Activating mutation in a mucolipin transient receptor potential channel leads to melanocyte loss in varitint–waddler mice (original) (raw)
Abstract
Transient receptor potential (TRP) genes of the mucolipin subfamily (TRPML1–3 and MCOLN1–3) are presumed to encode ion channel proteins of intracellular endosomes and lysosomes. Mutations in human TRPML1 (mucolipin 1/MCOLN1) result in mucolipidosis type IV, a severe inherited neurodegenerative disease associated with defective lysosomal biogenesis and trafficking. A mutation in mouse TRPML3 (A419P; TRPML3_Va_) results in the varitint–waddler (Va) phenotype. Va mice are deaf, exhibit circling behavior due to vestibular defects, and have variegated/dilute coat color as a result of pigmentation defects. Prior electrophysiological studies of presumed TRPML plasma membrane channels are contradictory and inconsistent with known TRP channel properties. Here, we report that the Va mutation produces a gain-of-function that allows TRPML1 and TRPML3 to be measured and identified as inwardly rectifying, proton-impermeant, Ca2+-permeant cation channels. TRPML3 is highly expressed in normal melanocytes. Melanocyte markers are lost in the Va mouse, suggesting that their variegated and hypopigmented fur is caused by severe alteration of melanocyte function or cell death. TRPML3_Va_ expression in melanocyte cell lines results in high resting Ca2+ levels, rounded, poorly adherent cells, and loss of membrane integrity. We conclude that the Va phenotype is caused by mutation-induced TRPML3 gain-of-function, resulting in cell death.
Keywords: calcium channel, lysosome, mucolipidosis, TRPML, hair
TRPML1 is a putative intracellular ion channel that colocalizes with late endosomal/lysosomal markers (1–3). TRPML2 and TRPML3 are less well understood but are also presumed to be primarily intracellular channels that mediate ion fluxes across endosomal membranes (4). TRPML3 is mutated in varitint–waddler (Va and VaJ) mice (5). Mice homozygous or heterozygous for the Va (A419P) mutation are deaf and exhibit circling behavior indicative of a vestibular defect. Heterozygotes display a variegated and dilute coat color, whereas homozygotes are almost white and have reduced viability (6). A second mutation in TRPML3 arising in the original Va stock (A419P; I362T) results in a less-severe (VaJ) phenotype (7).
Mucolipin channel function is unclear because of conflicting data from heterologously expressed, presumed TRPML currents. TRPML1 was claimed to underlie nonselective outwardly rectifying monovalent (8) or proton-conducting (9) currents, but neither of these results were reproduced by others (1). Here, we show that the A419P mutation in the presumed segment (S)4–S5 linker results in a constitutively active, inwardly rectifying cation channel that can be measured across the cell's plasma membrane. Mutation of the corresponding amino acids in TRPML1 results in similar overall conductance properties. WT TRPML3 is highly expressed in melanocytes, but these cells are lost in Va/Va mice, as assessed by the disappearance of melanocyte markers. Consistent with the toxicity of TRPML3_Va_ expression in melanocyte cell lines, we hypothesize that the loss of fur color is caused by the loss of functioning melanocytes.
Results
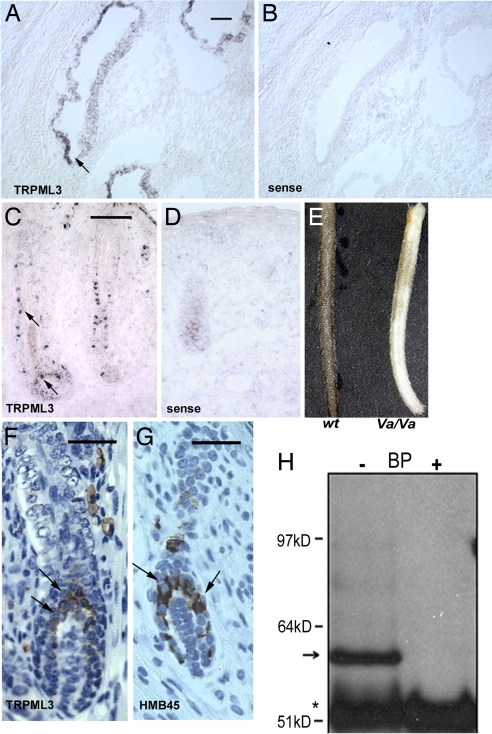
TRPML3 mRNA is abundant in the cochlea (particularly the stria vascularis) (Fig. 1 A and B) and hair follicles of postnatal day (P)0 mice (Fig. 1C). In hair follicles, we observed prominent staining in a subset of cells along the hair shaft and in the hair bulb (Fig. 1 C and D). The distribution of TRPML3 at P0 is similar to the distribution of melanocytes that later migrate to the bottom of the hair follicle and localize to the hair bulb (10). We generated antibodies that were specific to TRPML3, as shown by immunohistochemistry and Western blotting [supporting information (SI) Fig. 6]. Both homozygous (Fig. 1E) and heterozygous Va mice exhibit a fur color defect. TRPML3-specific immunolabeling of skin sections from P4 WT mice showed specific cytoplasmic staining of cells in the hair follicles, particularly in cells of the upper region of the hair bulb (Fig. 1F), consistent with the location of mature melanocytes at this developmental stage (11). Staining of the same skin sections with a melanocyte-specific marker suggests that the TRPML3-positive cells were melanocytes (Fig. 1G). We confirmed the expression of TRPML3 in the mouse melanocyte cell line, melan-a2. A ≈59-kDa band corresponding to the predicted size of mouse TRPML3 was detected by Western blotting in immunoprecipitates from melan-a2 cell lysates (Fig. 1H; see controls in SI Fig. 6).
