Sex Differences in Medial and Lateral Orbitofrontal Cortex Hypoperfusion in Cocaine-Dependent Men and Women (original) (raw)
. Author manuscript; available in PMC: 2007 Sep 20.
Abstract
Background
The different clinical trajectories of cocaine-dependent men and women may be a consequence of distinct neurobiological substrates. Hypoperfusion of the orbitofrontal cortex (OFC) has previously been reported in individuals addicted to cocaine and has been posited as a biological mediator of relapse due to impulsivity or impaired decision making.
Objective
This study assessed regional cerebral blood flow (rCBF) between abstinent cocaine-dependent men and women and sex-matched healthy controls.
Methods
Cocaine-dependent subjects were abstinent from cocaine for 11 to 28 days and had no other major mental health or substance use disorders. rCBF was assessed with single photon emission computed tomography after administration of a placebo saline infusion. A resting scan was also obtained in a subset of cocaine-dependent and control men.
Results
In the 35 cocaine-dependent and 37 healthy control subjects examined, a sex-by-group effect was observed for the left lateral (P = 0.001), right lateral (P = 0.002), and medial (P < 0.02) OFC. Cocaine-dependent men demonstrated significantly lower right and left lateral, but not medial, OFC rCBF compared with sex-matched healthy controls after placebo infusion (P ≤ 0.001). Similar bilateral OFC decreases were observed in male cocaine-dependent subjects at rest. In contrast, cocaine-dependent women showed lower rCBF in the medial, but not lateral, OFC relative to sex-matched healthy controls after placebo infusion (P < 0.01). Male cocaine-dependent subjects also showed decreased rCBF (P < 0.01) in the bilateral anterolateral temporal cortex and anterior cingulate, whereas decreased rCBF was observed in female cocaine-dependent subjects in the bilateral superior frontal gyri. Large and diffuse areas of increased rCBF were observed after placebo infusion in cocaine-dependent men, but not in women, relative to sex-matched healthy controls.
Conclusions
rCBF appears to be reduced in the bilateral OFC in cocaine-dependent men and in the medial OFC in cocaine-dependent women. Sex differences in the medial and lateral OFC rCBF may be relevant to understanding relapse characteristics differentiating men and women addicted to cocaine.
Keywords: cocaine-related disorders, female, gender, orbitofrontal cortex, single-photon emission-computed tomography
INTRODUCTION
Epidemiologic, etiologic, and clinical differences have been observed between men and women suffering from cocaine addiction. Women are less likely to be diagnosed with cocaine use disorders1 and tend to initiate use of cocaine at an older age than do men.2,3 Women dependent on cocaine express more craving when exposed to cocaine cues,4,5 and experience a heightened vulnerability to the physical, psychiatric, and social consequences of substance use.6 In light of the growing literature demonstrating discrete differences in the brains of men and women, neurobiological substrates may underlie the different clinical trajectories of men and women with addictive disorders.7,8 In nonaddicted subjects, for example, investigators have reported sex differences in brain structure,9 basal measures of brain functioning,10,11 and functional measures of neural responding.12 Several studies have now documented basal neural differences in cocaine-dependent men and women, including a relative absence of cerebral perfusion defects,13 less frontal cortex neuronal loss,14 and better white-matter blood flow15 in women relative to men. Women dependent on cocaine also differ from similarly dependent men in their neural response to cue-induced craving16 and stress.17
One of the most consistent findings in substance use disorders, and particularly in cocaine-dependent individuals, is reduced activity of the orbitofrontal cortex (OFC) in the addicted population.18–20 The OFC assesses the motivational value of prospective rewards, assuring that stimuli most advantageous to survival are chosen and that stimuli most detrimental to survival are avoided. Persons with damaged cortex in the region of the OFC tend to make both rapid and irresponsible decisions, although memory, learning, language, and attention are preserved. In the presence of such lesions, established behaviors are not altered in response to a reversal of reward contingencies21 and decision making does not appropriately reflect long-term consequences.22
Volkow et al23–25 have reported that men addicted to cocaine, methamphetamine, or alcohol exhibit decreased energy utilization in the OFC, suggesting that the heightened impulsivity and impaired decision making observed in the addicted person may be a consequence of attenuated OFC activity.18–20 Alterations in OFC energy utilization or regional cerebral blood flow (rCBF) have also been reported in individuals with substance use disorders during decision-making tasks.26,27 Consistent with Volkow et al,24 we have observed a decrease in right and left lateral OFC rCBF after saline infusion in 10 cocaine-dependent men relative to sex-matched healthy controls.28 Lower medial29,30 and right lateral30 OFC gray-matter tissue density has also been observed in cocaine-dependent individuals relative to healthy controls. However, other investigators have not reported a similar perfusion deficit in this addicted population.31,32 Because decreased perfusion of the OFC is frequently cited as the biological underpinning of the impulsiveness and impaired decision making ubiquitous in addicted individuals, confirmation of this finding is essential.
In a previous study using single photon emission computed tomography (SPECT) and t-image analytic techniques, we reported that the lateral OFC deficits observed in cocaine-dependent male subjects28 did not extend to the female subjects.33 Because the presence or absence of OFC deficits in female cocaine-dependent subjects may have important clinical implications, we further assessed potential sex differences in OFC rCBF in cocaine-dependent subjects by directly comparing the OFC rCBF response after saline infusion in an expanded pool of male and female cocaine-only addicted subjects and sex- and age-similar healthy controls using statistical parametric mapping (SPM). Our saline infusion data were derived from a single-blind study designed to compare procaine-induced rCBF changes to placebo (saline). As procaine can be anxiogenic, we further considered that the attenuated OFC rCBF after saline infusion in our previous report of male subjects might have reflected group differences in the anticipation of a possible procaine administration. A third additional SPECT scan was therefore also obtained in a subset of male cocaine-dependent and control subjects while at rest.
SUBJECTS AND METHODS
Subjects
Twenty-eight cocaine-dependent men, 10 cocaine-dependent women, 18 healthy male controls, and 19 healthy female controls were studied. Three cocaine-dependent men were excluded owing to methodologic problems during scanning. Subjects were 24 to 45 years old (per study criteria), and underwent a medical history and physical examination, the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) Structured Clinical Interview,34 clinical laboratory tests, urine drug screen, electrocardiogram, and clinical magnetic resonance imaging (MRI). Lifetime cocaine and other substance use history was obtained from cocaine-dependent subjects using the Timeline Follow-Back interview.35 After complete description of the study to the subjects, written informed consent was obtained. Subjects were financially compensated for their participation. Approval for the study was obtained from the institutional review board of the University of Texas Southwestern Medical Center and the VA North Texas Health Care System, both in Dallas, Texas.
Cocaine-dependent subjects were recruited from persons requesting treatment for cocaine dependence in Dallas, Texas, at the VA Medical Center, Homeward Bound, Inc., and The Salvation Army. Addicted subjects were hospitalized as soon as possible after their last reported use of cocaine and remained in a structured residential unit until the study was completed. Exclusion criteria included present use of any central nervous system (CNS) active medications; a lifetime history for affective, anxiety, or schizophrenic disorder or organic brain syndrome experienced before the onset of a substance abuse diagnosis or after a period of at least 3 months’ abstinence; a substance use disorder (other than cocaine or nicotine) within the previous 12 months; or a lifetime history of withdrawal from alcohol, sedative-hypnotics, or opioids. All women were premenopausal, and a negative pregnancy test was obtained in all female subjects before SPECT scanning.
Exclusion criteria for healthy controls included the medical criteria as noted for the cocaine-dependent subjects, as well as a lifetime history of any other DSM-IV Axis I disorder (except nicotine or caffeine abuse or dependence). Healthy controls with a first-degree relative with an Axis I disorder or ≥2 second-degree relatives with a substance use disorder were also excluded.
