Prevalence of oesophageal eosinophils and eosinophilic oesophagitis in adults: the population‐based Kalixanda study (original) (raw)
Abstract
Background
Eosinophilic oesophagitis may be increasing but the prevalence in the general population remains unknown. Our aim was to assess this and the presence of eosinophils in the distal oesophageal epithelium in the community.
Methods
Oesophagogastroduodenoscopy was performed in a random sample (n = 1000) of the adult Swedish population (mean age 54 years, 49% men). Oesophageal biopsy samples were obtained from 2 cm above, and at, the Z‐line. Any eosinophil infiltration of the epithelium was defined as “eosinophils present”. Definite eosinophilic oesophagitis was defined as ⩾20, probable as 15–19, and possible as 5–14 eosinophils/high‐power field (HPF, at magnification ×40) in oesophageal biopsy specimens.
Results
Eosinophils were present in 48 subjects (4.8%, 95% CI 3.5 to 6.1%, mean age 54 years, 63% men), in 54% without troublesome reflux symptoms. Definite eosinophilic oesophagitis was present in four subjects (0.4%, 95% CI 0.01 to 0.8%, mean age 51 years, 75% men) and probable eosinophilic oesophagitis in seven subjects (0.7%, 95% CI 0.2 to 1.2%, mean age 58 years, 43% men). Erosive oesophagitis (OR = 2.99, 95% CI 1.58 to 5.66) and absence of dyspepsia (OR = 0.23, 95% CI 0.07 to 0.75) and Helicobacter pylori infection (OR = 0.41, 95% CI 0.19 to 0.92) were independent predictors for “eosinophils present”. Definite eosinophilic oesophagitis was associated with dysphagia (2/66 vs 2/926, p = 0.025), and probable eosinophilic oesophagitis with narrowing of the oesophageal lumen (2/15 vs 5/978, p = 0.005).
Conclusions
Oesophageal eosinophils were present in nearly 5% of the general population; approximately 1% had definite or probable eosinophilic oesophagitis. Oesophageal eosinophils may be a manifestation of reflux disease in adults, but the condition is as likely to be asymptomatic and go unrecognised.
Keywords: eosinophilic oesophagitis, epidemiology, gastro‐oesophageal reflux disease, population‐based
Eosinophilic oesophagitis was first described in a single case of achalasia.1 Eosinophilic oesophagitis has arbitrarily been defined as the presence of more than 20 eosinophils per high‐power field (HPF) in the oesophageal epithelium,2,3 although some have used lower cut‐offs.4,5,6 It is thought to be a rare inflammatory condition in adults7 with an estimated prevalence 0.2–3 in 10 000.8 However, the normal cut‐off that should be used to define eosinophilic oesophagitis and “presence of oesophageal epithelial eosinophils” is unclear because of the absence of population‐based data. The epidemiology of eosinophilic oesophagitis may also be changing; several case reports and case series suggest that either the incidence is increasing or the disease is now recognised more often.9,10
According to available reports, eosinophilic oesophagitis typically presents in young men with dysphagia; food impaction may occur in two‐thirds and heartburn in a quarter.10,11 Endoscopic findings can include a normal oesophagus.2,12 Reports of abnormalities include oesophageal rings or a corrugated appearance, oedema or fragility, narrowing or stricturing of the oesophagus especially more proximally, and whitish papules or exudates.4,6,10 In an 11‐year follow‐up of 30 adult patients, no malignant potential was associated with this disease.12 The number of people with eosinophilic oesophagitis who do not consult for medical care is not known.
The underlying aetiopathogenesis of eosinophilic oesophagitis remains obscure. In up to 50% of cases, it can be associated with allergic disorders and may represent “asthma of the oesophagus”.10,13,14 Data on its association with gastro‐oesophageal reflux disease (GORD) are conflicting, and no population‐based information is available.8,15
The prevalence of oesophageal epithelial eosinophils and eosinophilic oesophagitis in the general population remains unknown, as endoscopy is required for diagnosis. The aims of this study were to: (1) determine the presence of eosinophils in the distal oesophageal epithelium and the prevalence of eosinophilic oesophagitis in a random sample of an adult Swedish population; (2) determine the association of oesophageal epithelial eosinophils with upper gastrointestinal symptoms and GORD.
Methods
Study samples and design
The Kalixanda study, described in detail elsewhere16,17,18, was performed in two communities in northern Sweden. The distribution of age and sex in this population (n = 28 988) is similar to that of Sweden as a whole.16 A random sample of 2860 adults was surveyed using a validated postal questionnaire assessing gastrointestinal symptoms, the Abdominal Symptom Questionnaire (ASQ).19,20 An oesophagogastroduodenoscopy was performed in a representative sample of 1000 of the responders to the ASQ.