Fig. 1.
TRPML3 is highly expressed in the cochlea and melanocytes in skin hair follicles. (A–D) In situ hybridization of mTRPML3 in P0 mice. (B and D) Sense probe. (A) TRPML3 in the stria vascularis of the cochlea. (Scale bar, 100 μm.) (C) TRPML3 expression in a whisker hair follicle. Note the individual cells stained by the antisense probe. (Scale bar, 100 μm.) (E) Skin color differences in WT and Va/Va 6-week-old littermates. (F and G) Immunohistochemical staining with TRPML3-specific antibodies (F) and melanocyte-specific antibodies (HMB45) (G) on paraffin-embedded skin sections of P4 WT mice; antibody staining is brown, and the nuclei are blue. TRPML3-specific staining is visible in the cytoplasm of cells in the upper region and sides of the hair bulb (arrows). (Scale bar, 50 μm.) (G) Melanocytes in similar locations in the hair bulb. (Scale bar, 50 μm.) (H) Immunoprecipitation using anti-TRPML3 antibody was performed in the absence or presence of blocking peptide (BP) from lysates of melan-a2 cells. The TRPML3 band is ≈59 kDa (arrow). The asterisk indicates ab heavy chains; molecular mass markers are shown to the left.
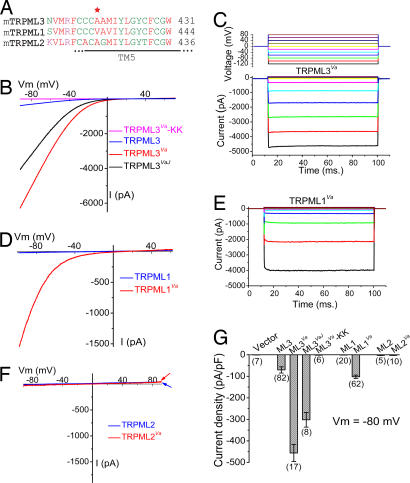
The Va mutation is located in the putative S4–S5 linker (Fig. 2A), corresponding to a similar region in the well studied Kv channels that links movement of the S1–S4 voltage-sensor/ligand domain to a hydrophobic pocket in the S6 gate of the S5–S6 ion conducting pore (12). Alanine substitution by proline (A863P) in an analogous region of hNaV1.7 produces neuronal hyperexcitability and neuropathy (13). We compared WT, Va, and VaJ TRPML3 in heterologously expressing HEK293T cells. For WT TRPML3 (Fig. 2B), only small nonselective currents were measured (−70 ± 14 pA/pF at −80 mV, n = 82) (Fig. 2G). Much larger inwardly rectifying currents were seen in TRPML3_Va_-transfected cells (Fig. 2B); TRPML3_Va_-mediated (I_TRPML3-Va) was −456 ± 40 pA/pF (−80 mV; n = 17), ≈6-fold larger than ITRPML3. Similar results were obtained in transfected melanoma B16 cells (data not shown). The double mutant, ITRPML3-VaJ, was ≈4-fold larger than ITRPML3. The current–voltage relationship (I–V) and reversal potentials were similar for all three TRPML3 proteins, suggesting that the Va mutation increased channel activity without altering its pore properties. Replacement of two negatively charged acidic amino acids (D458 and D459) by basic amino acids (KK) in the putative pore region of TRPML3 completely eliminated the inwardly rectifying current of both TRPML3 and TRPML3_Va (TRPML3-KK, TRPML3_Va_-KK, Fig. 2 B and G), suggesting that the overall architecture of TRPML is similar to other TRP (and _K_V) channels. TRPML3_Va_-expressing cells also displayed a strong inwardly rectifying current in response to voltage steps (Fig. 2C). _I_TRPML3-Va did not inactivate significantly with changes in voltage or time. In addition, _I_TRPML3-Va exhibited no cumulative slow inactivation upon repeated depolarization. Thus, TRPML3_Va_is constitutively active at resting membrane potentials.
Fig. 2.
Large constitutively active inwardly rectifying currents in expressed TRPML1 and TRPML3 Va mutants. (A) Alignment of TRPML (TRPML1, TRPML2, and TRPML3) protein sequences near the Va locus. The red asterisk indicates Ala-419 in TRPML3, which is a proline in Va mice. TM5, putative transmembrane S5. (B) Representative whole-cell currents in HEK293T cells transfected with WT TRPML3, TRPML3_Va_, TRPLM3_VaJ_, and TRPML3_Va_–KK constructs. Currents were elicited by voltage ramps (−100 to +100 mV, 400 ms) with a 4-s interval between ramps in standard extracellular solution (see Materials and Methods). Holding potential (HP) = 0 mV. (C) Large inwardly rectifying whole-cell current elicited by voltage steps (−120 mV to +80 mV, 20 mV increments, step duration = 90 ms) in TRPML3_Va_-expressing cells. HP = 0 mV. _I_TRPML3-Va is instantaneously activated at negative potentials. (D) Representative whole-cell currents in TRPML1- and TRPML1_Va_-expressing HEK293T cells. (E) I_TRPML1-Va elicited by voltage steps. (F) No significant current was measured in HEK293T cells transfected with TRPML2 or TRPML2_Va. (G) Average inward current densities of TRPML channels at −80 mV normalized by cell capacitance (pF). (n) = number of cells.
