The Fibronectin Domain ED-A Is Crucial for Myofibroblastic Phenotype Induction by Transforming Growth Factor-β1 (original) (raw)
Abstract
Transforming growth factor-β1 (TGFβ1), a major promoter of myofibroblast differentiation, induces α-smooth muscle (sn) actin, modulates the expression of adhesive receptors, and enhances the synthesis of extracellular matrix (ECM) molecules including ED-A fibronectin (FN), an isoform de novo expressed during wound healing and fibrotic changes. We report here that ED-A FN deposition precedes α-SM actin expression by fibroblasts during granulation tissue evolution in vivo and after TGFβ1 stimulation in vitro. Moreover, there is a correlation between in vitro expression of α-SM actin and ED-A FN in different fibroblastic populations. Seeding fibroblasts on ED-A FN does not elicit per se α-SM actin expression; however, incubation of fibroblasts with the anti-ED-A monoclonal antibody IST-9 specifically blocks the TGFβ1-triggered enhancement of α-SM actin and collagen type I, but not that of plasminogen activator inhibitor-1 mRNA. Interestingly, the same inhibiting action is exerted by the soluble recombinant domain ED-A, but neither of these inhibitory agents alter FN matrix assembly. Our findings indicate that ED-A–containing polymerized FN is necessary for the induction of the myofibroblastic phenotype by TGFβ1 and identify a hitherto unknown mechanism of cytokine-determined gene stimulation based on the generation of an ECM-derived permissive outside in signaling, under the control of the cytokine itself.
Keywords: fibroblast, α-SM actin, extracellular matrix, wound healing, fibrosis
Acquisition of smooth muscle (SM)1 cell features by fibroblastic cells is observed during morphogenetic processes, wound healing, organ fibrosis, and stroma reaction to epithelial cancer (for review see Grinnell, 1994; Desmoulière and Gabbiani, 1996). α-SM actin– expressing myofibroblasts have long been recognized as suppliers of the driving force for granulation tissue contraction (Gabbiani et al., 1972), a mandatory function for an efficient and rapid wound closure. Moreover, these cells are involved in the pathogenesis of several fibrotic diseases, being responsible for tissue retraction and overproduction of extracellular matrix (ECM) components, such as collagen type I (Zhang et al., 1994). During development and under normal conditions, myofibroblasts are accountable for the generation of the structural and functional complexity of fundamental physiological units such as the glomerulus (Soriano, 1994) and the lung alveolar sack (Boström et al., 1996). In addition, myofibroblasts are typical components of the stroma reaction to epithelial tumors where they secrete proteolytic enzymes and growth factors which may activate cancer cell invasive behavior (for review see Rønnov-Jessen et al., 1996). The role of factors regulating the generation of the myofibroblastic phenotype remains largely unknown. Transforming growth factor-β1 (TGFβ1) is the prototype of a large family of cytokines that control cell proliferation, differentiation, motility, and apoptosis, exerting their functions both during embryogenesis, in terms of pattern formation and tissue specification, and in the adult organism, where they orchestrate complex phenomena such as inflammation, tissue repair, and neoplastic transformation (Roberts and Sporn, 1993; Kingsley, 1994; Massagué et al., 1997). It is well accepted that TGFβ1, which is known to stimulate α-SM actin synthesis by fibroblasts (Desmoulière et al., 1993; Rønnov-Jessen and Petersen, 1993), upregulates fibrillar collagen and fibronectin (FN) expression (Ignotz and Massagué, 1986; Roberts et al., 1986).
FN, a 440-kD dimeric glycoprotein widely distributed in plasma and in ECM, is expressed at high levels in healing wounds (Kurkinen et al., 1980). Each FN subunit is formed by a series of repeating homologous modules and contains binding sites for cell surface receptors and for other ECM components. FN polymorphism is due to alternative splicing of the type III segments ED-A, ED-B, and IIICS. Recently, a novel splicing variant lacking the IIICS region and the segments I-10 and III-15 has been characterized (MacLeod et al., 1996). Two of these alternative spliced segments, namely ED-A and ED-B, are included in the so-called cellular FN (Hynes, 1990; Kosmehl et al., 1996; MacLeod et al., 1996). Previous in situ hybridization studies have demonstrated that granulation tissue fibroblasts show a FN splicing pattern consisting of ED-A and ED-B domains, similar to that found in the embryo (ffrench-Constant et al., 1989). In vitro, TGFβ1 increases total FN levels by preferentially promoting accumulation of the ED-A FN isoform (Balza et al., 1988; Borsi et al., 1990; Kocher et al., 1990). For this reason we hypothesized that ED-A FN, interacting with a not yet characterized cell surface receptor, could transduce signals initiated by TGFβ1 and/or synergize with them, behaving as a crucial intermediary for the induction of myofibroblastic features, such as α-SM actin and collagen type I expression. Moreover, it has been previously suggested that ED-A FN modulates hepatic stellate cells to α-SM actin–expressing myofibroblast-like cells (Jarnagin et al., 1994).
It is well known that integrin-mediated adhesion to ECM regulates transmission of activated growth factor receptor tyrosine kinases and that convergence of integrin and growth factor-dependent pathways is required for the proper stimulation of gene expression, cell growth and differentiation (Clarke and Brugge, 1995; Schwartz, 1997; Schlaepfer and Hunter, 1998). The TGFβ serine/threonine kinase receptors signal from cell membrane to the nucleus mainly through the SMAD family of signal transducers (Heldin et al., 1997). However, it is not yet known whether and how ECM can influence TGFβ effects on target cells. Here we provide a molecular dissection of the ECM-generated pathway that needs to be activated for the induction of the myofibroblastic phenotype by TGFβ.
Materials and Methods
Antibodies
We used an affinity-purified fibronectin polyclonal antibody recognizing both cellular and plasma FN (Sigma Chemical Co., St. Louis, MO) and three mouse IgG1 mAbs selectively raised against different domains of FN isoforms (Borsi et al., 1987; Carnemolla et al., 1987, 1989, 1992): (a) IST-4, to the fifth FN type III domain shared by cellular and plasma FN; (b) IST-9, against the ED-A FN type III domain of cellular FN; and (c) BC-1, recognizing a cryptic epitope within the seventh FN type III domain, which is unmasked only when the ED-B domain is included in the cellular FN molecule. Anti–αSM-1, an IgG2a mAb, against α-SM actin (Skalli et al., 1986), and DIA-900, an IgG1 mAb, against the 6× His tag (Dianova, Hamburg, Germany), were also used. For control purposes, irrelevant antibodies of the same isotypes were used.
Purification of FNs and Production of Recombinant ED-A Domain
Plasma (ED-A −, ED-B −) and cellular (ED-A +, ED-B +) FNs were purified from human plasma and from the conditioned medium of the SV-40–transformed embryonic human lung WI-38-VA cell line as previously reported (Zardi et al., 1987). The presence or the absence of ED-A and ED-B in purified FNs was further verified by Western blotting with IST-9 and BC-1 mAbs (Borsi et al., 1987; Carnemolla et al., 1987, 1989).
The 270-bp cDNA sequence coding for the complete amino acid sequence of the ED-A domain (Kornblihtt et al., 1984) was generated by PCR amplification starting from the full-length cellular FN cDNA clone pFH111 (gift of F.E. Baralle, International Centre for Genetic Engineering and Biotechnology, Trieste, Italy) and using Pwo Pyrococcus woesei DNA polymerase (Boehringer Mannheim, Mannheim, Germany) and the following primers: (a) 5′ CTCGGATCCAACATTGATCGCCCTAAA 3′, which covers a FN sequence from base 5084 to base 5101 and includes the underlined BamHI restriction site, and (b) 5′ CTCGGATCCAATAGCTGTGGACTGGGT 3′, which covers a FN sequence from base 5342 to base 5359 and includes the underlined BamHI restriction site. PCR product was isolated, digested with BamHI restriction enzymes, and then cloned in the pQE-12 expression vector with a 3′ 6× His tag (QIAGEN Inc., Santa Clarita, CA). Escherichia coli were transformed with this construct and the 6× His COOH-terminal–tagged recombinant ED-A (rED-A) protein was purified using a Ni-NTA resin column (QIAGEN Inc.) according to the manufacturer. 6× His-tagged rED-A was then dialyzed against PBS and sterilized through a 0.22-μm filter. After filtration, protein concentration was established by analyzing absorbance at a 280-nm wavelength. Protein purity and size were verified by Coomassie blue staining after SDS-PAGE on a 10% polyacrylamide gel. Immunological protein reactivity was investigated by Western blotting with mAb IST-9.
In Vivo Experimental Procedures
Excisional wound granulation tissue was generated as previously described (Darby et al., 1990). In brief, on day 0, eight-week-old female Wistar rats were anaesthetized and a 2 × 2-cm skin wound was made on the middorsal surface. Granulation tissue samples were collected at 4, 7, and 12 d after wounding.
All procedures involving animals were reviewed and approved by the Animal Care Committee at the University of Geneva. These procedures conform the guidelines as established in the Guide for the Care and Use of Laboratory Animals and Public Health Service Policy on Human Care and Use of Laboratory Animals.
Double Indirect Immunofluorescence and Confocal Laser Scanning Microscopy Analysis
Tissue samples were embedded in OCT 4583 (Miles Scientific, Naperville, IL) and snap frozen in precooled liquid isopentane. 4-μm serial sections were fixed for 5 min in acetone at −20°C, air dried for 2 h at room temperature, sequentially incubated with anti–αSM-1, revealed by a TRITC-tagged goat anti–mouse IgG2a (Jackson ImmunoResearch Labs, Inc., West Grove, PA), and then with IST-9, followed by a dichlorotriazinyl amino fluorescein-labeled goat anti–mouse IgG1 (Jackson ImmunoResearch Labs, Inc.).
