The Small GTPases Rho and Rac Are Required for the Establishment of Cadherin-dependent Cell–Cell Contacts (original) (raw)
Abstract
Cadherins are calcium-dependent cell–cell adhesion molecules that require the interaction of the cytoplasmic tail with the actin cytoskeleton for adhesive activity. Because of the functional relationship between cadherin receptors and actin filament organization, we investigated whether members of the Rho family of small GTPases are necessary for cadherin adhesion. In fibroblasts, the Rho family members Rho and Rac regulate actin polymerization to produce stress fibers and lamellipodia, respectively. In epithelial cells, we demonstrate that Rho and Rac are required for the establishment of cadherin-mediated cell–cell adhesion and the actin reorganization necessary to stabilize the receptors at sites of intercellular junctions. Blocking endogenous Rho or Rac selectively removed cadherin complexes from junctions induced for up to 3 h, while desmosomes were not perturbed. In addition, withdrawal of cadherins from intercellular junctions temporally precedes the removal of CD44 and integrins, other microfilament-associated receptors. Our data showed that the concerted action of Rho and Rac modulate the establishment of cadherin adhesion: a constitutively active form of Rac was not sufficient to stabilize cadherindependent cell–cell contacts when endogenous Rho was inhibited. Upon induction of calcium-dependent intercellular adhesion, there was a rapid accumulation of actin at sites of cell–cell contacts, which was prevented by blocking cadherin function, Rho or Rac activity. However, if cadherin complexes are clustered by specific antibodies attached to beads, actin recruitment to the receptors was perturbed by inhibiting Rac but not Rho. Our results provide new insights into the role of the small GTPases in the cadherin-dependent cell– cell contact formation and the remodelling of actin filaments in epithelial cells.
Adhesion of cells to their neighbors is an important process for the differentiation of multicellular organisms. During embryonic development and adult life, the cadherin superfamily of cell–cell adhesion receptors has been implicated in many processes such as cell segregation, proliferation, differentiation, and invasive behavior (for review see Takeichi, 1991, 1993). In epithelial tissues, cadherins are required for the assembly of cells into multiple layers and the establishment and maintenance of the epithelial phenotype (Rodriguez-Boulan and Nelson, 1989; Wheelock and Jensen, 1992; Hodivala and Watt, 1994; Lewis et al., 1994). Functional cadherin receptors can influence the reorganization of the microfilament, microtubule, and intermediate cytoskeleton, the distribution of cytoplasmic and transmembrane proteins in a polarized fashion, and the formation of gap junctions and other adhesive structures (desmosomes and tight junctions; Green et al., 1987; Magee et al., 1987; Gumbiner et al., 1988; Mege et al., 1988; McNeill et al., 1990; Jongen et al., 1991; Zamansky et al., 1991; Lewis et al., 1994; Amagai et al., 1995).
Cadherins are transmembrane molecules that bind to the same type of cadherin in adjacent cells (homophilic binding; Takeichi, 1991). Their adhesive function requires calcium ions and the association of the cytoplasmic tail with the actin cytoskeleton, which is mediated by the interaction with intracellular proteins called catenins (α-catenin and the armadillo proteins β-catenin, plakoglobin, and p120; for review see Cowin and Burke, 1996). In addition to their role in cadherin-mediated adhesion, the armadillo proteins also bind to other cytoplasmic and nuclear partners and participate in signal transduction events (Rubinfeld et al., 1993, 1996; Su et al., 1993; Behrens et al., 1996; for review see Gumbiner, 1995). Binding to different partners (including the cadherin cytoplasmic tail) occurs via repeats of 42 amino acids, a new protein–protein interaction motif (armadillo repeats; Peifer et al., 1994_a_ ). It is believed that the association of cadherin–catenin complexes with the cytoskeleton does not affect the binding of the cadherin extracellular domains (Wheelock et al., 1987; Bixby and Zhang, 1990) but rather participates in the clustering of the receptors at sites of cell–cell contacts, providing strength to adhesion (Kemler, 1993; Brieher et al., 1996).
In spite of the importance of cadherin function for many developmental processes and tumor metastasis, the model postulated for the regulation of cadherin-mediated cell– cell adhesion is still very fragmented. It is not known how the association of the different catenins with the cadherin tail is regulated, how these complexes cluster at sites of cell– cell contacts, or how the recruitment of cytoskeletal and signaling proteins to intercellular junctions is achieved. Recent results provide insights on some of these issues, as the translocation of the catenins from intracellular pools to the membrane may be regulated by phosphorylation (Bradley et al., 1993; Hinck et al., 1994; Peifer et al., 1994_b_ ; Balsamo et al., 1996; Munemitsu et al., 1996; Rubinfeld et al., 1996). However, induction of calcium-dependent cell–cell contacts does not alter the proportion of catenins associated to cadherin complexes nor their phosphorylation levels in keratinocytes (Braga et al., 1997). In other cell lines, increased tyrosine phosphorylation of β-catenin correlates with conditions that perturb cell–cell adhesion, such as the expression of oncogenes (v-src, ras) and treatment with orthovanadate or growth factors (epidermal growth factor and hepatocyte growth factor/scatter factor; HGF/SF; Matsuyoshi et al., 1992; Volberg et al., 1992; Behrens et al., 1993; Shibamoto et al., 1994; Kinch et al., 1995; Takeda et al., 1995). Yet no specific kinase nor the important phosphorylation sites in the catenin sequences have been identified.
Because cadherin function is dependent on the interaction with the actin cytoskeleton, we sought to investigate whether the Rho family of small GTPases is required for cadherin-mediated adhesiveness. Members of the Rho subfamily belong to the Ras GTPase superfamily and provide a link between growth factor signaling and reorganization of the actin cytoskeleton (for review see Machesky and Hall, 1996; Zigmond, 1996). Studies in fibroblasts revealed that members of the Rho family modulate different aspects of actin organization and cell morphology, i.e., stress fibers, lamellipodia, and filopodia are regulated by Rho, Rac, and Cdc42, respectively (Ridley and Hall, 1992; Ridley et al., 1992; Nobes and Hall, 1995). In addition, Rho and Rac modulate the formation of focal complexes, sites of clustering of integrin receptors that mediate adhesion to substratum (Ridley and Hall, 1992; Ridley et al., 1992; Nobes and Hall, 1995). However, additional functions have been assigned to these GTPases, as their role in different cell types is investigated and specific targets identified. In the past few years, the Rho proteins have been implicated in the regulation of endocytosis, secretion, cytokinesis, ras transformation, and cell proliferation (for review see Ridley, 1996).
Evidence in the literature suggests that these small GTPases may also participate in the organization of the actin cytoskeleton in epithelial cells. First, armadillo repeats are also found in smgGDS, an exchange factor for small GTPases (Hiraoka et al., 1992; Peifer et al., 1994_a_ ). Second, during Drosophila development, Cdc42 is required for the elongation and maintenance of the polarized cell shape (Eaton et al., 1995), while Rac1 participates in the accumulation of actin and myosin at intercellular junctions (Eaton et al., 1995; Harden et al., 1995). Finally, inactivation of Rho by treatment with C3 transferase affects the organization and permeability of tight junctions in polarized epithelia (Nusrat et al., 1995). Collectively, these results indicate that the small GTPases may be required for the maintenance of the cytoskeletal organization in fully differentiated epithelia.
Here we show for the first time that Rho and Rac activities are required for the establishment of cadherin-mediated adhesion and the actin reorganization necessary to stabilize the receptors at junctions. Although the changes in the actin cytoskeleton induced by cell–cell or cell–substratum adhesion are well documented, the mechanisms via which actin remodelling is achieved are not well understood (for review see Zigmond, 1996). Our results demonstrate that upon induction of intercellular adhesion, actin is rapidly recruited to sites of stable cell–cell contacts, in a process that requires functional E-cadherin receptors and endogenous Rac. We present evidence for the specificity of Rho and Rac in modulating cadherin function and discuss possible roles for the small GTPases in the establishment of calcium-dependent cell–cell adhesion in epithelial cells.
Materials and Methods
Cells
Normal human keratinocytes isolated from newborn foreskin (strains kb and z; passages 3–6) were cultured on a mitomycin C-treated feeder layer of 3T3 fibroblasts in DME/F12 medium (1:3 mixture of DME and Ham's F12 medium) supplemented with 10% fetal calf serum, 1.8 × 10−4 M adenine, 5 μg/ml insulin, 0.5 μg/ml hydrocortisone, 10−10 M cholera toxin, and 10 ng/ml epidermal growth factor (standard medium, 1.8 mM calcium) as described before (Hodivala and Watt, 1994; Rheinwald, 1989). Low calcium medium (0.1 mM calcium) contained the same formula as the standard medium, but calcium salts were omitted, and the divalent cations present in the fetal calf serum were chelated with Chelex 100 resin (BioRad Laboratories, Richmond, CA; Hodivala and Watt, 1994). Keratinocytes were seeded in standard calcium medium, transferred to low calcium medium after 2–3 d, and cultured until cells were confluent. Cells were seeded on glass coverslips in 4-well plates (3 × 104 cells/well) or 8-well glass slides (105 cells/slide) and cultured at 37°C in 5% CO2 atmosphere.
