The Parkinson's disease gene DJ-1 is also a key regulator of stroke-induced damage (original) (raw)
Abstract
Recent evidence has indicated that common mechanisms play roles among multiple neurological diseases. However, the specifics of these pathways are not completely understood. Stroke is caused by the interruption of blood flow to the brain, and cumulative evidence supports the critical role of oxidative stress in the ensuing neuronal death process. DJ-1 (PARK7) has been identified as the gene linked to early-onset familial Parkinson's disease. Currently, our work also shows that DJ-1 is central to death in both in Vitro and in Vivo models of stroke. Loss of DJ-1 increases the sensitivity to excitotoxicity and ischemia, whereas expression of DJ-1 can reverse this sensitivity and indeed provide further protection. Importantly, DJ-1 expression decreases markers of oxidative stress after stroke insult in Vivo, suggesting that DJ-1 protects through alleviation of oxidative stress. Consistent with this finding, we demonstrate the essential role of the oxidation-sensitive cysteine-106 residue in the neuroprotective activity of DJ-1 after stroke. Our work provides an important example of how a gene seemingly specific for one disease, in this case Parkinson's disease, also appears to be central in other neuropathological conditions such as stroke. It also highlights the important commonalities among differing neuropathologies.
Keywords: neurodegeneration, oxidative stress, ischemia, neuroprotection
Stroke is one of the major leading causes of death and disability in North America (1, 2). It is caused by the interruption of the brain blood supply due to occlusion (ischemic) or rupture of blood vessels (hemorrhagic) leading to neuronal dysfunction and death. Although, the complete nature of the complex intra-/extracellular signals that regulate neuronal injury remains to be clarified, a growing body of evidence supports the essential role of oxidative stress in initiation and progression of the injury process (3, 4).
Reactive oxygen species (ROS), free radicals that are normal by-products of oxygen metabolism, are produced in excess during the course of ischemia/reperfusion by a variety of mechanisms such as aberrant electron transport in injured mitochondria (5), calcium influx (6), and inflammatory reactions (7). ROS rapidly react with proteins, lipids, and DNA and cause damage and if severe, cell death. Equally important, they likely activate specific signaling pathways that initiate adaptive or death responses (8). This ability to control ROS is critical in stroke, and neuronal damage occurs if the “oxidant–antioxidant” balance is disturbed in favor of excess oxidative stress during ischemia/reperfusion (9, 10). Therefore, ROS management plays a central role in the pathogenesis of stroke.
DJ-1 (PARK7), a protein originally discovered as an oncogene (11), was also identified as an autosomal recessive gene of Parkinson's disease (PD) (12). The physiological role of DJ-1 remains unclear; however, the protective role of DJ-1 against oxidative stress has been shown in several pathological disease models both in vitro and in vivo (13–15). For instance, previous published data from our laboratory demonstrate the neuroprotective activity of DJ-1 in models of PD induced by the dopaminergic toxins rotenone and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) (16). Our results also suggested that DJ-1 may respond to selective initiators of damage, in particular those that involve oxidative stress. This finding was demonstrated by the observation that DJ-1 loss did not sensitize cultured cells to DNA damage- or staurosporine-induced death. These results are consistent with the notion that DJ-1 itself is modified by oxidation (17, 18) and that DJ-1 modifies the transcription of antioxidant enzymes in various cell lines, likely through the regulation of Nrf2, a transcription factor that coordinates a variety of antioxidant enzymes (19). However, whether this result occurs in neurons is unknown.
If the main mechanism of action of DJ-1 is through its ability to handle oxidative stress, then DJ-1 should also be central to other models of neuronal injury where oxidative damage plays a paramount role, including stroke. The question of whether DJ-1 plays this role is of particular interest because there is increasing evidence of the cross-talk between various neurodegenerative diseases. For example, small “silent” strokes are thought to predispose patients to cognitive impairments later in life (20, 21). A significant population of PD patients also suffer from cognitive defects (22). Clearly, mechanisms/genes defined for one neurodegenerative condition might also be central in multiple models of neuropathology. To test these hypotheses, we examined whether the PD gene DJ-1 may be important in stroke-induced damage. In this work, we demonstrate that DJ-1 deficiency sensitizes brains to ischemic damage in vivo, whereas DJ-1 expression does the converse. DJ-1-mediated protection is also accompanied by decreased markers of oxidative damage. Finally, the protective activity depends on cysteine-106 in DJ-1, a residue shown to be critical in the ability of DJ-1 to handle oxidative stress. Our results indicate that a classic PD gene is also central to the ability of the brain to respond to stroke, likely mediated through its capacity to manage ROS.
Results
DJ-1-Null Cerebellar Granule Neurons (CGNs) Are More Sensitive to Excitotoxicity in Model of Death in Vitro.
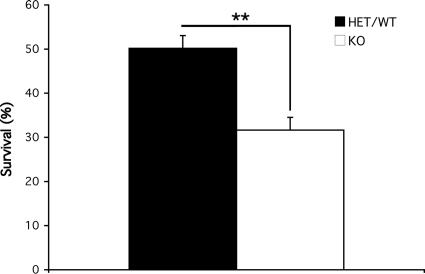
To evaluate whether DJ-1 plays any role in stroke-related injury, we first utilized an in vitro model of glutamate-induced excitotoxicity. This mechanism of death is critical in stroke. Neuronal death occurs through stimulation of glutamate receptors, subsequent influx of calcium ions, overproduction of ROS, and oxidative damage (23). As is shown in Fig. 1, DJ-1 null neurons are significantly more sensitive to glutamate-induced excitotoxicity than littermate controls [0.2 ± 3.8% survival in wild-type (WT) or heterozygous (HET) vs. 31.6 ± 2.9% survival in DJ-1-deficient animals]. This evidence demonstrates the neuroprotective role of DJ-1 in excitotoxicity, at least in vitro, and is consistent with the proposed role of DJ-1 against oxidative stress. Also consistent with this finding, we observed that DJ-1-deficient neurons were also sensitive to hypoxic injury (data not shown).
