Sodium Benzoate, a Food Additive and a Metabolite of Cinnamon, Modifies T Cells at Multiple Steps and Inhibits Adoptive Transfer of Experimental Allergic Encephalomyelitis (original) (raw)
. Author manuscript; available in PMC: 2008 Jul 1.
Abstract
Experimental allergic encephalomyelitis (EAE) is the animal model for multiple sclerosis. This study explores a novel use of sodium benzoate (NaB), a commonly used food additive and a Food and Drug Administration-approved nontoxic drug for urea cycle disorders, in treating the disease process of relapsing-remitting EAE in female SJL/J mice. NaB, administered through drinking water at physiologically tolerable doses, ameliorated clinical symptoms and disease progression of EAE in recipient mice and suppressed the generation of encephalitogenic T cells in donor mice. Histological studies reveal that NaB effectively inhibited infiltration of mononuclear cells and demyelination in the spinal cord of EAE mice. Consequently, NaB also suppressed the expression of proinflammatory molecules and normalized myelin gene expression in the CNS of EAE mice. Furthermore, we observed that NaB switched the differentiation of myelin basic protein-primed T cells from Th1 to Th2 mode, enriched regulatory T cell population, and down-regulated the expression of various contact molecules in T cells. Taken together, our results suggest that NaB modifies encephalitogenic T cells at multiple steps and that NaB may have therapeutic importance in multiple sclerosis.
Multiple sclerosis (MS) is the most common human demyelinating disease of the CNS. Although the exact cause behind this disease is not completely understood, several studies on MS patients suggest that it is a T cell-mediated autoimmune response (1). In healthy human beings, autoimmune T cells are normally suppressed by a population of regulatory T cells (Tregs) (2). However, in patients with MS, myelin-reactive T cells overcome the usual restraining mechanism of Tregs, become activated, and target the CNS. Detection of a wide variety of proinflammatory molecules, such as proinflammatory cytokines (IL-1_β_, IFN-γ, IL-6, and TNF-α), proinflammatory chemokines (MCP-1/CCL2, and IP-10/CXCL10), proinflammatory enzymes (inducible NO synthase, cyclooxygenase 2), and proinflammatory transcription factors (NF-_κ_B, C/EBP), in CNS lesions of MS patients suggests that a complex interaction among autoimmune T cells, macrophages, and resident glial cells initiates a series of inflammatory cascades that ultimately led to the damage of myelin-synthesizing oligodendrocytes (3–7).
Experimental allergic encephalomyelitis (EAE) is an animal model of MS. Adoptively transferred EAE mimics the relapsing-remitting MS, the most common form of MS found in patients. In this model, neuroantigen-specific autoimmune T cells first contact a naive intact blood-brain barrier (BBB) and are able to extravasate through the BBB due to their activated status. These cells are retained in the CNS due to the presentation of appropriate Ag and undergo further activation (8). This is followed by the recruitment of non-Ag-specific lymphocytes and activated macrophages from the blood into this site, accompanied by activation of resident glial cells and further disruption of the BBB. Similar to MS, various proinflammatory molecules are also implicated in the disease process of EAE. This model is widely used to identify a new therapeutic approach against MS.
Sodium benzoate (NaB), the sodium salt of an aromatic carboxylic acid, is a component of Ucephan, a Food and Drug Administration-approved drug used in the treatment for hepatic metabolic defects associated with hyperammonemia, such as urea cycle disorder in children (9, 10). It is also widely used as a preservative in a broad range of foods and cosmetic products (11). It is nontoxic and can be administered as a solution in drinking water. It has been reported that a 2% solution of NaB in drinking water is safe for lifelong treatment in mice without any noticeable side effects (12). In accordance with the published reports (13, 14), a minor amount of benzoic acid, a direct metabolite of cinnamic acid (15), is also excreted in the urine of humans.
In this study, we provide the first evidence that NaB inhibits the disease process of the relapsing-remitting model of EAE (RR-EAE). NaB attenuates the trafficking, inflammation, and demyelination in adoptively transferred EAE mice by reducing the expression of contact molecules, switching the differentiation of Th cells, and enriching the population of Tregs. Our findings raise a strong possibility that NaB, a component of a prescribed drug for human urea cycle disorder and a widely used food preservative, may find further application in MS.
Materials and Methods
Reagents
NaB, sodium formate (NaFO), Solvent Blue 38, cresyl violet acetate, and lithium carbonate were purchased from Sigma-Aldrich. FITC-labeled hamster anti-mouse CD29 (integrin _β_1 chain) and goat anti-mouse CD49D (integrin _α_4 chain) mAbs were purchased from Chemicon International and BD Biosciences, respectively. PE-labeled rat anti-mouse CD4 and FITC-labeled rat anti-mouse CD25 mAbs were purchased from Chemicon International.
Induction of EAE
Specific pathogen-free female SJL/J mice (3–4 wk old) were purchased from Harlan Sprague Dawley.
By passive transfer of myelin basic protein (MBP)-reactive T cells
Donor mice were immunized s.c. with 400 µg bovine MBP and 60 µg Mycobacterium tuberculosis in IFA (16). Animals were killed 10–12 days postimmunization, and the draining lymph nodes were harvested. Single-cell suspensions were treated with RBC lysis buffer (Sigma-Aldrich), washed, and cultured at a concentration of 4–5 × 106 cells/ml in 6-well plates in RPMI 1640 supplemented with 10% FBS, 50 µg/ml MBP, 50 µM 2-ME, 2 mM l-glutamine, 100 U/ml penicillin, and 100 µg/ml streptomycin. On day 4, cells were harvested and resuspended in HBSS. A total of 2 × 107 viable cells in a volume of 200 µl was injected into the tail vein of naive mice. Pertussis toxin (150 ng/mouse; Sigma-Aldrich) was injected once via i.p. route on 0 days posttransfer (dpt) of cells. Cells isolated from donor mice immunized with CFA or IFA alone were not viable after 4 days in culture with MBP and therefore were not transferred.
By active immunization of MBP
Active immunization of MBP was performed as described earlier (16), with some modifications. In brief, female SJL/J mice were immunized s.c. on both sides of the flanks with 500 µg guinea pig MBP and 60 µg M. tuberculosis in IFA. Mice also received pertussis toxin (400 ng/mouse) via the i.p. route on 0 and 2 days of immunization. Control mice received PBS (vehicle), M. tuberculosis, IFA, and pertussis toxin.
Animal maintenance and experimental protocols were approved by Animal Care Committees of the University of Nebraska and the Rush University Medical Center. Animals were observed daily for clinical symptoms. Experimental animals were scored by a masked investigator as follows: 0, no clinical disease; 0.5, piloerection; 1, tail weakness; 1.5, tail paralysis; 2, hind limb weakness; 3, hind limb paralysis; 3.5, forelimb weakness; 4, forelimb paralysis; and 5, moribund or death.
T cell proliferation assay
T cells (2 × 105) cultured in 96-well U-bottom microtiter plates were stimulated with 50 µg/ml MBP in the presence or absence of different doses of NaB. Unstimulated cells were kept as controls. After 72 h of stimulation, cells were pulsed with [3H]thymidine (0.5 µCi/well) for another 24 h. The level of [3H]thymidine incorporation in cells was assessed as described earlier (17, 18).
Treatment with NaB and NaFO
Groups of mice were treated with NaB or NaFO through drinking water in a blinded fashion at doses ranging from 2.5 to 10 mg/ml from 0 dpt. Mice were also allowed to drink water containing NaB (5 mg/ml) at various phases of the disease (before acute phase and before chronic phase). Controls received normal drinking water without NaB. Statistical analysis was determined by the RS/1 multicomparison procedure using a one-way ANOVA and Dunnett's test for multiple comparisons with a common control group. Differences between means were considered significant when p values were <0.05.
Histological microscopy
On 18 dpt (first chronic phase), six mice from each of the following groups (control, vehicle-treated EAE, and EAE mice receiving NaB from 4 dpt) were anesthetized. After perfusion with PBS (pH 7.4) and then with 4% (w/v) paraformaldehyde solution in PBS, the whole spinal cord was dissected out from each mouse. The tissues were further fixed and then divided into two longitudinal halves: one-half of the spinal cord was used for histological staining as described previously (17), whereas the other half was used for myelin staining. For histological analysis, routine histology was performed to obtain perivascular cuffing and morphological details of spinal cord tissues of EAE mice. Paraformaldehydefixed tissues were embedded in paraffin, and serial sections (4 µm) were cut. Sections were stained with a conventional H&E staining method. Digital images were collected under bright-field setting, using a ×40 objective. Slides were assessed in a blinded fashion for inflammation by three examiners in different anatomical compartments (meninges and parenchyma). Inflammation was scored using the following scale as described. For meninges and parenchyma: 0, no infiltrating cells; 1, few infiltrating cells; 2, numerous infiltrating cells; and 3, widespread infiltration. For vessels: 0, no cuffed vessel; 1, one or two cuffed vessels per section; 2, three to five cuffed vessels per section; and 3, more than five cuffed vessels per section. At least six serial sections of each spinal cord from each of five mice per group were scored and statistically analyzed by ANOVA.