Antibodies
Antibodies used against E-cadherin were HECD-1 (mouse monoclonal; gift from M. Takeichi, Kyoto University, Japan; Shimoyama et al., 1989) and ECCD-2 (rat monoclonal; Hirai et al., 1989); antibody against P-cadherin was NCC-CAD-299 (mouse monoclonal, gift from S. Hirohashi, National Cancer Center Research Institute, Tokyo, Japan; Shimoyama et al., 1989). Other antibodies used were: anti-β-catenin (VB-2, rabbit anti-peptide antiserum; Braga et al., 1995); anti-talin (rabbit polyclonal, gift from K. Burridge, University of North Carolina, Chapel Hill, NC); anti-CD44 antibody (mouse monoclonal E1/2, gift from C. Isacke, Imperial College, London, UK; Isacke et al., 1986); anti-desmoplakin (mouse monoclonal 115F, gift from D. Garrod, Manchester University, Manchester, UK; Parrish et al., 1987); anti-β1 integrin (P5D2; Dittel et al., 1993); anti-integrin α3β1 (mouse monoclonal VM-2; Kaufmann et al., 1989). Secondary antibodies were bought from Jackson ImmunoResearch Laboratories (West Grove, PA): Cy5-conjugated donkey anti–mouse IgG; FITC-conjugated goat anti–mouse IgG; FITC-conjugated donkey anti–rat IgG; FITC-conjugated donkey anti–rabbit IgG.
Recombinant Proteins
Recombinant proteins were prepared as glutathione S-transferase fusion proteins in Escherichia coli, purified using glutathione Sepharose beads, thrombin cleaved, dialyzed, and concentrated essentially as described previously (Ridley et al., 1992). Active protein concentrations were determined by filter binding assay using [3H]GTP or [3H]GDP, and the activity of each batch of recombinant proteins was tested in fibroblasts as described (Ridley et al., 1992; Nobes and Hall, 1995). Recombinant proteins used were: C3 transferase, dominant negative N17Rac, constitutively active Rac (L61Rac), Cdc42 (L61Cdc42), and Rho (L63Rho; Ridley et al., 1992; Nobes and Hall, 1995). V12Rac74Rho, a chimeric protein that has the biological activity of Rho and yet is insensitive to C3 ADP ribosylation, was also used (Diekmann et al., 1995; Hotchin and Hall, 1995).
Microinjection
Confluent patches of keratinocytes were injected (4–8 adjacent cells/ patch; 6–8 patches/coverslip) using a microscope (Axiovert 135m; Zeiss, Inc., Thornwood, NY) attached to an Eppendorf Microinjection Unit (Microinjector model 5242; Micromanipulator model 5170; CO2 Controller model 3700, and Heat Controller model 3700). During microinjection, cells were maintained at 37°C in 5% CO2 atmosphere. Visualization of injected cells was achieved by mixing recombinant proteins with Dextran conjugated to FITC or Texas red (MW 10,000; Molecular Probes, Inc., Eugene, OR).
To evaluate the effect of recombinant proteins in the establishment of cadherin-mediated adhesion, keratinocytes were microinjected in low calcium medium and immediately transferred to standard calcium medium for 55 min to induce calcium-dependent cell–cell adhesion. In other experiments, to access the role of recombinant GTPases, keratinocytes grown in low calcium medium were placed in standard medium for 2 to 3 h, microinjected, and incubated in the same medium for 25 min.
Microinjection of Labeled Globular Actin
Actin was purified from rabbit muscle acetone powder as described before (Spudich and Watt, 1971). After the final centrifugation step, the top 2/3 of the actin was separated and dialyzed for 24 h against labeling and polymerization buffer (0.1 M sodium carbonate, pH 9.0, 0.1 M KCl, 2 mM MgCl2, 0.3 mM ATP, and 0.5 mM dithiotreitol). An aliquot of the polymerized actin (at 5 μg/ml) was added to Cy3 reactive dye for 30 min at room temperature. To remove the unbound dye and to depolymerize actin, the mixture was dialyzed for 48 h against Buffer G (2 mM Tris-HCl, pH 7.5, 0.2 mM ATP, 0.5 mM dithiotreitol, 2 mM CaCl2). After centrifugation of the labeled actin (120,000 g for 30 min) to remove polymer, the top 1/3 of each tube was removed. Aliquots were frozen at −70°C for use in microinjection experiments. Controls were performed to demonstrate that binding of the label to globular (G)1-actin did not perturb its incorporation into filamentous (F)-actin (data not shown).
Cy3-actin (15 μm final concentration) was injected into confluent patches of keratinocytes grown in low calcium medium, and cells were either maintained in the same medium or immediately transferred to standard medium for 20 min. Alternatively, keratinocytes were preincubated for 1 h with a cocktail of blocking functional antibodies against E- and P-cadherin (10 μg/ml HECD-1 and 4 μg/ml NCC-CAD-299) or control IgG (14 μg/ml rabbit anti–mouse IgG; Sigma Chemical Co., St. Louis, MO). Cells were microinjected with Cy3-actin and subsequently incubated in standard medium for 20 min. To evaluate the effects of recombinant proteins in actin turnover after induction of cell–cell contacts, Cy3-actin was coinjected with C3 transferase or N17Rac followed by a 20-min incubation in standard medium.
Immunostaining
After microinjection, cells were fixed in 3% paraformaldehyde for 10 min at room temperature, rinsed in PBS, and permeabilized in 0.1% Triton X-100 diluted in 10% fetal calf serum, for 10 min at room temperature. In some experiments, cells were blocked and permeabilized in 0.1% Triton X-100 diluted in 50% fetal calf serum. Incubations with the antibodies were for 1 h at room temperature; all antibodies were diluted in 10% fetal calf serum. Staining with Texas red- or FITC–phalloidin (Sigma Chemical Co.) was performed for 30 min. After staining, coverslips were mounted in gelvatol.
E-cadherin was detected using the mouse monoclonal HECD-1 followed by FITC-conjugated anti–mouse IgG. Double labeling was performed by sequential incubations with anti-cadherin antibody (ECCD-2), FITC-conjugated anti–rat IgG, anti-desmoplakin (115F), and Cy5-conjugated anti–mouse IgG. No crossreactivity was observed between these two conjugates. In some experiments, keratinocytes were double labeled with cadherins and a mouse monoclonal antibody against CD44 (E1/2) or β1 integrins (P5D2) in the same order as described above.
Confocal images were obtained using a laser scanner (MRC 1024; BioRad, Richmond, CA) attached to a Nikon microscope (Optiphot 2). To avoid leakage between the different filters when double labeling experiments were analyzed, the laser was optimized for each fluorophore, and images were collected separately. Pictures were processed using the Adobe Photoshop program.
Clustering of F-Actin
Polystyrene beads (Sigma Chemical Co., average 11.9 μm diam) were coated with mouse anti–E-cadherin mAb (HECD-1, 1 μg/μl) or mouse anti-integrin mAb (VM-2, anti-α3β1 integrin, 1 μg/μl) overnight at 4°C (Miyamoto et al., 1995). Beads were blocked with 10 mg/ml heat-denatured BSA (ICN, Irvine, CA) for 1 h at room temperature, followed by 3 washes in PBS. Immediately before use, beads were resuspended in standard medium and kept at 37°C.
Keratinocytes were microinjected with different recombinant GTPases, and after 15 min, the coated bead suspension was incubated with the cells (105 beads/coverslip) for 45 min at 37°C in 5% CO2 atmosphere. After three washes in PBS, cells were fixed in 3% paraformaldehyde, permeabilized, and labeled with phalloidin–Texas red. Controls for recruited cytoplasmic proteins to the coated beads (104 beads/coverslip) was performed by immunostaining with rabbit antibodies against β-catenin or talin as described. Images were taken with the confocal microscope, in a level 3–4 μm above the base of the beads in contact with the cells. Positive beads for actin clustering were scored when clear, distinct dots of F-actin were visible around the beads and expressed as a percentage of total attached beads. Between 40 and 200 beads were counted for the group (noninjected, C3, N17Rac, or buffer injected) with each type of antibody coating (i.e., anti-cadherin or anti-integrin). Only beads in which contour was visible with the phalloidin labeling were included in the analyses. Beads located on intercellular junctions were not considered in the analysis whenever actin recruitment to the beads overlapped cell–cell contact sites, due to the high concentration of actin at these sites. Statistical analysis (Student's t test) was performed assuming unequal variance.
Results
Rho and Rac Are Necessary for the Establishment of Cadherin-mediated Adhesion
Confluent patches of keratinocytes grown in low calcium medium were microinjected with C3 transferase to block endogenous Rho or dominant negative Rac (N17Rac), to inhibit endogenous Rac. The role of dominant negative Cdc42 (N17Cdc42) was not assessed, due to the lack of an assay to test the activity of the recombinant protein. After injection, cells were immediately transferred to standard calcium medium for 55 min to allow calcium-dependent cell–cell contacts to establish (Fig. 1). Cadherin-mediated adhesion was evaluated by the presence of E-cadherin immunopositive staining at intercellular junctions (Fig. 1, B, D, F, and H, arrows). After incubation for 1 h, injected cells were spread and still touching each other (Fig. 1, A, C, E, and G), indicating that there was not a general breakdown of the cytoskeleton. Patches were not considered in the analysis when microinjected cells retracted, or when the control noninjected neighbors did not show uniform cadherin staining at sites of cell–cell contacts. Our results showed that the cells microinjected with C3 or N17Rac were unable to form stable cadherin adhesion (Fig. 1, B and D, respectively). On the other hand, injection of constitutively active Rho (L63Rho; Fig. 1 F, arrowhead), Rac (L61Rac; Fig. 1 H, arrowhead), or Cdc42 (L61Cdc42; data not shown) did not prevent cadherin localization at intercellular junctions. Our data suggested that activation of Rho and Rac is required for the establishment of cadherin-dependent cell–cell contacts.
Figure 1.
