Protein Kinase C–Dependent Mobilization of the α6β4 Integrin from Hemidesmosomes and Its Association with Actin-Rich Cell Protrusions Drive the Chemotactic Migration of Carcinoma Cells (original) (raw)
Abstract
We explored the hypothesis that the chemotactic migration of carcinoma cells that assemble hemidesmosomes involves the activation of a signaling pathway that releases the α6β4 integrin from these stable adhesion complexes and promotes its association with F-actin in cell protrusions enabling it to function in migration. Squamous carcinoma-derived A431 cells were used because they express α6β4 and migrate in response to EGF stimulation. Using function-blocking antibodies, we show that the α6β4 integrin participates in EGF-stimulated chemotaxis and is required for lamellae formation on laminin-1. At concentrations of EGF that stimulate A431 chemotaxis (∼1 ng/ml), the α6β4 integrin is mobilized from hemidesmosomes as evidenced by indirect immunofluorescence microscopy using mAbs specific for this integrin and hemidesmosomal components and its loss from a cytokeratin fraction obtained by detergent extraction. EGF stimulation also increased the formation of lamellipodia and membrane ruffles that contained α6β4 in association with F-actin. Importantly, we demonstrate that this mobilization of α6β4 from hemidesmosomes and its redistribution to cell protrusions occurs by a mechanism that involves activation of protein kinase C-α and that it is associated with the phosphorylation of the β4 integrin subunit on serine residues. Thus, the chemotactic migration of A431 cells on laminin-1 requires not only the formation of F-actin–rich cell protrusions that mediate α6β4-dependent cell movement but also the disruption of α6β4-containing hemidesmosomes by protein kinase C.
Keywords: integrins, cell movement, PKC, hemidesmosomes, cytoskeleton
Chemotactic migration is essential for embryonic development, tissue homeostasis, and the immune response 29 36 55. It is also a major factor in the pathogenesis of many human diseases 21 30 54. This complex and poorly understood process involves dynamic and coordinated interactions among integrins, chemoattractant receptors and the actin cytoskeleton. The net result of these interactions is localized actin polymerization in the direction of the chemoattractant gradient coupled with the establishment of traction forces necessary for migration 14 36 52. The chemotactic migration of epithelial-derived cells is of particular significance to the mechanism of wound healing and carcinoma invasion 21 30 54. However, the mechanisms involved in epithelial migration are not well understood. For example, an explanation needs to be provided for how integrin function is altered in response to stimuli that induce epithelial migration. At the very least, the hypothesis can be formulated that integrin function differs in stably adherent epithelial cells in comparison to migrating epithelial cells such as those cells at a wound edge or in an invasive carcinoma.
The α6β4 integrin is ideal for studying differences in integrin function between stably adherent and migrating epithelial cells. This integrin, which is a receptor for the laminins (reviewed in 34), is essential for the organization and maintenance of epithelial architecture. In many epithelia, α6β4 mediates the formation of stable adhesive structures termed hemidesmosomes that link the intermediate filament cytoskeleton with the extracellular matrix 4 23. In addition to α6β4, the classical hemidesmosome contains at least three other known proteins: BPAG-11, BPAG-2, and HD1/plectin 23. A second type of hemidesmosome has been described that contains only α6β4 and HD1/plectin 57. Recent studies have provided evidence that α6β4 is linked to intermediate filaments through HD1/plectin, and that this interaction is critical for hemidesmosomal formation 37 45 47. BPAG-1 has also been shown to link the hemidesmosome to intermediate filaments 24. The importance of the α6β4 integrin in hemidesmosome function and epithelial architecture has been reinforced by the generation of β4-nullizygous mice. The most obvious defect in these mice is a loss of hemidesmosomes and detachment of the epidermis 16 58. In striking contrast to its function in normal epithelia, α6β4 can stimulate carcinoma migration and invasion through its ability to interact with the actin cytoskeleton and mediate the formation and stabilization of lamellae 44. This dynamic function of α6β4 in enhancing the migration of invasive carcinoma cells is quite distinct from its role in maintaining stable adhesive contacts in normal epithelia by associating with intermediate filaments. In fact, we have established that the ability of α6β4 to stimulate carcinoma migration and invasion depends upon its activation of distinct signaling pathways including PI3-K 51 and cAMP-specific phosphodiesterase 39. In essence, our studies have defined an integrin-mediated mechanism of carcinoma invasion that involves the stimulation of chemotactic migration by the association of α6β4 with F-actin and the activation of a specific signaling pathway by this integrin.
The above observations raise the important issue of whether migratory stimuli influence the localization and cytoskeletal interactions of α6β4. Such changes could provide a mechanism to account for the dichotomy of α6β4 function in stably adherent and migrating cells. To address this issue, we used squamous carcinoma-derived A431 cells for several reasons. A431 cells express α6β4, as well as the EGF receptor that is known to stimulate their chemotactic migration and in vitro invasion 32. In addition, a previous report indicated that activation of the EGF receptor can disassemble hemidesmosomes in 804G bladder carcinoma cells 31. The results obtained in our study indicate that EGF, at concentrations that stimulate A431 chemotaxis, mobilizes the α6β4 integrin from hemidesmosomes and increases the formation of α6β4-containing lamellipodia and membrane ruffles. Importantly, we also demonstrate that this mobilization of α6β4 occurs by a protein kinase C (PKC)–dependent mechanism, and that it involves the phosphorylation of the β4 integrin subunit on serine residues.
Materials and Methods
Cells and Reagents
The A431 squamous carcinoma cell line was obtained from the American Type Culture Collection and maintained in DMEM with 10% fetal calf serum, at 37°C in a humidified atmosphere containing 5% CO2.
The following antibodies were used in this study: mouse mAb 2B7 (integrin α6–specific) was prepared in our laboratory 50; rat GoH3 mAb (integrin α6–specific) was purchased from Immunotech; rat mAb 439-9B (integrin β4–specific; reference 18) was provided by Dr. Rita Falcioni (Regina Elena Cancer Institute, Rome, Italy). A peptide-specific antiserum elicited against the last 20 amino acids of the carboxy terminus of the β4 subunit was prepared commercially. A rabbit polyclonal antibody specific for the EGF receptor was purchased from Santa Cruz Biotechnology. The phosphotyrosine-specific antibodies PY20 and 4G10 were purchased from Transduction Labs and Upstate Biotechnology Incorporated, respectively. Mouse monoclonal antibodies specific for BPAG-1 (R185), BPAG-2 (1D1), and HD1/plectin (121) 26 40 were a gift of Dr. Owaribe (Nagoya University, Japan). Anti-actin polyclonal antibody, pan-cytokeratin mAb, rat IgG, and mouse IgG were purchased from Sigma Chemical.
Laminin-1, prepared from the EHS sarcoma was provided by Dr. Hynda Kleinman (NIDR, Bethesda, MD). Collagen type I was purchased from Collagen Corp. Human recombinant EGF was purchased from Sigma Chemical. The PKC inhibitor Gö6976 was obtained from Alexis Corp. PMA was obtained from Calbiochem-Novabiochem.
Chemotaxis Assays
Chemotaxis was analyzed using 6.5-mm Transwell™ chambers, 8-μm pore size (Costar). The separating membrane was coated with laminin-1 (20 ug/ml) on both sides for 2 h at room temperature and then blocked with 1% albumin in PBS for 30 min. A431 cells (3 × 104) were resuspended in DMEM containing 0.1% albumin. In some experiments, antibodies (10 μg/ml of 2B7 or mouse IgG control) were added to the resuspended cells. The cells were added to the top wells of the Transwell™ chambers and allowed to settle on the filters for 1 h at 37°C, before EGF (1 ng/ml) was added to the lower chamber. In some cases, Gö6976 (1 μM) or vehicle alone (DMSO) was added 30 min before EGF stimulation. After a 2-h incubation, cells that had not migrated from the upper surface of the membrane were removed using cotton swabs and the remaining cells on the lower side of the membrane were fixed in methanol, dried, and stained with a 0.2% solution of crystal violet in 2% ethanol. Migration was quantified by digital analysis as described below.
Analysis of Lamellar Area
A431 cells were plated on laminin-1 for 1 h and either not stimulated or stimulated with EGF (1 ng/ml) for 15 min in the presence or absence of antibody (2B7 or IgG, 10 μg/ml). The antibodies were added 30 min before EGF stimulation. The lamellar area, defined as a characteristic flat and thin protrusion of the cell containing no vesicles, was measured using a Nikon Diaphot 300 inverted microscope with phase contrast optics. This microscope was connected to a CCD camera (Dage-MTI), a frame-grabber (Scion), and a 7600 Power Macintosh computer to capture the images. Images were collected and analyzed with IPlab Spectrum image analysis software. Lamellar area was determined by tracing the lamellae contour and quantifying the area digitally. For each individual experiment 50–80 cells were analyzed.
Indirect Immunofluorescence Microscopy
Bacteriological dishes were coated with 20 μg of laminin-1 or collagen type I for 2 h at room temperature and the dishes were then blocked with PBS containing 1% bovine serum albumin (BSA) for 30 min. A431 cells were resuspended in serum-free RPMI 1640 medium containing 10 mM Hepes and 0.1% BSA. The cells were plated at low density (2 × 104 cells/cm2) on the matrix-coated dishes and allowed to adhere for 1–2 h in a humidified atmosphere with 5% CO2 at 37°C. When indicated, Gö6976 (0.5–1 μM) in DMSO or vehicle alone was added 30 min before stimulation. The cells were then stimulated with either EGF (0.5–100 ng/ml) for 15 min or PMA (25–50 ng/ml) for 30 min.
Cells were fixed with a buffer containing 2% paraformaldehyde, 100 mM KCl, 200 mM sucrose, 1 mM EGTA, 1 mM MgCl2, 1 mM PMSF, and 10 mM Pipes at pH 6.8 for 15 min. In some cases, the cells were extracted before fixation with a buffer containing 0.2% Triton X-100, 100 mM KCl, 200 mM sucrose, 10 mM EGTA, 2 mM MgCl2, 200 μM sodium vanadate, 1 mM PMSF, and 10 mM Pipes at pH 6.8 for 1 min. After fixation, the cells were rinsed with PBS and incubated with a blocking solution that contained 1% albumin and 5% goat serum in PBS for 30 min. Cells that were to be stained for HD1/plectin, BPAG-1 or BPAG-2, were fixed with acetone/methanol 1:1 (vol/vol) instead of paraformaldehyde. Either primary antibodies or FITC phalloidin (20 μg/ml) in blocking solution were added to the fixed cells separately or in combination for 30 min. The cells were rinsed three times and either a fluorescein-conjugated donkey anti–mouse or a rhodamine-conjugated donkey anti–rat IgG (minimal cross-reaction inter-species; Jackson ImmunoResearch Laboratories) in blocking buffer (1:150) were used separately or in combination to stain the cells for 30 min. Cells were rinsed with PBS and mounted in a mixture (8:2) of glycerol and PBS (pH 8.5) containing 1% propylgallate. The dishes were cut into slides and examined by confocal microscopy.