Staining for myelin
Serial longitudinal sections of paraformaldehyde-fixed spinal cords were stained with Luxol fast blue for myelin as described elsewhere. Slides were assessed in a blinded fashion for demyelination by three examiners using the following scale: 0, normal white matter; 1, rare foci; 2, a few areas of demyelination; and 3, large areas of demyelination. At least six serial sections of each spinal cord from each of six mice per group were scored and statistically analyzed by ANOVA.
Isolation of splenic MBP-primed T cells
Female SJL/J mice were immunized with MBP as described above. Spleens were collected from these mice on day 10 postimmunization, and a single-cell suspension was prepared in RPMI 1640 containing 10% FBS, 2 mM l-glutamine, 50 µM 2-ME, 100 U/ml penicillin, and 100 µg/ml streptomycin. Cells cultured at a concentration of 4–5 × 106 cells/ml in 6-well plates were incubated with 50 µg/ml MBP for 4 days. The nonadherent cells were collected and used for RNA isolation and FACS analysis.
Semiquantitative RT-PCR analysis
Total RNA was isolated from splenic T cells and spinal cord by using a RNeasy Mini Kit (Qiagen) and from spleen and cerebellum by using an Ultraspec-II RNA reagent (Biotecx Laboratories) according to the manufacturer's protocol. To remove any contaminating genomic DNA, total RNA was digested with DNase. Semiquantitative RT-PCR was conducted as described earlier (19) using a RT-PCR kit from BD Clontech. In brief, 1 µg of total RNA was reverse transcribed using oligo(dT)12–18 as primer and Moloney murine leukemia virus reverse transcriptase (BD Clontech) in a 20-µl reaction mixture. The resulting cDNA was appropriately diluted, and diluted cDNA was amplified using Titanium TaqDNA polymerase and following primers. Amplified products were electrophoresed on a 1.8% agarose gel and visualized by ethidium bromide staining as follows: CD25: sense, 5′-AGCCAAGTAGGGTGTCTCTCAACC-3′ and antisense, 5′-GCCCAGGATACACAGTGAAGAACG-3′; CD4: sense, 5′-GAGAGTCAGCGGAGTTCTC-3′ and antisense, 5′-CTCACAGGTCAAAGTATTGTTG-3′; VCAM-1: sense, 5′-CAAGGGTGACCAGCTCATGA-3′ and antisense, 5′-TGTGCAGCCACCTGAGATCC-3′; E-selectin: sense, 5′-GGTAGTTGCACTTTCTGCGG-3′ and antisense, 5′-CCTTCTGTGGCATGTTC-3′; P-selectin: sense, 5′-ACGAGCTGGACGGACCCG-3′ and antisense, 5′-GGCTGGCACTCAAATTTACAG-3′; integrin-_β_2: sense, 5′-CTGCTGTGTCCCAGGAATGCACC-3′ and antisense, 5′-CCCGCCCAGCTTCTTGACGTTGT-3′; integrin _α_l: sense, 5′-TGCGGGCCCCAGACTTTTGCTAC-3′ and antisense, 5′-GGTCACAGGCCAAAAGGCTTCCC-3′; Foxp3: sense, 5′-CAGCTGCCTACAGTGCCCCTAG-3′ and antisense, 5′-CATTTGCCAGCAGTGGGTAG-3′; integrin-α_4: sense, 5′AACCGGGCACTCCTACAACCTGGAC-3′ and antisense, 5′-ACCCCCAGCCACTGGTTATCCCTCT-3′; inducible NO synthase (iNOS): sense, 5′-CCCTTCCGAAGTTTCTGGCAGCAGC-3′ and antisense, 5′-GGCTG TCAGAGCCTCGTGGCTTTGG-3′; IL-1_β: sense, 5′-CTCCATGAGCTTTGTACAAGG-3′ and antisense, 5′-TGCTGATGTACCAGTTGGGG-3′; IL-6: sense, 5′-GACAACTTTGGCATTGTGG-3′ and antisense, 5′-ATGCAGGGATGATGTTCTG-3′; integrin _β_1: sense, 5′GAGACATGTCAGACCTGCCTTGGCG-3′ and antisense, 5′-GGGATGATGTGGGGACCAGTAGGAC-3′; ICAM-1: sense, 5′-CTGGGCTTGGAGACTCAGTG-3′ and antisense, 5′-GTGTCGAGCTTTGGGATGGTA-3′; myelin oligodendrocyte glycoprotein (MOG): sense, 5′-CCTCTCCCTTCTCCTCCTTC-3′ and antisense, 5′-AGAGTCAGCACACCGGGGTT-3′; MBP: sense, 5′-TGGAGAGATTCACCGAGGAGA-3′ and antisense, 5′-TGAAGCTCGTCGGACTCTGAG-3′; proteolipid protein (PLP): sense, 5′-CTTTGCTTCCCTGGTGGCCA-3′ and antisense, 5′-TGTTGGCCTCTGGAACCCCT-3′; 2′,3′-cyclic nucleotide 3-phosphodiesterase (CNPase): sense, 5′-CTACCCTCCACGAGTGCAAGA-3′ and antisense, 5′-AGTCTAGTCGCCACGCTGTCT-3′; IL-4: sense, 5′-CATCCTGCTCTTCTTTCTCG-3′ and antisense, 5′-GGACTTGGACTCATTCATGGTGC-3′; IL-10: sense, 5′-GCACTGCTATGCTGCCTGCT-3′ and antisense, 5′-CCGATAAGGCTTGGCAACCC-3′; IFN-γ: sense, 5′-GCTGTTACTGCCACGGCACA-3′ and antisense, 5′-GGACCACTCGGATGAGCTCA-3′; and GAPDH: sense, 5′-GGTGAAGGTCGGTGTGAACG3′ and antisense, 5′-TTGGCTCCACCCTTCAAGTG-3′.
The relative expression of each gene with respect to GAPDH was measured after scanning the bands with a Fluor Chem 8800 Imaging System (Alpha Innotech).
Real-time PCR analysis
It was performed using the Applied Biosystems Prism 7700 sequence detection system as described earlier (19). In brief, reactions were performed in 96-well optical reaction plates on cDNA equivalent to 50 ng DNase-digested RNA in a volume of 25 ml, containing 12.5 ml TaqMan Universal Master mix and optimized concentrations of FAM-labeled probe, forward and reverse primers following the manufacturer's protocol. All primers and FAM-labeled probes for mouse genes and GAPDH were obtained from Applied Biosystems. The mRNA expressions of respective genes were normalized to the level of GAPDH mRNA. Data were processed by the Applied Biosystems Sequence Detection System 1.6 software and analyzed by ANOVA.
Assay of cytokines by ELISA
Splenocytes from MBP-immunized mice were cultured in tissue culture plates with 50 µg/ml MBP along with various doses of NaB. After 4 days of incubation, supernatants were collected to assay IFN-γ, IL-4, and IL-10 by high-sensitivity ELISA kits (BD Biosciences).
Flow cytometry
Surface expression of VLA-4 and the surface coexpression of CD4 and CD25 on splenic MBP-primed T cells were checked by single-color and two-color flow cytometry, respectively, as described previously (18). One × 106 cells suspended in RPMI 1640 medium/FBS were incubated in the dark with appropriately diluted FITC-labeled Abs to CD49d (integrin _α_4 chain) or CD29 (integrin _β_1 chain) for single color or with appropriately diluted FITC-labeled Abs to CD25 and PE-labeled Abs to CD4 for two color at 4°C for 1 h. Following incubation, the cell suspension was centrifuged, washed three times, and resuspended in 500 µl of RPMI 1640 medium/FBS. The cells were then analyzed through FACS (BD Biosciences). A minimum of 10,000 cells was accepted for FACS analysis. Cells were gated based on morphological characteristics. Apoptotic and necrotic cells were not accepted for FACS analysis.