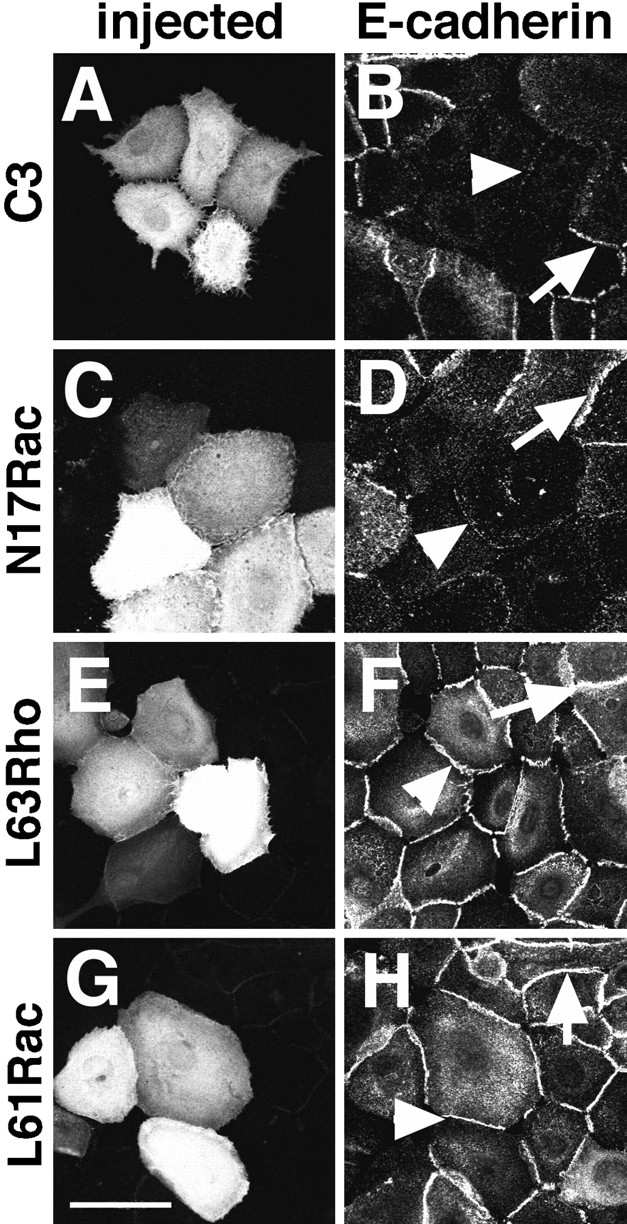
Establishment of cadherin-dependent cell–cell contacts requires Rho and Rac activities. Confluent patches of keratinocytes grown in low calcium medium were microinjected with different recombinant proteins and immediately transferred to standard medium for 55 min to induce calcium-dependent adhesion. Injected proteins were: C3 transferase to block endogenous Rho (A and B), dominant negative N17Rac (C and D), constitutively active L63Rho (E and F), or constitutively active L61Rac (G and H). Microinjected cells were identified by coinjection of Dextran–Texas red (A, C, E, and G). Cadherin-mediated adhesiveness was evaluated by the presence of immunopositive staining for E-cadherin at intercellular junctions (B, D, F, and H, arrows). Arrowheads (B and D) point to the absence of cadherin staining in the microinjected cells when Rho or Rac function was blocked. Arrowheads show the presence of cadherin-mediated adhesion between cells microinjected with activated Rho (F) or activated Rac (H). Bar, 50 μm.
Specificity of Rho and Rac on Cadherin Function
Because the formation of other adhesive structures in epithelial cells is delayed in the absence of cadherin function (Gumbiner et al., 1988; Mege et al., 1988; Amagai et al., 1995; Lewis et al., 1994), we could not test the specificity of Rho and Rac in the assay discussed above. To overcome this problem, calcium-dependent cell–cell adhesion was induced for 2–3 h in keratinocytes grown in low calcium medium before injection of C3 or N17Rac. After microinjection, cells were incubated for 25 min in standard medium, fixed, permeabilized, and double labeled for cadherins (Fig. 2, B, E, and H) and desmoplakin, a component of the desmosomal plaque (Fig. 2, C, F, and I). In the microinjected patches with C3 or N17Rac, desmoplakin was detected at intercellular junctions (Fig. 2, C and F, arrows), but E-cadherin was not (Fig. 2, B and E, arrows). The localization of both proteins was not affected by microinjection of buffer alone (Fig. 2, H and I, arrows). These results indicated that desmosomes are not affected by inhibiting the activity of endogenous Rho or Rac.
Figure 2.
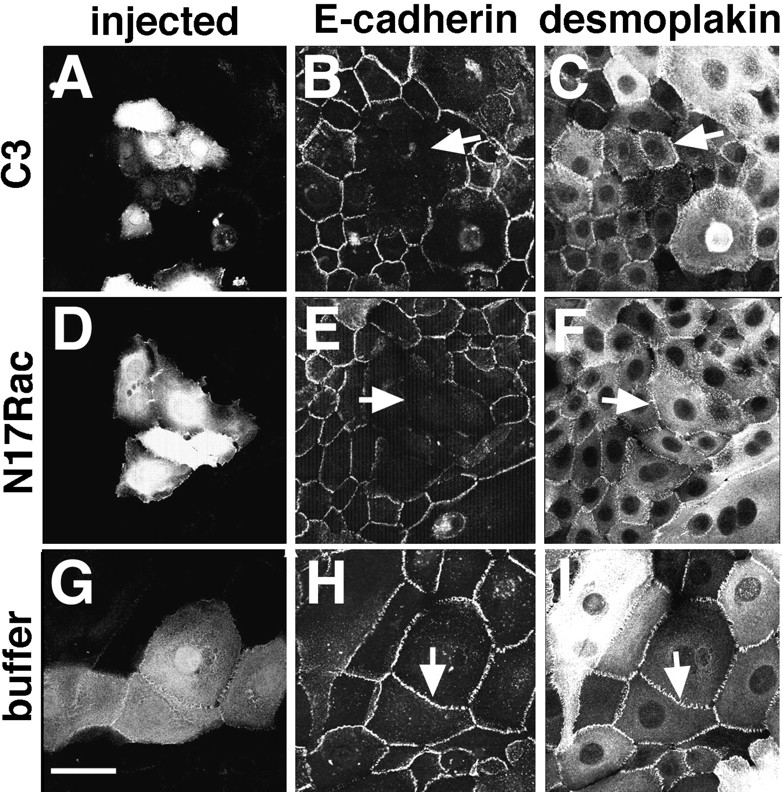
Inhibition of endogenous Rho and Rac perturbs stable cadherin-mediated adhesion but not desmosomes. Keratinocytes grown in low calcium medium were transferred to standard medium for up to 3 h before microinjection of C3 transferase (A–C), N17Rac (D–F), or buffer alone (G–I). Cells were incubated for 25 min in the same medium before fixation. A, D, and G show the microinjected cells, visualized by coinjection of Dextran–Texas red. Keratinocytes were double labeled for E-cadherin followed by FITC-conjugated anti–rat IgG (B, E, and H) and desmoplakin followed by Cy5conjugated anti–mouse IgG (C, F, and I). Arrows (C, F, and I) show immunopositive staining for desmoplakin in the microinjected cells; arrows in B and E show that cadherin staining is not detected at the intercellular junctions. The arrow in H indicates that cadherin immunostaining is not perturbed by microinjection of buffer. Bar, 50 μm.
We also investigated the effects of the recombinant proteins on other transmembrane receptors, CD44 and integrins (Fig. 3). Within the period of time examined, our data showed that E-cadherin was absent from cell–cell contacts in the C3 or N17Rac microinjected patches (Fig. 3, B and E, arrows), while the presence of CD44 was reduced but not completely eliminated (Fig. 3, C and F, arrows). Integrin localization at intercellular junctions (confocal sections taken at 2–3 μm above substratum) was unaffected within 30 min of blocking Rho (Fig. 3 I, arrow) or Rac function (data not shown).
Figure 3.
E-cadherin is selectively removed from stable cell–cell contacts. Keratinocytes grown in low calcium medium were transferred to standard medium for up to 3 h before microinjection of C3 transferase (A–C and G–I) or N17Rac (D–F). After incubation for 25 min, cells were fixed and injected cells identified by coinjection of Dextran–Texas red (A, D, and G). Keratinocytes were double labeled for E-cadherin followed by FITC-conjugated anti–rat IgG (B, E, and H) and CD44 (C and F) or β1-integrins (I) followed by Cy5-conjugated anti–mouse IgG. Arrows show immunopositive staining in the microinjected cells for CD44 (C and F) or integrins (I); arrows (B, E, and H) indicate the absence of cadherin staining at intercellular junctions of injected cells. Bar, 50 μm.
To evaluate whether both Rho and Rac activities are required for the establishment of cadherin-dependent adhesion, C3 and a constitutively active form of Rac (L61Rac) were coinjected into keratinocytes grown in low calcium medium (Fig. 4, C and D). Cells were immediately transferred to standard medium for 55 min, fixed, and stained for E-cadherin. Our results showed that L61Rac was unable to override the inhibition of cadherin function by C3 (Fig. 4 D, arrow), while injection of L61Rac alone did not prevent cadherin adhesion (Fig. 1, G and H, and data not shown). Unfortunately, we were unable to test whether constitutively active Rho can rescue the effect of Rac inactivation on cadherin localization. It seems that the dominant negative effect of N17Rac in keratinocytes requires a considerably high activity that is impaired by the dilution with other recombinant proteins. To show that the inhibition of cadherin function by C3 was due specifically to blocking endogenous Rho, C3 was coinjected with a Rho chimeric protein insensitive to ADP-rybosylation (V12Rac74Rho), and cell–cell contacts were induced for 55 min (Fig. 4, E and F). We observed that, by providing the exogenous chimeric Rho protein together with C3, E-cadherin immunostaining was restored at intercellular junctions (Fig. 4 F, arrow).
Figure 4.
