The Chemokine SDF-1 Is a Chemoattractant for Human CD34+ Hematopoietic Progenitor Cells and Provides a New Mechanism to Explain the Mobilization of CD34+ Progenitors to Peripheral Blood (original) (raw)
Abstract
Hematopoietic progenitor cells migrate in vitro and in vivo towards a gradient of the chemotactic factor stromal cell-derived factor-1 (SDF-1) produced by stromal cells. This is the first chemoattractant reported for human CD34+ progenitor cells. Concentrations of SDF-1 that elicit chemotaxis also induce a transient elevation of cytoplasmic calcium in CD34+ cells. SDF-1-induced chemotaxis is inhibited by pertussis toxin, suggesting that its signaling in CD34+ cells is mediated by seven transmembrane receptors coupled to Gi proteins. CD34+ cells migrating to SDF-1 include cells with a more primitive (CD34+/CD38− or CD34+/DR−) phenotype as well as CD34+ cells phenotypically committed to the erythroid, lymphoid and myeloid lineages, including functional BFU-E, CFU-GM, and CFU-MIX progenitors. Chemotaxis of CD34+ cells in response to SDF-1 is increased by IL-3 in vitro and is lower in CD34+ progenitors from peripheral blood than in CD34+ progenitors from bone marrow, suggesting that an altered response to SDF-1 may be associated with CD34 progenitor mobilization.
Entrance and exit of hematopoietic progenitor cells (HPC)1 to or from the bone marrow (BM) takes place during transplantation or mobilization of CD34+ progenitor cells, two procedures routinely used in the clinic today. For successful transplantation, donor HPC are given intravenously and must subsequently home to the BM. Conversely, during chemotherapy and/or growth factor–induced mobilization of BM progenitors, HPC increase 10–200-fold in the blood (1–4). The mechanisms and specific molecules involved in the experimental mobilization of HPC from the BM into peripheral blood (PB) or in the homing of HPC and in their trafficking through the BM are still unclear, but they might be similar to those utilized by HPC to recirculate and migrate during their maturation.
HPC in the BM are thought to be located within specific stroma niches (5). These specific environments provide soluble factors and cellular interactions required for HPC proliferation and differentiation. As HPC differentiate, they may move from one type of niche to another (6–8). In addition to their movement within the BM, HPC presumably move out from this organ as indicated by the detection of HPC in the PB of normal untreated adults (∼1% of the number present in the BM) (9, 10). Whether HPC circulate across, exit, or remain sessile within a BM niche may ultimately depend on the type and function of cell adhesion receptors on their surface.
It is well established that the function of adhesion molecules on the surface of leukocytes is critically regulated by activating events triggered by chemoattractants binding to specific receptors on the leukocytes. These activation events allow them to stick firmly to and, eventually, to emigrate through the vessel wall into the extravascular spaces (11–13). Leukocytes are ultimately attracted to extravascular sites via the generation of chemotactic gradients. Chemoattractants for HPC have not been described so far. Cells of the hematopoietic microenvironment (stromal cells) are ideal candidates for producing chemoattractants that could be involved in the homing, retention, and exit of HPC in hematopoietic organs.
In this report, we provide evidence that human CD34+ HPC are attracted by stromal cell-derived factor-1 (SDF-1), a chemoattractant produced by BM stromal cells. The chemotactic response to SDF-1 is lower in CD34+ HPC from PB collections than in CD34+ HPC from BM collections, suggesting that an altered response to SDF-1 may be associated with HPC mobilization.
Materials and Methods
PBPC Collections.
Mobilized PBPCs were collected by leukapheresis from seven patients being treated on protocols at the Dana-Farber Cancer Institute (14). Donor patients were being treated for advanced breast cancer (n = 6) or non-Hodgkin's lymphoma (n = 1) (stage III to stage IV). Mobilization regimens consisted of single-agent doxorubicin 90 mg/m2 (n = 3) or cyclophosphamide 6 g/m2 (n = 3) followed in either case by subcutaneous injection of 5 μg/kg of granulocyte–colony-stimulating factor (G-CSF) daily for 5 d (Neupogen, Amgen, Thousand Oaks, CA). PBPCs were collected on consecutive days at the time of hematological recovery after the chemotherapy-induced leukopenia. One patient had PBPC, collected at the beginning the fifth day of G-CSF treatment (5 μg/kg), which was given daily by subcutaneous injection without chemotherapy (labeled as a diamond in Fig. 4). PBPCs used in this study were all obtained from different subjects and from products collected either on the first (n = 1), second (n = 4), or third (n = 3) day of leukapheresis. All samples were obtained from the Dana Farber Cancer Institute blood component laboratory with the exception of one set of BM or PB from the same patient which was obtained from the hematology/oncology staff of The Hospital Universitario de Alcala and The Centro Ramon y Cajal (Madrid, Spain).
