Cytoplasmic Dynein and Dynactin Are Required for the Transport of Microtubules into the Axon (original) (raw)
Abstract
Previous work from our laboratory suggested that microtubules are released from the neuronal centrosome and then transported into the axon (Ahmad, F.J., and P.W. Baas. 1995. J. Cell Sci. 108: 2761–2769). In these studies, cultured sympathetic neurons were treated with nocodazole to depolymerize most of their microtubule polymer, rinsed free of the drug for a few minutes to permit a burst of microtubule assembly from the centrosome, and then exposed to nanomolar levels of vinblastine to suppress further microtubule assembly from occurring. Over time, the microtubules appeared first near the centrosome, then dispersed throughout the cytoplasm, and finally concentrated beneath the periphery of the cell body and within developing axons. In the present study, we microinjected fluorescent tubulin into the neurons at the time of the vinblastine treatment. Fluorescent tubulin was not detected in the microtubules over the time frame of the experiment, confirming that the redistribution of microtubules observed with the experimental regime reflects microtubule transport rather than microtubule assembly. To determine whether cytoplasmic dynein is the motor protein that drives this transport, we experimentally increased the levels of the dynamitin subunit of dynactin within the neurons. Dynactin, a complex of proteins that mediates the interaction of cytoplasmic dynein and its cargo, dissociates under these conditions, resulting in a cessation of all functions of the motor tested to date (Echeverri, C.J., B.M. Paschal, K.T. Vaughan, and R.B. Vallee. 1996. J. Cell Biol. 132: 617–633). In the presence of excess dynamitin, the microtubules did not show the outward progression but instead remained near the centrosome or dispersed throughout the cytoplasm. On the basis of these results, we conclude that cytoplasmic dynein and dynactin are essential for the transport of microtubules from the centrosome into the axon.
The microtubule array of the axon is tightly regulated. Each microtubule within the array is oriented with its plus end distal to the cell body of the neuron (Heidemann et al., 1981), and each microtubule has a consistent 13-protofilament lattice (Tilney et al., 1973). In typical nonneuronal cells, the polarity orientation and lattice structure of a microtubule are influenced by its nucleation from and attachment to the centrosome (Euteneuer and McIntosh, 1982; Brinkley, 1985; Evans et al., 1985). Interestingly, the microtubules within the axon are not attached to the centrosome and yet are tightly regulated with regard to their polarity orientation and lattice structure. A possible explanation for this apparent paradox is that microtubules destined for the axon are in fact nucleated at the centrosome within the cell body of the neuron, after which they are released and transported into the axon with their plus ends leading. In this model, axonal microtubules obtain their lattice structure from centrosomal nucleation and their polarity orientation from their specific transport by a microtubule-based motor protein (for reviews see Baas, 1996, 1997). Initial support for this model was derived from studies showing that γ-tubulin, a protein essential for microtubule nucleation within living cells, is absent from the axon and concentrated at the centrosome within neurons (Baas and Joshi, 1992), and from studies showing that experimental inhibition of microtubule nucleation at the centrosome compromises axon outgrowth (Ahmad et al., 1994).
Additional support for the model was derived from pharmacological studies from our laboratory. These studies were aimed at resolving the contribution of microtubule transport by suppressing the contribution of microtubule assembly. In one study, we cultured rat sympathetic neurons in the presence of various concentrations of vinblastine that were shown to prevent the total microtubule levels in the neurons from increasing as well as individual microtubules from elongating (Baas and Ahmad, 1993). Microtubules accumulated within the axons of these neurons and were concomitantly depleted from the cell body, suggesting a transfer of microtubule from one compartment of the neuron into the other. In all cases, the microtubules appeared within the axons with their plus ends distal to the cell body, indicating that new microtubule assembly is not required for the establishment of this pattern. In a subsequent study, we used nocodazole to experimentally deplete the neurons of microtubules and then rinsed out the drug for a few minutes to permit a burst of microtubule assembly from the centrosome (Ahmad and Baas, 1995). We then exposed the neurons to an appropriate concentration of vinblastine to suppress further microtubule assembly from occurring. Over time, there was a gradual depletion of microtubules from the centrosome and central region of the cell body and a concomitant accumulation of microtubules at the periphery of the cell body and within developing axons. On the basis of these two studies, we concluded that microtubules are actively transported from the centrosome into the axon, presumably by a motor protein that conveys them specifically with their plus ends leading.
If the observed redistribution of microtubules in our experimental regime is indeed the result of motor-driven microtubule transport, it should be possible to identify the specific motor protein that is responsible. Cytoplasmic dynein is a potentially attractive candidate. It has the appropriate directionality to transport microtubules with plus ends leading (Paschal and Vallee, 1987; Holzbaur and Vallee, 1994) and has already been shown to be capable of generating forces that may organize microtubules during mitotic spindle formation (Heald et al., 1996; see also McGrail and Hayes, 1997). Abundant evidence suggests that cytoplasmic dynein transports membranous organelles along the axon in the retrograde direction (see for example Schnapp and Reese, 1989). An additional role for cytoplasmic dynein in microtubule transport is consistent with the fact that most of the cytoplasmic dynein that moves anterogradely down the axon does so in the slow component of axonal transport, the component in which the cytoskeletal elements move (Dillman et al., 1996_a_ ).
To test whether cytoplasmic dynein is the motor that transports microtubules from the neuronal centrosome into the axon, we have now performed the pharmacological regime described above on neurons in which we inhibited cytoplasmic dynein function by disrupting dynactin. Dynactin, a complex of at least nine polypeptides that range from 24 to 150 kD (Gill et al., 1991; Paschal et al., 1993; Schafer et al., 1994; Karki and Holzbaur, 1995; Vaughan and Vallee, 1995), has been implicated in cytoplasmic dynein function in biochemical, genetic, and cell biological analyses (for reviews see Allan, 1996; Schroer, 1996; Vallee and Sheetz, 1996). The 50-kD subunit of dynactin, originally termed p50, was renamed dynamitin to reflect its capacity to dissociate the complex when present at high levels (Echeverri et al., 1996). The dissociation of dynactin by excess dynamitin disrupts all cytoplasmic dynein-dependent cellular events tested to date in nonneuronal cells, including chromosome alignment and spindle organization during prometaphase (Echeverri et al., 1996), as well as localization of membranous organelles and organization of microtubule arrays during interphase (Burkhardt et al., 1997). Our studies now show that microinjection of recombinant dynamitin into cultured neurons inhibits axon outgrowth and prevents the outward progression of microtubules observed with the pharmacological regime. On the basis of these results, we conclude that cytoplasmic dynein and dynactin are necessary for the transport of microtubules from the neuronal centrosome into the axon.
Materials and Methods
Cell Culture
Cultures of sympathetic neurons from the superior cervical ganglia of newborn rat pups were prepared as follows. After dissection, the ganglia were treated with 0.25% collagenase and 0.25% trypsin for 15 min and then triturated with a Pasteur pipet into a single cell dispersion. The cells were then plated onto “special dishes” that were prepared by adhering a glass coverslip to the bottom of a 35-mm plastic petri dish into which had been drilled a 1-cm-diam hole. For the preparation of these dishes, we used glass coverslips that had been photoetched with a pattern of demarcated boxes that assist in the relocation of individual cells (Bellco Glass, Inc., Vineland, NJ). Before plating the cells, the glass-bottomed well of the special dish was treated for 3 h with 1 mg/ml polylysine, rinsed extensively, and then treated with 10 μg/ml laminin (Sigma Chemical Co., St. Louis, MO) for 4 h. Cells were plated in medium consisting of Leibovitz' L-15 (Sigma Chemical Co.) supplemented with 0.6% glucose, 2 mM l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, 10% fetal bovine serum (Hyclone Labs, Logan, UT), and 100 ng/ml nerve growth factor (Upstate Biotechnology, Lake Placid, NY).