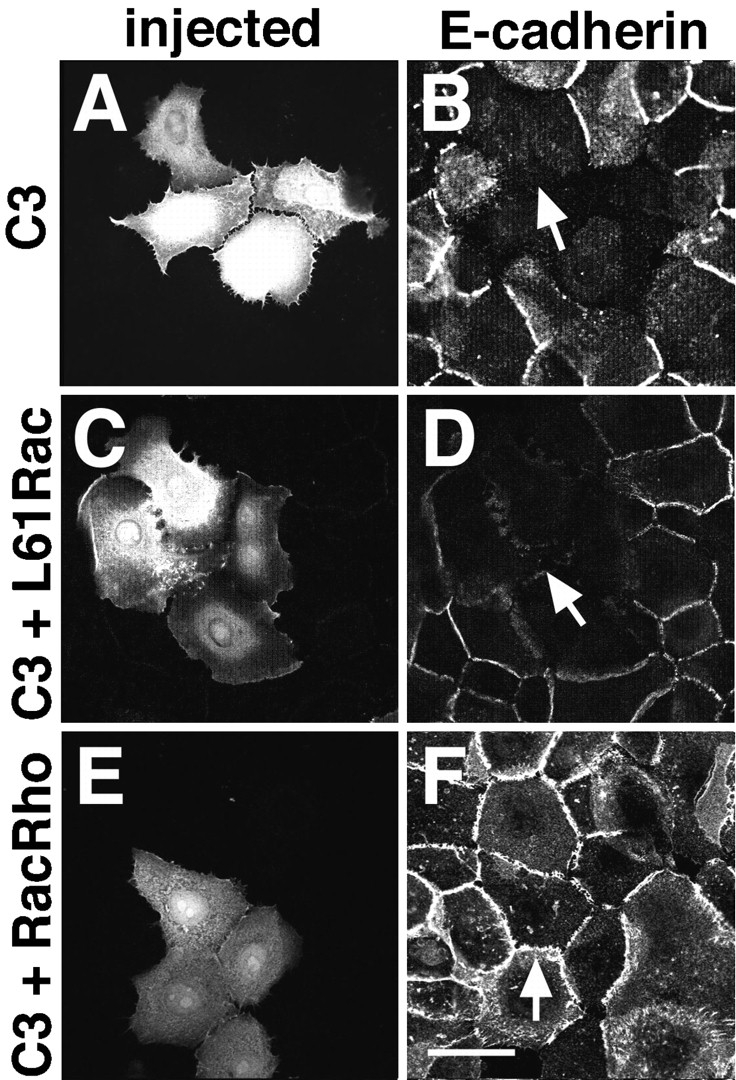
Both Rho and Rac are required for the establishment of cadherin-dependent cell–cell adhesion. Keratinocytes grown in low calcium medium were microinjected with C3 transferase alone (A and B) or coinjected with an activated form of Rac (L61Rac; C and D ). Alternatively, C3 was injected with a Rho chimera insensitive to C3 ADP ribosylation, V12Rac74Rho (RacRho; E and F). Cells were immediately transferred to standard medium and incubated for 55 min. Injected cells were visualized by coinjected Dextran–Texas red (A, C, and E). Arrows in B and D indicate that cadherin-dependent cell–cell contacts were absent in the microinjected cells. The arrow in F shows that the exogenous Rho chimera was able to restore cadherin immunostaining at the intercellular junctions. Bar, 50 μm.
Actin Is Recruited to E-cadherin Complexes upon Induction of Calcium-dependent Cell–Cell Adhesion
Epithelial cells show a characteristic arrangement of the actin cytoskeleton, which is dependent on their adhesion to neighbor cells. To test how the actin turnover was influenced after establishment of cell–cell contacts and the putative effect of the small GTPases, G-actin was purified from rabbit muscles and labeled with Cy3. Keratinocytes grown in low calcium medium were microinjected with Cy3-actin and incubated in different media for 20 min (Fig. 5). As shown before, cells maintained in low calcium medium after microinjection showed diffuse cadherin staining (Fig. 5 B; Gumbiner et al., 1988; Wheelock and Jensen, 1992; Hodivala and Watt, 1994; Braga et al., 1995). No incorporation of labeled actin into peripheral actin bundles and stress fibers was seen (Fig. 5 A), even though these structures were observed at the periphery of the cells as visualized by phalloidin staining (data not shown). In contrast, after induction of calcium-dependent cell–cell contacts, Cy3-actin was rapidly recruited to intercellular junctions (<10 min, data not shown), where it colocalized with E-cadherin staining (Fig. 5, C and D) and with stress fibers (data not shown). The time point of 20 min was chosen to clearly show incorporation of labeled actin at the membrane, as compared to cells maintained in low calcium medium. Blocking cadherin function with specific antibodies before the microinjection abolished calcium-induced accumulation of labeled actin and E-cadherin at sites of cell–cell contacts (Fig. 5, E and F), while preincubation with control IgG had no effect (Fig. 5, G and H).
Figure 5.
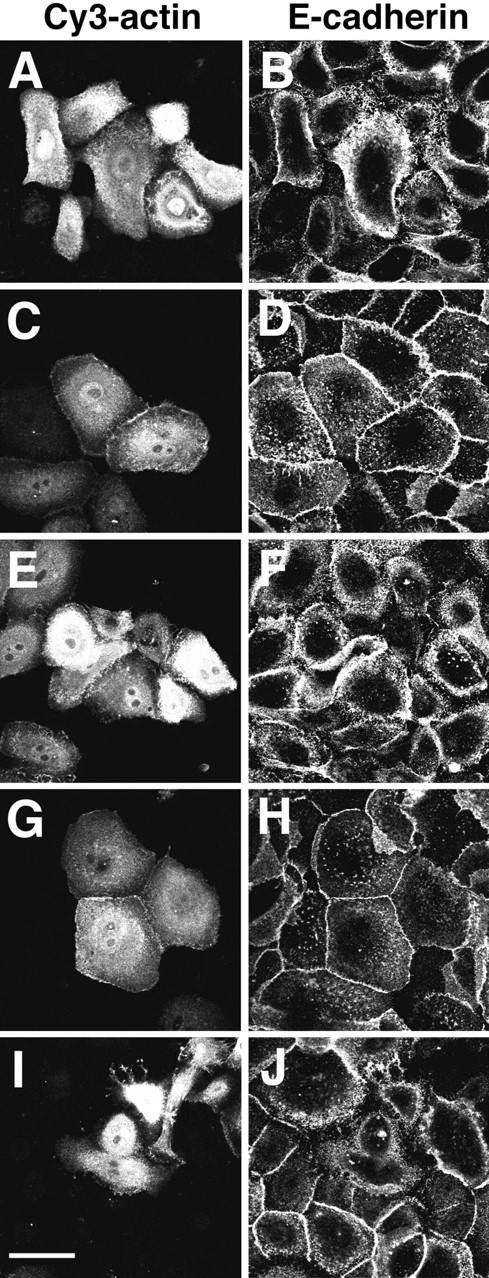
Cy3-actin is rapidly concentrated at sites of cadherinmediated adhesion upon induction of stable cell–cell contacts. Keratinocytes grown in low calcium medium were microinjected with Cy3-actin and either maintained in the same medium (A and B) or transferred to standard medium for 20 min (C–J). Control IgG (G and H) or blocking-functional antibodies against cadherins (E and F) were incubated with the cells before the microinjection of labeled actin. In other experiments, Cy3-actin was coinjected with C3 (I and J) before induction of cell–cell contacts for 20 min. The same results were obtained by coinjection of Cy3actin with N17Rac (data not shown). Microinjected cells were identified (A, C, E, G, and I), and immunostaining for E-cadherin was performed (B, D, F, H, and J). Bar, 50 μm.
When N17Rac (data not shown) or C3 transferase were coinjected with Cy3-actin, recruitment of labeled actin to cell–cell adhesion sites was also perturbed (Fig. 5, I and J). However, the optimal time for detection of labeled actin at the membrane after microinjection (20 min incubation) was not enough to completely abolish the establishment of cadherin-mediated adhesion by C3 or N17Rac (as opposed to 1 h shown in Figs. 1 and 4). Around 20% of the microinjected cells showed a few clusters of cadherins at intercellular contacts (data not shown). At these sites, Cy3actin was also present, confirming the dependence on functional cadherin complexes for actin accumulation.
Dissecting the Effects of Rho and Rac on the Cadherin-dependent Adhesion in Keratinocytes
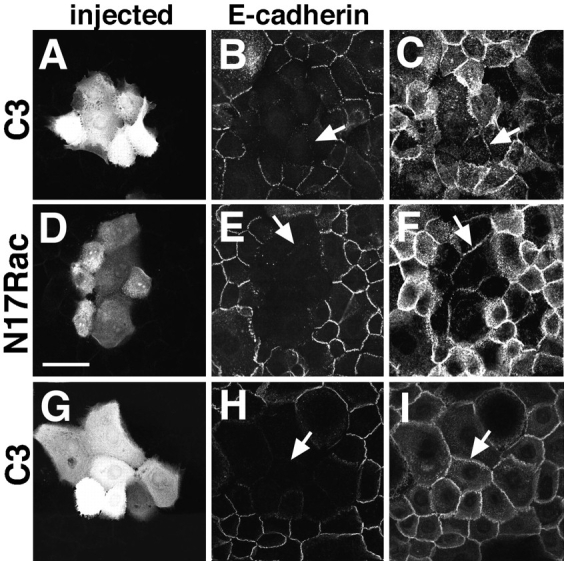
We observed that functional cadherin receptors, Rho and Rac, were required for the recruitment of actin to sites of cell–cell contacts (Fig. 5). It is possible that the small GTPases modulate the accumulation of actin directly or indirectly through regulating the clustering of cadherin complexes, an important step for actin filament reorganization. To investigate these possibilities, we used latex beads coated with a specific antibody against E-cadherin to cluster cadherin complexes (anti-cadherin beads). Control experiments showed that anti-cadherin beads were able to cluster actin around them and attached well to keratinocytes (50–100 beads/40× field; HECD-1 beads, Fig. 6 C), as opposed to BSA-coated beads (∼1.2 beads/40× field; Fig. 6 A). The catenins p120 (data not shown) and β-catenin were recruited to anti-cadherin beads (Fig. 6 D) but not to BSA beads (Fig. 6 B) nor anti-integrin α3β1 beads (VM-2 beads; Fig. 6 F). In addition, talin, a component of focal adhesion complexes, was not recruited to anti-cadherin beads (Fig. 6 H).