For the qualitative FN matrix assembly assay, after in vitro blocking experiments with the IST-9 mAb or the rED-A fragment (see below), cultured fibroblastic cells were rinsed in PBS, fixed in 4% paraformaldehyde for 15 min at room temperature, permeabilized with 0.1% Triton-X 100 for 5 min at room temperature, rinsed in PBS, and then stained in immunofluorescence. The primary monoclonal antibodies used were the affinity-purified rabbit polyclonal anti-FN antibody (Sigma Chemical Co.) alone or combined with DIA-900 (Dianova), and then revealed by a TRITC-tagged goat anti–rabbit and a dichlorotriazinyl amino fluorescein-labeled goat anti-mouse (both from Jackson ImmunoResearch Labs, Inc.), respectively.
Specimens were observed with a confocal laser scan fluorescence inverted microscope (model LSM 410; Carl Zeiss, Oberkochen, Germany) equipped with two lasers used simultaneously: (a) a helium laser (excitation wavelength at 543 nm) and (b) an argon-neon laser (excitation wavelength 488 nm). The appropriate combination of filters was used to separate excitation and emission spectra. The objective used was an immersion oil plan-neofluar 63×/1.4. Images of 512 × 512 pixels were stored on an erasable optical disk (Sony Corp., Tokyo, Japan) and then printed with a Kodak XLS8600 printer (Eastman Kodak Co., Rochester, NY) by means of dye thermic sublimation technique.
Cell Culture and Treatment
Passage 5 human fibroblasts obtained from explants of breast skin, palmar fascia, or Dupuytren's nodules were plated on Petri dishes containing Eagle's minimum essential medium (MEM; GIBCO AG, Basel, Switzerland) supplemented with Monomed (a defined serum-free medium containing insulin, transferrin, sodium selenite, 2-mercaptoethanol, 2-aminoethanol, sodium pyruvate, glutamine, and a BSA-oleic acid complex; Commonwealth Serum Laboratories, Melbourne, Australia), 100 U/ml penicillin, 100 mg/ml streptomycin, and 2 mM l-glutamine. Cell density was ∼1.5 × 104 cells/cm2. They were maintained at 37°C in a humid atmosphere of 5% CO2 and 95% air. Medium was removed 24 h after plating and fibroblasts were incubated for 1–6 d in MEM plus Monomed alone or containing 10 ng/ml of TGFβ1 (gift of A. Roberts, National Institutes of Health, Bethesda, MD, and purchased from Sigma Chemical Co.), or TGFβ2 (gift of A. Cox, Novartis, Basel, Switzerland). Passage 5 rat fibroblasts obtained from explants of subcutaneous tissue, lung, and dermis were plated on Petri dishes (1.5 × 104 cells/cm2) containing MEM (GIBCO AG) supplemented with Monomed (Commonwealth Serum Laboratories) and were cultured at 37°C in a humid atmosphere of 5% CO2 and 95% air for 4 d.
In blocking experiments with mAbs, 50, 150, or 300 μg of each anti-FN mAb were diluted in 1 ml of 2% gelatin (Sigma Chemical Co.) and then coated onto 6-cm Petri dishes; coatings of the same volume of 2% gelatin alone or containing equal amounts of irrelevant mAbs were used as controls. In blocking experiments with rED-A domain 50, 150, or 300 μg of this fragment were diluted in 1 ml of 2% gelatin (Sigma Chemical Co.) and then coated onto 6-cm Petri dishes. Cells were then plated on precoated Petri dishes containing MEM supplemented with Monomed (Commonwealth Serum Laboratories). Cell density and culture conditions were the same as above. Medium was removed 24 h after plating and fibroblasts were incubated for 24 h (extraction of total RNA) or for 3 d (extraction of proteins) in MEM plus Monomed alone or containing 10 ng/ml of TGFβ1 or TGFβ2.
The effects of cell adhesion on plasma and cellular FN were investigated as follows. 6-cm Petri dishes were coated with increasing concentrations of plasma FN or cellular FN (25, 50, and 100 μg/ml in PBS, pH 7.4). Proteins were allowed to bind overnight at 4°C. In some experiments the Petri dishes were rinsed and blocked for 2 h at 37°C with 3% heat-denatured BSA (RIA grade; Sigma Chemical Co.) in PBS, pH 7.4. In another set of experiments, the blocking step was omitted. Passage 5 human fibroblasts were resuspended in MEM (GIBCO AG) supplemented with Monomed (Commonwealth Serum Laboratories), 100 U/ml penicillin, 100 mg/ml streptomycin, and 2 mM l-glutamine, and then plated (1.5 × 104 cells/cm2) on Petri dishes precoated with plasma FN or cellular FN. They were maintained at 37°C in a humid atmosphere of 5% CO2 and 95% air for 1–4 d in MEM plus Monomed. All experiments were repeated at least five times and results were similar with all tested fibroblasts.
Western Blot Analysis
Cells were harvested and then extracted, or directly extracted on the dish with a buffer containing 1% SDS (Bio-Rad Laboratories AG, Glattbrugg, Switzerland), 1% dithiothreitol (Fluka Chemie AG, Buchs, Switzerland), 1 mM PMSF, 1 mM Nα-_p_-tosyl-l-arginine methyl ester (Sigma Chemical Co.) in 0.4 M Tris-HCl, pH 6.8, immediately sonicated, boiled for 5 min, and then centrifuged at 10,000 g for 20 min (model 5415C; Eppendorf Scientific, Inc., Hamburg, Germany). Protein content was determined according to Bradford (1976). Equal amounts of total proteins (15 μg for actin analysis and 50 μg for FN analysis) were fractionated by SDS-PAGE in acrylamide gels (5–20% gradient for actin analysis and 6% for FN analysis) and transferred to nitrocellulose filters (0.45 μm; Schleicher & Schuell, Dassel, Germany) as previously described (Serini and Gabbiani, 1996). Filters were then probed with mAbs IST-9, BC-1, anti–αSM-1, or the affinity-purified rabbit polyclonal anti-FN antibody (Sigma Chemical Co.). The secondary antibodies were either a goat anti–mouse IgG or a goat anti–rabbit IgG both conjugated with alkaline phosphatase (Promega Corp., Madison, WI). Specific binding was detected by the Problot Western Blot AP system (Promega Corp.).
Northern Blot Analysis
Total RNA was isolated from cultured cells by TRI REAGENT (Molecular Research Center, Inc., Cincinnati, OH), according to the manufacturer's instructions. 25 μg of total RNA per lane were denatured by glyoxal/DMSO treatment, separated by electrophoresis on a 1% agarose gel, and then transferred overnight onto an Electran nylon membrane (BDH, Poole, UK). RNA was immobilized on membrane by cross-linking in a Stratalinker UV light box (Stratagene, La Jolla, CA). To verify correct loading and transfer, filters were stained with 0.04% methylene blue in 0.5 M Na-acetate. Filters were then processed for hybridization with three different probes: (a) a 120-bp α-SM actin cDNA derived from the rat α-SM actin 3′-untranslated region and recognizing the human α-SM actin mRNA in one band at 1.7 kb (prepared in our laboratory by P. Neuville and T. Christen), (b) a 1,600-bp rat α1(I) collagen cDNA recognizing the human α1(I) collagen mRNA in two typical bands, one at 5.8 kb and the other one at 4.7 kb (Genovese et al., 1984), and (c) a 600-bp bovine plasminogen activator inhibitor type 1 (PAI-1) cDNA (gift of M.S. Pepper, Department of Morphology, University of Geneva, Switzerland) recognizing the human PAI-1 mRNA in two typical bands, one at 2.6 kb and the other one at 3.6 kb (Cicila et al., 1989). Probes were labeled by random priming using the MEGAPRIME DNA labeling system RPN 1606 (Amersham, Little Chalfont, UK). Prehybridization and hybridization were performed for 4 and 16 h, respectively, at 55°C in 5× standard saline citrate, 5× Denhardt's solution, 0.01% SDS, and 400 μg/ml denatured salmon sperm DNA. After hybridization, filters were washed twice for 15 min at 55°C in 5× standard saline citrate and 0.1% SDS. Northern blots were then exposed on Kodak film at −70°C (X-Omat SO-282).
Quantitation and Statistical Analysis
For quantitation, membranes and films corresponding to each Western and Northern blot experiment were scanned with an Arcus II scanner (Agfa, Mortsel, Belgium) and analyzed with the ImageQuant Software Version 3.3 (Molecular Dynamics, Sunnyvale, CA) obtaining the sum of the pixel values of band areas, as previously described (Bochaton-Piallat et al., 1998). Depending on the experiment, densitometric analysis results were presented as fold increase, percentage of the corresponding control, or percentage of induction inhibition (see Results) and expressed as arithmetical mean of all experiments performed ± SEM. For statistical evaluation, results were analyzed with Student's t test.
Results
ED-A FN Deposition Precedes α-SM Actin Expression during Granulation Tissue Evolution
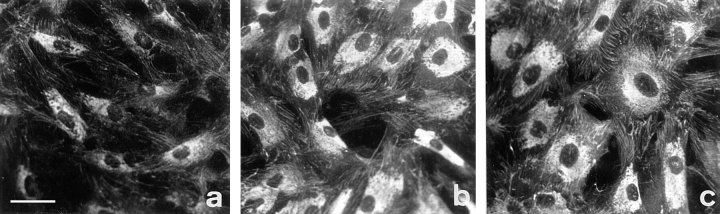
To assess the potential role of ED-A FN as an in vivo inducer of α-SM actin, first we have investigated both spatial and temporal relationships between ED-A FN and α-SM actin expression in a rat excisional model of wound repair. As previously reported (ffrench-Constant et al., 1989) ED-A FN was not present in fibroblasts of normal dermis under our conditions (data not shown). Fibroblastic cells containing cytoplasmic actin but not α-SM actin (Fig. 1, a and b) were abundant within the 4-d-old granulation tissue. At this time, ED-A FN was already expressed in huge amounts around them (Fig. 1 b). Only after this early ED-A FN deposition did α-SM actin start accumulating, evident around the seventh day (Fig. 1 c), and reached a maximal peak at the twelfth day (Fig. 1 d). Hence, during wound repair ED-A FN appearance precedes α-SM actin expression by fibroblastic cells.
Figure 1.
