EZH2 is a marker of aggressive breast cancer and promotes neoplastic transformation of breast epithelial cells (original) (raw)
Abstract
The Polycomb Group Protein EZH2 is a transcriptional repressor involved in controlling cellular memory and has been linked to aggressive prostate cancer. Here we investigate the functional role of EZH2 in cancer cell invasion and breast cancer progression. EZH2 transcript and protein were consistently elevated in invasive breast carcinoma compared with normal breast epithelia. Tissue microarray analysis, which included 917 samples from 280 patients, demonstrated that EZH2 protein levels were strongly associated with breast cancer aggressiveness. Overexpression of EZH2 in immortalized human mammary epithelial cell lines promotes anchorage-independent growth and cell invasion. EZH2-mediated cell invasion required an intact SET domain and histone deacetylase activity. This study provides compelling evidence for a functional link between dysregulated cellular memory, transcriptional repression, and neoplastic transformation.
Breast cancer is a leading cause of cancer-related death in women, accounting for ≈40,000 deaths per year in the United States (1). Despite advances in the early detection and treatment of breast cancer, mortality for those 20% of patients with recurrences and or metastases is ≈100% (2). Currently, the most important prognostic markers for patients with breast cancer that are used in the clinical setting are components of the staging system, such as primary tumor size and the presence of lymph node metastasis (3). However, the accuracy of these conventional indicators is not as precise as desired, leading to inefficient application of systemic therapy (4). Thus, there is a need for novel molecular predictors of tumor behavior at the time of diagnosis that will help guide clinical therapy decisions.
Few biomarkers of breast cancer progression have been proven to be clinically useful (4). Estrogen receptor (ER) and progesterone receptor (PR) are highly predictive of breast cancer patients that will benefit from endocrine therapy (5) but are weak prognostic factors (6). Other tumor markers that have been considered for prognostication in breast cancer include erbB2 amplification/overexpression, cathepsin D, and uPAR (4). The consensus, however, remains that new prognostic factors that are more precise and reliable are needed (7).
Through our gene expression profiling studies, we identified EZH2 as being overexpressed in metastatic prostate cancer (8). In clinically localized prostate cancer, EZH2 was found to be predictive of poor outcome postprostatectomy (i.e., biochemical recurrence or metastasis). EZH2 is a Polycomb Group (PcG) protein homologous to Drosophila Enhancer of Zeste and involved in gene silencing (9, 10). PcG proteins are presumed to function in controlling the transcriptional memory of a cell (9). Dysregulation of this gene silencing machinery can lead to cancer (9, 11, 12). In the context of prostate cancer, we provided evidence that EZH2 functions as a transcriptional repressor, and inhibition of EZH2 blocks prostate cell growth (8). Interestingly, several recent studies demonstrated that EZH2 has enzymatic activity and functions as a histone H3 methyltransferase (13–15).
Biochemical analysis indicates that PcG proteins belong to at least two multimeric complexes, PRC1 (16) and EED-EZH2 (Enx1) (17). These complexes are thought to heritably silence genes by acting at the level of chromatin structure. The EED protein interacts directly with type 1 histone deacetylases (HDACs) in mammalian cells (18), and in Drosophila (19), and this has been suggested to be part of the silencing mechanism. Furthermore, recent studies have demonstrated that EED/EZH2 complexes methylate H3-K9 and K27 in vitro, with a strong preference for K27 (13–15). Methylation of both H3-K9 (20) and H3-K27 is thought to be involved in targeting the PRC1 complex to specific genetic target loci.
By interrogating publicly available gene expression data sets, we identified EZH2 as being dysregulated in breast cancer. In the present study, we examined EZH2 mRNA transcript and protein level in normal breast and in breast cancer progression. Immunohistochemical analyses performed on a spectrum of breast cancer specimens demonstrated that high EZH2 levels were strongly associated with poor clinical outcome in breast cancer patients. EZH2 was an independent predictor of breast cancer recurrence and death and provided prognostic information above and beyond known clinical, pathologic, and biomarkers studied. Overexpression of EZH2 in normal breast epithelial cell lines produced a neoplastic phenotype characterized by anchorage-independent growth and cell invasion. Neoplastic transformation mediated by EZH2 depended on both the SET domain as well as HDAC activity. Importantly, we propose a biologic basis for the association of EZH2 and tumor aggressiveness in that high levels of EZH2 promote the invasive potential of carcinomas.
Methods
Selection of Patients and Tissue Microarray Development. Breast tissues for tissue microarray construction were obtained from the Surgical Pathology files at the University of Michigan with Institutional Review Board approval. A total of 280 cases (n = 917 tissue microarray samples) were reviewed by the study pathologist (C.G.K.) and arrayed in three high-density tissue microarrays, as described (21, 22). See Supporting Methods, which are published as supporting information on the PNAS web site, www.pnas.org, for details.
Immunohistochemical Studies. Immunohistochemistry was performed on the tissue microarrays (TMAs) by using standard biotin–avidin complex technique and a polyclonal antibody against EZH2 that was previously validated by immunoblot analysis (8). See Supporting Methods for detailed methodology. The TMAs were immunostained for ER and PR and for HER-2/neu by using well described and validated procedures (23). See Supporting Methods for details.