This study was approved by the Umeå University ethics committee and conducted in accordance with the revised (1998) Declaration of Helsinki.
Questionnaire and response rate
The ASQ has been validated and previously described in detail.16,19,20 An extended ASQ with added evaluation of symptom frequency (daily, weekly, past 3 months) was completed by the oesophagogastroduodenoscopy study sample immediately before the endoscopy.
A total of 2122 people completed the postal questionnaire (response rate 74%). The response rate for those eligible for investigation was 73%. In total, the sex and age distribution for the 1000 subjects (mean age 53.5 years, 49% male) closely reflected the 2122 responders to the postal questionnaire, as well as the Swedish population.16
At the oesophagogastroduodenoscopy visit, the participants were asked about their medical history as well as tobacco and alcohol use. A venous blood sample for Helicobacter pylori serology was also taken.
Endoscopy
Before the endoscopy, the three endoscopists were blinded to the medical history and symptoms. The three endoscopists, one gastroenterologist from Kalix and two general practitioners from Haparanda, were all experienced, each having previously performed 2500–6000 upper endoscopies. All three had been participating in regular quality assessment programmes in Sweden and/or in Finland. To achieve reliability of endoscopic assessment, the endoscopists participated in training sessions before and during the study, focusing on assessment of Barrett's oesophagus and oesophagitis. Furthermore, a predefined endoscopy protocol was applied.16,17,18
The gastro‐oesophageal junction was defined as the junction of the proximal gastric folds and the tubular oesophagus. In addition, hiatus hernia and abnormal endoscopic findings were recorded in the predefined protocol.
Definitions of reflux oesophagitis, reflux symptoms, dyspepsia, epithelial eosinophils in the oesophagus and eosinophilic oesophagitis
At endoscopy, erosive oesophagitis was graded according to the Los Angeles classification system.21,22
Gastro‐oesophageal reflux symptoms were defined as troublesome heartburn and/or acid regurgitation over the past three months assessed by the ASQ.17,23
Dyspepsia, also assessed by the ASQ, was defined as troublesome pain or discomfort expressed as one or more of the 11 listed modalities (burning sensation, aching, pain, tenderness, gripe, twinge, stitch, cramp, colic, sinking feeling and “butterflies”) indicated in the epigastric part of the abdomen, or reporting one or more of the symptoms “uncomfortable feeling of fullness”, “early satiety” or “nausea” (“upper abdominal bloating” not reported).16 The listed symptoms are as similar to those used in the Rome II definition of dyspepsia as possible, giving linguistic limitations.24 As proposed in the Rome II definition, the listed dyspeptic symptoms were not allowed to be associated with or relieved by defaecation.
Any eosinophil infiltration of the oesophageal epithelium was defined as “eosinophils present”. It was classified as low grade if 1–4 eosinophils/HPF (at ×40 magnification), possible eosinophilic oesophagitis if 5–14 eosinophils/HPF, probable eosinophilic oesophagitis if ⩾15–<20 eosinophils/HPF, and definite eosinophilic oesophagitis if ⩾20 eosinophils/HPF2 were noted.
Biopsies
At least two biopsy samples were taken from the following locations in the oesophagus: 2 cm above the Z‐line, at the Z‐line, and any abnormal areas. In the stomach, samples were obtained according to the recommendations of the updated Sydney System.25
Biopsy specimens were independently examined by two experienced gastrointestinal pathologists (MV and MS), who were unaware of the clinical data and endoscopic findings. In the initial analysis, the specimens were scored in a semi‐quantitative manner: none, mild, moderate and marked infiltration. Subsequently, for a precise cell count, specimens with any eosinophils on the first evaluation were reviewed by a third pathologist (MMW), who was also blinded to the clinical data and endoscopic findings, paired with specimens with no eosinophils on the first assessment, such that an independent review of eosinophil counts could be undertaken. This confirmed the absence of eosinophils in paired biopsy specimens without eosinophils on first assessment. In two cases, specimens from 2 cm above the Z‐line were missing. Biopsy specimens taken from the Z‐line were not available for evaluation in absolute number in six cases; five of these were classified as mild, and in one case there were no eosinophils in the initial analysis.
Biopsy specimens were stained with haematoxylin and eosin, and those from the stomach also with the Warthin‐Starry silver stain for identification of H pylori.
Histological features of the gastric mucosa, such as inflammation, intestinal metaplasia and atrophy, were recorded and scored according to the updated Sydney System.25 “Carditis” was defined as histological presence of lymphocytes and granulocytes in the biopsy specimens of the cardia of the stomach.
For identification of H pylori, histology and culture were used.26
Laboratory analysis
H pylori IgG antibodies were determined by electroimmunoassay (Pyloriset EIA‐G, Orion diagnostica, Espoo Finland).26
Statistical analysis
p Values were all two tailed, and the α level of significance was set at 0.05. The prevalences are presented as percentages with 95% CI. Likelihood ratio χ2 test and Fisher's exact test were used for testing differences in univariate analyses.