Va mutations were made in the homologous positions of TRPML1 and TRPML2. ITRPML1 was 1.2 ± 0.2 pA/pF (−80 mV, n = 20, Fig. 2 D and G), and not significantly different from the current density of vector-transfected HEK293T cells (0.9 ± 0.1 pA/pF, n = 9). In contrast, ITRPML1-Va was ≈80 times larger (−102 ± 8 pA/pF; n = 62). In the voltage step protocol (Fig. 2C), ITRPML1-Va inwardly rectified with little time dependence at negative potentials (Fig. 2E), although I_TRPML3-Va rectification was less steep than for ITRPML1-Va. No significant currents were detected in cells transfected with TRPML2 or TRPML2_Va (Fig. 2 F and G).
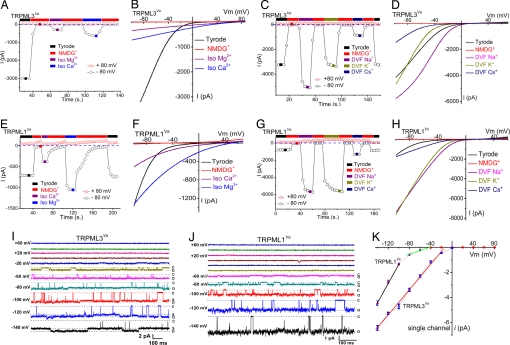
Replacement of bath cations by N_-methyl-d-glucamine (NMDG+) (pH 7.4) suggests that TRPML3_Va is a cation-selective ion channel (Fig. 3 A and B). In isotonic (105 mM) Mg2+ or Ca2+, a smaller, less-rectifying current reversed at very positive potentials (> +60 mV) (Fig. 3 A and B). Inwardly rectifying currents were also measured with monovalent cations in the absence of divalent cations [divalent-free (DVF); <10 nM] (Fig. 3 C_ and D). Cation substitution experiments showed that relative monovalent conductances were Na+ > K+ > Cs+. Similar results were also obtained with WT TRPML3 (data not shown). Thus, we conclude that TRPML3/TRPML3_Va is a nonselective cationic channel with permeability to Na+, K+, Ca2+, and Mg2+. In TRPML1_Va_-expressing cells, large inwardly rectifying currents were measured in isotonic Ca2+ and isotonic Mg2+ (Fig. 3 E and F); in contrast to TRPML3_Va_, Mg2+ currents were larger than Ca2+-mediated currents. Relative monovalent conductances for TRPML1_Va_ were: Na+ ≈ K+ > Cs+ (Fig. 3 G and H).
Fig. 3.
Permeation properties of TRPML1 and TRPML3 channels. (A) TRPML3_Va_ is permeable to both Ca2+ and Mg2+. Currents were initially recorded in external solution and elicited by repeated voltage ramps (−100 to +100 mV, 400 ms) with a 4-s interval between ramps. Data at −80 mV and +80 mV were plotted against time. No significant inward current was seen in the NMDG+ (Na+-free, Ca2+-free) solution. Switching the bath to isotonic (105 mM) Mg2+ or Ca2+ solution yielded smaller, but measurable, current. Divalent I_TRPML3-Va currents did not inactivate after repetitive voltage ramps. (B) Whole-cell I–V relations in the presence of isotonic [Ca2+]o and [Mg2+]o. Note the positive reversal potentials (more than +60 mV). (C) Monovalent permeability of I_TRPML3-Va in divalent-free (DVF, [Ca2+]i < 10 nM) solution. Large inwardly rectifying currents were elicited by DVF, Na+-containing and DVF, K+-containing solutions and were smaller in DVF Cs+ solution. (_D_) Representative _I–V_ relations of monovalent Na+, K+, and Cs+ currents in DVF conditions. (_E_ and _F_) _I_TRPML1-Va Ca2+ and Mg2+ permeability. Currents elicited by isotonic divalent solutions exhibit weaker voltage dependence than _I_TRPML1-Va in external solution. Divalent _I_TRPML1-Va currents did not inactivate over longer durations. (_G_ and _H_) Large inwardly rectifying monovalent _I_TRPML1-Va in DVF conditions. At − 80 mV, Na+ _I_TRPML1-Va in DVF solution was >9-fold larger than the current in external solution. (I) Single-channel currents in an inside-out patch excised from a TRPML3_Va_-expressing HEK293T cell. Pipette contained external solution; the bath was Cs+-based internal solution (147 mM Cs-Mes). A voltage step [−140 mV to + 80 mV; holding potential (HP) = 0 mV] applied to the inside-out patch elicited channel openings at negative potentials. C and O are closed and open levels, respectively. At negative potentials, substates (S) were frequently observed. (J) Single-channel TRPML1_Va currents. (K) TRPML3_Va single-channel conductance (−140 mV to −20 mV) was 49 ± 1 pS (n = 4). TRPML1_Va_ single-channel conductance was 76 ± 4 (−140 mV to −100 mV; n = 5) and 11 ± 0.4 pS (−80 mV to −40 mV; n = 5).