Statistical Analysis. Comparison of the intensity of EZH2 staining between normal breast, hyperplasia, ductal carcinoma in situ, invasive carcinoma, and metastases was carried out by calculating the median staining intensity for each case and applying the Wilcoxon rank test. A P value of <0.05 was considered significant. Overall survival was calculated from the date of surgical excision of the primary tumor to the date of death. Patients who died of or with the disease were included in the analysis. For disease-specific survival, data for patients who died from other causes were censored at the time of death. Overall survival and disease-specific survival curves were constructed by the Kaplan–Meier method. Clinical criteria for treatment failure were local recurrence and/or the development of metastases.
Univariate analyses of disease-specific survival were performed by using a two-sided log-rank test to evaluate EZH2 protein expression, age, tumor size, nodal status, stage, angiolymphatic invasion, ER status, PR status, and HER-2/neu status. To assess the influence of several variables simultaneously, a multivariable Cox proportional hazards model of statistically significant covariates was developed by removing nonsignificant parameters in a step-wise manner. Statistical significance in the Cox models was determined by Wald's test.
SYBR Green Quantitative Real-Time PCR. We performed SYBR green real-time quantitative PCR analysis on 19 laser-microdissected frozen breast tissues obtained from the frozen breast tissue bank in our institution with Institutional Review Board approval. See Supporting Methods for details.
Immunoblot Analysis. Protein extracts were prepared from normal and cancerous breast tissues and standard immunoblot analysis performed. See Supporting Methods for details.
Adenovirus Constructs. Adenoviral constructs were generated by in vitro recombination. In brief, the full-length EZH2 or SET domain deleted EZH2 (EZH2ΔSET) were inserted in an adenoviral shuttle plasmid [pACCMVpLpA(–)loxP-SSP]. Viruses were generated by transfection into the 293-complementation cell line. Virus was propagated in 911 cells and purified on a CsCl gradient. Multiplicities of infection were calculated, and purified viruses were stored in 10 mM Tris·HCl (pH 7.4)/137 mM NaCl/5 mM KCl/1 mM MgCl2 in 10% glycerol (by volume).
Cell Count. H16N2 were infected with EZH2 adenovirus. Cell counts were estimated by trypsinizing cells and analysis by Coulter counter at the indicated time points in triplicate.
Soft Agar Assay. A 0.6% (wt/vol) bottom layer of low melting point agarose in normal medium was prepared in six-well culture plates. On top, a layer of 0.6% agarose containing 1 × 105 stable transfected cells was placed (24). After 25 days, foci were stained with P-Iodonitrotetrazolium violet and counted.
HDAC Assay. HDAC activity assays were performed according to the manufacturer's instructions (Biomol, Plymouth Meeting, PA). See Supporting Methods for details.
Basement Membrane Matrix Invasion Assay. Cells were infected with vector, EZH2, and EZH2ΔSET adenovirus. Forty-eight hours after infection, the cells were trypsinized and seeded at equal numbers onto the basement membrane matrix 24-well culture plates [extracellular membrane (ECM); Chemicon] in the presence or absence of HDAC inhibitors suberoylanilide hydroxamic acid (SAHA) (7.5 μM) and trichostatin A (TSA) (0.5 μM). FBS was added to the lower chamber to act as a chemoattractant. After 48-h incubation, the noninvading cells and ECM were removed gently by cotton swab. The cells that are invaded that are present on the lower side of the chamber were stained, air dried, and photographed. The invaded cells were counted under the microscope. For colorimetric assay, the inserts were treated with 150 μl of 10% acetic acid, and absorbance was measured at 560 nm.
Sea Urchin (SU) Embryo Basement Membrane Invasion Assay. H16N2 cells were infected with vector, EZH2, and EZH2ΔSET adenovirus and trypsinized after 48 h. The infected cells alone or treated with HDAC inhibitors SAHA (7.5 μM) and TSA (0.5 μM) and analyzed for invasiveness by using the SU embryo basement membrane invasion assay (25). See Supporting Methods for details.
Chick Chorioallantoic Membrane (CAM) Invasion Assay. EZH2- and control virus-infected H16N2 cells were labeled with Fluoresbrite carboxylated polystyrene nanospheres of 48 nm diameter (Polysciences) as described (26). See Supporting Methods for details.
Results
EZH2 Transcript and Protein Expression Are Elevated in Breast Cancer. On the basis of our previous work characterizing EZH2 in prostate cancer (8), we were interested in determining whether EZH2 is dysregulated in breast cancer, which, similar to prostate cancer, is steroid hormone regulated. This was facilitated by our group's ongoing efforts to create a cancer microarray metaanalysis database (see www.ONCOMINE.org) stemming from our initial work in prostate (27). Of the five publicly available breast cancer gene expression datasets (28–32), only the Perou et al. (28) study had neoplastic and normal breast tissues to make comparisons between benign and cancer. Interestingly, in this dataset, we found that the EZH2 transcript was overexpressed significantly in invasive breast cancer and metastatic breast cancer relative to normal (P = 0.002, t test) (28).