Study Sessions
Study sessions were held at the Nuclear Medicine Center at the University of Texas Southwestern Medical Center. Cocaine-dependent men and women were both studied at a mean time point of 3 weeks’ abstinence (range, 11–28 days) at the time of the saline study. Saline administration was conducted as part of a study of the effects of procaine on rCBF. Every subject received both a saline and a procaine infusion. Saline administration was performed at ~12:00 PM and procaine was administered 48 hours later.28 Due to the strong subjective response to procaine, a fixed-order, single-blind design was employed, with saline always administered on the first study day. (The findings from the procaine scan performed on the second study day are not reported here.) After 30 minutes of rest following IV catheter insertion, a Symptom Checklist 90-R (SCL 90-R)36 or Brief Symptom Inventory (BSI)37 was obtained (basal measure). Two minutes later, saline was administered over 2 minutes followed by 20 mCi of technetium 99m hexamethyl-propyleneamine-oxime (99mTc-HMPAO) (Nycomed/Amersham, Princeton, New Jersey) over 30 seconds and 10-mL saline flush over 30 seconds. Infusion took place behind a curtain, and subjects were not aware of the exact time of the infusion. Four minutes after completion of the final infusion, the SCL 90-R/BSI was repeated. The SPECT scan was obtained 90 minutes after saline infusion. This perfusion image represents rCBF at the time of radiotracer administration and not at the time of the scan.
The SCL 90-R was used with the first 30 subjects, and the BSI for all subsequent subjects. The BSI uses 53 of the 90 questions in the SLC 90-R, shortening the time of questioning. Both questionnaires assess the same 9 dimensions plus 3 summary measures of symptom severity: positive symptom distress index (PSDI, severity of response); positive symptom total (PST, frequency of response); and global severity index (GSI, severity by frequency of response). To allow the joint analyses of both questionnaires in our study, we extracted the 53 BSI questions from the subjects who received the SCL 90-R. Raw nontransformed scores were used because our time frame of questioning has not been empirically validated.
We considered that the rCBF response after saline infusion could reflect the anticipation of possibly receiving procaine. A third “at rest” study was therefore obtained in a subset of subjects. The paradigm was exactly the same as that described for the saline infusion, except that subjects were informed that no medication would be administered. Fourteen addicted subjects (13 men, 1 woman) and 15 controls (10 men, 5 women) participated in the resting scan. Because only 1 addicted female subject was included in this third scan, only the results for the male subjects are reported. The third scan was obtained 5 to 7 days after the procaine scan in 11 addicted male subjects and 5 male controls. Five of the controls returned for a third scan between 8 and 70 months after their saline scan. Two addicted men were restudied 3 to 4 years after their saline scan when they were readmitted for cocaine use. These 2 men were 5 to 10 weeks abstinent at the time of their resting scan.
SPECT Imaging
SPECT images were acquired with a PRISM 3000S 3-headed SPECT camera (Picker International, Cleveland, Ohio) and using ultra high-resolution fan-beam collimators (reconstructed resolution of 6.5 mm) in a 128 × 128 matrix in 3° increments. 20mCi of 99mTc-HMPAO was administered for each scan, and total scan duration was 20 minutes. Image reconstruction was performed in the transverse domain using back-projection with a ramp filter. Voxels in reconstructed images were 1.9 mm3. Reconstructed images were smoothed with a fourth-order Butterworth 3-dimensional filter, and attenuation was corrected using a Chang first-order method with ellipse size adjusted for each slice.
Statistical Analysis
Demographic and Clinical Data
Data were analyzed with Statistical Package for the Social Sciences (SPSS) software, version 13.0 (SPSS Inc., Chicago, Illinois). Parametric statistics were used to examine the demographic characteristics of the sample and the substance use metrics of the addicted groups. Nominal data were analyzed via χ2 crosstabs analysis.
Image Analysis
Image analyses comprised 3 different activities: normalization, coregistration, and subtraction. SPECT images were resliced to 2 mm3 voxels, coregistered to Talairach space,38 and normalized to whole brain counts. Analyses of change in rCBF were measured relative to global cerebral blood flow. Thus, higher rCBF in the control group relative to the addicted group may reflect higher rCBF in the control group, lower rCBF in the addicted group, or some of both. Images were smoothed from their original resolution of 6.5 mm to a final resolution of 10 mm. Areas differing between addicted subjects and controls were overlaid on an MRI model in Talairach space. Voxel-wise analyses of saline-induced effects on rCBF were analyzed using SPM2 (Wellcome Department of Cognitive Neurology, University College, London, England) with a statistical threshold of P < 0.01 for voxel z scores and a cluster size no smaller than 50 voxels. Mean relative rCBF in 18 selected clusters was exported to SPSS to examine sex-by-group differences and the regions of interest (ROIs) relationships with subjective measures.
The selected clusters were chosen based on our a priori hypothesis, their relevance to the addictive disorders, and/or their prominence in the SPM analysis. Because of the numerous regions of increased rCBF in the cocaine-dependent men relative to the male controls (see “Results” section), we selected areas for ROI analysis from this comparison based on a concept proposed by Gusnard et al.39 They posit that the “resting” brain is involved in a number of mental processes, including self-referential attention and the awareness of one’s own physiological state and environmental milieu. Based primarily on findings from neuroimaging studies, Gusnard et al suggest these areas of basal activity include the dorsal medial, ventral medial, posterior medial, and posterior lateral cortices. Three of these areas—the posterior cingulate (representing the posterior medial cortex), the mesial OFC (representing the ventral medial cortex), and the right mesial aspect of the superior frontal gyrus and left middle frontal gyrus (representing the dorsal medial cortex)—were observed in our male comparison and therefore selected for ROI analysis. The right caudate was chosen from this comparison because of its significance in the addictive disorders.
Cluster Analysis
To explore sex-by-group (control and cocaine-dependent) differences, cluster boundaries for specific ROIs identified by the SPM comparisons were identified and the mean counts per voxel within each cluster were determined for each subject. Because of the high degree of intercorrelation between several of the selected ROIs (data not shown), a factor analysis with varimax rotation and Kaiser normalization was calculated across these regions with the goal of data reduction. The resultant rCBF factors were summarized by composite regression factors. Items were included with factor loadings of ≥0.5.
rCBF Response to Saline
A 1-way analysis of variance was conducted between the 4 groups (addicted male subjects, male controls, addicted female subjects, female controls) for the 18 identified ROIs. Significance was assessed at a Bonferroni adjusted α of 0.003 (0.05/18). Post hoc paired comparisons were made using the Tukey test, with P values of 0.05 considered significant. An analysis of covariance (ANCOVA) was performed to account for the effects of age.
Subjective Responses to Saline
Nonparametric methods were employed because the BSI data did not meet the assumptions of normality and homoscedasticity. The Mann-Whitney U test of independent samples was employed to assess between-group (ie, addicted male subjects vs male controls) differences on the 9 BSI domains and 3 summary factors. Comparisons were performed for basal and net BSI change (BSI after saline – basal BSI) scores. Within-group (all subjects, males vs females, and sex-by-group) comparisons of BSI scores before and after saline infusion were conducted with the Wilcoxon signed rank test. To control for the type I error rate, significance was assessed at a Bonferroni adjusted α of 0.004 (0.05/12).
Correlational Analysis
A Spearman rank correlation was conducted between the 5 analytically derived factors and 12 BSI scores, using a Bonferroni adjusted α of 0.0008 (0.05/60). Correlations between the 5 ROI factors and 4 measures of drug use history were performed with a Pearson product moment correlation, using a Bonferroni adjusted α of 0.0025 (0.05/20).
RESULTS
Demographic and Clinical Characteristics
The cocaine-dependent men and women in this study were more likely to be African American than were controls (Table I). Addicted men were significantly older than male controls (_t_41 = 3.60; P < 0.001), although the mean age difference between the 2 groups was only 6 years. Male and female cocaine-dependent subjects revealed no significant differences in recent and lifetime cocaine use, although women reported significantly more “days used” in the 90 days preceding treatment (P = 0.007). Lifetime nicotine consumption was greater for cocaine-dependent subjects than for controls. Nicotine use in the female cocaine-dependent group was skewed by 1 woman who reported 90 pack-years of smoking.
Table I.
