression of CXCR3 and cytoplasmic cytokines has been determined as reported in the Materials and Methods section. The figure shows that BAL T cells from lung allograft recipients with A1-A3 rejection episodes or BOS express CXCR3 and IFN-γ but not IL-4. CXCR3 was expressed at higher intensity by BAL T cells of patients with higher grade acute rejection (A2-A3) or BOS than in the patients with the lower grade rejection (A1) (P > 0.01 at the Kolmogorov-Smirnov analysis).
CXCR3 Expressed by Pulmonary T Cells from Patients with Allograft Rejection Mediates their Chemotaxis
To characterize the biological properties of CXCR3, highly purified T cells isolated from the BALs of patients with allograft rejection and T cell alveolitis were assessed for their migratory capabilities in response to different concentrations of CXCL10. The evaluation of the migratory capabilities of pulmonary T cells in normal patients or patients with no signs of rejection was prevented by the low recovery of pulmonary T cells from the BAL. For this reason, the 300-19 T cell lines expressing high levels of CXCR3 or not expressing CXCR3 were used as positive and negative controls for the in vitro chemotaxis assay.
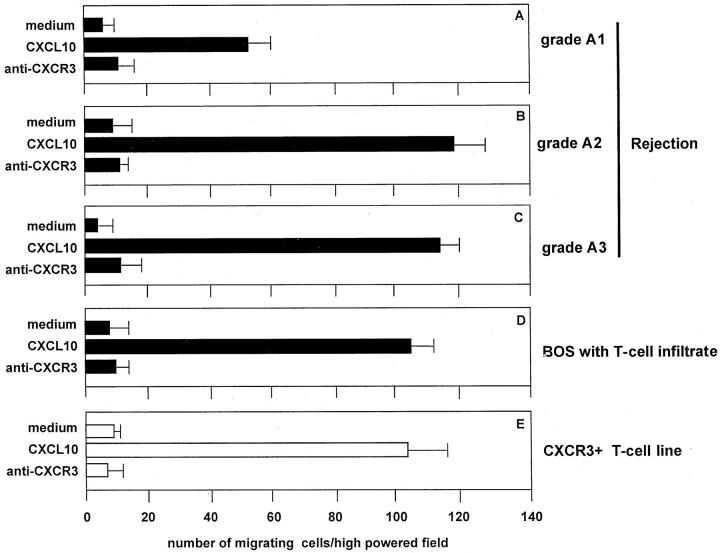
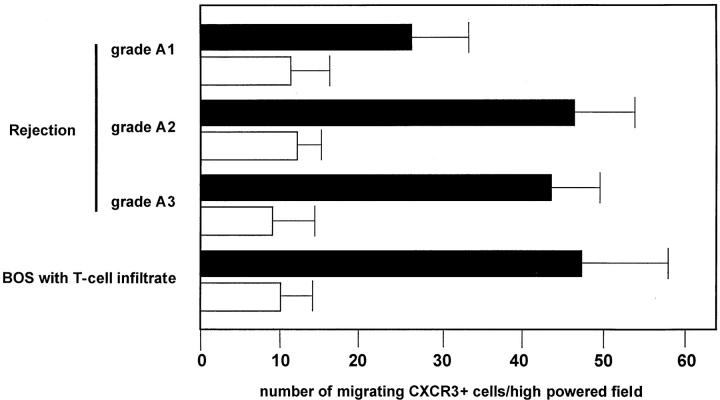
As shown in Figure 3 ▶ , pulmonary T cells purified from the BAL of patients with grade A1 to A3 and BOS exhibited a definite migratory capability in response to CXCL10. The migratory capability was influenced by CXCR expression. In fact, the blocking of the CXCR3 receptor with specific antibodies determined a marked inhibition of CXCL10-induced chemotaxis. These data suggest that pulmonary T cells infiltrating lung allografts express a functional CXCR3 receptor that induces migration in response to CXCL10.
Figure 3.