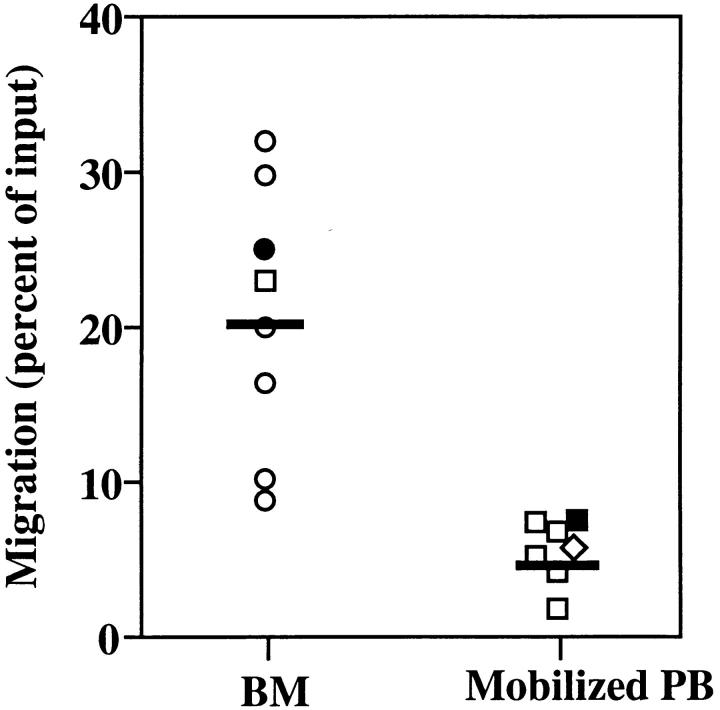
Figure 4.
Mobilized PB CD34+ cells are less responsive to SDF-1 induced chemotaxis than their BM counterparts. PB samples were obtained from solid tumor patients treated in the hematological recovery phase of chemotherapy + G-CSF treatment (n = 6, squares), or on day 5 of G-CSF treatment alone (n = 1, diamond), as described in Materials and Methods. BM samples were obtained from normal donors (n = 6; circles) or from solid tumor patient in remission (n = 1; square). Closed circles represent one set of CD34+ cells purified from BM or PB samples obtained the same day of treatment from the same tumor patient treated in the hematological recovery phase of chemotherapy + G-CSF treatment. Symbols indicate the percentage of CD34+ cells migrating to SDF-1 (300 ng/ml) in a transendothelial assay. Each symbol represents one different preparation from a different donor. The horizontal bars indicate the average percentage of migration for the bone marrow and the mobilized peripheral blood CD34+cell samples, respectively. Data for PB CD34+ cells are the following: average 5.1% of input, SEM 0.58. Data for BM CD34+ cells are the following: average 20% of input, SEM 3.4; P <0.01. The proportion of cells migrating to control media in these transendothelial assays ranged from 0.05 to 0.5% percent of input, resulting in a low accuracy in detecting few cells (<1,000) by flow cytometry and a large variability of chemotactic indexes (CI). Therefore, no statistically significant differences were found between the CI of BM and PB CD34+ cells to SDF-1, although a tendency was observed for lower CI in PB CD34+ cells (median CI for BM CD34+, 48 [SEM 8]; for PB CD34+, 24 [SEM 16]).
All leukapheresis procedures were performed using the Cobe Spectra (Lakewood, CO) cell separator. Colony-forming unit– cell (CFC) assays were performed on all leukapheresis components using methylcellulose-based media with recombinant cytokines (Stem Cell Technologies, Vancouver, Canada) to control for the presence of HPC in the PBPC. Colonies were classified as CFU-GM, CFU-MIX, and BFU-E colonies according to standardized morphologic criteria, as previously described (14). The average content in CFC per 106 MNC from the leukapheresis used in this study were the following: CFU-GM, 5.1 × 105 (SD 3.3), CFU-MIX 8.2 × 104 (SD 6.3), and BFU-E 4.5 × 105 (SD 1.2), respectively.
BM and Umbilical Cord Blood Samples.
Umbilical cord blood (CB) was collected following normal deliveries at the Brigham and Women's Hospital according to institutional guidelines for discarded material (protocol no. 92-5142-02). BM was collected from normal donors (n = 6) and from one patient with breast cancer at the time of harvest for allogeneic or autologous transplantation at the Dana-Farber Cancer Institute (labeled as a square in Fig. 4).
Purification of CD34+ Cells.
