Follicular B Helper T Cells Express Cxc Chemokine Receptor 5, Localize to B Cell Follicles, and Support Immunoglobulin Production (original) (raw)
Abstract
Chemokines and their receptors have been identified as major regulators controlling the functional organization of secondary lymphoid organs. Here we show that expression of CXC chemokine receptor 5 (CXCR5), a chemokine receptor required for B cell homing to B cell follicles, defines a novel subpopulation of B helper T cells localizing to follicles. In peripheral blood these cells coexpress CD45RO and the T cell homing CC chemokine receptor 7 (CCR7). In secondary lymphoid organs, CD4+CXCR5+ cells lose expression of CCR7, which allows them to localize to B cell follicles and germinal centers where they express high levels of CD40 ligand (CD40L), a costimulatory molecule required for B cell activation and inducible costimulator (ICOS), a recently identified costimulatory molecule of the CD28 family. Thus, when compared with CD4+CD45RO+CXCR5− cells, CD4+CD45RO+CXCR5+ tonsillar T cells efficiently support the production of immunoglobulin (Ig)A and IgG. In contrast, analysis of the memory response revealed that long-lasting memory cells are found within the CD4+CD45RO+CXCR5− population, suggesting that CXCR5+CD4 cells represent recently activated effector cells. Based on the characteristic localization within secondary lymphoid organs, we suggest to term these cells “follicular B helper T cells” (TFH).
Keywords: CXC chemokine receptor 5, CC chemokine receptor 7, T cell homing, germinal centers, T helper cells
Introduction
To efficiently combat invading pathogens, lymphocytes continuously circulate through lymphoid and nonlymphoid tissue to mount an adaptive immune response. Lymphocyte trafficking does not occur at random, but is subjected to tightly controlled mechanisms allowing selective entry of lymphocytes into secondary lymphoid organs and into functional compartments within these organs 1. Recent data provide strong experimental evidence that chemokines and their receptors are mandatory for the homeostatic regulation of lymphocyte trafficking and functional compartmentalization of lymphoid organs 2. The majority of B and T lymphocytes have to interact with specialized high endothelial venules (HEVs) and pass through them in order to enter lymphoid organs. We recently demonstrated that CC chemokine receptor 7 (CCR7) is indispensable for the migration of naive T cells through these venules. In addition, B cells exploit CCR7 to efficiently enter LNs and Peyer's patches 3. Once B cells are within lymphoid organs, they use CXC chemokine receptor 5 (CXCR5 [4]) to follow a follicular chemokine gradient of B lymphocyte chemoattractant (BLC [5]), which guides them to the B cell–rich follicles. BLC is a ligand for CXCR5 which, according to a new classification of chemokines, has been renamed to CXCL13 6. In addition to the chemokine system, various members of the lymphotoxin (LT)/TNF family and their receptors were found to substantially participate in lymphoid organ organogenesis 7. Their effects on regular lymphoid organ architecture can be attributed, at least in part, to their ability to induce the expression of various chemokines in lymphoid organs including BLC, secondary lymphoid tissue chemokine (SLC), and EBV-induced molecule 1 ligand chemokine (ELC 8). In addition, it was recently shown that chemokines can induce a positive feedback loop on LT expression. Data derived from BLC gene-targeted mice demonstrate that BLC induces B cells to upregulate membrane-bound LTα1β2, a cytokine known to induce follicular dendritic cell (FDC) development and the expression of BLC 5. Taken together, these data demonstrate a pivotal role for CXCR5 and its ligand in directing FDCs and B cells to form primary and secondary B cell follicles.
In contrast to B cells, recently entered T cells do not migrate to follicles but stay in close contact with antigen-presenting dendritic cells (DCs) to screen these cells for presented peptides recognized by the T cell antigen receptor. In most cases, no specific peptide is encountered and the majority of T cells leave the LN within a few hours. However, if T cells are activated by antigen-presenting cells, a proliferation and differentiation program is initiated giving rise to effector T cells either migrating to places of inflammation or to the B cell follicles to participate in the formation of germinal centers (GCs [9, 10]). A fraction of activated T cells develop into memory cells, allowing a fast and effective immune response once rechallenged with the same antigen 11. Based on the expression of the chemokine homing receptor CCR7, it has been suggested that T memory cells fall into one of two pools: CCR7− effector memory T cells (TEM) endowed to migrate to inflamed tissue, and CCR7+ central memory T cells (TCM) with the potential to home to lymphoid organs 12. We now show that CXCR5+ Th cells represent a novel subpopulation of Th cells that localize to B cell follicles and GCs to provide the support that B cells need during their differentiation program in order to efficiently produce Igs.
Materials and Methods
Abs.
In addition to Abs described elsewhere 13, mAbs directed against CD95, IL-2, and IFN-γ, and mAbs specific for various human Igs were purchased from BD PharMingen. mAbs specific for human BLC/CXCL13 and ELC/CCL19 were obtained from R&D Systems; the anti-CCR7 mAb (clone 3D12) was produced in our laboratory and has been described previously 12.