Results
NaB inhibits clinical symptoms and disease severity in female SJL/J mice
Because NaB is highly water soluble and nontoxic, we first examined whether administration of NaB in drinking water could inhibit the clinical symptoms and disease severity in adoptively transferred EAE mice. Therefore, groups of mice were treated with different doses of NaB in drinking water from 0 dpt. One group of mice was given NaFO in drinking water (10 mg/ml) as a negative control, whereas the positive control mice received only drinking water. Clinical scores were observed on each day after transfer, and because the relapsing-remitting type of EAE is associated with multiple chronic phase peaks following acute phase peak, we continued our observation until 51 dpt. Although NaB at a dose of 2.5 mg/ml was not effective in inhibiting clinical symptoms, at doses of 5 and 10 mg/ml, this drug dramatically inhibited clinical symptoms in acute as well as chronic phases of EAE (Fig. 1A). Only piloerection was observed as the highest clinical symptom in most of the mice that received NaB at 5 and 10 mg/ml. In contrast, NaFO having a similar structure but without the benzene ring was unable to inhibit the clinical symptoms of EAE (Fig. 1A), suggesting the specificity of the effect. These findings suggest that NaB is capable of inhibiting clinical symptoms and disease severity in the acute as well as chronic phases of EAE.
FIGURE 1.
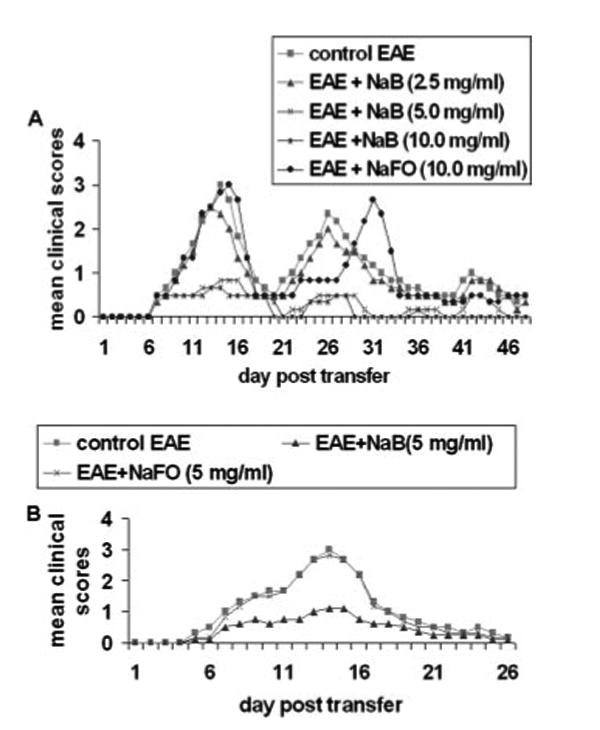
Dose-dependent inhibition of clinical symptoms of EAE in female SJL/J mice by NaB. A, Female SJL/J mice were induced EAE by adoptive transfer of MBP-primed T cells. From 0 dpt, mice received drinking water containing different concentrations of NaB. Control EAE mice received normal drinking water. One group of mice also received drinking water containing 10 mg/ml NaFO. B, EAE was induced in female SJL/J mice by direct immunization of MBP. From day 0 of immunization, mice received drinking water containing either NaB or NaFO. Each group of mice (n = 6) was examined for clinical symptoms every day.
Effect of NaB on clinical symptoms and disease severity of actively induced EAE
Because NaB inhibited the clinical symptoms and severity of adoptively transferred EAE, we examined whether this drug was also able to suppress EAE induced by active immunization. Mice received drinking water containing 5 mg/ml NaB from day 0 of active induction of EAE. Clinical symptoms of NaB-treated EAE animals were compared with that of normal drinking water-treated and NaFO-treated EAE animals. Consistent with the inhibition of clinical symptoms in the adoptive transfer model (Fig. 1A), NaB suppressed clinical symptoms, compared with the normal drinking water group in the actively induced model (Fig. 1B). In contrast, NaFO was unable to inhibit the disease process of actively induced EAE (Fig. 1B) suggesting the specificity of the effect.
NaB inhibits the encephalitogenicity of MBP-primed T cells
Because MBP-primed T cells are encephalitogenic and adoptive transfer of these T cells induces EAE, we were interested to investigate whether NaB is capable of inhibiting the proliferation and encephalitogenicity of MBP-primed T cells. T cells isolated from the lymph nodes of MBP-primed mice proliferated in response to MBP, and the maximum proliferation was observed at 50 or 100 µg/ml MBP (17). However, pretreatment of MBP-primed T cells with NaB dose-dependently inhibited the proliferation in response to MBP (Fig. 2A). Next, MBP-primed T cells and NaB-treated MBP-primed T cells were adoptively transferred to recipient mice. Another group of mice received NaFO-treated MBP-primed T cells. Our results showed that groups of mice that received NaB-treated MBP-primed T cells exhibited significantly reduced clinical symptoms and disease severity compared to mice receiving either MBP-primed T cells or NaFO-treated MBP-primed T cells (Fig. 2B). These results suggest that NaB inhibits the encephalitogenicity of MBP-primed T cells.
FIGURE 2.
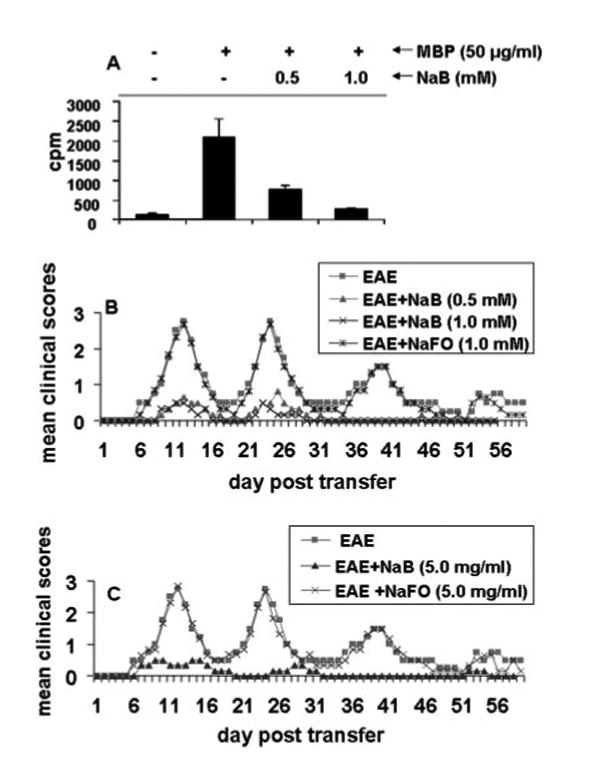
Inhibition of proliferation and encephalitogenicity of MBP-primed T cells by NaB. A, T cells suspended in RPMI 1640 containing 10% FBS were treated with different concentrations of MBP in the presence or absence of NaB. After 4 days of incubation, proliferation was assayed. Data are mean ± SD of three different experiments, p < 0.001 vs MBP. B, MBP-primed T cells were treated with either NaB or NaFO for 4 days during in vitro stimulation by MBP. Then female SJL/J recipient mice were induced with EAE by adoptive transfer of either MBP-primed T cells, NaB-treated MBP-primed T cells, or NaFO-treated MBP-primed T cells. C, Donor mice were immunized with MBP, IFA, and M. tuberculosis. From day 0 of immunization, mice were treated with either NaB or NaFO in drinking water (5 mg/ml). On day 12 of immunization, mice were sacrificed, and total lymph node cells were further primed with MBP (50 µg/ml) for 4 days. A total of 2 × 107 viable MBP-primed T cells was adoptively transferred to naive mice. Six mice were used in each group.
NaB inhibits the generation of encephalitogenic T cells in vivo in donor mice
Because in vitro treatment of MBP-primed T cells with NaB inhibited the encephalitogenicity of MBP-primed T cells, we next investigated whether NaB treatment in donor mice was capable of inhibiting the generation of encephalitogenic T cells in vivo. Therefore, donor mice were given either NaB or NaFO in drinking water at 5 mg/ml, and T cells from these donor mice were primed with MBP for 4 days and transferred adoptively to recipient mice. In the control group, EAE was induced by the adoptive transfer of MBP-primed T cells from donor mice that were given only drinking water. Our results showed that mice that received T cells from NaB-treated donor mice exhibited dramatically less clinical symptoms and disease severity compared with the control EAE group and the NaFO-treated EAE group (Fig. 2C). These results suggest that NaB is capable of inhibiting the generation of encephalitogenic T cells in donor mice and that this inhibitory effect is specific.
NaB inhibits disease progression in the adoptively transferred model of EAE
Next, we were encouraged to investigate whether NaB could inhibit the disease progression in adoptively transferred EAE mice. To achieve this goal, mice were treated with NaB in two different groups. In the first group, mice were treated with NaB (5 mg/ml drinking water) from the onset of the acute phase (5 dpt). The results in Fig. 3A clearly show that the inhibitory effect of NaB on the clinical symptoms was observed within 4 days of treatment (from 9 dpt). There was further marked inhibition on subsequent days of treatment and this inhibition was maintained throughout the duration of the experiment (Fig. 3A). In contrast, NaFO had no such inhibitory effect (Fig. 3A). In the second group, NaB treatment began from the onset of the relapsing phase (16 dpt) and was continued until 40 dpt. Fig. 3B clearly shows that NaB, but not NaFO, in this instance also halted disease progression. Similar to the first instance, the inhibitory effect of NaB was manifested only after 5 days of treatment (21 dpt) through drinking water. The EAE disease severity in the NaB-treated group was always below or around stage 0.5 from 22 dpt until the end of the study (40 dpt; Fig. 3B). These results clearly suggest that NaB can control the ongoing relapsing-remitting EAE when administered either early (at the onset of acute disease) or late (at the onset of relapsing disease).