In multivariate analysis, a main effect logistic regression model after model improvement, adjusted for age and sex, was used. The results are shown as odds ratios (ORs) with 95% CI. The reference group was given an OR of 1. The goodness of fit of the models was judged from the Pearson χ2 test and degrees of freedom, which should be about equal. The fit of the model was considered to be acceptable with a p value ⩾0.05. The Intercooled Stata 8 program27 was used for the analyses.
Age, sex, education, hiatus hernia, erosive oesophagitis, obesity, reflux symptoms, dyspepsia, alarm symptoms (weight loss, dysphagia, anaemia, haematochezia), diagnosed asthma or allergy, tobacco use (smoking or snuff), alcohol, use of non steroidal anti‐inflammatory drugs or aspirin, use of antacids/alginates, H2‐receptor antagonists or proton pump inhibitors, use of asthma or allergy drugs, current H pylori infection, serology positive but no current infection of H pylori, chemical gastritis, corpus dominant H pylori gastritis, atrophy of mucosa, and dichotomised histological presence of carditis of the stomach were the dependent variables analysed in the logistic regression models as possible predictors for oesophageal eosinophils being present.
Results
Endoscopy
Oesophagitis was found in 155 cases (109 Los Angeles grade A, 39 grade B, three grade C, two grade D, and missing classification in two cases). Narrowing of the oesophageal lumen was found in 17 subjects, in seven of them without erosive oesophagitis.
Prevalence of epithelial eosinophils present in the oesophagus and prevalence of definite eosinophilic oesophagitis
Epithelial eosinophils were present in the oesophagus in 48 subjects (4.8%, 95% CI 3.5 to 6.1%, mean age 53.8 years, 62.5% men; table 1). About half of these (n = 26) had no troublesome reflux symptoms, four had asthma or allergy, and three reported dysphagia. Proton pump inhibitors had been taken by two, histamine‐2 receptor blockers by two, and antacids by another six during the 3 months before the endoscopy. They had significantly fewer doctor consultations (22/614 vs 26/336, p = 0.010) than those without eosinophils; two had consulted for gastrointestinal complaints, but none for upper gastrointestinal complaints in the year before the endoscopy.
Table 1 Prevalence (n, %) of eosinophils present, low‐grade eosinophil counts (ie, 1–4 eosinophils/HPF), and possible (5–14/HPF), probable (15–19/HPF) or definite (⩾20/HPF) eosinophilic oesophagitis at both oesophageal sites evaluated (2 cm above the Z‐line and at the Z‐line) in the Kalixanda study population, mean age and proportion of men.
| N (%) (95% CI) | Mean age (years) | Proportion of men (%) | |
|---|---|---|---|
| Kalixanda study population | 1000 | 53.5 | 48.8 |
| Eosinophils present at both 2 cm above and at the Z‐line* | |||
| Eosinophils present | 48 (4.8) (3.5 to 6.1) | 53.8 | 62.5 |
| 1–4 Eosinophils /HPF | 10 (1.0) (0.4 to 1.6) | 59.5 | 50.0 |
| Possible EO | 25 (2.5) (1.5 to 3.5) | 49.9 | 72.0 |
| Probable EO | 7 (0.7) (0.2 to 1.2) | 58.4 | 42.9 |
| Definite EO | 4 (0.4) (0.0 to 0.8) | 50.8 | 75.0 |
| Eosinophils present 2 cm above the Z‐line* | |||
| Eosinophils present | 26 (2.6) (1.6 to 3.6) | 53.2 | 76.9 |
| 1–4 Eosinophils/HPF | 18 (1.8) (1.0 to 2.6) | 58.1 | 72.2 |
| Possible EO | 5 (0.5) (0.1 to 0.9) | 42.4 | 100 |
| Probable EO | 2 (0.2) (0.0 to 0.5) | 45.5 | 50 |
| Definite EO | 1 (0.1) (0.0 to 0.3) | 35.0 | 100 |
| Eosinophils present at the Z‐line* | |||
| Eosinophils present | 47 (4.7) (3.4 to 6.0) | 53.8 | 63.8 |
| 1–4 Eosinophils/HPF | 9 (0.9) (0.3 to 1.5) | 57.0 | 55.6 |
| Possible EO | 24 (2.4) (1.5 to 3.3) | 50.9 | 70.8 |