Demographic and clinical characteristics of male and female cocaine-dependent individuals and healthy controls by subject group.
| Test Statistic | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Male Control (n = 18) | Male Cocaine (n = 25) | Female Control (n = 19) | Female Cocaine (n = 10) | F | t | χ2 | DF | P |
| Age, mean (SD), y | 33 (7) | 39 (4) | 33 (7) | 36 (7) | 5.4 | 3,68 | 0.002 | ||
| Race, no. (%) | 29.3 | 9 | <0.001 | ||||||
| White | 11 (61) | 4 (16) | 11 (58) | 3 (30) | |||||
| Black | 2 (11) | 20 (80) | 4 (21) | 7 (70) | |||||
| Hispanic | 3 (17) | 1 (4) | 3 (16) | 0 | |||||
| Asian | 2 (11) | 0 | 1 (5) | 0 | |||||
| Cigarette pack-years, mean (SD) | 0.04 (0.2) | 9 (9) | 0.1 (0.3) | 21 (30) | 9.17 | 3,66 | <0.001 | ||
| Cocaine use, mean (SD) | |||||||||
| Days used in 90 days | 46 (27) | 75 (25) | 2.88 | 32 | 0.007 | ||||
| Years used | 13 (5) | 10 (8) | 1.46 | 32 | 0.15 | ||||
| Dollars spent—lifetime | 212,000(212,000 (212,000(411,000) | 225,000(225,000 (225,000(305,000) | |||||||
| Days used—lifetime | 1714 (1438) | 1945 (1828) | 0.40 | 33 | 0.69 | ||||
| Days abstinent at testing | 19 (4) | 20 (4) | 0.59 | 33 | 0.56 |
rCBF Response to Saline
Male Controls Versus Male Cocaine-Dependent Subjects
The rCBF response after saline infusion was compared between sex-matched cocaine-dependent and control subjects (P < 0.01) (Figure 1). Regions of difference are described by cluster size and uncorrected z scores. Cocaine-dependent men showed a decrease in rCBF, relative to male controls, in the right lateral OFC and anterolateral temporal cortex (cluster size = 1337; z = 5.04; P < 0.001) and left lateral OFC and anterolateral temporal cortex (cluster size = 877; z = 4.17; P < 0.001) (Figures 1 and 2). Addicted male subjects also revealed a decrease in the rostral anterior cingulate rCBF compared with male controls (cluster size = 231; z = 3.50; P < 0.001).
Figure 1.
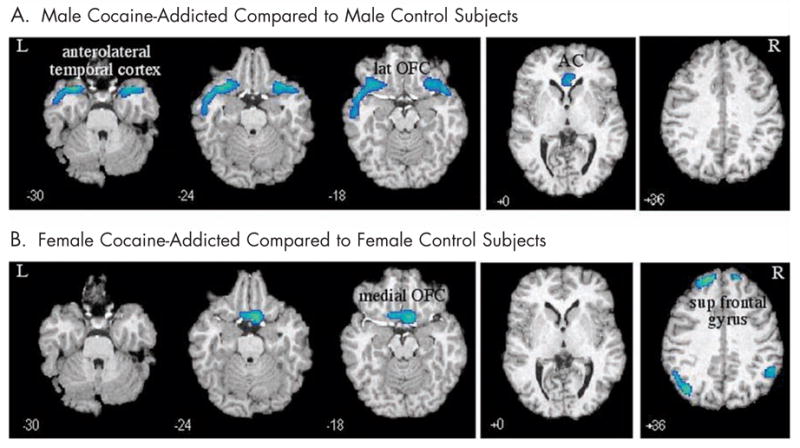
Decreases in regional cerebral blood flow in (A) male and (B) female cocaine-dependent subjects compared with healthy sex-matched controls. Talairach coordinate (z-axis) noted. L = left; lat = lateral; OFC = orbitofrontal cortex; AC = anterior cingulate; R = right; sup = superior.
Figure 2.
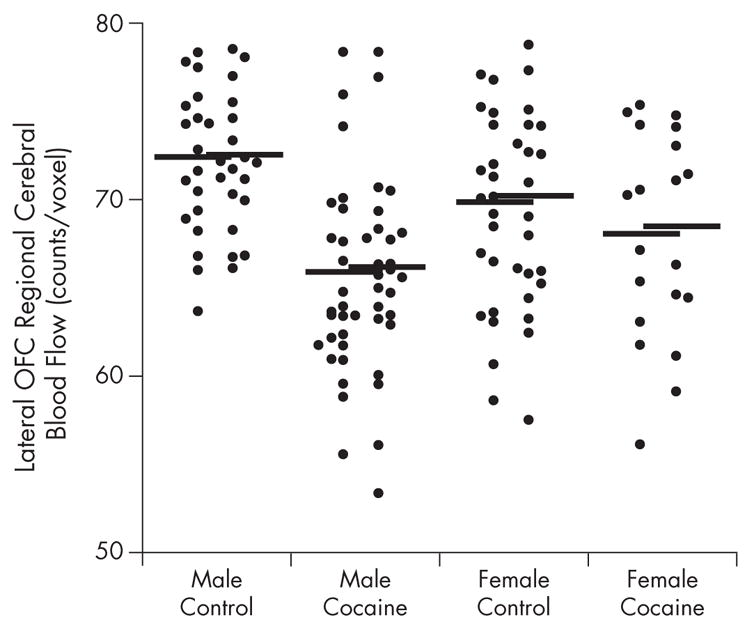
Counts per voxel in right and left lateral orbitofrontal cortex (OFC) of male and female cocaine-dependent and healthy control subjects. Horizontal lines denote mean for group. Left column represents counts of left lateral OFC; right column represents counts of right lateral OFC.
In contrast to the relatively isolated regions of decreased rCBF, cocaine-dependent subjects showed large and diffuse areas of increased rCBF relative to controls (Figure 3). These regions included the posterior cingulate, left and right superior frontal gyrus, middle frontal gyrus, medial OFC, cerebellum, right thalamus, and a large area on the left incorporating the left insula, Brodman area (BA) 41, thalamus, parahippo-campus, and putamen.
Figure 3.
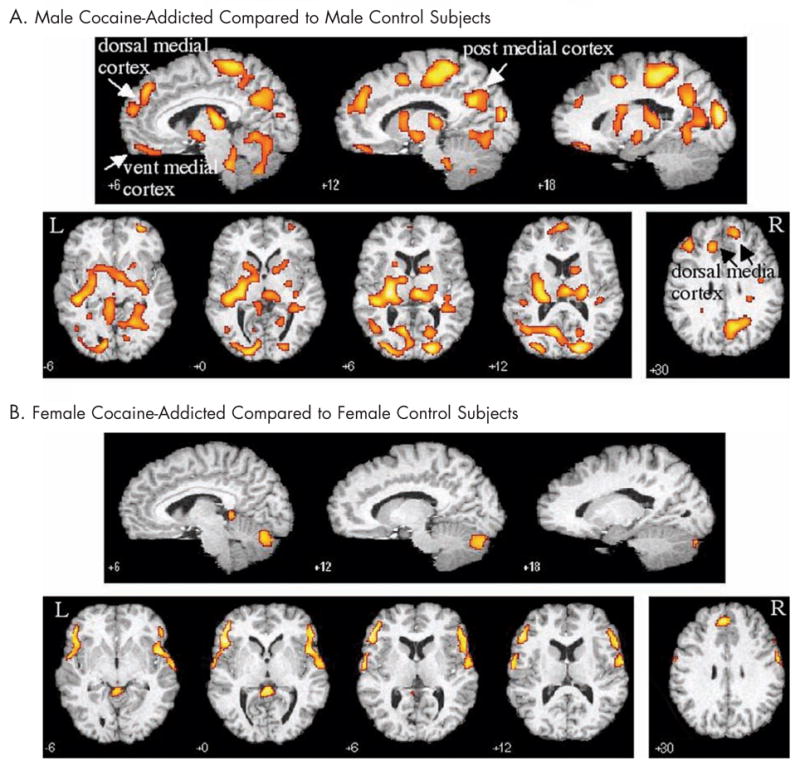
Increases in regional cerebral blood flow in (A) male and (B) female cocaine-dependent subjects relative to healthy sex-matched controls. Talairach coordinate (z-axis for transverse, y-axis for sagittal) noted. Post = posterior; vent = ventral; L = left; R = right.
Female Controls Versus Female Cocaine-Dependent Subjects
Female cocaine-dependent subjects showed decreases (Figures 1 and 4) relative to sex-matched controls, in the medial OFC (primarily BA 11) (cluster size = 410; z = 3.67; P < 0.001), right (cluster size = 410; z = 3.67; P < 0.001) and left (cluster size = 409; z = 3.67; P < 0.001) superior frontal gyrus, and the right (cluster size = 201; z = 2.88; P < 0.002) and left (cluster size = 201; z = 3.38; P < 0.001) parietal cortex.