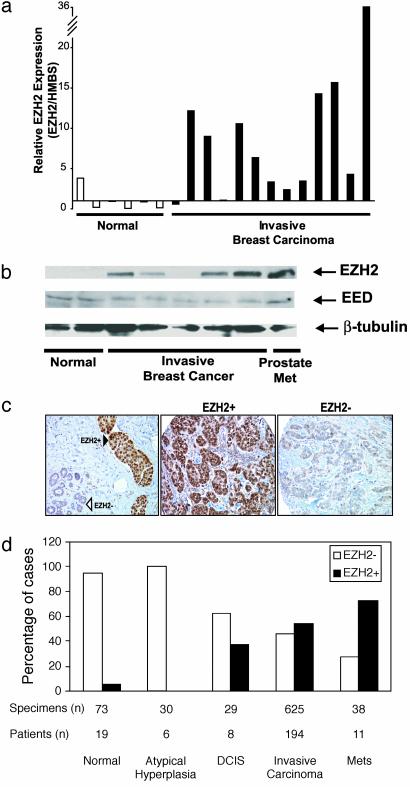
To validate these DNA microarray results, we carried out SYBR green quantitative real-time PCR on 19 laser-capture microdissected normal and invasive breast cancers. As predicted, levels of EZH2 mRNA were increased an average of 7.5-fold in invasive carcinomas compared with normal breast epithelial cells (t test, P = 0.0085) (Fig. 1_a_). To confirm that EZH2 is elevated at the protein level in invasive breast cancer, we analyzed normal breast and breast cancer tissue extracts by immunoblot analysis. Consistent with the transcript data, invasive breast cancer expressed high levels of EZH2 protein relative to normal (Fig. 1_b_). Importantly, EED, a PcG protein that forms a complex with EZH2, did not exhibit similar protein dysregulation.
Fig. 1.
EZH2 mRNA transcript and protein levels are elevated in breast cancer. (a) Quantitative SYBR green RT-PCR of EZH2 transcript in laser-capture microdissected normal and breast cancer epithelia. Each sample was performed in duplicate, and a ratio was calculated relative to the housekeeping gene hydroxymethylbilane synthase (HMBS). (b) Immunoblot analysis of EZH2 and EED in breast tissue extracts. Metastatic (Met) prostate cancer was used as a positive control. β-Tubulin was included as a loading control. (c) Representative breast tissue sections stained with an antibody to EZH2. (Left) Normal breast epithelia (open triangle) and adjacent intravascular breast cancer emboli (filled triangle). (Center) An invasive breast cancer expressing high levels of EZH2. (Right) An invasive breast cancer expressing low levels of EZH2. (d) Tissue microarray analysis of EZH2 expression in breast cancer progression. Tumor specimens were stratified into high EZH2 expressors (filled bars, scored 3 or 4) and low EZH2 expressors (open bars, scored 1 or 2). The y axis represents the percentage of patients in each category.
Using high-density tissue microarrays, we next evaluated the expression of EZH2 protein in a wide range of breast tissues (280 patients, n = 917 samples) to characterize its expression in situ by immunohistochemistry. EZH2 protein expression was observed primarily in the nucleus (Fig. 1_c_), as reported previously (33). Invasive breast cancer that expressed high levels of EZH2 (scores 3–4, EZH2+) and those that expressed low levels of EZH2 (scores 1–2, EZH2–) were readily apparent (Fig. 1_c_ Center and Right). There was a remarkable staining difference between tumor cells that form intravascular emboli and adjacent normal breast epithelia (Fig. 1_c_ Left). Consistent with our mRNA transcript data, EZH2 protein levels were elevated in invasive carcinoma relative to normal or atypical hyperplasia (Wilcoxon test, P < 0.0001, Fig. 1_d_). As in the case of metastatic prostate cancer (8), breast cancer metastases expressed high levels of EZH2 (Fig. 1_d_). Median EZH2 staining intensities of normal, atypical hyperplasia, ductal carcinoma in situ (DCIS), invasive carcinoma, and metastases were 1.47 (SE 0.61), 2 (SE 0), 2.38 (SE 0.52), 2.74 (SE 0.99), and 3.09 (SE 1.04), respectively (Fig. 1_d_). Interestingly, increased EZH2 protein and transcript were already present in DCIS, a precursor of invasive carcinoma (Fig. 1_d_).
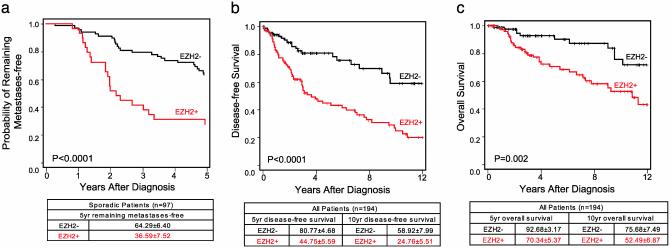
Prognostic Value of EZH2 in Breast Cancer. To investigate whether EZH2 mRNA expression levels are associated with outcome, we analyzed the published van't Veer et al. (30) breast cancer gene expression dataset, which contains outcome information on 78 sporadic invasive carcinomas <5 cm with negative lymph nodes. We found that the levels of EZH2 transcript expression were significantly higher in invasive carcinomas that metastasized within 5 years of primary diagnosis when compared with invasive carcinomas that did not metastasize (Wilcoxon rank test _P_ = 0.01, Fig. 2_a_). By Kaplan–Meier analysis, high EZH2 expression [>1.26 (log10 ratio >0.1)] was associated significantly with the development of metastasis within 5 years of primary diagnosis (log rank P < 0.0001). Multivariable Cox hazards regression analysis showed that EZH2 mRNA expression was an independent predictor of the development of metastases with a hazard ratio of 2.02 (95% confidence interval 1.08–3.76, P = 0.03).
Fig. 2.
High EZH2 levels are associated with aggressive breast cancer. (a) Kaplan–Meier analysis of metastasis-free survival according to EZH2 mRNA transcript levels as measured using DNA microarrays by van't Veer et al. (30). Kaplan–Meier analysis of disease-specific (b) and overall (c) survival according to EZH2 protein levels as assessed by immunohistochemical analysis. Patients grouped on the basis of high (+) or low (–) EZH2 expression levels. P values were calculated by using the log-rank test.