CD34+ cell selection was performed by immunoadsorption using the CellPro (Bothell, WA) Ceprate LC system as follows. CB, BM, or PB samples were diluted 1:2 with PBS containing sodium citrate 0.6%, 1% FCS (JRH Biosciences, Lenexa, KS), then layered onto Histopaque1077 (Sigma Chemical Co., St. Louis, MO) and centrifuged at 400 g for 30 min. After collecting the mononuclear interface, the cells were incubated with a biotinylated mAb to CD34 (8G12), washed three times with PBS, 0.2% BSA, and purified using the immunoaffinity column (Ceprate-LC34; CellPro), according to manufacturer's instructions. After elution, cells were washed twice in IMDM (Biowhittaker, Walkersville, MD). Purity of the CD34+ cell-enriched fraction ranged between 85–95% for CB samples, and between 90–95% for BM and PB preparations. The cloning efficiency for CD34+ cells in a CFC was, on average, 8% for BM cells, 9.5% for mobilized PB cells, and 14% for CB cells.
Stromal Cell–conditioned Media and Purification of SDF-1.
Conditioned media from BM-derived mouse stromal cell lines MS-5 (gift from Professor K.J. Mori, Kyoto, Japan) (15), CBRBM,D#1, CBR-BM,L#21, CBR-BM,D#10, CBR-BM,L#19 (16), or NIH-3T3 embryonic BM fibroblasts, were grown in IMDM 10% FCS until confluent. The medium was replaced with serum-free medium (Ultraculture, Biowhittaker), collected after 4–5 d of culture, and passed though a 0.45-μm filter.
The SDF-1 was purified from conditioned medium of the MS-5 cell line as described in detail elsewhere (17). In brief, the conditioned media was passed through a 0.22 μm filter and applied to a 5 ml HiTrap heparin column (Pharmacia) on the Pharmacia FPLC system. Active material was eluted from the column with a linear gradient of NaCl (0.4–2.0 M) and then applied to a 1 ml SP Sepharose HP cation exchange column (Pharmacia). Active fractions were then eluted with a linear gradient of NaCl (0.15–1.0 M) and applied to a Vydac C4 reverse-phase HPLC column (Vydac, Hesperia, CA). Active material eluted as a single peak with an acetonitrile gradient (0–80%) in trifluoroacetic acid, was lyophilized and resuspended in 10 mM sodium phosphate, pH 7.3, 150 mM NaCl. The purified protein migrated as a single band in SDS-PAGE of 8 kD. The protein was identified as SDF-1 by NH2-terminal amino acid sequencing, amino acid composition, and mass spectrometry (17). SDF-1 protein concentration was determined using a BCA protein assay (Pierce).
Chemokines and Cytokines.
For chemotactic assays, the following chemokines obtained from Peprotec were used at the indicated optimal concentration: human macrophage inflammatory protein-1 (MIP-1) α and β (50 ng/ml), human and mouse macrophage chemotactic protein-1 (MCP-1) (25 ng/ml), eotaxin (100 ng/ml), RANTES (50 ng/ml), IL-8 (25 ng/ml). Human stem cell factor (gift from Immunex) was used at 100 ng/ml.
Chemotactic Assays.
All assays were performed in duplicate using 5-μm pore filters (Transwell, 24-well cell clusters; Costar, Boston, MA). For transendothelial migration assays, 2 × 104 cells of the mouse endothelial cell line b-End-3 (18) were layered on the filters and grown in 10% FCS IMDM for 2 d before the performance of each assay. Immediately before each assay, bare filters or endothelial cell–covered filters were rinsed in serum-free media and the supernatant was aspirated. 1.5 × 105 CD34+ cells in 100 μl were loaded into each Transwell filter. Filters were then carefully transferred to another well containing 600 μl dilutions of supernatant (1:2) or purified chemokines in serum-free media, previously incubated for 15 min at 37°C in 7.5% CO2. After 3.5 h, the upper chamber was carefully removed and the cells in the bottom chamber resuspended and divided in aliquots for FACS® analysis (cell counting and immunophenotyping) and CFC assays. 10% of the input cell population (1.5 × 104) was also seeded in duplicate wells in 600 μl of serum-free media for the same period of time (but not subjected to any chemotaxis assay) to be used as control for FACS® counting.
Results obtained with transendothelial chemotaxis assays using the b-End-3 cell line were similar to those obtained in a limited series of experiments using HUVECS to coat the transwell inserts (data not shown).
Cell Counting and Immunophenotyping of CD34+ Populations.