Fig. 1.
Increased sensitivity to glutamate-induced excitotoxicity in the absence of DJ-1. CGNs were harvested from DJ-1+/− or +/+ (n = 7) and DJ-1−/− (n = 4) 7- to 9-day-old mice pups. Seven days after plating, they were treated with 50 μM l-glutamate for 45 min, washed, and incubated in 37°C for 2 h. Then cells were lysed, and nuclei were fixed and counted on a hemocytometer slide to assess their viability. Each data point is the mean ± SEM of three independent cultures, **, P < 0.01. KO, knockout.
Oxidation-Sensitive Cysteine-106 Residue in DJ-1 Is Essential for Its Neuroprotective Activities in Vitro.
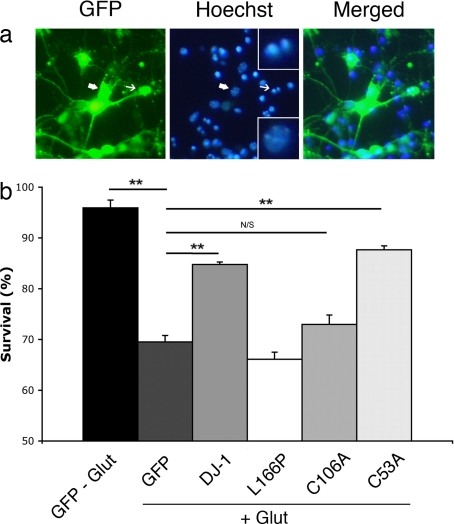
We next determined the converse effects of DJ-1 expression on excitotoxic death. As shown in Fig. 2, we expressed GFP alone, GFP with WT DJ-1, or the mutant of DJ-1 (L166P) associated with familial PD. These constructs were targeted to CGNs by recombinant adenoviral vectors (rAV) before glutamate-induced excitotoxicity. Survival was assessed, and it was noted that expression of WT DJ-1 protects from excitotoxic death (84.8 ± 0.5% vs. 69.5 ± 1.3%), whereas the PD mutant form of DJ-1 (66.1 ± 1.4%) proved to display no protective function. Taken together, the evidence indicates that although loss of DJ-1 sensitizes to excitotoxic injury, gain of DJ-1 function is protective.
Fig. 2.
Cysteine-106 is essential for neuroprotective function of DJ-1 after glutamate-induced excitotoxicity in vitro. CGNs, harvested from CD-1 WT pups, were targeted by rAV vectors carrying the indicated constructs along with GFP, 5 days after plating, and treated with l-glutamate at day 7 in vitro (as described in Materials and Methods). (a) Viability of virally infected neurons was assessed based on nuclear morphology under UV microscopy (representative cells are shown). Condensed or fragmented nuclei (indicated by thin arrows) were scored as dead versus intact nuclei considered as alive (indicated by thick arrows). (Upper Inset) High magnification of fragmented nuclei. (Lower Inset) High magnification of intact nuclei. (b) Quantification of neuronal survival after treatment with l-glutamate compared with nontreated GFP-expressing neurons. Each data point is the mean ± SEM of three independent cultures (one-way ANOVA followed by Tukey's least significant difference test; **, P < 0.01; N/S, not significant).
As was reported previously, DJ-1 responds to oxidative stressors through isoelectric pH (pI) shift to a more acidic form of the molecule (24). Moreover, different independent groups of investigators have demonstrated that the cysteine residue in position 106 is particularly sensitive to oxidative modification and stress (25, 26). To evaluate the importance of this and other cysteine residues in the ability of DJ-1 to protect against excitotoxicity in vitro, we also evaluated the ability of C106A or C53A mutant forms of DJ-1 to protect. The latter cysteine mutant is not thought to be modified by as much as cysteine-106 upon oxidative stress (18). As shown in Fig. 2, the mutant C53A form of DJ-1 was still protective compared with control (87.7 ± 0.8% vs. 84.8 ± 0.5%), whereas the C106A mutant failed to enhance survival (73.0 ± 1.9%), indicating the importance of cysteine-106 in mediating protection against excitotoxic death.
DJ-1-Deficient Mice Are Sensitive to Brain Ischemia.