Figure 6.
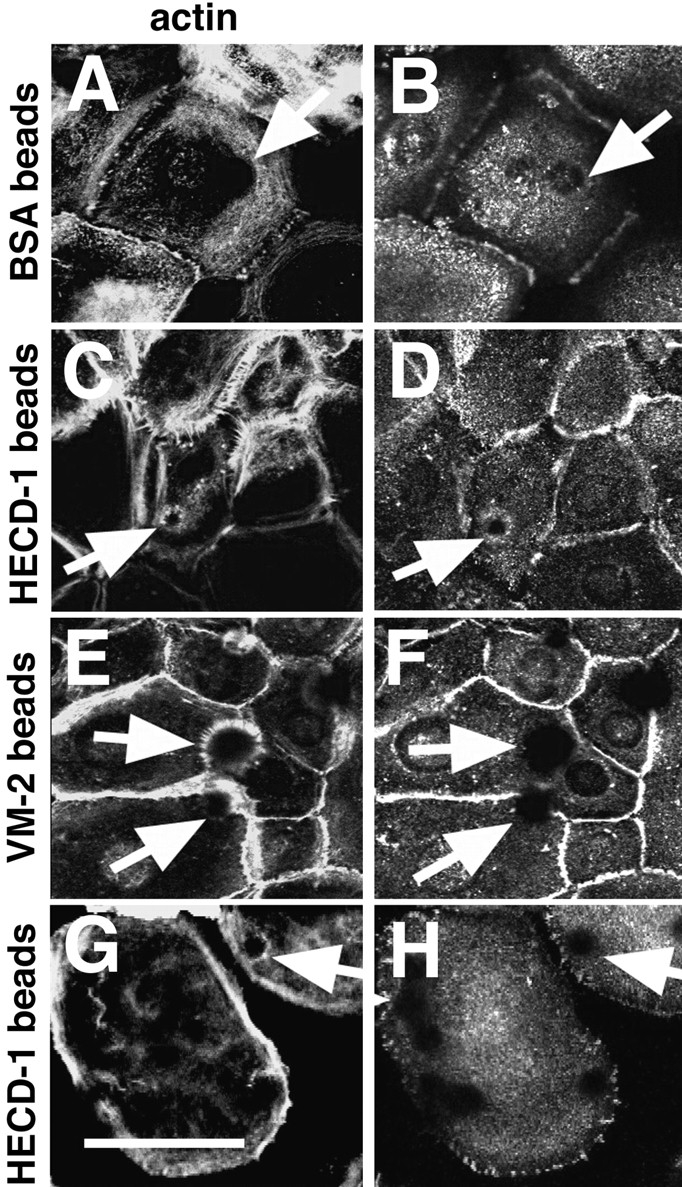
Recruitment of actin and β-catenin, but not talin, to latex beads coated with anti-cadherin antibodies. Keratinocytes were incubated for 1 h with a suspension of latex beads coated with BSA (A and B), anti–E-cadherin mAb (C, D, G, and H), or anti-integrin α3β1 mAb (E and F). Cells were double labeled with phalloidin (A, C, E, and G) and anti–β-catenin (B, D, and F) or anti-talin (H) rabbit polyclonal antibodies. A less confluent area is shown in G and H to demonstrate talin staining at focal contact sites. Arrows point to attached beads. Bar, 50 μm.
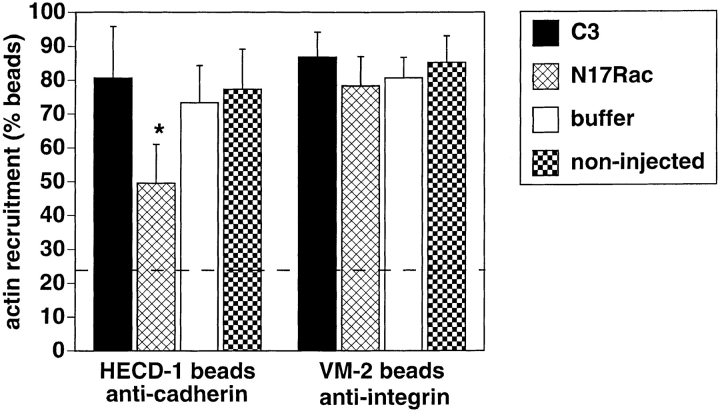
Keratinocytes were microinjected with C3 (Fig. 7, A and B), N17Rac (Fig. 7, C and D), or buffer alone (Fig. 7, E and F), and, after 15 min, cells were incubated with the anti-cadherin beads for an additional 45 min and stained with phalloidin (Fig. 7, B, D, and F). Experiments were also performed using beads coated with a monoclonal antibody against α3β1 integrins (data not shown). Quantitation of the attached beads with recruited actin was performed as described in Materials and Methods and shown in Fig. 8. In our hands, ∼20% of the few BSA-coated beads able to attach to keratinocytes showed weak phalloidin dots around them, and this proportion of beads with recruited actin was considered our base line (negative or nonspecific binding, Fig. 8). In contrast, ∼80% of the attached anti-cadherin and anti-integrin beads showed distinctive and intense phalloidin staining around them (data not shown; Figs. 7 and 8). Microinjection of C3 or buffer alone did not affect the proportion of beads positively labeled with actin as compared with the noninjected area (Figs. 7, B and F, arrows, and 8). As controls for the inhibition of Rho activity by C3, the effects on the actin cytoskeleton were observed in the phalloidin-stained cells, and disruption of cadherin function by C3 microinjection was evaluated in parallel coverslips. Cells microinjected with dominant negative Rac protein, however, showed a significant reduction on the proportion of anti-cadherin beads able to recruit actin, when compared to the keratinocytes injected with C3 or buffer (Figs. 7 D, arrows, and 8; Student's t test, P < 0.001). Although N17Rac reduced slightly the proportion of anti-integrin beads able to recruit actin, this reduction was not significant when compared to the buffer controls (Fig. 8, Student's _t_ test, _P_ > 0.1). These results suggested that endogenous Rac, but not Rho, is necessary for the recruitment of actin to clustered cadherin complexes.
Figure 7.
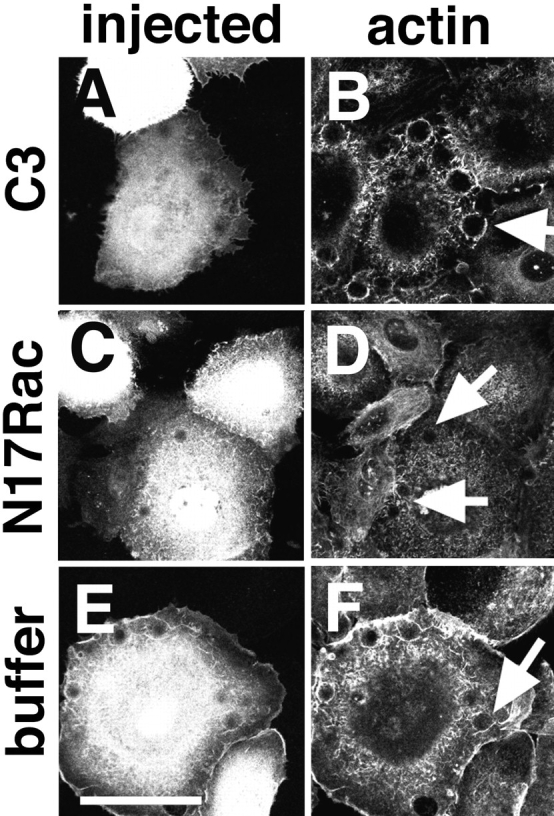
Actin recruitment to beads coated with anti-cadherin antibodies is perturbed by N17Rac. Keratinocytes grown in low calcium medium were microinjected with C3 transferase (A and B), N17Rac (C and D), or buffer alone (E and F). After 15 min, a suspension of latex beads coated with anti-cadherin antibodies was incubated with the cells for an additional 45 min. Injected cells were identified by coinjection of Dextran–FITC (A, C, and E); keratinocytes were labeled with phalloidin–Texas red (B, D, and F, actin). Arrows in B and F show beads on the microinjected cells with clustered actin around them. Arrows in D show beads on N17Rac-injected cells; top arrow shows a bead negative for actin recruitment. Bar, 50 μm.
Figure 8.
Quantitation of actin recruitment to beads coated with anti-cadherin antibodies (HECD-1 beads) or anti-α3β1 integrins (VM-2 beads). The percentage of beads with recruited actin on the microinjected cells and on control noninjected cells was quantified from experiments similar to the one shown in Fig. 7 and expressed as percentage of total attached beads (see Materials and Methods). Around 20% of the few BSA-coated beads able to attach to keratinocytes showed some weak phalloidin staining, and this was considered our baseline (negative or nonspecific binding, dashed line). Error bars represent standard deviation. *, statistically significant (Student's t test, P < 0.001).