Chemotactic activity of CXCL10 on BAL T cells purified from three representative patients with A1-A3 rejection episodes and a patient with active BOS. The assays were performed using a modified Boyden chamber in triplicate and data are given as mean ± SD. CXL10 shows significant chemotactic activity on BAL T cells (A–D) and the CXCR3+ T cell clone (E). The number of BAL T cells migrating in response to CXCL10 was higher in patients with A2-A3 grade rejection than in patients with lower grade rejection (A1). Because of the limited number of patients evaluated the difference was not significant.
Immunohistochemical and Flow Cytometry Analysis of the Expression of CXCL10 by Pulmonary Immunoinflammatory Cells during Lung Allograft Rejection Episodes
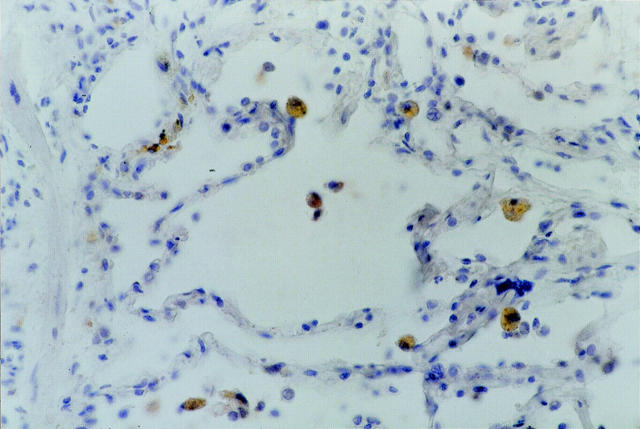
In a second set of experiments we evaluated which inflammatory cells infiltrating lung allograft express CXCL10 during rejection episodes. The expression of CXCL10 was observed in macrophage-infiltrating lung biopsies of patients with histological evidence of A1-A3 rejection (Figure 4) ▶ or with BOS; interestingly, also a few epithelial cells expressed CXCL10.
Figure 4.
Immunohistochemistry for CXCL10 expression by AMs infiltrating transbronchial biopsy in a representative lung allograft recipient with a rejection episode classified as grade A2. The figure shows that several AMs are strongly marked by the anti-CXCL10 antibody. Scattered epithelial cells also bear CXCL10. Original magnification, ×400.
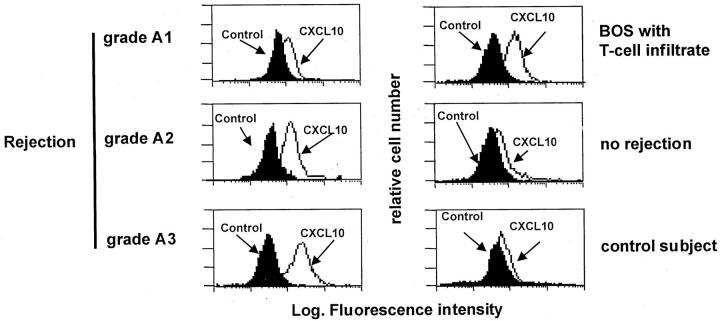
Flow cytometry analysis confirmed the selective expression of CXCL10. As shown in Figure 5 ▶ , macrophages isolated from the BAL of recipients with rejection episodes or BOS expressed CXCL10.
Figure 5.
Flow cytometry profile of AMs recovered from three representative patients with A1-A3 rejection episodes, a patient with active BOS, a patient showing no rejection signs, and a control patient. The expression of cytoplasmic cytokines was determined as reported in the Material and Methods section. The figure shows that AMs from lung allograft recipients with A1-A3 rejection episodes or BOS express CXCL10.