By using our breast cancer tissue microarray data, we were in the position to evaluate clinical and pathology associations of EZH2 protein levels in breast cancer. In our cohort of 236 consecutive breast cancer patients (n = 712 samples), 194 had complete follow-up information. Clinicopathologic characteristics of the patients can be found in Table 1. The median age of the study population was 56 years (ranging from 26 to 89 years). After a median follow-up of 3.2 years (range 17 days to 15.8 years), 42 of the 194 patients (21.6%) died of breast cancer. The 5- and 10-year disease-specific survival rates for the entire cohort of patients were 60.28% and 38.66%, respectively. The association between EZH2 protein levels and clinical characteristics is shown in Table 3, which is published as supporting information on the PNAS web site. EZH2 expression was strongly associated with standard pathology predictors of clinical outcome, including tumor diameter (P = 0.002) and stage of disease (P < 0.0001). Higher EZH2 levels were also significantly associated with decreasing age (P = 0.0003), negative ER status (P = 0.0001), negative PR status (P < 0.0001), and lymph node status (P = 0.001), but not HER2/neu overexpression. Hazard ratios of recurrence or metastasis according to EZH2 status were 2.92 (P < 0.0001).
Table 1. Demographics of patients with clinical follow-up used in this study.
| Parameter | Value |
|---|---|
| No. of patients | 194 |
| Median age, years (range) | 56 (26-89) |
| Follow-up/years, median (range) | 3.2 years (17 d-16 years) |
| Pathologic stage, no. (%) | |
| I | 78 (40) |
| II | 66 (34) |
| III | 32 (16) |
| IV | 18 (10) |
| Tumor size, cm (range) | 2 (0.3-6.7) |
| Lymph node status, no. (%) | |
| Negative | 99 (56) |
| Positive | 78 (44) |
| ER status | |
| Negative, no. (%) | 67 (36) |
| Positive, no. (%) | 120 (64) |
| PR status | |
| Negative, no. (%) | 86 (45) |
| Positive, no. (%) | 107 (55) |
| HER-2/neu status | |
| Negative, no. (%) | 163 (85) |
| Positive, no. (%) | 28 (15) |
The results of the univariate analysis are shown in Table 4, which is published as supporting information on the PNAS web site. As expected, at the univariate level, lymph node status, tumor diameter, and stage of disease were associated with disease-specific and overall survival. Hormone receptor status was inversely associated with outcome. We found a strong association between EZH2 protein levels and patient outcome. Higher EZH2 protein levels were associated with a shorter disease-free interval after initial surgical treatment, lower overall survival, and a high probability of disease-specific death (or death due to breast cancer) (Fig. 2 b and c). The 10-year disease-free survival for patients with tumors expressing high EZH2 levels was 24.76% and, by contrast, 58.92% for low levels of EZH2 (log rank P < 0.0001, Fig. 2_b_). High EZH2 expression was associated with disease-specific survival in patients with lymph node-negative disease (log rank P = 0.007). EZH2 expression was associated with disease-specific survival in patients with stage I and II disease (log rank, P = 0.037 and P = 0.048, respectively), but not in patients with advanced stage (stages III and IV). EZH2 was not associated with survival in patients with positive lymph nodes. The strong inverse association between high EZH2 protein expression and negative ER status (Kruskal–Wallis test, P = 0.001, Table 3) prompted us to investigate whether the prognostic utility of EZH2 depends on ER status. Kaplan–Meier analysis showed that EZH2 levels were strongly associated with outcome in both ER-positive and -negative invasive carcinomas (see Fig. 5, which is published as supporting information on the PNAS web site). Thus, our data suggest that EZH2 has prognostic utility independently of ER status.
The best multivariable model predictive of disease-specific survival included positive lymph nodes, high EZH2 expression, and negative PR status (Table 2). High EZH2 expression was a strong independent predictor of outcome providing survival information above other independent prognostic features, with a hazard ratio of 2.04 and a 95% confidence interval of 1.17–3.57, P = 0.01. Tumor size, angiolymphatic invasion, and ER status, identified as having strong associations with EZH2 at the univariate level, were not independently associated with outcome at the multivariable level.
Table 2. Independent factors predictive of death from breast cancer.