Figure 4.
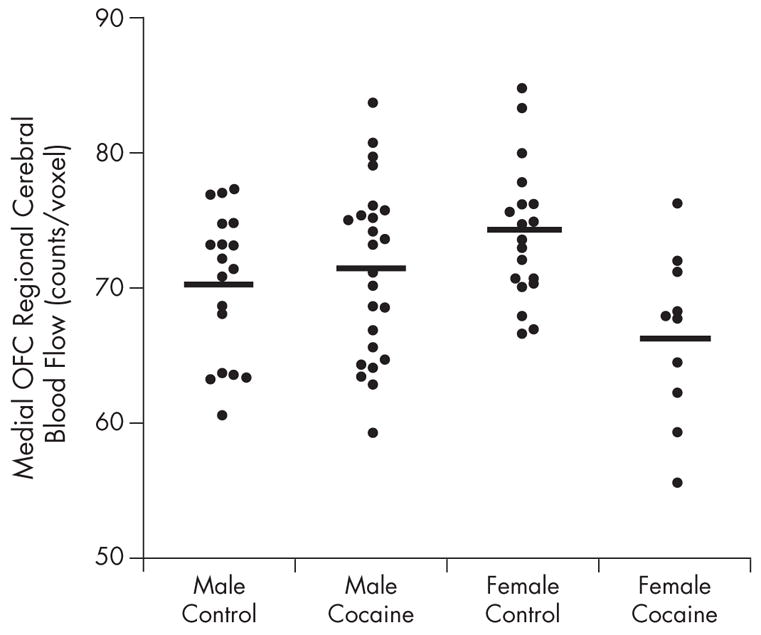
Counts per voxel in the medial orbitofrontal cortex (OFC) of male and female cocaine-dependent and healthy control subjects. Horizontal lines denote mean for group.
Increases in rCBF after saline infusion in the addicted subjects relative to the control female group were isolated and circumscribed compared with that of males (Figure 3). Relative increases in rCBF were observed in a region comprising the right middle frontal gyrus and extending into the superior aspect of the anterolateral temporal cortex (cluster size = 1538; z = 3.55; P < 0.001) and a similar region on the left (cluster size = 1334; z = 3.71; P < 0.001), anterior cingulate (cluster size = 218; z = 3.35; P < 0.001), left postcentral gyrus (cluster size = 156; z = 2.81; P < 0.002), and several regions in the cerebellum.
ROI Analyses
Eighteen ROIs were selected to assess sex-by-group comparisons (see “Methods” section) (Tables II and III). Differences were observed between all clusters, although 5 regions did not meet our more rigorous Bonferroni adjusted α of 0.003. Post hoc analyses demonstrated expected differences in the male and female group comparisons in all regions, and in no case did a cluster identified in the male comparison differ between females and visa versa. Of particular relevance to our a priori hypothesis was the decreased rCBF in right and left lateral OFC in the male cocaine-dependent subjects (P ≤ 0.001), but not the female cocaine-dependent subjects, and decreased rCBF in the medial OFC in the addicted female subjects (P < 0.02), but not in the addicted male subjects. Male controls and female controls did not significantly differ in any of the selected ROIs. Because the cocaine-dependent men were significantly older than the male controls, an ANCOVA was performed between the 10 selected male ROIs. The right and left lateral OFC and anterolateral temporal cortices, as well as the right posterior cingulate, remained different at P < 0.005. The other 5 regions were significant at P < 0.05. To assess the role of cigarette use on the results, correlations of the selected clusters with cigarette pack-years were performed within both the male and female cocaine-dependent groups. There were no significant correlations between pack-years and cluster rCBF.
Table II.
Areas of decreased regional cerebral blood flow after saline administration in male and female cocaine-dependent and healthy control subjects (mean [SD] counts per voxel).
| One-Way ANOVA | Post Hoc Comparisons by Sex* | |||||||
|---|---|---|---|---|---|---|---|---|
| Region of Interest | Male Control (n = 18) | Male Cocaine (n = 25) | Female Control (n = 19) | Female Cocaine (n = 10) | F (3,68) | P | Male Control vs Cocaine | Female Control vs Cocaine |
| Decrease in male cocaine-dependent subjects relative to male controls (Figure 1A) | ||||||||
| L lateral OFC | 72 (4) | 65 (5) | 69 (5) | 68 (6) | 6.1 | 0.001 | <0.001 | 0.93 |
| R lateral OFC | 72 (4) | 66 (5) | 69 (6) | 68 (6) | 5.4 | 0.002 | 0.001 | 0.92 |
| L anterolateral temporal cortex | 71 (4) | 65 (4) | 70 (4) | 71 (4) | 10.0 | <0.001 | <0.001 | 0.99 |
| R anterolateral temporal cortex | 69 (3) | 64 (5) | 67 (4) | 66 (5) | 7.5 | <0.001 | <0.001 | 1.0 |
| Anterior cingulate | 72 (6) | 66 (6) | 70 (4) | 70 (7) | 4.8 | 0.004 | 0.004 | 1.0 |
| Decrease in female cocaine-dependent subjects relative to female controls (Figure 1B) | ||||||||
| Medial OFC | 70 (5) | 71 (6) | 74 (5) | 66 (6) | 3.6 | 0.017 | 0.99 | 0.01 |
| L superior frontal gyrus | 63 (4) | 66 (4) | 65 (4) | 59 (3) | 7.1 | <0.001 | 0.13 | 0.004 |
| R superior frontal gyrus | 62 (3) | 64 (4) | 6 (4) | 58 (3) | 5.8 | 0.001 | 0.74 | 0.001 |
Table III.
Areas of increased regional cerebral blood flow after saline administration in male and female cocaine-dependent and healthy control subjects (mean [SD] counts per voxel).
| One-Way ANOVA | Post Hoc Comparisons by Sex* | |||||||
|---|---|---|---|---|---|---|---|---|
| Region of Interest | Male Control (n = 18) | Male Cocaine (n = 25) | Female Control (n = 19) | Female Cocaine (n = 10) | F (3,68) | P | Male Control vs Cocaine | Female Control vs Cocaine |
| Increase in male cocaine-dependent subjects relative to male controls (Figure 3A) | ||||||||
| R caudate | 75 (3) | 80 (5) | 78 (5) | 78 (5) | 3.3 | 0.02 | 0.001* | 1.0 |
| Medial OFC | 64 (4) | 70 (6) | 66 (6) | 66 (6) | 3.8 | 0.01 | 0.009* | 1.0 |
| R superior frontal gyrus | 79 (2) | 83 (3) | 80 (3) | 81 (3) | 6.0 | 0.001† | 0.001* | 0.93 |
| L middle frontal gyrus | 75 (3) | 79 (4) | 76 (4) | 78 (4) | 5.1 | 0.003 | 0.004* | 0.41 |
| R posterior cingulate | 92 (23) | 97 (4) | 93 (4) | 94 (5) | 6.0 | 0.001† | 0.001* | 0.98 |
| Increase in female cocaine-dependent subjects relative to female controls (Figure 3B) | ||||||||
| L middle frontal cortex | 66 (3) | 67 (4) | 64 (4) | 71 (4) | 7.1 | <0.001† | 0.96 | <0.001* |
| R middle frontal cortex | 64 (4) | 63 (4) | 62 (4) | 69 (5) | 6.1 | <0.001† | 0.98 | <0.001* |
| L anterolateral temporal cortex | 58 (5) | 57 (4) | 58 (4) | 65 (5) | 7.8 | <0.001† | 0.82 | 0.003* |
| R anterolateral temporal cortex | 60 (4) | 59 (4) | 60 (4) | 66 (4) | 8.6 | <0.001† | 0.97 | 0.001* |
| Anterior cingulate | 86 (3) | 88 (4) | 86 (2) | 90 (4) | 4.3 | 0.008 | 0.25 | 0.02* |
ROI Factor Analysis
Five factors emerged from the factor analysis of the 18 selected ROIs. Two ROIs—the anterior cingulate (from female comparison) and the right caudate (from male comparison)—were deleted from the model because they did not meet our minimum item loading criteria. The 5 factors (FAC1–FAC5) explained 72.6% of the total variance: FAC1 = 19.0%; FAC2 = 16.1%; FAC3 = 14.7%; FAC4 = 12.9%; and FAC5 = 9.9% (Table IV). Item loading by sex was consistent for all 5 factors. The direction of the loading (increase or decrease) was consistent for all factors except FAC5.