Single-channel properties of TRPML channels were measured in inside-out patch recordings. Similar to whole-cell currents, TRPML3_Va_ and TRPML1_Va_-mediated single-channel currents were inwardly rectifying (Fig. 3 I–K and SI Fig. 7). Channel openings with large amplitudes were recorded at negative potentials, whereas no openings were resolved at positive potentials. At very negative potentials (less than −80 mV), both TRPML1_Va_ and TRPML3_Va_ channels were usually open (P_open > 0.7). For TRPML3_Va, the mean open and closed times at −120 mV were 282 ± 116 and 2.1 ± 0.9 ms, respectively. For TRPML1_Va_, the mean open and closed times at −120 mV were 113 ± 41 and 6.1 ± 2.6 ms, respectively. The single-channel conductance for TRPML3_Va_ (−140 mV to −20 mV) was 49 ± 1 pS. Single-channel TRPML1_Va_ conductances were 76 ± 4 pS (−140 mV to −100 mV), and 11 ± 0.4 pS (−80 mV to −40 mV), respectively.
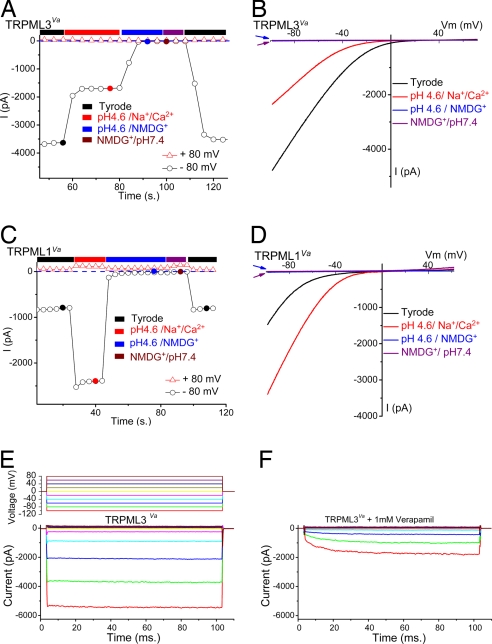
TRPML channels are believed to be primarily targeted to endosomes or lysosomes (3, 14). A salient feature of acidified endosomes and lysosomes is low-intravesicular pH. Also, previous reports suggested that TRPML1 was permeant to protons (9). We measured TRPML channels at low extracellular pH (analogous to the luminal pH exposure of the channel's intravesicular surface). Because protons also activated an endogenous Cl− conductance that was strongly outwardly rectifying (15), gluconate− or Mes− was usually used to replace most of the Cl− ([remaining Cl−]o = 5–10 mM) for all low-pH bath solutions. In the presence of permeable cations (low-pH “Tyrode's solution”), pH 4.6 inhibited _I_TRPML3-Va by 67 ± 2% (n = 7) (Fig. 4 A and B). No significant inward current was measured when NMDG+ replaced all cations in the bath solution at pH 4.6, suggesting that TRPML3 was not significantly permeant to protons. In contrast to _I_TRPML3-Va, ITRPML1-Va was potentiated by acid (pH 4.6) (Fig. 4 C and D) by 2.9 ± 0.2-fold (n = 5), but again no significant current was observed when NMDG+ replaced all of the cations in the low-pH bath solution (Fig. 4 C and D). A small inward current (<30 pA) was observed in both TRPML-transfected and nontransfected control cells (SI Fig. 8). Thus, TRPML1 is a proton-impermeant, nonselective cation channel modulated by pH. These results are also in disagreement with a previous report on a current probably misidentified as TRPML1 in which an outwardly rectifying current was inhibited by acid (16).
Fig. 4.
TRPML1 and TRPML3 pH dependence and pharmacological block. (A and B) TRPML3_Va_ is a proton-impermeant cation channel inhibited by low extracellular pH. (A) I_TRPML3-Va in external solution was inhibited by ≈60% when the pH of the bath solution was decreased to 4.6. Replacement of permeable cations (Na+/Ca2+/Mg2+/K+) by NMDG+ at pH 4.6 eliminated most inward current. (B) Representative currents in external, pH 4.6, low-pH NMDG+, and pH 7.4 NMDG+ solutions. The amplitudes of NMDG+ currents at pH 7.4 (blue arrow) and pH 4.6 (red arrow) are similar. (C and D) TRPML1_Va is a proton-impermeant cation channel strongly enhanced by low extracellular pH. (C) _I_TRPML1-Va in external solution increased ≈3-fold by acidification to pH 4.6. No significant inward current was seen in NMDG+ solutions (pH 7.4 and pH 4.6). (D) Representative whole-cell currents in pH 4.6 external, low-pH NMDG+, and pH 7.4 NMDG+ solutions. Note that the NMDG+-elicited currents at pH 7.4 (blue arrow) and pH 4.6 (red arrow) are almost indistinguishable. (E) Large inwardly rectifying whole-cell current in a TRPML3_Va_-expressing cell (protocol, Top) in external solution. (F) Verapamil (1 mM) inhibits and slows activation of currents at negative potentials.