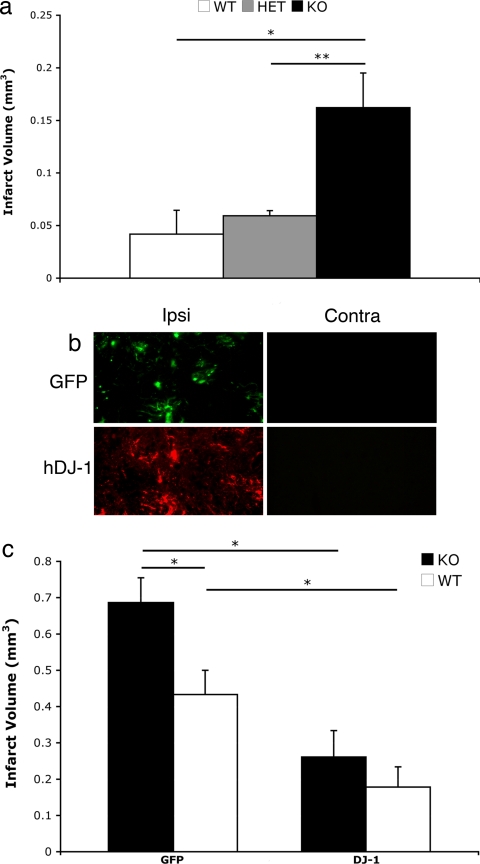
Our findings in the in vitro models of excitotoxicity provided strong rationale to examine whether DJ-1 is necessary to protect brain tissue against ischemia-induced damage in vivo. To examine this hypothesis, we used striatal focal ischemia model by injecting endothlin-1 in the striatum of DJ-1-deficient, heterozygote, and WT control mice. Endothelin-1 is a vasopressive protein that induces local vasoconstriction around the site of injection, resulting in ischemia-induced tissue damage in affected areas (27). We injected endothelin-1 in two points, 0.4 mm apart in the striatum at coordinates as described in Materials and Methods. Animals were killed 7 days after surgery, perfused, and brains were processed as described. The area of damage was determined as presented in Fig. 3b. Endothelin-1 injection in DJ-1-deficient animals produced a significantly larger infarct size (≈4 times larger) than either heterozygote or WT control groups. This finding, in line with in vitro results, strongly supports the idea of involvement of DJ-1 in neuroprotection in ischemia-induced stroke.
Fig. 3.
DJ-1 protects brain tissue from endothelin-induced focal ischemia in vivo. Infarct volume in cortex and striatum of mouse brains after acute ischemic stroke is shown. (a) Nonvirally injected mice of DJ-1 colony were assessed for infarct volume after endothelin-induced focal ischemia and were analyzed according to their genotype (WT, n = 3; HET, n = 3; KO, n = 4). There is a significant difference between the animals of the DJ-1-null background vs. either the WT or HET group but no significant difference between the latter two. (b) Expression of either GFP or human DJ-1 was assessed in the ipsi- and contralateral sides of the brains of DJ-1 KO mice (compared with the injected side). (c) Mice of DJ-1-null or WT genotype from the DJ-1 colony were injected with adenoviral vectors expressing either GFP (KO, n = 5; WT, n = 7) or DJ-1 (KO, n = 6; WT, n = 8). Animals in each group received the same previously mentioned stroke model procedure. Seven days later, brain infarct volume was measured as described. There were significant differences between DJ-1-null + GFP-injected and DJ-1-null + DJ-1-injected as well as an additional protection seen in the WT overexpressing DJ-1 as opposed to overexpressing GFP. No significant changes were observed between the WT overexpressing GFP and the KO overexpressing DJ-1. Each data point represents mean ± SEM for a representative population of three to eight mice (one-way ANOVA followed by Tukey's least significant difference test; *, P < 0.05; **, P < 0.01).
Induction of DJ-1 Can Reverse Ischemia-Induced Damage Sensitivity in DJ-1-Deficient Mice in Vivo.
Germ-line DJ-1 deficiency might have long-term secondary consequences not directly related to a DJ-1-mediated mechanism of action. To support the importance of DJ-1, we next examined whether induced expression of DJ-1 reverses the sensitization phenotype of DJ-1-null mice in response to endothelin-1-induced ischemic brain damage. We expressed WT DJ-1 in the striatum of DJ-1-null and WT controls by adenoviral vectors a week before initiation of ischemic insult, as described in Materials and Methods. Results shown in Fig. 3c indicate that DJ-1 expression reverses the sensitivity to ischemic damage induced by endothelin-1 in DJ-1-null mice and protect them to levels that are not significantly different from WT DJ-1 mice. Furthermore, it was observed that DJ-1 overexpression in the control group provided additional protection against ischemic injury. It must be noted that the degree of sensitization induced by DJ-1 deficiency in control GFP-expressing mice is less than that observed in naïve nonvirally injected animals (Fig. 3). This effect may be caused by the response of animals to viral infection. Nonetheless, taken together, these findings strongly support the role of DJ-1 in protecting neuronal cells against the ischemic damage model of stroke.
Cysteine-106 Is Essential for the Protective Role of DJ-1 in Ischemic Brain Injury.