Immunohistology and Flow Cytometry.
Flow cytometry and immunohistology were done as described previously 4 13. In addition, immunohistology using anti-BLC/CXCL13 mAb and anti-ELC/CCL19 was done by enhancing signals with the tyramide signal amplification system (NEN Life Science Products/Dupont).
Cytokine Production.
PBLs were isolated by Ficoll density centrifugation and stained with anti-CD4–FITC, anti-CD45RO–PE, and anti-CXCR5–biotin/streptavidin–Cychrome (clone RF8B2). Using flow cytometry (FACS Vantage™; Becton Dickinson), CD4+CD45RO+ cells were sorted into two fractions: CXCR5− and CXCR5+ cells. Sorted cells were cultured in RPMI/10% FCS (105 cells/500 μl) and stimulated with plastic-bound anti-CD3 mAb (20 μg/ml). After 24 h, cell culture supernatants were collected and stored at −80°C. The presence of various cytokines within the supernatants was analyzed using various cytokine-specific ELISAs (Endogen). Detection of intracellular cytokines was done as described elsewhere 12.
Ab Production.
As described for peripheral blood T cells above, CD4+CD45RO+CXCR5+ and CD4+CD45RO+CXCR5− cells were also isolated from tonsils. In addition, CD19+ B cells were isolated from the same tonsils by means of flow cytometry sorting. To assess the capability of both T cell subsets to support Ig secretion and isotype switching, B cells were cultured either alone or in the presence of the same number of sorted T cells for 11 d. Supernatants were collected and analyzed for the presence of various Ig isotypes using pairs of isotype-specific Abs and ELISA (BD PharMingen).
Memory Response.
Using Ficoll, PBLs were isolated from individuals who had not been immunized with tetanus toxin (TT) during the last 2 yr. Cells were plated for 2 h in RPMI/5% auto- logous serum. Nonadherent lymphocytes were withdrawn, and adherent macrophages were collected, irradiated (50 Gy), and stored on ice until further use. Lymphocytes were stained with CD4, CD45RO, and CXCR5 Abs and sorted into the two fractions described above. Sorted T cells were titrated and cocultured with irradiated autologous macrophages (100,000/well) in RPMI/5% autologous serum in the presence or absence of TT (1:800; Wyeth). In some experiments, sorted T cells were also cultured with irradiated heterologous macrophages. After 6 d of incubation, [3H]thymidine was added to the medium (0.025 μCi/well) for 18 h. Cells were harvested and the amount of incorporated [3H]thymidine was determined.
CCR7 Downregulation.
White blood cells (5 × 106/ml) were incubated at 37°C (RPMI/10% fetal bovine serum/5 mM Hepes, pH 7.4) in the presence or absence of 300 ng/ml of ELC/CCL19 (R&D Systems). After various incubation periods, aliquots were withdrawn, stored on ice, and stained with anti-CD4 and anti-CCR7 mAbs. Expression levels of CCR7 on CD4+ T cells were determined by flow cytometry.
Determination of Inducible Costimulator Expression by Reverse Transcription PCR.
Total RNA from CD4+CD45RO+ CXCR5+ cells was isolated with Trizol (Life Technologies). cDNA was generated with Superscript II reverse transcriptase (Boehringer) using random hexamer primers. Primers were designed based on the inducible costimulator (ICOS) cDNA sequence (sequence data available from EMBL/GenBank/DDBJ under accession no. AF218312). The PCR was performed using 5 U Taq polymerase, 1.5 mM MgCl, and 0.6 μM of each primer (primer A, 5′-TGCCATTCTCAGTTATCC; primer B, 5′-ACATGTATTCACCGTTAGG) in an amplifier (model 225; MJ Research) applying the following parameters: denaturation at 94°C for 4 min, and addition of Taq polymerase at 88°C followed by 35 amplification cycles with 15 s denaturation at 94°C, annealing at 55°C for 30 s, and a 1-min extension at 72°C. Using the cDNA as template, these primers generated a 298-bp product.
Results
Peripheral Blood CXCR5+ CD4 Cells Are a Subpopulation of TCM.
CXCR5 expression was studied on peripheral blood and tonsillar T cells. Flow cytometry on PBLs demonstrated that the vast majority of CD4+CXCR5+ cells coexpress CD45RO 13 and CCR7 (Fig. 1 A), thus identifying them as a subpopulation of the recently described TCM 12. In contrast, >50% of CD4+CXCR5+ cells isolated from tonsils lack CCR7, suggesting that most T cells downregulate CCR7 once they are within lymphoid organs (Fig. 1 B). As ligand-induced receptor internalization might contribute to this process, we exposed PBLs to ELC. As shown in Fig. 1 C, CCR7 gets downregulated on peripheral blood T cells to ∼50–60% of the initial levels. This observation suggests that in addition to ligand-induced receptor uptake, other mechanisms such as TCR activation contribute to CCR7 downregulation. When analyzing the expression of ELC within the tonsil, we found this chemokine expressed not only on DCs (not shown) as reported earlier 14, but also on the HEVs. High power magnification revealed that ELC polarizes to the luminal site of the HEVs (Fig. 1 D). This observation indicates that, in addition to SLC/CCL21, ELC/CCL19 may contribute to transendothelial migration.