FIGURE 3.
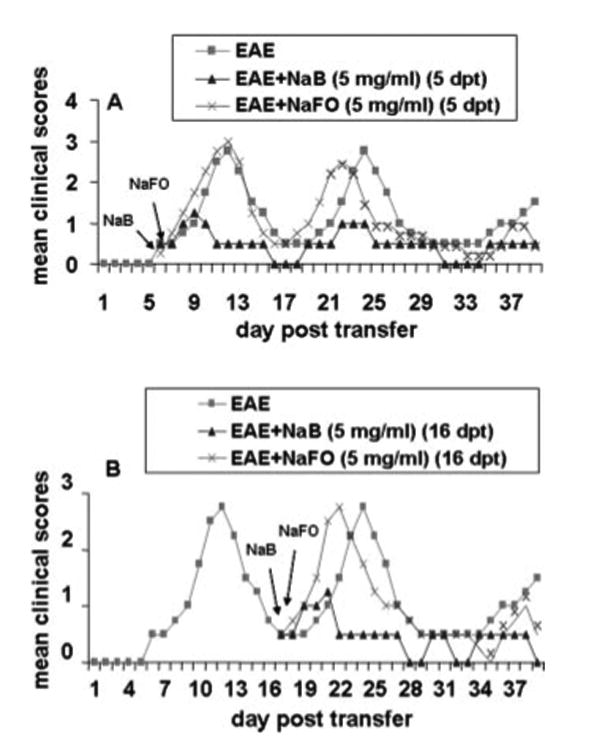
Treatment of adoptively transferred EAE in SJL/J mice by NaB. EAE was induced in female SJL/J mice by adoptive transfer of MBP-primed T cells. A, One group of EAE mice (n = 6) was treated with either NaB or NaFO in drinking water from the onset of the acute phase (5 dpt). B, The other group of EAE mice (n = 6) were treated with either NaB or NaFO in drinking water from the onset of the relapsing phase (16 dpt). Mice were examined for clinical symptoms every day until 40 dpt.
NaB inhibits the infiltration of mononuclear cells, inflammation, and demyelination in the CNS of EAE
Because NaB inhibited the disease process of adoptively transferred EAE, we were prompted to investigate mechanistic details by which NAB is capable of doing so. It is believed that EAE, as well as MS, is caused by the infiltration of autoreactive T cells and associated mononuclear cells, such as macrophages, into the CNS followed by broad-spectrum inflammatory events. We investigated whether NaB attenuated infiltration and inflammation in adoptively transferred EAE mice. Mice receiving NaB-containing drinking water from 5 dpt (onset of the acute phase) were sacrificed on 12 dpt. H&E staining (Fig. 4A, top) in the longitudinal section of spinal cord of EAE mice showed widespread infiltration of inflammatory cells into the spinal cord. In contrast, NaB treatment through drinking water markedly inhibited the infiltration of inflammatory cells into the spinal cord of EAE mice (Fig. 4A). In contrast, NaFO was unable to inhibit the infiltration of inflammatory cells (Fig. 4A). Quantitation of the relative level of inflammation in Fig. 4B shows that NaB, but not NaFO, dramatically reduced the infiltration and the appearance of cuffed vessels in the spinal cord sections of RR-EAE mice.
FIGURE 4.
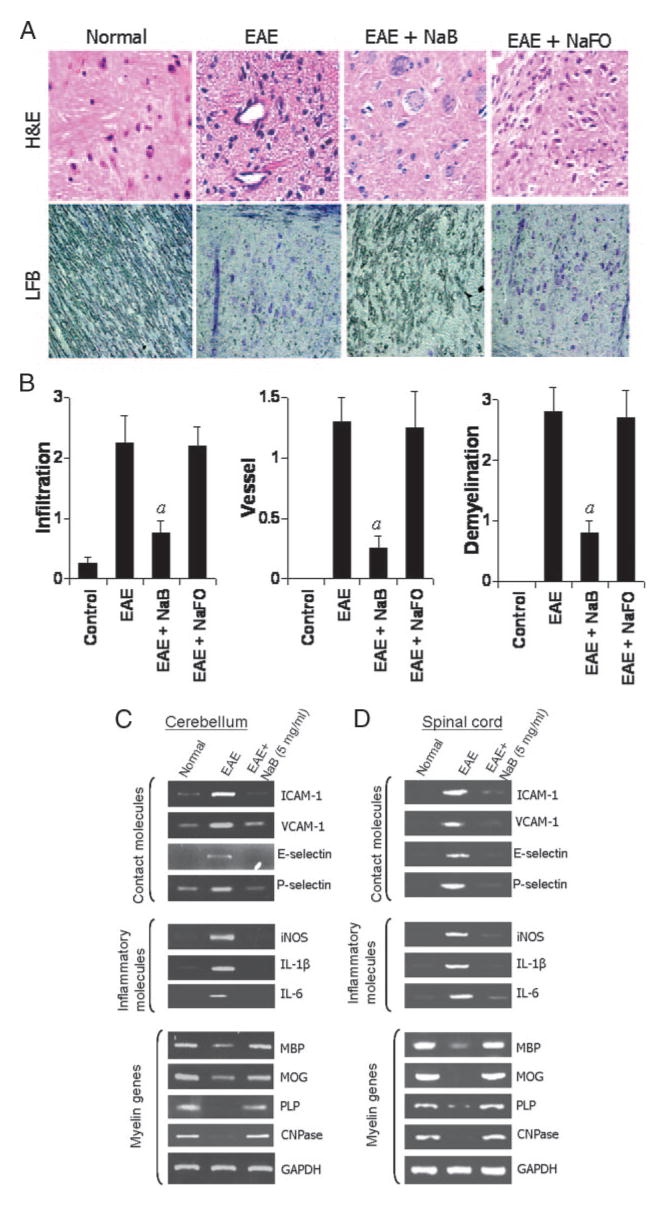
NaB inhibits infiltration of mononuclear cells, inflammation, and demyelination in the CNS of EAE mice. A, Longitudinal sections of the spinal cord isolated from control EAE (9 dpt) and either NaB- or NaFO-treated EAE mice (9 dpt receiving NaB/NaFO from 4 dpt) were stained with H&E (top) and Luxol fast blue (bottom). Digital images were collected under a bright-field setting using a ×40 objective. B, Infiltration, cuffed vessel, and demyelination were represented quantitatively by using a scale as described in Materials and Methods. Data are expressed as the mean ± SD of five different mice; p < 0.001 vs EAE. Cerebellum (C) and spinal cord (D) of normal, EAE and NaB-treated EAE mice were analyzed for ICAM-1, VCAM-1, E-selectin, and P-selectin (top), iNOS, IL-1_β_, and IL-6 (middle), MBP, MOG, PLP, and CNPase (bottom), and GAPDH mRNAs by semiquantitative RT-PCR. Results represent three independent experiments.
Infiltration is mediated by adhesion molecules such as ICAM-1, VCAM-1, E-selectin, and P-selectin which are expressed in the endothelium of BBB, as well as in glial cells in the CNS parenchyma. Therefore, we examined the effect of NaB on the expression of these molecules in the CNS (spinal cord and cerebellum) of EAE mice. Our semiquantitative RT-PCR data revealed marked expression of these adhesion molecules in both the spinal cord and cerebellum of EAE mice compared with control mice. However, NaB treatment through drinking water effectively inhibited the expression of these contact molecules in both cerebellum and spinal cord of EAE mice (Fig. 4, C and D, top). Because infiltration was inhibited, we next examined whether NaB was capable of inhibiting the expression of proinflammatory molecules in the CNS of EAE mice. Marked expression of proinflammatory molecules like iNOS, IL-1_β_, and IL-6 was observed in the cerebellum as well as spinal cord of EAE mice compared with control mice (Fig. 4, C and D, middle). However, NaB treatment through drinking water dramatically reduced the expression of these proinflammatory molecules in the cerebellum as well as the spinal cord of EAE mice (Fig. 4, C and D, middle).