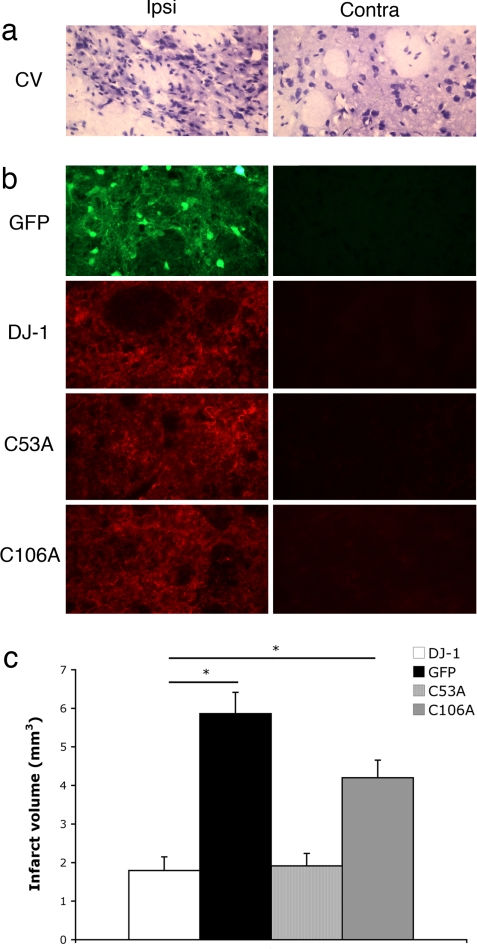
Our in vitro experiments indicated the requirement of the oxidation-sensitive cysteine-106 in the neuroprotective activity of DJ-1 in excitotoxicity. To evaluate the significance of this residue in the protective function of DJ-1 in our adult stroke models of injury in vivo, we evaluated the effects of DJ-1, WT and mutants, in our model of endothelin-induced damage. In this case, we used the rat model, which, based on our previous experience, produces larger infarct volumes in response to endothelin-1. This model facilitates the precise evaluation of the protective effects of DJ-1. In addition, we also used the adeno-associated viral (AAV) vectors to express wild-type DJ-1, C106A and C53A mutants, or GFP control in rat brains. AAV is known to be an effective, neurotrophic, and reliable method of gene delivery that produces a stronger and broader gene expression than with adenovirus (28). We injected AAV vectors 2 weeks before endothelin-1 injection to allow sufficient expression of the proteins of interest (Fig. 4b). One week after the endothelin-1 injection, we transcardially perfused the rats and prepared the brains for cryostat sectioning. Brain serial sections were examined to evaluate the volume of infarct (Fig. 4a). As demonstrated in Fig. 4c, the volume of infarct is significantly reduced in WT DJ-1- and C53A mutant-expressing brains compared with GFP-expressing brains (1.79 ± 0.36 and 1.91 ± 0.33 vs. 5.86 ± 0.55, respectively). Importantly, however, the protective effect of C106A was as greatly compromised compared with WT DJ-1 (4.20 ± 0.45 vs. 1.79 ± 0.36, respectively). These results clearly demonstrate that cysteine-106 is essential for protective function of DJ-1 in this model of ischemic stroke.
Fig. 4.
Cysteine-106 is essential for neuroprotective function of DJ-1 after focal ischemia in vivo. Infarct volume in the striatum after injection of endothelin-1 in WT rats overexpressing AAVs was assessed. (a) Representative sections of damaged (ipsilateral to the endothelin-1 injection) and healthy (contralateral) cells were stained with cresyl violet, and damaged areas were characterized through shrunken/condensed nuclear staining (dead cell) vs. healthy neurons with smooth, round nuclei and visible nucleoli (alive). (b) Sections from the injected hemisphere overlapping the infarcted area were stained for respective virally expressing protein. (c) Infarct volume was measured by cresyl violet staining between different virally injected groups (n = 4 for GFP and DJ-1; n = 3 for C53A and C106A). Each data point represents mean ± SEM for a representative population of rats. There were significant differences between DJ-1 and GFP overexpressing rats as well as C53A and GFP but not C106A and GFP overexpressing rats (one-way ANOVA followed by Tukey's least significant difference test; *, significant). No significant changes were observed between DJ-1 and C53A overexpressing rats.
DJ-1-Mediated Protection Is Associated with Reduced Markers of Oxidative Stress.
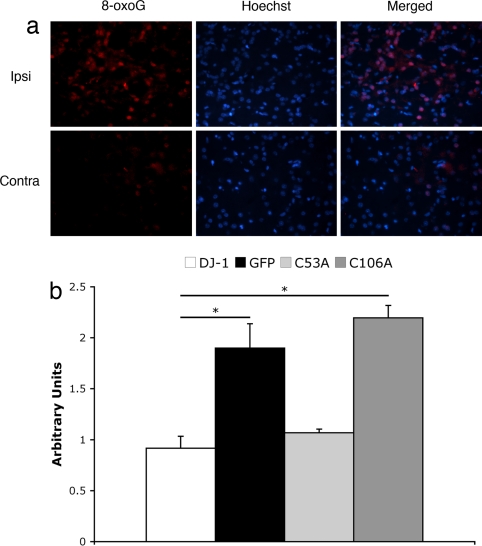
Ischemia/reperfusion is believed to provoke neuronal damage by increasing production of ROS. To evaluate the level of ROS after ischemic insult, we used 8-oxoguanine (8-oxoG), an oxidized by-product of purine base of DNA, as an indicator of oxidative damage (29). We performed a preliminary time course and observed that 8-oxoG reactivity in tissue reaches its maximum at 48 h after ischemia (data not shown). If DJ-1 acts to promote survival through its antioxidant properties, we would expect that ROS level and consequent oxidative damage after stroke would decrease in the presence of WT or neuroprotective mutants of DJ-1. To test this possibility, we assessed the level of 8-oxoG reactivity by immunohistofluorescence staining and comparing signal intensity between injected vs. noninjected hemispheres in the presence of overexpressed GFP, WT, or mutant forms of DJ-1, 48 h after ischemia. Densitometry analysis presented in Fig. 5 clearly indicates that WT DJ-1 as well as C53A mutant effectively reduce poststroke ROS (0.92 ± 0.12 and 1.07 ± 0.04, respectively) compared with the measurements in C106A and GFP, which are comparable (2.20 ± 0.12 vs. 1.90 ± 0.24, respectively).
Fig. 5.