Table IV.
Factor (FAC) analysis of selected regions of interest (ROIs) for 35 male and female cocaine-dependent subjects and 37 healthy male and female controls.
| FAC Correlation Coefficients | |||||
|---|---|---|---|---|---|
| ROIs with FAC Loadings of ≥0.5 | FAC1 | FAC2 | FAC3 | FAC4 | FAC5 |
| L lateral OFC (male decreased) | 0.86 | ||||
| R lateral OFC (male decreased) | 0.79 | ||||
| L anterolateral temporal cortex (male decreased) | 0.72 | ||||
| R anterolateral temporal cortex (male decreased) | 0.72 | ||||
| L middle frontal cortex (female increased) | 0.85 | ||||
| R middle frontal cortex (female increased) | 0.89 | ||||
| L anterolateral temporal cortex (female increased) | 0.69 | ||||
| R anterolateral temporal cortex (female increased) | 0.57 | ||||
| L superior frontal gyrus (female decreased) | 0.82 | ||||
| R superior frontal gyrus (female decreased) | 0.82 | ||||
| Medial OFC (female decreased) | 0.52 | 0.54 | |||
| L middle frontal gyrus (male increased) | 0.84 | ||||
| R superior frontal gyrus (male increased) | 0.75 | ||||
| R posterior cingulate (male increased) | 0.62 | ||||
| Medial OFC (male increased) | −0.65 | ||||
| Anterior cingulate (male decreased) | 0.65 |
rCBF Response During Rest
In each resting scan, mean counts per voxel were obtained for the 10 ROIs described previously in the male comparison after saline infusion (Table V). There was a significant correlation between the left (r = 0.94; P < 0.001) and right (_r_ = 0.88; _P_ < 0.001) lateral OFC rCBF in the saline and resting scans. With the exception of the left middle frontal gyrus (_r_ = 0.14) and the right caudate (_r_ = 0.54), all other saline and resting scan ROIs were significantly correlated (_r_ > 0.6; P < 0.005) (Table V). Independent t tests between these 10 ROIs revealed 3 regions that met our adjusted α of P < 0.005: the left (t = 4.95; P < 0.001) and right (t = 3.61; P = 0.002) lateral OFC and the right posterior cingulate (t = 3.71; P = 0.002). Statistical trends were noted in the right anterolateral temporal cortex, anterior cingulate, and right superior frontal gyrus.
Table V.
Comparisons between selected regions of interest after saline administration and during rest in male cocaine-dependent and healthy control subjects.
| Correlation: Saline vs Rest | Mean (SD) Counts/Voxel in Resting Scan | Independent t Test Controls vs Patients | ||||
|---|---|---|---|---|---|---|
| Region of Interest | r | P* | Male Controls (n = 10) | Addicted Male Subjects (n = 12) | t (2,20) | P* |
| Decrease in cocaine-dependent males relative to male controls (Figure 1A) | ||||||
| L lateral OFC | 0.94 | <0.001 | 72 (4) | 62 (5) | 4.95 | <0.001 |
| R lateral OFC | 0.88 | <0.001 | 69 (4) | 63 (5) | 3.61 | 0.002 |
| L anterolateral temporal cortex | 0.82 | <0.001 | 69 (4) | 66 (5) | 1.63 | 0.12 |
| R anterolateral temporal cortex | 0.80 | <0.001 | 65 (3) | 62 (4) | 2.21 | 0.04 |
| Anterior cingulate | 0.90 | <0.001 | 72 (7) | 65 (5) | 2.97 | 0.008 |
| Increase in cocaine-dependent males relative to male controls (Figure 3A) | ||||||
| R caudate | 0.54 | 0.01 | 77 (4) | 80 (5) | 1.52 | 0.14 |
| Medial OFC | 0.94 | <0.001 | 64 (2) | 68 (8) | 1.58 | 0.13 |
| R superior frontal gyrus | 0.71 | <0.001 | 81 (2) | 83 (2) | 2.70 | 0.01 |
| L middle frontal gyrus | 0.14 | 0.53 | 78 (2) | 77 (3) | 0.73 | 0.47 |
| R posterior cingulate | 0.63 | 0.002 | 95 (3) | 99 (3) | 3.71 | 0.001 |
Subjective Response to Saline
Brief Symptom Inventory
Before saline administration, male cocaine-dependent subjects demonstrated significantly higher (P < 0.004) BSI scores than did male controls on GSI, PSDI, and obsessive compulsive, phobic anxiety, and psychoticism indexes (Table VI). Only paranoid ideation was significantly higher in female cocaine-dependent subjects relative to female controls (P < 0.01), although somatization, GSI, and PSDI revealed strong trends toward significance. BSI scores before and after saline administration revealed no within-group differences in total (all subjects), by group, or in sex-by-group.
Table VI.
Mean (SD) Brief Symptom Inventory37 scores before saline administration in male and female cocaine-dependent and healthy control subjects.
| Male Control vs Male Cocaine | Female Control vs Female Cocaine | |||||||
|---|---|---|---|---|---|---|---|---|
| Male Control (n = 18) | Male Cocaine (n = 25) | Female Control (n = 19) | Female Cocaine (n = 10) | U | P* | U | P* | |
| Somatization | 0.10 (0.15) | 0.15 (0.29) | 0.04 (0.08) | 0.20 (0.19) | 220.0 | 0.89 | 43.0 | 0.006 |
| Obsessive compulsive | 0.07 (0.16) | 0.33 (0.35) | 0.25 (0.92) | 0.17 (0.28) | 109.5 | 0.002 | 74.0 | 0.20 |
| Interpersonal sensitivity | 0.04 (0.13) | 0.17 (0.30) | 0.03 (0.08) | 0.23 (0.36) | 168.5 | 0.07 | 73.5 | 0.13 |
| Depression | 0.03 (0.07) | 0.13 (0.25) | 0.01 (0.04) | 0.12 (0.25) | 159.0 | 0.05 | 80.0 | 0.19 |
| Anxiety | 0.13 (0.19) | 0.26 (0.29) | 0.11 (0.15) | 0.12 (0.14) | 164.5 | 0.11 | 88.0 | 0.72 |
| Hostility | 0 | 0.10 (0.18) | 0 | 0.08 (0.14) | 144.0 | 0.005 | 66.5 | 0.01 |
| Phobic anxiety | 0 | 0.14 (0.20) | 0.02 (0.06) | 0.02 (0.06) | 126.0 | 0.001 | 94.5 | 0.96 |
| Paranoid ideation | 0.04 (0.11) | 0.30 (0.43) | 0.04 (0.08) | 0.22 (0.30) | 137.5 | 0.01 | 46.0 | 0.004 |
| Psychoticism | 0.01 (0.05) | 0.29 (0.42) | 0.01 (0.05) | 0.12 (0.21) | 116.5 | 0.001 | 70.5 | 0.06 |
| GSI | 0.05 (0.07) | 0.19 (0.21) | 0.03 (0.04) | 0.14 (0.16) | 102.0 | 0.002 | 36.5 | 0.006 |
| PST | 2.6 (3.5) | 8.8 (9.1) | 1.6 (2.1) | 6.8 (7.9) | 113.5 | 0.005 | 41.0 | 0.01 |
| PSDI | 0.56 (0.51) | 1.06 (0.57) | 0.53 (0.51) | 1.12 (0.74) | 96.0 | <0.001 | 44.5 | 0.009 |
Correlation Between BSI and ROI Factors
In the combined male group, 6 significant relationships (all, P < 0.001) were observed between FAC1 and obsessive compulsive (ρ = −0.517), depression (ρ = −0.494), psychoticism (ρ = −0.492), GSI (ρ = −0.578), PST (ρ = −0.534), and PSDI (ρ = −0.622). Although not statistically significant, strong trends were noted between FAC1 and phobic anxiety (ρ = −0.475) and paranoid ideation (ρ = −0.488). These relationships appeared to be secondary to the higher BSI scores and lower FAC1 scores in the cocaine-dependent men, as significant correlations were not evident within either male group when considered separately. To determine if subjective assessments influenced observed differences between cluster rCBF, the 5 regions in FAC1 were assessed with ANCOVA for these 6 significant BSI measures. After ANCOVA, all of the group differences persisted (P < 0.005), suggesting that the rCBF differences were not attributable to the subjects’ emotional state.