It is believed that the infiltration of blood mononuclear cells and associated neuroinflammation plays an important role in CNS demyelination observed in MS patients and EAE animals. Therefore, we examined whether NAB protected EAE mice from demyelination. Our semiquantitative RT-PCR data reveal a marked loss of myelin genes like MBP, MOG, PLP, and CNPase in both the spinal cord and cerebellum of EAE mice compared with control mice (Fig. 4, C and D, bottom). However, significant restoration of myelin gene mRNA expression was observed in NaB-treated EAE mice (Fig. 4, C and D, bottom). To confirm this finding further, we stained longitudinal sections of spinal cord with Luxol fast blue for myelin and observed widespread demyelination zones in the white matter of the spinal cord of EAE mice (Fig. 4, A, bottom, and B). However, NaB treatment via drinking water remarkably restored the myelin level in spinal cord of RR-EAE mice (Fig. 4A, bottom and Fig. 4B). In contrast, NaFO was unable to restore myelin level in spinal cord of EAE mice (Fig. 4B). Taken together, these results suggest that NaB inhibits the infiltration of mononuclear cells, inflammation, and demyelination in the CNS of EAE mice.
NaB inhibits VLA-4 and LFA-1 integrins in MBP-primed splenic T cells
Because all of these inflammatory and demyelination events happening in the CNS of MS patients and EAE animals are primarily caused by infiltration of self-reactive T cells, we were interested in investigating whether NaB could modify these T cells at some level and thereby prevent their infiltration into the CNS. During trafficking, several contact molecules on the surface of T cells interact with adhesion molecules present on BBB endothelial cells and glial cells. For example, VLA-4 and LFA-1 present on the surface of T cells interact with endothelial and glial VCAM-1 and ICAM-1, respectively. Therefore, we first examined the effect of NaB on the expression of two important contact molecules, VLA-4 and LFA-1 integrins in MBP-primed splenic T cells. Semiquantitative RT-PCR results in Fig. 5A and quantitative real-time PCR results in Fig. 5B show marked expression of _α_4 and _β_1 constituting VLA-4 and _α_l and _β_2 constituting LFA-1 integrins in MBP-primed splenic T cells compared with normal splenic T cells. However, NaB dose-dependently inhibited the expression of _α_4, _β_1, _α_l, and _β_2 in MBP-primed splenic T cells with maximum inhibition observed at a 0.5 or 1.0 mM concentration (Fig. 5, A and B). Because these molecules are present on the cell surface, we also confirmed our finding by FACS analysis. We observed an increase in surface expression of _α_4 and _β_1 integrins in MBP-primed splenic T cells compared with control splenic T cells (Fig. 5C). Consistent with its effect on mRNA expression, NaB markedly inhibited the surface protein expression of _α_4 and _β_1 integrins on MBP-primed splenic T cells (Fig. 5C).
FIGURE 5.
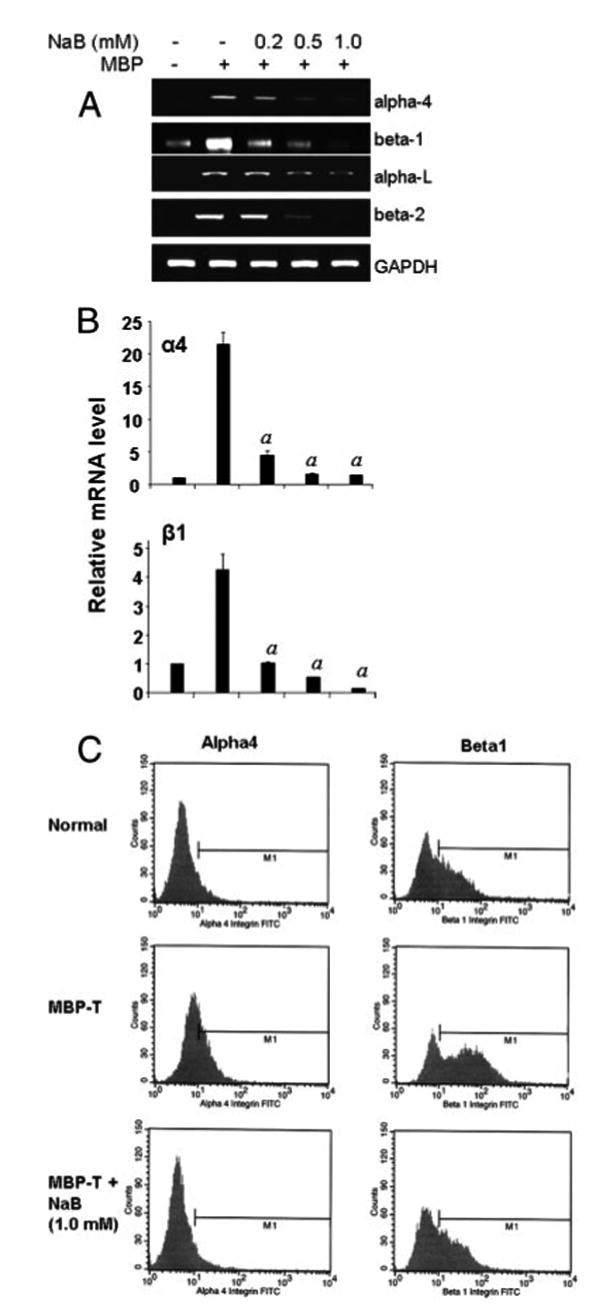
NaB inhibits VLA-4 and LFA-1 integrins in MBP-primed splenic T cells. Splenocytes from donor mice were activated by MBP in the presence or absence of different doses of NaB for 4 days, followed by analysis of _α_4, _β_1, _α_l, and _β_2 mRNAs in T cells by semiquantitative RT-PCR (A) and quantitative real-time PCR (B). Data are mean ± SD of three different experiments; p < 0.001 vs MBP only. C, Normal, MBP-primed T cells and NaB- treated MBP-primed T cells were incubated with appropriately diluted FITC-conjugated anti-_α_4 and _β_1 Abs for 1 h followed by FACS analysis. Results represent three independent experiments.
NaB inhibits the expression of VLA-4 and LFA-1 integrins in vivo in spleens of donor mice
Because NaB treatment blocked the expression of VLA-4 and LFA-1 in MBP-primed splenic T cells, we next examined whether NaB is capable of inhibiting these integrins in vivo in the spleen as well. Therefore, a group of donor mice (n = 5) received NaB (5 mg/ml) via drinking water from day 0 of immunization and, after 10 days of treatment, spleens were collected for RNA isolation followed by semiquantitative RT-PCR and real-time PCR analysis. One group of donor mice was also given NaFO (5 mg/ml) in drinking water as a negative control. Expectedly, we observed a marked increase in the mRNA expression of _α_4, _β_1, _α_l, and _β_2 in spleens of MBP-immunized donor mice compared to normal mice (Fig. 6). However, NaB treatment resulted in significant inhibition of mRNA expression of _α_4, _β_1, _α_l, and _β_2 in the spleens of donor mice (Fig. 6). In contrast, NaFO was unable to inhibit the mRNA expression of VLA-4 and LFA-1 subunits in spleens of donor mice (Fig. 6).
FIGURE 6.
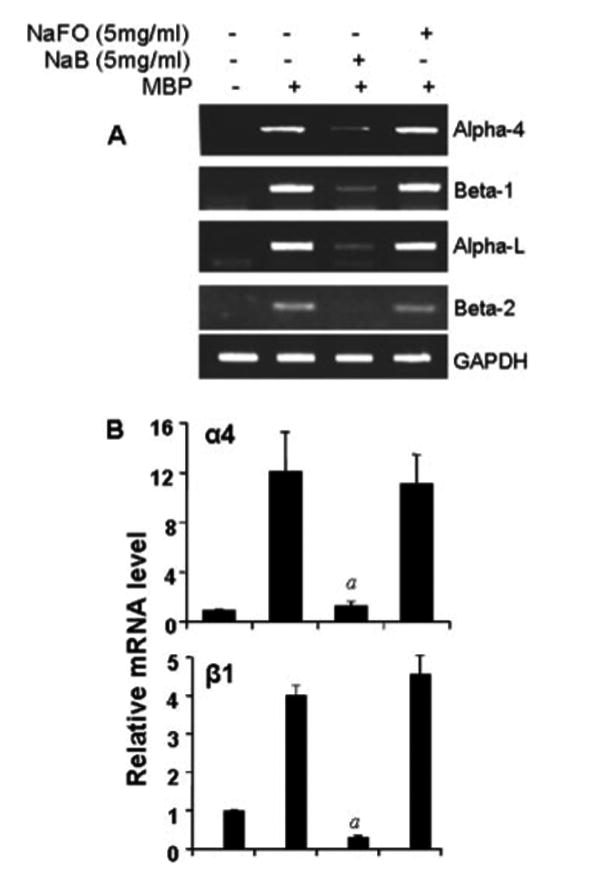
NaB inhibits the expression of VLA-4 and LFA-1 integrins in donor spleen. Spleens from normal mice, donor mice, and NaB- or NaFO-treated donor mice were analyzed for _α_4, _β_1, _α_l, and _β_2 mRNAs by semiquantitative RT-PCR (A) and quantitative real-time PCR (B). Data are mean ± SD of three different experiments; p < 0.001 vs MBP (donor) spleen.