Pharmacological Regime for Revealing Microtubule Transport
In a previous report, we described a pharmacological regime for revealing the transport of microtubules from the centrosome first to the periphery of the neuronal cell body and then into developing axons (Ahmad and Baas, 1995; see introduction). In this regime, nocodazole (Aldrich Chemical Co., Milwaukee, WI) was introduced into the cultures 30 min after plating at a final concentration of 10 μg/ml, and the cultures were returned to the incubator. After 6 h, the cultures were rinsed twice with warm drug-free medium, placed in a third rinse of warm drug-free medium, and then returned to the incubator. After 3 min of recovery, vinblastine sulfate (Sigma Chemical Co.) was added to a final concentration of 50 nM, and the cultures were once again returned to the incubator for various periods of time. Cultures were then prepared for immunofluorescence visualization of microtubule distribution at various points during this regime. In the present study, we wished to confirm that the relocation of microtubules observed with this regime is truly the result of microtubule transport rather than of new microtubule assembly that might have occurred even in the presence of the drug. To resolve this issue, we introduced fluorescent (rhodamine-labeled) tubulin into the neuron concomitantly with the addition of the vinblastine. By “concomitantly,” we mean that the vinblastine was added first, and then two to three cells per dish were rapidly injected. Most cells were injected within 3 s of the addition of the drug, and no cells were injected at times greater than 3 min. The fluorescent tubulin was prepared as described by Keating et al. (1997) and was injected at a concentration of 4 mg/ml and a volume of roughly 0.1 picoliters. After 30 min, sufficient time for the microtubules to vacate the central region of the cell body and accumulate at cell periphery, the cultures were extracted, fixed, and prepared for immunofluorescence microscopy so that an image of the total microtubule mass within the cell could be visualized side-by-side with an image of any fluorescent tubulin that had incorporated into polymer during the vinblastine treatment.
Preparation of Bacterially Expressed Dynamitin
Bacterial expression of human dynamitin protein was carried out using the pET14b system (Novagen, Madison, WI) as previously described (Echeverri et al., 1996). Recombinant protein was purified by one of two methods, both of which gave similar results. In the first method, the recombinant protein was expressed with a thrombin-cleavable His-tag at its NH2 terminus. Nickel-affinity purification and subsequent removal of the His-tag were carried out with a commercially available kit according to the manufacturer's instructions (Novagen). In the other method, the recombinant protein was expressed without the His-tag and was purified by MonoQ ion-exchange chromatography, followed by size fractionation using gel filtration chromatography (see Whitman et al., 1997). If necessary, the protein obtained with either of these methods was then concentrated either by using Centricon-30 concentrators (Amicon, Beverly, MA) or by a salt bump from a MonoQ column on the SMART system. The protein was aliquoted and then frozen in liquid nitrogen at a final concentration of 3 mg/ml in a buffer containing 50 mM Kpipes, pH 7.0, 100 mM KCl, 1 mM EGTA, 0.1% BME, and 10% glyclerol. This buffer showed no deleterious effects on the health of a number of different cell types, including primary rat sympathetic neurons, when microinjected into their cytoplasm. The aliquots were thawed just before use, and the protein was microinjected without further dilution at a volume of roughly 0.1 picoliters per cell. Microinjection was carried out on the environmentally regulated stage of an inverted microscope using the Eppendorf (Hamburg, Germany) microinjection system. The purity of the recombinant protein was confirmed by SDS-PAGE.
Experimental Regimes Involving Recombinant Dynamitin
Three experimental regimes involving recombinant dynamitin were used. First, we wanted to confirm that microinjection of recombinant dynamitin effectively inactivates a known function of cytoplasmic dynein and that this response is not obtained if the protein is denatured by boiling before microinjection. For this, we examined the distribution of Golgi elements, reported to be regulated by cytoplasmic dynein (Corthesy-Theulaz et al., 1992; Fath et al., 1994; Burkhardt et al., 1997), in uninjected cells, cells that had been microinjected with the recombinant protein, and cells that had been microinjected with the recombinant protein after boiling. Second, having confirmed the usefulness of the recombinant dynamitin as a tool for studying cytoplasmic dynein function, we wished to use it to ascertain the effects of inactivating cytoplasmic dynein on axon outgrowth. For this, we microinjected either the viable or denatured dynamitin protein into freshly plated neurons that had been permitted to attach to the glass coverslip for roughly 30 min. The cells were then monitored over time and the extent of axon outgrowth was recorded using either a thermal video-printer (Sony, Japan) or a cooled CCD (Photometrics, Tucson, AZ) to obtain higher resolution images. Axon outgrowth per cell was measured as the sum of the length of all axons extended by an individual neuron. Third, we investigated the potential role of cytoplasmic dynein in transporting microtubules outward from the neuronal centrosome using a modification of the pharmacological regime described above (Ahmad and Baas, 1995). For this, either the native or denatured dynamitin protein was microinjected into a portion of the neurons in the dish after the fifth hour in nocodazole.
Fluorescence Microscopy
For visualization of Golgi elements, we used a modification of the procedure of Letourneau and Wire (1995). Cultures were fixed and extracted simultaneously for 15 min in a microtubule-stabilizing buffer termed PHEM (60 mM Pipes, 25 mM Hepes, 10 mM EGTA, 2 mM MgCl2, pH 6.9) containing 0.2% glutaraldehyde and 0.1% Triton X-100, rinsed in PHEM, treated for 15 min with 10 mg/ml sodium borohydride to reduce autofluorescence, incubated for 15 min in a blocking solution containing 5 mg/ml BSA in PBS, incubated for 1 h at 37°C with 75 μg/ml rhodamine-conjugated wheat germ agglutinin (Sigma Chemical Co.), rinsed in PBS, and mounted in a medium containing 100 mg/ml DABCO and 1 mg/ml _p_-phenylenediamine in 90% glycerol and 10% PBS (to reduce photobleaching).
For visualization of microtubules, we used our previously described procedure (Ahmad and Baas, 1995). Cultures were rinsed briefly in PHEM and then extracted for 3 min with 0.5% Triton X-100 in PHEM containing 10 μM taxol (provided as a gift from the National Cancer Institute, Bethesda, MD). This treatment removes unassembled tubulin while preserving microtubules (Black et al., 1986). The cultures were then fixed by the addition of an equal volume of PHEM containing 8% paraformaldehyde and 0.3% glutaraldehyde. After 10 min of fixation, the cultures were rinsed twice in PBS, treated three times for 5 min each in PBS containing 10 mg/ml sodium borohydride, and rinsed again in PBS. Cultures were then treated for 30 min in a blocking solution containing 2% normal goat serum and 1% BSA in PBS and exposed overnight at 4°C to the primary antibody diluted in blocking solution. The following morning, the cultures were rinsed three times in PBS, treated again for 30 min in blocking solution, exposed for 1 h at 37°C to the second antibody diluted in blocking solution, rinsed four times for 5 min each in PBS, and mounted in the same mounting medium described above for the Golgi studies. The primary antibody was a mouse monoclonal antibody against β-tubulin used at 1:500 and purchased from Amersham Corp. (Arlington Heights, IL). For the studies on cells into which rhodamine-labeled tubulin had been injected, the second antibody was a fluorescein-conjugated goat anti–mouse used at 1:100. For all other experiments, the second antibody was a Cy-3–conjugated goat anti–mouse used at 1:100. Both second antibodies were purchased from Jackson ImmunoResearch (West Grove, PA).
For the studies on fluorescent-tubulin incorporation, the cells were visualized with a cooled CCD to optimize sensitivity and improve our ability to visualize any incorporation of fluorescent tubulin into microtubules that might have occurred. For all other studies, a confocal microscope was used to optimize resolution. Optical sections through each cell were obtained using the LSM 410 laser scanning confocal microscope (Carl Zeiss, Inc., Thornwood, NY). The optical sections were 0.5 μm in width, and all sections composing an individual cell were examined. Estimates of total microtubule levels within individual neurons were routinely obtained using either the Zeiss system software or NIH Image software (provided free of charge from the National Institutes of Health, Bethesda, MD) to confirm the efficacy of the vinblastine treatment.