Figure 1.
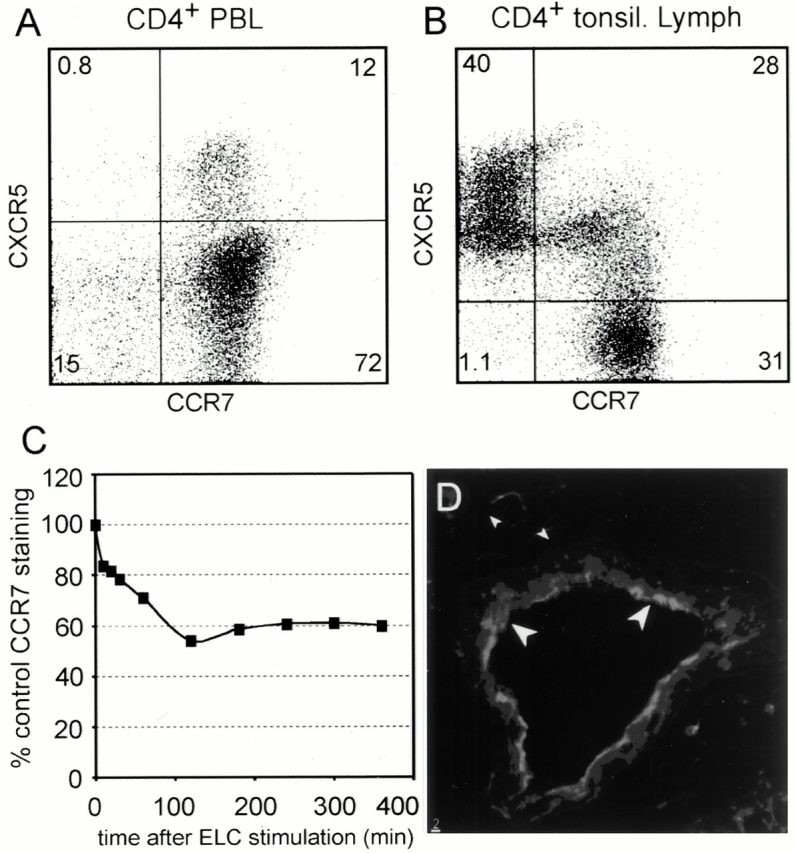
The expression of CXCR5, CCR7, and ELC/CCL19. Peripheral blood cells (A) or tonsillar cells (B) were stained with anti-CD4, anti-CXCR5, and anti-CCR7, and analyzed by flow cytometry as indicated. (C) PBLs were incubated at 37°C with 300 ng/ml ELC/CCL19. At the time points indicated, cells were removed, placed on ice, and stained with anti-CD4 and anti-CCR7 mAbs. The expression of CCR7 on CD4+ cells was analyzed and is shown as the percentage of control staining of cells not exposed to ELC. (D) Tonsillar cryostat sections were stained using anti-ELC mAb and the tyramide signal amplification. Arrowheads indicate that ELC polarized to the luminal site of HEVs.
CXCR5+ CD4 Cells Localize to B Cell Follicles and GCs to Express ICOS and CD40 Ligand.
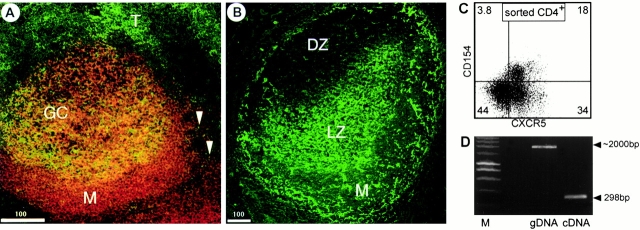
We next studied expression of CXCR5 on T cells using tonsillar cryosections applying anti-CXCR5 and anti-CD4 mAbs. CXCR5, known to be required for the formation of B cell follicles 4, is highly expressed on all B cells of the follicular mantle and on T cells that are closely associated with B cells in the light zone of GCs, the compartment of B cell affinity ma-turation. In addition, some T cells that are closely related to B cell follicles, but outside these B cell–rich areas, also express CXCR5 (Fig. 2 A), thus confirming our earlier observations 4. When using Abs specific for BLC/CXCL13, a ligand for CXCR5 that induces migration of T cells in vitro 15, we observed that expression of this chemokine is restricted to the follicular mantle and the light zone of the GC. Based on the reticular appearance of the staining, BLC+ cells seem to be mainly FDCs (Fig. 2 B).
Figure 2.