The combined female group did not show significant correlations between FAC1 and any BSI measures (ρ = −0.005 to −0.334). Correlations were also observed at a less restricted level of significance (P < 0.01) between FAC1 and phobic anxiety (ρ = 0.475; P < 0.002) and paranoid ideation (ρ = 0.488; P < 0.001) and FAC4 and phobic anxiety (ρ = 0.417; P = 0.005) in the male sample, and FAC2 and obsessive compulsive (ρ = 0.495; P = 0.006), GSI (ρ = 0.494; P < 0.007), and PST (ρ = 0.508; P < 0.005) in the female sample.
Relationship Between Cocaine Use and ROI Factors
There were no significant relationships between the 5 ROI factors and drug use metrics in the total patient sample or by sex (Table I). The only noteworthy trend was between FAC3 and “days used” in the 90 days before treatment (r = −0.433) for the total group.
DISCUSSION
Our findings support previous reports of decreased OFC activity in substance-dependent subjects. We have further isolated the decrease in OFC rCBF to the bilateral OFC in male, but not in female, cocaine-dependent subjects. In contrast, a decrease in medial OFC is observed in female, but not in male, cocaine-dependent subjects. Increases in rCBF following saline were also sex-specific, with diffuse regions of increased rCBF observed in male, but not in female, cocaine-dependent subjects relative to sex-matched healthy controls.
The anatomical distinctions between the medial and lateral OFC may have clinical relevance. Bolla et al,40 for example, examined healthy subjects with positron emission tomography during performance of a gambling task.41 Whereas male subjects activated the bilateral OFC, women activated the medial OFC, suggesting sex differences in OFC responsiveness and/or utilization during a decision-making task. Elliott et al42 have suggested that the medial OFC is involved in the assessment of reward value, based on the familiarity, or “rightness,” of a stimuli. In contrast, the lateral OFC is primarily involved in the suppression of previously rewarded responses. O’Doherty et al,43 for example, describe a functional MRI 2-choice response reversal task in healthy subjects in which medial OFC activation correlated with the amount of money won (eg, reward value, or “rightness”) and activation of the lateral OFC correlated with the amount of money lost. Lateral OFC activation associated with monetary loss presumably reflected a change in response contingencies.
A decrease in the perfusion of medial OFC may leave female cocaine-dependent subjects less responsive to the rewarding effects (or “rightness”) of a stimulus (reward insensitivity). This may produce a muted response to the rewarding effects of cocaine, nondrug rewards, or both. In male cocaine-dependent subjects, a perfusion deficit in the lateral OFC may attenuate their ability to alter behaviors in response to changing contingencies; that is, they would not respond appropriately to the adverse outcomes of stimuli (ie, cocaine) previously associated with reward (punishment insensitivity).
Volkow et al24,44–46 reported a decrease in glucose metabolism in the lateral and/or medial OFC of abstinent cocaine-dependent men at rest, although the OFC anatomical region was not consistently defined. Although our work supports these findings in the lateral OFC, we did not observe a similar decrease in the medial OFC in men. Tucker et al32 and Ernst et al15 also showed regional perfusion differences in male and female cocaine-addicted subjects during rest. However, Tucker et al only identified broad areas of increased (in female cocaine-dependent subjects relative to controls) and decreased (in male cocaine-dependent subjects relative to controls) perfusion, and neither encompassed the OFC. Methodologic differences between these studies make comparison with ours difficult, including the assessment of patients with much shorter (4 days32) or longer (>4 months15) periods of abstinence, the possible inclusion of subjects taking CNS active medications, and ROIs that were manually defined.15
Other studies support our general findings that cocaine-dependent women show less neural disruption than their male counterparts, although these studies did not isolate specific differences in OFC activation in cocaine-dependent men and women. Using SPECT imaging, for example, Levin et al13 found deficits in the frontal, temporal, and basal ganglia regions in cocaine-dependent men but not in cocaine-dependent women. Compared to sex-matched controls, male cocaine-dependent subjects showed a higher frequency of frontal “defects” (56%) than did female cocaine-dependent subjects (0%). Similarly, King et al47 found that whereas the electroencephalogram (EEG) of male cocaine-dependent subjects significantly differed from controls, the EEG of female cocaine-dependent subjects did not differ from sex-matched controls. Female cocaine-dependent subjects also showed less neuronal injury and glial activation in the frontal cortex compared with their male counterparts.14 Sex differences between cocaine-dependent men and women may result from premorbid differences or the menstrual/estrous-related pharmacokinetic48 or pharmacodynamic48–51 effects of cocaine.
Increased cerebral perfusion has previously been reported in the frontal, temporo-parietal, and/or parietal regions of cocaine-dependent men and women relative to controls.15,52 In our study, areas associated with heightened self-awareness (ie, dorsal medial, posterior medial, and ventral medial cortex39) were significantly elevated in the male (but not in the female) cocaine-dependent subjects, suggesting that the males may have been more focused on their internal states and external environment. This may have been due to either a basal hyper-awareness or in expectation of procaine administration. However, rCBF measures of the dorsal and ventral medial cortex (FAC4) did not correlate with BSI measures (except phobic anxiety) before saline administration.
Methodologic strengths of our study include a carefully selected patient and control population, excluding many confounds common in substance abuse research. Cocaine-dependent subjects were studied during a restricted period of verified abstinence, thus avoiding the rapid fluctuations in neural activity that occur during the first few days of cocaine abstinence as well as the more gradual changes that may develop with extended abstinence. The cocaine-dependent group excluded subjects with a lifetime history of other Axis I disorders and had limited histories of other drug use.
Methodologic weaknesses include the administration of saline, which may have increased anxiety during ligand uptake. However, similar findings were observed in the right and left lateral OFC during rest. Other potential weaknesses included the assessment of comparatively few addicted female subjects relative to controls, although 9 of the 10 female cocaine-dependent subjects showed medial OFC rCBF below the mean of the female controls. The imbalance in the number of female controls and addicted female subjects reflects the difficulty in recruiting cocaine-using–only female participants. All recruited female controls were included in the analysis based on the concept that our power to find differences would be greatest with the largest number of subjects per group. Because we confined subjects to a specific period of abstinence, menstrual phase was not controlled. Nevertheless, the within-group findings in the female subjects were relatively consistent.
Limits of spatial resolution and the ability to only determine relative measures of limbic activation are inherent in our SPECT methodology and camera. However, the reported differences were quite large and well within our limits of resolution, and our finding that OFC rCBF is lower in cocaine-dependent subjects relative to controls is similar to the reports of others using absolute measures of energy utilization.24 It should also be noted that the SPECT technique utilized assessed relative, not absolute, differences between groups. Although both gray29 and white53,54 matter are reduced in cocaine-dependent subjects relative to controls, no discrete structural lesions (by MRI) were observed in any of the subjects in this study. However, structural changes in gray and/or white matter may, at least in part, explain the decreases in rCBF observed in the cocaine-dependent group. Groups were not matched on other potential confounds, such as nicotine use, race, or socioeconomic status.
Lastly, we had previously reported that OFC rCBF did not differ between 10 female cocaine-dependent subjects and matched female controls when assessed by _t_-image analysis.33 A retrospective analysis of this comparison, again performed by _t_-image analysis, showed a difference in medial OFC rCBF when the P value was increased to 0.05 (from 0.01). Thus, differences from our previous report may be secondary to the image analytic technique utilized or the increased power of a larger number of female controls.