Discussion
Epithelial cells adhere tightly to their neighbors, an essential characteristic for epithelial functions such as a barrier between two compartments and polarized secretion. Investigation on the regulation of intercellular adhesion is important for understanding (a) how epithelial differentiation is achieved and (b) how the breakdown of adhesive structures leads to the invasive phenotype in carcinomas. Cadherin-mediated adhesion is central to these issues, and we present evidence for the first time that the small GTPases Rho and Rac are required for cadherin function.
Cadherin receptors become competent to bind when the extracellular concentration of calcium ions is raised to 2 mM. Under these conditions, the receptors cluster at sites of cell–cell contacts and promote a major reorganization of microfilaments into peripheral, circumferential stress fibers in epithelial cells (see Gumbiner et al., 1988). Abolishing cadherin function by many different ways inhibits the accumulation of cadherins at intercellular junctions and the rearrangement of the cytoskeleton (for review see Takeichi, 1991). Here we demonstrate that blocking the function of endogenous Rho and Rac prevents the establishment of cadherin-mediated cell–cell contacts in keratinocytes. Constitutively active forms of Rho or Rac do not disturb the presence of cadherin receptors at these sites within 1 h of induction of calcium-dependent intercellular adhesion. These results indicate that the establishment of stable cadherin-mediated adhesion requires the activation of Rho proteins.
Cadherin complexes are selectively removed from junctions induced for 2–3 h by microinjection of C3 or N17Rac. Desmosomes, another calcium-dependent adhesive structure, are not affected by blocking Rac or Rho function. While desmosomes contain different members of the cadherin family (desmocollins and desmogleins), they do not interact with microfilaments but with keratin filaments (for review see Cowin and Burke, 1996). Two additional transmembrane receptors that associate indirectly with actin filaments were used as controls: CD44 and integrins, which may participate in cell–cell adhesion and localize at intercellular junctions in epithelial cells (Lokeshwar et al., 1994; Tsukita et al., 1994). At the period of time examined after blocking endogenous Rho or Rac (30 min), cadherin receptors are removed from cell–cell contacts, while the presence of CD44 at these sites is reduced but not completely eliminated. Integrin localization at the junctions is not perturbed. The spread morphology of the microinjected cells at the time point examined suggests that there was not a general breakdown of the cytoskeleton or cell– cell contacts, in agreement with published reports (Ridley et al., 1995). Taken together, our data indicate that the elimination of E-cadherin from junctions by inhibiting Rho or Rac function is specific and temporally precedes the removal of other microfilament-associated transmembrane proteins from the same sites.
It is possible that longer incubations may also lead to the removal of CD44 and integrins from the lateral domain of epithelial cells. This could potentially result directly from the inactivation of the Rho proteins (Hirao et al., 1996) or indirectly, via a dependence on cadherin-mediated adhesion or a widespread disruption of actin filaments. Based on our results, we are currently investigating whether the activitity of Rho proteins is also required for the maintenance of cadherin adhesion in stable and fully mature junctions as opposed to contacts induced for 2–3 h.
The next question is whether both Rho and Rac are necessary for cadherin function. Our data show that a constitutively active form of Rac (L61Rac) is not sufficient to induce cadherin-mediated adhesion when endogenous Rho is blocked by C3 transferase. In fact, Rho is revealed as an essential component: when C3 is coinjected with a Rho chimeric protein insensitive to its inhibitory effects, the presence of cadherins at intercellular junctions is restored. These results suggest that the concerted action of Rho and Rac modulate the establishment of cadherin adhesion, and that the two small GTPases may probably be required for distinct processes (see below). It is also possible that Rac can activate Rho, similarly to the hierarchy observed among the small GTPases in fibroblasts (Ridley et al., 1992; Nobes and Hall, 1995). However, the same hierarchy may not be observed in different cell types, and this is an open question in epithelial cells.
How can the small GTPases interfere with cadherin function? At least three possibilities can be envisaged. First, Rho and Rac may regulate the availability of cadherin receptors at the surface, perhaps by preventing their recycling. Interestingly, Rho and Rac have been shown to interfere with endocytosis in other cell types (Schmalzing et al., 1995; Lamaze et al., 1996). We observe, however, that clathrin-dependent endocytosis of transferrin receptor is not blocked by microinjection of C3 or N17Rac in keratinocytes (data not shown). It is possible that cadherin receptors may be recycled by a clathrin-independent pathway, which may be sensitive to blocking Rho and Rac function. Further experiments are required to explore this alternative.
Two other possibilities of how Rho and Rac regulate cadherin function are closely linked: the small GTPases may be required for the recruitment of actin to the receptors and/or the clustering of cadherin complexes at sites of cell–cell adhesion. To test these alternatives, we used two different approaches. First, after microinjection of Cy3- labeled actin, keratinocytes maintained in low calcium medium for 20 min show no detectable turnover or accumulation of actin at the membrane or in peripheral stress fibers. In contrast, induction of calcium-dependent cell–cell contacts promotes a rapid accumulation of Cy3-actin at intercellular junctions, in a process dependent on functional E-cadherin, Rho and Rac. These results are similar to the requirements for focal adhesion formation: in addition to Rho/Rac GTPases, integrin receptors and extracellular matrix are also necessary (Hotchin and Hall, 1995).
Secondly, subsequent experiments indicate that if cadherin complexes are clustered by the use of antibodies attached to beads, recruitment of actin to the receptors is specifically perturbed by blocking Rac function, but not Rho. Recruitment of actin to beads coated with an anti- integrin antibody is not significantly affected by inhibiting endogenous Rho or Rac. Two alternatives can be raised to explain our results. As inhibition of Rho can perturb cadherin function, it is possible that Rho may be involved in the clustering of cadherins and/or the recruitment of cytoplasmic proteins to sites of cell–cell contacts (Fincham et al., 1996). Since clustering of receptors is thought to be necessary for actin recruitment, this would account for the observed effect of C3 transferase in the incorporation of labeled actin at intercellular junctions (Fig. 5). It has also been suggested that Rho can participate in the integrin- dependent aggregation of lymphocytes (Tominaga et al., 1993) and in the clustering of integrin receptors at focal contacts, by inactivation of myosin light chain phosphatase (Chrzanowska-Wodnicka and Burridge, 1996; Kimura et al., 1996). Alternatively or concomitantly, Rho can regulate the formation of stress fibers in epithelia, which may help stabilize adhesion between neighbor cells.
We demonstrate that one possible role of Rac in modulating cadherin-mediated cell–cell contacts is in the accumulation of actin to clustered cadherin complexes. Our results are consistent with published data in which expression of dominant negative Rac in Drosophila perturbs the incorporation of actin and myosin at the intercellular junctions of fully polarized epithelia (Eaton et al., 1995; Harden et al., 1995). We have extended these findings to show that this process requires functional cadherin receptors. In addition, the involvement of Rac in the recruitment of actin to the cell cortex has also been reported in fibroblasts (Machesky and Hall, 1997), and in platelets, Rac can regulate actin assembly via uncapping of filamentous actin (Hartwig et al., 1995).
In conclusion, we present evidence that the establishment of cadherin-dependent cell–cell contacts can be regulated by the small GTPases Rho and Rac. The challenge now is to translate this functional relationship into molecular interactions between components of the cadherin complexes and Rho or Rac. It is known that both GTPases can localize at sites of cell–cell contacts (Adamson et al., 1992, and data not shown), and good candidates for interactions in the cadherin complexes are α-catenin and α-actinin, which are able to bind and bundle actin filaments (Knudsen et al., 1995; Rimm et al., 1995). Alternatively, Rho and Rac could exert their effects indirectly, via activation of kinases and/or phosphatases that could potentially regulate cadherin function (Brady-Kalnay et al., 1995; Balsamo et al., 1996; Machesky and Hall, 1996). Exciting possibilities are now opened to investigate the ability of functional cadherin receptors to trigger signaling pathways, which will ultimately lead to epithelial differentiation.
Acknowledgments
We thank Tina Bridges for the preparation of recombinant proteins and Drs. S. Hirohashi, M. Takeichi, C. Isacke, K. Burridge, and D. Garrod for generous gift of antibodies. We would also like to thank the generous support of Cancer Research Campaign and D. Drechsel and E. Caron for stimulating discussions.
Footnotes
1. Abbreviations used in this paper: F- and G-actin, filamentous and globular actin.
L. Machesky is recipient of a Medical Research Council Career Development Award.
N.A. Hotchin's present address is School of Biochemistry, University of Birmingham, Birmingham, B15 2TT, U.K.