CXCR5+CD4 cells localize to follicles and express costimulatory molecules. Tonsillar cryostat sections were stained with anti-CD4 (green) and anti-CXCR5 (red; A) or with anti-BLC/CXCL13 (B). M, marginal zone; T, T zone; DZ, dark zone; LZ, light zone. (C) CD4 cells were sorted from tonsils, activated with PMA (50 ng/ml) for 15 min, transferred to ice, and stained with the Abs indicated. (D) CD4+CD45RO+CXCR5+ cells were sorted from tonsils and subjected to reverse transcription PCR using ICOS-specific primers (lane labeled cDNA). Using genomic DNA (gDNA) as a template, the PCR yielded a fragment of ∼2 kb in size, indicating an intronic sequence between the primers used and demonstrating the purity of the cDNA. M, marker.
As CXCR5+ Th cells reside in GCs, tonsillar CD4 cells were isolated by MACS and tested for the presence of intracellular stores of CD154 (CD40 ligand [CD40L]), a molecule known to be crucial for B cell activation and Ab class switching 16. After a 15-min activation with PMA (50 ng/ml)/ionomycin (800 ng/ml), a process known to translocate these molecules to the cell surface, we found a large fraction of CD4+CXCR5+ cells to express CD154 (Fig. 2 C).
It has been recently demonstrated that ICOS, a novel member of the CD28 family of costimulatory molecules, is also highly expressed on Th cells located in follicles and GCs 17. Therefore, we were interested in whether CXCR5+ CD4 cells coexpress ICOS. Indeed, using reverse transcription PCR on sorted CD4+CD45RO+ CXCR5+ tonsillar cells, we found high levels of ICOS-specific transcripts in this cell population (Fig. 2 D). In addition, preliminary data obtained by flow cytometry applying ICOS-specific Abs (provided by Dr. Kroczek, Robert Koch Institute, Berlin, Germany) demonstrate a marked correlation between the expression of CXCR5 and ICOS.
CXCR5+ CD4 Cells Produce Few Cytokines but Support Ig Production.
As CXCR5+ CD4 cells express costimulatory molecules, we were interested to see whether CXCR5− and CXCR5+ helper cells produce different sets of cytokines. According to the expression of CXCR5, peripheral blood CD4+CD45RO+ cells were sorted into two fractions: CXCR5+ and CXCR5− cells. After a 24-h stimulation period the cytokine profile was analyzed. Within the scope of our experiments we noticed no differences with regard to IL-2 production. However, we observed decreased levels of IL-10, and found dramatically reduced levels of IFN-γ, IL-5 (Fig. 3A and Fig. B), and IL-4 (not shown) in the CXCR5+ population compared with the CXCR5− fraction. These findings suggest that CD4+ CXCR5+ cells represent a non-Th1/Th2 subset of effector T cells that is involved in B cell help.
Figure 3.
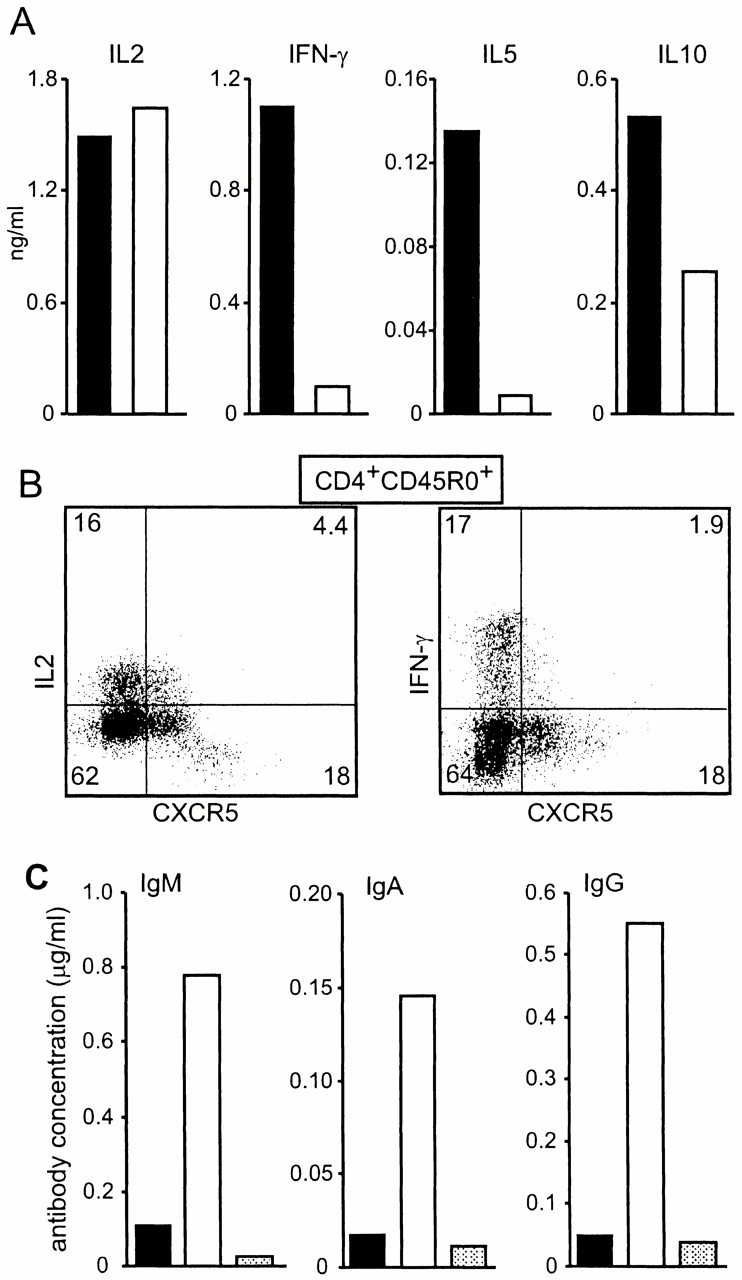
CXCR5+CD4 cells support Ig production. PBLs were sorted into two fractions: CD4+CD45RO+CXCR5− (black bars) and CD4+CD45RO+CXCR5+ (white bars). Sorted cells were stimulated with plastic-bound anti-CD3 for 24 h and the amount of cytokines produced was determined by ELISA. Similar results were obtained in two additional experiments. (B) Peripheral blood CD4+CD45RO+ cells were sorted, activated with PMA/ionomycin, and examined for intracellular production of cytokines. (C) Tonsillar CD19+ B cells were sorted and cocultured for 11 d with sorted CD4 cells from the same tonsil before Ab production was analyzed by ELISA. CD4+CD45RO+CXCR5−, black bars; CD4+CD45RO+CXCR5+, white bars; no T cells, shaded bars. Similar results were obtained in a second experiment