| Probable EO | 6 (0.6) (0.1 to 1.1) | 58.8 | 50.0 |
| Definite EO | 3 (3.0) (0.0 to 0.6) | 56.0 | 66.7 |
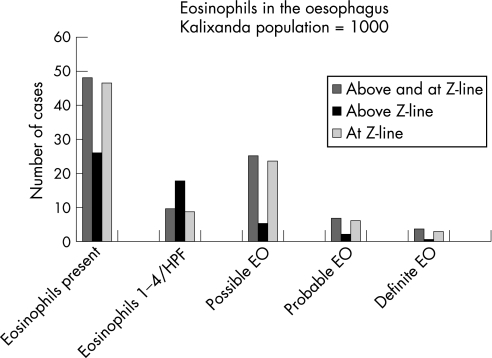
On evaluation of all oesophageal sites biopsied, definite eosinophilic oesophagitis was present in four cases (0.4%, 95% CI 0.01 to 0.8%, mean age 50.7 years, three men; figs 1 and 2). None of these had consulted a doctor for gastrointestinal symptoms in the previous year or received treatment for eosinophilic oesophagitis. Three of them reported troublesome reflux symptoms, but none had erosive oesophagitis. Probable eosinophilic oesophagitis was present in seven subjects (0.7%, 95% CI 0.2 to 1.2%, mean age 58.4 years, 42.9% men) and possible eosinophilic oesophagitis in 25 subjects (2.5%, 95% CI 1.5 to 3.5%, mean age 49.9 years, 72% men). Erosive oesophagitis was present in two, and troublesome reflux symptoms were reported by three of those with probable eosinophilic oesophagitis; the corresponding figures for those with possible eosinophilic oesophagitis were 13 for oesophagitis and 10 for troublesome reflux symptoms, respectively. There were 10 subjects (1.0%, 95% CI 0.4 to 1.6, mean age 59.5 years, 50.0% men) with low eosinophil count/HPF (ie, 1–4). Of these, erosive oesophagitis was present in one, and six reported troublesome reflux symptoms.
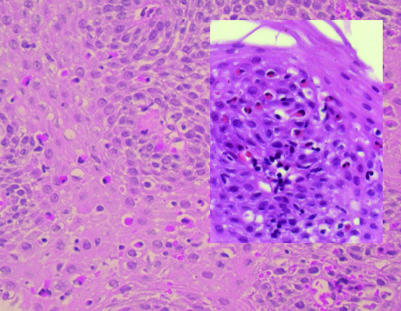
Figure 1 Squamous oesophageal epithelium taken 2 cm above the Z line from a male non‐consulting subject from the Kalixanda study. Eosinophilic oesophagitis with eosinophils throughout the epithelium, >20 eosinophils per high‐power field (HPF) (original magnification ×40). Inset shows probable eosinophilic oesophagitis with 14 eosinophils per HPF (original magnification ×40).
Figure 2 Number of subjects in the Kalixanda study population with eosinophils present in the oesophagus, with low eosinophil count/high‐power field (HPF) (ie, 1–4) and possible, probable or definite eosinophilic oesophagitis (EO).
Prevalence of epithelial eosinophils present and prevalence of definite eosinophilic oesophagitis at 2 cm above the Z‐line in the oesophagus
Epithelial eosinophils were present 2 cm above the Z‐line in 26 subjects (2.6%, 95% CI 1.6 to 3.6, mean age 53.2 years, 76.9% men; table 1). Mean number of eosinophils/HPF was 4.8, and the median was 3. Low‐grade counts of eosinophils were found in 18 subjects (1.8%, 95% CI 1.0 to 2.6, mean age 58.1 years, 72.2% men). Possible eosinophilic oesophagitis was found in five subjects (0.5%, 95% CI 0.1 to 0.9, mean age 42.4 years, all men), and probable eosinophilic oesophagitis in two (0.2% 95% CI 0.0 to 0.5%, mean age 45.5 years, one male). The one 35‐year‐old male subject with definite eosinophilic oesophagitis 2 cm above the Z‐line reported current asthma and dysphagia.
Prevalence of epithelial eosinophils present and prevalence of definite eosinophilic oesophagitis at the Z‐line in the oesophagus
At the Z‐line, there were 47 subjects with eosinophils present (4.7%, 95% CI 3.4 to 6.0, mean age 53.8 years, 63.8% men; table 1). The mean number of eosinophils/HPF was 9.5, and the median was 8. Low‐grade counts of eosinophils were found in nine (0.9%, 95% CI 0.3 to 1.5, mean age 57 years, 55.6% men), possible eosinophilic oesophagitis in 24 (2.4%, 95% CI 1.5 to 3.3%, mean age 50.9 years, 70.8% men), probable eosinophilic oesophagitis in six (0.6%, 95% CI 0.1 to 1.1%, mean age 58.8 years, 50% men), and definite eosinophilic oesophagitis in three (0.3%, 95% CI 0.0 to 0.6, mean age 56 years, two men). In 25 of the 26 subjects with eosinophils present at 2 cm above the Z‐line, eosinophils were also found to be present at the Z‐line.