Cysteine-106 is crucial for the regulation of ROS end product, 8-oxoGafter focal ischemia in vivo. Rats subjected to AAV injection overexpressing DJ-1, GFP, C53A, and C106A, as indicated, were subjected to ischemic insult by endothelin-1 and were killed after 48 h. Brains were collected and processed to be stained for 8-oxoG as well as Hoechst 33258. Representative pictures were taken of sections surrounding injection area, and colocalizing 8-oxoG/Hoescht neurons (n >50) were evaluated by densitometry compared with the contralateral side. (a) Representative sections show expression of ROS marker 8-oxoG on the ipsi- and contralateral sides of the endothelin injection. (b) Quantification of densitometry results. Arbitrary units represent the ratio of signal density for the injected vs. noninjected (internal control) sides. Each data point represents mean ± SEM for a representative population of three or four rats per group. There is a significant difference between DJ-1 and GFP overexpressing groups as well as DJ-1 and C106A overexpressing rats (one-way ANOVA; followed by Tukey's least significant difference test; *, significant). No significant changes were observed between DJ-1 and C53A groups.
Discussion
There is increasing evidence that common mechanistic elements may play central roles in a variety of neurodegenerative and neuropathological conditions. Chief among these elements is ROS. Free radical damage has been implicated in arguably every neuropathological condition described. The importance of ROS is underscored by the elegant system of enzymes developed by the cell to manage and balance ROS under normal conditions and times of stress. These antioxidant enzymes include catalases, glutathione peroxidases, thioredoxin reductases, and superoxide dismutases (30).
A more recently identified protein linked to ROS is DJ-1. DJ-1 was first identified by many groups in the context of fertility and oncogenesis (11, 31). However, more recent excitement was generated with its identification as a familial PD gene (12). The reason why loss of DJ-1 leads to PD is still unknown. However, growing evidence suggested a link to oxidative stress (17, 24, 32). For example, we had previously shown that DJ-1 deficiency protects against direct peroxide-induced death but not death initiated by DNA damage or staurosporine (16). The possible mechanisms for its link to oxidative stress will be discussed in more detail below. However, if the link between DJ-1 and oxidative stress was valid, one logical prediction would be that DJ-1 should play important roles in other non-PD-related neuropathological conditions also associated with ROS. In this regard, stroke-induced damage is an obvious test candidate because ROS is strongly induced after ischemic damage, and amelioration of ROS has been shown to prevent ischemic injury (33). Accordingly, in the present work, we demonstrate the critical impact of DJ-1 in in vitro and in vivo models of ischemic stroke. Our results indicate that (i) DJ-1 provides protection against excitotoxicity and ischemic brain injury and (ii) antioxidant activity of DJ-1 is essential to its neuroprotective role against ischemia-induced neuronal damage.
We initially examined the role of DJ-1 in glutamate-induced excitotoxicity as a critical pathogenic event during stroke (34). Our data show that DJ-1-null CGNs are significantly more sensitive to glutamate treatment than the controls harvested from littermate pups. Consistent with this observation, overexpression of human DJ-1 in mouse CGNs considerably protects them against glutamate-induced death. This sensitivity also extends to adult models of stroke injury in vivo where we observed that DJ-1-deficient mice displayed larger infarct volumes than control littermates. More importantly, this sensitivity was reversible through induction of human DJ-1. The importance of DJ-1 in modifying survival is also underscored by the observation that expression of DJ-1 alone is protective against ischemic stress, both in vitro and in vivo. Importantly, expression of human DJ-1 provides additional protection, even in DJ-1 WT mice and rats. Our results are significant because they show how a gene classically associated with one neurological disease, in this case PD, also plays pivotal roles in other conditions such as stroke. Our results are consistent with increasing evidence of commonalities among neuropathologies. These commonalities extend not only to signaling elements including those classically associated with death such as JNKs, p53, but broader mechanistic elements such as mitochondrial damage, endoplasmic reticulum stress, and of course free radical damage to name just a few (35–38). In further support of this view, there appears to be overlap between etiologies and even pathologies of a number of diseases. For example, strokes predispose patients to dementia (21). PD patients have symptoms not classically associated with movement such as dementia and mood disorders. Accordingly, in the context of the present work, it would be interesting to determine whether PD patients with DJ-1 deficiency are also more sensitive to other neuropathologies such as stroke.
There are several lines of evidence to support the role of DJ-1 in handling ROS in stroke. First, expression of DJ-1 diminishes markers of oxidative stress induced by stroke insult. In this regard, our results show that the 8-oxoG epitope, which represents that the oxidized form of guanosine, is increased after stroke and reduced after DJ-1 expression (Fig. 5). Second, we also show that mutants of DJ-1 with known defects in responsiveness to oxidative stress do not protect against stroke. In this regard, the C106A mutant of DJ-1 failed to protect against ischemic damage, both in vitro and in vivo. The cyteine-106 residue was initially reported to be the most sensitive amino acid residue to hydrogen peroxide oxidation (18). Subsequent studies have shown that this residue to also critical for the protective properties of DJ-1 in response to oxidative stress (25). Our results are consistent with this observation and give further support to the importance of DJ-1 and its ability to handle oxidative stress in stroke.