α-SM actin and ED-A FN expression in granulation tissues at different times after wounding examined by confocal laser scanning microscopy. Sections of 4- (a and b), 7- (c), and 12-d-old (d) granulation tissue were double stained for total actin (a, red) or α-SM actin (b–d, red) and ED-A FN (a–d, green). (a) Fibroblastic cells showing an important cytoplasmic staining for total actin are already present and interact with ED-A FN (yellow staining, corresponding to the overlay of red and green staining) which appears de novo in huge amounts as early as 4 d after wounding within granulation tissue stroma. (b) At 4 d α-SM actin is localized only in connection with SM-cells of small blood vessels, but not in connection with fibroblasts. (c) A 7-d-old wound tissue shows focal α-SM actin staining of fibroblasts within an ED-A FN-rich extracellular network. (d) 12-d-old granulation tissue fibroblastic cells show wide positivity for α-SM actin. α-SM actin and ED-A FN are colocalized (c and d; yellow); areas of colocalization are more abundant in 12- (d) than in 7-d-old (c) granulation tissue. Bar, 50 μM.
Levels of ED-A FN and α-SM Actin Expression Are Related in Different Fibroblastic Populations; ED-A Precedes α-SM Actin Induction by TGFβ1
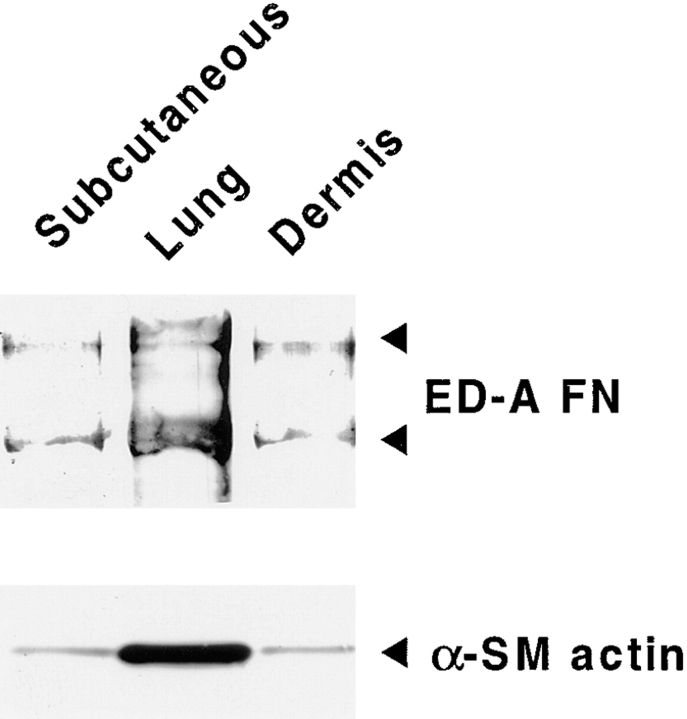
Although cultured fibroblastic cells from different origins display a roughly uniform morphology, they are heterogeneous in terms of growth, gene expression, and cell behavior (for review see Sappino et al., 1990). Indeed, when grown in vitro, fibroblasts from diverse organs can express different levels of α-SM actin (Desmoulière et al., 1992; Xu et al., 1997; Dugina et al., 1998). Therefore, we compared the expression of ED-A FN with that of α-SM actin in rat fibroblasts cultured from different tissues. Densitometric scanning of Western blots showed that α-SM actin content was similar in subcutaneous and dermal fibroblasts (Fig. 2) and was 11.3-fold higher in lung fibroblasts. Quantitative changes of ED-A FN expressed by fibroblastic populations from different organs mirrored α-SM actin expression pattern (Fig. 2), being similar in subcutaneous and dermal fibroblasts and 6.9-fold higher in lung fibroblasts. Thus, the ability to synthesize different amounts of ED-A FN by fibroblastic populations isolated from various tissues is proportional to their degree of myofibroblastic differentiation.
Figure 2.
ED-A FN and α-SM actin expression in cultured rat fibroblasts isolated from different tissues. Passage 5 rat fibroblast obtained from subcutaneous tissue, lung, and dermis were cultured for 4 d in absence of serum, then ED-A FN or α-SM actin expression were evaluated by Western blot analysis on equal amounts of total protein extracts. The levels of ED-A FN expression parallel those of α-SM actin.
We have previously shown (Desmoulière et al., 1993) that one-week stimulation with TGFβ1 (10 ng/ml) induces a two- to threefold increase of α-SM actin expression in cultured fibroblasts. By a more precise time course analysis (Fig. 3), we revealed a 2.3-fold increase in α-SM actin only after 72 h of TGFβ1 treatment and a plateau (2.6-fold) at the fourth day. Similar profiles were obtained in time- dependent increase of α-SM actin transcript (data not shown). These data suggest that α-SM actin upregulation by TGFβ1 could be the result of an indirect or synergizing effect, mediated by one or more intermediary molecules induced by TGFβ1 itself, such as ED-A FN. Indeed, continue fibroblast stimulation with TGFβ1 caused a fivefold ED-A FN increase within the first 24 h and a further increase (6.5-fold) after 48–72 h (Fig. 3). Therefore, during TGFβ1 treatment of cultured fibroblastic cells, the rise of ED-A FN precedes and then parallels α-SM actin increase. All together, these data are compatible with a role for ED-A FN as intermediary between α-SM actin and its positive regulator TGFβ1.
Figure 3.
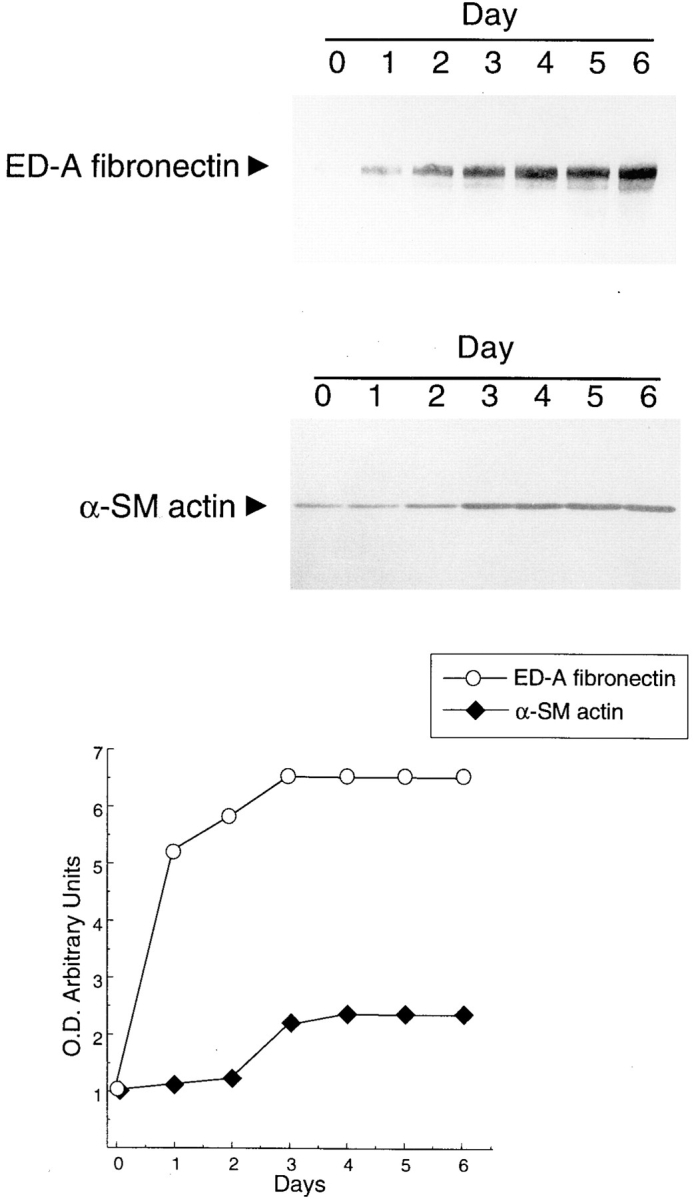
Time course analysis of α-SM actin and ED-A FN expression modulation by TGFβ1 in cultured human subcutaneous fibroblasts. Fibroblasts were incubated with 10 ng/ml of TGFβ1 and total proteins were extracted after different time of continuous stimulation. Immunoblotting after SDS-PAGE shows that TGFβ1 induces a clear-cut α-SM actin increase only after 72 h of treatment, whereas ED-A FN response to TGFβ1 precedes and then parallels α-SM actin increase. Tracks were loaded with equal amounts of total proteins. These data are the means of five independent experiments; the SEMs, which are not represented in the figure, were always lower than 5% of the values.