NaB switches Th1 to Th2 cytokine expression in MBP-primed splenic T cells
It is known that MS is a Th1-mediated autoimmune disease and that switching of Th1 to a Th2 phenotype is a way to ameliorate the disease. Therefore, we examined whether NaB could facilitate the switching of Th1 to the Th2 phenotype. IFN-γ is the major cytokine that characterizes the Th1 phenotype, whereas the Th2 phenotype is commonly characterized by the secretion of cytokines like IL-4 and IL-10. In this study, our semiquantitative RT-PCR data show that splenic T cells isolated from normal female SJL/J mice expressed IL-10 and IL-4 and that MBP priming inhibited the expression of these cytokines in splenic T cells (Fig. 7A). In contrast, MBP-primed splenic T cells expressed higher amounts of IFN-γ compared to normal splenic T cells (Fig. 7B). However, NaB dose-dependently increased the mRNA expression of IL-4 and IL-10 and inhibited the mRNA expression of IFN-γ in MBP-primed splenic T cells (Fig. 7, A and B). Under similar treatment conditions, NaFO had no effect on the expression of either Th1 (IL-4 and IL-10) or Th2 (IFN-γ) cytokines (Fig. 7, A and B), suggesting the specificity of the effect. Because these cytokines are secretory proteins, we further verified our findings by ELISA experiment and, as expected, results also supported our RT-PCR data (Fig. 7C). Normal splenic T cells produced higher amounts of IL-4 and IL-10 and lower amounts of IFN-γ than MBP-primed T cells (Fig. 7C). NaB stimulated the production of Th2 (IL-4 and IL-10) cytokines while suppressing the production of IFN-γ (a Th1 cytokine) in MBP-primed splenic T cells (Fig. 7C).
FIGURE 7.
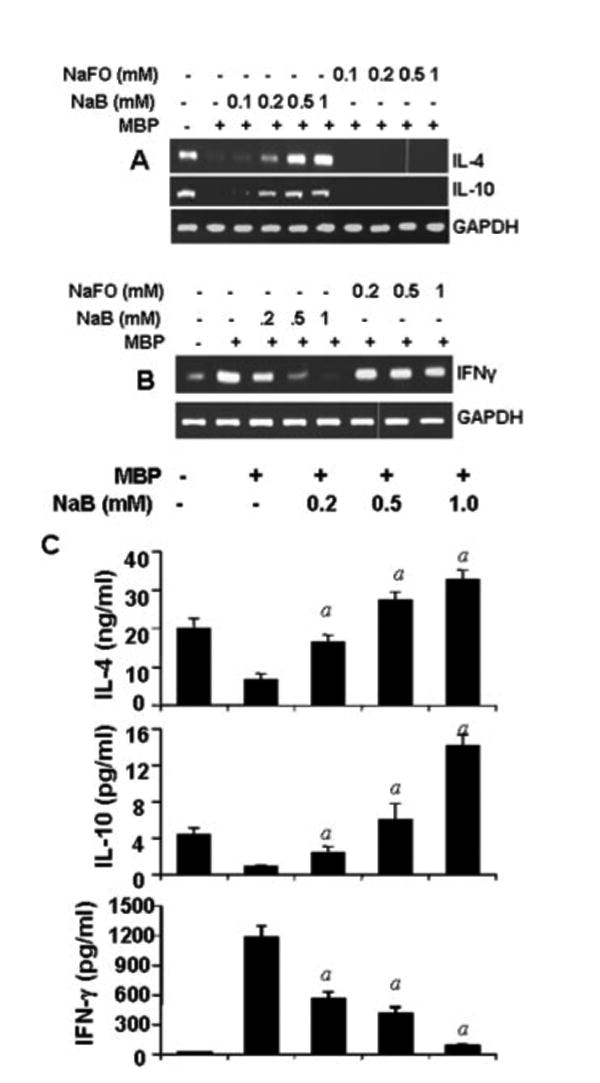
NaB switches from the Th1 to Th2 response. Splenocytes isolated from MBP-immunized mice were stimulated with MBP in the presence or absence of different doses of NaB or NaFO. After 72 h of stimulation, the expression of Th2 (IL-4 and IL-10; A) and Th1 (IFN-γ; B) cytokine mRNAs was monitored by semiquantitative RT-PCR. After 96 h of stimulation, supernatants were assayed for Th1 and Th2 cytokines by ELISA (C). Data are mean ± SD of three different experiments; p < 0.001 vs MBP.
NaB enriches the Treg population in MBP-primed splenic T cells
There is increasing evidence that regulatory Tregs play vital roles in autoimmune diseases. A major population of Tregs is characterized by CD4+CD25+ T cells that also express a transcription factor, Foxp3. Because NaB suppressed the clinical symptoms of EAE in mice, we examined whether NaB had any effect on Tregs. Interestingly, our semiquantitative RT-PCR data show that NaB dose-dependently increased the expression of Foxp3 as well as CD25 in MBP-primed splenic T cells (Fig. 8A). In contrast, the expression of CD4 remained unchanged (Fig. 8A). FACS analysis also demonstrates that MBP-primed splenic T cells contained fewer numbers of CD4+CD25+ cells compared with normal splenic T cells (Fig. 8B). Consistent with the effect of NaB on mRNA expression of CD25 and Foxp3 in MBP-primed splenic T cells, this drug increased the population of CD4+CD25+ cells in MBP-primed T cells (Fig. 8B), suggesting that NaB increases the abundance of Tregs.
FIGURE 8.
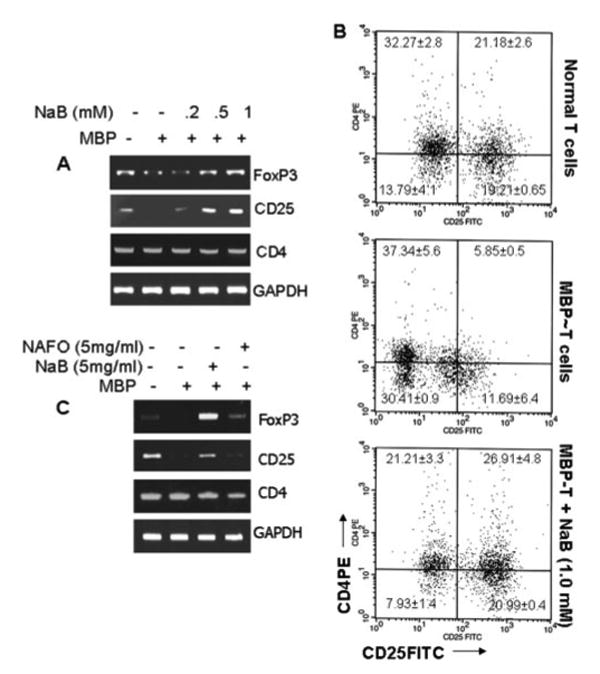
NaB enriches Treg character in MBP-primed T cells as well as in donor spleen. A, Normal, MBP-primed and NaB-treated, MBP-primed T cells were analyzed for Foxp3, CD25, and CD4 mRNAs by semiquantitative RT-PCR. B, Normal, MBP-primed, and NaB-treated MBP-primed T cells were incubated with appropriately diluted FITC-conjugated anti-CD25 and PE-conjugated anti-CD4 for 1 h followed by FACS analysis. Percentage of cells in various quadrants has been mentioned. C, Normal spleen, donor spleen and NaB- or NaFO-treated donor spleen were analyzed for Foxp3, CD25, and CD4 mRNAs by semiquantitative RT-PCR. Results represent three independent experiments.
NaB increases both Foxp3 and CD25 in the spleen of donor mice
Because NaB treatment of MBP-primed splenic T cells enriched the Treg population, we next examined whether NaB increased the Treg population in vivo. Therefore, donor mice were treated with NaB and NaFO from day 0 of immunization as mentioned above, and, on day 10 of immunization, the expression of Foxp3 and CD25 was analyzed in spleens. Significant up-regulation of both Foxp3 and CD25 was observed in the spleen of MBP-immunized donor mice that received drinking water containing NaB compared to donor mice receiving normal drinking water (Fig. 8C). In contrast, treatment of donor mice with NaFO had no effect on the splenic expression of Foxp3 and CD25 (Fig. 8C). As observed in MBP-primed splenic T cells, NaB had no effect on the expression of CD4 in vivo in the spleens of donor mice. Because Foxp3 and CD25 are well-known markers of CD4+ Tregs, we may conclude that NaB increases the abundance of Treg character in vivo as well.