For determination of the proportion of CD34+ cells that showed a chemotactic response, migrating cells (recovered from the lower chamber) were labeled with anti-CD34 PE (Becton Dickinson, Mountain View, CA) and anti-CD45 FITC (Dako, Carpinteria, CA) mAbs and analyzed in parallel from duplicate wells. CD34+ cells were defined as positive events in the low SSC region, CD34+/CD45dull windows. To obtain absolute values of migratory cells, flow cytometric counts for each sample were obtained during a constant, predetermined period of time and were compared with duplicate flow cytometric counts obtained from the control wells that were set up with 10% of the input population and were not subjected to chemotaxis assays. Data were expressed as a percentage of the input population or as chemotactic index (the ratio between the cells that migrated to the chemotactic stimuli and the cells that migrated to the control media). For immunophenotyping, cells from chemotaxis assays were labeled with anti-CD34 PE and either anti-CD38 FITC, anti-CD45RA FITC, anti-CD33 FITC (from Dako), anti-HLA-DR FITC, antiCD10 FITC (Becton Dickinson), and analyzed by flow cytometry. All stainings were performed on ice, for 25 min, in PBS containing 3% FCS, 1% BSA (Sigma), 0.1% sodium azide in 96-well V-bottomed plates. Propidium iodide (PI) (Sigma) to 1 μg/ml was added to each sample before the analysis. Samples were collected on a FACScan® flow cytometer (Becton Dickinson) and data was acquired in listmode with Cell Quest software (Becton Dickinson).
Assay for CFC.
Appropriate amounts of cells from the chemotaxis assays were plated in duplicate in 1 ml methylcellulose assay–based media (19) containing 2.5 U/ml rHu erythropoietin (R.W. Johnson Pharma Research Institute, Springhouser, PA), 150 ng/ml rHu stem cell factor, 200 U/ml rHu IL-3, 40 ng/ml of PIXY321, (all gift of Immunex, Seattle, WA). Colonies were scored for CFU-GM, CFU-MIX, and BFU-E after incubation at 37°C, 5% CO2. The total number of CFC present in the sample was calculated by multiplying for the fraction of the migrated cells analyzed in the CFC assay.
In Vivo Migration of Transplanted FDCP-MIX Progenitor Cells to SDF-1-injected Mouse Spleen.
The mouse progenitor cell line FDCP-MIX (20) was cultured in Fischer's medium supplemented with 20% horse serum and 5% X-63–IL-3 conditioned medium (21). To distinguish FDCP-MIX progenitor cells in transplantation experiments from host progenitor cells, 15–20 × 106 FDCP-MIX cells were labeled with the fluorescent membrane/dye PKH-26 (Sigma) following the instructions of the manufacturer. In preliminary experiments, we determined that 72 h after labeling, PKH-26-labeled FDCP-MIX cells could still be detected by their fluorescent signal and distinguished from nonlabeled host progenitor cells. For these series of experiments PKH26-labeled FDCP-MIX cells were plated in a modified colony assay containing 0.9% methylcellulose, IMDM, 20% horse serum, 2% X-63–IL-3-conditioned medium and 2 mM l-Glutamine. At 72 h from initiation of the culture, clusters of approximately 32 cells were observed; all of them were also detected by fluorescence microscopy. After 72 h, the sensitivity in detecting fluorescent clusters was consistently reduced, owing to dilution of the PKH-26 membrane dye into daughter cells (>5 cell divisions). After 5 d of culture, all clusters had reached the size of colonies (>50 cells) but had lost most of the initial detectable fluorescent signal (data not shown). Female C57BL/6 mice were used as recipients for transplantation. To deliver SDF-1 into their spleen, the mice were anesthetized and the spleen carefully exposed through a postero–lateral left incision. SDF-1 in PBS, MIP-1α in PBS, or PBS alone (75–100 μl vol) were injected intrasplenically using an insulin needle. After the body wall was sutured, the mice were injected intravenously with 2–3 × 106 FDCP-MIX cells labeled with PKH-26 as described above. 3 h after the injection, the mice were sacrificed by CO2 inhalation and the spleens collected. 5 × 105 splenocytes were plated in duplicate in the clonogenic assay for FDCP-MIX described above. After 72 h, the plates were scored by counting fluorescent colonies (∼32 cells) on an inverted fluorescent microscope and the number of FDCPMIX clonogenic progenitors per spleen calculated by correcting the counts obtained in the assay for the total number of splenocytes.
Calcium Efflux Assay.
Purified BM or PB CD34+ cells (purity ∼95%) were loaded with Fluo-3 (Molecular Probes) (7 μm in HBSS), for 20 min at 37°C, then diluted 1:5 with RPMI and further incubated for 20 min. After two washes, the cells (1 × 106/ml) were resuspended in Hepes-buffered saline (HS) containing 137 mM NaCl, 5 mM KCl, 1 mM Na2HPO4, 5 mM glucose, 1 mM CaCl2, 0.5 mM MgCl2, 1 g/l BSA, and 10 mM Hepes pH 7.4. The cells were then incubated for 10 min at 37°C, vortexed, and analyzed on a FACScan® for basal levels of fluorescence. After stimulation with a chemokine, fluorescence changes were monitored over time for up to 150 s. Data were analyzed by gating on low/SSC, medium/high FSC cells and calculating the mean fluorescence intensity and the percentage of responding cells (fluorescence above threshold) every 4 s.