Supernatants Obtained by Cultured Macrophages Show Biological Activity on the CXCR3+ T Cell Line and CXCR3 Ligands May Be Demonstrated in the Fluid Component of BAL
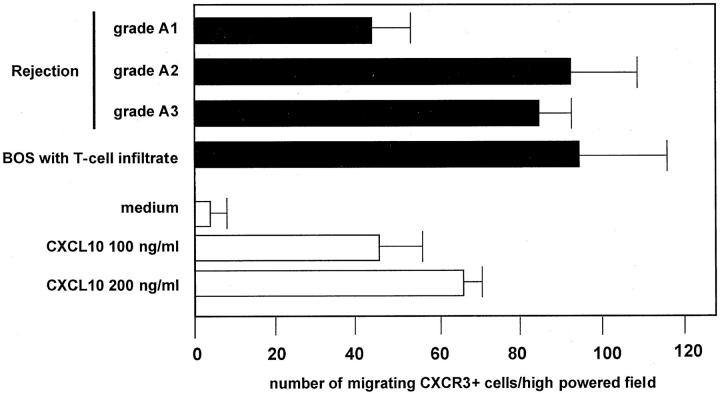
Cell-free supernatants were obtained from 24-hour cultured AMs and tested for their capabilities of inducing T cell migration. Supernatants obtained from AMs of patients with acute rejection episodes or BOS exerted significant chemotactic activity on the CXCR3+ cell line (Figure 6) ▶ ; the CXCR3-negative cell line did not migrate in the presence of supernatants (data not shown). The addition of an anti-CXCL10 neutralizing antibody (Figure 6) ▶ , but not of a control antibody, inhibited chemotactic activities of supernatants (data not shown). The inhibitory activity shown by the neutralizing antibody was not complete (range, 46 to 79%). This suggests that other CXCR3 ligands, including CXCL9 and CXL11, are likely to be present in conditioned media and interact with the CXCR3+ T cell clone, mediating its migration. When macrophages were cultured in the presence of IFN-γ the chemotactic activity of cell-free supernatants was higher than the levels obtained from unstimulated AMs (46.7 ± 12.3 versus 58.0 ± 9.1 number of migrating CXCR3+ cells/high-powered field; mean ± SD of pooled results obtained in different lung allograft recipients; P < 0.01). Again, the chemotactic activity of conditioned medium obtained by IFN-γ stimulated AMs was only partially inhibited by the pretreatment with the anti-CXCL10 antibody, suggesting the presence of other CXCR3 ligands in the supernatants. As a confirmation, the addition of an anti-CXCL9 antibody also inhibited chemotactic activities of supernatants (data not shown). Taken together these data suggest the need to evaluate the role of other non-ERL chemokines in the pathogenesis of allograft rejection.
Figure 6.
Chemotactic activity of supernatants obtained from 24-hour cultures of AMs from three representative patients with A1-A3 rejection episodes and a patient with active BOS. Supernatants obtained from AMs of lung allograft recipients with rejection episodes or with BOS exerted chemotactic activity on the CXCR3 + 300-19 T-cell line that was inhibited by a neutralizing anti-CXCL10 antibody (white bars). The inhibitory activity was not complete, indicating that AMs release other proteins into supernatants that are able to interact with the CXCR3+ T cell clone.
To evaluate whether CXCR3 ligands are released in the pulmonary microenvironment in vivo, the fluid component of nine BAL samples was evaluated for chemotactic activity on the CXCR3+ cell line (Figure 7) ▶ . A definite biological activity was demonstrated in the samples of patients with acute rejection or BOS; this activity was partially inhibited by an anti-CXCL10 neutralizing antibody indicating the presence of other CXCR3 ligands in the fluid component of BAL.
Figure 7.
The fluid components of the BAL obtained from lung allograft recipients with rejection episodes or with BOS exert chemotactic activity on the CXCR3 + 300-19 T cell line, indicating the presence of CXCR3 ligand(s). For a comparison, the figure shows the migratory response of the 300-19 T cell line in response to rhCXL10 (white bars). The assays were performed in triplicate, and data are given as mean ± SD.
Discussion
This study provides the first evidence that alloimmune T cells trafficking into lung allografts express CXCR3 and migrate in response to CXCL10. In addition, the chemokine is expressed and actively released by graft-infiltrating macrophages during rejection episodes in the lung microenvironment.