Results
The Pharmacological Regime Reveals Microtubule Transport
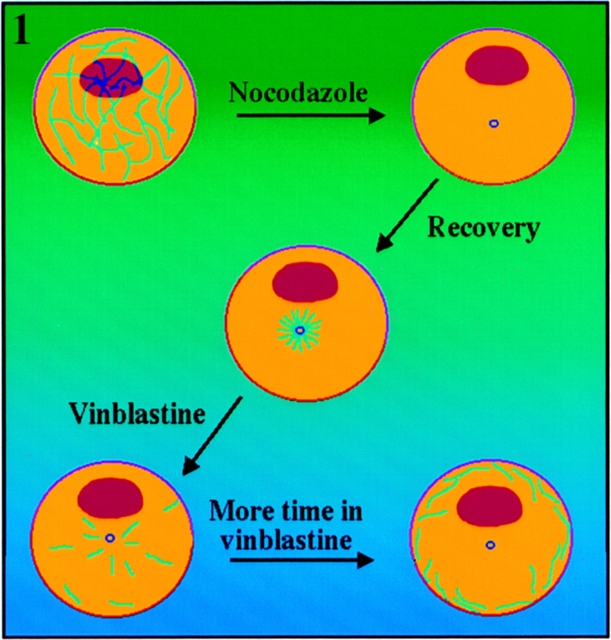
In a previous report, we described the following experimental regime, summarized in Fig. 1 (see Ahmad and Baas, 1995). Freshly plated rat sympathetic neurons are treated for 6 h with nocodazole, which typically diminishes their microtubule mass to less than 1% of control levels (the drug is less effective in ∼10% of the cells). The drug is then rinsed out, and microtubules are permitted to reassemble for 3 min. Virtually all of the microtubules reassemble from the centrosome, and these microtubules are typically shorter than 2–3 μm in length, although somewhat longer microtubules are occasionally seen as well. In a small number of cells (∼10%), significantly higher levels of microtubule polymer are found throughout the cell body, presumably because these cells correspond to those in which the nocodazole treatment was less effective in depolymerizing microtubules. After 3 min of recovery, the cells are then exposed to 50 nM vinblastine for various periods of time. By 15 min, the central region of the cell body is empty or nearly empty of microtubules, with most of the microtubules loosely packed at its periphery. By 30 min, virtually all of the microtubules are tightly packed at the periphery of the cell body. Most of the cells do not grow axons, but in the small number that do, the outward progression of microtubules continues into the axons. In cells examined at all points after the addition of vinblastine, there is a variability in the length of the individual microtubules that is generally similar to the variability observed during the initial recovery from nocodazole. When the microtubules redistribute from the centrosome, they sometimes tend to appear longer on average, but careful inspection suggests that this is due to the overlap of shorter microtubules. Quantitative analyses on microtubule mass suggest that no microtubule assembly occurs in the presence of the vinblastine, and hence that the redistribution of microtubules owes to microtubule transport (Ahmad and Baas, 1995). To confirm that this conclusion is correct, we have now performed studies to ascertain whether fluorescent tubulin can incorporate into microtubules during the vinblastine phase of this experimental regime.
Figure 1.
Schematic illustration of our pharmacological strategy for revealing the progression of microtubules outward from the neuronal centrosome (Ahmad and Baas, 1995; see Results for details).
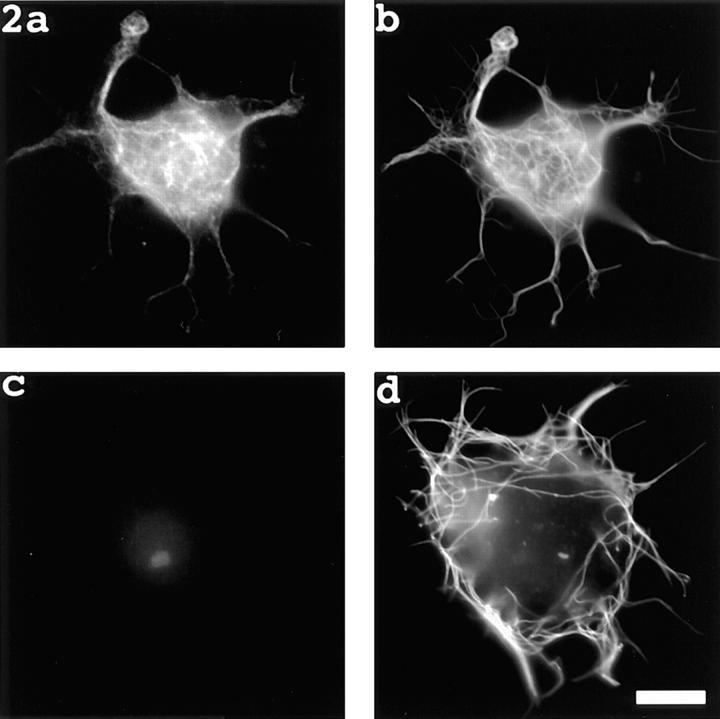
Fig. 2, a and b, shows a neuron treated with nocodazole, rinsed free of the drug, immediately injected with fluorescent tubulin, and then prepared for immunofluorescence visualization of microtubules 30 min later. The rhodamine image (depicting fluorescent tubulin that had incorporated into microtubule polymer; Fig. 2 a) and the fluorescein image (immunofluorescence image of total microtubule mass; Fig. 2 b) are similar in appearance, indicating complete or near-complete incorporation of fluorescent tubulin into polymer. This result was obtained with all 24 cells treated in this fashion. Fig. 2, c and d, show a neuron treated with nocodazole, rinsed free of the drug, simultaneously exposed to 50 nM vinblastine and injected with fluorescent tubulin (see Materials and Methods), and then prepared for immunofluorescence visualization of microtubules 30 min later. While the immunofluorescence image shows the expected peripheral concentration of microtubules (Fig. 2 d), the rhodamine image shows only faint background fluorescence and a fluorescent “scar” made by the injection (Fig. 2 c). No fluorescent microtubule polymer was detected in this cell, or in any of the other 18 neurons treated in this fashion. On the basis of these results, we conclude that the 50 nM concentration of vinblastine used in this regime is sufficient to inhibit microtubule assembly from occurring over the time frame of the experiment.
Figure 2.
Evidence that the pharmacological strategy reveals microtubule transport, not microtubule assembly. a and b show a neuron treated with nocodazole, rinsed free of the drug, immediately injected with fluorescent tubulin, and then prepared for immunofluorescence visualization of microtubules 30 min later. The rhodamine image (depicting fluorescent tubulin that had incorporated into microtubule polymer [_a_]) and the fluorescein image (immunofluorescence image of total microtubule mass [_b_]) are similar in appearance, indicating complete or near-complete incorporation of fluorescent tubulin into polymer. c and d show a neuron treated with nocodazole, rinsed free of the drug, simultaneously exposed to 50 nM vinblastine, injected with fluorescent tubulin (see Materials and Methods), and then prepared for immunofluorescence visualization of microtubules 30 min later. The immunofluorescence image shows the expected peripheral concentration of microtubules (d). The rhodamine image shows only faint background fluorescence and a fluorescent “scar” made by the injection (c). No fluorescent microtubule polymer was detected. Bar, 5 μm.
Microinjection of Recombinant Dynamitin Inhibits Cytoplasmic Dynein Function in Cultured Neurons
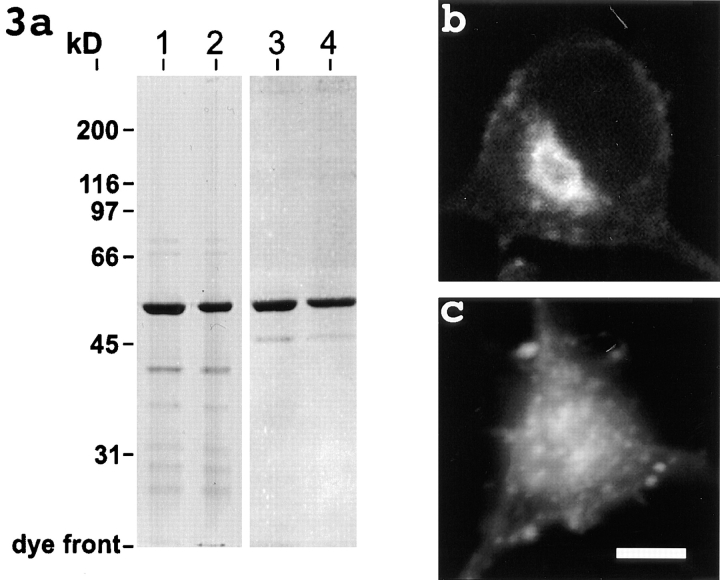
In previous studies, overexpression of dynamitin in nonneuronal cells was shown to dissociate dynactin and inhibit multiple forms of cytoplasmic dynein-based transport. This resulted in mitotic arrest (Echeverri et al., 1996), mislocalization of certain types of membranous organelles, and abnormalities in microtubule organization during interphase (Burkhardt et al., 1997). To produce a similar but more acute inactivation of cytoplasmic dynein function in the present study, we have prepared and purified bacterially expressed human dynamitin and microinjected it directly into cultured rat sympathetic neurons. As a negative control, we microinjected cells with dynamitin preparations that had been denatured by boiling. SDS-PAGE analyses confirmed the purity of the protein preparations (Fig. 3 a).