Ex Vivo Culture of CD34+ Cells.
CD34+ cells (0.4 × 106/ well) were plated in a 24-well plate in IMDM containing 10% FCS containing 10−5 M 2-ME, 2 mM l-Glutamin, 50 U/ml penicillin, 50 μg/ml Streptomycin in the presence of either rHu IL-3 (30 ng/ ml), rHu G-CSF (50 ng/ml), rHu SCF (100 ng/ml), or mouse SDF-1 (100 ng/ml). After 18 h, cells were collected, washed once, and subjected to a chemotaxis assay as described before. The percentage of migratory CD34+ cells after culture in the presence of the different cytokines was performed as described above.
Results
Stromal Cell–derived SDF-1 Is a Chemoattractant for Human CD34+ Progenitor Cells.
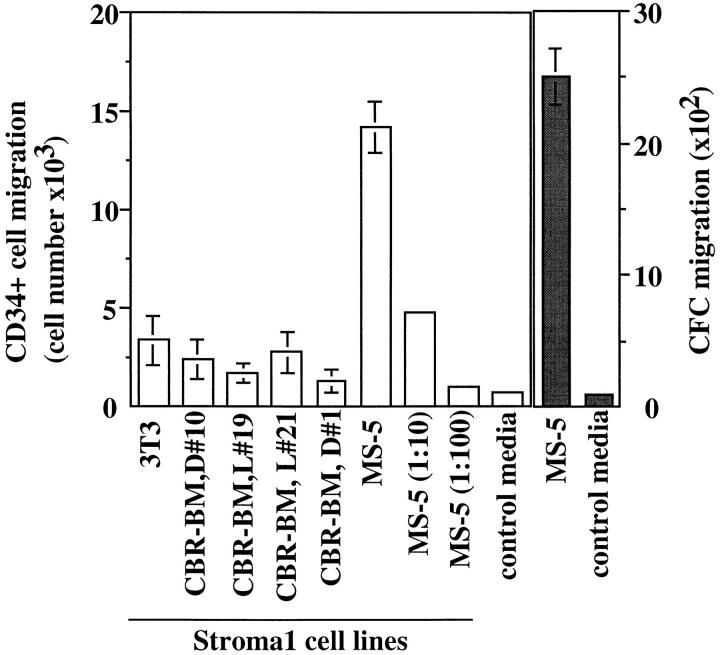
We have used an in vitro chemotactic assay to study the migratory response of human CD34+ progenitor cells to different stimuli. Since cells of the microenvironment of the BM may be involved in the trafficking of HPC, we first assayed the chemotactic response of CD34+ cells to conditioned media from different BM stromal cell lines. Conditioned media from MS-5 stromal cells (22) and, to a lesser extent, from other BM stromal cell lines (16), were able to induce the chemotaxis in vitro of human CD34+ progenitor cells (Fig. 1). Migratory cells were evaluated both phenotypically by the expression of CD34 and functionally by their ability to form hematopoietic colonies in methylcellulose assays (CFC).
Figure 1.
BM stromal cells produce a chemoattractant for human CD34+ progenitor cells. CD34+ cells from human cord blood (CB) (2.0 × 105/well) were assayed for their capacity to migrate in a Boyden chamber (5 μm bare filters), towards conditioned media from different BM stromal cell lines. The migratory population was evaluated phenotypically as the number of CD34+ cells that migrated (left, open bars), and functionally as the number of clonogenic progenitors (CFC) that migrated to the conditioned media (right, closed bars). Bars and columns indicate the duplicate and mean of one representative experiment out of three.
In these initial experiments, it was determined that the conditioned media of MS-5 cells also contained a chemotactic activity for lymphocytes (17). Because lymphocytes are more easily obtained and isolated than CD34+ progenitor cells, they were used as indicator cells during the purification of chemoattractant activity(s) present in the conditioned media of MS-5 stromal cells. The purification of this activity was performed by sequential heparin, cation exchange, and reverse-phase HPLC chromatography. The chemoattractant activity coeluted with a single protein peak in reverse-phase HPLC and migrated as a single band in SDS-PAGE of 8 kD (17). The protein was identified by NH2-terminal sequencing (17) as SDF-1 (23–25). Electrospray and matrix-assisted laser desorption mass spectrometry gave a mass that was consistent with that predicted for SDF-1 (17). MS-5-derived SDF-1 purified to homogeneity-attracted human CD34+ cells in vitro with similar efficacy to the original MS-5-conditioned medium (Fig. 2 A). At present, we do not know whether additional factors are present in the MS-5-conditioned media that could also induce chemotaxis of CD34+ progenitor cells.