TRP pharmacology is still in its infancy, and few specific blockers have been identified. TRPML1 and TRPML3 were not inhibited by 2APB (200 μM), SKF 96365 (50 μM), amiloride (100 μM), ruthenium red (10 μM), or nifedipine (100 μM). However, at high concentrations of verapamil (1 mM), TRPML3 activation was slowed 10-fold from τ = 0.1 ± 0.01 ms (n = 4, −100 mV) to τ = 1 ± 0.1 ms (n = 4; Fig. 4 E and F). Similar effects were observed for ITRPML1-Va.
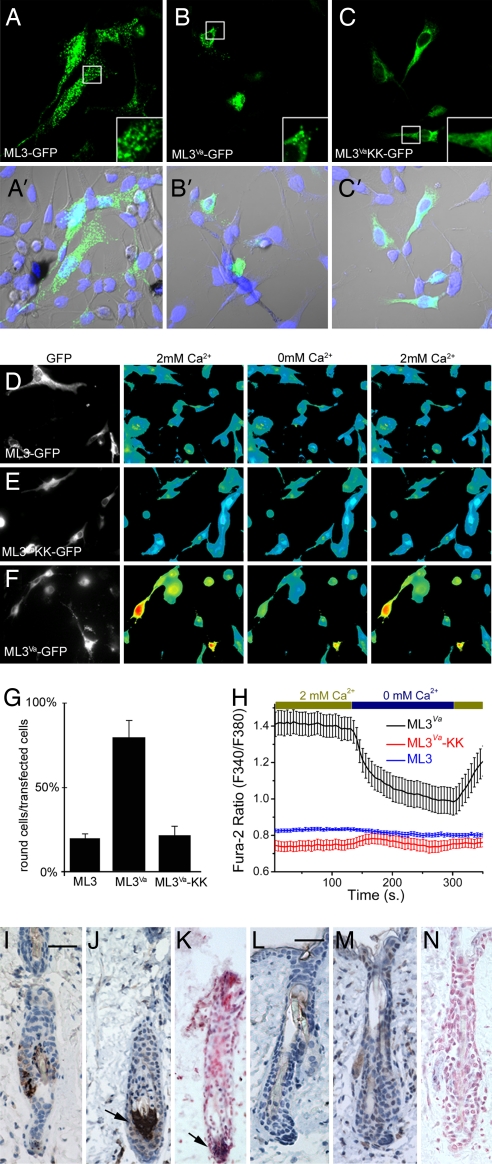
TRPML3 is natively expressed in melan-a2 cells (see Fig. 1H). To determine whether TRPML3 mutants differed in their trafficking or localization, we transfected melan-a2 cells with N-terminal GFP-tagged TRPML3 (Fig. 5A), TRPML3_Va_ (Fig. 5B), and TRPML3_Va_-KK (Fig. 5C). TRPML3 was present in widespread vesicular compartments (Fig. 5A), whereas TRPML3_Va_ appeared to be partially mislocalized within the cell (Fig. 5B). More strikingly, most of the TRPML3_Va_-transfected cells rounded up (80 ± 9%) and were stained by the Ethidium bromide homodimer EthD-1 (71 ± 22%), suggesting a loss of membrane integrity (Fig. 5G and SI Fig. 6). The frequency of round cells and EthD-1-positive cells was significantly less in TRPML3-transfected cells (19 ± 3% and 5 ± 7%, respectively (Fig. 5G and SI Fig. 6), suggesting that the constitutive activity of TRPML3_Va_ was toxic.
Fig. 5.