Figure 3.
SDS-PAGE gel showing the purity of the recombinant dynamitin preparations (a) and micrographs showing Golgi distribution in a control neuron (b) and a neuron 6 h after microinjection with recombinant dynamitin (c). a shows Coomassie blue staining of native (lanes 1 and 3) and boiled (lanes 2 and 4) dynamitin preparations purified either by nickel-affinity (lanes 1 and 2) or by two-step chromatography (lanes 3 and 4). The analyses confirm the presence of a single major band of the expected size, 50 kD, common to both preparations. In b, the control neuron shows a discrete Golgi apparatus located near the nucleus. In c, the Golgi elements are dispersed throughout the injected cell. Bar, 5 μm.
Before studying the effects of the recombinant dynamitin on microtubule transport, we initially wished to confirm in neurons the effects of the protein on a known function of cytoplasmic dynein. Neurons are terminally postmitotic cells, and for this reason we could not investigate its effects on the formation or functioning of the mitotic spindle. However, neurons have a prominent Golgi apparatus located near the nucleus at the center of the cell body, and several lines of evidence indicate that the presence of a discrete Golgi apparatus results from the transport of dispersed Golgi elements along microtubules (Rogalski and Singer, 1984; Ho et al., 1989; Lococq et al., 1989) by cytoplasmic dynein (Corthesy-Theulaz et al., 1992; Fath et al., 1994; Burkhardt et al., 1997). Hence, the dispersion of the Golgi apparatus can be used as a reliable indicator for the inactivation of cytoplasmic dynein. To visualize the Golgi apparatus, we used a fluorescent lectin that labels Golgi elements and, to a lesser extent, other membranes of the cell (see Letourneau and Wire, 1995). We noted variable degrees of Golgi dispersion within the first few hours of dynamitin injection and dramatic and consistent Golgi dispersion by the sixth hour in over 50 cells analyzed in four different experiments. Fig. 3 b shows a typical cultured neuron with a discrete Golgi apparatus located near the nucleus. Fig. 3 c shows a neuron 6 h after microinjection with recombinant dynamitin; the Golgi apparatus is dispersed. The Golgi apparatus of cells injected with boiled dynamitin protein was indistinguishable from that of uninjected control cells (not shown). These results indicate that the viable dynamitin protein but not the denatured protein disrupts a known function of cytoplasmic dynein function in cultured neurons.
Recombinant Dynamitin Inhibits Axon Outgrowth
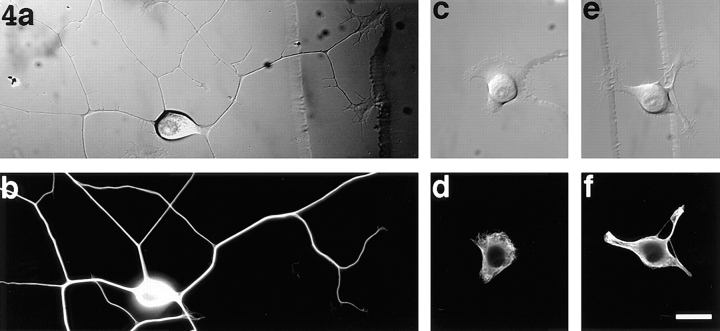
If microtubule transport is essential for establishing the microtubule array of a developing axon, and if cytoplasmic dynein is required for the transport of microtubules into the developing axon, we would expect inhibition of cytoplasmic dynein function to be deleterious to axon outgrowth. To test this prediction, we measured the outgrowth of axons from uninjected neurons, neurons that had been injected with the viable dynamitin protein, and neurons that had been injected with the boiled protein. The neurons were injected 30 min after plating, after they had attached to the glass coverslip but before they had begun to grow axons. A total of at least 100 neurons were examined under each experimental condition. Uninjected neurons and neurons injected with the boiled dynamitin protein started to extend axons within the first hour of plating, while the neurons injected with the viable dynamitin protein showed no axon outgrowth during this time. Neurons injected with the boiled dynamitin were slightly slower to initiate axons than the uninjected cells, probably because of the trauma of injection. (This same result was obtained when buffer alone was injected.) The axons extended by the uninjected neurons and the neurons injected with the boiled protein continued to grow, reaching lengths exceeding hundreds of microns (370 ± 80 and 290 ± 60 μm, respectively) by 7 h (Fig. 4 a). Immunofluorescence analyses of these neurons showed intense staining for microtubules in the cell bodies and axons (Fig. 4 b). By comparison, 109 of the 112 neurons injected with the native dynamitin protein showed either no axon outgrowth or substantially reduced levels of outgrowth even after 9 h, the longest time at which they were observed. Three of the neurons extended axons similar in length to controls. Of the 109 neurons that showed deleterious effects on axon outgrowth, 81 showed no axon outgrowth whatsoever (Fig. 4 c). These cells extended only broad lamellipodia into which the microtubules splayed but did not form dense bundles as they did within the control axons (Fig. 4 d). Some of the dynamitin-injected cells (28 of the 112) extended short processes with a broad flat appearance, as opposed to the thin, cylindrical appearance characteristic of control axons (Fig. 4 e). The microtubules within these processes were more splayed apart and less paraxial than those within the control axons (Fig. 4 f). The average length of the processes extended by these 109 dynamitin-injected neurons was 17 ± 5 μm, a reduction of ∼95.5% compared with the uninjected neurons and roughly a reduction of 94.1% compared with the neurons injected with boiled protein. Thus excess recombinant dynamitin resulted in a dramatic inhibition of axon outgrowth in over 97% of the cells into which it was microinjected.
Figure 4.
Effects on axon outgrowth of recombinant dynamitin. (a) A neuron 7 h after plating. Hundreds of microns of axons have grown. (b) An immunofluorescence image showing the dense microtubule array of the same cell after extraction and fixation. (c) A typical neuron 9 h after microinjection with the recombinant dynamitin. The cell has extended a broad lamellipodium but no axons. (d) An immunofluorescence image of the microtubule array within this cell. The microtubules splayed into the lamellipodia but did not form dense bundles as they did within the axons of control neurons. (e) One of a small number of neurons injected with recombinant dynamitin that extended processes (shown 9 h after microinjection). In these cases, the processes were significantly shorter than the axons of control neuron and also had a broad flat appearance that was rather different from the thin cylindrical shape of the control axons. (f) An immunofluorescence image of the microtubule array within this cell. The microtubules within these processes are more spayed apart and less paraxial than those within the control axons. Bar, 10 μm.
Recombinant Dynamitin Inhibits the Outward Progression of Microtubules from the Centrosome
The inhibition of axon outgrowth that we observed in cells injected with the recombinant dynamitin is consistent with the hypothesis that cytoplasmic dynein is required for microtubule transport into the axon. However, inhibition of axon outgrowth is also consistent with the inactivation of other known functions of the motor. For example, cytoplasmic dynein is known to function in the retrograde transport of endocytosed materials, such as growth factors that are essential for axon growth. Therefore, to test whether cytoplasmic dynein is necessary for the transport of microtubules, we needed to use an assay for microtubule transport that is independent of axon outgrowth or other known functions of the motor. Toward this end, we used the pharmacological assay described above.
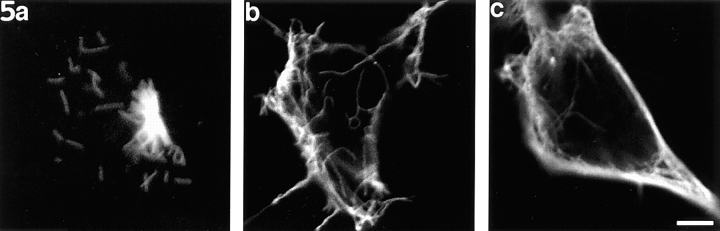
Neurons that had been microinjected with the boiled dynamitin protein (Fig. 5) exhibited the same microtubule behaviors that we previously observed in uninjected neurons. 3 min after nocodazole removal, a dense radial array of short microtubules was apparent at the centrosome (Fig. 5 a). A few unattached microtubules of roughly the same length were also sometimes apparent. In the cell shown in Fig. 5 a, the microtubules are 1–2.5 μm in length, but sometimes these lengths were as long as ∼5 μm. After exposure to 50 nM vinblastine for 15 min, microtubules were essentially absent from the central region of the cell body and became loosely packed near its periphery (Fig. 5 b). Note that the microtubules in the cell shown in Fig. 5 b are slightly longer than those shown in Fig. 5 a, but they are not outside of the range of microtubule lengths identified in other cells before vinblastine treatment. After 30 min in vinblastine, the microtubules were also at the cell periphery but were now more tightly packed (Fig. 5 c). In 50 cells examined at each of these time points, all of the cells showed this peripheral concentration of microtubules. Only two cells at each time point failed to show the absence or near absence of microtubules from the central region of the cell body. At times exceeding 1 h, some of the cells extended short axons, and in these cases, the microtubules continued to vacate the cell body as they began to appear within the axons (data not shown). Though the microtubule nucleating center was usually centrally located, it could also sometimes be found near the edge of the cell, as was the case with the neuron shown in Fig. 5 a.