| Parameter | P value | Hazard ratio | 95% confidence interval for hazard ratio | |
|---|---|---|---|---|
| EZH2 positive (vs. negative) | 0.01 | 2.04 | 1.17 | 3.57 |
| Positive lymph nodes (≥4, 1-3, 0) | <0.0001 | 1.9 | 1.4 | 2.57 |
| PR positive (vs. negative) | 0.02 | 0.54 | 0.32 | 0.91 |
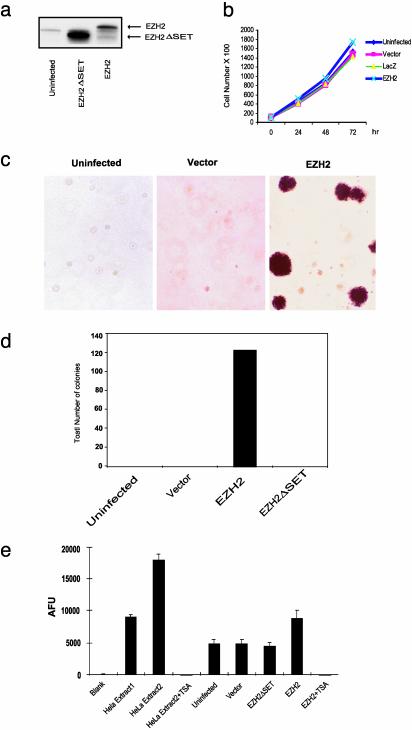
EZH2 Overexpression Promotes Anchorage-Independent Growth and HDAC Activity in Normal Breast Epithelial Cells. To study the function of dysregulated EZH2 expression in breast epithelial cells, we generated adenovirus constructs expressing EZH2. We also generated an adenovirus expressing a mutant version of EZH2 in which the C-terminal SET domain is truncated (EZH2ΔSET). Normal immortalized breast epithelial cells (H16N2) (34) were infected with EZH2 and EZH2ΔSET expressing viruses and protein expression demonstrated in Fig. 3_a_. Overexpression of EZH2 in breast epithelial cells did not significantly enhance cell proliferation in tissue culture (Fig. 3_b_). Interestingly, EZH2 overexpression markedly promoted colony formation in soft agar relative to EZH2ΔSET and vector controls (Fig. 3 c and d). In fact, colonies were present only in EZH2-infected H16N2 cells, supporting the notion that EZH2 can facilitate anchorage-independent growth. As in our previous study with prostate cells (8), overexpression of EZH2 in breast carcinoma cells induced transcriptional repression of a cohort of target genes (data not shown). Previous studies have demonstrated that the EED–EZH2 complex recruits type I HDACs (18). To determine whether overexpression of EZH2 modulates HDACs, we measured HDAC enzymatic activity in breast epithelial cell lysates. Overexpression of EZH2 but not the EZH2ΔSET mutant increased total HDAC activity in breast epithelial cells. This activity was completely abrogated in the presence the HDAC inhibitor TSA.
Fig. 3.
Anchorage-independent growth mediated by EZH2. (a) Immunoblot analysis of breast cell line H16N2 infected with adenovirus encoding EZH2 or EZH2 Δ SET mutant. (b) Ectopic overexpression of EZH2 does not significantly enhance growth of breast epithelial cells in culture. H16N2 cells were infected with EZH2 adenovirus and controls, and cells were counted at indicated time points. LacZ adenovirus and vector adenovirus were used as controls. (c) EZH2 expression enhances anchorage-independent growth in vitro. H16N2 cells were infected with EZH2, EZH2 Δ SET, or vector adenoviruses. Anchorage-independent growth was determined by assaying colony formation in soft agar as described in Methods. After 25 days, the plates were stained and photographed. (d) Quantitation of soft agar colonies from experiments described in c. Colonies from three wells were quantitated for each condition. (e) EZH2 induces HDAC activity in breast epithelial cells. HDAC activity was measured in extracts from H16N2 cells infected with indicated viruses ± treatment with TSA (1.0 μM). As indicated by the manufacturer (Biomol), nuclear extracts from HeLa cells were used as positive controls. Extract 2 had 2-fold more HDAC activity than Extract 1. AFU, arbitrary fluorescence units.
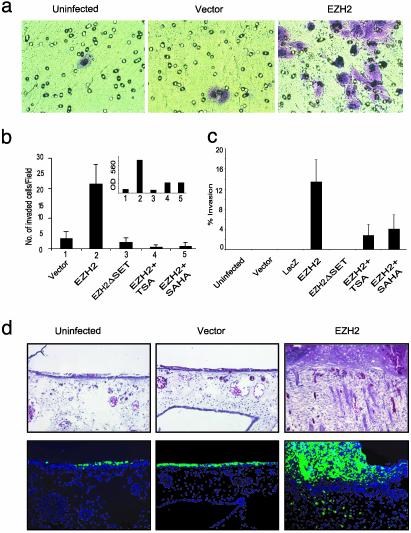
Dysregulated EZH2 Orchestrates the Invasive Potential of Breast Epithelial Cells. We next assessed the biological function of EZH2 in the context of cancer cell invasion. We observed that overexpression of EZH2 in breast epithelial cells promotes invasion in a reconstituted basement membrane invasion chamber assay (Fig. 4 a and b). The control experiments that included EZH2ΔSET mutant and vector did not exhibit similar proinvasive properties. Importantly, EZH2-mediated invasion was attenuated with inclusion of the HDAC inhibitors TSA and SAHA. Cell invasion was quantitated by both cell counting and colorimetry (Fig. 4_b_). Next, we used SU-ECM (25, 35) as invasion substrates to examine the invasive properties of EZH2 expressing breast epithelial cells. The SU-ECM assay has advantages over the reconstituted basement membrane assay in that it is a uniform, biological, serum-free basement membrane that closely mimics the type of extracellular matrix that cells encounter in vivo. As with the reconstituted basement membrane assay, EZH2 overexpression in the SU-ECM assay supported similar findings regarding the invasive potential of EZH2 and its requirement for HDAC activity (Fig. 4_c_).
Fig. 4.
EZH2 orchestrates cell invasion both in vitro and in vivo.(a) A reconstituted basement membrane invasion chamber assay (Chemicon) was used to assess breast epithelial cell lines infected with EZH2 and control adenoviruses. Representative fields of invaded and stained cells are shown. (b) The numbers of invaded cells were counted in six fields, and the mean values were determined. Quantitation by colorimetry (absorbance at 560 nm) is shown in Inset.(c) EZH2-mediated invasion of SU-ECM. H16N2 cells were infected with EZH2, EZH2 Δ SET, or control adenoviruses. (d) EZH2 overexpression mediates invasion of breast epithelial cells in a CAM assay. (Upper) CAM tissues stained with hematoxylin/eosin. Arrows indicate the cells that have invaded the CAM. Because cells were labeled with Fluoresbrite carboxylated polystyrene nanospheres, they could also be visualized by fluorescence (Lower).