As recently reviewed, 17 BAL associated with transbronchial biopsies may be useful for understanding the natural course of lung transplantation. During the first 3 months after pulmonary transplantation, elevated total cell counts in BAL and neutrophilic alveolitis are common features, representing the cellular response to the graft. Lymphocytic alveolitis with a decreased CD4/CD8 ratio suggests acute rejection, but is also detectable in viral pneumonia and obliterative bronchiolitis. In the case of a combined lymphocytosis and neutrophilia without any evidence of infection, BOS should be taken into account. It is herein shown that graft-infiltrating T cells express CXCR3 whenever a T cell alveolitis occurs. Episodes of grade A2 or greater rejection were associated with a striking expression of this chemokine receptor by pulmonary lymphocytes as well as with cases of patients suffering from BOS and showing BAL lymphocytosis and neutrophilia. We have also shown CXCR3 expression in allograft recipients with clinical signs of viral pneumonia associated with a lymphocytic alveolitis (data not shown).
CXCR3 expression is likely to represent a common mechanism that is involved in the recruitment of activated T cells in the pulmonary microenvironment of lung allograft recipients. The continuous recruitment of Th1-type CXCR3+ T cells might play a role not only in the pathogenesis of acute rejection but also in favoring the persistence of local hypersensitivity reactions that in turn set the stage for the development of the fibroproliferative process. In this context, because acute rejection and viral infection represent major risk factors for BOS, 3,18 it may be speculated that the fibroproliferative response represents a relative resolution process of the persistent CXCR3+ T-cell chronic inflammation, which may partially recover obviously at the expense of airway fibrosis. 19 This hypothesis may have important therapeutic implications. Because of the role of CXCR3 expression in the migration of T cells into lung allografts, strategies to block CXCR3/CXCL10 interactions are likely to be successful in preventing the development of BOS. It is also important to note that such pathogenetic hypothesis we suggest is not specific for allograft reaction, because CXCR3/CXCL10 interactions have been found to be important in mediating CTL response 20 and T cell reactions taking place in the lung of patients with another Th1 disorder that is followed by severe tissue injury and remodeling, ie, sarcoidosis 16
A second issue raised by our data deals with mechanisms that regulate CXCR3/CXCL10 interactions in lung allografts. Recent data, obtained in the animal model, have shown a distinct pattern of cytokines and chemokine gene expression in the development of posttransplant airway obliteration. 21 Allografts show a strong and persistent Th1-type response (expression of IL-2 and IFN-γ genes), even after fibrous airway obliteration is complete, suggesting that an ongoing Th1-mediated alloimmune process persists until late in the fibroproliferative stage. Other investigators have shown that Th1, Th2, and cytotoxic lymphocyte subtypes contribute to the development of obliterative bronchiolitis in the heterotopic mouse trachea model. 22 Our data indicating that graft-infiltrating T cells show a dominant expression of IFN-γ suggest a putative role for IFN-γ in regulating CXCL10/CXCR3 interactions in the pulmonary milieu. The recent evidence that detection of IFN-γ in BAL cells significantly correlates with early acute rejection, 23 coupled to the present finding that in the presence of IFN-γ graft-infiltrating macrophages increased their release of chemotactic factor, further supports the concept that IFN-γ plays a regulatory role in the recruitment of CXCR3+ pulmonary T cells. IL-15 is another ideal candidate that may favor T cell accumulation in lung allografts. In fact, IL-15, a potent inducer of CXC-chemokines on T cells, 24 may favor the expression of CXCR3 in the lungs of patients with other Th1-mediated hypersensitivity reactions. 25 Because our preliminary data indicate that this molecule in actively released during rejection episodes (data not shown), it is conceivable that IFN-γ, IL-15, and IFN-γ-inducible CXC chemokines act in concert to sustain Th1 inflammatory responses in lung allografts. Accordingly, it has been recently reported that CXCL10 selectively up-regulates human T-cell cytokine synthesis, with enhancement selectively targeted toward the promotion of IFN-γ expression (Th1-like). 26