Detergent Extractions
To obtain a fraction enriched in cytokeratins 9 19 35, A431 cells (2 × 106) were incubated on laminin-1–coated dishes as described above for 1–2 h. In some cases, Gö6976 (1 μM) or vehicle alone was added 30 min before stimulation. EGF (0.5–2 ng ml) was added and the cells incubated at 37°C for an additional 15 min. The cells were initially extracted with a buffer containing 0.2% Triton X-100, 100 mM KCl, 200 mM sucrose, 10 mM EGTA, 2 mM MgCl2, 200 μM sodium vanadate, 1 mM PMSF, and 10 mM Pipes at pH 6.8 for 1 min (membrane/soluble fraction). The cells were rinsed several times before adding a second buffer containing 1% Tween-40, 0.5% deoxycholate, 10 mM NaCl, 2 mM MgCl2, 20 mM Tris-HCl, pH 7.5, for 10 min (cytoskeletal fraction). The cells were rinsed and the residual fraction was extracted with a third buffer containing SDS 0.4%, 10 mM NaCl, 20 mM Tris-HCl, pH 7.5, sonicated and then boiled before adding Triton X-100 to a final concentration of 1% (vol/vol; cytokeratin fraction). The samples were immunoprecipitated with the β4-specific polyclonal antibody, resolved by SDS-PAGE (6 or 8%) and immunoblotted with the same polyclonal antibody. Immune complexes were detected using a secondary antibody conjugated to horseradish peroxidase and visualized by enhanced chemiluminescence (Amersham, Inc.).
Analysis of Protein Phosphorylation
To examine tyrosine phosphorylation using phosphotyrosine-specific antibodies, A431 cells were plated on laminin-1–coated dishes for 1 h at 37°C as described above. Cells were then stimulated with EGF (1–100 ng/ml) for 15 min, extracted with RIPA buffer containing 0.1% SDS, 1% Triton X-100, 0.5% deoxycholate, 150 mM NaCl, 1 mM EGTA, 1 mM EDTA, 50 mM sodium pyrophosphate, 100 mM sodium fluoride, 1 mM sodium vanadate, 1 mM PMSF, 10 ug/ml each of leupeptin, pepstatin A, and aprotinin, and 50 mM Tris-HCl, pH 7.5. The samples were immunoprecipitated using the 439-9B antibody, resolved by SDS-PAGE and immunoblotted using a combination of both the PY20 and 4G10 phospho-specific antibodies. Immune complexes were detected using a secondary antibody conjugated to horseradish peroxidase and visualized by enhanced chemiluminescence (Amersham, Inc.). Subsequently, the membranes were stripped and immunoblotted with the β4-specific polyclonal antibody.
For metabolic radiolabeling with 32PO4, A431 cells (2 × 106) were plated on laminin-1–coated dishes for 30 min as described above. Subsequently, the medium was removed and replaced with a phosphate-deficient medium (GIBCO). After a 1-h incubation in this medium, 32PO4 [0.5–2.0 mCi/ml (NEN)] was added and the cells were incubated for an additional 2 h. When indicated, Gö6976 (0.5–1 μM) in DMSO or vehicle alone was added 30 min before stimulation. The cells were then stimulated with either EGF (0.5–100 ng/ml) for 15 min or PMA (25–50 ng/ml) for 30 min. The cells were extracted with RIPA buffer as described above and the extracts were immunoprecipitated with 439-9B antibody, resolved by PAGE (6% gels) and transferred to PVDF membranes (Immobilon-P; Micropore). The membranes were exposed to x-ray film, developed, and then immunoblotted with the β4-specific polyclonal antibody to control for equivalent amounts of protein in the samples. A quantitative analysis of the relative intensities of the radioactive bands was made using a 2D electronic counter (Instant Imager; Packard, Meriden, CT). For phosphoamino-acid analysis, the area of the membrane that contained the β4 subunit was excised with a razor, acid hydrolyzed, and the hydrolysate was separated using 2D-thin layer chromatography following standard techniques 49 and exposed to x-ray film.
Construction, Analysis, and Expression of PKC-α cDNAs
Wild-type and myristoylated PKC-α constructs were generated by PCR using a bovine PKC-α cDNA as a template. The wild-type PKC-α was subcloned into the pCMV5 mammalian expression vector using the EcoRI and XhoI sites. The FLAG epitope (DYKDDDDK) was added to the carboxy terminus of the PKC-α cDNA by PCR. The myristoylated PKC-α cDNA was constructed by adding the relevant PCR fragment of bovine PKC-α to the XbaI and EcoRI sites of pCMV6 and by adding the Src myristoylation site (MYPYDVPDYA) at the amino terminus. All sequences were confirmed by DNA sequencing.
To assess the activity of the PKC-α cDNAs, human embryonic kidney 293T cells were transfected with 1 μg of either the vector alone (pCMV5), PKC-α-FLAG cDNA, or myristoylated PKC α-FLAG cDNA using calcium phosphate for 6 h. Subsequently, the cells were cultured in the absence of serum for 24 h and extracted in a 1% NP-40 buffer 13. The PKC-α proteins were immunoprecipitated using a FLAG M2 mAb (Sigma Chemical Co.) and a mixture of protein A and G beads. The beads were washed stringently and immune complex kinase assays were performed on the washed beads using MBP as the substrate (see reference 13 for details). No lipid cofactors were added to the reaction mix. The kinase assay was resolved on a 12.5% SDS gel and phosphorylated MBP was detected by autoradiography. Relevant expression of PKC-α was detected by immunoblotting total cell extracts with a PKC-α specific polyclonal Ab (Santa Cruz).
To analyze the effects of PKC-α expression on A431 cells, these cells were transfected with 5 μg of each construct using Superfect (Qiagen) according to manufacturer's guidelines. After 24 h, the cells were fixed as described above and double stained with anti-FLAG mAb and either GoH3 mAb or mAb 121.
Results
EGF Stimulation of A431 Cells Redistributes the Localization of the α6β4 Integrin from Hemidesmosomes to Lamellipodia and Membrane Ruffles
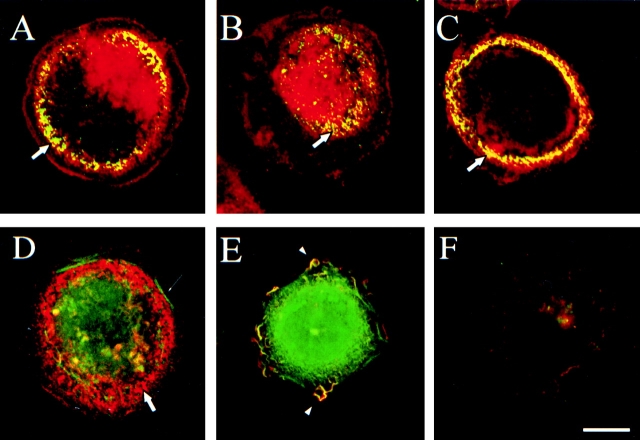
Indirect immunofluorescence microscopy revealed that the α6β4 integrin on the ventral surface of A431 cells plated on either laminin-1 (Fig. 1A–D) or collagen (not shown) is localized primarily in discrete structures and plaques in areas that exclude stress fibers. This pattern of staining for α6β4 is characteristic of its localization in hemidesmosomes 11 46. Moreover, a distinct colocalization of α6β4 with the hemidesmosomal components BPAG-1 (Fig. 1 A), BPAG-2 (Fig. 1 B), and HD1/plectin (Fig. 1 C) was evident in these structures. These data establish that α6β4 is localized in structures that are characteristic of hemidesmosomes on the ventral surface of adherent A431 cells.
Figure 1.
The α6β4 integrin redistributes from hemidesmosomes to lamellipodia and ruffles after EGF stimulation. Indirect immunofluorescence analysis of A431 cells plated on laminin-1–coated dishes for 2 h and either stimulated with EGF (E and F; 1 ng/ml) or left without treatment (A–D). All cells were processed for double-staining with the α6-specific rat mAb GoH3 (red) and either a mouse mAb specific for one of the following hemidesmosome components (green): BPAG-1 (A), BPAG-2 (B), HD1/plectin (C and F), or (green) FITC-phalloidin (D and E). In these photomicrographs, the yellow color is indicative of colocalization of two antigens. All images correspond to confocal sections taken at the basal plane of the cell except E that was taken at a suprabasal plane. Thick arrows indicate hemidesmosomes, arrowheads indicate lamellipodia and ruffles, and thin arrow indicates F-actin stress fibers. Bar, 10 μm.
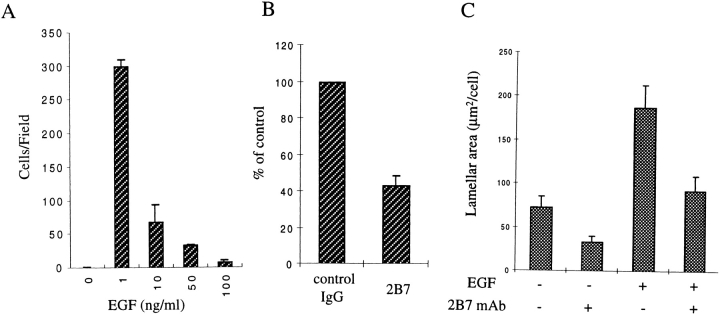
Our previous data established that α6β4 participates in carcinoma migration through its ability to interact with the actin cytoskeleton 44. For this reason, we examined the involvement of this integrin in the chemotactic migration of A431 cells towards EGF because these cells express high levels of the EGF receptor and this growth factor is known to stimulate their migration 32 53. As shown in Fig. 2 A, relatively low concentrations of EGF (1 ng/ml) stimulated a robust migration response in A431 cells. However, EGF did not stimulate significant migration when used at higher concentrations (>10 ng/ml) (Fig. 2 A). The effect of EGF on cell migration was mostly chemotactic in nature because the chemokinetic element represented <20% of the total migration (measured as the migration occurring in the presence of EGF in both chambers to disrupt gradients at the optimal dose of 1 ng/ml; data not shown). The fact that A431 cells express α6β4 and no α6β1 integrin (1, 17, and data not shown) enabled us to use function-blocking, α6-subunit specific antibodies to examine the contribution of α6β4 to A431 chemotaxis. Treatment of A431 cells with the 2B7 mAb inhibited chemotaxis toward EGF on laminin-1 by 60% (Fig. 2 B). This mAb did not inhibit the attachment of the cells to laminin-1 (data not shown), indicating a distinct function for α6β4 in the chemotactic migration of A431 cells. Lamellipodial protrusions are thought to be critical in cell migration and are the basis for generating lamellae, which are larger protrusions that are associated with the direction the cell migrates 36 52. Indeed, A431 cells displayed a striking increase in lamellipodia and ruffle formation for sustained periods of time in response to treatment with EGF at a concentration that stimulate optimal chemotaxis (1 ng/ml) (Fig. 1 E). High concentrations of EGF (>5 ng/ml), which were inefficient in stimulating chemotaxis, caused the cells to round up quickly after a short period of protrusive activity, leaving behind numerous retraction fibers (data not shown). For this reason, we used low concentrations of EGF to stimulate A431 cells in subsequent experiments (0.5–2 ng/ml). The importance of α6β4 in the formation of such protrusions is supported by the fact that the lamellar area of A431 cells was substantially reduced by pretreatment with the 2B7 mAb before plating on laminin-1 (Fig. 2 C).