To examine the role of EZH2-mediated invasion in an in vivo setting, we used a CAM assay. In this model, EZH2 overexpressing breast epithelial cells are labeled with fluorescent beads, seeded in duplicate on CAMs, of 10-day-old chicken embryos and incubated. At time of harvest, frozen sections were made from the CAM tissues and examined by fluorescent and light microscopy after hematoxylin/eosin staining. EZH2 overexpressing breast epithelial cells consistently promoted invasion of the CAM (a representative experiment is shown in Fig. 4_d_).
Discussion
In the present study, we characterized the expression pattern of EZH2 transcript and protein in a wide spectrum of breast disease and assessed the utility of EZH2 as a prognostic marker in patients with breast cancer. EZH2 is significantly increased in invasive carcinoma and breast cancer metastases at both the transcript and protein levels when compared with normal breast tissues. Cells forming intravascular tumor emboli had strikingly increased EZH2 expression (Fig. 1_c_ Left), suggesting that EZH2 may play a role in vascular invasion and breast cancer metastasis. In vitro and in vivo experiments in which EZH2 was ectopically overexpressed in normal mammary epithelial cell lines provide biological evidence that EZH2 can mediate anchorage-independent growth and cell membrane invasion, hallmarks of cancer (36). This is especially intriguing in that EZH2, which targets transcriptional repression of target genes, presumably mediates an invasive cancer phenotype.
To test the clinical utility of EZH2 protein expression as a prognostic biomarker of breast cancer progression, we evaluated the associations between EZH2 and survival afer treatment. At the univariate level, EZH2, tumor stage, tumor size, the presence of axillary lymph node metastases, and hormone receptor status were all significantly associated with survival. In a multivariable Cox regression analysis, high EZH2 expression and lymph node metastasis were independent predictors of outcome. The single best multivariable model included high EZH2 levels, positive lymph nodes, and negative PR status. In silico analysis of the cDNA expression profiling of breast cancer performed by van't Veer et al. (30) showed that high EZH2 levels were associated with the development of metastasis within 5 years of primary diagnosis in patients with sporadic invasive carcinomas. These findings support the potential clinical utility of incorporating EZH2 into clinical nomograms to help determine the risk of cancer progression.
A major limitation of our analysis is its retrospective nature, which precludes an accurate analysis of survival in the context of hormonal or adjuvant treatment. In our patient cohort, 88% ER-positive tumors received hormonal treatment. Thus, we critically evaluated the prognostic significance of EZH2, taking into account tumor ER status. EZH2 was strongly associated with clinical outcome in hormone-dependent and -independent breast cancer patients, indicating that the prognostic power of EZH2 is independent of ER status. Future studies will test the model developed in this study on a validation cohort to confirm these initial observations.
The prognostic significance of EZH2 as biomarker for aggressive breast cancer is likely linked to its biological functions. EZH2 is a member of a group of polycomb proteins that are involved in maintaining heritable gene expression profiles and thus regulate cell type identity. Thus, dysregulation of the transcriptional machinery of a cell may result in loss of cell type identity and neoplastic transformation. Here we provide biological evidence that dysregulated EZH2 promotes oncogenic transformation. Overexpression of EZH2 in breast epithelial cells induced anchorage-independent growth and cell invasion. Invasive properties of EZH2 overexpressing cells were demonstrated in both in vitro assays (i.e., basement membrane invasion chamber and SU-ECM assays) as well as in an in vivo assay (i.e., CAM). EZH2 overexpression induced HDAC enzymatic activity in breast epithelial cells. Interestingly, EZH2-mediated cell invasion are abrogated by the HDAC inhibitors TSA and SAHA, implying that EZH2-mediated invasion requires HDAC activity. Previous reports have shown that type I HDACs are recruited to the EZH2-EED PcG complex (18). Our group and other groups have found that EZH2-mediated gene silencing requires an intact SET domain and recruitment of HDAC activity (8), and that inhibition of HDAC activity blocked the transcriptional repressor functions of EZH2. Several HDAC inhibitors, including SAHA, have been shown to have promise clinically as antitumorigenic agents (37). Thus, we suggest that inhibitors of HDAC may be useful therapeutic compounds in EZH2 overexpressing tumors. In addition, the HDAC activity induced by EZH2 may explain the intriguing strong association between EZH2 protein expression and negative ER, and one might speculate that EZH2 may transcriptionally repress ER. Further investigation in this area may be warranted.
Several recent studies provide strong evidence that EZH2 has inherent activity as a histone H3 methyltransferase, which may represent the mechanism of PcG silencing (10, 13–15). Cao et al. (13) present evidence that the specific target of EZH2 is lysine 27 on the histone H3 N-terminal tail (13). If EZH2 plays a role in breast cancer progression, its inherent methyltransferase activity may serve as an attractive therapeutic target. Together, these studies suggest that the transcriptional memory machinery of a cell may have a role in cancer progression.