Please address all correspondence to Vania M.M. Braga, MRC-Laboratory for Molecular Cell Biology, University College London, Gower Street, WCIE 6BT, London, U.K. Tel.: (44) 171-380-7814; Fax: (44) 171380-7805; E-mail: v.braga@ucl.ac.uk
References
- Adamson P, Paterson HF, Hall A. Intracellular localization of the p21rhoproteins. J Cell Biol. 1992;119:617–627. doi: 10.1083/jcb.119.3.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amagai M, Fujimori T, Masunaga T, Shimizu H, Nishikawa T, Shimizu N, Takeichi M, Hashimoto T. Delayed assembly of desmosomes in keratinocytes with disrupted classic cadherin-mediated cell adhesion by a dominant negative mutant. J Investig Dermatol. 1995;104:27–32. doi: 10.1111/1523-1747.ep12613462. [DOI] [PubMed] [Google Scholar]
- Balsamo J, Leung T, Ernst H, Zanin MKB, Hoffman S, Lilien J. Regulated binding of a PTP1B-like phosphatase to N-cadherin: control of cadherin mediated adhesion by dephosphorylation of β-catenin. J Cell Biol. 1996;134:801–813. doi: 10.1083/jcb.134.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens J, Vakaet L, Winterhager E, Van Roy F, Mareel MM, Birchmeier W. Loss of epithelial differentiation and gain of invasiveness correlates with tyrosine phosphorylation of the E-cadherin/β-catenin complex in cells transformed with a temperature-sensitive v-srcgene. J Cell Biol. 1993;120:757–766. doi: 10.1083/jcb.120.3.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens J, von Kries JP, Kuhl M, Bruhn L, Wedlich D, Grosschedel R, Birchmeier W. Functional interaction of β-catenin with the transcription factor LEF-1. Nature (Lond) 1996;382:638–642. doi: 10.1038/382638a0. [DOI] [PubMed] [Google Scholar]
- Bixby JL, Zhang R. Purified N-cadherin is a potent substrate for the rapid induction of neurite outgrowth. J Cell Biol. 1990;110:1253–1260. doi: 10.1083/jcb.110.4.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley RS, Cowin P, Brown AMC. Expression of Wnt-1 in PC12 cells results in modulation of plakoglobin and E-cadherin and increased cellular adhesion. J Cell Biol. 1993;123:1857–1865. doi: 10.1083/jcb.123.6.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady-Kalnay S, Rimm DL, Tonks NK. Receptor protein tyrosine phosphatase PTPμ associates with cadherins and catenins in vivo. J Cell Biol. 1995;130:977–986. doi: 10.1083/jcb.130.4.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga VMM, Hodivala KJ, Watt FM. Calcium-induced changes in distribution and solubility of cadherins and their associated cytoplasmic proteins in human keratinocytes. Cell Adhes Commun. 1995;3:201–215. doi: 10.3109/15419069509081287. [DOI] [PubMed] [Google Scholar]
- Braga, V.M.M, N. Hajibagheri, and F.M. Watt. 1997. Calcium-induced intercellular adhesion of keratinocytes does not involve accumulation of β1 integrins at cell–cell contact sites and does not involve changes in the levels or phosphorylation of catenins. Cell Adhesion Com. In press. [DOI] [PubMed]
- Brieher WM, Yap AS, Gumbiner BM. Lateral dimerization is required for the homophilic binding activity of C-cadherin. J Cell Biol. 1996;135:487–496. doi: 10.1083/jcb.135.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrzanowska-Wodnicka M, Burridge K. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J Cell Biol. 1996;133:1403–1415. doi: 10.1083/jcb.133.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowin PM, Burke B. Cytoskeleton-membrane interactions. Curr Opin Cell Biol. 1996;8:56–65. doi: 10.1016/s0955-0674(96)80049-4. [DOI] [PubMed] [Google Scholar]
- Diekmann D, Nobes CD, Burbelo PD, Abo A, Hall A. Rac GTPase interacts with GAPs and target proteins through multiple effector sites. EMBO (Eur Mol Biol Organ) J. 1995;14:5297–5305. doi: 10.1002/j.1460-2075.1995.tb00214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittel BN, McCarthy JB, Wayner EA, LeBien TW. Regulation of human B-cell precursor adhesion to bone marrow stromal cells by cytokines that exert opposing effects on the expression of vascular cell adhesion molecule-1 (VCAM-1) Blood. 1993;81:2272–2282. [PubMed] [Google Scholar]
- Eaton S, Auvinen P, Luo L, Jan YN, Simons K. CDC42 and Rac 1 control different actin-dependent processes in the Drosophilawing disc epithelium. J Cell Biol. 1995;131:151–164. doi: 10.1083/jcb.131.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fincham VJ, Unlu M, Brunton VG, Pitts JD, Wyke JA, Frame MC. Translocation of Src kinase to the cell periphery is mediated by the actin cytoskeleton under the control of the Rho family of small G proteins. J Cell Biol. 1996;135:1551–1564. doi: 10.1083/jcb.135.6.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green KJ, Geiger B, Jones JCR, Talian JC, Goldman RD. The relationship between intermediate filaments and microfilaments before and during the formation of desmosomes and adherens-type junctions in mouse epidermal cells. J Cell Biol. 1987;104:1389–1402. doi: 10.1083/jcb.104.5.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbiner BM. Signal transduction by β-catenin. Curr Biol. 1995;7:634–640. doi: 10.1016/0955-0674(95)80104-9. [DOI] [PubMed] [Google Scholar]
- Gumbiner B, Stevenson B, Grimaldi A. The role of the cell adhesion molecule uvomorulin in the formation and maintenance of the epithelial junctional complex. J Cell Biol. 1988;107:1575–1587. doi: 10.1083/jcb.107.4.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harden N, Loh HY, Chia W, Lim L. A dominant inhibitory version of the small GTP-binding protein Rac disrupts cytoskeletal structures and inhibits developmental cell shape changes in Drosophila. Development (Camb) 1995;121:903–914. doi: 10.1242/dev.121.3.903. [DOI] [PubMed] [Google Scholar]
- Hartwig JH, Bokoch GM, Carpenter CL, Jammey PA, Taylor LA, Toker A, Stossel TP. Thrombin receptor ligation and activated Rac uncap actin filament barbed ends through phosphoinositide synthesis in permeabilized human platelets. Cell. 1995;82:643–653. doi: 10.1016/0092-8674(95)90036-5. [DOI] [PubMed] [Google Scholar]
- Hinck L, Nelson JW, Papkoff J. Wnt-1 modulates cell–cell adhesion in mammalian cells by stabilizing β-catenin binding to the cell adhesion protein cadherin. J Cell Biol. 1994;124:729–741. doi: 10.1083/jcb.124.5.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai Y, Nose A, Kobayashi S, Takeichi M. Expression and role of E- and P-cadherin adhesion molecules in embryonic histogenesis. II. Skin morphogenesis. Development (Camb) 1989;105:271–277. doi: 10.1242/dev.105.2.271. [DOI] [PubMed] [Google Scholar]
- Hirao M, Sato N, Kondo T, Yonemura S, Monden M, Sasaki T, Takai Y, Tsukita S, Tsukita S. Regulation mechanism of ERM (Erzin/Radixin/Moesin) protein/plasma membrane association: possible involvement of phosphatidylinositol turnover and Rho-dependent signalling pathways. J Cell Biol. 1996;135:37–51. doi: 10.1083/jcb.135.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraoka K, Kaibuchi K, Ando S, Musha T, Takaishi K, Mizuno T, Asada M, Menard L, Tomhave E, Didsbury J, et al. Both stimulatory and inhibitory GDP/GTP exchange proteins, smgGDS and rhoGDI, are active on multiple small GTP-binding proteins. Biochem Biophys Res Comm. 1992;182:921–930. doi: 10.1016/0006-291x(92)91820-g. [DOI] [PubMed] [Google Scholar]
- Hodivala KJ, Watt FM. Evidence that cadherins play a role in the downregulation of integrin expression that occurs during keratinocyte terminal differentiation. J Cell Biol. 1994;124:589–600. doi: 10.1083/jcb.124.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotchin NA, Hall A. The assembly of integrin adhesion complexes requires both extracellular matrix and intracellular rho/rac GTPases. J Cell Biol. 1995;131:1857–1865. doi: 10.1083/jcb.131.6.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isacke CM, Sauvage CA, Hyman R, Lesley J, Schulte R, Trowbridge IS. Identification and characterization of the human Pgp-1 glycoprotein. Immunogenetics. 1986;23:326–332. doi: 10.1007/BF00398797. [DOI] [PubMed] [Google Scholar]
- Jongen WMF, Fitzgerald DJ, Asamoto M, Piccoli C, Slaga TJ, Gros D, Takeichi M, Yamasaki H. Regulation of connexin 43-mediated gap junctional intercellular communication by Ca2+in mouse epidermal cells is controlled by E-cadherin. J Cell Biol. 1991;114:545–555. doi: 10.1083/jcb.114.3.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann R, Frosch D, Westphal C, Weber L, Klein CE. Integrin VLA-3: ultrastructural localization at cell–cell contact sites of human cell cultures. J Cell Biol. 1989;109:1807–1815. doi: 10.1083/jcb.109.4.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemler R. From cadherins to catenins: cytoplasmic protein interactions and regulation of cell adhesion. Trends Genet. 1993;9:317–321. doi: 10.1016/0168-9525(93)90250-l. [DOI] [PubMed] [Google Scholar]
- Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, Yamamori B, Feng J, Nakano T, Okawa K, et al. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase) Science (Wash DC) 1996;273:245–248. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- Kinch MS, Clark GJ, Der CJ, Burridge K. Tyrosine phosphorylation regulates the adhesion of ras-transformed breast epithelia. J Cell Biol. 1995;130:461–471. doi: 10.1083/jcb.130.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen K, Soler AP, Johnson KR, Wheelock MJ. Interaction of α-actinin with the cadherin/catenin cell-cell adhesion complex via α-catenin. J Cell Biol. 1995;130:67–77. doi: 10.1083/jcb.130.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamaze C, Chuang TH, Terlecky LJ, Bokoch GM, Schmid SL. Regulation of receptor-mediated endocytosis by Rho and Rac. Nature (Lond) 1996;382:177–179. doi: 10.1038/382177a0. [DOI] [PubMed] [Google Scholar]