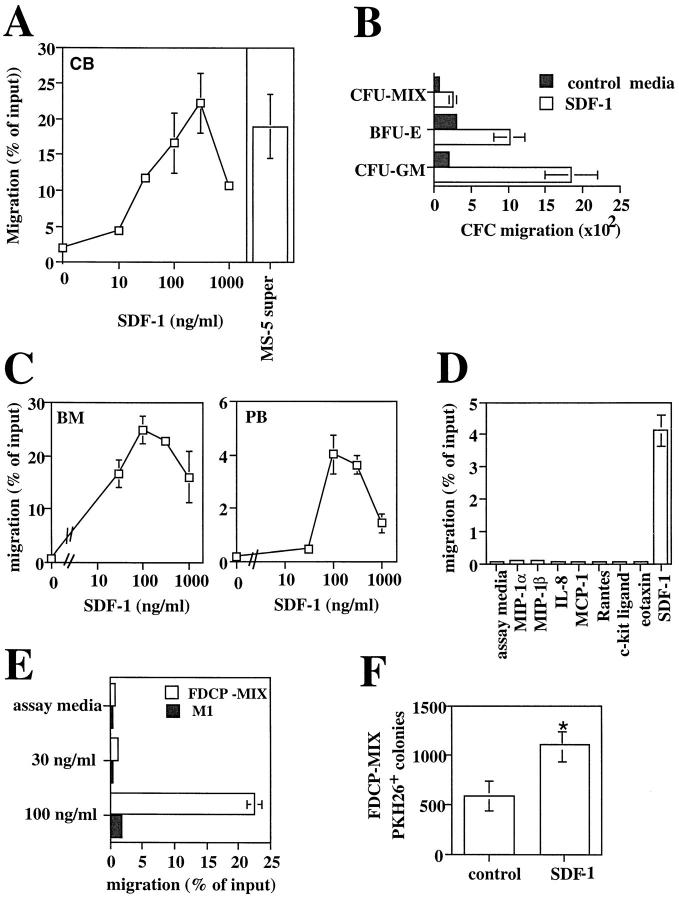
Figure 2.
Stromal-derived SDF-1 is a chemoattractant for human and mouse hematopoietic progenitor cells. (A) Chemotaxis assay of cord blood (CB) CD34+ cells in response to various concentration of SDF-1 (10, 30, 100, 300, 1,000 ng/ml). Results represent the average and the range of three experiments performed in duplicates. Data are expressed as the percent of input cells that migrated. The percentage of migration of CB CD34+ to undiluted MS-5 supernatant in the same experiments was used as control and is shown as a bar diagram on the right (n = 3). (B) Chemotactic response to SDF-1 of clonogenic progenitors (CFC). The graph shows the number of CFU-GM, BFU-E, and CFU-MIX progenitors from human CB CD34+ cells that migrated to 300 ng/ml of SDF-1 or control media in a chemotaxis assay. (C) Transendothelial chemotaxis in vitro of human bone marrow (BM) or mobilized peripheral blood (PB) CD34+ cells in response to different concentration of SDF-1. Results show the average and the range of three experiments performed in duplicates. (D) Transendothelial chemotaxis of mobilized PB CD34+ cells in response to SDF-1 or to various chemoattractants and cytokines at the concentrations described in the Materials and Methods. (E) Transendothelial chemotaxis in vitro of the mouse progenitor cell lines FDCP-MIX and M1 in response to SDF-1. (F). In vivo delivery of SDF-1 into mouse spleens increases the seeding of intravenously transplanted FDCP-MIX cells in this organ. Experimental mice were injected intrasplenically with SDF-1 and control mice were injected with MIP-1α or with PBS and then transplanted i.v. with PKH-26-labeled FDCP-MIX cells, as described in Materials and Methods (three experimental mice and three control mice per experiment; three separate experiments performed). 3 h after the injection, mice were killed and 5 × 105 splenocytes plated in duplicate in a clonogenic assay for FDCP-MIX. After 72 h, the plates were counted on an inverted fluorescent microscope and scored for the number of fluorescent colonies. Results show the significant increase in FDCP-MIX clonogenic precursors per SDF-1 injected spleens relative to control injected spleens. Data shown are from three experiments: *P <0.005.