Figure 2.
The α6β4 integrin functions in EGF-induced chemotaxis and lamellae formation of A431 cells on laminin-1. (A) The optimal concentration of EGF that stimulates chemotaxis was determined in a 2-h assay using a modified Boyden chamber coated with laminin-1 as described in Materials and Methods. Data represent the mean number of cells/microscopic field (± SE) that migrated through the separating membrane. (B) The ability of the α6-specific mAb 2B7 or IgG control to inhibit the chemotactic migration of A431 cells toward EGF (1 ng/ml) was analyzed using the method described in A. Data shown represent the percentage of cells that migrated relative to the control. (C) A431 cells were plated on laminin-1 for 1 h and either not stimulated or stimulated with EGF (1 ng/ml) for 15 min in the presence of 10 μg of either 2B7 Ab or control IgG (-). The antibodies were added 30 min before EGF stimulation. The lamellar area was analyzed using an image analysis system as described in Materials and Methods. The data shown represent the mean lamellar area ± SE.
The findings that α6β4 is localized in hemidesmosomes in adherent A431 cells and that EGF stimulated their α6β4-dependent migration raised the possibility that EGF also altered the localization and cytoskeletal interactions of this integrin. Under these conditions of EGF stimulation, a striking change in the localization of α6β4 was apparent. Specifically, this integrin was substantially reduced in hemidesmosomes on the ventral surface (Fig. 1D and Fig. E, and Fig. 7, left panels), but it was readily apparent in the lamellipodia and ruffles that are formed in response to EGF stimulation (Fig. 1 E). We observed also that EGF stimulation results in a reduction of HD1/plectin staining in hemidesmosomes, indicating a disassembly of hemidesmosome structure (Fig. 1 F).
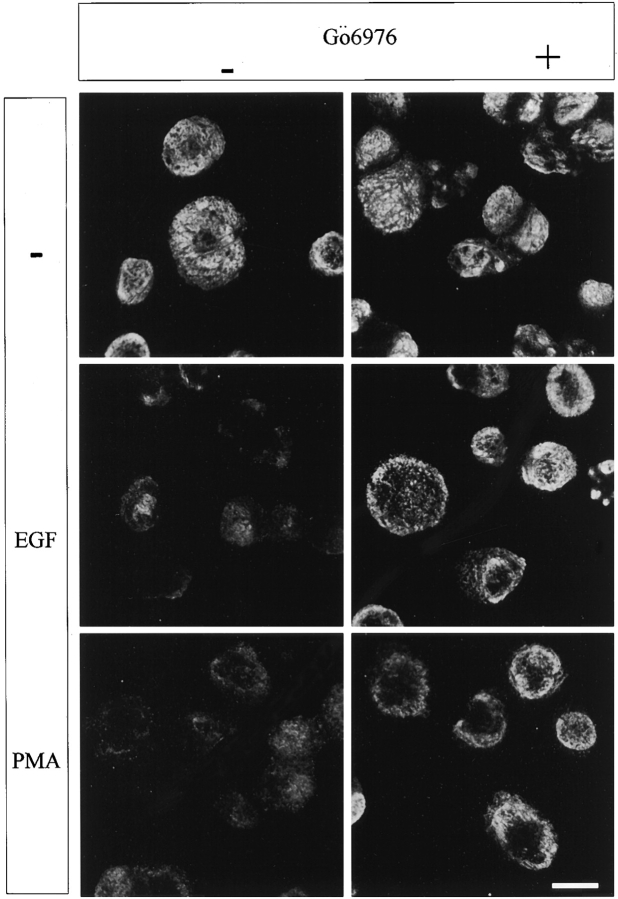
Figure 7.
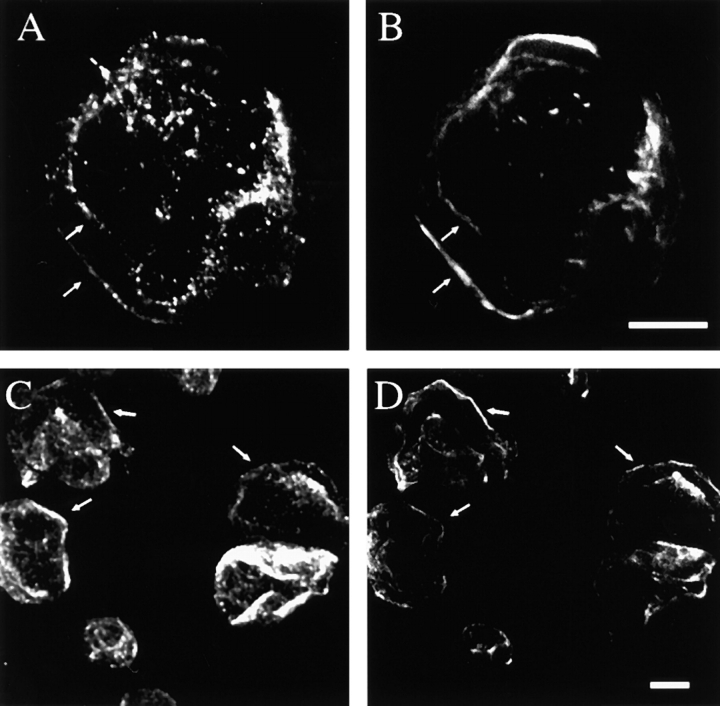
Inhibition of conventional PKCs blocks the EGF/PMA-induced redistribution of α6β4 from hemidesmosomes. A431 cells plated on laminin-1 for 2 h were incubated with Gö6976 (1 μM) or vehicle (DMSO) alone for 30 min before stimulating with EGF (1 ng/ml) for 15 min or PMA (25 ng/ml) for 30 min. The cells were extracted with Triton X-100 buffer before fixation to enhance visualization of hemidesmosomes. The fixed cells were stained with the α6-specific antibody GoH3 and a rhodamine-conjugated anti–rat IgG, and then analyzed using indirect immunofluorescence. Confocal images shown were taken at the basal surface of the cells. Bar, 10 μm.
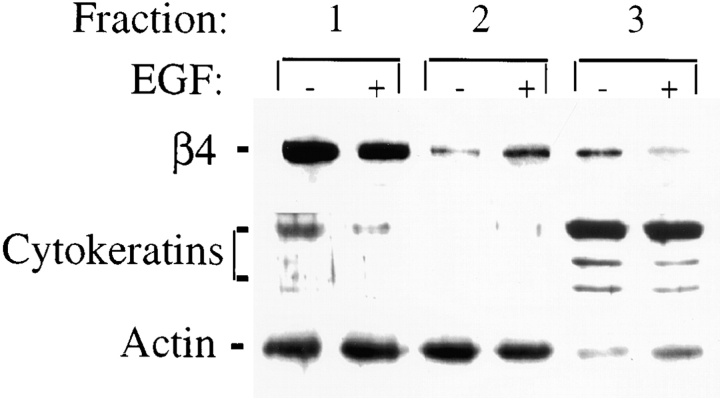
Our observation that α6β4 is redistributed from hemidesmosomes to lamellipodia and membrane ruffles in response to EGF stimulation prompted us to examine its association with cytokeratins and F-actin in more detail using an in situ extraction scheme that solubilizes proteins to an extent that correlates with their cytoskeletal associations 9 19. Specifically, membrane, actin, and cytokeratin fractions were obtained from A431 cells that had been either left untreated or stimulated with EGF using sequentially a Triton X-100 buffer (fraction 1, membrane), a two-detergent buffer (1.0% Tween-40/0.5% deoxycholate, fraction 2, actin) that removes the bulk of the actin cytoskeleton but not cytokeratins and associated proteins, and a third buffer containing SDS that solubilizes cytokeratins and associated proteins (fraction 3, cytokeratin; references 9, 19). The relative amount of α6β4 present in each fraction was detected by immunoprecipitation and subsequent immunoblotting with β4-specific antibodies. The relative distribution of actin and cytokeratin among the three fractions was also determined to assess the efficiency of the fractionation (Fig. 3). As expected, the cytokeratins were present largely in fraction 3 and actin was distributed between fractions 1 and 2, which represent the G-actin and F-actin pools, respectively. Importantly, EGF stimulation did not alter this relative distribution of cytoskeletal proteins among the three fractions. However, as shown in Fig. 3, EGF stimulation resulted in a substantial reduction in the amount of α6β4 in fraction 3 (cytokeratin) and an increase in the amount of α6β4 in the actin fraction in comparison to unstimulated cells. Densitometric analysis of the β4-specific bands in this figure revealed an approximate 63% reduction of α6β4 in the cytokeratin fraction and a 48% increase in the actin fraction. These observations provide evidence that the mobilization of α6β4 from hemidesmosomes that we detected by indirect immunofluorescence microscopy is associated with a disruption in its association with cytokeratins and an increase in its association with F-actin.
Figure 3.
EGF promotes the release of α6β4 integrin from an insoluble cytokeratin fraction and increases its association with an actin fraction. A431 cells were plated on laminin-1 for 1 h and either left untreated or stimulated with EGF (1 ng/ml) for 15 min. The cells were then sequentially extracted to obtain membrane, actin, and cytokeratin fractions (fractions 1, 2, and 3, respectively) as described in Materials and Methods. After solubilization, α6β4 was immunoprecipitated from each fraction using the GoH3 mAb, resolved by SDS-PAGE and detected by immunoblotting using a β4-specific polyclonal antibody. Actin and cytokeratins were detected in each fraction by immunoblotting using an anti-actin polyclonal and pan-cytokeratin monoclonal Abs, respectively. Note that the β4 subunit is detected as a doublet under these conditions.
The localization of α6β4 in membrane ruffles and lamellipodia that form in response to EGF stimulation prompted us to explore the possibility of its association with F-actin in these structures because we had previously observed such an association in colon carcinoma cells 44. We found that the colocalization of α6β4 with F-actin in cell protrusions detected by immunofluorescence was retained in a significant number of protrusions after extraction of EGF-stimulated cells with a Triton X-100 buffer that preserves the actin cytoskeleton (Fig. 4A and Fig. B). However, extraction of these EGF-stimulated cells with the Tween-40/DOC buffer described above eliminated both the F-actin and α6β4 staining (data not shown). Taken together, these findings indicate that EGF stimulates a dissociation of α6β4 from cytokeratin-associated hemidesmosomes, as well as the formation of lamellipodia and ruffles that contain α6β4 in association with F-actin.
Figure 4.
The α6β4 integrin associates with F-actin in response to EGF. A431 cells were plated on laminin-1 for 1 h before stimulation with EGF (1 ng/ml) for 15 min. The cells were extracted with a Triton X-100–containing buffer and then fixed as described in Materials and Methods. The fixed cells were double-stained for indirect immunofluorescence analysis using (A and C) GoH3 mAb and (B and D) FITC-phalloidin. Arrows indicate points of colocalization. Bars, 10 μm.