The exact mechanism by which DJ-1 and the cysteine-106 residue protect neurons from ROS stress is unclear. Direct quenching and in vitro catalase activity of DJ-1 has been reported previously (39). Whether this activity is central to diseases such as stroke is unknown. DJ-1 is also suggested to enhance prosurvival signals such as AKT (39, 40). However, its relevance in neurodegeneration again is unclear. Recent data also indicate that DJ-1 can stabilize the transcription factor, Nrf2 (19). This finding is particularly interesting because Nrf2 is known to coordinate the expression of a number of antioxidant genes. Interestingly, we have shown that DJ-1 can modify activation of an important antioxidant enzyme Prdx2 (41). Whether and how these mechanisms impact on DJ-1, PD, or stroke will be of intense interest. Taken together, our findings in this work provide direct evidence to support the neuroprotective role of DJ-1 in mammalian animal model of brain ischemic injury. Given the central nature of ROS in stroke, DJ-1 may be an important new target in the prevention of stroke damage.
Materials and Methods
Cell Culture.
Primary cultures of CGNs were obtained from postnatal day 7–9 CD1 or DJ-1 null colony mouse pups, as described previously (42).
Viral Constructs.
Recombinant AAV (rAAV1) vectors were constructed by subcloning cDNA sequences (NheI–PmeI fragment) of WT human DJ-1 or mutated forms (C53A and C106A) into the SpeI–EcoRV sites of the AM/CBA-pl-WPRE-bGH plasmid. The virus was then generated and purified as described (43). For adenovirus (AV) construction, the same sequences were subcloned into the pAdTrack vector under a cytomegalovirus (CMV) promoter. The construct also contains a second CMV promoter that separately controls expression of GFP. The construct was then used to generate recombinant AV, as described (44).
Adenoviral Infection and Glutamate Excitotoxicity.
Five days after plating, CGNs were infected with adenovirus-expressing GFP along with DJ-1 WT or DJ-1 mutants (L166P, C53A, C106A) or by GFP itself as control (multiplicity of infection, 20). On day 7, l-glutamate was added to the wells to a final concentration of 50 μM for 45 min and then washed three times with conditioned medium and incubated for 2 h in 37°C. This step was performed in the presence or absence of 10 μM MK801. All cultures were fixed in 4% paraformaldehyde [containing 0.2% picric acid in 0.1 M phosphate buffer (pH 6.9)], and their nuclei were stained with Hoechst 33258 (final concentration of 0.5 μg/ml). The total number of GFP-positive neurons that had healthy nuclei (round intact nuclei vs. shrunken condensed or fragmented nuclei) per well was evaluated and compared with the number of GFP-positive live neurons in control wells.
DJ-1-Null Mice, Colony Maintenance, and Genotyping.
DJ-1-deficient mice were generated by deletion of exons 3–5 and replacement with a NeoTD cassette, maintained, and genotyped by PCR as described previously by Kim et al. (16). Animals were maintained at 25°C on a 12-h/12-h light/dark cycle with access to standard rodent laboratory chow and water. All animal procedures were in the accordance with the guidelines of the Canadian Council and Care of Animals in Research and the Canadian Institutes of Health Research and were approved by the University of Ottawa Animal Care Veterinary Services.
Intrastriatal Viral Injection and Focal Ischemia Induction.
Surgical procedures were performed as described by Rashidian et al. (45). Briefly, mice of the DJ-1 colony (C57BL/6 background 12 generations, 8–12 weeks old) or male Wistar rats weighing 75–100 g (Charles River) were anesthetized with a mixture of 2–2.5% isoflurane and 1 liter/min oxygen, and after proper sterilization and wellness procedures, they were placed in a stereotaxic frame. Stereotaxic intrastriatal AV (1 μl, 1 × 107 particles per injection, infusion rate, 0.25 μl/min) or rAAV injection [3 μl, premixed with 30% (vol/vol) mannitol, 1.2 × 109 particles per injection, infusion rate, 0.15 μl/min) for mice (left side, from bregma: +0.5 mm anterioposterior, +1.9 mm lateral, −2.9 mm deep) and rats (left side, from bregma: +0.9 mm anterioposterior, +2.8 mm lateral, −5.8 mm deep) were performed 7 and 14 days before ischemic insult, respectively. To induce ischemic insult in rats, we injected 1 μl of vasoconstrictor, endothelin-1 (1 mg/ml dissolved in ddH2O; Calbiochem) into the same virally injected striatal region (infusion rate of 0.125 μl/min). For mice, the endothelin-1 injection was repeated twice, in two points of the left striatum distanced 0.4 mm apart (from bregma: +0.5 mm anterioposterior, +2.1 and +1.7 mm lateral, −2.9 mm deep) for a total volume of 2 μl.
Histological Staining and Infarct Volume Assessment.
Animals were anesthetized and transcardially perfused 7 days after endothelin-1 injection with 0.9% saline followed by 4% paraformaldehyde (PFA). Brains were then removed, fixed overnight in 4% PFA, and dehydrated over a course of 5 days in 10% (wt/vol) sucrose in 0.1 M phosphate buffer. Coronal sections of 14 μm were obtained by using a Microm HM500 cryomicrotome. Serial sections were taken starting at bregma +1.54 mm and ended at bregma −0.94 mm to include the whole striatal area. One of five consecutive sections of the striatum was collected, refixed in 4% PFA, and stained with 0.2% cresyl violet. The damaged tissue surface area, determined on nuclear morphology (areas with condensed/shrunken nuclei were considered infarcted), was outlined by using Northern Eclipse imaging software (Empix Imaging), and infarct volume was assessed by multiplying the area of damage by the thickness of section as well as the gap between selected slices. All microscopic and imaging studies were performed by using a Zeiss microscope (Axiophot).
Immunohistochemistry.