Figure 5.
Neurons at various stages of the pharmacological regime designed to reveal the outward progression of microtubules from the centrosome. The cells shown here were injected with the boiled recombinant dynamitin before rinsing out the nocodazole (see Results for details). The results were indistinguishable from those obtained on uninjected cells. (a) A neuron that had been treated with nocodazole, microinjected with the boiled protein, rinsed free of the drug for 3 min, and then prepared for immunofluorescence visualization of microtubules. A dense radial array of short microtubules is apparent at the centrosome, as are a few unattached microtubules of roughly the same length. (b) A neuron treated in similar fashion and then exposed to 50 nM vinblastine for 15 min. Microtubules are essentially absent from the central region of the cell body and are loosely packed beneath its periphery. (c) A neuron exposed to vinblastine for 30 min. The microtubules are more tightly packed at cell periphery. The microtubules vary somewhat in length from cell to cell, with some of the microtubules appearing longer in the cell shown in b than those in the cell shown in a. However, the differences are no greater than those observed among different cells before or after vinblastine treatment. Bar, 3 μm.
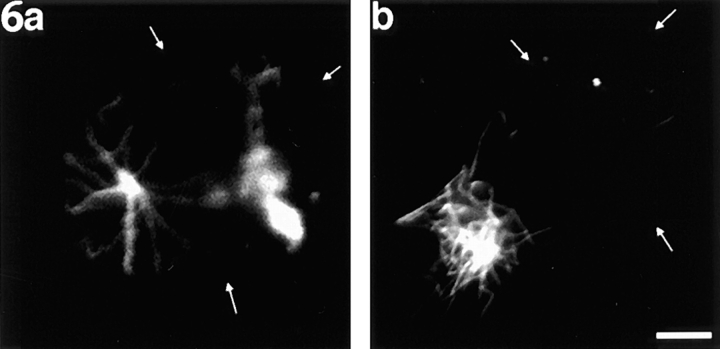
By contrast with control cells, neurons that had been microinjected with the native dynamitin protein showed differences in microtubule organization at all stages of the experimental regime after microinjection, even after the initial 3-min recovery from nocodazole. In some of the cells, the individual microtubules emanating from the centrosome had somewhat greater lengths than those within corresponding control cells (Fig. 6 a). This is reminiscent of similar observations on mitotic nonneuronal cells induced to overexpress dynamitin (Echeverri et al., 1996; our unpublished data), and also of recent observations of abnormally elongated cytoplasmic microtubules in dynein-deficient yeast (Carminati and Stearns, 1997). In other cells, the nucleating center appeared somewhat more diffuse than in similarly treated control cells (Fig. 6 b), also reminiscent of similar observations in dynamitin-overexpressing nonneuronal cells (Burkhardt et al., 1997). Some of the dynamitin-injected neurons displayed somewhat lower apparent levels of microtubule polymer than control cells recovered for the same amount of time, even though individual microtubules within these same cells may have been somewhat longer than those in control cells (compare for example the cell shown in Fig. 6 a with the cell shown in Fig. 5 a). For this reason, we increased the recovery time from 3 to 7.5 min to obtain microtubule levels that were more consistently similar to the levels obtained in control cultures recovered for 3 min.
Figure 6.
Examples of neurons treated with nocodazole, injected with recombinant dynamitin, rinsed free of the drug for 3 min, and then immunostained to reveal microtubules. (a) A neuron with a discrete site of nucleation similar to control neurons. The microtubules are fewer and somewhat longer than the microtubules in similarly treated control neurons. (b) A neuron in which the site of nucleation is somewhat more diffuse than in a similarly treated control (uninjected or injected with boiled dynamitin protein) neuron, but the level of microtubule reassembly is similar. Small white arrows indicate the perimeter of the cell body where it is not apparent from the immunofluorescence image. Bar, 3 μm.
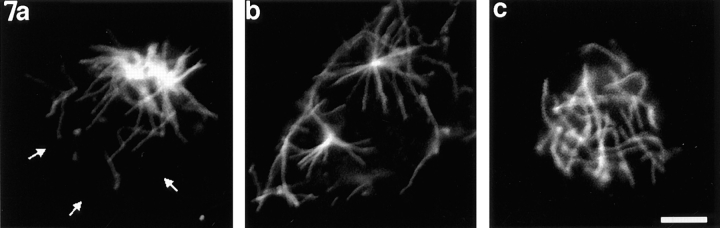
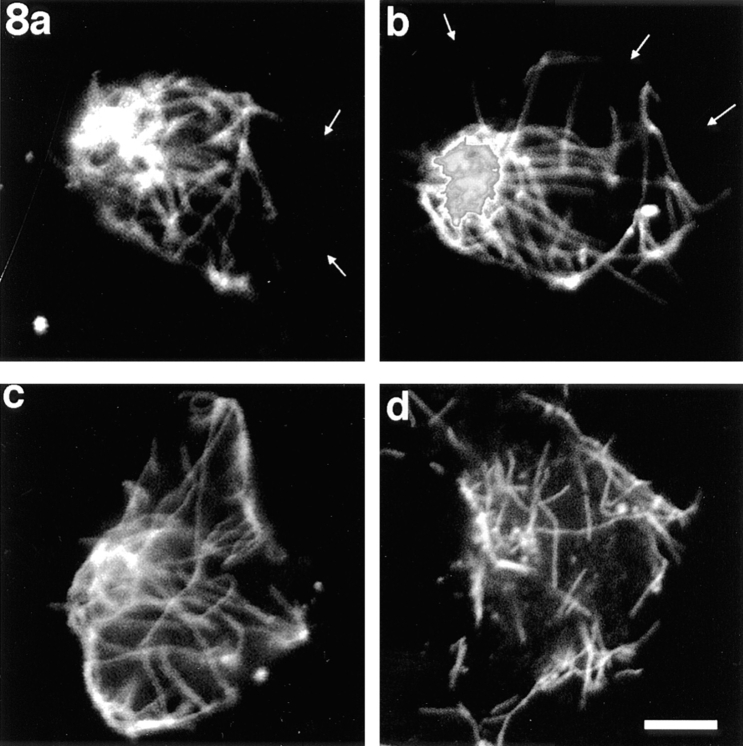
Neurons incubated in vinblastine for 15 or 30 min after introducing the recombinant dynamitin and rinsing out the nocodazole were markedly different from similarly treated control neurons with regard to microtubule distribution. Over 70 neurons were analyzed at each of these time points. At both time points, approximately half of the cells showed microtubules still clustered at their apparent nucleation sites (Figs. 7, a and b, and 8, a and b), while the other half showed microtubules distributed throughout their cytoplasm (Figs. 7 c and 8, c and d). In the former group, the apparent nucleation sites were located closer to the edge of the cell than to its center. In addition, most of the apparent nucleation sites had the somewhat more diffuse appearance mentioned above (Figs. 7 a and 8, a and b). In some neurons, the nucleation site had completely split into two relatively discrete and spatially segregated sites (Fig. 7 b). Most importantly, in no case did we observe the characteristic peripheral concentration and corresponding depletion of microtubules from cell center observed in comparable uninjected cells or cells injected with the boiled protein. Thus, inhibition of cytoplasmic dynein function by recombinant dynamitin inhibits the outward progression of microtubules from the neuronal centrosome.
Figure 7.