Purified human CB CD34+ progenitor cells responded in chemotactic assays to concentrations of SDF-1 as low as 30 ng/ml. On average, 20% of the CD34+ population migrated to SDF-1 at the optimal concentration of 300 ng/ml (Fig. 2 A). In contrast, no chemotactic response of CD34+ cells was observed with other chemokines, including MIP-1α (26), that can be produced by stromal cells and are well documented to exert other effects than chemotaxis on CD34+ progenitor cells (27, 28; data not shown). The presence of clonogenic progenitors among the CD34+ cells that migrated to SDF-1 was confirmed by CFC assays (Fig. 2 B). Both BFU-E erythroid and CFU-GM myeloid progenitors present in the CD34+ population exhibited a marked chemotactic activity towards SDF-1. Their relative proportions within the total CFC population did not change after migration (compared with their relative proportions in the input population), indicating that the effect of SDF-1 is similar on both myeloid and erythroid progenitors. Multipotent clonogenic progenitors (CFU-MIX) were also capable of chemotaxis in vitro to SDF-1, but their proportion within the total CFC population was reduced by 50% after migration (data not shown; Fig. 2 B).
Migration across the sinusoidal endothelial barrier is an important event in the trafficking of HPC inside or outside the BM. We determined CD34+ cells were capable of migrating in a transendothelial assay. For these assays, we used the mouse endothelial cell line b-End-3 (18). These cells allowed simple and reproducible establishment of an homogeneous cells layer (90–95% confluence at the time of the assay). Mouse endothelial cells have been used extensively in the past to study adhesion and transmigration of human leukocytes based on the high functional equivalence of the majority of human adhesion molecules and their mouse homologs (29–31). We found that both BM and mobilized PB CD34+ cells responded in a transendothelial assay to SDF-1 at the optimal concentration of 100–300 ng/m (Fig. 2 C). Chemically synthesized SDF-1 elicited optimal chemotactic response on these CD34 populations at the same concentration range (data not shown). MCP-1, MIP-1α, MIP-1β, IL-8, Rantes, eotaxin, and c-kit ligand (SCF) were all ineffective in a similar assay (Fig. 2 D).
Moreover, SDF-1 elicited chemotaxis on the mouse primitive progenitor cell line FDCP-MIX (20). 20% of the input cells migrated to 100 ng/ml of SDF-1 (Fig. 2 E ). In contrast, the mouse myeloid progenitor cell line M1 (32) showed no chemotactic response to the same concentration of SDF-1.
To determine the ability of SDF-1 to attract progenitor cells in vivo, we injected SDF-1 intrasplenically and measured the lodging in the spleen of intravenously transplanted FDCP-MIX progenitor cells. To distinguish transplanted progenitor cells from the endogenous ones, FDCP-MIX cells were labeled before injection with the membrane dye PKH-26. The number of FDCP-MIX clonogenic precursors that seeded in SDF-1-injected spleens was significantly greater (2.5-fold; P <0.005) than that found in PBS or mMIP-1α-injected spleens from control mice (Fig. 2 F ).
To test whether specific subpopulations of CD34+ cells had markedly different chemotactic responses to SDF-1, we performed immunophenotyping of CD34+ cells that migrated towards SDF-1 and compared them with the input CD34+ population (Table 1). No changes were observed in the proportion of more primitive CD34+/DR− or CD34+/CD38− (33) cells after migration. Similarly, CD34+/CD33+ cells (34), CD34+/CD10+ cells, (35), and CD34+/CD7+ cells (36; data not shown), enriched for myeloid, B, and T cell progenitors, respectively, were equally represented in the input and migratory populations. CD34+ cells that migrated to SDF-1 were more enriched in committed CD45RA+ cells (37); this enrichment was more evident in PB than in BM CD34+ cells.
Table 1.
Immunophenotype of CD34+ Cells Migrating to SDF-1
| CD34+/CD38− | CD34+/HLA-DR− | CD34+/CD45RA+ | CD34+/CD10+ | CD34+/CD33+ | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Input | Migrated | Input | Migrated | Input | Migrated | Input | Migrated | Input | Migrated | |
| BM | 3.7 ± 0.9 | 4.3 ± 0.5 | 2.9 ± 0.6 | 2.9 ± 0.7 | 54.5 ± 6 | 66.9 ± 4.6 | 15.8 | 13.6 | 58.8 | 66.5 |
| PB | 6.5 | 3.5 | 1.38 | 1.07 | 31.1 | 67.8 | 2.6 ± 1 | 3.0 ± 0.9 | 83 ± 10 | 79 ± 9 |
| CB | 2.6 ± 0.2 | 2.5 ± 0.2 | ND | ND | 30.5 | 46.1 | ND | ND | ND | ND |
We studied whether the action of SDF-1 on CD34+ progenitors induced other responses (i.e., proliferation or differentiation) in addition to chemotaxis. SDF-1 did not display colony-stimulating factor activity on BM CD34+ cells either alone, or in association with IL-3 or SCF, at the concentrations of 50 and 200 ng/ml (data not shown).
SDF-1 Induces Calcium Mobilization and Pertussis Toxindependent Chemotaxis in CD34+ Cells.