In summary, we discovered that EZH2 is a promising biomarker of aggressive breast cancer, not only extending our initial observations in prostate cancer but also suggesting that EZH2 (and thus the cell memory machinery) may have a role in carcinoma progression in malignancies from hormonally regulated tissues. Clinically, our retrospective studies suggest that EZH2 levels can be used to identify patients with breast cancer of a more aggressive phenotype, thereby enhancing our prognostic knowledge. Although our results are promising, EZH2 expression needs to be validated in relationship to outcome in the context of carefully controlled clinical trials. If confirmed, application of EZH2 immunohistochemical analysis should be technically straightforward and feasible. In addition to the potential prognostic utility of EZH2, we also provide a biologic mechanism for its association with aggressive cancers, by mediating anchorage-independent growth and cell invasion.
Supplementary Material
Supporting Information
Acknowledgments
We thank M. Wicha for helpful suggestions and S. Merajver for helpful discussions, substantive revisions of the text, and support of C.G.K.'s research. We also thank S. Ethier (University of Michigan, Ann Arbor) for breast epithelial cell lines, T. Jenuwein (Research Institute of Molecular Pathology, Vienna) for the EZH2 plasmid, K. Hamer for antibody production and purification, J. Wolf for tissue microarray construction, S. Markwart for technical assistance, S. Bhagavathula for database support, and T. Lamigan and the Vector Core for adenovirus generation. A.M.C. is a Pew Foundation Scholar, and S.A.T. is supported by the Medical Scientist Training Program. This work was supported in part by Department of Defense Grants DAMD17-02-1-0490 and DAMD17-02-1-491 (to C.G.K.); a Munn Grant from the University of Michigan (to C.G.K.); a Breast Cancer Research Foundation grant (to A.M.C.); a grant from the Mary Kay Ash Foundation (to A.M.C.); a grant from the V Foundation (to A.M.C.); National Institutes of Health Grant RO1 CA97063 (to A.M.C., D.G., and M.A.R.); and National Institutes of Health Grant P50CA69568 (to A.M.C., D.G., and M.A.R.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: ER, estrogen receptor; PR, progesterone receptor; PcG, Polycomb Group; HDAC, histone deacetylase; TSA, trichostatin A; ECM, extracellular membrane; SU, sea urchin; CAM, chicken chorioallantoic membrane; SAHA, suberoylanilide hydroxamic acid.
References
- 1.Jemal, A., Murray, T., Samuels, A., Ghafoor, A., Ward, E. & Thun, M. J. (2003) CA Cancer J. Clin. 53**,** 5–26. [DOI] [PubMed] [Google Scholar]
- 2.Ellis, M., Hayes, D. & Lippman, M. (2000) in Diseases of the Breast, eds. Harris, J., Lippman, M. E. & Morrow, M. (Lippincott–Raven, Philadelpha), pp. 749–798.
- 3.Hayes, D. F., Isaacs, C. & Stearns, V. (2001) J. Mammary Gland Biol. Neoplasia 6**,** 375–392. [DOI] [PubMed] [Google Scholar]
- 4.Hayes, D. F., Trock, B. & Harris, A. L. (1998) Breast Cancer Res. Treat. 52**,** 305–319. [DOI] [PubMed] [Google Scholar]
- 5.Honig, S. (1996) in Diseases of the Breast, eds. Harris, J. L. M., Morrow, M. & Hellman, S. (Lippincott–Raven, Philadelphia), pp. 461–485.
- 6.Clark, G. M. (1996) in Diseases of the Breast, eds. Harris. J. L. M.., Morrow, M. & Hellman, S. (Lippincott–Raven, Philadephia), pp. 461–485.
- 7.Hayes, D. F. (2000) Eur. J. Cancer 36**,** 302–306. [DOI] [PubMed] [Google Scholar]
- 8.Varambally, S., Dhanasekaran, S. M., Zhou, M., Barrette, T. R., Kumar-Sinha, C., Sanda, M. G., Ghosh, D., Pienta, K. J., Sewalt, R. G., Otte, A. P., et al. (2002) Nature 419**,** 624–629. [DOI] [PubMed] [Google Scholar]
- 9.Laible, G., Wolf, A., Dorn, R., Reuter, G., Nislow, C., Lebersorger, A., Popkin, D., Pillus, L. & Jenuwein, T. (1997) EMBO J. 16**,** 3219–3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Satijn, D. P. & Otte, A. P. (1999) Biochim. Biophys. Acta 1447**,** 1–16. [DOI] [PubMed] [Google Scholar]
- 11.Jacobs, J. J., Kieboom, K., Marino, S., DePinho, R. A. & van Lohuizen, M. (1999) Nature 397**,** 164–168. [DOI] [PubMed] [Google Scholar]
- 12.Jacobs, J. J., Scheijen, B., Voncken, J. W., Kieboom, K., Berns, A. & van Lohuizen, M. (1999) Genes Dev. 13**,** 2678–2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cao, R., Wang, L., Wang, H., Xia, L., Erdjument-Bromage, H., Tempst, P., Jones, R. S. & Zhang, Y. (2002) Science 298**,** 1039–1043. [DOI] [PubMed] [Google Scholar]