The α6β4 Integrin Colocalizes with the EGF Receptor and Phosphotyrosine in Membrane Ruffles and Lamellipodia
Our findings that EGF stimulation mobilizes α6β4 from hemidesmosomes and promotes α6β4-dependent chemotaxis suggested a possible association between α6β4 and the EGF receptor. To address this possibility, EGF-stimulated A431 cells were stained for both α6β4 and the EGF receptor. As shown in Fig. 5 A, a striking colocalization of these two receptors was evident in membrane ruffles and lamellipodia. The specificity of this colocalization is evidenced by the finding that another surface protein, the HLA antigen, was not present in these F-actin–rich structures (data not shown). We were unable, however, to detect a specific, physical association between α6β4 and the EGF receptor by coimmunoprecipitation (data not shown).
Figure 5.
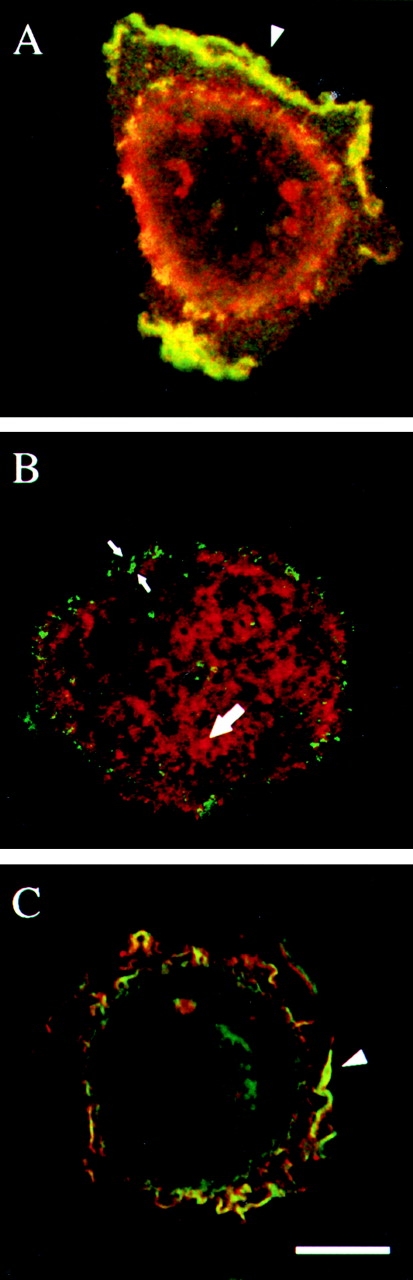
The α6β4 integrin colocalizes with the EGF receptor and phosphotyrosine in ruffles and lamellipodia but not in hemidesmosomes. A431 cells were plated on laminin-1 for 1 h and then either stimulated with EGF (1 ng/ml) for 15 min (A and C) or left untreated (B). All cells were processed for double-staining with the α6-specific rat mAb GoH3 (red) and mouse Abs specific for either the EGFR (A, green) or phosphotyrosine (PY20; B and C, green). Yellow depicts colocalization of two antigens. Arrowheads indicate colocalization in lamellipodia and ruffles, and small arrows indicate focal adhesion-like structures. Large arrow indicates hemidesmosome area. Bar, 10 μm.
Given the fact that the EGF receptor is a tyrosine kinase and the report that EGF stimulation of A431 cells results in a substantial increase in the tyrosine phosphorylation of the β4 integrin subunit 31, we explored the distribution of phosphotyrosine in both EGF-stimulated, as well as unstimulated, A431 cells using indirect immunofluorescence. In unstimulated cells, most of the phosphotyrosine staining on the ventral surface is seen at the cell periphery in radial arrangements similar to focal adhesions (Fig. 5 B). A consistent colocalization of either α6β4 (Fig. 5 B) or EGFR (data not shown) with phosphotyrosine in these structures was not evident. Moreover, significant phosphotyrosine staining was not evident in hemidesmosomes (Fig. 5 B). EGF stimulation, however, resulted in the colocalization of phosphotyrosine and α6β4 in lamellipodia and ruffles (Fig. 5 C). From these results, we can conclude that α6β4 is associated with more phosphotyrosine-containing proteins in cell protrusions than in hemidesmosomes.
EGF Stimulation Induces the Phosphorylation of the β4 Integrin Subunit on Serine Residues
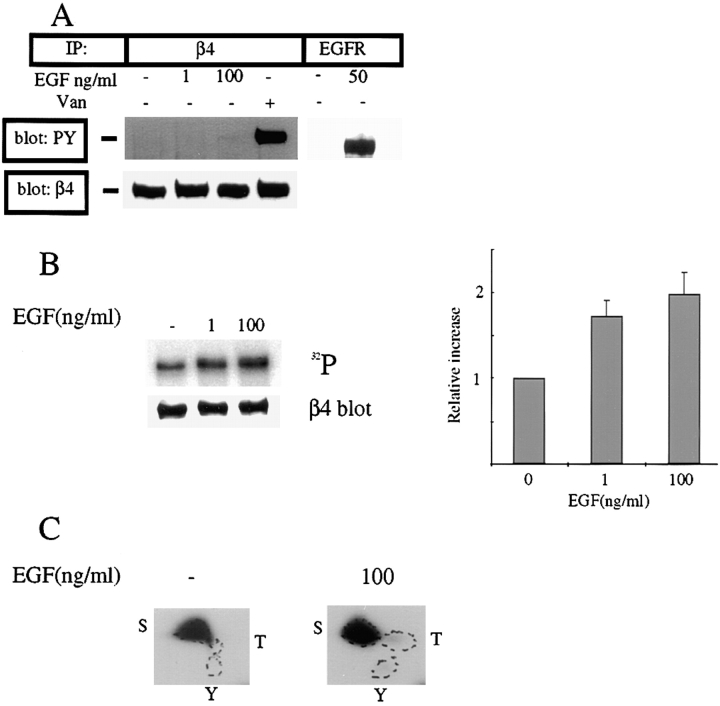
The colocalization of α6β4 with phosphotyrosine in the lamellipodia and ruffles of EGF-stimulated A431 cells prompted us to examine the phosphorylation of α6β4 induced by EGF. For this purpose, α6β4 was immunoprecipitated from stimulated A431 cells with the 439-9B mAb and the immunoprecipitates were blotted with two phosphotyrosine-specific Abs (PY20 and 4G10). In these experiments, the cells were extracted with RIPA buffer because we observed a nonspecific interaction between α6β4 and the EGFR using a Triton X-100 buffer (data not shown). Under these conditions, we detected no phosphotyrosine in the β4 subunit in response to the concentration of EGF (1 ng/ml) that induced α6β4 redistribution to lamellipodia and ruffles, and that stimulated optimal A431 chemotaxis (Fig. 6 A). Moreover, even high concentrations of EGF (100 ng/ml) that induced a rapid rounding-up of adherent A431 cells and did not stimulate chemotaxis (Fig. 2 A) induced only a marginal increase, at best, in the phosphotyrosine content of the β4 subunit as detected by these antibodies (Fig. 6 A). Similar results were obtained with other α6 and β4-specific mAbs including GoH3, 2B7, A9, as well as a β4-specific polyclonal antibody (data not shown). These findings are in contrast to the report that EGF stimulation induces a substantial increase in the tyrosine phosphorylation of the β4 subunit in A431 cells 31.
Figure 6.
EGF stimulates serine phosphorylation of the β4 subunit. (A) Analysis of tyrosine phosphorylation using phosphotyrosine-specific antibodies. A431 cells were plated on laminin-1 for 1 h and then either stimulated with EGF (1–100 ng/ml) for 15 min or left untreated. Detergent (RIPA) extracts were immunoprecipitated using the β4 antibody 439-9B, resolved by SDS-PAGE, and immunoblotted using a combination of the PY20 and 4G10 phosphotyrosine-specific antibodies as described in Materials and Methods. Positive controls shown for tyrosine phosphorylation are the EGF receptor immunoprecipitated from EGF-stimulated A431 cells and α6β4 immunoprecipitated from A431 cells that had been pretreated with pervanadate before extraction. Both immunoprecipitates were blotted with PY20 and 4G10 as described. (B) 32PO4 metabolic labeling and analysis of EGF-stimulated A431 cells. A431 cells plated on laminin-1 were labeled with 32PO4 as described in Materials and Methods, and stimulated with EGF for 15 min at the concentrations indicated. Cells were then extracted, immunoprecipitated with 439-9B, blotted with the β4-specific polyclonal antibody, and exposed to x-ray film (left panel). The relative intensities of the radiolabeled β4 bands were quantified using an electronic counter as shown in the bar graph in the right panel. The data shown were obtained from three separate experiments (± SE). After exposure, the membranes were probed with the β4-specific antibody to corroborate equal loading of the samples (left panel). (C) Phosphoamino-acid analysis of 32PO4-labeled β4. The radiolabeled band that corresponded to the β4 subunit obtained from cells stimulated with either 0 or 100 ng/ml EGF was excised from the PVDF membrane, acid hydrolyzed, and then separated by two-dimensional thin-layer electrophoresis as described in Materials and Methods. The plate was then exposed to x-ray film. S, phosphoserine; T, phosphothreonine; Y, phosphotyrosine.
To exclude the possibility that the phosphotyrosine-specific Abs we used were unable to detect significant tyrosine phosphorylation of the β4 subunit after EGF stimulation, A431 cells were labeled metabolically with 32P-orthophosphate and then stimulated with EGF. As shown in Fig. 6 B, EGF stimulation did increase the phosphorylation of the β4 subunit substantially with half-maximal phosphorylation observed at ∼1 ng/ml of EGF. The discrepancy between the phosphotyrosine-specific Ab results and the metabolic labeling results prompted us to do a phosphoamino acid analysis of the radiolabeled β4 subunit. Surprisingly, we found that the β4 subunit is phosphorylated almost exclusively on serine (Fig. 6 C). Indeed, both the basal and EGF-induced increases in β4 phosphorylation that we detected in Fig. 6 B result from serine phosphorylation (Fig. 6 C). This phosphoamino-acid analysis in conjunction with the phosphotyrosine antibody data provide convincing evidence that EGF stimulation induces significant phosphorylation of the β4 subunit on serine but not tyrosine residues.