TGFβ1 Induction of Myofibroblastic Phenotype Requires a Permissive ED-A FN–derived Outside In Signaling
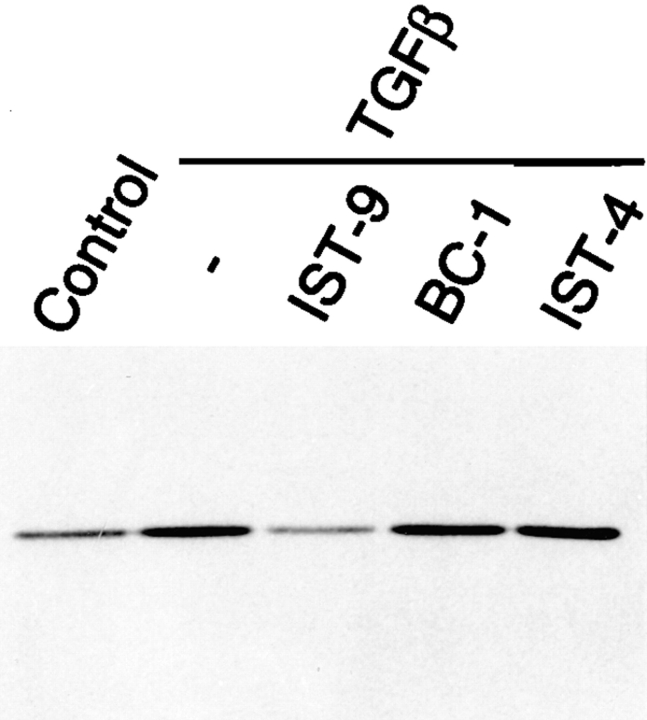
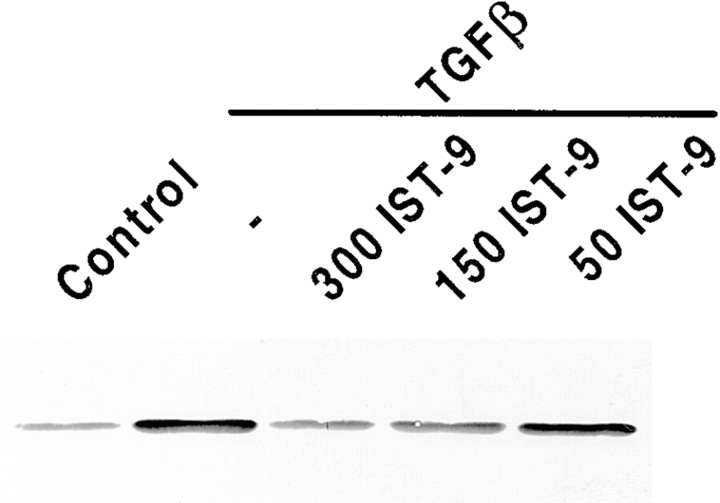
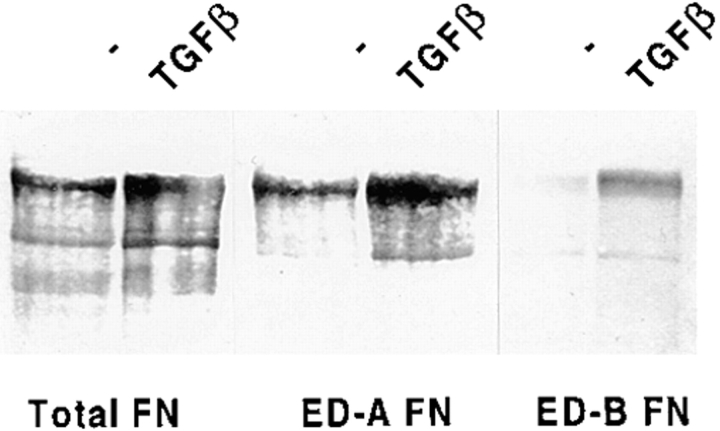
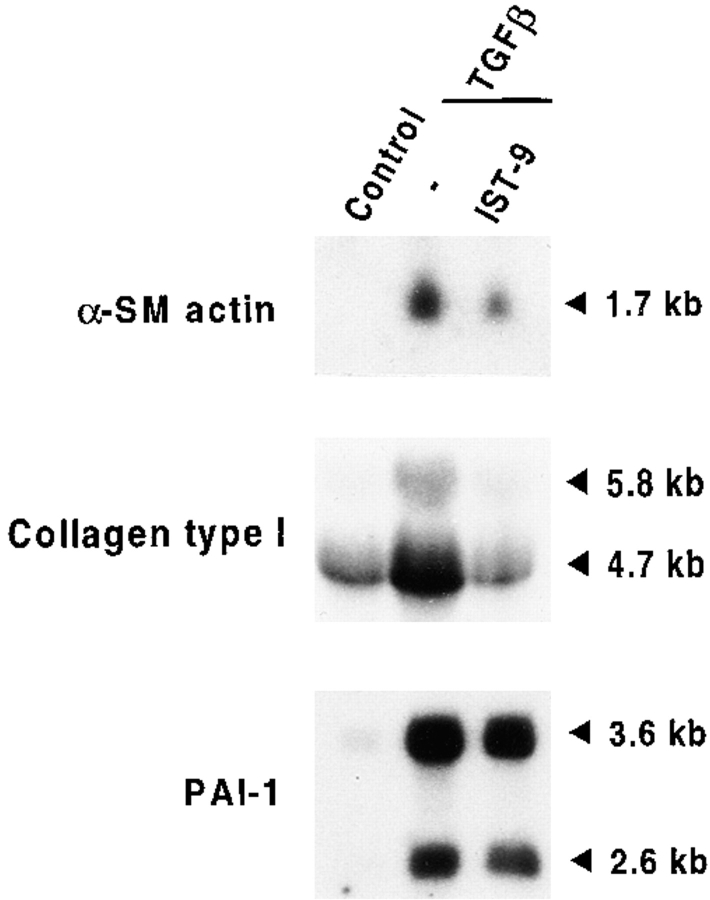
To directly investigate the role of ED-A FN as intermediary between α-SM actin and TGFβ1, we stimulated with TGFβ1 fibroblastic cells plated on gelatin containing specific blocking mAbs raised against different type III domains of FN (Borsi et al., 1987; Carnemolla et al., 1989, 1992). When examined after 72 h of TGFβ1 treatment (densitometric analysis values being expressed as percentages of the corresponding control), fibroblasts seeded on gelatin showed the expected α-SM actin increase (217 ± 32%) compared with control cells. Plating fibroblasts on gelatin containing IST-9, an IgG1 against the ED-A domain of cellular FN, led to a complete inhibition of α-SM actin induction (77 ± 13%, P < 0.001), whereas neither BC-1 (an IgG1 against the ED-B containing FN isoform), nor IST-4 (an IgG1 against the fifth type III domain of both cellular and plasma FN) were active in this regard (220 ± 29% and 221 ± 26%, respectively; Fig. 4). Similar results were obtained by stimulating cells with TGFβ2 (data not shown), which is as effective as TGFβ1 in upregulating α-SM actin both in vivo and in vitro (Serini and Gabbiani, 1996). The action of IST-9 was dose dependent (50–300 μg/ml; Fig. 5). Densitometric analysis revealed that the percentage of inhibition of α-SM actin induction by TGFβ1 was 25 ± 5% for 50 μg/ml, 59 ± 8% for 150 μg/ml, and 96 ± 15% for 300 μg/ml. It is known that TGFβ secreted and proteolytically activated by cultured fibroblasts induces a limited myofibroblastic differentiation (Masur et al., 1996); seeding cells on gelatin containing only IST-9 resulted in a slight lowering of the basal α-SM actin expression levels (data not shown). As previously described TGFβ1 is able to induce the insertion within FN not only of the ED-A, but also of the ED-B domain (Balza et al., 1988). Indeed, Western blot analysis revealed that treatment of fibroblasts with TGFβ1 induced an increase of both ED-A (3.5 ± 0.3-fold) and ED-B (7.3 ± 0.5-fold) FN isoforms (Fig. 6). This, together with our blocking experiment data, further confirms the role played by ED-A FN during myofibroblast formation.
Figure 4.
ED-A FN antibodies block TGFβ1 induction of α-SM actin in cultured human subcutaneous fibroblasts. Cells were seeded on simple gelatin or on gelatin containing blocking monoclonal antibodies against different FN type III domains and then stimulated with TGFβ1 (10 ng/ml) for 3 d; α-SM actin was then analyzed by Western blotting on equal amounts of total proteins. Note that inhibition of α-SM actin induction by TGFβ1 was obtained only when fibroblasts were plated on gelatin containing 300 μg/ml of IST-9.
Figure 5.
The inhibition of TGFβ1 induction of α-SM actin by IST-9 is dose dependent. Cells were seeded on simple gelatin or on gelatin containing different amounts (50, 150, and 300 μg/ml) of IST-9 and then stimulated with TGFβ1 (10 ng/ml) for 3 d; α-SM actin was then analyzed by Western blotting on equal amounts of total proteins.
Figure 6.
TGFβ1 induces the insertion of both ED-A and ED-B domain within cellular FN. Passage 5 human subcutaneous fibroblasts were stimulated with TGFβ1 (10 ng/ml) for 3 d; total FN, ED-A FN, and ED-B FN were then evaluated by Western blot analysis on equal amounts of total proteins. Treatment with TGFβ1 determined an increase of total FN and of both ED-A and ED-B FN isoforms when compared with control cells.
We then tested whether TGFβ-regulated genes other than α-SM actin are dependent on the ED-A FN–driven signaling. First, we selected collagen type I because its production represents a hallmark of myofibroblastic transition and a key pathogenetic event in the progression of fibrotic diseases (Border and Noble, 1994; Zhang et al., 1994). Northern blot analysis revealed that as expected, IST-9 treatment inhibited the TGFβ1-induced increase of α-SM actin at the mRNA level by 68 ± 12% (P < 0.001; Fig. 7); moreover, IST-9 mAb inhibited by 95 ± 17% the TGFβ1 stimulation of collagen type I mRNA (P < 0.001; Fig. 7). Hence, collagen type I mRNA induction by TGFβ requires a functionally active ED-A domain within the cellular FN molecule, similar to α-SM actin. The next gene analyzed was the PAI-1 which plays a crucial role both in the regulation of extracellular matrix-degrading enzymes and in the production of active TGFβ1 (Lund et al., 1987; Keski-Oja et al., 1988). Blocking the ED-A domain with IST-9 mAb did not counteract significantly the increase of PAI-1 mRNA level induced by TGFβ1 (Fig. 7). Thus, TGFβ1 regulation of PAI-1 expression differs from that of the two main myofibroblastic markers, α-SM actin and collagen type I.
Figure 7.
ED-A FN antibodies block TGFβ1 induction of α-SM actin and collagen type I, but not of PAI-1 mRNA in cultured human subcutaneous fibroblasts. Under the same experimental conditions described in Fig. 4, cells treated with TGFβ1 for 24 h contain higher levels of α-SM actin, collagen type I, and PAI-1 mRNA when compared with untreated cells; treatment with IST-9 mAb dramatically inhibits α-SM actin and collagen type I induction, but not that of PAI-1.