As CXCR5+ CD4 cells migrate to B cell follicles in lymphoid organs where they express ICOS, which is known to superinduce IL-10, a B cell differentiation factor, and CD40L, we looked into whether these cells support B cells to secret Ig. To that end, tonsillar CD4+CD45RO+ cells were sorted into two fractions as described above for peripheral blood cells and cultured in the presence of sorted CD19+ B cells isolated from the same tonsil. As shown in Fig. 3 C, B cells produced comparable low amounts of both IgA and IgG when cultured for 11 d without T cells or with the CXCR5− fraction. In contrast, coculture of B cells with CXCR5+CD45RO+ CD4 cells resulted in an ∼10-fold increase in the production of IgA and IgG Abs. With regard to the production of IgM, differences between the two Th cell subsets were less obvious. Compared with B cell cultures without T cells, addition of CXCR5− CD4 cells induced a 5-fold increase in IgM, whereas addition of CXCR5+ cells yielded a 15-fold increase in the production of this isotype. These data suggest that CD4+CXCR5+ cells represent the classical Th cells supplying B cells with the costimulatory signals required for Ig production.
Memory CD4 Cells Are within the CXCR5− Fraction.
As nearly all CD4+CXCR5+ T cells coexpress CD45RO 13, a molecule found on memory T cells, we were interested in studying whether the expression of CXCR5 is associated with T cell memory. Memory function analysis was performed on sorted peripheral blood T cells derived from donors who had not been immunized with TT during the last 2 yr. Coculture of sorted T cells with irradiated autologous antigen-presenting cells loaded with TT revealed that memory cells were principally found within the CXCR5− fraction (Fig. 4 A). In contrast, both populations responded equally well to allogenic antigen-presenting cells (Fig. 4 B), demonstrating that CXCR5+ T cells have the capability to respond to foreign antigens. As these data suggest that CXCR5+ T cells might represent finally differentiated effector T cells prone to be removed by apoptosis, we stained tonsillar T cells with anti-CD95 mAb. Interestingly, 94% of the CD4+CXCR5+ cells expressed CD95, whereas <30% of the CD4+CXCR5− cells were associated with this molecule (Fig. 4 C). This observation indicates that CXCR5+ T cells might be particularly prone to die by apoptosis, which might help to explain why we found long-lived memory cells within the CXCR5− subset.
Figure 4.
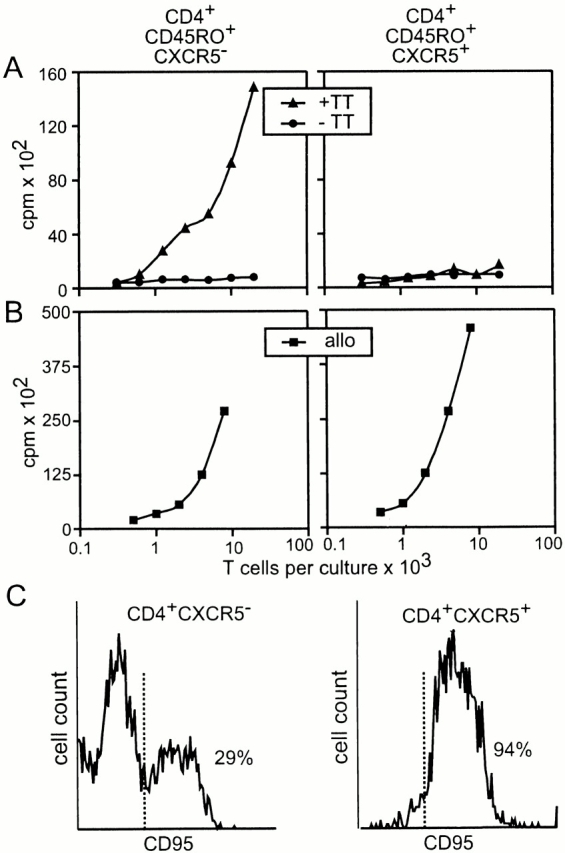
Memory response in CD4+CD45RO+CXCR5− cells. (A) PBLs were sorted as indicated and cultured with autologous serum and irradiated autologous macrophages in the presence or absence of TT for 7 d. For the last 24 h of culture, [3H]thymidine was added to the medium and the amount of incorporated 3H was determined (every point represents the mean of 12 replicas). Similar results were obtained in a second experiment. In a third experiment, we also observed a weak proliferative response in the TT-treated CXCR5+ fraction at the highest T cell count tested. (B) The same as described for A, with the exception that TT was not added and allogenic (allo) macrophages were substituted for autologous macrophages. Data shown are representative of three experiments. (C) CXCR5− and CXCR5+ tonsillar CD4 cells were analyzed for the expression of CD95 by flow cytometry.