Activation of PKC Redistributes α6β4 from Hemidesmosomes to Cell Protrusions and Induces Phosphorylation of the β4 Subunit on Serine Residues
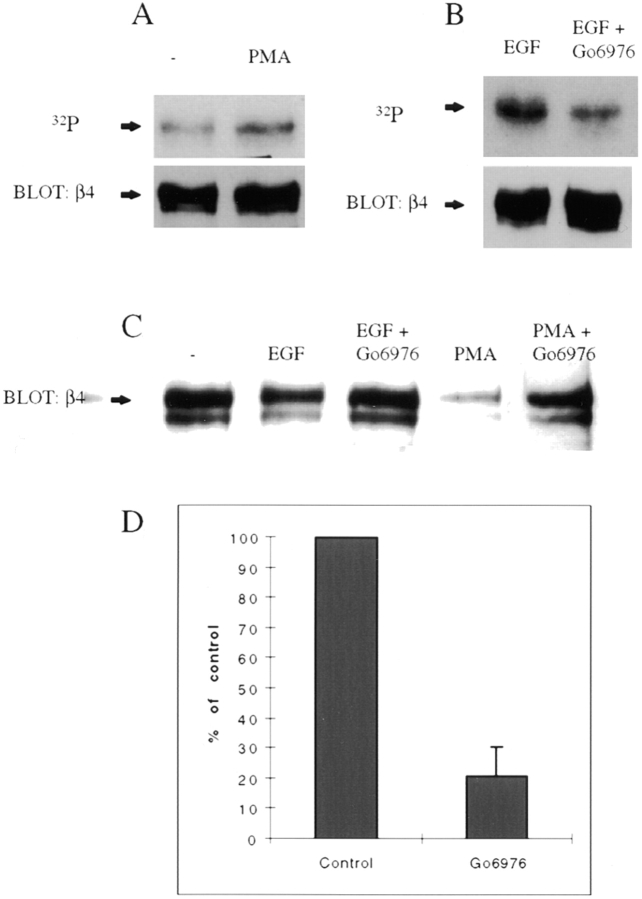
The above findings indicated that EGF activates a serine protein kinase that is involved in β4 phosphorylation and that could also be involved in the redistribution of α6β4 from hemidesmosomes to lamellipodia and membrane ruffles. We hypothesized that a likely candidate for this kinase is PKC because its activation by EGF is well documented 2. As an initial test of this hypothesis, we examined the effect of PMA stimulation on α6β4 localization in A431 cells. PMA stimulation mobilized α6β4 from hemidesmosomes (Fig. 7, left panels), and increases the formation of α6β4-containing lamellipodia and ruffles (data not shown) as assessed by indirect immunofluorescence microscopy. These data were substantiated biochemically by analyzing the amount of β4 that remains associated with the cytokeratin fraction after stimulation with PMA, using the detergent extraction procedure described above. PMA stimulation markedly reduced the amount of α6β4 in the cytokeratin fraction in comparison to unstimulated cells (Fig. 8 C). Consistent with a role of PKC-dependent phosphorylation in the redistribution of α6β4, we found that PMA stimulation itself increased the phosphorylation of the β4 subunit significantly as assessed by 32P-orthophosphate labeling (Fig. 8 A). The fact that we detected no tyrosine phosphorylation of β4 in response to PMA stimulation using the phosphotyrosine-specific antibodies (data not shown) indicates that the increase in 32P-orthophosphate labeling can be attributed to serine phosphorylation.
Figure 8.
Protein kinase C mediates the effects of EGF on β4 serine phosphorylation, release of α6β4 from a cytokeratin fraction, and chemotaxis. (A) A431 cells plated on laminin-1 were labeled with 32PO4 as described in Materials and Methods and then stimulated with either PMA or vehicle (DMSO) alone. Cells were then extracted, immunoprecipitated with 439-9B mAb, blotted with the β4-specific antibody, and exposed to x-ray film. (B) A431 cells plated on laminin-1 were labeled with 32PO4 and then incubated with either Gö6976 (1 μM) or vehicle (DMSO) alone for 30 min before stimulation with EGF (100 ng/ml) for 15 min. The cells were then extracted and processed as in A. In A and B, the lower panel is an immunoblot analysis of β4 content that verifies equal loading of the samples. (C) Inhibition of EGF-induced release of α6β4 from a cytokeratin fraction. A431 cells plated on laminin-1 were incubated with Gö6976 (1 μM) or vehicle (DMSO) alone for 30 min before stimulating with EGF (1 ng/ml) for 15 min or PMA (50 ng/ml) for 30 min. The cells were then extracted with a buffer that solubilizes the actin cytoskeleton but preserves the cytokeratin network. After solubilization, α6β4 was immunoprecipitated from this residual fraction, resolved by SDS-PAGE, and detected by immunoblotting using a β4-specific polyclonal antibody as described in Materials and Methods. (D) Gö6976 inhibits EGF-stimulated chemotactic migration; chemotaxis was assessed in a modified Boyden chamber as described in Fig. 2. Gö6976 (1 μM) or vehicle alone was added 30 min before stimulation with EGF (1 ng/ml).
If PKC activation is required for the mobilization of α6β4 from hemidesmosomes, inhibition of PKC activity should inhibit this process. To establish this causality, we used Gö6976, an inhibitor of the conventional isoforms of PKC (α, β, γ) 33 42. The effects of this inhibitor on α6β4 localization were profound. As shown in Fig. 7Gö6976 blocked the release of α6β4 from hemidesmosomes on the ventral surface of A431 cells in response to either EGF or PMA stimulation. Consistent with a role for serine phosphorylation in the release of α6β4 from hemidesmosomes, we found that Gö6976 reduced the EGF-stimulated phosphorylation of the β4 subunit by ∼50% (Fig. 8 B), a reduction that corresponds to the level of β4 phosphorylation observed in the absence of EGF stimulation (see Fig. 6 B). Moreover, Gö6976 inhibited the EGF-induced dissociation of α6β4 from the cytokeratin fraction as assessed by the detergent extraction approach described above (Fig. 8 C). This inhibition was evident for both EGF and PMA-stimulated cells (Fig. 8 C).
The above data suggested the participation of a conventional PKC isoform in the disassembly of the hemidesmosome and mobilization of α6β4 integrin. To obtain additional evidence for PKC involvement, we examined the possibility that activation of PKC-α, a widely distributed conventional PKC isoform, was sufficient to disassemble hemidesmosomes in the absence of EGF stimulation. For this purpose, we constructed a constitutively active PKC-α cDNA that contained the Src myristoylation site at its amino terminus. This myristoylated PKC-α exhibited a high level of in vitro kinase activity relative to the wild-type enzyme (Fig. 9). Subsequently, we expressed both the myristoylated and wild-type PKC-α cDNAs in A431 cells and analyzed the effect of these cDNAs on hemidesmosome structure. As shown in Fig. 10A431 cells that expressed myristoylated PKC-α, as evidenced by expression of the epitope tag (FLAG), showed a striking reduction in hemidesmosomes. More specifically, expression of both HD-1 and α6β4 was markedly reduced on the basal surface of these cells. In contrast, the cells that expressed the wild-type PKC-α, showed little change in the formation of hemidesmosomes. These results suggest that activation of PKC-α is sufficient to cause redistribution of the α6β4 integrin and other components of the hemidesmosome.
Figure 9.
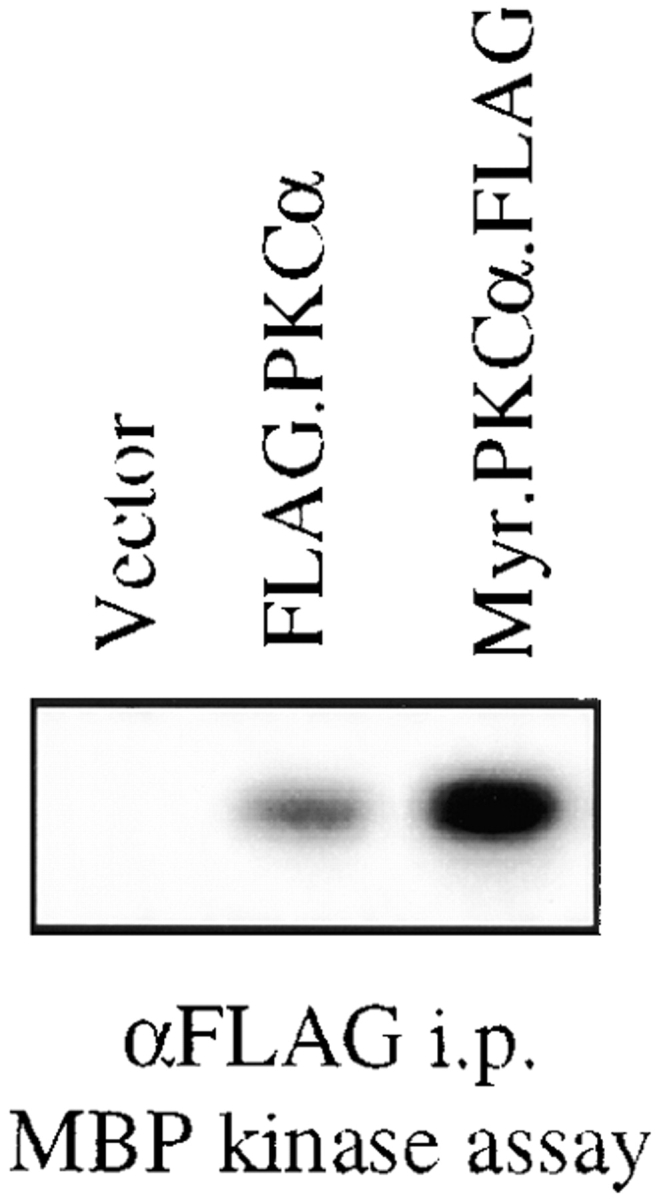
Analysis of wild-type and myristoylated PKC-α kinase activity. 293T cells were transfected with either the pCMV5 vector alone, the PKC-α-FLAG cDNA, or the myristoylated PKC-α-FLAG cDNA as described in Materials and Methods. These PKC proteins were immunoprecipitated using a FLAG-specific Ab. Expression of PKC-α was confirmed by immunoblotting these precipitates with a PKC-α specific Ab and this information was used to normalize PKC-α expression for the kinase assays (data not shown). Immune complex kinase assays were performed using MBP as the substrate as described in Materials and Methods.
Figure 10.
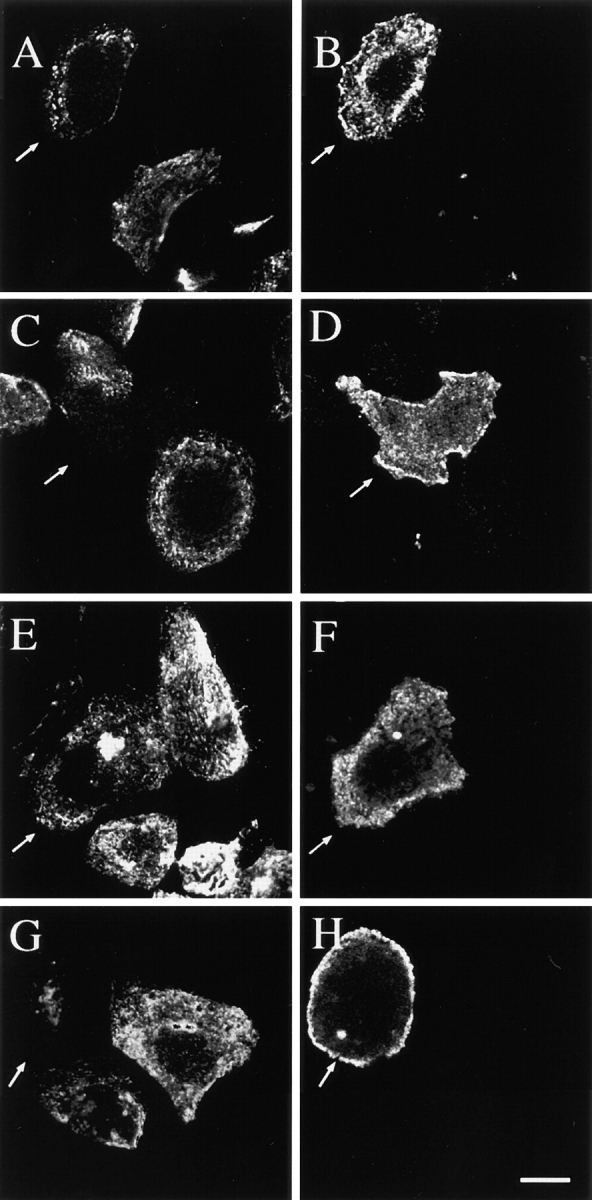
Activation of PKC-α is sufficient to induce disassembly of the hemidesmosome and release of α6β4 integrin. A431 cells were transfected with the PKC-α-FLAG cDNA (A, B, E, and F) or the myristoylated PKC-α-FLAG cDNA (C, D, G, and H) as described in Materials and Methods. After 24 h, the cells were fixed and the presence of hemidesmosomes was assessed by double immunofluorescence using either mAb 121 (HD-1; A and C) and an anti-FLAG mAb (B and D), or GoH3 mAb (α6β4; E and G) and an anti-FLAG mAb (F and H). Arrows indicate FLAG-positive cells. Note the absence of hemidesmosomes in cells that express the myristoylated PKC-α. Bar, 10 μm.