A general finding in the present study is that addicted male subjects demonstrate greater neurobiological disruption relative to addicted female subjects. These observations are consistent with much of the existing literature reviewed previously in this article. Yet, clinical studies suggest women run a more severe course of substance dependence compared with men.55–57 These seemingly disparate findings may suggest a disconnect between imaging assessments of CNS dysfunction and the presumed associated addictive behaviors. Alternately, patient populations meeting the rigorous study inclusion and exclusion criteria of neuroimaging studies may not appropriately reflect clinical populations. A third interpretation is that medial OFC perfusion deficits may result in heightened psycho-pathology relative to lateral OFC deficits, thus worsening the course of addiction in cocaine-dependent women relative to men.
CONCLUSIONS
Lowered OFC perfusion in cocaine-dependent subjects appears to be in sex-specific anatomically discrete regions. Distinct roles have been suggested for the mesial and lateral OFC in signaling reward value. Future studies are required to replicate these findings in male and female cocaine-dependent subjects and to assess the relevance of sex-specific differences in OFC rCBF to their clinical presentation and course.
Acknowledgments
This study was funded by National Institute on Drug Abuse grants DA10218 and DA11434 and supported by the VA North Texas Health Care System. GE Healthcare is acknowledged for its generous contribution of 99mTc-HMPAO. The authors would like to thank the staffs of the Substance Abuse Team at the Dallas VA Medical Center and Homeward Bound, Inc., for their support in the screening and recruitment of patients. Deanna Alexander and Dr. Sylva Frock are thanked for their recruitment, assessment, and monitoring of participants.
References
- 1.Hughes TL, Day LE, Marcantonio RJ, Torpy E. Gender differences in alcohol and other drug use among young adults. Subst Use Misuse. 1997;32:317–342. doi: 10.3109/10826089709055853. [DOI] [PubMed] [Google Scholar]
- 2.O’Malley PM, Johnston LD, Bachman JG. Adolescent substance use. Epidemiology and implications for public policy. Pediatr Clin North Am. 1995;42:241–260. doi: 10.1016/s0031-3955(16)38945-3. [DOI] [PubMed] [Google Scholar]
- 3.Van Etten ML, Neumark YD, Anthony JC. Male-female differences in the earliest stages of drug involvement. Addiction. 1999;94:1413–1419. doi: 10.1046/j.1360-0443.1999.949141312.x. [DOI] [PubMed] [Google Scholar]
- 4.Robbins SJ, Ehrman RN, Childress AR, O’Brien CP. Comparing levels of cocaine cue reactivity in male and female outpatients. Drug Alcohol Depend. 1999;53:223–230. doi: 10.1016/s0376-8716(98)00135-5. [DOI] [PubMed] [Google Scholar]
- 5.Elman I, Karlsgodt KH, Gastfriend DR. Gender differences in cocaine craving among non-treatment-seeking individuals with cocaine dependence. Am J Drug Alcohol Abuse. 2001;27:193–202. doi: 10.1081/ada-100103705. [DOI] [PubMed] [Google Scholar]
- 6.Greenfield SF, Manwani SG, Nargiso JE. Epidemiology of substance use disorders in women. Obstet Gynecol Clin North Am. 2003;30:413–446. doi: 10.1016/s0889-8545(03)00072-x. [DOI] [PubMed] [Google Scholar]
- 7.McCance-Katz EF, Carroll KM, Rounsaville BJ. Gender differences in treatment-seeking cocaine abusers—implications for treatment and prognosis. Am J Addict. 1999;8:300–311. doi: 10.1080/105504999305703. [DOI] [PubMed] [Google Scholar]
- 8.Hernandez-Avila CA, Rounsaville BJ, Kranzler HR. Opioid-, cannabis- and alcohol-dependent women show more rapid progression to substance abuse treatment. Drug Alcohol Depend. 2004;74:265–272. doi: 10.1016/j.drugalcdep.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 9.Luders E, Narr KL, Thompson PM, et al. Gender differences in cortical complexity. Nat Neurosci. 2004;7:799–800. doi: 10.1038/nn1277. [DOI] [PubMed] [Google Scholar]
- 10.Andreason PJ, Zametkin AJ, Guo AC, et al. Gender-related differences in regional cerebral glucose metabolism in normal volunteers. Psychiatry Res. 1994;51:175–183. doi: 10.1016/0165-1781(94)90037-x. [DOI] [PubMed] [Google Scholar]
- 11.Gur RC, Mozley LH, Mozley PD, et al. Sex differences in regional cerebral glucose metabolism during a resting state. Science. 1995;267:528–531. doi: 10.1126/science.7824953. [DOI] [PubMed] [Google Scholar]
- 12.Cahill L, Haier RJ, White NS, et al. Sex-related difference in amygdala activity during emotionally influenced memory storage. Neurobiol Learn Mem. 2001;75:1–9. doi: 10.1006/nlme.2000.3999. [DOI] [PubMed] [Google Scholar]
- 13.Levin JM, Holman BL, Mendelson JH, et al. Gender differences in cerebral perfusion in cocaine abuse: Technetium-99m-HMPAO SPECT study of drug-abusing women. J Nucl Med. 1994;35:1902–1909. [PubMed] [Google Scholar]
- 14.Chang L, Ernst T, Strickland T, Mehringer CM. Gender effects on persistent cerebral metabolite changes in the frontal lobes of abstinent cocaine users. Am J Psychiatry. 1999;156:716–722. doi: 10.1176/ajp.156.5.716. [DOI] [PubMed] [Google Scholar]
- 15.Ernst T, Chang L, Oropilla G, et al. Cerebral perfusion abnormalities in abstinent cocaine abusers: A perfusion MRI and SPECT study. Psychiatry Res. 2000;99:63–74. doi: 10.1016/s0925-4927(00)00056-1. [DOI] [PubMed] [Google Scholar]
- 16.Li CS, Kemp K, Milivojevic V, Sinha R. Neuro-imaging study of sex differences in the neuropathology of cocaine abuse. Gend Med. 2005;2:174–182. doi: 10.1016/s1550-8579(05)80046-4. [DOI] [PubMed] [Google Scholar]
- 17.Li CS, Kosten TR, Sinha R. Sex differences in brain activation during stress imagery in abstinent cocaine users: A functional magnetic resonance imaging study. Biol Psychiatry. 2005;57:487–494. doi: 10.1016/j.biopsych.2004.11.048. [DOI] [PubMed] [Google Scholar]
- 18.London ED, Ernst M, Grant S, et al. Orbitofrontal cortex and human drug abuse: Functional imaging. Cereb Cortex. 2000;10:334–342. doi: 10.1093/cercor/10.3.334. [DOI] [PubMed] [Google Scholar]
- 19.Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: Involvement of the orbitofrontal cortex. Cereb Cortex. 2001;10:318–325. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]
- 20.Rilling LM, Adinoff B. Cognitive dysfunction in cocaine abuse: Evidence for impairments in impulse control and decision-making. In: Cooper EF, editor. Progress in Cocaine Abuse Research. Hauppauge, NY: Nova Science Publishers; In press. [Google Scholar]
- 21.Hornak J, O’Doherty J, Bramham J, et al. Reward-related reversal learning after surgical excisions in orbito-frontal or dorsolateral prefrontal cortex in humans. J Cogn Neurosci. 2004;16:463–478. doi: 10.1162/089892904322926791. [DOI] [PubMed] [Google Scholar]
- 22.Bechara A. Neurobiology of decision-making: Risk and reward. Semin Clin Neuropsychiatry. 2001;6:205–216. doi: 10.1053/scnp.2001.22927. [DOI] [PubMed] [Google Scholar]
- 23.Volkow ND, Hitzemann R, Wang GJ, et al. Decreased brain metabolism in neurologically intact healthy alcoholics. Am J Psychiatry. 1992;149:1016–1022. doi: 10.1176/ajp.149.8.1016. [DOI] [PubMed] [Google Scholar]
- 24.Volkow ND, Fowler JS, Wang GJ, et al. Decreased dopamine D2 receptor availability is associated with reduced frontal metabolism in cocaine abusers. Synapse. 1993;14:169–177. doi: 10.1002/syn.890140210. [DOI] [PubMed] [Google Scholar]