Forty-eight hours after endothelin-1 administration, animals in the indicated groups were perfused, and brains were fixed as described before. Free-floating coronal cryosections of 14 μm were obtained (bregma +2.70 mm and ended at bregma −2.12 mm). Sections were then blocked in 2% normal goat serum with 1% BSA and incubated with 8-oxoG (1:300; Chemicon) monoclonal anti-mouse antibody overnight at 4°C. After three washes with PBS, tissue was stained with secondary CY3-conjugated secondary antibody (1:300; Jackson ImmunoResearch) for 1 h at room temperature. Slides were washed, and coverslips were mounted with GelMount Aqueous Mounting Medium (Sigma).
Statistical Analysis.
All statistical variance was carried out by using Student's t test (two-tailed) or one-way ANOVA followed by Tukey's post hoc least significant difference test. Significance: *, P < 0.05; **, P < 0.01 unless otherwise stated.
Acknowledgments
This work was supported by Heart and Stroke Foundation Ontario, Parkinson's Society of Canada, Canadian Institutes of Health Research, Parkinson's Disease Foundation, U.S. Army, and the Parkinson's Research Consortium (to D.S.P.). H.A. is a recipient of a Focus on Stroke Scholarship. D.S.P. is a Heart and Stroke Career Investigator.
Footnotes
The authors declare no conflict of interest.
References
- 1.Centers for Disease Control Prevention (CDC) MMWR Morb Mortal Wkly Rep. 2007;56:469–474. [Google Scholar]
- 2.Hunter DJW, Grant HJ, Purdue MPH, Spasoff RA, Dorland JL, Bains N. Chronic Dis Can. 2004;25:138–146. [PubMed] [Google Scholar]
- 3.Alexandrova M, Bochev P, Markova V, Bechev B, Popova M, Danovska M, Simeonova V. J Clin Neurosci. 2004;11:501–506. doi: 10.1016/j.jocn.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 4.Schaller B, Graf R. J Cereb Blood Flow Metab. 2004;24:351–371. doi: 10.1097/00004647-200404000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Adam-Vizi V. Antioxid Redox Signal. 2005;7:1140–1149. doi: 10.1089/ars.2005.7.1140. [DOI] [PubMed] [Google Scholar]
- 6.Starkov AA, Chinopoulos C, Fiskum G. Cell Calcium. 2004;36:257–264. doi: 10.1016/j.ceca.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 7.Cho S, Park EM, Febbraio M, Anrather J, Park L, Racchumi G, Silverstein RL, Iadecola C. J Neurosci. 2005;25:2504–2512. doi: 10.1523/JNEUROSCI.0035-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sugawara T, Chan PH. Antioxid Redox Signal. 2003;5:597–607. doi: 10.1089/152308603770310266. [DOI] [PubMed] [Google Scholar]
- 9.Islekel S, Islekel H, Guner G, Ozdamar N. Res Exp Med (Berl) 1999;199:167–176. doi: 10.1007/s004330050121. [DOI] [PubMed] [Google Scholar]
- 10.Piantadosi CA, Zhang J. Stroke. 1996;27:327–332. doi: 10.1161/01.str.27.2.327. [DOI] [PubMed] [Google Scholar]
- 11.Nagakubo D, Taira T, Kitaura H, Ikeda M, Tamai K, Iguchi-Ariga SM, Ariga H. Biochem Biophys Res Commun. 1997;231:509–513. doi: 10.1006/bbrc.1997.6132. [DOI] [PubMed] [Google Scholar]
- 12.Bonifati V, Rizzu P, van Baren MJ, Schaap O, Breedveld GJ, Krieger E, Dekker MC, Squitieri F, Ibanez P, Joosse M, et al. Science. 2003;299:256–259. doi: 10.1126/science.1077209. [DOI] [PubMed] [Google Scholar]
- 13.Martinat C, Shendelman S, Jonason A, Leete T, Beal MF, Yang L, Floss T, Abeliovich A. PLoS Biol. 2004;2:e327. doi: 10.1371/journal.pbio.0020327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taira T, Saito Y, Niki T, Iguchi-Ariga SM, Takahashi K, Ariga H. EMBO Rep. 2004;5:213–218. doi: 10.1038/sj.embor.7400074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park J, Kim SY, Cha GH, Lee SB, Kim S, Chung J. Gene. 2005;361:133–139. doi: 10.1016/j.gene.2005.06.040. [DOI] [PubMed] [Google Scholar]
- 16.Kim RH, Smith PD, Aleyasin H, Hayley S, Mount MP, Pownall S, Wakeham A, You-Ten AJ, Kalia SK, Horne P, et al. Proc Natl Acad Sci USA. 2005;102:5215–5220. doi: 10.1073/pnas.0501282102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mitsumoto A, Nakagawa Y, Takeuchi A, Okawa K, Iwamatsu A, Takanezawa Y. Free Radic Res. 2001;35:301–310. doi: 10.1080/10715760100300831. [DOI] [PubMed] [Google Scholar]
- 18.Kinumi T, Kimata J, Taira T, Ariga H, Niki E. Biochem Biophys Res Commun. 2004;317:722–728. doi: 10.1016/j.bbrc.2004.03.110. [DOI] [PubMed] [Google Scholar]