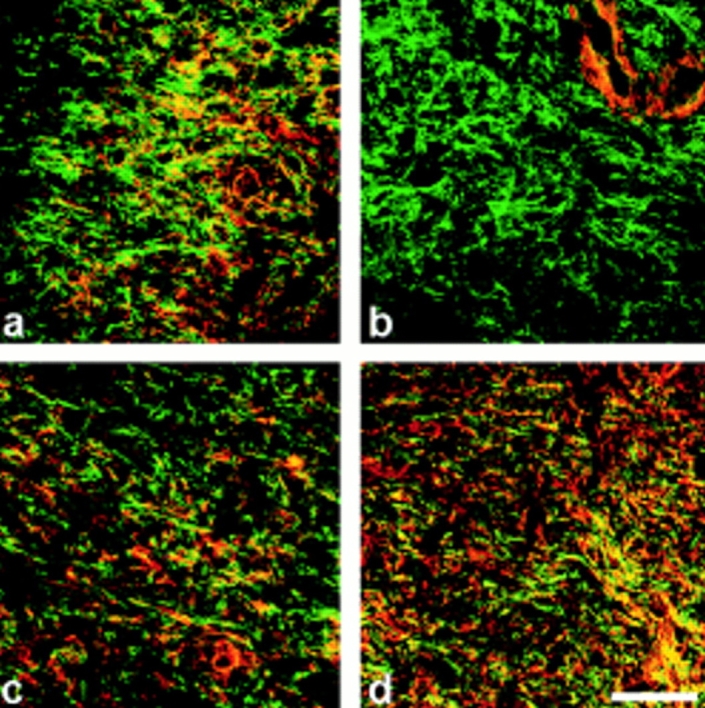
To investigate whether ED-A FN is not only necessary for TGFβ1 activity on fibroblasts, but also sufficient to cause their modulation to α-SM actin expressing myofibroblasts, cells were plated on Petri dishes precoated with increasing amounts of ED-A–negative plasma FN or ED-A–containing cellular FN (refer to Materials and Methods). 1–4 d after plating, no changes in α-SM actin expression were noted at any dose used (data not shown). Hence, ED-A FN does not directly stimulate the conversion of cultured fibroblasts to myofibroblasts. Next, we studied the influence of soluble ED-A FN on fibroblast modulation into α-SM actin–expressing myofibroblasts. For this purpose we used the isolated human rED-A domain (refer to Materials and Methods). Cells were plated on gelatin containing carrier solution or rED-A domain and stimulated with TGFβ1. After 72 h of TGFβ1 treatment, fibroblasts seeded on gelatin-containing carrier solution showed the usual upregulation in α-SM actin expression compared with control cells (Fig. 8). Plating fibroblasts on gelatin-containing rED-A domain resulted in a slight decrease (15 ± 3%, P < 0.001) of α-SM actin basal expression levels and in a clear-cut inhibition (61 ± 5%, P < 0.001) of α-SM actin induction by TGFβ1 (Fig. 8). The effects exerted by the rED-A domain mimicked the results obtained using the mAb IST-9 (data not shown; refer to Fig. 4), suggesting that these two approaches affect the same biological mechanism. These results can be interpreted in different ways. One is that the exogenous ED-A domain interferes with cellular FN matrix assembly. Indeed, FN matrix assembly can be disrupted using FN fragments containing critical domains (e.g., III-1 domain or α5β1-binding domain) or antibodies against these domains (McDonald, 1994). Moreover, TGFβ1 is known to increase the assembly of FN by human fibroblasts (Allen-Hoffmann et al., 1988). To test this hypothesis, we monitored TGFβ1-induced FN matrix assembly by cultured fibroblasts in the presence of IST-9 mAb or rED-A fragment (as described above) by means of double immunofluorescence staining, using the polyclonal FN antibody, IST-9 mAb, or the antibody against the 6× His tag of rED-A. As expected, TGFβ1 increased the expression and assembly of cellular FN when compared with control (Fig. 9, a and b); however, neither IST-9 mAb (Fig. 9 c) nor rED-A (data not shown) blocked basal and TGFβ1-stimulated FN matrix expression and assembly (Fig. 9, a and b). Interestingly, whereas IST-9 mAb colocalized with FN fibrils within the assembled matrix, rED-A did not, as revealed by a monoclonal directed against its 6× His tag (data not shown). These results indicate that the inhibition of the TGFβ1 induction of the myofibroblastic phenotype by IST-9 mAb and rED-A domain is not due to an inhibition or a perturbation of FN matrix assembly. Our data are compatible with the possibility that IST-9 mAb acts by preventing the ED-A domain to interact with a hypothetical receptor. Soluble rED-A domain would compete for the binding of the same receptor. In any event, our results suggest that in order to be permissive for the action of TGFβ1, the ED-A domain should be incorporated within the assembled and polymerized FN molecule.
Figure 8.
Addition of rED-A domain blocks TGFβ1 induction of α-SM actin in cultured human subcutaneous fibroblasts. Cells were seeded on simple gelatin or on gelatin containing 300 μg/ml of rED-A domain and then stimulated or not with TGFβ1 (10 ng/ml) for 3 d; α-SM actin was then analyzed by Western blotting on equal amount of total protein extracts. Treatment of fibroblasts with rED-A slightly lowered basal α-SM actin expression levels and inhibited α-SM actin induction by TGFβ1.
Figure 9.
FN matrix assembly evaluation on TGFβ1 stimulated fibroblasts after IST-9 mAb treatment. Subcutaneous fibroblasts were plated on simple gelatin (a and b) or on gelatin containing IST-9 mAb (c) and then stimulated with TGFβ1 (10 ng/ml) for 3 d (b and c). Cells were then stained with an anti-total FN rabbit polyclonal antibody. When compared with control (a), TGFβ1 stimulation determines an increase in FN expression and assembly by fibroblasts (b). IST-9 mAb treatment (c) does not interfere with TGFβ1 action. Bar, 25 μM.
Discussion
TGFβ1 is presently considered as the main inducer of the myofibroblastic phenotype, being able to upregulate α-SM actin as well as collagen expression in fibroblasts both in vitro and in vivo (Border and Ruoslahti, 1992; Sporn and Roberts, 1992; Desmoulière et al., 1993; Rønnov-Jessen and Petersen, 1993; Zhang et al., 1994). Many data point to TGFβ as a key cytokine in controlling tissue repair, and disregulation of its production may be a cause of tissue fibrosis (Border and Ruoslahti, 1992; Sporn and Roberts, 1992; Border and Noble, 1994). When compared with other cytokines, a distinctive feature of TGFβ is the ability to control cell adhesion and migration by modulating the adhesion molecule repertoire (Zambruno et al., 1995) as well as the synthesis of ECM components such as FN and collagen (Ignotz and Massagué, 1986; Roberts et al., 1986). Furthermore, expression of TGFβ1 gene has been shown to be influenced by ECM molecules (Streuli et al., 1986), suggesting a feedback loop in vivo. However, the mechanisms by which ECM influences TGFβ effects on target cells are not yet fully characterized.
We demonstrate here that ED-A FN deposition precedes α-SM actin expression both in vivo, during granulation tissue evolution, and in vitro, during TGFβ1 fibroblast stimulation. Moreover, the degree of myofibroblastic differentiation exhibited by fibroblasts cultured from different organs is proportional to the different amounts of ED-A FN they produce. Furthermore, selectively blocking the ED-A domain of cellular FN by IST-9 mAb inhibits α-SM actin and collagen type I mRNA induction by TGFβ1 in cultured fibroblasts. In contrast, TGFβ1 upregulation of PAI-1 is not influenced by ED-A FN, indicating that the PAI-1 gene is regulated differently than α-SM actin and collagen type I. Interestingly, it has been shown that the increase of collagen type I and actin mRNA induced by TGFβ1 is dependent on protein synthesis, whereas the induction of PAI-1 transcript is not (Lund et al., 1987; Keski-Oja et al., 1988; Penttinen et al., 1988). Hence, the synthesis of an intermediary protein such as ED-A FN is necessary for the stimulation by TGFβ1 of at least some morphofunctional genes, i.e., α-SM actin and collagen type I. Thus, it appears that TGFβ1 regulates gene expression through different mechanisms, possibly according to the biological functions exerted by the corresponding proteins.
Hautmann and colleagues (1997) have identified a TGFβ response element in the α-SM actin promoter that drives the stimulation of α-SM actin gene expression in concert with two CArG elements in rat sn cells. We demonstrate here that, at least in fibroblastic cells, the presence of functional ED-A FN is mandatory for α-SM actin induction by TGFβ. The ED-A FN signaling is necessary but not sufficient for α-SM actin–positive regulation by TGFβ. Taken together, these observations suggest that TGFβ activation of α-SM actin expression results from the cooperation of two signal transduction pathways raised respectively by TGFβ and ED-A FN.
The observation that treatment of fibroblasts with the soluble rED-A domain inhibits TGFβ1 induction of the myofibroblastic phenotype without interfering with FN assembly (similar to treatment with IST-9 mAb) allows to hypothesize the existence of an hitherto unknown specific receptor interacting with the ED-A domain. It is worth noting that a TGFβ-dependent morphogenic process, i.e., the cellular condensation event that occurs during chondrogenesis (Leonard et al., 1991), has been recently shown to be spatiotemporally correlated to and to depend upon the ED-A domain insertion in cellular FN; moreover, this crucial step in skeletal pattern formation was similarly inhibited by the soluble rED-A domain and an anti–ED-A antibody (Gehris et al., 1997). All together, these data suggest that the ED-A–dependent signaling described here is a general mechanism used by TGFβ to finely regulate the correct execution of different morphogenetic programs.
Cell binding activity of FN was originally localized in the tenth type III repeat to the amino acid sequence RGD (Pytela et al., 1987) and in the alternative spliced IIICS region to the amino acid sequence LDV (Humphries et al., 1988). The recent demonstrations that activated β1 integrins mediate cell adhesion and spreading on recombinant FN type III repeats lacking RGD have expanded the possibility for integrin-dependent cell–ECM interactions (Chi-Rosso et al., 1997; Moyano et al., 1997). Xia and Culp (1994) have shown that the isolated rED-A domain promotes cell adhesion, whereas Manabe et al. (1997) have demonstrated that cells adhere and migrate more actively on the ED-A FN probably because ED-A induces a conformational change of FN, which in turn increases the accessibility of the RGD motif to integrin α5β1. We hypothesize that the ED-A domain interacting with its receptor acts in conjunction with the integrin-binding sites of FN to switch on a qualitatively different and more complex signaling. Moreover, we show here that to exert its permissive function on TGFβ activity, the ED-A domain must be presented within the proper molecular context, that is the polymerized FN molecule; indeed isolated the rED-A domain inhibits this function. Thus, the ED-A receptor is able to bind its domain independently of the molecular context, but generates intracellular signals only in a conformationally sensitive manner. In this respect, two recent reports (Shrivastava et al., 1997; Vogel et al., 1997) outline an intriguing new paradigm for ECM signaling: the receptor tyrosine kinases DDR1 and DDR2 bind to and are activated by collagen in a conformation-dependent way. It is proposed that signals generated by the activation of these receptors act in concert with signals generated by binding of ECM molecules to classical integrins. Further work along these lines may help in identifying the hypothetical ED-A cell-surface receptor.