Elevated [Ca2+]i in TRPML3_Va_-expressing cells and melanocyte cell death. (A–C) Melan-a2 cells were transfected with GFP-tagged TRPML3 (A and A′), TRPML3_Va_ (B and B′), and TRPML3_Va_-KK (C and C′) constructs and assayed after 48 h. Note punctate distribution of TRPML3 in melan-a2 cells (A Inset). (B) Round morphology of TRPML3_Va_-expressing cells. (C and C Inset) Cells expressing the TRPML3_Va_-KK mutant attach and spread; the fluorescence signal is not punctate. (A′_–_C′) Cells were stained with TO-PRO3 nuclear stain. (D–F) Melan-a2 cells were transfected with GFP-tagged TRPML3 (D), TRPML3_Va_-KK (E), and TRPML3_Va_ (F) constructs; [Ca2+]i was measured after 20 h. Resting [Ca2+]i in TRPML3- and TRPML3_Va_-KK-transfected cells was similar to nontransfected cells. In contrast, [Ca2+]i was significantly elevated in TRPML3_Va_-transfected cells in 2 mM extracellular [Ca2+]. Representative data are from four to eight experiments. (G) Quantification of round vs. spread cells after transfection with the indicated construct (80 ± 9% round cells for TRPML3_Va_ vs. 19 ± 3% round cells for WT TRPML3). The levels of KK mutant were similar to those of WT (19 ± 3% round cells for TRPML3 vs. 22 ± 6% round cells for TRPML3_Va_-KK transfected cells; n = 3 experiments). (H) In TRPML3_Va_-transfected cells, Fura-2 ratios in 2 mM [Ca2+]o (1.4 ± 0.06) drop when [Ca2+]o is reduced (nominal 0 mM Ca2+, 1 mM EGTA; 0.99 ± 0.07). [Ca2+]i did not change significantly after removal of extracellular Ca2+ for TRPML3 and TRPML3_Va_-KK transfected cells (0.82 + 0.01 and 0.75 + 0.03). (I, J, L, and M). Representative immunohistochemical staining, using tyrosinase- (I and L) and TRPML3- (J and M) specific antibodies of paraffin-embedded skin sections from 6-week-old WT (I–K) and Va/Va (L–N) mice. (K and N) Sections were also stained for TRPML3 by in situ hybridization. Antibody staining is brown; nuclei are stained blue. The in situ signal is visible as a blue precipitate with nuclei stained in red. (Scale bar, 50 μm.) (I) Tyrosinase staining of WT hair follicles indicates that melanocytes are primarily in the bulb of the hair follicle. (J) Staining with a TRPML3-specific antibody indicates that TRPML3-positive cells are localized to a similar region in adult mouse hair follicle. (K) TRPML3-specific in situ hybridization; TRPML3 antibody and the in situ probe stain similar regions in the hair follicle bulb. (L and M) Immunohistochemical staining, using tyrosinase- and TRPML3-specific antibodies, was performed side by side with the WT slides. Neither tyrosinase- nor TRPML3- specific signal was detected in the hair bulb of Va/Va mice. In the WT skin sections, ≈26% of the hair follicles were labeled by the tyrosinase antibody (90/347) or by the TRPML3 specific antibody (66/255). No tyrosinase- or TRPML3-specific staining was detected in Va/Va mouse hair follicle bulbs (≈300 hair follicles were analyzed). (N) TRPML3 is absent in hair follicle bulbs from Va/Va skin sections (in situ hybridizations).
To determine whether cell toxicity resulted from channel-mediated cation influx, melan-a2 cells were transfected with the nonfunctional TRPML3_Va_-KK pore mutant (Fig. 5C). Although also mislocalized compared with the WT expression (Fig. 5 C vs. A), the number of dying cells was significantly reduced when compared with the TRPML3_Va_-expressing cells (Fig. 5G). The toxicity of constitutively active TRPML3 could result from sustained cation influx across the plasma membrane or from equilibration of cations across the late endosomal/lysosomal membranes. TRPML3 and TRPML3_Va_-KK transfected melan-a2 cell resting [Ca2+] levels were similar to nontransfected cells (Fig. 5 D and E), whereas TRPML3_Va_-expressing cells showed significantly higher [Ca2+]i (Fig. 5F). Removal of extracellular Ca2+ reduced [Ca2+]i in TRPML3_Va_-expressing cells, but not in TRPML3 and TRPML3_Va_-KK-transfected cells. We conclude that the constitutive activity of expressed plasma membrane TRPML3_Va_ induced Ca2+ overload and cell death (Fig. 5H).
Skin sections from WT or Va homozygous littermates were stained with TRPML3 antibody or tyrosinase to label melanocytes (10). In WT skin sections, 26% of hair follicles contained melanocytes (Fig. 5 I and J). The presence of TRPML3-expressing cells in the hair bulb was further confirmed by in situ hybridization (Fig. 5K). In contrast, no melanocytes were detected in the hair follicles of skin sections derived from the homozygous Va mouse (Fig. 5 L and M), nor was the hair bulb labeled by the TRPML3-specific in situ probe (Fig. 5N). We conclude most melanocytes are lost from hair follicles of the 6-week-old Va mice, resulting in lighter fur and skin color (Fig. 1E).
Discussion
The primarily intracellular localization of TRPML ion channels had prevented the direct study of mucolipin currents. The varitint–waddler mouse, identified >60 years ago, provides a fortuitous window into the workings of the TRPML family. Based on past work, mutations in the S4/S5 linker junction alter channel gating (17). Analogous mutations in TRPML1 and TRPML3 result in large conductances across the plasma membrane, even at resting membrane potentials. TRPML1 and TRPML3, like most TRP ion channels, are cation-nonselective and Ca2+-permeant; no H+ conductance was detected in this study. Constitutive activity on the plasma membrane is a deleterious gain-of-function, killing melanocytes over time and thus reducing hair and skin pigmentation. These results agree with of the observed loss of melanocytes in the cochlea of Va/Va mice (18). It is likely that cell loss due to mutant TRPML3 in the cochlea and vestibular apparatus underlies the deafness and the circling behavior of Va/Va mice. The low viability of Va/Va mice will require further study. A more important question left unanswered is whether the primary function of TRPML3 is endosomal/lysosomal, on the plasma membrane, or both. Interestingly, several TRP ion channels appear to function on both intracellular membranes and the plasma membrane, and rapid translocation can occur in response to signaling molecules (19).
Materials and Methods
Molecular Biology.