PKC Is Essential for α6β4-mediated Chemotaxis
A key implication of the above findings is that PKC activity is required for EGF-stimulated chemotaxis of A431 cells because the PKC-dependent redistribution of α6β4 is a necessary event in the mechanism of chemotaxis. We tested this implication by analyzing the effect of Gö6976 on the chemotactic response of A431 cells to EGF. The results in Fig. 8 D reveal that Gö6976 inhibited EGF-stimulated chemotaxis by >80%. It is important to note that Gö6976 at the concentrations used did not inhibit the attachment or spreading of A431 cells (see Fig. 7). These results support the involvment of PKC in the chemotactic signal elicited by EGF, and they substantiate the importance of a regulated and dynamic redistribution of the α6β4 integrin in chemotactic migration.
Discussion
The data we present provide insight into the mechanism of cell migration, especially the migration of epithelial and carcinoma cells. Recent work by our group has established that α6β4 participates in the chemotactic migration of carcinoma cells by interacting with F-actin at their leading edges and regulating essential signaling pathways 39 44 51. This function underlies the contribution of this integrin to carcinoma invasion (reviewed in 43). An issue that needed to be resolved, however, is the relationship between α6β4 function in the hemidesmosomes of epithelial-derived cells and its ability to promote the migration of these cells. Epithelial cells use hemidesmosomes to anchor to the basal lamina. These multi-protein structures connect the substratum with cytokeratins to form rigid adhesion complexes. The α6β4 integrin is an essential component of hemidesmosomes and it is necessary for mediating their adhesive function 4 16 23. Given this ability of α6β4-containing hemidesmosomes to generate stable adhesive contacts, it is reasonable to assume that their function needs to be disrupted to facilitate cell migration similar to the disruption of focal contacts and reduction in the strength of cell-substratum adhesion that occurs during fibroblast migration 41 59. In fact, there is evidence that epithelial cells lose their hemidesmosomes when they are induced to migrate in response to wounding 22 46, and that metastatic carcinoma cells often lack hemidesmosomes 43. Also, the results obtained in our study were foreshadowed in a previous study that found that EGF stimulation disrupted α6β4-associated hemidesmosomes in 804G bladder carcinoma cells 31. Our findings demonstrate that a chemotactic stimulus will not only disassemble hemidesmosomes but also promote the formation of α6β4-containing lamellipodia and membrane ruffles, thus establishing a mechanism for the dichotomy of α6β4 function in stably adherent and migrating epithelial-derived cells. An important implication of this model is that chemotactic factors can drive the migration of invasive carcinoma cells by mobilizing α6β4 and disassembling hemidesmosomes.
The redistribution of α6β4 from hemidesmosomes to lamellipodia and ruffles that we observed in response to EGF stimulation, implied the existence of EGF-mediated signaling events responsible for this redistribution. Indeed, an important finding in this study is that redistribution is dependent on the activity of PKC and that it is associated with serine phosphorylation of the β4 subunit. The serine phosphorylation of β4 by EGF stimulation provided an important clue in our identification of the kinase activity involved in the redistribution of α6β4. It is well established that EGF can activate PKC through the PLC-γ–mediated formation of diacylglycerol and inositol trisphosphate 25 38. In fact, there is evidence that PLC-γ participates in EGF-stimulated cell migration 12, as well as in the motility induced by other growth factors such as PDGF 28 and IGF-1 3. Based on this knowledge, we found that PMA is able to mimic the effect of EGF by altering the localization of α6β4 from hemidesmosomes to lamellipodia and ruffles and stimulating the phosphorylation of the β4 subunit on serine residues.
Most likely, the conventional PKC isoform, PKC-α, is involved in the redistribution of α6β4 and the disassembly of hemidesmosomes. Gö6976, a specific inhibitor of the conventional PKC isoforms (α, β, γ) 33 42, was able to impede the mobilization of α6β4 from hemidesmosomes and inhibit the EGF-stimulated phosphorylation of the β4 subunit. Indeed, hemidesmosomes were well preserved in EGF-stimulated A431 cells that had been pretreated with Gö6976. Consistent with the notion that the preservation of hemidesmosomes impedes cell migration, Gö6976 also inhibited the chemotactic response of A431 cells to EGF. In addition to these data, we observed that activation of PKC-α by expression of a constitutively active, myristoylated form of the enzyme, was sufficient to induce the redistribution of α6β4 and disassembly of hemidesmosomes.
An issue that remains to be addressed is the nature of the signaling pathway that links the EGF receptor to activation of PKC-α and the mobilization of α6β4 from hemidesmosomes. As mentioned above, PLC-γ is a likely intermediary 12 25 38. However, we observed that the PLC-γ inhibitor U73122 (1 μM) had rather drastic effects on the morphology of A431 cells and could not be used to assess the involvement of this phospholipase in the dynamic behavior of α6β4. Similarly, other widely used inhibitors such as wortmannin induced morphological changes in A431 cells even in the absence of EGF stimulation. We did observe, however, a partial inhibition of the EGF-induced redistribution of α6β4 with the MEK inhibitor PD98059 (data not shown). Although this observation needs to be established more rigorously, it does suggest the possible involvement of the MAP kinase pathway in the EGF-induced mobilization of α6β4 from hemidesmosomes. Interestingly, PD98059 can abrogate EGF-induced focal adhesion disassembly and cell motility in fibroblasts 59.
Although we cannot exclude a role for tyrosine phosphorylation of the β4 subunit in the EGF-induced chemotaxis of A431 cells, the data we obtained argue strongly against such a role primarily because we did not detect tyrosine phosphorylation of β4 using EGF at concentrations that promote optimal chemotaxis. In fact, when we used much higher concentrations of EGF (100 ng/ml) we detected an additional increase in β4 serine phosphorylation, but only a marginal increase, at best, in β4 tyrosine phosphorylation. These results contrast with a previous study by Mainiero et al. 31 that reported a striking increase in the tyrosine phosphorylation of the β4 subunit in A431 cells stimulated with high concentrations of EGF (10–250 ng/ml). At this point, we are unable to explain the discrepancies between our findings and those of Mainiero et al. 31. It is worth noting, however, that in our study, the use of such high concentrations of EGF to stimulate A431 cells failed to induce chemotaxis and caused the cells to round-up, making it difficult to correlate phosphorylation of β4 with cell migration under these conditions.
The EGF-stimulated phosphorylation of the β4 subunit on serine residues that we identified is novel and it provides an impetus for investigating the nature of this phosphorylation and its role in regulating the cytoskeletal interactions of the α6β4 integrin in more detail. For example, the extremely large β4 cytoplasmic domain contains multiple consensus motifs for PKC phosphorylation based on our analysis of the human β4 cDNA sequence using Prosite (data not shown). The presence of these motifs supports the possibility that PKC may phosphorylate α6β4 directly. It is also worth considering the possibility that PKC may activate another downstream serine kinase that is involved in β4 phosphorylation because there are also consensus sites in the β4 sequence for other serine kinases such as casein kinase II. Another important issue to be studied is whether phosphorylation of the β4 subunit is essential for either its mobilization from hemidesmosomes or its recruitment into lamellipodia and ruffles. The data we provide in this study provide a strong correlation of serine phosphorylation with these events. Definitive proof of involvement will require identification of the specific serine residue(s) in the β4 subunit that are phosphorylated by EGF stimulation and the subsequent mutational analysis of these sites. The possibility that PKC-dependent serine phosphorylation also influences other components of hemidesmosomes should be considered. For example, there is evidence that PKC can mobilize BPAG-2 from hemidesmosomes 27, and that it can phosphorylate HD1/plectin and weaken its interaction with intermediate filaments 20.
The EGF-induced release of α6β4 from hemidesmosomes and its association with F-actin in lamellipodia and membrane ruffles are probably independent events. This idea is derived from our finding that Gö6976, an inhibitor of the conventional PKC isoforms, did not block the EGF-induced formation of α6β4-containing membrane ruffles and lamellipodia (data not shown), even though it prevented α6β4 mobilization from hemidesmosomes (Fig. 7). This observation suggests that the PKC-dependent mobilization of α6β4 from hemidesmosomes is independent of the recruitment of α6β4 into the lamellipodia and ruffles that are formed in response to EGF stimulation. As we have shown, however, Gö6976 did inhibit EGF-induced chemotactic migration. Collectively, these findings underscore the hypothesis that the increased formation of cell protrusions in the form of ruffles and lamellipodia is not enough to generate movement and that the destabilization of hemidesmosome function mediated by PKC is an essential component of the migration process.
An interesting issue that arises is the role that EGF plays in the recruitment of α6β4 to the lamellipodia and ruffles. Most likely, EGF stimulates the formation of new cell protrusions that contain α6β4 rather than promoting the preferential incorporation of α6β4 into such structures. This assumption is based on our finding that the few lamellipodia that form in the absence of EGF do incorporate α6β4, and they have a similar intensity of α6β4 expression as those lamellipodia that are formed in response to EGF stimulation (for example, see Fig. 1). Thus, we suggest that α6β4 is transported to and concentrated at the leading edges by mechanisms intrinsic to lamellipod formation, and that EGF increases the number of protrusions while providing a pool of α6β4 liberated from hemidesmosomes. One possible mechanism by which α6β4 could be recruited to the leading edge is exemplified by the transient association of small aggregates of β1 integrins with the actin cytoskeleton that occurs in neuronal growth cones. These aggregates of β1 integrins are transported on the dorsal surface in a directed way to the leading edge 48. Although we do not have direct data to support a transient association of α6β4 with the actin cytoskeleton, the fact that only a fraction of the α6β4 in lamellipodia is resistant to extraction with a Triton X-100 buffer (e.g., compare the extracted lamellipodia of Fig. 4 to the unextracted ones of Fig. 5) may be the reflection of a dynamic equilibrium attained by the constant association and dissociation of α6β4 with F-actin. Another model for the recruitment of membrane proteins into motile structures involves the concentration of recycling proteins in ruffles by directed exocytosis induced by EGF through a Rac-dependent pathway 7. In this model, the ability of membrane proteins to be recycled is essential for their recruitment into ruffles. This model is of particular interest to our findings because there is evidence that α6β4 is recycled on the cell surface 6, and we have implicated Rac in the α6β4-dependent migration and invasion of carcinoma cells 51. Moreover, the recycling of the population of α6β4 that is released from hemidesmosomes by EGF could provide a pool for newly forming ruffles and lamellipodia.