Examples of neurons treated with nocodazole, injected with recombinant dynamitin, rinsed free of drug for 7.5 min, exposed to vinblastine for 15 min, and then immunostained to reveal microtubules. In no such case did the microtubules vacate the central region of the cell body and concentrate beneath the cell periphery, as was the case with similarly treated uninjected neurons or neurons injected with boiled protein. (a) A neuron in which most of the microtubules remained attached to their apparent nucleation site, which had become rather diffuse in appearance. (b) A neuron in which the nucleation site had split into two, and most of the microtubules remained attached to these sites. A few unattached microtubules were present at cell periphery, but the vast majority were not. (c) A neuron in which the microtubules were dispersed throughout the cytoplasm and not attached to any apparent nucleation sites. Small white arrows indicate the perimeter of the cell body where it is not apparent from the immunofluorescence image. Bar, 4 μm.
Discussion
The present study represents the latest in our ongoing efforts to understand the mechanisms by which the axonal microtubule array is established. There are essentially two schools of thought on this issue, one based on the active transport of microtubules, and the other based on the concept of stationary microtubules (for reviews see Baas and Brown, 1997; Hirokawa et al., 1997). Results from our laboratory support the first idea and suggest a model whereby microtubules destined for the axon are nucleated and then released from the centrosome within the cell body of the neuron, after which they are actively transported into the axon by a microtubule-based motor protein. A technical challenge in testing this model is distinguishing the contribution of microtubule transport from that of microtubule assembly. To accomplish this, two recent studies from our laboratory have used the pharmacological agent vinblastine to suppress microtubule assembly and thereby reveal the contribution of microtubule transport. In the first of these studies, we demonstrated that neurons cultured in the presence of nanomolar levels of vinblastine continue to grow axons, and that microtubules accumulate within the axons while concomitantly vacating the cell body (Baas and Ahmad, 1993). In the second study, a nocodazole- recovery regime was used to obtain a synchronized burst of microtubules from the centrosome before exposing the neurons to vinblastine. After the addition of vinblastine, we documented an outward progression of the microtubules from the centrosome into the axon (Ahmad and Baas, 1995). These results were interpreted in terms of the active transport of microtubules.
Our interpretation of these results has recently been challenged by Miller and Joshi (1996), who questioned whether the vinblastine treatments used in our studies were actually effective in suppressing microtubule assembly. These authors exposed cultured neurons to vinblastine, injected them with fluorescent tubulin, waited various periods of time, extracted them to remove free tubulin, and then determined whether any of the fluorescent tubulin had incorporated into microtubule polymer. They found that vinblastine concentrations of 1 or 4 nM permitted fluorescent tubulin to incorporate into polymer segments that grew by fractions of a micron per minute. However, they did not determine whether the incorporation of fluorescent tubulin represents the net assembly of new microtubule polymer or merely subunit exchange with the soluble tubulin pool. It is well documented that vinblastine does not entirely inhibit microtubules from exchanging subunits with the soluble tubulin pool, even when used at concentrations sufficient to suppress the addition of new polymer (Wilson and Jordan, 1994; Ahmad and Baas, 1995; Tanaka et al., 1995; for more discussion see Baas, 1997; Baas and Brown, 1997). Moreover, Miller and Joshi did not use the 50 nM vinblastine concentration used in our studies, nor did they use our specific experimental regime. In the present study, we have established that no detectable fluorescent tubulin incorporates into microtubule polymer in our experimental regime using the 50 nM vinblastine concentration. As suggested above, if fluorescent tubulin had incorporated into the microtubules, this would not have proven that any consequential microtubule assembly occurred in the presence of the drug. However, the fact that there was little or no incorporation shows unequivocally that our results cannot be attributed to microtubule assembly. Thus, as originally concluded, the outward progression of microtubules observed with our regime must be the result of microtubule transport.
If microtubule transport plays such a key role in organizing neuronal microtubules, then what is the role of the centrosome? In many cell types the centrosome anchors the microtubules at their minus ends, resulting in a radial array of uniformly oriented microtubules (for review see Brinkley, 1985). With regard to neurons and other cell types that establish microtubule arrays at sites distal to the centrosome, we have suggested that the centrosome acts as a kind of “generator” of microtubules, nucleating them with the proper lattice structure and then releasing them for transport into other regions of the cell (Baas, 1996). In support of this view, sophisticated templates for microtubule nucleation have now been identified within the pericentriolar material. These templates consist of γ-tubulin to nucleate the microtubule, in combination with several other proteins that form ring structures ideally suited to constrain its lattice structure (Moritz et al., 1995; Zheng et al., 1995). In addition, a novel protein called katanin has been localized in at least one cell type to a region just outside of the pericentriolar material (McNally et al., 1996). Katanin is a microtubule-severing protein, and its distribution around the pericentriolar material can readily explain how a microtubule is released so that it can be transported away from the centrosome. Thus, the machinery exists within cells to release centrosomal microtubules, and it is provocative to speculate that microtubule release may be more active in cell types that must deploy their microtubules. In strong support of this view, Keating and colleagues (1997) have now directly observed microtubule release and transport from the centrosome of living epithelial cells, close cousins to the neuron.
Cytoplasmic Dynein and Microtubule Transport
The main goal of the present studies was to test the hypothesis that cytoplasmic dynein is the motor protein that transports microtubules from the neuronal centrosome into the axon. Cytoplasmic dynein is a molecular motor that translocates toward the minus end of the microtubule. Assuming that the motor is tethered to another structure with greater resistance to movement than the microtubule itself, cytoplasmic dynein would have the appropriate properties to transport microtubules with their plus ends leading away from the centrosome. Our analyses, using an excess of dynamitin to inhibit cytoplasmic dynein function, indicate that the redistribution of microtubules from the neuronal centrosome depends on the activity of this motor. When cytoplasmic dynein function is inhibited, the microtubules are not conveyed to cell periphery but instead remain near the centrosome or dispersed throughout the cytoplasm of the neuron. These results provide an additional line of evidence indicating that motor-driven transport is the means by which microtubules relocate from the neuronal centrosome, and also demonstrate that cytoplasmic dynein and the dynactin complex are essential components of the molecular machinery by which this transport occurs.
There is an additional component of the transport machinery that our studies have not addressed, namely the structures or “substrate” against which cytoplasmic dynein generates its forces. As the motor domain of the molecule interacts with the microtubule, the cargo domain interacts via dynactin with another structure in the cytoplasm such as a membranous element, another type of cytoskeletal element, or another microtubule. If the microtubule is immobilized (for example by its attachment to the centrosome), then this other structure will be transported toward the minus end of the microtubule. However, as noted above, if the microtubule is not tethered to the centrosome, and if the other structure has a greater resistance to movement, then it is the microtubule itself that will be transported. One possibility is that the released microtubules are transported relative to the same kinds of membranous organelles that move toward the minus ends of microtubules that are attached to the centrosome. When the microtubule is attached to the centrosome, it has the greater resistance to movement, but when the microtubule is released, the organelles have a greater resistance to movement, and the microtubule moves toward cell periphery. Thus, the redistribution of microtubules and the direction of microtubule transport reported here may be a simple manifestation of the activity of cytoplasmic dynein and the conservation of momentum. A related possibility is that the microtubules are transported relative to one specific membranous structure, such as the extensive network of endoplasmic reticulum within the neuron (see Baas and Brown, 1997).
A very different possibility is that microtubules are transported away from the centrosome by generating forces against other cytoskeletal elements within the neuron. In the recent studies of Heald and collaborators (1996), short microtubules were observed to move relative to longer microtubules during the formation of artificial microtubule spindles in vitro. However, in these studies, the short microtubules moved with minus ends leading toward the minus ends of the longer microtubules, indicating that the short microtubules were conveyed as cargo. In our studies, it is unlikely that individual microtubules move by generating forces against one another because their transport is quite efficient even at relatively low microtubule densities. In addition, most of the microtubules are roughly similar in length, and none appear to be immobilized. Thus it is difficult to imagine how forces could be generated between microtubules to translocate virtually the entire microtubule mass from the center of the cell to its periphery. A more likely possibility is that the microtubules are moving relative to the actin-based cytomatrix. This idea was first proposed several years ago (McQuarrie et al., 1986) and is supported by recent studies showing that cytoplasmic dynein and dynactin are anterogradely transported down the axon at the same rate as the actin-based cytomatrix (Dillman et al., 1996_a_ ,b). On this basis, the Pfister laboratory has proposed that cytoplasmic dynein interacts via dynactin with the actin filaments that move down the axon, leaving the motor domain of the dynein molecule available to transport microtubules. The fact that the microtubules are transported somewhat more slowly than the actin filaments probably owes to an intermittent association of microtubules with the motor complex. A potential concern with this model is raised by studies showing that actin and dynactin do not directly interact (Holleran et al., 1996). However, it is possible that an indirect interaction between actin and dynactin is mediated by yet another protein, such as fodrin (see McQuarrie et al., 1986).