Discussion
Molecular mechanisms underlying the process of lymphocyte homing have been extensively studied during the last few years. Data derived from plt mice (paucity of LN T cells 18), a mouse carrying a mutation in one of the SLC gene loci 19, and observations made on CCR7 gene-targeted mice 3 clearly demonstrate that trans-HEV migration of T cells, and to a lower extent migration of B cells, requires the interaction of HEV-expressed SLC/CCL21 and ELC/CCL19 with its cognate receptor CCR7. Once inside the lymphoid organ, T cells migrate to the DCs to screen these cells for foreign peptides presented to them. The molecular mechanisms underlying this migratory process are poorly understood. The data shown here provide evidence that this might also be mediated by the interaction of T cell–expressed CCR7 with CCL19, produced by interdigitating DCs. More important, compared with other chemokine receptors involved in T cell trafficking such as CXCR4, CCR7 is rather resistant towards ligand-induced receptor internalization. After exposure to its ligand, 50% of surface CXCR4 is removed within 10 min 20, whereas a 2-h period was required to observe comparable effects on CCR7 (Fig. 1 C). This slow kinetic of CCR7 downregulation would enable T cells to follow a new ELC/CCL19 gradient build-up by DCs after having exploited the same chemokine/chemokine receptors for transendothelial migration. DC-activated T cells then migrate to the outer T zone, which represents the primary site for Th cell cognate help to B cells specific for the same antigen 9. Although it is currently unknown what cues direct activated T cells to the outer T zone, we recently demonstrated that B cells use CCR7 to stay in this area for some hours to increase the probability of meeting and interacting with T cells 3. Some of the activated B cells migrate to extrafollicular foci, again by unknown mechanisms, to rapidly produce Abs of relatively low affinity. In the second/parallel phase of the immune response, CD4+ T and B cells migrate to B cell follicles to form GCs. Data derived from mice deficient for CXCR5 or deficient for its ligand, BLC/CXCL13, show a profound lack in attracting B cells to follicles containing FDCs, demonstrating that this chemokine/chemokine receptor controls B cell migration to follicles 4 5. In addition, data derived from various mouse models support the idea that Th cell migration to follicles is controlled by the same mechanisms that direct B cell migration to these areas. Walker et al_._ recently demonstrated that CD28-dependent OX40 ligation of Th cells is closely linked to upregulation of CXCR5 and the appearance of these cells in B cell follicles 21. Once they start to express CXCR5, T cells become increasingly unresponsive to ELC/CCL19 and SLC/CCL21 22. These mechanisms, together with ligand-induced receptor internalization, might release T cells from the ELC/SLC gradients of the T cell–rich area. Interestingly, transgenic mice that constitutively express OX40 ligand on DCs show a strong increase in the numbers of CXCR5+ T cells and have a high frequency of CD4 T cells locating to B cell areas 23. Although there is strong evidence that T cells need CXCR5 to migrate to the follicles, expression of this receptor on T cells does not inevitably result in follicular localization of CXCR5+ CD4 cells, as demonstrated by adoptive transfer experiments 22. However, as in vivo activation of T cells with antigen plus adjuvant initiates T cell homing to follicles 22, it seems likely that in addition to CXCR5 expression, T cells require a second signal to be directed to follicles. As up to 10% of GC cells represent antigen-specific CD4 cells 24, one is tempted to speculate that this signal is provided at the time of T–B cell interaction, as this would privilege antigen-specific cells to migrate to the follicles. Once within the follicles, a large proportion of the activated T cells has lost CCR7 but expresses ICOS 17 and has intracellular stores of CD154 (Fig. 2 C). ICOS has been shown to induce production of various cytokines from recently activated T cells and to critically participate in T cell–dependent immune responses 17 25. Furthermore, the interaction of CD154 with its receptor, CD40, is an essential step during a T cell–dependent B cell response. Data derived from CD40- and CD154-deficient mice demonstrate that this interaction regulates proliferation of B cells, Ig class switching, and Ab production 16. The data presented here show that CXCR5+ CD4 cells homing to B cell follicles express ICOS, CD154, and IL-10. Consequently, when coculturing sorted tonsillar T cells with B cells, we observed Ig production only within the CXCR5+ fraction, thus identifying this cell population as the classical Th cells. Our observation of long-lived memory cells not being present within the pool of peripheral recirculating CXCR5+ CD4 cells is in accordance with the idea that CXCR5+ CD4 cells represent recently activated effector cells. Given the fact that GCs usually disappear within <4 wk, together with the observation that virtually all CXCR5+ tonsillar CD4 cells coexpress CD95, which renders them highly susceptible towards apoptosis, it seems likely that CXCR5+ CD4 cells do not contribute to the long-lived memory pool. However, we cannot currently exclude that some CXCR5+ CD4 cells lose CXCR5 expression, thus reverting to a phenotype (i.e., CXCR5−CD45RO+) associated with T cell memory. Independent of their origin, a considerable part of the long-lived central memory CD4 cells has to maintain or regain sufficient levels of CCR7 to continuously recirculate through secondary lymphoid organs in order to initiate a second wave of antigen-specific T cells once rechallenged with previously encountered antigens.