Predictors for epithelial eosinophils present at 2 cm above the Z‐line in the oesophagus
The prevalence of epithelial eosinophils present at 2 cm above the Z‐line in subjects with troublesome reflux symptoms over the past 3 months was 3.0% (95% CI 1.9 to 4.1) and in those without 2.3% (95% CI 1.4 to 3.2) (p = 0.52). In subjects with erosive oesophagitis, the prevalence was 6.6% (95% CI 5.1 to 8.1) and in those without 1.9% (95% CI 1.1 to 2.7) (p = 0.003).
Compared with those without epithelial eosinophils present, subjects with eosinophils present at 2 cm above the Z‐line were more often men (20/468 vs 6/506, p = 0.003), were more likely to have erosive oesophagitis (10/145 vs 16/829, p = 0.004), and had significantly fewer dyspeptic symptoms (1/221 vs 25/753, p = 0.007) and less carditis (5/386 vs 20/581, p = 0.035) in the univariate analysis. There was no significant association with troublesome reflux symptoms (table 2).
Table 2 Prevalence of significant predictors for eosinophils present in the distal oesophagus, eosinophils present at 2 cm above the Z‐line, eosinophils present at the Z‐line, and eosinophils present only at the Z‐line in the Kalixanda study population.
| Kalixanda study population (n = 1000) | Eosinophils present (n = 48) | Eosinophils present 2 cm above the Z‐line (n = 26) | Eosinophils present at the Z‐line (n = 47) | Eosinophils present only at the Z‐line (n = 22) | |
|---|---|---|---|---|---|
| Male sex (%) | 48.8 | 62.5 | 76.9 | 63.8 | 45.5 |
| p Value | NS (0.051) | 0.003 | 0.034 | NS | |
| Gastro‐oesophageal reflux symptoms (%) | 40 | 45.8 | 46.2 | 44.7 | 45.5 |
| p Value | NS | NS | NS | NS | |
| Dyspepsia (%) | 22.2 | 6.3 | 3.9 | 6.4 | 9.1 |
| p Value | 0.002 | 0.007 | 0.003 | NS | |
| Erosive oesophagitis (%) | 15.5 | 37.5 | 38.5 | 38.3 | 36.4 |
| p Value | <0.001 | 0.009 | <0.001 | 0.013 | |
| Hiatus hernia (%) | 23.9 | 39.6 | 38.5 | 40.4 | 40.9 |
| p Value | 0.013 | NS | 0.010 | NS | |
| Oesophageal ulcer (%) | 2.2 | 12.5 | 7.7 | 12.8 | 18.2 |
| p Value | <0.001 | NS | <0.001 | 0.001 | |
| Narrowing of the oesophageal lumen (%) | 1.7 | 6.3 | 3.9 | 6.4 | 9.1 |
| p Value | 0.044 | NS | 0.042 | NS (0.051) | |
| H pylori* (%) | 33.9 | 16.7 | 19.2 | 17.0 | 13.6 |
| p Value | 0.006 | NS | 0.008 | 0.029 | |
| Carditis (%) | 39.4 | 23.4 | 20.0 | 23.9 | 27.3 |
| p Value | 0.017 | 0.035 | 0.023 | NS |
Male sex (OR = 3.02, 95% CI 1.18 to 7.72) and erosive oesophagitis (OR = 2.84, 95% CI 1.24 to 6.50) remained independent predictors in the multivariate analysis by logistic regression.
Predictors for epithelial eosinophils present at the Z‐line in the oesophagus
At the Z‐line the prevalence of epithelial eosinophils present in subjects with troublesome reflux symptoms over the past three months was 5.3% (95% CI 3.9 to 6.7), and in those without 4.3% (95% CI 3.0 to 5.6) (p = 0.51). In those with erosive oesophagitis, the prevalence was 11.6% (95% CI 9.6 to 13.6), and in those without 3.4% (95% CI 2.3 to 4.5) (p<0.001).
In the univariate analysis, eosinophils present at the Z‐line were associated with male sex (30/458 vs 17/495, p = 0.034), erosive oesophagitis (18/137 vs 29/816, p<0.001), hiatus hernia (19/220 vs 28/733, p = 0.010), oesophageal ulcer (6/16 vs 41/937, p<0.001) and narrowing of the oesophageal lumen (3/14 vs 44/939, p = 0.042). There was a negative association with H pylori infection (8/331 vs 39/622, p = 0.008), carditis (11/380 vs 35/566, p = 0.023) and dyspeptic symptoms (3/219 vs 44/734, p = 0.003) compared with those without epithelial eosinophils present (table 2). In the multivariate analysis by logistic regression, erosive oesophagitis (OR = 2.54, 95% CI 1.23 to 5.21), oesophageal ulcer (OR = 4.39, 95% CI 1.39 to 13.88) and having less dyspepsia (OR = 0.22, 95% CI 0.07 to 0.73) remained independent predictors.