If the mobilization of α6β4 from hemidesmosomes constitutes one essential component of the EGF-stimulated migration of A431 cells, the second component of migration in which α6β4 participates is the actual process of migration itself. Indeed, a function for α6β4 in A431 migration is supported by its localization in lamellipodia and ruffles and, more directly, by our finding that an α6-specific mAb inhibited both chemotactic migration and lamellae formation on laminin-1. These results are consistent with our previous work on colon carcinoma cells that established a role for α6β4 in the formation and stabilization of lamellae and filopodia 44. In this study, we observed that protruding filopodia, which anchor to the substrate using α6β4, are frequently followed by the extension of the lamella towards the anchoring point. This function of α6β4 may relate to the concept of a molecular clutch that anchors actin bundles to the substrate providing traction tracks for myosin II motors 36. Because a retrograde flow of actin filaments from the periphery towards the nucleus occurs in most motile cells, the net effect of this clutch action on actin polymerization would also be to generate a forward protrusive force, as has been recently shown in a growth cone model 56. In addition to these mechanical functions, we have defined at least two distinct signaling pathways regulated by α6β4 that are essential for lamellae formation, chemotactic migration and invasion of carcinoma cells. These pathways involve PI3-kinase/Rac 51 and a cAMP-specific phosphodiesterase 39. It is also worth noting that we have observed apparent ligand-independent effects of α6β4 on lamellae formation and chemotactic migration. For example, expression of α6β4 in a breast carcinoma cell line stimulates lamellae formation and chemotaxis on collagen in response to a chemoattractant, processes that are not inhibited by α6-specific Abs 39. The implication of these findings is that α6β4 can regulate key signaling pathways independently of its adhesive functions.
The colocalization of the EGFR, α6β4, and phosphotyrosine in membrane ruffles and lamellipodia suggests the presence of an active signaling complex involved in cell migration. In fact, there is evidence that the activated form of the EGFR is localized in ruffles and lammelipodia, and that these structures are also sites of PLC-γ activation stimulated by EGF 10 15. Several other EGFR substrates, such as ezrin, spectrin, and calpactin II are recruited to membrane ruffles and have been detected in their phosphorylated form in these structures 5 8. The fact that we observed a striking colocalization of α6β4 with the EGF receptor in lamellipodia and ruffles suggests that these two receptors may interact to facilitate their ability to signal chemotactic migration. This possibility, which remains to be demonstrated for α6β4 and the EGFR, is supported by the recent finding that the α6β4 and α6β1 integrins associate with the erbB-2 receptor in several carcinoma cell lines 18. Nonetheless, it is evident from our data that activation of the EGFR has a profound effect on the localization of α6β4, and the close proximity of these two receptors in F-actin–rich areas, along with the other signaling molecules mentioned above, suggests cooperation in their signaling of chemotactic migration.
In summary, our findings describe a mechanism for the chemotactic migration of carcinoma cells that assemble hemidesmosomes. An important implication of our findings is that the mobilization of the α6β4 integrin from hemidesmosomes by a PKC-dependent mechanism, is an essential step in the migration process, presumably because it releases the strong and stable adhesion mediated by hemidesmosomes and allows for the dynamic adhesive interactions that are required for migration. Importantly, we also demonstrate that the α6β4 integrin can associate with F-actin in lamellipodia and membrane ruffles, and participate in the migration process itself. Collectively, our results explain how this integrin can mediate both stable adhesion and cell migration. They also suggest that growth and motility factors that are known to promote tumor progression may function, in part, by changing the cytoskeletal interactions and localization of this unique receptor.
Acknowledgments
We thank Bao-Kim Nguyen for technical help. Valuable discussions were had with Robin Bachelder, Rita Falcioni, Kathy O'Connor, and Leslie Shaw.
This work was supported by National Institutes of Health grants CA80789 and CA44704, and the Harvard Digestive Diseases Center.
Footnotes
1.used in this paper: BPAG, bullous pemphigoid antigen; EGFR, EGF receptor; PKC, protein kinase C; PLC, phospholipase C; PI3K, phosphoinositide 3-OH kinase
References
- Ardini E., Tagliabue E., Magnifico A., Buto S., Castronovo V., Colnaghi M.I., Menard S. Co-regulation and physical association of the 67-kDa monomeric laminin receptor and the α6β4 integrin. J. Biol. Chem. 1997;272:2342–2345. doi: 10.1074/jbc.272.4.2342. [DOI] [PubMed] [Google Scholar]
- Boonstra J., Rijken P., Humbel B., Cremers F., Verkleij A., van Bergen en Henegouwen P. The epidermal growth factor. Cell Biol. Int. 1995;19:413–430. doi: 10.1006/cbir.1995.1086. [DOI] [PubMed] [Google Scholar]
- Bornfeldt K.E., Raines E.W., Nakano T., Graves L.M., Krebs E.G., Ross R. Insulin-like growth factor-I and platelet-derived growth factor-BB induce directed migration of human arterial smooth muscle cells via signaling pathways that are distinct from those of proliferation. J. Clin. Invest. 1994;93:1266–1274. doi: 10.1172/JCI117081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borradori L., Sonnenberg A. Hemidesmosomes—roles in adhesion, signaling and human diseases. Curr. Opin. Cell Biol. 1996;8:647–656. doi: 10.1016/s0955-0674(96)80106-2. [DOI] [PubMed] [Google Scholar]
- Bretscher A. Rapid phosphorylation and reorganization of ezrin and spectrin accompany morphological changes induced in A-431 cells by epidermal growth factor. J. Cell Biol. 1989;108:921–930. doi: 10.1083/jcb.108.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher M.S. Circulating integrinsα5β1, α6β4 and Mac-1, but not α3β1, α4β1 or LFA-1. EMBO (Eur. Mol. Biol. Organ.) J. 1992;11:405–410. doi: 10.1002/j.1460-2075.1992.tb05068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher M.S., Aguado-Velasco C. EGF induces recycling membrane to form ruffles. Curr. Biol. 1998;8:721–724. doi: 10.1016/s0960-9822(98)70281-7. [DOI] [PubMed] [Google Scholar]
- Campos-Gonzalez R., Kanemitsu M., Boynton A.L. Epidermal growth factor induces the accumulation of calpactin II on the cell surface during membrane ruffling. Cell Motil. Cytoskel. 1990;15:34–40. doi: 10.1002/cm.970150106. [DOI] [PubMed] [Google Scholar]
- Capco D.G., Wan K.M., Penman S. The nuclear matrixthree-dimensional architecture and protein composition. Cell. 1982;29:847–858. doi: 10.1016/0092-8674(82)90446-9. [DOI] [PubMed] [Google Scholar]
- Carpentier J.L., White M.F., Orci L., Kahn R.C. Direct visualization of the phosphorylated epidermal growth factor receptor during its internalization in A-431 cells. J. Cell Biol. 1987;105:2751–2762. doi: 10.1083/jcb.105.6.2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter W.G., Kaur P., Gil S.G., Gahr P.J., Wayner E.A. Distinct functions for integrins α3β1 in focal adhesions and α6β4/bullous pemphigoid antigen in a new stable anchoring contact (SAC) of keratinocytesrelation to hemidesmosomes. J. Cell Biol. 1990;111:3141–3154. doi: 10.1083/jcb.111.6.3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P., Xie H., Sekar M.C., Gupta K., Wells A. Epidermal growth factor receptor-mediated cell motilityphospholipase C activity is required, but mitogen-activated protein kinase activity is not sufficient for induced cell movement. J. Cell Biol. 1994;127:847–857. doi: 10.1083/jcb.127.3.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou M.M., Hou W., Johnson J., Graham L.K., Lee M.H., Chen C.S., Newton A.C., Schaffhausen B.S., Toker A. Regulation of protein kinase C zeta by PI 3-kinase and PDK-1. Curr. Biol. 1998;8:1069–1077. doi: 10.1016/s0960-9822(98)70444-0. [DOI] [PubMed] [Google Scholar]
- Condeelis J. Life at the leading edgethe formation of cell protrusions. Annu. Rev. Cell Biol. 1993;9:411–444. doi: 10.1146/annurev.cb.09.110193.002211. [DOI] [PubMed] [Google Scholar]
- Diakonova M., Payrastre B., van Velzen A.G., Hage W.J., van Bergen en Henegouwen P.M., Boonstra J., Cremers F.F., Humbel B.M. Epidermal growth factor induces rapid and transient association of phospholipase C-gamma 1 with EGF-receptor and filamentous actin at membrane ruffles of A431 cells. J. Cell Sci. 1995;108:2499–2509. doi: 10.1242/jcs.108.6.2499. [DOI] [PubMed] [Google Scholar]
- Dowling J., Yu Q.C., Fuchs E. Beta-4 integrin is required for hemidesmosome formation, cell adhesion and cell survival. J. Cell Biol. 1996;134:559–572. doi: 10.1083/jcb.134.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcioni R., Sacchi A., Resau J., Kennel S.J. Monoclonal antibody to human carcinoma-associated protein complexquantitation in normal and tumor tissue. Cancer Res. 1988;48:816–821. [PubMed] [Google Scholar]
- Falcioni R., Antonini A., Nistico P., Di Stefano S., Crescenzi M., Natali P.G., Sacchi A. α6β4 and α6β1 integrins associate with ErbB-2 in human carcinoma cell lines. Exp. Cell Res. 1997;236:76–85. doi: 10.1006/excr.1997.3695. [DOI] [PubMed] [Google Scholar]
- Fey E.G., Wan K.M., Penman S. Epithelial cytoskeletal framework and nuclear matrix-intermediate filament scaffoldthree-dimensional organization and protein composition. J. Cell Biol. 1983;98:1973–1984. doi: 10.1083/jcb.98.6.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foisner R., Traub P., Wiche G. Protein kinase A- and protein kinase C-regulated interaction of plectin with lamin B and vimentin. Proc. Natl. Acad. Sci. USA. 1991;88:3812–3816. doi: 10.1073/pnas.88.9.3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gailit J., Clark R.A. Wound repair in the context of extracellular matrix. Curr. Opin. Cell Biol. 1994;6:717–725. doi: 10.1016/0955-0674(94)90099-x. [DOI] [PubMed] [Google Scholar]