Full-length mouse TRPML1, TRPML2, and TRPML3 were cloned from a mouse macrophage cDNA library and cloned into the EGFP-C2 vector (Clontech) or pcDNA3 containing an HA tag at the 5′end.
Antibodies and Immunoprecipitation.
Rabbit polyclonal antibodies (Chemicon) were raised against N-terminal (amino acids 5–20) and C-terminal (amino acids 528–547) peptides of TRPML3 and affinity-purified (Pierce). TRPML3 antibodies did not recognize overexpressed TRPML1 or TRPML2. Goat anti-tyrosinase antibody was from Santa Cruz Biotechnologies, and mouse HMB45 was from Dako.
HA-TRPML3-transfected cells or melan-a2 cells were solubilized in lysis buffer (50 mM Tris·Cl, pH 8.0/150 mM NaCl/1% Triton X-100) supplemented with protease inhibitor mixture (Roche) and immunoprecipitated with TRPML3 CT antibody.
Immunocytochemistry and Confocal Microscopy.
Cells were fixed with 4% formaldehyde, permeabilized with 0.2% Triton X-100, and blocked by 10% goat serum in PBS. Cells were labeled with TRPML3 antibodies and Cy5-conjugated goat anti-rabbit IgG (Invitrogen) or TO-PRO3 (Invitrogen). Confocal images were obtained with a 60 × 1.4 N.A. objective, zoom 1 (Olympus Fluoview 1000).
Transfection.
Melan-a2 cells were transfected by using electroporation (Amaxa) and assayed 20–48 h after transfection. HEK293T cells and B16 cells were transfected by using Lipofectamine (Invitrogen) and assayed 24 h after transfection.
In situ hybridization was performed by using a 650-bp fragment from the 3′ UTR of mouse TRPML3 cloned into the pCR4-Topo vector. Automated ISH was performed on paraffin-embedded sections (Discovery System; Ventana Medical Systems). Detection was performed by biotinylated anti-digoxigenin antibody (Biogenex) followed by streptavidin-alkaline-phosphatase conjugate and visualized by nitroblue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate substrate reaction (Ventana BlueMap detection kit). Slides were counterstained by nuclear fast red (Vector Laboratories). Frozen section staining was performed as described in ref. 20.
Mice.
C57BL/6 and heterozygous Mcoln_Va_ (TRPML3_Va_) were obtained from The Jackson Laboratory. The breeding scheme for the Mcoln_Va_ line was +/+ × Va/+ or Va/+ × Va/+, and mice were genotyped as described in ref. 5.
Immunohistochemistry.
Formalin-fixed paraffin-embedded tissue sections (5μ) were pretreated with 10 mM citrate, pH 6.0 (Zymed; decloaking chamber, BioCare Medical). Slides were pretreated with peroxidase block (Dako) for 5 min. Primary antibodies were diluted 1:250 (ML3NT) and 1:100 (tyrosinase and HMB45). Slides were washed, and anti-rabbit horseradish peroxidase-conjugated antibody (Envision detection kit, Dako) was applied. Immunoperoxidase staining was developed by using a DAB chromagen kit (Dako) and counterstained with hematoxylin.
Electrophysiology.
Recordings were performed in transiently transfected HEK293T cells. No significant functional difference was observed between EGFP-tagged and HA-tagged TRPML_Va_ constructs. Transfected cells, cultured at 37°C, were plated onto glass coverslips and recorded 24 h after transfection. Unless otherwise stated, the pipette solution contained 147 mM Cs, 120 mM methane-sulfonate, 8 mM NaCl, 10 mM EGTA, 2 mM Mg-ATP, and 20 mM Hepes (pH 7.2; free [Ca2+]i < 10 nM). Standard extracellular bath Tyrode's solution contained 153 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 20 mM Hepes, and 10 mM glucose (pH 7.4). Low-pH external solution contained 150 mM Na-gluconate, 5 mM KCl, 2 mM CaCl2, 1 mM MgCl2,10 mM Hepes, and 10 mM Mes (pH 4.6). NMDG+ solution contained 160 mM NMDG·Cl, 20 mM Hepes, and 10 mM glucose (pH 7.4). Low-pH NMDG+ solution contained 150 mM NMDG, 10 mM glucose, 10 mM Mes, 10 Hepes (pH adjusted to 4.6, using methanesulfonic acid). DVF monovalent solutions contained 30 mM glucose, 20 mM Hepes, 10 mM EDTA, 160 mM KCl, NaCl, or CsCl (pH 7.4; free Ca2+ < 10 nM). Data were collected by using an Axopatch 2A and Digidata 1440 and pClamp software, Version 10.0 (Axon Instruments). Whole-cell currents and single-channel recordings were digitized at 10 kHz and filtered at 2 kHz. Capacity current was nulled; series resistance compensation was 60–85%. All experiments were conducted at room temperature (≈21–23°C). All recordings were analyzed with pCLAMP10 (Axon Instruments) and Origin software, Version 7.5 (OriginLab).
Ca2+ Imaging.
Twenty hours after transfection, melan-a2 cells were loaded with 5 μM Fura-2AM and 0.02% pluronic acid at 37°C for 60 min, then washed for 10–30 min. GFP fluorescence was monitored at 440 nm (F440). Fura-2 ratios (F340/F380) were determined [Ca2+]i, and experiments were repeated four to eight times; representative traces are shown in figures.