- 25.Volkow ND, Chang L, Wang GJ, et al. Low level of brain dopamine D2 receptors in methamphetamine abusers: Association with metabolism in the orbitofrontal cortex. Am J Psychiatry. 2001;158:2015–2021. doi: 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]
- 26.Paulus MP, Hozack NE, Zauscher BE, et al. Behavioral and functional neuroimaging evidence for prefrontal dysfunction in methamphetamine-dependent subjects. Neuropsychopharmacology. 2002;26:53–63. doi: 10.1016/S0893-133X(01)00334-7. [DOI] [PubMed] [Google Scholar]
- 27.Bolla KI, Eldreth DA, London ED, et al. Orbitofrontal cortex dysfunction in abstinent cocaine abusers performing a decision-making task. Neuroimage. 2003;19:1085–1094. doi: 10.1016/s1053-8119(03)00113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adinoff B, Devous MD, Sr, Best SM, et al. Limbic responsiveness to procaine in cocaine-dependent subjects. Am J Psychiatry. 2001;158:390–398. doi: 10.1176/appi.ajp.158.3.390. [DOI] [PubMed] [Google Scholar]
- 29.Franklin TR, Acton PD, Maldjian JA, et al. Decreased gray matter concentration in the insular, orbitofrontal, cingulate, and temporal cortices of cocaine patients. Biol Psychiatry. 2002;51:134–142. doi: 10.1016/s0006-3223(01)01269-0. [DOI] [PubMed] [Google Scholar]
- 30.Matochik JA, London ED, Eldreth DA, et al. Frontal cortical tissue composition in abstinent cocaine abusers: A magnetic resonance imaging study. Neuroimage. 2003;19:1095–1102. doi: 10.1016/s1053-8119(03)00244-1. [DOI] [PubMed] [Google Scholar]
- 31.Childress AR, Mozley PD, McElgin W, et al. Limbic activation during cue-induced cocaine craving. Am J Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tucker KA, Browndyke JN, Gottschalk PC, et al. Gender-specific vulnerability for rCBF abnormalities among cocaine abusers. Neuroreport. 2004;15:797–801. doi: 10.1097/00001756-200404090-00011. [DOI] [PubMed] [Google Scholar]
- 33.Adinoff B, Devous MD, Best SE, et al. Regional cerebral blood flow in female cocaine-dependent subjects following limbic activation. Drug Alcohol Depend. 2003;71:255–268. doi: 10.1016/s0376-8716(03)00138-8. [DOI] [PubMed] [Google Scholar]
- 34.First MB, Spitzer RL, Gibbon M, et al. Structured Clinical Interview for DSM-IV Axis I Disorders—Non-Patient Edition (SCID-I/NP, Version 2.0) New York, NY: New York State Psychiatric Institute; 1996. [Google Scholar]
- 35.Sobell MB, Sobell LC. Behavioral Treatment of Alcohol Problems. New York, NY: Plenum Press; 1978. [Google Scholar]
- 36.Derogatis LR, Rickels K, Rock AF. The SCL-90 and the MMPI: A step in the validation of a new self-report scale. Br J Psychiatry. 1976;128:280–289. doi: 10.1192/bjp.128.3.280. [DOI] [PubMed] [Google Scholar]
- 37.Derogatis LR, Melisaratos N. The Brief Symptom Inventory: An introductory report. Psychol Med. 1983;13:595–605. [PubMed] [Google Scholar]
- 38.Talairach J, Tournoux P. Co-planar stereotactic atlas of the human brain. Stuttgart, Germany: Georg Thieme Verlag; 1988. [Google Scholar]
- 39.Gusnard DA, Raichle ME, Raichle ME. Searching for a baseline: Functional imaging and the resting human brain. Nat Rev Neurosci. 2001;2:685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- 40.Bolla KI, Eldreth DA, Matochik JA, Cadet JL. Sex-related differences in a gambling task and its neurological correlates. Cereb Cortex. 2004;14:1226–1232. doi: 10.1093/cercor/bhh083. [DOI] [PubMed] [Google Scholar]
- 41.Bechara A, Damasio H, Tranel D, Anderson SW. Dissociation of working memory from decision making within the human prefrontal cortex. J Neurosci. 1998;18:428–437. doi: 10.1523/JNEUROSCI.18-01-00428.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Elliott R, Dolan RJ, Frith CD. Dissociable functions in the medial and lateral orbitofrontal cortex: Evidence from human neuroimaging studies. Cereb Cortex. 2000;10:308–317. doi: 10.1093/cercor/10.3.308. [DOI] [PubMed] [Google Scholar]
- 43.O’Doherty J, Kringelbach ML, Rolls ET, et al. Abstract reward and punishment representations in the human orbitofrontal cortex. Nat Neurosci. 2001;4:95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- 44.Volkow ND, Fowler JS, Wolf AP, et al. Changes in brain glucose metabolism in cocaine dependence and withdrawal. Am J Psychiatry. 1991;148:621–626. doi: 10.1176/ajp.148.5.621. [DOI] [PubMed] [Google Scholar]
- 45.Volkow ND, Hitzemann R, Wang GJ, et al. Long-term frontal brain metabolic changes in cocaine abusers [published correction appears in Synapse. 1992;12:86] Synapse. 1992;11:184–190. doi: 10.1002/syn.890110303. [DOI] [PubMed] [Google Scholar]
- 46.Volkow ND, Wang GJ, Hitzemann R, et al. Recovery of brain glucose metabolism in detoxified alcoholics. Am J Psychiatry. 1994;151:178–183. doi: 10.1176/ajp.151.2.178. [DOI] [PubMed] [Google Scholar]
- 47.King DE, Herning RI, Gorelick DA, Cadet JL. Gender differences in the EEG of abstinent cocaine abusers. Neuropsychobiology. 2000;42:93–98. doi: 10.1159/000026678. [DOI] [PubMed] [Google Scholar]
- 48.Lukas SE, Sholar M, Lundahl LH, et al. Sex differences in plasma cocaine levels and subjective effects after acute cocaine administration in human volunteers. Psychopharmacology (Berl) 1996;125:346–354. doi: 10.1007/BF02246017. [DOI] [PubMed] [Google Scholar]
- 49.Kaufman MJ, Levin JM, Maas LC, et al. Cocaine-induced cerebral vasoconstriction differs as a function of sex and menstrual cycle phase. Biol Psychiatry. 2001;49:774–781. doi: 10.1016/s0006-3223(00)01091-x. [DOI] [PubMed] [Google Scholar]
- 50.Festa ED, Quinones-Jenab V. Gonadal hormones provide the biological basis for sex differences in behavioral responses to cocaine. Horm Behav. 2004;46:509–519. doi: 10.1016/j.yhbeh.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 51.Roth ME, Cosgrove KP, Carroll ME. Sex differences in the vulnerability to drug abuse: A review of preclinical studies. Neurosci Biobehav Rev. 2004;28:533–546. doi: 10.1016/j.neubiorev.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 52.Gottschalk PC, Kosten TR. Cerebral perfusion defects in combined cocaine and alcohol dependence. Drug Alcohol Depend. 2002;68:95–104. doi: 10.1016/s0376-8716(02)00109-6. [DOI] [PubMed] [Google Scholar]
- 53.Bartzokis G, Goldstein IB, Hance DB, et al. The incidence of T2-weighted MR imaging signal abnormalities in the brain of cocaine-dependent patients is age-related and region-specific. AJNR Am J Neuroradiol. 1999;20:1628–1635. [PMC free article] [PubMed] [Google Scholar]
- 54.Lim KO, Choi SJ, Pomara N, et al. Reduced frontal white matter integrity in cocaine dependence: A controlled diffusion tensor imaging study. Biol Psychiatry. 2002;51:890–895. doi: 10.1016/s0006-3223(01)01355-5. [DOI] [PubMed] [Google Scholar]
- 55.Griffin ML, Weiss RD, Mirin SM, Lange U. A comparison of male and female cocaine abusers. Arch Gen Psychiatry. 1989;46:122–126. doi: 10.1001/archpsyc.1989.01810020024005. [DOI] [PubMed] [Google Scholar]
- 56.Lex BW. Some gender differences in alcohol and poly-substance users. Health Psychol. 1991;10:121–132. doi: 10.1037//0278-6133.10.2.121. [DOI] [PubMed] [Google Scholar]
- 57.Haseltine FP. Gender differences in addiction and recovery. J Women’s Health Gend Based Med. 2000;9:579–583. doi: 10.1089/15246090050118080. [DOI] [PubMed] [Google Scholar]