Unlike Jarnagin et al. (1994) and Van De Water et al. (Van De Water, L., C. Reimer, L. Plantefaber, R.O. Hynes, and J.H. Peters. 1996. Abstract from Wound Repair in Context, Keystone Symposia, Taos, NM), we were unable to induce α-SM actin expression by simply plating cells on ED-A–containing cellular FN. This discrepancy may be related to the different cell types (hepatic stellate cells versus fibroblasts) or alternatively to the different sources of FN the authors used in their assay system, e.g., endothelial cell-derived ECM and commercial FNs. Indeed, an increasing number of cytokines, including TGFβ, have been found associated with the ECM proteins and both latent and active form of TGFβ have been found to bind cellular FN of many cell types, endothelial cells included (Taipale and Keski-Oja, 1997). Moreover, many commercial sources of FN contain TGFβ activity (Fava et al., 1987). Our data show that ED-A FN is necessary, but not sufficient, to induce myofibroblastic differentiation and that it exerts a permissive effect on TGFβ activity.
It is well accepted that ED-A FN is synthesized by mesenchymal cells which are driven toward an α-SM actin– positive phenotype by TGFβ in many physiological and pathological settings, such as wound healing (Gabbiani et al., 1972; Darby et al., 1990), Dupuytren's disease (Berndt et al., 1995), organ fibrosis (Schürch et al., 1992), developing aorta (Glukhova et al., 1990), arterial intimal thickening (Glukhova et al., 1989), and stroma reaction to epithelial cancers (Pujuguet et al., 1996). Our results support that in such different systems ED-A FN acts as a necessary ECM molecule that allows TGFβ to induce SM differentiation.
Pathological deposition within tissues of ECM components results in fibrosis, which may alter irreversibly the function of the involved organ. Recently it has been demonstrated that α-SM actin–expressing myofibroblasts are the main collagen type I synthesizing cells during fibrosis (Zhang et al., 1994). Blocking TGFβ1 with antibodies or with decorin was therapeutic in many models of fibrotic disease (Border and Noble, 1994) and, where investigated, was associated to a significant attenuation of ED-A FN deposition (Isaka et al., 1996; Giri et al., 1997). However, extreme reduction of TGFβ1 levels or mutation in its receptors could be unfavorable, causing autoimmune-like disease (Shull et al., 1992; Kulkarni et al., 1993) and leading to malignant transformation (Marx, 1995). ED-A FN could be considered as a potential target for therapy since: (a) it is an extracellular, easily reachable molecule; and (b) in contrast to TGFβ1, it drops to low levels in adult tissues (Kornblihtt et al., 1996) decreasing the probability that its blocking results in side effects. Thus, the outside in signal described here represents another possible target for pharmacological studies on myofibroblastic activities during developmental and pathological events. Notably, recent studies support the feasibility of in vivo targeting alternatively spliced exons of FN present in pathological tissues but not in their normal counterparts (Mariani et al., 1997_a_ ,b; Neri et al., 1997).
Abbreviations used in this paper
ECM
extracellular matrix
FN
fibronectin
MEM
Eagle's minimum essential medium
PAI-1
plasminogen activator inhibitor-1
rED-A
recombinant ED-A
SM
smooth muscle
TGFβ
transforming growth factor β
Footnotes
A. Roberts (National Institutes of Health, Bethesda, MD), D.A. Cox (Novartis, Basel, Switzerland), F.E. Baralle (International Centre for Genetic Engineering and Biotechnology, Trieste, Italy), and M.S. Pepper (University of Geneva, Geneva, Switzerland) are gratefully acknowledged for providing recombinant human TGFβ1, recombinant human TGFβ2, full-length cellular FN cDNA clone and PAI-1 cDNA probe, respectively. We thank R.B. Low (University of Vermont, Burlington, VT) and L. Trusolino (University of Torino Medical School, Candiolo, Italy) for critically reading this manuscript, F. Gabbiani for skillful technical assistance, J.C. Rumbeli and E. Denkinger for photographic work, and M. Vitali for typing the manuscript (all four from University of Geneva).
This work was partially supported by grants from the Swiss National Science Foundation (31-40372.94 and 31-50568.97) and Associazione Italiana per la Ricerca sul Cancro (AIRC) funds. G. Serini was supported by the Fondation pour des Bourses d'Etudes Italo-Suisses (Lausanne, Switzerland).
Address all correspondence to Giulio Gabbiani, Department of Pathology, CMU, University of Geneva, 1 rue Michel-Servet, 1211 Geneva 4, Switzerland. Tel.: (41) 22-70-25-742. Fax: (41) 22-70-25-746. E-mail: giulio.gabbiani@medecine.unige.ch
G. Serini's present address is Molecular Histology Unit (2A1), Department of Biological and Technical Research (DIBIT), H San Raffaele Scientific Institute, Via Olgettina 58, 20132 Milan, Italy.
References
- Allen-Hoffmann BL, Crankshaw CL, Mosher DF. Transforming growth factor β increases cell surface binding and assembly of exogenous (plasma) fibronectin by normal human fibroblasts. Mol Cell Biol. 1988;8:4234–4242. doi: 10.1128/mcb.8.10.4234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balza E, Borsi L, Allemmanni G, Zardi L. Transforming Growth Factor β regulates the levels of different fibronectin isoforms in normal cultured fibroblasts. FEBS (Fed Eur Biochem Soc) Lett. 1988;228:42–44. doi: 10.1016/0014-5793(88)80580-5. [DOI] [PubMed] [Google Scholar]
- Berndt A, Kosmehl H, Mandel U, Gabler U, Luo X, Celada D, Zardi L, Katenkamp D. TGFβ and bFGF synthesis and localization in Dupuytren's disease (nodular palmar fibrosis) relative to cellular activity, myofibroblast phenotype and oncofetal variants of fibronectin. Histochemical J. 1995;27:1014–1020. [PubMed] [Google Scholar]
- Bochaton-Piallat ML, Gabbiani G, Pepper MS. Plasminogen activator expression in rat arterial smooth muscle cells depends on their phenotype and is modulated by cytokines. Circ Res. 1998;82:1086–1093. doi: 10.1161/01.res.82.10.1086. [DOI] [PubMed] [Google Scholar]
- Border WA, Ruoslahti E. Transforming growth factor-β in disease: the dark side of tissue repair. J Clin Invest. 1992;90:1–7. doi: 10.1172/JCI115821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Border WA, Noble NA. Transforming growth factor-β in tissue fibrosis. N Engl J Med. 1994;331:1286–1292. doi: 10.1056/NEJM199411103311907. [DOI] [PubMed] [Google Scholar]
- Borsi L, Carnemolla B, Castellani P, Rossellini C, Vecchio D, Allemmanni G, Chang SE, Taylor-Papadimitriou J, Pande H, Zardi L. Monoclonal antibodies in the analysis of fibronectin isoforms generated by alternative splicing of mRNA precursors in normal and transformed human cells. J Cell Biol. 1987;104:595–600. doi: 10.1083/jcb.104.3.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsi L, Castellani P, Risso AM, Leprini A, Zardi L. Transforming growth factor β regulates the splicing pattern of fibronectin messenger RNA precursor. FEBS (Fed Eur Biochem Soc) Lett. 1990;261:175–178. doi: 10.1016/0014-5793(90)80664-5. [DOI] [PubMed] [Google Scholar]
- Boström H, Willetts K, Pekny M, Levéen P, Lindahl P, Hedstrand H, Pekna M, Hellström M, Gebre-Medhin S, Schalling M, Nilsson M, Kurland S, Törnell J, Heath JK, Betsholtz C. PDGF-A signaling is a critical event in lung alveolar myofibroblast development and alveogenesis . Cell. 1996;85:863–873. doi: 10.1016/s0092-8674(00)81270-2. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye-binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Carnemolla B, Borsi L, Zardi L, Owens RJ, Baralle FE. Localization of the cellular-fibronectin-specific epitope recognized by the monoclonal antibody IST-9 using fusion proteins expressed in E. Coli. FEBS (Fed Eur Biochem Soc) Lett. 1987;215:269–273. doi: 10.1016/0014-5793(87)80160-6. [DOI] [PubMed] [Google Scholar]
- Carnemolla B, Balza E, Siri A, Zardi L, Nicotra MR, Bigotti A, Natali PG. A tumor-associated fibronectin isoform generated by alternative splicing of messenger RNA precursors. J Cell Biol. 1989;108:1139–1148. doi: 10.1083/jcb.108.3.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnemolla B, Borsi L, Zardi L, Owens RJ, Baralle FE. Localization of the cellular-fibronectin-specific epitope recognized by the monoclonal antibody IST-9 using fusion proteins expressed in E. Coli. FEBS (Fed Eur Biochem Soc) Lett. 1987;215:269–273. doi: 10.1016/0014-5793(87)80160-6. [DOI] [PubMed] [Google Scholar]
- Carnemolla B, Leprini A, Allemanni G, Saginati M, Zardi L. The inclusion of the type III repeat ED-B in the fibronectin molecule generates conformational modification that unmask a cryptic sequence . J Biol Chem. 1992;267:24689–24692. [PubMed] [Google Scholar]
- Chi-Rosso G, Gotwals PJ, Yang J, Ling L, Jiang K, Chao B, Baker DP, Burkly LC, Fawell SE, Koteliansky VE. Fibronectin type III repeats mediate RGD-independent adhesion and signaling through activated β1 integrins. J Biol Chem. 1997;272:31447–31452. doi: 10.1074/jbc.272.50.31447. [DOI] [PubMed] [Google Scholar]
- Cicila GT, O'Connell TM, Hahn WC, Rheinwald JG. Cloned cDNA sequence for the human mesothelial protein ‘mesosecrin' discloses its identity as a plasminogen activator inhibitor (PAI-1) and a recent evolutionary change in transcript processing. J Cell Sci. 1989;94:1–10. doi: 10.1242/jcs.94.1.1. [DOI] [PubMed] [Google Scholar]
- Clarke EA, Brugge JS. Integrins and signal transduction pathways: the road taken. Science. 1995;268:233–239. doi: 10.1126/science.7716514. [DOI] [PubMed] [Google Scholar]
- Darby I, Skalli O, Gabbiani G. Alpha-smooth muscle actin is transiently expressed by myofibroblasts during experimental wound healing. Lab Invest. 1990;63:21–29. [PubMed] [Google Scholar]
- Desmoulière A, Rubbia-Brandt L, Abdiu A, Walz T, Macieira-Coelho A, Gabbiani G. Alpha-smooth muscle actin is expressed in a subpopulation of cultured and cloned fibroblasts and is modulated by gamma-interferon. Exp Cell Res. 1992;201:64–73. doi: 10.1016/0014-4827(92)90348-c. [DOI] [PubMed] [Google Scholar]
- Desmoulière A, Geinoz A, Gabbiani F, Gabbiani G. Transforming growth factor-β1 induces α-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol. 1993;122:103–111. doi: 10.1083/jcb.122.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmoulière, A., and G. Gabbiani. 1996. The role of the myofibroblast in wound healing and fibrocontractive diseases. In The Molecular and Cellular Biology of Wound Repair. R.A.F. Clark, editor. Plenum Press, New York. 391–423.