In summary, this study adds weight to the notion that the pattern of expressed chemokine receptors functionally characterizes T cell populations 26. It was demonstrated that CXCR5 is expressed on T cells situated in GCs and follicles, which express costimulatory molecules (CD40L, ICOS) required for B cell maturation and survival. Based on the cytokines expressed, CXCR5+ T cells do not fall into the Th1/Th2 classification but represent an effector subset that is involved in B cell help.
As these cells show all characteristics required for efficient B cell help within lymphoid follicles, we suggest calling these cells “follicular B helper T cells” (TFH).
Acknowledgments
We would like to thank U. Höpken and R. Schmidt-Ulrich for their critical reading of the manuscript.
Footnotes
Abbreviations used in this paper: BLC, B lymphocyte chemoattractant; CCR7, CC chemokine receptor 7; CXCR5, CXC chemokine receptor 5; DC, dendritic cell; ELC, EBV-induced molecule 1 ligand chemokine; FDC, follicular DC; GC, germinal center; HEV, high endothelial venule; ICOS, inducible costimulator; LT, lymphotoxin; SLC, secondary lymphoid tissue chemokine; TCM, central memory T cells; TT, tetanus toxin.
References
- Butcher E.C., Picker L.J. Lymphocyte homing and homeostasis. Science. 1996;272:60–66. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- Cyster J.G. Chemokines and cell migration in secondary lymphoid organs. Science. 1999;286:2098–2102. doi: 10.1126/science.286.5447.2098. [DOI] [PubMed] [Google Scholar]
- Förster R., Schubel A., Breitfeld D., Kremmer E., Renner-Müller I., Wolf E., Lipp M. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 1999;99:23–33. doi: 10.1016/s0092-8674(00)80059-8. [DOI] [PubMed] [Google Scholar]
- Förster R., Mattis E.A., Kremmer E., Wolf E., Brem G., Lipp M. A putative chemokine receptor, BLR1, directs B cell migration to defined lymphoid organs and specific anatomic compartments of the spleen. Cell. 1996;87:1037–1047. doi: 10.1016/s0092-8674(00)81798-5. [DOI] [PubMed] [Google Scholar]
- Ansel K.M., Ngo V.N., Hyman P.L., Luther S.A., Forster R., Sedgwick J.D., Browning J.L., Lipp M., Cyster J.G. A chemokine-driven positive feedback loop organizes lymphoid follicles. Nature. 2000;406:309–314. doi: 10.1038/35018581. [DOI] [PubMed] [Google Scholar]
- Zlotnik A., Yoshie O. Chemokinesa new classification system and their role in immunity. Immunity. 1999;12:121–127. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]
- Chaplin D.D., Fu Y. Cytokine regulation of secondary lymphoid organ development. Curr. Opin. Immunol. 1998;10:289–297. doi: 10.1016/s0952-7915(98)80167-2. [DOI] [PubMed] [Google Scholar]
- Ngo V.N., Korner H., Gunn M.D., Schmidt K.N., Riminton D.S., Cooper M.D., Browing J.L., Sedgwick J.D., Cyster J.G. Lymphotoxin alpha/beta and tumor necrosis factor are required for stromal cell expression of homing chemokines in B and T cell areas of the spleen. J. Exp. Med. 1999;189:403–412. doi: 10.1084/jem.189.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garside P., Ingulli E., Merica R.R., Johnson J.G., Noelle R.J., Jenkins M.K. Visualization of specific B and T lymphocyte interactions in the lymph node. Science. 1998;281:96–99. doi: 10.1126/science.281.5373.96. [DOI] [PubMed] [Google Scholar]
- Austrup F., Vestweber D., Borges E., Lohning M., Brauer R., Herz U., Renz H., Hallmann R., Scheffold A., Radbruch A., Hamann A. P- and E-selectin mediate recruitment of T-helper-1 but not T-helper-2 cells into inflamed tissues. Nature. 1997;385:81–83. doi: 10.1038/385081a0. [DOI] [PubMed] [Google Scholar]
- Dutton R.W., Bradley L.M., Swain S.L. T cell memory. Annu. Rev. Immunol. 1998;16:201–223. doi: 10.1146/annurev.immunol.16.1.201. [DOI] [PubMed] [Google Scholar]
- Sallusto F., Lenig D., Forster R., Lipp M., Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- Förster R., Emrich T., Kremmer E., Lipp M. Expression of the G-protein-coupled receptor BLR1 defines mature recirculating B cells and a subset of T memory helper cells. Blood. 1994;84:830–840. [PubMed] [Google Scholar]