- 14.Czermin, B., Melfi, R., McCabe, D., Seitz, V., Imhof, A. & Pirrotta, V. (2002) Cell 111**,** 185–196. [DOI] [PubMed] [Google Scholar]
- 15.Muller, J., Hart, C. M., Francis, N. J., Vargas, M. L., Sengupta, A., Wild, B., Miller, E. L., O'Connor, M. B., Kingston, R. E. & Simon, J. A. (2002) Cell 111**,** 197–208. [DOI] [PubMed] [Google Scholar]
- 16.Shao, Z., Raible, F., Mollaaghababa, R., Guyon, J. R., Wu, C. T., Bender, W. & Kingston, R. E. (1999) Cell 98**,** 37–46. [DOI] [PubMed] [Google Scholar]
- 17.Sewalt, R. G., van der Vlag, J., Gunster, M. J., Hamer, K. M., den Blaauwen, J. L., Satijn, D. P., Hendrix, T., van Driel, R. & Otte, A. P. (1998) Mol. Cell. Biol. 18**,** 3586–3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van der Vlag, J. & Otte, A. P. (1999) Nat. Genet. 23**,** 474–478. [DOI] [PubMed] [Google Scholar]
- 19.Tie, F., Furuyama, T., Prasad-Sinha, J., Jane, E. & Harte, P. J. (2001) Development (Cambridge, U.K.) 128**,** 275–286. [DOI] [PubMed] [Google Scholar]
- 20.Sewalt, R. G., Lachner, M., Vargas, M., Hamer, K. M., den Blaauwen, J. L., Hendrix, T., Melcher, M., Schweizer, D., Jenuwein, T. & Otte, A. P. (2002) Mol. Cell. Biol. 22**,** 5539–5553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dhanasekaran, S. M., Barrette, T. R., Ghosh, D., Shah, R., Varambally, S., Kurachi, K., Pienta, K. J., Rubin, M. A. & Chinnaiyan, A. M. (2001) Nature 412**,** 822–826. [DOI] [PubMed] [Google Scholar]
- 22.Perrone, E. E., Theoharis, C., Mucci, N. R., Hayasaka, S., Taylor, J. M., Cooney, K. A. & Rubin, M. A. (2000) J. Natl. Cancer Inst. 92**,** 937–939. [DOI] [PubMed] [Google Scholar]
- 23.Camp, R. L., Charette, L. A. & Rimm, D. L. (2000) Lab. Invest. 80**,** 1943–1949. [DOI] [PubMed] [Google Scholar]
- 24.Zhou, M., Chinnaiyan, A. M., Kleer, C. G., Lucas, P. C. & Rubin, M. A. (2002) Am. J. Surg. Pathol. 26**,** 926–931. [DOI] [PubMed] [Google Scholar]
- 25.Livant, D. L., Linn, S., Markwart, S. & Shuster, J. (1995) Cancer Res. 55**,** 5085–5093. [PubMed] [Google Scholar]
- 26.Morris, V. L., Koop, S., MacDonald, I. C., Schmidt, E. E., Grattan, M., Percy, D., Chambers, A. F. & Groom, A. C. (1994) Clin. Exp. Metastasis 12**,** 357–367. [DOI] [PubMed] [Google Scholar]
- 27.Rhodes, D. R., Barrette, T. R., Rubin, M. A., Ghosh, D. & Chinnaiyan, A. M. (2002) Cancer Res. 62**,** 4427–4433. [PubMed] [Google Scholar]
- 28.Perou, C. M., Sorlie, T., Eisen, M. B., van de Rijn, M., Jeffrey, S. S., Rees, C. A., Pollack, J. R., Ross, D. T., Johnsen, H., Akslen, L. A., et al.. (2000) Nature 406**,** 747–752. [DOI] [PubMed] [Google Scholar]
- 29.Hedenfalk, I., Duggan, D. D., Chen, Y., Radmacher, M., Bittner, M., Simon, R., Meltzer, P., Gusterson, B. A., Esteller, M., Kallioniemi, O. P., et al. (2001) New Engl. J. Med. 344**,** 539–548. [DOI] [PubMed] [Google Scholar]
- 30.van't Veer, L. J., Dai, H., van de Vijver, M. J., He, Y. D., Hart, A. A., Mao, M., Peterse, H. L., van der Kooy, K., Marton, M. J., Witteveen, A. T., et al. (2002) Nature 415**,** 530–536. [DOI] [PubMed] [Google Scholar]
- 31.Gruvberger, S., Ringner, M., Chen, Y., Panavally, S., Saal, L. H., Borg, A., Ferno, M., Peterson, C. & Meltzer, P. S. (2001) Cancer Res. 61**,** 5979–5984. [PubMed] [Google Scholar]
- 32.Sorlie, T., Perou, C. M., Tibshirani, R., Aas, T., Geisler, S., Johnsen, H., Hastie, T., Eisen, M. B., van de Rijn, M., Jeffrey, S. S., et al. (2001) Proc. Natl. Acad. Sci. USA 98**,** 10869–10874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raaphorst, F. M., van Kemenade, F. J., Blokzijl, T., Fieret, E., Hamer, K. M., Satijn, D. P., Otte, A. P. & Meijer, C. J. (2000) Am. J. Pathol. 157**,** 709–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ignatoski, K. M., Lapointe, A. J., Radany, E. H. & Ethier, S. P. (1999) Endocrinology 140**,** 3615–3622. [DOI] [PubMed] [Google Scholar]
- 35.Livant, D. L., Brabec, R. K., Pienta, K. J., Allen, D. L., Kurachi, K., Markwart, S. & Upadhyaya, A. (2000) Cancer Res. 60**,** 309–320. [PubMed] [Google Scholar]
- 36.Hanahan, D. & Weinberg, R. A. (2000) Cell 100**,** 57–70. [DOI] [PubMed] [Google Scholar]
- 37.Huang, L. & Pardee, A. B. (2000) Mol. Med. 6**,** 849–866. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information