- Lewis JE, Jensen PJ, Wheelock MJ. Cadherin function is required for human keratinocytes to assemble desmosomes and stratify in response to calcium. J Investig Dermatol. 1994;102:870–877. doi: 10.1111/1523-1747.ep12382690. [DOI] [PubMed] [Google Scholar]
- Lokeshwar VB, Fregien N, Bourguignon LY. Ankyrin-binding domain of CD44 (GP85) is required for the expression of hyaluronic acidmediated adhesion function. J Cell Biol. 1994;126:1099–1109. doi: 10.1083/jcb.126.4.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machesky LM, Hall A. Rho: A connection between membrane receptor signalling and the cytoskeleton. Trends Cell Biol. 1996;6:304–310. doi: 10.1016/0962-8924(96)10026-x. [DOI] [PubMed] [Google Scholar]
- Machesky, L.M., and A. Hall. 1997. Role of actin polymerization and adhesion to extracellular matrix in Rac- and Rho-induced cytoskeletal reorganization. J. Cell Biol. In press. [DOI] [PMC free article] [PubMed]
- Magee AI, Lytton NA, Watt FM. Calcium-induced changes in cytoskeleton and motility of cultured human keratinocytes. Exp Cell Res. 1987;172:43–53. doi: 10.1016/0014-4827(87)90091-7. [DOI] [PubMed] [Google Scholar]
- Matsuyoshi N, Hamaguchi M, Taniguchi S, Nagafuchi A, Tsukita S, Takeichi M. Cadherin-mediated cell-cell adhesion is perturbed by v-srctyrosine phosphorylation in metastatic fibroblasts. J Cell Biol. 1992;118:703–714. doi: 10.1083/jcb.118.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeill H, Ozawa M, Kemler R, Nelson WJ. Novel function of the cell adhesion molecule uvomorulin as an inducer of cell surface polarity. Cell. 1990;62:309–316. doi: 10.1016/0092-8674(90)90368-o. [DOI] [PubMed] [Google Scholar]
- Mege R-M, Matsuzaki F, Gallin WJ, Goldberg JI, Cunningham BA, Edelman GM. Construction of epithelioid sheets by transfection of mouse sarcoma cells with cDNAs for chicken cell adhesion molecules. Proc Natl Acad Sci USA. 1988;85:7274–7278. doi: 10.1073/pnas.85.19.7274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto S, Akiyama S, Yamada KM. Synergistic roles for receptor occupancy and aggregation in integrin transmembrane function. Science (Wash DC) 1995;267:883–885. doi: 10.1126/science.7846531. [DOI] [PubMed] [Google Scholar]
- Munemitsu S, Albert I, Rubinfeld B, Polakis P. Deletion of an amino-terminal sequence stabilizes β-catenin in vivo and promotes hyperphosphorylation of the adenomatous polyposis coli tumor supressor protein. Mol Cell Biol. 1996;16:4088–4094. doi: 10.1128/mcb.16.8.4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobes C, Hall A. Rho, Rac, and Cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- Nusrat A, Giry M, Turner JR, Colgan SP, Parkos CA, Carnes D, Lemichez E, Boquet P, Madara JL. Rho protein regulates tight junctions and perijunctional actin organization in polarized epithelia. Proc Natl Acad Sci USA. 1995;92:10629–10633. doi: 10.1073/pnas.92.23.10629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrish EP, Steart PV, Garrod DR, Weller RO. Antidesmosomal monoclonal antibody in the diagnosis of intracranial tumours. J Pathol. 1987;153:265–273. doi: 10.1002/path.1711530311. [DOI] [PubMed] [Google Scholar]
- Peifer M, Berg S, Reynolds AM. A repeating amino acid motif shared by proteins with diverse cellular roles. Cell. 1994a;76:789–791. doi: 10.1016/0092-8674(94)90353-0. [DOI] [PubMed] [Google Scholar]
- Peifer M, Pai L-M, Casey M. Phosphorylation of the Drosophila adherens junction protein armadillo: roles for Wingless signal and Zestewhite 3 kinase. Dev Biol. 1994b;166:543–556. doi: 10.1006/dbio.1994.1336. [DOI] [PubMed] [Google Scholar]
- Rheinwald, J.G. 1989. Methods for clonal growth and serial cultivation of normal human epidermal keratinocytes and mesothelial cells. In Cell Growth and Division. A Practical Approach. R. Baserga, editor. IRL Press, Oxford. 81–94.
- Ridley AJ. Rho: theme and variations. Curr Biol. 1996;6:1256–1264. doi: 10.1016/s0960-9822(02)70711-2. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Paterson HF, Johnston CL, Diekman D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Comoglio PM, Hall A. Regulation of scatter factor/ hepatocyte growth factor responses by Ras, Rac and Rho in MDCK cells. Mol Cell Biol. 1995;15:1110–1122. doi: 10.1128/mcb.15.2.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimm DL, Koslov ER, Kebriaei P, Cianci CD, Morrow JS. Alpha 1(E)-catenin is an actin-binding and -bundling protein mediating the attachment of F-actin to the membrane adhesion complex. Proc Natl Acad Sci USA. 1995;92:8813–8817. doi: 10.1073/pnas.92.19.8813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Boulan I, Nelson WJ. Morphogenesis of the polarized epithelial cell phenotype. Science (Wash DC) 1989;245:718–725. doi: 10.1126/science.2672330. [DOI] [PubMed] [Google Scholar]
- Rubinfeld B, Souza B, Albert I, Muller O, Chamberlain SH, Masiarz FR, Munemitsu S, Polakis P. Association of the APC gene product with β-catenin. Science (Wash DC) 1993;262:1731–1733. doi: 10.1126/science.8259518. [DOI] [PubMed] [Google Scholar]
- Rubinfeld B, Albert I, Porfiri E, Fiol C, Munemitsu S, Polakis P. Binding of GSK3β to the APC-β-catenin complex and regulation of complex assembly. Science (Wash DC) 1996;272:1023–1026. doi: 10.1126/science.272.5264.1023. [DOI] [PubMed] [Google Scholar]
- Schmalzing G, Richter H-P, Hansen A, Schwartz W, Just I, Aktories K. Involvement of the GTP binding protein Rho in constitutive endocytosis in Xenopus laevisoocytes. J Cell Biol. 1995;130:1319–1332. doi: 10.1083/jcb.130.6.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibamoto S, Hayakawa M, Takeuchi K, Hori T, Oku N, Miyazawa K, Kitamura N, Takeichi M, Ito F. Tyrosine phosphorylation of β-catenin and plakoglobin enhanced by hepatocyte growth factor and epidermal growth factor in human carcinoma cells. Cell Adhes Commun. 1994;1:295–305. doi: 10.3109/15419069409097261. [DOI] [PubMed] [Google Scholar]
- Shimoyama Y, Yoshida T, Terada M, Shimosato Y, Abe O, Hirohashi S. Molecular cloning of human Ca2+-dependent cell-cell adhesion molecule homologous to mouse placental cadherin: its low expression in human placental tissues. J Cell Biol. 1989;109:1787–1794. doi: 10.1083/jcb.109.4.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich JA, Watt OS. The regulation of rabbit skeletal muscle contraction I. Biochemical studies of the interaction of the tropomyosintroponin complex with actin and the protein fragments of myosin. J Biol Chem. 1971;246:4866–4872. [PubMed] [Google Scholar]
- Su L-K, Vogelstein B, Kinzler KW. Association of the APC tumor suppressor protein with catenins. Science (Wash DC) 1993;262:1734–1737. doi: 10.1126/science.8259519. [DOI] [PubMed] [Google Scholar]
- Takeda H, Nagafuchi A, Yonemura S, Tsukita S, Behrens J, Birchmeier W, Tsukita S. V-srckinase shifts the cadherin-based cell adhesion from the strong to the weak state and β-catenin is not required for the shift. J Cell Biol. 1995;131:1839–1847. doi: 10.1083/jcb.131.6.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeichi M. Cadherin cell adhesion receptors as a morphogenetic regulator. Science (Wash DC) 1991;251:1451–1455. doi: 10.1126/science.2006419. [DOI] [PubMed] [Google Scholar]
- Takeichi M. Cadherins in cancer: implications for invasion and metastasis. Curr Opin Cell Biol. 1993;5:806–811. doi: 10.1016/0955-0674(93)90029-p. [DOI] [PubMed] [Google Scholar]
- Tominaga T, Sugie K, Hirata M, Morii N, Fukata J, Uchida A, Imura H, Narumiya S. Inhibition of PMA-induced, LFA-1-dependent lymphocyte aggregation by ADP ribosylation of the small molecular weight GTP binding protein, rho. J Cell Biol. 1993;120:1529–1537. doi: 10.1083/jcb.120.6.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukita S, Oishi K, Sato N, Sagara J, Kawai A, Tsukita S. ERM family members as molecular linkers between the cell surface glycoprotein CD44 and actin-based cytoskeletons. J Cell Biol. 1994;126:391–401. doi: 10.1083/jcb.126.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volberg T, Zick Y, Dror R, Sabanay I, Gilon C, Levitzki A, Geiger G. The effect of tyrosine specific protein phosphorylation on the assembly of adherens-type junctions. EMBO (Eur Mol Biol Organ) J. 1992;11:1733–1742. doi: 10.1002/j.1460-2075.1992.tb05225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheelock MJ, Jensen PJ. Regulation of keratinocyte intercellular junction organization and epidermal morphogenesis by E-cadherin. J Cell Biol. 1992;117:415–425. doi: 10.1083/jcb.117.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheelock MJ, Buck CA, Bechtol KB, Damsky CH. Soluble 80kd fragment of cell-CAM 120/80 disrupts cell-cell adhesion. J Cell Biochem. 1987;34:187–202. doi: 10.1002/jcb.240340305. [DOI] [PubMed] [Google Scholar]
- Zamansky GB, Nguyen U, Chou I-N. An immunofluorescence study of the calcium-induced coordinated reorganization of microfilaments, keratin intermediate filaments, and microtubules in cultured human epidermal keratinocytes. J Investig Dermatol. 1991;97:985–994. doi: 10.1111/1523-1747.ep12491899. [DOI] [PubMed] [Google Scholar]
- Zigmond SH. Signal transduction and actin filament organization. Curr Opin Cell Biol. 1996;8:66–73. doi: 10.1016/s0955-0674(96)80050-0. [DOI] [PubMed] [Google Scholar]