Predictors for epithelial eosinophils present, definite and probable eosinophilic oesophagitis, combining all oesophageal sites biopsied
On evaluation of both oesophageal sites biopsied (the Z‐line and 2 cm above), epithelial eosinophils present were associated positively with erosive oesophagitis (18/137 vs 30/815, p<0.001), hiatus hernia (19/220 vs 29/732, p = 0.013), narrowing of the oesophageal lumen (3/14 vs 45/938, p = 0.044) and oesophageal ulcer (6/16 vs 42/936, p<0.001), and negatively with dyspepsia (3/219 vs 45/733, p = 0.002), H pylori infection (8/331 vs 40/621, p = 0.006) and carditis (11/380 vs 36/565, p = 0.017) in the univariate analysis (table 2).
In the multivariate analysis by logistic regression, erosive oesophagitis (OR = 2.99, 95% CI 1.58 to 5.66) and the absence of dyspepsia (OR = 0.23, 95% CI 0.07 to 0.75) and H pylori infection (OR = 0.41, 95% CI 0.19 to 0.92) remained independent predictors for eosinophils present in the oesophagus.
In the univariate analysis, on evaluation of both oesophageal sites biopsied, definite eosinophilic oesophagitis was associated with dysphagia (2/66 vs 2/926, p = 0.025) and probable eosinophilic oesophagitis with narrowing of the oesophageal lumen (2/15 vs 5/978, p = 0.005) compared with those without epithelial eosinophils present. Multivariate analysis was not appropriate because of the small number of cases.
Discussion
We have studied the prevalence of oesophageal epithelial eosinophils and eosinophilic oesophagitis in a representative random cohort of the Swedish general population. The prevalence of oesophageal epithelial eosinophils in adults was nearly 5 in 100. A histological diagnosis of definite eosinophilic oesophagitis was made in one case (0.1%) 2 cm above the Z‐line and in three cases (0.3%) at the Z‐line. Whereas eosinophils in the oesophageal epithelium at 2 cm above the Z‐line were not associated with GORD symptoms, they were independently associated with erosive oesophagitis and male sex. Similarly, eosinophils at the Z‐line were not associated with GORD symptoms, but they were associated positively with erosive oesophagitis and oesophageal ulcer, and negatively with dyspepsia.
The importance of eosinophilic oesophagitis in adults has increased since the first major report on 12 adult patients was published in 1993.2 The aetiology of eosinophilic oesophagitis and its prevalence are not known,5,7 but recent case reports have implied that the prevalence may be increasing.5,9 This study could not address the incidence of eosinophilic oesophagitis, but provides, for the first time to our knowledge, reasonably robust current community prevalence estimates. Our findings for the prevalence of oesophageal epithelial eosinophils and histological eosinophilic oesophagitis are higher than previously appreciated. This may be explained by the fact that we took biopsy samples from every subject and even from oesophagus with normal appearance. On the other hand, we did not obtain mid or proximal oesophageal specimens, and hence the prevalence of oesophageal epithelial eosinophils may be higher if the condition is patchy.
It is of particular interest to note that “silent” oesophageal epithelial eosinophils occur commonly; in this study, 50% of the subjects with eosinophil infiltrates in the oesophagus had no troublesome oesophageal symptoms. Furthermore, none of those with definite or probable eosinophilic oesophagitis had consulted a doctor for symptoms of this disease. Surprisingly, the presence of oesophageal eosinophils was associated with fewer dyspeptic symptoms, at least partly due to the fact that the definition of dyspepsia was more strict because symptoms associated with defaecation were excluded.
In 1985, Lee 28 reported on 11 patients with obvious oesophageal eosinophil infiltration, 10 of whom had reflux oesophagitis. Since then, the connection between GORD and eosinophilic oesophagitis has been under debate.2,5,8,29 In a recent systematic review, pathological acid reflux was found in only 10% of cases of eosinophilic oesophagitis.10 Similarly, we did not find a significant association between troublesome GORD symptoms and eosinophils in the oesophageal epithelium, possibly because of a lack of statistical power. However, oesophageal epithelial eosinophils were associated with erosive oesophagitis, hiatus hernia, oesophageal ulcer, and narrowing of the oesophageal lumen, consistent with other clinical reports of eosinophils being present in the distal oesophagus in GORD.29 The results from this study support the concept that eosinophils in the oesophageal epithelium may be a manifestation of erosive oesophagitis, although this might also reflect a non‐specific association between mucosal injury and oesophageal eosinophils. However, the optimal eosinophil cut‐off for identifying GORD remains unclear, and to establish this link more firmly, controlled studies of acid suppression therapy combined with oesophageal pH testing will be needed. Those with eosinophils present 2 cm above the Z‐line were more often men, and those with >4 eosinophils/HPF were younger, suggesting either a different severity of the disease or a different pathogenesis (perhaps true eosinophilic oesophagitis or reactive infiltration of eosinophils at the Z‐line due to GORD).