Reagents.
2APB, verapamil, nifedipine, amiloride, and SKF96365 were prepared in stock solution containing dimethylsulfulfonate (DMSO; < 0.1% final).
Data Analysis.
Group data are presented as mean ± SEM. Statistical comparisons were made with analysis of variance and the ANOVA test. P < 0.05 was considered statistically significant.
Supplementary Material
Supporting Information
Acknowledgments
We thank the core facility of the Brigham and Women's Hospital for help with the immunohistochemistry and in situ hybridization, Dr. Urs Berger for assistance, Dr. Bimal Desai for his stimulating discussion throughout the project, and members of the Clapham and Xu laboratories for discussion and helpful comments. H.X. was supported by the startup funds from the Department of MCDB and Biological Science Scholar Program, the University of Michigan.
Abbreviations
[Ca2+]
Ca2+ concentration
[X]i
intracellular concentration of an ion X
[X]o
extracellular concentration of an ion X
DVF
divalent-free
I–V
current–voltage
KK
lysine lysine
P_n_
postnatal day n
S_n_
segment n.
Footnotes
The authors declare no conflict of interest.
References
- 1.Pryor PR, Reimann F, Gribble FM, Luzio JP. Traffic. 2006;7:1388–1398. doi: 10.1111/j.1600-0854.2006.00475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Venkatachalam K, Hofmann T, Montell C. J Biol Chem. 2006;281:17517–17527. doi: 10.1074/jbc.M600807200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vergarajauregui S, Puertollano R. Traffic. 2006;7:337–353. doi: 10.1111/j.1600-0854.2006.00387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Song Y, Dayalu R, Matthews SA, Scharenberg AM. Eur J Cell Biol. 2006;85:1253–1264. doi: 10.1016/j.ejcb.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 5.Di Palma F, Belyantseva IA, Kim HJ, Vogt TF, Kachar B, Noben-Trauth K. Proc Natl Acad Sci USA. 2002;99:14994–14999. doi: 10.1073/pnas.222425399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cloudman AM, Bunker LE. J Hered. 1945;36:258–263. [Google Scholar]
- 7.Lane PW. J Hered. 1972;63:135–140. doi: 10.1093/oxfordjournals.jhered.a108252. [DOI] [PubMed] [Google Scholar]
- 8.Kiselyov K, Chen J, Rbaibi Y, Oberdick D, Tjon-Kon-Sang S, Shcheynikov N, Muallem S, Soyombo A. J Biol Chem. 2005;280:43218–43223. doi: 10.1074/jbc.M508210200. [DOI] [PubMed] [Google Scholar]
- 9.Soyombo AA, Tjon-Kon-Sang S, Rbaibi Y, Bashllari E, Bisceglia J, Muallem S, Kiselyov K. J Biol Chem. 2006;281:7294–7301. doi: 10.1074/jbc.M508211200. [DOI] [PubMed] [Google Scholar]
- 10.Botchkareva NV, Botchkarev VA, Gilchrest BA. J Investig Dermatol Symp Proc. 2003;8:76–79. doi: 10.1046/j.1523-1747.2003.12176.x. [DOI] [PubMed] [Google Scholar]
- 11.Nishimura EK, Granter SR, Fisher DE. Science. 2005;307:720–724. doi: 10.1126/science.1099593. [DOI] [PubMed] [Google Scholar]
- 12.Long SB, Campbell EB, MacKinnon R. Science. 2005;309:897–903. doi: 10.1126/science.1116269. [DOI] [PubMed] [Google Scholar]
- 13.Harty TP, Dib-Hajj SD, Tyrrell L, Blackman R, Hisama FM, Rose JB, Waxman SG. J Neurosci. 2006;26:12566–12575. doi: 10.1523/JNEUROSCI.3424-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zeevi DA, Frumkin A, Bach G. Biochim Biophys Acta. 2007;1772:851–858. doi: 10.1016/j.bbadis.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 15.Xu H, Jin J, DeFelice LJ, Andrews NC, Clapham DE. PLoS Biol. 2004:2–E50. doi: 10.1371/journal.pbio.0020050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raychowdhury MK, Gonzalez-Perrett S, Montalbetti N, Timpanaro GA, Chasan B, Goldmann WH, Stahl S, Cooney A, Goldin E, Cantiello HF. Hum Mol Genet. 2004;13:617–627. doi: 10.1093/hmg/ddh067. [DOI] [PubMed] [Google Scholar]
- 17.Clapham DE. Nature. 2003;426:517–524. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- 18.Cable J, Steel KP. Hear Res. 1998;123:125–136. doi: 10.1016/s0378-5955(98)00107-5. [DOI] [PubMed] [Google Scholar]
- 19.Ramsey IS, Delling M, Clapham DE. Annu Rev Physiol. 2006;68:619–647. doi: 10.1146/annurev.physiol.68.040204.100431. [DOI] [PubMed] [Google Scholar]
- 20.Xu H, Delling M, Jun JC, Clapham DE. Nat Neurosci. 2006;9:628–635. doi: 10.1038/nn1692. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information