- Ngo V.N., Tang H.L., Cyster J.G. Epstein-Barr virus–induced molecule 1 ligand chemokine is expressed by dendritic cells in lymphoid tissues and strongly attracts naive T cells and activated B cells. J. Exp. Med. 1998;188:181–191. doi: 10.1084/jem.188.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn M.D., Ngo V.N., Ansel K.M., Ekland E.H., Cyster J.G., Williams L.T. A B-cell homing chemokine made in lymphoid follicles activates Burkitt's lymphoma receptor-1. Nature. 1998;391:799–803. doi: 10.1038/35876. [DOI] [PubMed] [Google Scholar]
- Grewal I.S., Flavell R.A. CD40 and CD154 in cell-mediated immunity. Annu. Rev. Immunol. 1998;16:111–135. doi: 10.1146/annurev.immunol.16.1.111. [DOI] [PubMed] [Google Scholar]
- Hutloff A., Dittrich A.M., Beier K.C., Eljaschewitsch B., Kraft R., Anagnostopoulos I., Kroczek R.A. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature. 1999;397:263–266. doi: 10.1038/16717. [DOI] [PubMed] [Google Scholar]
- Gunn M.D., Tangemann K., Tam C., Cyster J.G., Rosen S.D., Williams L.T. A chemokine expressed in lymphoid high endothelial venules promotes the adhesion and chemotaxis of naive T lymphocytes. Proc. Natl. Acad. Sci. USA. 1998;95:258–263. doi: 10.1073/pnas.95.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassileva G., Soto H., Zlotnik A., Nakano H., Kakiuchi T., Hedrick J.A., Lira S.A. The reduced expression of 6Ckine in the plt mouse results from the deletion of one of two 6Ckine genes. J. Exp. Med. 1999;190:1183–1188. doi: 10.1084/jem.190.8.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amara A., Gall S.L., Schwartz O., Salamero J., Montes M., Loetscher P., Baggiolini M., Virelizier J.L., Arenzana-Seisdedos F. HIV coreceptor downregulation as antiviral principleSDF-1alpha–dependent internalization of the chemokine receptor CXCR4 contributes to inhibition of HIV replication. J. Exp. Med. 1997;186:139–146. doi: 10.1084/jem.186.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker L.S., Gulbranson-Judge A., Flynn S., Brocker T., Raykundalia C., Goodall M., Forster R., Lipp M., Lane P. Compromised OX40 function in CD28-deficient mice is linked with failure to develop CXC chemokine receptor 5–positive CD4 cells and germinal centers. J. Exp. Med. 1999;190:1115–1122. doi: 10.1084/jem.190.8.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansel K.M., McHeyzer-Williams L.J., Ngo V.N., McHeyzer-Williams M.G., Cyster J.G. In vivo–activated CD4 T cells upregulate CXC chemokine receptor 5 and reprogram their response to lymphoid chemokines. J. Exp. Med. 1999;190:1123–1134. doi: 10.1084/jem.190.8.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocker T., Gulbranson-Judge A., Flynn S., Riedinger M., Raykundalia C., Lane P. CD4 T cell traffic controlin vivo evidence that ligation of OX40 on CD4 T cells by OX40-ligand expressed on dendritic cells leads to the accumulation of CD4 T cells in B follicles. Eur. J. Immunol. 1999;29:1610–1616. doi: 10.1002/(SICI)1521-4141(199905)29:05<1610::AID-IMMU1610>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Gulbranson-Judge A., MacLennan I. Sequential antigen-specific growth of T cells in the T zones and follicles in response to pigeon cytochrome c. Eur. J. Immunol. 1996;26:1830–1837. doi: 10.1002/eji.1830260825. [DOI] [PubMed] [Google Scholar]
- Coyle A.J., Lehar S., Lloyd C., Tian J., Delaney T., Manning S., Nguyen T., Burwell T., Schneider H., Gonzalo J.A. The CD28-related molecule ICOS is required for effective T cell-dependent immune responses. Immunity. 2000;13:95–105. doi: 10.1016/s1074-7613(00)00011-x. [DOI] [PubMed] [Google Scholar]
- Sallusto F., Lanzavecchia A., Mackay C.R. Chemokines and chemokine receptors in T-cell priming and Th1/Th2-mediated responses. Immunol. Today. 1998;19:568–574. doi: 10.1016/s0167-5699(98)01346-2. [DOI] [PubMed] [Google Scholar]