The relationship between the fibroangiogenetic response associated with the bronchiolitis obliterans and the local release of chemokines is another important aspect that deserves further investigation. The CXC chemokine family contains members that on a structural/functional basis exhibit either angiogenic or angiostatic biological activity. In particular, it is known that a variety of ELR-containing chemokines are chemotactic for endothelial cells whereas non-ELR CXC chemokines, including CXCL9 and CXCL10 are not only nonangiogenic themselves, but they inhibit the angiogenic effects of other ELR chemokines. 27 As an example, IL-8/CXCL8, which is expressed in the lung of patients evolving toward BOS, 28 has markedly increased angiogenetic activity compared to the IFN-γ-induced CXCL10 in idiopathic pulmonary fibrosis. 29 Furthermore, Th2 cytokines (IL-4 and IL-13) have been involved in the pathogenesis of idiopathic pulmonary fibrosis whereas Th1 cytokines (IFN-γ) seem to have a protective role. 30 Our findings and the data of DiGiovine and colleagues 28 suggest that the production of ordinarily antifibrotic agents (IFN-γ and CXCL10) and profibrotic molecules, as CXCL8/IL-8 may be paradoxically associated during local fibroproliferative repair processes taking place in chronic lung rejection. These data are consistent with findings obtained in animal models: although IFN-γ has antifibrotic activity in experimental granuloma models, 31 a persistent Th1 immune response (IL-2 and IFN-γ) characterizes the development of posttransplant airway obliteration observed in the rat tracheal transplant model. 21
Thus, events mediating fibroangiogenesis are likely to be different in the posttransplant airway obliteration and in diffuse lung diseases characterized by an evolution toward fibrosis. Further studies aimed at evaluating the complex interactions between Th1/Th2 cytokines, CXC chemokines, and CC chemokines may help to clarify this issue. In particular, given the ability of other ligands of CXCR3 (CXCL9 and CXCL11) to favor T cell recruitment, studies are in progress in our laboratories to evaluate the functional importance of these IFN-γ inducible CXC chemokines during the different phases of the rejection process. The production of CC chemokines should be also investigated. In a murine model it has been shown that CCL5 and monocyte chemotactic protein-1 (MCP-1/CCL2) are induced late after transplantation, whereas up-regulation of macrophage inflammatory protein-2 (MIP-2α/CXCL2) occurs early after transplant. 21 Studies in lung allograft recipients have also demonstrated that RANTES/CCL5 contributes to the intrapulmonary accumulation of immune cells during acute rejection 32 and CMV pneumonia after lung transplantation. 33
In this context, it is likely that chemokines may be redundant in their action on different lung cells. For instance, CCL2 and CCL5 interacting with CCR1/CCR2 or CCR1/CCR3/CCR5, respectively, may be chemoattractant for different cell targets that have been involved in the pathogenesis of both acute and chronic complications of lung transplantation, including macrophages, T lymphocytes, neutrophils, mast cells, and eosinophils. 17,34-37 The issue is complicated by the fact that lung macrophages do not represent the only chemokine source in the lung. As an example, Th1 or Th2 cytokines in the lung tissue may polarize lung fibroblasts to produce either CCL5 or eotaxin (CCL11) 38 and the T-cell-specific chemokines CXCL9, CXCL10, and CXCL11 may be produced by pulmonary epithelial cells. 39 Given the heterogeneous pattern of pulmonary infiltrate during rejection episodes, a full understanding of the interdependence of the local hyperproduction of chemoattractant molecules may help to clarify not only events leading to acute rejection but also why chronic rejection is associated with a Th1 immune response and evolves toward bronchiolitis obliterans.
Despite the complexity and redundancy of the chemokine system, it is easy to anticipate that further information on chemokine biology will provide pharmacological opportunities to improve long-term survival in transplant recipients. Our findings clearly indicate the effects of CXCR3/CXCL10 interactions on allograft rejection, suggesting that targeting CXCR3 and its ligand with engineered molecules might have therapeutic utility in down-modulating T cell recruitment and thus T-cell-specific inflammatory responses taking place in the pulmonary parenchyma. Considering the importance of T cells in the alloimmune responses, further studies are needed in animal models to explore the therapeutic potential of CXCR3 or CXCL10 antagonists with the ultimate goal of offering new clues for immune intervention in human diseases.