- Dugina V, Alexandrova A, Chaponnier C, Vasiliev J, Gabbiani G. Rat fibroblasts cultured from various organs exhibit differences in alpha-smooth muscle actin expression, cytoskeletal pattern, and adhesive structure organization. Exp Cell Res. 1998;238:481–490. doi: 10.1006/excr.1997.3868. [DOI] [PubMed] [Google Scholar]
- Fava RA, McClure DB. Fibronectin-associated transforming growth factor. J Cell Physiol. 1987;131:184–189. doi: 10.1002/jcp.1041310207. [DOI] [PubMed] [Google Scholar]
- ffrench-Constant C, Van De Water L, Dvorak HF, Hynes RO. Reappearance of an embryonic of fibronectin splicing during wound healing in the adult rat. J Cell Biol. 1989;109:903–914. doi: 10.1083/jcb.109.2.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbiani G, Hirschel BJ, Ryan GB, Statkov PR, Majno G. Granulation tissue as contractile organ. A study of structure and function. J Exp Med. 1972;135:719–734. doi: 10.1084/jem.135.4.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehris AL, Stringa E, Spina J, Desmond ME, Tuan RS, Bennett VD. The region encoded by the alternatively spliced exon IIIA in mesenchymal fibronectin appears essential for chondrogenesis at the level of cellular condensation. Dev Biol. 1997;190:191–205. doi: 10.1006/dbio.1997.8693. [DOI] [PubMed] [Google Scholar]
- Genovese C, Rowe D, Kream B. Construction of DNA sequences complementary to rat alpha 1 and alpha 2 collagen mRNA and their use in studying the regulation of type I collagen synthesis by 1,25-dihydroxyvitamin D. Biochemistry. 1984;23:6210–6216. doi: 10.1021/bi00320a049. [DOI] [PubMed] [Google Scholar]
- Giri SN, Hyde DM, Braun RK, Gaarde W, Harper JR, Pierschbacher MD. Antifibrotic effect of decorin in a bleomycin hamster model of lung fibrosis. Biochem Pharmacol. 1997;54:1205–1216. doi: 10.1016/s0006-2952(97)00343-2. [DOI] [PubMed] [Google Scholar]
- Glukhova MA, Frid MG, Shekhonin BV, Vasilevskaya TD, Grunwald J, Saginati M, Koteliansky VE. Expression of extra domain-A fibronectin sequence in vascular smooth muscle cells is phenotype dependent . J Cell Biol. 1989;109:357–366. doi: 10.1083/jcb.109.1.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glukhova MA, Frid MG, Shekhonin BV, Balabanov YV, Koteliansky VE. Expression of fibronectin variants in vascular and visceral smooth muscle cells in development. Dev Biol. 1990;141:193–202. doi: 10.1016/0012-1606(90)90114-x. [DOI] [PubMed] [Google Scholar]
- Grinnell F. Fibroblasts, myofibroblasts, and wound contraction. J Cell Biol. 1994;124:401–404. doi: 10.1083/jcb.124.4.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hautmann MB, Madsen CS, Owens GK. A transforming growth factor β (TGFβ) control element drives TGFβ-induced stimulation of smooth muscle α-actin gene expression in concert with two CArG elements. J Biol Chem. 1997;272:10948–10956. doi: 10.1074/jbc.272.16.10948. [DOI] [PubMed] [Google Scholar]
- Heldin CH, Miyazono K, ten Dijke P. TGFβ signalling from cell membrane to nucleus through SMAD proteins. Nature. 1997;390:465–471. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- Humphries MJ, Akiyama SK, Komoriya A, Olden K, Yamada KM. Neurite extension of chicken peripheral nervous system neurons on fibronectin: relative importance of specific adhesion sites in the central cell-binding domain and the alternatively spliced type III connecting segment. J Cell Biol. 1988;106:1289–1297. doi: 10.1083/jcb.106.4.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes, R.O. 1990. Fibronectins. In Springer's Series in Molecular Biology. A. Rich, editor. Springer-Verlag, Inc., New York. 546 pp.
- Ignotz RA, Massagué J. Transforming growth factor-β stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J Biol Chem. 1986;261:4337–4345. [PubMed] [Google Scholar]
- Isaka Y, Brees DK, Ikegaya K, Kaneda Y, Imai E, Noble NA, Border WA. Gene therapy by skeletal muscle expression of decorin prevents fibrotic disease in rat kidney. Nature Med. 1996;2:418–423. doi: 10.1038/nm0496-418. [DOI] [PubMed] [Google Scholar]
- Jarnagin WR, Rockey DC, Koteliansky VE, Wang SS, Bissell DM. Expression of variant fibronectin in wound healing: cellular source and biological activity of the EIIIA fibronectin segment in rat hepatic fibrogenesis. J Cell Biol. 1994;127:2037–2048. doi: 10.1083/jcb.127.6.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keski-Oja J, Raghow R, Sawdey M, Loskutoff DJ, Postlethwaite AE, Kang AH, Moses HL. Regulation of mRNAs for type-1 plasminogen activator inhibitor, fibronectin, and type I procollagen by transforming growth factor-beta. Divergent responses in lung fibroblasts and carcinoma cells. J Biol Chem. 1988;263:3111–3115. [PubMed] [Google Scholar]
- Kingsley DM. The TGF-β superfamily: new members, new receptors, and new genetic tests of function in different organisms. Genes Dev. 1994;8:133–146. doi: 10.1101/gad.8.2.133. [DOI] [PubMed] [Google Scholar]
- Kocher O, Kennedy SP, Madri JA. Alternative splicing of endothelial cell fibronectin mRNA in the IIICS region. Am J Pathol. 1990;137:1509–1524. [PMC free article] [PubMed] [Google Scholar]
- Kornblihtt AR, Vibe-Pedersen K, Baralle FE. Human fibronectin: cell specific alternative mRNA splicing generates polypeptide chains differing in the number of internal repeats. Nucleic Acids Res. 1984;12:5853–5868. doi: 10.1093/nar/12.14.5853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornblihtt AR, Pesce CG, Alonso CR, Cramer P, Srebrow A, Werbajh S, Muro AF. The fibronectin gene as a model for splicing and transcription studies. FASEB (Fed Am Soc Exp Biol) J. 1996;10:248–257. doi: 10.1096/fasebj.10.2.8641558. [DOI] [PubMed] [Google Scholar]
- Kosmehl H, Berndt A, Katenkamp D. Molecular variants of fibronectin and laminin: structure, physiological occurrence and histopathological aspects. Virchows Arch. 1996;429:311–322. doi: 10.1007/BF00198435. [DOI] [PubMed] [Google Scholar]
- Kulkarni AB, Huh CG, Becker D, Lyght M, Flanders KC, Roberts AB, Sporn MB, Ward JM, Karlsson S. Transforming growth factor β1null mutation causes excessive inflammatory response and early death. Proc Natl Acad Sci USA. 1993;90:770–774. doi: 10.1073/pnas.90.2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurkinen M, Vaheri A, Roberts PJ, Stenman S. Sequential appearance of fibronectin and collagen in experimental granulation tissue. Lab Invest. 1980;43:47–51. [PubMed] [Google Scholar]
- Leonard CM, Fuld HM, Frenz DA, Downie SA, Massague J, Newman SA. Role of transforming growth factor-beta in chondrogenic pattern formation in the embryonic limb: stimulation of mesenchymal condensation and fibronectin gene expression by exogenenous TGF-β and evidence for endogenous TGF-β-like activity. Dev Biol. 1991;145:99–109. doi: 10.1016/0012-1606(91)90216-p. [DOI] [PubMed] [Google Scholar]
- Lund LR, Riccio A, Andreasen PA, Nielsen LS, Kristensen P, Laiho M, Saksela O, Blasi F, Dano K. Transforming growth factor-β is a strong and fast acting positive regulator of the level of type-1 plasminogen activator inhibitor mRNA in WI-38 human lung fibroblasts. EMBO (Eur Mol Biol Organ) J. 1987;6:1281–1286. doi: 10.1002/j.1460-2075.1987.tb02365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod JN, Burton-Wurster N, Gu DN, Lust G. Fibronectin mRNA splice variant in articular cartilage lacks bases encoding the V, III-15, and I-10 protein segments. J Biol Chem. 1996;271:18954–18960. doi: 10.1074/jbc.271.31.18954. [DOI] [PubMed] [Google Scholar]
- Manabe R, Oh-e N, Maeda T, Fukuda T, Sekiguchi K. Modulation of cell-adhesive activity of fibronectin by the alternatively spliced EDA segment. J Cell Biol. 1997;139:295–307. doi: 10.1083/jcb.139.1.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariani G, Lasku A, Balza E, Gaggero B, Motta C, Di Luca L, Dorcaratto A, Viale GA, Neri D, Zardi L. Tumor targeting potential of the monoclonal antibody BC-1 against oncofetal fibronectin in nude mice bearing human tumor implants. Cancer. 1997a;80:2378–2384. doi: 10.1002/(sici)1097-0142(19971215)80:12+<2378::aid-cncr7>3.3.co;2-0. [DOI] [PubMed] [Google Scholar]