There are other reasons why a model invoking the actin cytomatrix is potentially attractive. Over the years, there have been several lines of evidence suggesting that microtubules and actin filaments are functionally linked in some way (for review see Gavin, 1997). One possibility is that force generation between these two polymer systems by cytoplasmic dynein is a common theme across cell types that is exaggerated in the neuron. Some support for this idea derives from the fact that disrupting actin filaments with drugs such as cytochalasin and disrupting cytoplasmic dynein function appear to have many of the same effects on microtubules. In both cases, there can be notable changes in tension on the microtubules as well as abnormal increases in microtubule length (Echeverri et al., 1996; Heidemann, 1996; Carminati and Stearns, 1997; Gavin, 1997; our present results). In addition, it is not difficult to imagine how the actin cytomatrix could confer directionality on the transport of microtubules. Like microtubules, actin filaments are polar structures, and the progression of microtubules outward from the centrosome might be the result of the polarity orientation of the actin filaments. Alternatively, the microtubules may move outward because the actin cytomatrix is denser near the cell membrane. In support of this view, studies on nematode axons have shown that the distal ends of the microtubules tend to tilt off the longitudinal axis toward the cell membrane (Chalfie and Thompson, 1979).
The past few years have seen a revolution with regard to the role of molecular motor proteins in organizing microtubules. While early work focused on the role of motor proteins in transporting membranous elements along microtubules, it is now clear that motor proteins also play fundamental roles in organizing microtubules themselves. For example, there is abundant evidence that cytoplasmic dynein and a variety of kinesin-related motor proteins are essential for the formation and functioning of the mitotic spindle, the most fundamental of all microtubule arrays (for review see Barton and Goldstein, 1996). Studies from our laboratory indicate that postmitotic neurons do not abandon the principles that underlie microtubule organization within dividing cells. For example, we have shown that the kinesin-related protein known as CHO1/MKLP1, previously thought to be mitosis-specific, continues to be expressed in postmitotic neurons, where it plays a key role in transporting microtubules into the dendrite (Sharp et al., 1997). The present studies on cytoplasmic dynein demonstrate that at least one other motor has critical roles in organizing microtubules both in the mitotic spindle and in the postmitotic neuron. We would conclude that the transport of microtubules by motor proteins such as cytoplasmic dynein is a theme used across cell types, and that neurons capitalize on this theme to elaborate their elongate processes.
Figure 8.
Examples of neurons treated with nocodazole, injected with recombinant dynamitin, rinsed free of drug for 7.5 min, and then exposed to vinblastine for 30 min. In no such case did the microtubules vacate the central region of the cell body and concentrate beneath the cell periphery, as was the case with similarly treated uninjected neurons or neurons injected with boiled protein. (a and b) Neurons in which some of the microtubules appear to be attached to a rather diffuse nucleation site. Other microtubules were dispersed. (c and d) Neurons in which the microtubules do not appear to be attached to discernible nucleation sites, but rather appear to be dispersed throughout the cytoplasm. Fluorescence intensity is saturating within the densest cluster of microtubules in b. Small white arrows indicate the perimeter of the cell body where it is not apparent from the immunofluorescence image. Bar, 4 μm.
Acknowledgments
We are grateful to Dr. Anthony Hyman (EMBL, Heidelberg, Germany) for sharing with us some of his recombinant dynamitin, which we used in several of our experiments. We thank Drs. J. Richard McIntosh (University of Colorado, Boulder, CO), E. Vaisberg (University of Colorado), and K. Kevin Pfister (University of Virginia, Charlottesville, VA) for providing antibodies and helpful advice during the early phases of this project. We thank Crist Cook for technical assistance.
This work was supported by grants from the National Institutes of Health to P.W. Baas and R.B. Vallee.
Footnotes
Send all correspondence to Dr. Peter W. Baas, Department of Anatomy and Program in Cellular and Molecular Biology, The University of Wisconsin Medical School, 1300 University Avenue, Madison, WI 53706. Tel.: (608) 262-7307. Fax: (608) 262-7306. E-mail: pwbaas@facstaff.wisc.edu
References
- Ahmad FJ, Baas PW. Microtubules released from the neuronal centrosome are transported into the axon. J Cell Sci. 1995;108:2761–2769. doi: 10.1242/jcs.108.8.2761. [DOI] [PubMed] [Google Scholar]
- Ahmad FJ, Joshi HC, Centonze VE, Baas PW. Inhibition of microtubule nucleation at the neuronal centrosome compromises axon growth. Neuron. 1994;12:271–280. doi: 10.1016/0896-6273(94)90270-4. [DOI] [PubMed] [Google Scholar]
- Allan V. Motor proteins: a dynamic duo. Curr Biol. 1996;6:630–633. doi: 10.1016/s0960-9822(09)00434-5. [DOI] [PubMed] [Google Scholar]
- Baas PW. The neuronal centrosome as a generator of microtubules for the axon. Curr Top Dev Biol. 1996;33:281–298. doi: 10.1016/s0070-2153(08)60341-5. [DOI] [PubMed] [Google Scholar]
- Baas PW. Microtubules and axonal growth. Curr Opin Cell Biol. 1997;9:29–36. doi: 10.1016/s0955-0674(97)80148-2. [DOI] [PubMed] [Google Scholar]
- Baas PW, Ahmad FJ. The transport properties of axonal microtubules establish their polarity orientation. J Cell Biol. 1993;120:1427–1437. doi: 10.1083/jcb.120.6.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas PW, Brown A. Slow axonal transport: the polymer transport model. Trends Cell Biol. 1997;7:380–384. doi: 10.1016/S0962-8924(97)01148-3. [DOI] [PubMed] [Google Scholar]
- Baas PW, Joshi HC. γ-Tubulin distribution in the neuron: implications for the origins of neuritic microtubules. J Cell Biol. 1992;119:171–178. doi: 10.1083/jcb.119.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton NR, Goldstein LSB. Going mobile: microtubule motors and chromosome segregation. Proc Natl Acad Sci USA. 1996;93:1735–1742. doi: 10.1073/pnas.93.5.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black MM, Keyser P, Sobel E. Interval between the synthesis and assembly of cytoskeletal proteins in cultured neurons. J Neurosci. 1986;6:1004–1012. doi: 10.1523/JNEUROSCI.06-04-01004.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkley BR. Microtubule organizing centers. Annu Rev Cell Biol. 1985;1:145–172. doi: 10.1146/annurev.cb.01.110185.001045. [DOI] [PubMed] [Google Scholar]