Discussion
Although MS could be monophasic, majority of MS patients (>80%) experience relapsing-remitting symptoms. The RR-EAE is a widely used model for studying the disease process of RR-MS and, in particular, very much effective for examining novel therapeutic approaches against RR-MS. In our present study, we show the first evidence that NaB, a proven nontoxic compound and a widely used food preservative, inhibits the disease process of RR-EAE. Our conclusion is based on the following observations. First, adoptively transferred MBP-primed T cells were unable to induce clinical symptoms of EAE in female SJL/J mice receiving proper doses of NaB via drinking water. In contrast, MBP-primed T cells induced EAE in mice receiving drinking water containing NaFO, a NaB analog without the benzene ring, or normal drinking water. Second, NaB, but not NaFO, inhibited the progression of RR-EAE when administered via drinking water in either the early or late stage of the disease process. Third, adoptive transfer of MBP-primed T cells and NaFO-treated MBP-primed T cells, but not that of NaB-treated MBP-primed T cells, induced the clinical symptoms of EAE in female SJL/J mice. Fourth, treatment of donor mice with NaB, but not NaFO, also inhibited the generation of encephalitogenic T cells. Finally, clinical treatment of EAE animals with drinking water containing NaB was also capable of inhibiting the invasion of mononuclear cells into the spinal cord as well as the expression of inflammatory molecules (iNOS, IL-1_β_, IL-6) and restored myelination and the expression of myelin genes within the CNS.
Interaction of encephalitogenic T cells with BBB endothelium and entry of these cells into the CNS parenchyma are critical steps in the pathogenesis of EAE. It has been found that integrins like _α_4_β_1 (VLA-4) and _α_l_β_2 (LFA-1) on T cells bind to counterreceptor VCAM-1 and ICAM-1 on the endothelial cells and probably lead to the extravasation of lymphocytes (20, 21). Up-regulation of ICAM-1 and VCAM-1 was reported on cerebral vessels during EAE preceding the perivascular infiltration by lymphocytes and the onset of disease (22). Our studies have firmly shown that NaB down-regulates ICAM-1 and VCAM-1 expression in the CNS of EAE mice. Correspondingly, suppression of VLA-4 and LFA-1 integrins by NaB was also observed in MBP-primed T cells, as well as in the spleen of donor mice. These results suggest that NaB prevents adhesion and transendothelial migration of encephalitogenic lymphocytes by suppressing the expression of integrins and adhesion molecules. Similarly, the role of E- and P-selectins in the extravasation of T lymphocytes into the CNS is also evident when complete inhibition of rolling and arrest of Th1 cells in inflamed brain was observed with anti-E- and P-selectin Abs (23). Interestingly, NaB was also found to inhibit the expression of E- and P-selectins in the cerebellum as well as in the spinal cord of EAE mice.
CD4+ T cells secreting Th1 cytokines (e.g., IFN-γ) were more potent in transferring disease than other myelin-specific T cells (24). It has been shown that neuroantigen-primed T cells are mainly enriched in Th1 cells, and, after priming, they extravasate into the CNS and become further activated or polarized toward Th1 (16, 25, 26). However, T cells secreting Th2 cytokines (IL-4 and IL-10) appeared to ameliorate EAE. Taken together, the widely accepted concept is that a Th1 cell response to myelin Ag is destructive for EAE, whereas a Th2 response is protective (27). Consistent with this concept, in this study, we have demonstrated that NaB was effective in switching MBP-primed T cells from the Th1 to Th2 mode. Therefore, it suggests that, by inhibiting the activation and differentiation of Th1 cells, NaB might prevent the extravasation of neuroantigen-primed T cells into the CNS. Interestingly, VLA-4 and LFA-1 integrins are also involved to drive Th1 responses (28). Therefore, NaB-mediated down-regulation of VLA-4 and LFA-1 integrins might facilitate T cells to acquire Th2 phenotype.
Although autoreactive lymphocytes are normally deleted in the thymus, yet some self-reactive T cells are found in the periphery of normal healthy individuals (29). However, these T cells usually do not attack organs that produce self-Ags. Therefore, a state of self-tolerance is maintained in a normal individual. Recently, this has been shown to be mediated by Tregs, which are characterized by the expression of Foxp3, a transcription factor, and CD25 (IL-2R_α_-chain). Although there are many types of Tregs, CD4+CD25+ Tregs are known to be the key regulatory cell type that prevents the activation and differentiation of non-Tregs including auto-reactive T cells (30). In autoimmune diseases, autoreactive T cells somehow overcome the resistance provided by the Tregs and therefore undergo activation and proliferation. Interestingly, we found that NaB significantly up-regulated the expression of Foxp3 and CD25 and also the abundance of CD4+CD25+ Tregs, which were severely depleted in MBP-primed T cells. However, the expression of CD4 in MBP-primed T cells, as well as in the donor spleen, remained unchanged. Stimulation of Foxp3 and CD25 by NaB strongly suggest that this compound may exhibit its protective action in EAE by enriching CD4+CD25+ Tregs.
Theoretically, Tregs should suppress the proliferation of both Th1 and Th2 (31). However, in our study, NaB suppresses the Th1 response while stimulating the Th2 response. It is also possible as CD25+CD4+ Tregs may contribute to Th2 polarization. For example, McKee and Pearce (32) have demonstrated that CD25+CD4+ Tregs contribute to Th2 polarization during helminth infection by suppressing the development of the Th1 response. Similarly, Kohm et al. (33) have shown that supplementation of CD4+CD25+ Tregs by adoptive transfer before active and adoptive EAE induction significantly reduces the severity of clinical disease, potentially by promoting a disease-protective Th2 immune response. However, the underlying mechanisms are not known. Therefore, whether suppression of the Th1 response and stimulation of the Th2 response by NaB is a direct effect of NaB or an indirect effect via Tregs or both needs further study.
Although there is no effective therapy against MS, different forms of IFN-β have been currently used to treat this disease. However, NaB has several advantages over IFN-β. First, IFN-β has a number of side effects, including flu-like symptoms, menstrual disorders in women, decrease in neutrophil count and white blood cell count, increase in aspartate aminotransferase and alanine aminotransferase levels, and development of neutralizing Abs to IFN-β (34). However, NaB is fairly nontoxic and a widely used food preservative. It is a direct metabolite of cinnamic acid, found in the commonly used food spice cinnamon. In addition, this drug also has a long clinical history as a treatment for conditions associated with hyperammonemia, such as urea cycle disorders in children. Second, MS patients are treated with IFN-β through painful injections, which often lead to injection site reactions, such as skin necrosis. However, NaB can be taken through food and drinking water or milk, the least painful route. In addition, the human body is capable of metabolizing cinnamon to NaB. Third, IFN-β is costly, whereas NaB is very economical.
In summary, we have demonstrated that NaB, a commonly used food additive and a Food and Drug Administration-approved drug for urea cycle disorders, inhibits the expression of integrins on neuroantigen-primed T cells, switches the differentiation from Th1 to Th2, enriches Treg populations, and blocks the disease process of EAE, suggesting that this drug may be used for therapeutic intervention in MS.
Abbreviations used in this paper
MS
multiple sclerosis
Tregs
regulatory T cell
EAE
experimental allergic encephalomyelitis
NaB
sodium benzoate
RR-EAE
relapsing-remitting model of EAE
NaFO
sodium formate
dpt
days post-transfer
BBB
blood-brain barrier
MOG
myelin oligodendrocyte glycoprotein
iNOS
inducible NO synthase
PLP
proteolipid protein
CNPase
2′,3′-cyclic nucleotide 3-phosphodiesterase
Footnotes
This study was supported by grants from the National Multiple Sclerosis Society (RG3422A1/1) and National Institutes of Health (NS39940 and NS48923).
Disclosures
The authors have no financial conflict of interest.