- Mariani G, Lasku A, Pau A, Villa G, Motta C, Calcagno G, Taddei GZ, Castellani P, Syrigos K, Dorcaratto A, Epenetos AA, Zardi L, Viale GA. A pilot pharmacokinetic and immunoscintigraphic study with the technetium-99m-labeled monoclonal antibody BC-1 directed against oncofetal fibronectin in patients with brain tumors. Cancer. 1997b;80:2484–2489. doi: 10.1002/(sici)1097-0142(19971215)80:12+<2484::aid-cncr20>3.3.co;2-l. [DOI] [PubMed] [Google Scholar]
- Marx J. DNA repair defect tied to mutated TGF-β receptor gene. Science. 1995;268:1276–1277. doi: 10.1126/science.7761848. [DOI] [PubMed] [Google Scholar]
- Massagué J, Hata A, Liu F. TGF-β signalling through the Smad pathway. Trends Cell Biol. 1997;7:187–192. doi: 10.1016/S0962-8924(97)01036-2. [DOI] [PubMed] [Google Scholar]
- Masur SK, Dewal HS, Dinh TT, Erenburg I, Petridou S. Myofibroblasts differentiate from fibroblasts when plated at low density. Proc Natl Acad Sci USA. 1996;93:4219–4223. doi: 10.1073/pnas.93.9.4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald JA. Extracellular matrix assembly. Methods Enzymol. 1994;245:518–531. doi: 10.1016/0076-6879(94)45026-9. [DOI] [PubMed] [Google Scholar]
- Moyano JV, Carnemolla B, Dominguez-Jimenez C, Garcia-Gila M, Albar JP, Sanchez-Aparicio P, Leprini A, Querze G, Zardi L, Garcia-Pardo A. Fibronectin type III5 repeat contains a novel cell adhesion sequence, KLDAPT, which binds activated α4β1 and α4β7 integrins. J Biol Chem. 1997;272:24832–24836. doi: 10.1074/jbc.272.40.24832. [DOI] [PubMed] [Google Scholar]
- Neri D, Carnemolla B, Nissim A, Leprini A, Querze G, Balza E, Pini A, Tarli L, Halin C, Neri P, Zardi L, Winter G. Targeting by affinity-matured recombinant antibody fragments of an angiogenesis associated fibronectin isoform. Nat Biotechnol. 1997;15:1271–1275. doi: 10.1038/nbt1197-1271. [DOI] [PubMed] [Google Scholar]
- Penttinen RP, Kobayashi S, Bornstein P. Transforming growth factor β increases mRNA for matrix proteins both in the presence and in the absence of changes in mRNA stability. Proc Natl Acad Sci USA. 1988;85:1105–1108. doi: 10.1073/pnas.85.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujuguet P, Hammann A, Moutet M, Samuel JL, Martin F, Martin M. Expression of fibronectin ED-A+ and ED-B+isoforms by human and experimental colorectal cancer. Am J Pathol. 1996;148:579–592. [PMC free article] [PubMed] [Google Scholar]
- Pytela R, Pierschbacher MD, Argrves WS, Suzuki S, Ruoslahti E. Arg-Gly-Asp adhesion receptors. Methods Enzymol. 1987;144:475–489. doi: 10.1016/0076-6879(87)44196-7. [DOI] [PubMed] [Google Scholar]
- Roberts AB, Sporn MB, Assoian AK, Smith JM, Roche NS, Wakefield LM, Heine UI, Liotta LA, Falanga V, Kehrl JH, Fauci AS. Transforming growth factor type-β: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. . Proc Natl Acad Sci USA. 1986;83:4167–4171. doi: 10.1073/pnas.83.12.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AB, Sporn MB. Physiological actions and clinical applications of transforming growth factor β (TGFβ) Growth Factors. 1993;8:1–9. doi: 10.3109/08977199309029129. [DOI] [PubMed] [Google Scholar]
- Rønnov-Jessen L, Petersen OW. Induction of α-smooth muscle actin by transforming growth factor-β1 in quiescent human breast gland fibroblasts. Implications for myofibroblast generation in breast neoplasia. Lab Invest. 1993;68:696–707. [PubMed] [Google Scholar]
- Rønnov-Jessen L, Petersen OW, Bissell MJ. Cellular changes involved in conversion of normal to malignant breast: importance of the stromal reaction. Physiol Rev. 1996;76:69–125. doi: 10.1152/physrev.1996.76.1.69. [DOI] [PubMed] [Google Scholar]
- Sappino AP, Schurch W, Gabbiani G. Differentiation repertoire of fibroblastic cells: expression of cytoskeletal proteins as marker of phenotypic modulations. Lab Invest. 1990;63:144–161. [PubMed] [Google Scholar]
- Schlaepfer DD, Hunter T. Integrin signalling and tyrosine phosphorylation: just the FAKs? . Trends Cell Biol. 1998;8:151–157. doi: 10.1016/s0962-8924(97)01172-0. [DOI] [PubMed] [Google Scholar]
- Schwartz MA. Integrins, oncogenes, and anchorage independence. J Cell Biol. 1997;139:575–578. doi: 10.1083/jcb.139.3.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schürch, W., T.A. Seemayer, and G. Gabbiani. 1992. Myofibroblast. In Histology for Pathologists. S.S. Sternberg, editor. Raven Press, New York. 109–144.
- Serini G, Gabbiani G. Modulation of α-smooth muscle actin expression in fibroblasts by transforming growth factor-β isoforms: an in vivo and in vitro study. Wound Rep Reg. 1996;4:278–287. doi: 10.1046/j.1524-475X.1996.40217.x. [DOI] [PubMed] [Google Scholar]
- Shrivastava A, Radziejewski C, Campbell E, Kovac L, McGlynn M, Ryan TE, Davis S, Goldfarb MP, Glass DJ, Lemke G, Yancopoulos GD. An orphan receptor tyrosine kinase family whose members serve as nonintegrin collagen receptors. Mol Cell. 1997;1:25–34. doi: 10.1016/s1097-2765(00)80004-0. [DOI] [PubMed] [Google Scholar]
- Shull MM, Ormsby I, Kier AB, Pawlowski S, Diebold DJ, Yin M, Allen R, Sidman C, Proetzel G, Calvin D, Annunziata N, Doetschman T. Targeted disruption of the mouse transforming growth factor-β1 gene results in multifocal inflammatory disease. Nature. 1992;359:693–699. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalli O, Ropraz P, Trzeciak A, Benzonana G, Gillessen D, Gabbiani G. A monoclonal antibody against α-smooth muscle actin: a new probe for smooth muscle differentiation. J Cell Biol. 1986;103:2787–2796. doi: 10.1083/jcb.103.6.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P. Abnormal kidney development and hematological disorders in PDGF β-receptor mutant mice. Genes Dev. 1994;8:1888–1896. doi: 10.1101/gad.8.16.1888. [DOI] [PubMed] [Google Scholar]
- Sporn MB, Roberts AB. Transforming growth factor β: recent progress and new challenges. J Cell Biol. 1992;119:1017–1021. doi: 10.1083/jcb.119.5.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streuli CH, Schmidhauser C, Kobrin M, Bissel MJ, Derynck R. Extracellular matrix regulates expression of the TGFβ1 gene. J Cell Biol. 1993;120:253–260. doi: 10.1083/jcb.120.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taipale J, Keski-Oja J. Growth factors in the extracellular matrix. FASEB (Fed Am Soc Exp Biol)J. 1997;11:51–59. doi: 10.1096/fasebj.11.1.9034166. [DOI] [PubMed] [Google Scholar]
- Vogel W, Gish GD, Alves F, Pawson T. The discoidin domain receptor tyrosine kinases are activated by collagen. Mol Cell. 1997;1:13–23. doi: 10.1016/s1097-2765(00)80003-9. [DOI] [PubMed] [Google Scholar]
- Xia P, Culp LA. Adhesion activity in fibronectin's alternatively spliced domain ED-A (EIIIA) and its neighboring type III repeats: oncogene-dependent regulation. Exp Cell Res. 1994;213:253–265. doi: 10.1006/excr.1994.1197. [DOI] [PubMed] [Google Scholar]
- Xu, G., M. Redard, G. Gabbiani, P. Neuville. Cellular retinol-binding protein-1 is transiently expressed in granulation tissue fibroblasts and differentially expressed in fibroblasts cultured from different organs. Am. J. Pathol. 151: 1741–1749. [PMC free article] [PubMed]
- Zambruno G, Marchisio PC, Marconi A, Vaschieri C, Melchiori A, Giannetti A, De Luca M. Transforming growth factor-β1 modulates β1 and β5 integrin receptors and induces de novo expression αvβ6 heterodimer in normal keratinocytes: implication for wound healing. J Cell Biol. 1995;129:853–865. doi: 10.1083/jcb.129.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zardi L, Siri A, Carnemolla B, Cosulich E, Viale G, Santi L. A simplified procedure for the preparation of antibodies to serum fibronectin. J Immunol Methods. 1980;34:155–165. doi: 10.1016/0022-1759(80)90169-6. [DOI] [PubMed] [Google Scholar]
- Zhang K, Rekhter MD, Gordon D, Phan SH. Myofibroblasts and their role in lung collagen gene expression during pulmonary fibrosis. A combined immunohistochemical and in situhybridization study. Am J Pathol. 1994;145:114–125. [PMC free article] [PubMed] [Google Scholar]