The main strengths of this study are that it assessed a representative sample of the general population using endoscopy with extensive biopsies, validated symptom questionnaires and a predefined endoscopy protocol. We believe that the findings are generalisable to western Caucasian populations. The main limitation is data were collected at a single point in time, which does not allow the assignment of cause and effect. The natural history of eosinophilic oesophagitis in the community needs to be defined, although follow‐up of 30 adults from an outpatient clinic for a mean of 7 years suggested that, although dysphagia continues to be a troublesome problem in those with eosinophilic oesophagitis, nutritional status is not affected.12 Another limitation of the present study is that 24 h oesophageal pH monitoring was not available. The relatively small number of cases eosinophilic oesophagitis was another limitation. The slight over‐representation of younger subjects reporting symptoms, including troublesome symptoms of gastro‐oesophageal reflux,16 is a limitation, but was controlled for in the risk analysis. We used any report of troublesome gastro‐oesophageal reflux symptoms over the past 3 months to identify symptoms of GORD, and hence we may have overlooked subjects with some reflux symptoms that were not troublesome to them. However, defining the symptoms as troublesome is in accordance with the patient‐centred Montreal definition of GORD.30
In conclusion, oesophageal epithelial eosinophils were present in nearly 5% of the general adult population of Sweden; 0.4% had marked infiltration consistent with definite eosinophilic oesophagitis, and 0.7% had probable eosinophilic oesophagitis. Asymptomatic low‐grade counts of epithelial eosinophils (<15/HPF) may be more common than has been estimated, but are of uncertain clinical significance. The presence of oesophageal epithelial eosinophils may be a manifestation of reflux disease, and appears to be independently associated with erosive oesophagitis in adults.
Acknowledgements
This study was supported in part by the Swedish Research Council, the Swedish Society of Medicine, Mag‐tarm sjukas förbund, Norrbotten County Council (Sweden), and Astra Zeneca R&D (Sweden). We thank Else‐Maj Sundbaum Lomakka, Åsa Storskrubb, Timo Tanner and Bo Wikström for their participation and skilful help.
Abbreviations
ASQ - Abdominal Symptom Questionnaire
GORD - gastro‐oesophageal reflux disease
HPF - high‐power field
Footnotes
Competing interests: None.
References
- 1.Landres R T, Kuster G G, Strum W B. Eosinophilic esophagitis in a patient with vigorous achalasia. Gastroenterology 1978741298–1301. [PubMed] [Google Scholar]
- 2.Attwood S E, Smyrk T C, Demeester T R.et al Esophageal eosinophilia with dysphagia. A distinct clinicopathologic syndrome. Dig Dis Sci 199338109–116. [DOI] [PubMed] [Google Scholar]
- 3.Fox V L, Nurko S, Furuta G T. Eosinophilic esophagitis: it's not just kid's stuff. Gastrointest Endosc 200256260–270. [DOI] [PubMed] [Google Scholar]
- 4.Vasilopoulos S, Murphy P, Auerbach A.et al The small‐caliber esophagus: an unappreciated cause of dysphagia for solids in patients with eosinophilic esophagitis. Gastrointest Endosc 20025599–106. [DOI] [PubMed] [Google Scholar]
- 5.Potter J W, Saeian K, Staff D.et al Eosinophilic esophagitis in adults: an emerging problem with unique esophageal features. Gastrointest Endosc 200459355–361. [DOI] [PubMed] [Google Scholar]
- 6.Remedios M, Campbell C, Jones D M.et al Eosinophilic esophagitis in adults: clinical, endoscopic, histologic findings, and response to treatment with fluticasone propionate. Gastrointest Endosc 2006633–12. [DOI] [PubMed] [Google Scholar]
- 7.Croese J, Fairley S K, Masson J W.et al Clinical and endoscopic features of eosinophilic esophagitis in adults. Gastrointest Endosc 200358516–522. [DOI] [PubMed] [Google Scholar]
- 8.Straumann A, Beglinger C. Eosinophilic esophagitis: the endoscopist's enigma. Gastrointest Endosc 20066313–15. [DOI] [PubMed] [Google Scholar]
- 9.Straumann A, Simon H U. Eosinophilic esophagitis: escalating epidemiology? J Allergy Clin Immunol 2005115418–419. [DOI] [PubMed] [Google Scholar]