- 19.Clements CM, McNally RS, Conti BJ, Mak TW, Ting JP. Proc Natl Acad Sci USA. 2006;103:15091–15096. doi: 10.1073/pnas.0607260103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hachinski V. Stroke. 2007;38:1396. doi: 10.1161/01.STR.0000260101.08944.e9. [DOI] [PubMed] [Google Scholar]
- 21.Merino JG, Hachinski V. Curr Atheroscler Rep. 2002;4:285–290. doi: 10.1007/s11883-002-0007-5. [DOI] [PubMed] [Google Scholar]
- 22.Williams-Gray CH, Foltynie T, Brayne CE, Robbins TW, Barker RA. Brain. 2007;130:1787–1798. doi: 10.1093/brain/awm111. [DOI] [PubMed] [Google Scholar]
- 23.Juurlink BH, Sweeney MI. Neurosci Biobehav Rev. 1997;21:121–128. doi: 10.1016/s0149-7634(96)00001-2. [DOI] [PubMed] [Google Scholar]
- 24.Mitsumoto A, Nakagawa Y. Free Radic Res. 2001;35:885–893. doi: 10.1080/10715760100301381. [DOI] [PubMed] [Google Scholar]
- 25.Meulener MC, Xu K, Thomson L, Ischiropoulos H, Bonini NM. Proc Natl Acad Sci USA. 2006;103:12517–12522. doi: 10.1073/pnas.0601891103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Canet-Aviles RM, Wilson MA, Miller DW, Ahmad R, McLendon C, Bandyopadhyay S, Baptista MJ, Ringe D, Petsko GA, Cookson MR. Proc Natl Acad Sci USA. 2004;101:9103–9108. doi: 10.1073/pnas.0402959101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Windle V, Szymanska A, Granter-Button S, White C, Buist R, Peeling J, Corbett D. Exp Neurol. 2006;201:324–334. doi: 10.1016/j.expneurol.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 28.Tenenbaum L, Chtarto A, Lehtonen E, Velu T, Brotchi J, Levivier M. J Gene Med. 2004;6(Suppl 1):S212–S222. doi: 10.1002/jgm.506. [DOI] [PubMed] [Google Scholar]
- 29.Lodovici M, Casalini C, Cariaggi R, Michelucci L, Dolara P. Free Radic Biol Med. 2000;28:13–17. doi: 10.1016/s0891-5849(99)00194-x. [DOI] [PubMed] [Google Scholar]
- 30.Hou ST, MacManus JP. Int Rev Cytol. 2002;221:93–148. doi: 10.1016/s0074-7696(02)21011-6. [DOI] [PubMed] [Google Scholar]
- 31.Wagenfeld A, Gromoll J, Cooper TG. Biochem Biophys Res Commun. 1998;251:545–549. doi: 10.1006/bbrc.1998.9512. [DOI] [PubMed] [Google Scholar]
- 32.Lavara-Culebras E, Paricio N. Gene. 2007;400:158–165. doi: 10.1016/j.gene.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 33.Margaill I, Plotkine M, Lerouet D. Free Radic Biol Med. 2005;39:429–443. doi: 10.1016/j.freeradbiomed.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 34.Arundine M, Tymianski M. Cell Mol Life Sci. 2004;61:657–668. doi: 10.1007/s00018-003-3319-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Culmsee C, Mattson MP. Biochem Biophys Res Commun. 2005;331:761–777. doi: 10.1016/j.bbrc.2005.03.149. [DOI] [PubMed] [Google Scholar]
- 36.Borsello T, Forloni G. Curr Pharm Des. 2007;13:1875–1886. doi: 10.2174/138161207780858384. [DOI] [PubMed] [Google Scholar]
- 37.Lin MT, Beal MF. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 38.Paschen W, Mengesdorf T. Cell Calcium. 2005;38:409–415. doi: 10.1016/j.ceca.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 39.Yang Y, Gehrke S, Haque ME, Imai Y, Kosek J, Yang L, Beal MF, Nishimura I, Wakamatsu K, Ito S, et al. Proc Natl Acad Sci USA. 2005;102:13670–13675. doi: 10.1073/pnas.0504610102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gorner K, Holtorf E, Waak J, Pham TT, Vogt-Weisenhorn DM, Wurst W, Haass C, Kahle PJ. J Biol Chem. 2007;282:13680–13691. doi: 10.1074/jbc.M609821200. [DOI] [PubMed] [Google Scholar]
- 41.Qu D, Rashidian J, Mount MP, Aleyasin H, Parsanejad M, Lira A, Haque E, Zhang Y, Callaghan S, Daigle M, Rousseaux MW, Slack RS, Albert PR, Vincent I, Woulfe JM, Park DS. Neuron. 2007;55:37–52. doi: 10.1016/j.neuron.2007.05.033. [DOI] [PubMed] [Google Scholar]
- 42.O'Hare MJ, Hou ST, Morris EJ, Cregan SP, Xu Q, Slack RS, Park DS. J Biol Chem. 2000;275:25358–25364. doi: 10.1074/jbc.M001725200. [DOI] [PubMed] [Google Scholar]
- 43.Zolotukhin S, Potter M, Zolotukhin I, Sakai Y, Loiler S, Fraites TJ, Jr, Chiodo VA, Phillipsberg T, Muzyczka N, Hauswirth WW, Flotte TR, et al. Methods. 2002;28:158–167. doi: 10.1016/s1046-2023(02)00220-7. [DOI] [PubMed] [Google Scholar]
- 44.He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B. Proc Natl Acad Sci USA. 1998;95:2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rashidian J, Iyirhiaro G, Aleyasin H, Rios M, Vincent I, Callaghan S, Bland RJ, Slack RS, During MJ, Park DS. Proc Natl Acad Sci USA. 2005;102:14080–14085. doi: 10.1073/pnas.0500099102. [DOI] [PMC free article] [PubMed] [Google Scholar]