Acknowledgments
We thank Mr. Martin Donach for his help in the preparation of the manuscript and Mrs. Alessandra Dubrovic for the technical assistance in the preparation of lung biopsies for immunohistochemical analysis.
Footnotes
Address reprint requests to Gianpietro Semenzato, M.D., Università degli Studi di Padova, Dip. Medicina Clinica e Sperimentale, Immunologia Clinica, Via Giustiniani 2, 35128 Padova, Italy. E-mail: giansem@ux1.unipd.it.
Supported by grants from the M.U.R.S.T ex40%.
References
- 1.Arcasoy SM, Kotloff RM: Lung transplantation. N Engl J Med 1999, 340:1081-1091 [DOI] [PubMed] [Google Scholar]
- 2.Trulock EP: Lung transplantation. Am J Respir Crit Care Med 1997, 155:789-818 [DOI] [PubMed] [Google Scholar]
- 3.Heng D, Sharples LD, McNeil K, Stewart S, Wreghitt T, Wallwork J: Bronchiolitis obliterans syndrome: incidence, natural history, prognosis, and risk factors. J Heart Lung Transplant 1998, 17:1255-1263 [PubMed] [Google Scholar]
- 4.Kelly K, Hertz MI: Obliterative bronchiolitis. Clin Chest Med 1997, 18:319-338 [DOI] [PubMed] [Google Scholar]
- 5.Kelly KE, Hertz MI, Mueller DL: T-cell and major histocompatibility complex requirements for obliterative airway disease in heterotopically transplanted murine tracheas. Transplantation 1998, 66:764-771 [DOI] [PubMed] [Google Scholar]
- 6.Semenzato G: Chemotactic cytokines: from the molecular level to clinical use. Sarcoidosis Vasc Diffuse Lung Dis 1998, 15:131-133 [PubMed] [Google Scholar]
- 7.Kim CH, Broxmeyer HE: Chemokines: signal lamps for trafficking of T and B cells for development and effector function. J Leukoc Biol 1999, 65:6-15 [DOI] [PubMed] [Google Scholar]
- 8.Baggiolini M: Chemokines and leukocyte traffic. Nature 1998, 392:565-568 [DOI] [PubMed] [Google Scholar]
- 9.Luster A: Chemokines-chemotactic cytokines that mediate inflammation. N Engl J Med 1998, 228:436-445 [DOI] [PubMed] [Google Scholar]
- 10.Zlotnik A, Morales J, Hedrick JA: Recent advances in chemokines and chemokine receptors. Crit Rev Immunol 1999, 19:1-47 [PubMed] [Google Scholar]
- 11.Farber JM: Mig and IP-10: CXC chemokines that target lymphocytes. J Leukoc Biol 1997, 61:246-257 [PubMed] [Google Scholar]
- 12.Loetscher M, Gerber B, Loetscher P, Jones SA, Piali L, Clark-Lewis I, Baggiolini M, Moser B: Chemokine receptor specific for IP-10 and Mig: structure, function and expression in activated T-lymphocytes. J Exp Med 1996, 184:963-969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murphy PM, Baggiolini M, Charo IF, Hebert CA, Horuk R, Matsushima K, Miller LH, Oppenheim JJ, Power CA: International union of pharmacology. XXII. Nomenclature for chemokine receptors. Pharmacol Rev 2000, 52:145-176 [PubMed] [Google Scholar]
- 14.Yousem SA, Berry GJ, Cagle PT, Chamberlain D, Husain AN, Hruban RH, Marchevsky A, Ohori NP, Ritter J, Stewart S, Tazelaar HD: Revision of the 1990 working formulation for the classification of pulmonary allograft rejection: Lung Rejection Study Group. J Heart Lung Transplant 1996, 15:1-15 [PubMed] [Google Scholar]