- Burkhardt JK, Echeverri CJ, Nilsson T, Vallee RB. Overexpression of the dynamitin (p50) subunit of the dynactin complex disrupts dynein-dependent maintenance of membrane organelle distribution. J Cell Biol. 1997;139:469–484. doi: 10.1083/jcb.139.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carminati JL, Stearns T. Microtubules orient the mitotic spindle in yeast through dynein-dependent interactions with the cell cortex. J Cell Biol. 1997;138:629–641. doi: 10.1083/jcb.138.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalfie M, Thompson JN. Organization of neuronal microtubules in the nematode Caenorhabditis elegans. . J Cell Biol. 1979;93:15–23. doi: 10.1083/jcb.82.1.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corthesy-Theulaz I, Pauloin A, Pfeffer SR. Cytoplasmic dynein participates in the centrosomal localization of the Golgi complex. J Cell Biol. 1992;118:1333–1345. doi: 10.1083/jcb.118.6.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillman JF, III, Dabney LP, Pfister KK. Cytoplasmic dynein is associated with slow axonal transport. Proc Natl Acad Sci USA. 1996a;93:141–144. doi: 10.1073/pnas.93.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillman JF, III, Dabney LP, Karki S, Paschal BM, Holzbaur ELF, Pfister KK. Functional analysis of dynactin and cytoplasmic dynein in slow axonal transport. J Neurosci. 1996b;16:6742–6752. doi: 10.1523/JNEUROSCI.16-21-06742.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echeverri CJ, Paschal BM, Vaughan KT, Vallee RB. Molecular characterization of the 50-kD subunit of dynactin reveals function for the complex in chromosome alignment and spindle organization during mitosis. J Cell Biol. 1996;132:617–633. doi: 10.1083/jcb.132.4.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euteneuer U, McIntosh JR. Structural polarity of kinetochore microtubules in PTK2 cells. J Cell Biol. 1982;98:338–345. doi: 10.1083/jcb.89.2.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans L, Mitchison T, Kirschner M. Influence of the centrosome on the structure of nucleated microtubules. J Cell Biol. 1985;100:1185–1191. doi: 10.1083/jcb.100.4.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fath KR, Trimbur GM, Burgess DR. Molecular motors are differentially distributed on Golgi membranes from polarized epithelial cells. J Cell Biol. 1994;126:661–675. doi: 10.1083/jcb.126.3.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavin RH. Microtubule-microfilament synergy in the cytoskeleton. Int Rev Cytol. 1997;173:207–242. doi: 10.1016/s0074-7696(08)62478-x. [DOI] [PubMed] [Google Scholar]
- Gill SRT, Schroer TA, Szilak I, Steuer ER, Sheetz MP, Cleveland DW. Dynactin, a conserved, ubiquitously expressed component of an activator of vesicle motility mediated by cytoplasmic dynein. J Cell Biol. 1991;115:1639–1650. doi: 10.1083/jcb.115.6.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heald R, Tournebize R, Blank T, Sandaltzopoulos R, Becker P, Hyman A, Karsenti E. Self-organization of microtubules into bipolar spindles around artificial chromosomes in Xenopus egg extracts. Nature. 1996;382:420–425. doi: 10.1038/382420a0. [DOI] [PubMed] [Google Scholar]
- Heidemann SR. Cytoplasmic mechanisms of axonal and dendritic growth in neurons. Int Rev Cytol. 1996;165:235–296. doi: 10.1016/s0074-7696(08)62224-x. [DOI] [PubMed] [Google Scholar]
- Heidemann SR, Landers JM, Hamborg MA. Polarity orientation of axonal microtubules. J Cell Biol. 1981;91:661–665. doi: 10.1083/jcb.91.3.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa N, Terada S, Funakoshi T, Takeda S. Slow axonal transport: the subunit transport model. Trends Cell Biol. 1997;7:384–388. doi: 10.1016/S0962-8924(97)01133-1. [DOI] [PubMed] [Google Scholar]
- Ho WC, Allan VJ, Van Meer G, Berger EG, Kreis TE. Reclustering of scattered Golgi elements along microtubules. Eur J Cell Biol. 1989;48:250–263. [PubMed] [Google Scholar]
- Holleran EA, Tokito MK, Karki S, Holzbaur ELF. Centractin (ARP1) associates with spectrin revealing a potential mechanism to link dynactin to intracellular organelles. J Cell Biol. 1996;135:1815–1829. doi: 10.1083/jcb.135.6.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzbaur ELF, Vallee RB. Dyneins: molecular structure and cellular function. Annu Rev Cell Biol. 1994;10:339–372. doi: 10.1146/annurev.cb.10.110194.002011. [DOI] [PubMed] [Google Scholar]
- Karki S, Holzbaur ELF. Affinity chromatography demonstrates a direct binding between cytoplasmic dynein and the dynactin complex. J Biol Chem. 1995;270:28806–28811. doi: 10.1074/jbc.270.48.28806. [DOI] [PubMed] [Google Scholar]
- Keating TJ, Momcilovic D, Rodionov VI, Borisy GG. Microtubule release from the centrosome. Proc Natl Acad Sci USA. 1997;94:5078–5083. doi: 10.1073/pnas.94.10.5078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letourneau PC, Wire JP. Three-dimensional organization of stable microtubules and the Golgi apparatus in the somata of developing chick sensory neurons. J Neurocytol. 1995;24:207–223. doi: 10.1007/BF01181535. [DOI] [PubMed] [Google Scholar]
- Lococq JM, Berger EM, Warren G. Mitotic Golgi fragments in HeLa cells and their role in the reassembly pathway. J Cell Biol. 1989;109:463–474. doi: 10.1083/jcb.109.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrail M, Hayes TS. The microtubule motor cytoplasmic dynein is required for spindle orientation during germline cell divisions and oocyte differention in Drosophila. . Development (Camb) 1997;124:2409–2419. doi: 10.1242/dev.124.12.2409. [DOI] [PubMed] [Google Scholar]
- McNally FJ, Okawa K, Iwamatsu A, Vale RD. Katanin, the microtubule-severing ATP-ase, is concentrated at centrosomes. J Cell Sci. 1996;109:561–567. doi: 10.1242/jcs.109.3.561. [DOI] [PubMed] [Google Scholar]
- McQuarrie IG, Brady ST, Lasek RJ. Diversity in the axonal transport of structural proteins: major differences between optic and spinal axons in the rat. J Neurosci. 1986;6:1593–1605. doi: 10.1523/JNEUROSCI.06-06-01593.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KE, Joshi HC. Tubulin transport in neurons. J Cell Biol. 1996;133:1355–1368. doi: 10.1083/jcb.133.6.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritz M, Braunfeld MB, Sedat JW, Alberts B, Agard DA. Microtubule nucleation by γ-tubulin–containing rings in the centrosome. Nature. 1995;378:638–640. doi: 10.1038/378638a0. [DOI] [PubMed] [Google Scholar]
- Paschal BM, Vallee RB. Retrograde transport by the microtubule associated protein MAP1C. Nature. 1987;330:181–183. doi: 10.1038/330181a0. [DOI] [PubMed] [Google Scholar]
- Paschal BM, Holzbaur ELF, Pfister KK, Clark S, Meyer DI, Vallee RB. Characterization of a 50 kD polypeptide in cytoplasmic dynein preparations reveals a complex with p150-Gluedand a novel actin. J Biol Chem. 1993;268:15318–15323. [PubMed] [Google Scholar]
- Rogalski AA, Singer SJ. Association of elements of the Golgi apparatus with microtubules. J Cell Biol. 1984;99:1092–1100. doi: 10.1083/jcb.99.3.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer DA, Gill SR, Cooper JA, Hauser JE, Schroer TA. Ultrastructural analysis of the dynactin complex: an actin-related protein is a component of a filament that resembles F-actin. J Cell Biol. 1994;126:403–412. doi: 10.1083/jcb.126.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroer TA. Structure and function of dynactin. Semin Cell Dev Biol. 1996;7:321–328. [Google Scholar]
- Schnapp BJ, Reese TS. Dynein is the motor for retrograde axonal transport of organelles. Proc Natl Acad Sci USA. 1989;86:1548–1552. doi: 10.1073/pnas.86.5.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp DJ, Yu W, Ferhat L, Kuriyama R, Rueger DC, Baas PW. Identification of a motor protein essential for dendritic differentiation. J Cell Biol. 1997;138:833–843. doi: 10.1083/jcb.138.4.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka E, Ho T, Kirschner MW. The role of microtubule dynamics in growth cone motility and axonal growth. J Cell Biol. 1995;128:139–155. doi: 10.1083/jcb.128.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney LG, Bryan J, Bush DJ, Fujiwara K, Mooseker MS, Murphy DB, Snyder DH. Microtubules: evidence for 13 protofilaments. J Cell Biol. 1973;59:267–275. doi: 10.1083/jcb.59.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee R, Sheetz MP. Targeting of motor proteins. Science. 1996;271:1539–1544. doi: 10.1126/science.271.5255.1539. [DOI] [PubMed] [Google Scholar]
- Vaughan, K.T., and R.B. Vallee. 1995. Cytoplasmic dynein binds dynactin through a direct interaction between the intermediate chains and p150_glued. J. Cell Biol._ 131:1507–1516. [DOI] [PMC free article] [PubMed]
- Whitman, T., R. Heald, and A.A. Hyman. 1997. Production of active P50/dynamitin in bacteria. Methods Cell Biol. In press.
- Wilson, L., and M.A. Jordan. 1994. Pharmacological probes of microtubule function. In Microtubules. J.S. Hyams and C.W. Lloyd, editors. 59–83.
- Zheng Y, Wong ML, Alberts B, Mitchison T. Nucleation of microtubule assembly by a γ-tubulin-containing ring complex. Nature. 1995;378:578–583. doi: 10.1038/378578a0. [DOI] [PubMed] [Google Scholar]