- Gipson I.K., Spurr-Michaud S., Tisdale A., Elwell J., Stepp M.A. Redistribution of the hemidesmosome components α6β4 integrin and bullous pemphigoid antigens during epithelial wound healing. Exp. Cell Res. 1993;207:86–98. doi: 10.1006/excr.1993.1166. [DOI] [PubMed] [Google Scholar]
- Green K.J., Jones J.C.R. Desmosomes and hemidesmosomes—structure and function of molecular components. FASEB J. 1996;10:871–881. doi: 10.1096/fasebj.10.8.8666164. [DOI] [PubMed] [Google Scholar]
- Guo L., Degenstein L., Dowling J., Yu Q.C., Wollmann R., Perman B., Fuchs E. Gene targeting of BPAG1abnormalities in mechanical strength and cell migration in stratified epithelia and neurologic degeneration. Cell. 1995;81:233–243. doi: 10.1016/0092-8674(95)90333-x. [DOI] [PubMed] [Google Scholar]
- Hepler J.R., Nakahata N., Lovenberg T.W., DiGuiseppi J., Herman B., Earp H.S., Harden T.K. Epidermal growth factor stimulates the rapid accumulation of inositol (1,4,5)-trisphosphate and a rise in cytosolic calcium mobilized from intracellular stores in A431 cells. J. Biol. Chem. 1987;262:2951–2956. [PubMed] [Google Scholar]
- Hieda Y., Nishizawa Y., Uematsu J., Owaribe K. Identification of a new hemidesmosomal protein, HD1a major, high molecular mass component of isolated hemidesmosomes. J. Cell Biol. 1992;116:1497–1506. doi: 10.1083/jcb.116.6.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitajima Y., Owaribe K., Nishizawa Y., Jokura Y., Yaoita H. Phorbol ester- and calcium-induced reorganization of 180-kDa bullous pemphigoid antigen on the ventral surface of cultured human keratinocytes as studied by immunofluorescence and immunoelectron microscopy. Exp. Cell Res. 1992;203:17–24. doi: 10.1016/0014-4827(92)90034-6. [DOI] [PubMed] [Google Scholar]
- Kundra V., Escobedo J.A., Kazlauskas A., Kim H.K., Rhee S.G., Williams L.T., Zetter B.R. Regulation of chemotaxis by the platelet-derived growth factor receptor-beta. Nature. 1994;367:474–476. doi: 10.1038/367474a0. [DOI] [PubMed] [Google Scholar]
- Lauffenburger D.A., Horwitz A.F. Cell migration—a physically integrated molecular process. Cell. 1996;84:359–369. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- Liotta L.A., Stracke M.L., Aznavoorian S.A., Beckner M.E., Schiffmann E. Tumor cell motility. Semin. Cancer Biol. 1991;2:111–114. [PubMed] [Google Scholar]
- Mainiero F., Pepe A., Yeon M., Ren Y.L., Giancotti F.G. The intracellular functions of α6β4 integrin are regulated by EGF. J. Cell Biol. 1996;134:241–253. doi: 10.1083/jcb.134.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malliri A., Symons M., Hennigan R.F., Hurlstone A.F.L., Lamb R.F., Wheeler T., Ozanne B.W. The transcription factor AP-1 is required for EGF-induced activation of Rho-like GTPases, cytoskeletal rearrangements, motility, and in vitro invasion of A431 cells. J. Cell Biol. 1998;143:1087–1099. doi: 10.1083/jcb.143.4.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martiny-Baron G., Kazanietz M.G., Mischak H., Blumberg P.M., Kochs G., Hug H., Marme D., Schachtele C. Selective inhibition of protein kinase C isozymes by the indolocarbazole Go 6976. J. Biol. Chem. 1993;268:9194–9197. [PubMed] [Google Scholar]
- Mercurio A.M. Laminin receptors—achieving specificity through cooperation. Trends Cell Biol. 1995;5:419–423. doi: 10.1016/s0962-8924(00)89100-x. [DOI] [PubMed] [Google Scholar]
- Messier J.M., Shaw L.M., Chafel M., Matsudaira P., Mercurio A.M. Fimbrin localized to an insoluble cytoskeletal fraction is constitutively phosphorylated on its headpiece domain in adherent macrophages. Cell Motil. Cytoskel. 1993;25:223–233. doi: 10.1002/cm.970250303. [DOI] [PubMed] [Google Scholar]
- Mitchison T.J., Cramer L.P. Actin-based cell motility and cell locomotion. Cell. 1996;84:371–379. doi: 10.1016/s0092-8674(00)81281-7. [DOI] [PubMed] [Google Scholar]
- Niessen C.M., Hulsman E.H., Rots E.S., Sanchez-Aparicio P., Sonnenberg A. Integrin α6β4 forms a complex with the cytoskeletal protein HD1 and induces its redistribution in transfected COS-7 cells. Mol. Biol. Cell. 1997;8:555–566. doi: 10.1091/mbc.8.4.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984;308:693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- O'Connor K.L., Shaw L.M., Mercurio A.M. Release of cAMP gating by the α6β4 integrin stimulates lamellae formation and the chemotactic migration of invasive carcinoma cells. J. Cell Biol. 1998;143:1749–1760. doi: 10.1083/jcb.143.6.1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owaribe K., Nishizawa Y., Franke W.W. Isolation and characterization of hemidesmosomes from bovine corneal epithelial cells. Exp. Cell Res. 1991;192:622–630. doi: 10.1016/0014-4827(91)90084-8. [DOI] [PubMed] [Google Scholar]
- Palecek S.P., Loftus J.C., Ginsberg M.H., Lauffenburger D.A., Horwitz A.F. Integrin-ligand binding properties govern cell migration speed through cell-substratum adhesiveness. Nature. 1997;385:537–540. doi: 10.1038/385537a0. [DOI] [PubMed] [Google Scholar]
- Qatsha K.A., Rudolph C., Marme D., Schachtele C., May W.S. Go 6976, a selective inhibitor of protein kinase C, is a potent antagonist of human immunodeficiency virus 1 induction from latent/low-level-producing reservoir cells in vitro. Proc. Natl. Acad. Sci. USA. 1993;90:4674–4678. doi: 10.1073/pnas.90.10.4674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovitz I., Mercurio A.M. The integrin α6β44 and the biology of carcinoma. Biochem. Cell Biol. 1996;74:811–821. doi: 10.1139/o96-087. [DOI] [PubMed] [Google Scholar]
- Rabinovitz I., Mercurio A.M. The integrin α6β4 functions in carcinoma cell migration on laminin-1 by mediating the formation and stabilization of actin-containing motility structures. J. Cell Biol. 1997;139:1873–1884. doi: 10.1083/jcb.139.7.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezniczek G.A., de Pereda J.M., Reipert S., Wiche G. Linking integrin α6β4-based cell adhesion to the intermediate filament cytoskeletondirect interaction between the beta4 subunit and plectin at multiple molecular sites. J. Cell Biol. 1998;141:209–225. doi: 10.1083/jcb.141.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddelle K.S., Hopkinson S.B., Jones J.C. Hemidesmosomes in the epithelial cell line 804Gtheir fate during wound closure, mitosis and drug induced reorganization of the cytoskeleton. J. Cell Sci. 1992;103:475–490. doi: 10.1242/jcs.103.2.475. [DOI] [PubMed] [Google Scholar]
- Sanchez-Aparicio P., Martinez de Velasco A.M., Niessen C.M., Borradori L., Kuikman I., Hulsman E.H., Fassler R., Owaribe K., Sonnenberg A. The subcellular distribution of the high molecular mass protein, HD1, is determined by the cytoplasmic domain of the integrin beta 4 subunit. J. Cell Sci. 1997;110:169–178. doi: 10.1242/jcs.110.2.169. [DOI] [PubMed] [Google Scholar]
- Schmidt C.E., Dai J., Lauffenburger D.A., Sheetz M.P., Horwitz A.F. Integrin-cytoskeletal interactions in neuronal growth cones. J. Neurosci. 1995;15:3400–3407. doi: 10.1523/JNEUROSCI.15-05-03400.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sefton, B.M. 1997. Phosphoamino Acid Analysis. In Current Protocols in Molecular Biology. John Wiley & Sons, Inc., New York. 18.3.1–8. [DOI] [PubMed]
- Shaw L.M., Mercurio A.M. Regulation of α6β1 integrin laminin receptor function by the cytoplasmic domain of the α6 subunit. J. Cell Biol. 1993;123:1017–1025. doi: 10.1083/jcb.123.4.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw L.M., Rabinovitz I., Wang H.H., Toker A., Mercurio A.M. Activation of phosphoinositide 3-OH kinase by the α6β4 integrin promotes carcinoma invasion. Cell. 1997;91:949–960. doi: 10.1016/s0092-8674(00)80486-9. [DOI] [PubMed] [Google Scholar]
- Sheetz M.P. Cell migration by graded attachment to substrates and contraction. Semin. Cell Biol. 1994;5:149–155. doi: 10.1006/scel.1994.1019. [DOI] [PubMed] [Google Scholar]
- Slieker L.J., Martensen T.M., Lane M.D. Synthesis of epidermal growth factor receptor in human A431 cells. Glycosylation-dependent acquisition of ligand binding activity occurs post-translationally in the endoplasmic reticulum. J. Biol. Chem. 1986;261:15233–15241. [PubMed] [Google Scholar]
- Stetler-Stevenson W.G., Aznavoorian S., Liotta L.A. Tumor cell interactions with the extracellular matrix during invasion and metastasis. Annu. Rev. Cell Biol. 1993;9:541–573. doi: 10.1146/annurev.cb.09.110193.002545. [DOI] [PubMed] [Google Scholar]
- Stossel T.P. On the crawling of animal cells. Science. 1993;260:1086–1094. doi: 10.1126/science.8493552. [DOI] [PubMed] [Google Scholar]
- Suter D.M., Errante L.D., Belotserkovsky V., Forscher P. The Ig superfamily cell adhesion molecule, ApCAM, mediates growth cone steering by substrate-cytoskeletal coupling. J. Cell Biol. 1998;141:227–240. doi: 10.1083/jcb.141.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uematsu J., Nishizawa Y., Sonnenberg A., Owaribe K. Demonstration of type II hemidesmosomes in a mammary gland epithelial cell line, BMGE-H. J. Biochem. 1994;115:469–476. doi: 10.1093/oxfordjournals.jbchem.a124361. [DOI] [PubMed] [Google Scholar]
- Vanderneut R., Krimpenfort P., Calafat J., Niessen C.M., Sonnenberg A. Epithelial detachment due to absence of hemidesmosomes in integrin beta-4 null mice. Nat. Genet. 1996;13:366–369. doi: 10.1038/ng0796-366. [DOI] [PubMed] [Google Scholar]
- Xie H., Pallero M.A., Gupta K., Chang P., Ware M.F., Witke W., Kwiatkowski D.J., Lauffenburger D.A., Murphy-Ullrich J.E., Wells A. EGF receptor regulation of cell motilityEGF induces disassembly of focal adhesions independently of the motility-associated PLCgamma signaling pathway. J. Cell Sci. 1998;111:615–624. doi: 10.1242/jcs.111.5.615. [DOI] [PubMed] [Google Scholar]