- 15.Costabel U, Danel C, Haslam P, Higgenbottam T, Klech H, Pohl W, Rennard S, Rossi G, Rust M, Semenzato G: Technical recommendations and guidelines for bronchoalveolar lavage (BAL). Report of the European Society of Pneumology Task Group on BAL. Eur Respir J 1989, 2:561-585 [PubMed] [Google Scholar]
- 16.Agostini C, Cassatella M, Zambello R, Trentin L, Gasperini S, Perin A, Piazza F, Siviero M, Facco M, Dziejman M, Chilosi M, Qin S, Luster AD, Semenzato G: Involvement of the IP-10 chemokine in sarcoid granulomatous reactions. J Immunol 1998, 161:6413-6420 [PubMed] [Google Scholar]
- 17.Tiroke AH, Bewig B, Haverich A: Bronchoalveolar lavage in lung transplantation. State of the art. Clin Transplant 1999, 13:131-157 [DOI] [PubMed] [Google Scholar]
- 18.Girgis RE, Tu I, Berry GJ, Reichenspurner H, Valentine VG, Conte JV, Ting A, Johnstone I, Miller J, Robbins RC, Reitz BA, Theodore J: Risk factors for the development of obliterative bronchiolitis after lung transplantation. J Heart Lung Transplant 1996, 15:1200-1208 [PubMed] [Google Scholar]
- 19.Ward C, Snell GI, Zheng L, Orsida B, Whitford H, Williams TJ, Walters EH: Endobronchial biopsy and bronchoalveolar lavage in stable lung transplant recipients and chronic rejection. Am J Respir Crit Care Med 1998, 158:84-91 [DOI] [PubMed] [Google Scholar]
- 20.Agostini C, Facco M, Siviero M, Carollo D, Galvan S, Cattelan AM, Zambello R, Trentin L, Semenzato G: CXC chemokines IP-10 and Mig expression and direct migration of pulmonary CD8+/CXCR3+ T cells in the lungs of patients with HIV infection and T-cell alveolitis. Am J Respir Crit Care Med 2000, 162:1466-1473 [DOI] [PubMed] [Google Scholar]
- 21.Boehler A, Bai XH, Liu M, Cassivi S, Chamberlain D, Slutsky AS, Keshavjee S: Upregulation of T-helper 1 cytokines and chemokine expression in post-transplant airway obliteration. Am J Respir Crit Care Med 1999, 159:1910-1917 [DOI] [PubMed] [Google Scholar]
- 22.Neuringer IP, Walsh SP, Mannon RB, Gabriel S, Aris RM: Enhanced T cell cytokine gene expression in mouse airway obliterative bronchiolitis. Transplantation 2000, 69:399-405 [DOI] [PubMed] [Google Scholar]
- 23.Moudgil A, Bagga A, Toyoda M, Nicolaidou E, Jordan SC, Ross D: Expression of gamma-IFN mRNA in bronchoalveolar lavage fluid correlates with early acute allograft rejection in lung transplant recipients. Clin Transplant 1999, 13:201-207 [DOI] [PubMed] [Google Scholar]
- 24.Perera LP, Goldman CK, Waldmann TA: IL-15 induces the expression of chemokines and their receptors in T lymphocytes. J Immunol 1999, 162:2606-2612 [PubMed] [Google Scholar]
- 25.Agostini C, Trentin L, Perin A, Facco M, Siviero M, Piazza F, Basso U, Adami F, Zambello R, Semenzato G: Regulation of alveolar macrophage-T cell interactions during Th1-type sarcoid inflammatory process. Am J Physiol 1999, 277:L240-L250 [DOI] [PubMed] [Google Scholar]
- 26.Gangur V, Simons FE, Hayglass KT: Human IP-10 selectively promotes dominance of polyclonally activated and environmental antigen-driven IFN-gamma over IL-4 responses. FASEB J 1998, 12:705-713 [DOI] [PubMed] [Google Scholar]