- 10.Sgouros S N, Bergele C, Mantides A. Eosinophilic esophagitis in adults: a systematic review. Eur J Gastroenterol Hepatol 200618211–217. [DOI] [PubMed] [Google Scholar]
- 11.Desai T K, Stecevic V, Chang C H.et al Association of eosinophilic inflammation with esophageal food impaction in adults. Gastrointest Endosc 200561795–801. [DOI] [PubMed] [Google Scholar]
- 12.Straumann A, Spichtin H P, Grize L.et al Natural history of primary eosinophilic esophagitis: a follow‐up of 30 adult patients for up to 11.5 years. Gastroenterology 20031251660–1669. [DOI] [PubMed] [Google Scholar]
- 13.Arora A S, Yamazaki K. Eosinophilic esophagitis: asthma of the esophagus? Clin Gastroenterol Hepatol 20042523–530. [DOI] [PubMed] [Google Scholar]
- 14.Furuta G T, Straumann A. Review article: the pathogenesis and management of eosinophilic oesophagitis. Aliment Pharmacol Ther 200624173–182. [DOI] [PubMed] [Google Scholar]
- 15.Attwood S E. Eosinophils and gut dysmotility. Eur J Gastroenterol Hepatol 200517891–892. [DOI] [PubMed] [Google Scholar]
- 16.Aro P, Ronkainen J, Storskrubb T.et al Valid symptom reporting at upper endoscopy in a random sample of the Swedish adult general population, The Kalixanda study. Scand J Gastroenterol 2004391280–1288. [DOI] [PubMed] [Google Scholar]
- 17.Ronkainen J, Aro P, Storskrubb T.et al High prevalence of gastroesophageal reflux symptoms and esophagitis with or without symptoms in the general adult Swedish population: a Kalixanda study report. Scand J Gastroenterol 200540275–285. [DOI] [PubMed] [Google Scholar]
- 18.Ronkainen J, Aro P, Storskrubb T.et al Prevalence of Barrett's esophagus in the general population: an endoscopic study. Gastroenterology 20051291825–1831. [DOI] [PubMed] [Google Scholar]
- 19.Agréus L, Svärdsudd K, Nyrén O.et al Reproducibility and validity of a postal questionnaire. The abdominal symptom study. Scand J Prim Health Care 199311252–262. [DOI] [PubMed] [Google Scholar]
- 20.Aro P, Ronkainen J, Storskrubb T.et al Validation of the translation and cross‐cultural adaptation into Finnish of the Abdominal Symptom Questionnaire, the Hospital Anxiety and Depression Scale and the Complaint Score Questionnaire. Scand J Gastroenterol 2004391201–1208. [DOI] [PubMed] [Google Scholar]
- 21.Armstrong D, Bennett J R, Blum A L.et al The endoscopic assessment of esophagitis: a progress report on observer agreement. Gastroenterology 199611185–92. [DOI] [PubMed] [Google Scholar]
- 22.Lundell L R, Dent J, Bennett J R.et al Endoscopic assessment of oesophagitis: clinical and functional correlates and further validation of the Los Angeles classification. Gut 199945172–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klauser A G, Schindlbeck N E, Müller‐Lissner S A. Symptoms in gastro‐oesophageal reflux disease. Lancet 1990335205–208. [DOI] [PubMed] [Google Scholar]
- 24.Talley N, Stanghellini V, Heading R.et al Functional Gastroduodenal Disorders: A working team report for the ROME II consensus on functional gastrointestinal disorders. Gut 199945(Suppl 11)1137–1142. [Google Scholar]
- 25.Dixon M F, Genta R M, Yardley J H.et al Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol 1996201161–1181. [DOI] [PubMed] [Google Scholar]
- 26.Storskrubb T, Aro P, Ronkainen J.et al A negative Hp. serology test is a reliable tool for exclusion of premalignant gastric conditions. A report on histology & Hp. detection in the general adult population. Scand J Gastroenterol 200540302–311. [DOI] [PubMed] [Google Scholar]
- 27.StataCorp Stata Statistical Software: Release 8. 0: College Station, TX, Stata Corporation 2003
- 28.Lee R G. Marked eosinophilia in esophageal mucosal biopsies. Am J Surg Pathol 19859475–479. [DOI] [PubMed] [Google Scholar]
- 29.Steiner S J, Gupta S K, Croffie J M.et al Correlation between number of eosinophils and reflux index on same day esophageal biopsy and 24 hour esophageal pH monitoring. Am J Gastroenterol 200499801–805. [DOI] [PubMed] [Google Scholar]
- 30.Vakil N, van Zanten S V, Kahrilas P.et al The Montreal definition and classification of gastroesophageal reflux disease: a global evidence‐based consensus. Am J Gastroenterol 20061011900–1920. [DOI] [PubMed] [Google Scholar]