References
- 1.Martin R, McFarland HF, McFarlin DE. Immunological aspects of demyelinating diseases. Annu Rev Immunol. 1992;10:153–187. doi: 10.1146/annurev.iy.10.040192.001101. [DOI] [PubMed] [Google Scholar]
- 2.Walsh PT, Taylor DK, Turka LA. Tregs and transplantation tolerance. J Clin Invest. 2004;114:1398–1403. doi: 10.1172/JCI23238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benveniste EN. Role of macrophages/microglia in multiple sclerosis and experimental allergic encephalomyelitis. J Mol Med. 1997;75:165–173. doi: 10.1007/s001090050101. [DOI] [PubMed] [Google Scholar]
- 4.Du C, Khalil MW, Sriram S. Administration of dehydroepiandro-steronesuppresses experimental allergic encephalomyelitis in SJL/J mice. J Immunol. 2001;167:7094–7101. doi: 10.4049/jimmunol.167.12.7094. [DOI] [PubMed] [Google Scholar]
- 5.Parkinson JF, Mitrovic B, Merrill JE. The role of nitric oxide in multiple sclerosis. J Mol Med. 1997;75:174–186. doi: 10.1007/s001090050102. [DOI] [PubMed] [Google Scholar]
- 6.Pahan K, Schmid M. Activation of nuclear factor-κB in the spinal cord of experimental allergic encephalomyelitis. Neurosci Lett. 2000;287:17–20. doi: 10.1016/s0304-3940(00)01167-8. [DOI] [PubMed] [Google Scholar]
- 7.Bruck W, Stadelmann C. Inflammation and degeneration in multiple sclerosis. Neurol Sci. 2003;24 5:S265–S267. doi: 10.1007/s10072-003-0170-7. [DOI] [PubMed] [Google Scholar]
- 8.Becher B, Bechmann I, Greter M. Antigen presentation in autoimmun and CNS inflammation: how T lymphocytes recognize the brain. J Mol Med. 2006;84:532–543. doi: 10.1007/s00109-006-0065-1. [DOI] [PubMed] [Google Scholar]
- 9.Scaglia F, Carter S, O'Brien WE, Lee B. Effect of alternative pathway therapy on branched chain amino acid metabolism in urea cycle disorder patients. Mol Genet Metab. 2004;81 1:S79–S85. doi: 10.1016/j.ymgme.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 10.Leonard JV, Morris AA. Urea cycle disorders. Semin Neonatol. 2002;7:27–35. doi: 10.1053/siny.2001.0085. [DOI] [PubMed] [Google Scholar]
- 11.Nair B. Final report on the safety assessment of benzyl alcohol, benzoic acid, and sodium benzoate. Int J Toxicol. 2001;20 3:23–50. doi: 10.1080/10915810152630729. [DOI] [PubMed] [Google Scholar]
- 12.Toth B. Lack of tumorigenicity of sodium benzoate in mice. Fundam Appl Toxicol. 1984;4:494–496. doi: 10.1016/0272-0590(84)90208-2. [DOI] [PubMed] [Google Scholar]
- 13.Bridges JW, French MR, Smith RL, Williams RT. The fate of benzoic acid in various species. Biochem J. 1970;118:47–51. doi: 10.1042/bj1180047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kubota K, Ishizaki T. Dose-dependent pharmacokinetics of benzoic acid following oral administration of sodium benzoate to humans. Eur J Clin Pharmacol. 1991;41:363–368. doi: 10.1007/BF00314969. [DOI] [PubMed] [Google Scholar]
- 15.Abd El-Mawla AM, Schmidt W, Beerhues L. Cinnamic acid is a precursor of benzoic acids in cell cultures of Hypericum androsaemum L. but not in cell cultures of Centaurium erythraea RAFN. Planta. 2001;212:288–293. doi: 10.1007/s004250000394. [DOI] [PubMed] [Google Scholar]
- 16.Dasgupta S, Jana M, Zhou Y, Fung YK, Ghosh S, Pahan K. Antineuroinflammatory effect of NF-κB essential modifier-binding domain peptides in the adoptive transfer model of experimental allergic encephalomyelitis. J Immunol. 2004;173:1344–1354. doi: 10.4049/jimmunol.173.2.1344. [DOI] [PubMed] [Google Scholar]
- 17.Dasgupta S, Zhou Y, Jana M, Banik NL, Pahan K. Sodium phenylacetate inhibits adoptive transfer of experimental allergic encephalomyelitis in SJL/J mice at multiple steps. J Immunol. 2003;170:3874–3882. doi: 10.4049/jimmunol.170.7.3874. [DOI] [PubMed] [Google Scholar]
- 18.Dasgupta S, Jana M, Liu X, Pahan K. Myelin basic protein-primed T cells induce nitric oxide synthase in microglial cells: implications for multiple sclerosis. J Biol Chem. 2002;277:39327–39333. doi: 10.1074/jbc.M111841200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roy A, Fung YK, Liu X, Pahan K. Up-regulation of microglial CD11b expression by nitric oxide. J Biol Chem. 2006;281:14971–14980. doi: 10.1074/jbc.M600236200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vajkoczy P, Laschinger M, Engelhardt B. α4-integrin-VCAM-1 binding mediates G protein-independent capture of encephalitogenic T cell blasts to CNS white matter microvessels. J Clin Invest. 2001;108:557–565. doi: 10.1172/JCI12440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Von Andrian UH, Mackay CR. T-cell function and migration: two sides of the same coin. N Engl J Med. 2000;343:1020–1034. doi: 10.1056/NEJM200010053431407. [DOI] [PubMed] [Google Scholar]
- 22.Steffen BJ, Butcher EC, Engelhardt B. Evidence for involvement of ICAM-1 and VCAM-1 in lymphocyte interaction with endothelium in experimental autoimmune encephalomyelitis in the central nervous system in the SJL/J mouse. Am J Pathol. 1994;145:189–201. [PMC free article] [PubMed] [Google Scholar]
- 23.Piccio L, Rossi B, Colantonio L, Grenningloh R, Gho A, Ottoboni L, Homeister JW, Scarpini E, Martinello M, Laudanna C, et al. Efficient recruitment of lymphocytes in inflamed brain venules requires expression of cutaneous lymphocyte antigen and fucosyltransferase-VII. J Immunol. 2005;174:5805–5813. doi: 10.4049/jimmunol.174.9.5805. [DOI] [PubMed] [Google Scholar]
- 24.Zamvil SS, Steinman L. The T lymphocyte in experimental allergic encephalomyelitis. Annu Rev Immunol. 1990;8:579–621. doi: 10.1146/annurev.iy.08.040190.003051. [DOI] [PubMed] [Google Scholar]
- 25.Liu H, MacKenzie-Graham AJ, Palaszynski K, Liva S, Voskuhl RR. “Classic” myelin basic proteins are expressed in lymphoid tissue macrophages. J Neuroimmunol. 2001;116:83–93. doi: 10.1016/s0165-5728(01)00284-3. [DOI] [PubMed] [Google Scholar]
- 26.Martino G, Hartung HP. Immunopathogenesis of multiple sclerosis: the role of T cells. Curr Opin Neurol. 1999;12:309–321. doi: 10.1097/00019052-199906000-00010. [DOI] [PubMed] [Google Scholar]
- 27.Steinman L. Multiple sclerosis: a coordinated immunological attack against myelin in the central nervous system. Cell. 1996;85:299–302. doi: 10.1016/s0092-8674(00)81107-1. [DOI] [PubMed] [Google Scholar]
- 28.Mittelbrunn M, Molina A, Escribese MM, Yanez-Mo M, Escudero E, Ursa A, Tejedor R, Mampaso F, Sanchez-Madrid F. VLA-4 integrin concentrates at the peripheral supramolecular activation complex of the immune synapse and drives T helper 1 responses. Proc Natl Acad Sci USA. 2004;101:11058–11063. doi: 10.1073/pnas.0307927101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coffer PJ, Burgering BM. Forkhead-box transcription factors and their role in the immune system. Nat Rev Immunol. 2004;4:889–899. doi: 10.1038/nri1488. [DOI] [PubMed] [Google Scholar]
- 30.O'Garra A, Vieira PL, Vieira P, Goldfeld AE. IL-10-producing and naturally occurring CD4+ Tregs: limiting collateral damage. J Clin Invest. 2004;114:1372–1378. doi: 10.1172/JCI23215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu D, Liu H, Komai-Koma M, Campbell C, McSharry C, Alexander J, Liew FY. CD4+CD25+ regulatory T cells suppress differentiation and functions of Th1 and Th2 cells, Leishmania major infection, and colitis in mice. J Immunol. 2003;170:394–399. doi: 10.4049/jimmunol.170.1.394. [DOI] [PubMed] [Google Scholar]
- 32.McKee AS, Pearce EJ. CD25+CD4+ cells contribute to Th2 polarization during helminth infection by suppressing Th1 response development. J Immunol. 2004;173:1224–1231. doi: 10.4049/jimmunol.173.2.1224. [DOI] [PubMed] [Google Scholar]
- 33.Kohm AP, Carpentier PA, Anger HA, Miller SD. Cutting edge: CD4+CD25+ regulatory T cells suppress antigen-specific autoreactive immune responses and central nervous system inflammation during active experimental autoimmune encephalomyelitis. J Immunol. 2002;169:4712–4716. doi: 10.4049/jimmunol.169.9.4712. [DOI] [PubMed] [Google Scholar]
- 34.Miller A. Current and investigational therapies used to alter the course of disease in multiple sclerosis. South Med J. 1997;90:367–375. doi: 10.1097/00007611-199704000-00001. [DOI] [PubMed] [Google Scholar]