- 27.Belperio JA, Keane MP, Arenberg DA, Addison CL, Ehlert JE, Burdick MD, Strieter RM: CXC chemokines in angiogenesis. J Leukoc Biol 2000, 68:1-8 [PubMed] [Google Scholar]
- 28.DiGiovine B, Lynch JP, III, Martinez FJ, Flint A, Whyte RI, Iannettoni MD, Arenberg DA, Burdick MD, Glass MC, Wilke CA, Morris SB, Kunkel SL, Strieter RM: Bronchoalveolar lavage neutrophilia is associated with obliterative bronchiolitis after lung transplantation: role of IL-8. J Immunol 1996, 157:4194-4202 [PubMed] [Google Scholar]
- 29.Keane MP, Arenberg DA, Lynch JP, III, Whyte RI, Iannettoni MD, Burdick MD, Wilke CA, Morris SB, Glass MC, DiGiovine B, Kunkel SL, Strieter RM: The CXC chemokines, IL-8 and IP-10, regulate angiogenic activity in idiopathic pulmonary fibrosis. J Immunol 1997, 159:1437-1443 [PubMed] [Google Scholar]
- 30.Agostini C, Siviero M, Semenzato G: Immune effector cells in idiopathic pulmonary fibrosis. Curr Opin Pulmonary Med 1997, 3:348-355 [DOI] [PubMed] [Google Scholar]
- 31.Hesse M, Cheever AW, Jankovic D, Wynn TA: NOS-2 mediates the protective anti-inflammatory and antifibrotic effects of the Th1-inducing adjuvant, IL-12, in a Th2 model of granulomatous disease. Am J Pathol 2000, 157:945-955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Belperio JA, Burdick MD, Keane MP, Xue YY, Lynch JP, III, Daugherty BL, Kunkel SL, Strieter RM: The role of the CC chemokine, RANTES, in acute lung allograft rejection. J Immunol 2000, 165:461-472 [DOI] [PubMed] [Google Scholar]
- 33.Monti G, Magnan A, Fattal M, Rain B, Humbert M, Mege JL, Noirclerc M, Dartevelle P, Cerrina J, Simonneau G, Galanaud P, Emilie D: Intrapulmonary production of RANTES during rejection and CMV pneumonitis after lung transplantation. Transplantation 1996, 61:1757-1762 [DOI] [PubMed] [Google Scholar]
- 34.Bewig B, Stewart S, Bottcher H, Bastian A, Tiroke A, Hirt S, Haverich A: Eosinophilic alveolitis in BAL after lung transplantation. Transpl Int 1999, 12:266-272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Henke JA, Golden JA, Yelin EH, Keith FA, Blanc PD: Persistent increases of BAL neutrophils as a predictor of mortality following lung transplant. Chest 1999, 115:403-409 [DOI] [PubMed] [Google Scholar]
- 36.Zheng L, Walters EH, Ward C, Wang N, Orsida B, Whitford H, Williams TJ, Kotsimbos T, Snell GI: Airway neutrophilia in stable and bronchiolitis obliterans syndrome patients following lung transplantation. Thorax 2000, 55:53-59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yousem SA: The potential role of mast cells in lung allograft rejection. Hum Pathol 1997, 28:179-182 [DOI] [PubMed] [Google Scholar]
- 38.Teran LM, Mochizuki M, Bartels J, Valencia EL, Nakajima T, Hirai K, Schroder JM: Th1- and Th2-type cytokines regulate the expression and production of eotaxin and RANTES by human lung fibroblasts. Am J Respir Cell Mol Biol 1999, 20:777-786 [DOI] [PubMed] [Google Scholar]
- 39.Sauty A, Dziejman M, Taha RA, Iarossi AS, Neote K, Garcia-Zepeda EA, Hamid Q, Luster AD: The T cell-specific CXC chemokines IP-10, Mig, and I-TAC are expressed by activated human bronchial epithelial cells. J Immunol 1999, 162:3549-3558 [PubMed] [Google Scholar]