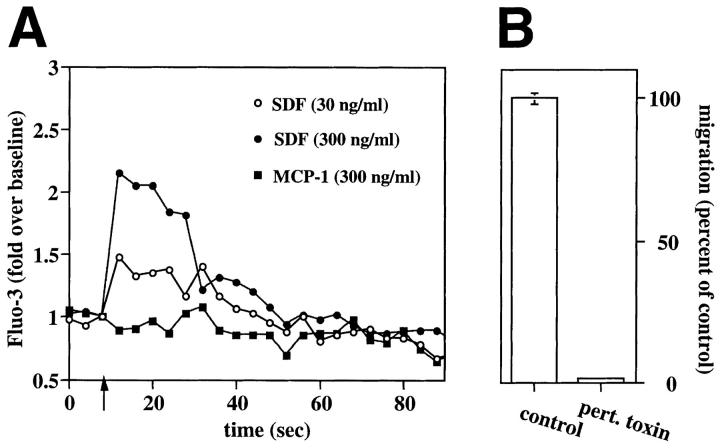
Mobilization of intracellular calcium is an early event in the response to chemokine signals (38). We tested the ability of SDF-1 to induce modulation of calcium in human BM CD34+cells in a flow cytometric assay. SDF-1 (300 ng/ml) induced a rapid, transient flux of intracellular calcium, which returned to basal levels within 60 s (Fig. 3 A). No response was elicited by human MCP-1 at either 300 ng/ml (Fig. 3 A) or 30 ng/ml (data not shown). The proportion of CD34+ cells fluxing calcium in response to SDF-1 ranged between 15–25% (data not shown). Mobilized PB CD34+ cells also showed a transient rise in intracellular calcium upon SDF-1 stimulation (data not shown). To our knowledge, these results demonstrate that human CD34+ hematopoietic progenitor cells are capable of modulating intracytoplasmatic calcium in response to an external ligand.
Figure 3.
SDF-1 induces calcium fluxes in human CD34+ cells and the associated chemotaxis is pertussis toxin sensitive. (A) SDF-1 induces calcium fluxes in human CD34+cells. Human BM CD34+ cells were loaded with the calcium-sensitive dye Fluo-3. Changes in Fluo-3 emission in response to SDF-1 (30 ng/ml, open circles; 300 ng/ml, closed circles) or human MCP-1 (300 ng/ml, open squares) were monitored over time by flow cytometry. (B) The chemotaxis of human CD34+ to SDF-1 is pertussis toxin sensitive. The chemotactic response of human BM CD34+ cells to SDF-1 (300 ng/ml) after a 2-h incubation in 10% FCS IMDM with (100 ng/ml; pert toxin) or without pertussis toxin (control). Results shown are the mean (and duplicates) of the percentage of the migration of the control samples (which are considered as 100%). Actual percentage of migration in the control ranged from 18–25% of input. Data are representative of three separate experiments.
Chemokines induce calcium mobilization and chemotaxis upon interaction with specific receptors that are coupled through heterotrimeric G proteins to signal-transducing pathways. To determine whether the response of CD34+ cells to SDF-1 involved interaction(s) with a receptor that was coupled to G proteins, we preincubated CD34+ cells with pertussis toxin, a potent inhibitor of Gαi-proteins (39). This pretreatment inhibited by 95% the transient flux of intracellular calcium to SDF-1 (data not shown), as well as abrogated almost completely the in vitro chemotactic response of CD34+ cells to SDF-1 (Fig. 3 B).
Mobilized PB CD34+ Cells Are Less Responsive to SDF1-induced Chemotaxis than BM CD34+ Cells.
In current transplantation protocols, CD34+ progenitor cells resident in the BM are currently mobilized to the PB for harvesting (40– 43) and subsequently used for transplantation (44). We compared the chemotactic responses to SDF-1 of BM resident and mobilized blood circulating CD34+ cells from patients receiving high dose chemotherapy and/or G-CSF treatment. In a transendothelial assay, mobilized PB CD34+ cells showed significantly lower chemotaxis (fourfold) than their BM counterparts (Fig. 4). The lower proportion of PB CD34+ cells migrating in response to SDF-1 was found at all concentrations of SDF-1 tested (data not shown; see Fig. 2 C). The same result was found in one experiment that was performed with BM and PB CD34+ cells collected from the same patient at the same timepoint after mobilization (indicated as solid symbols in Fig. 4). These results indicate that the lower response of PB CD34+ cells to SDF-1 correlates with their extramedullary location rather than with an indirect effect of the mobilization regime (which, in principle, should have had the same effect on BM CD34+ cells).
Chemotaxis of CD34+ Cells to SDF-1 Is Enhanced by IL-3 ex vivo Treatment.
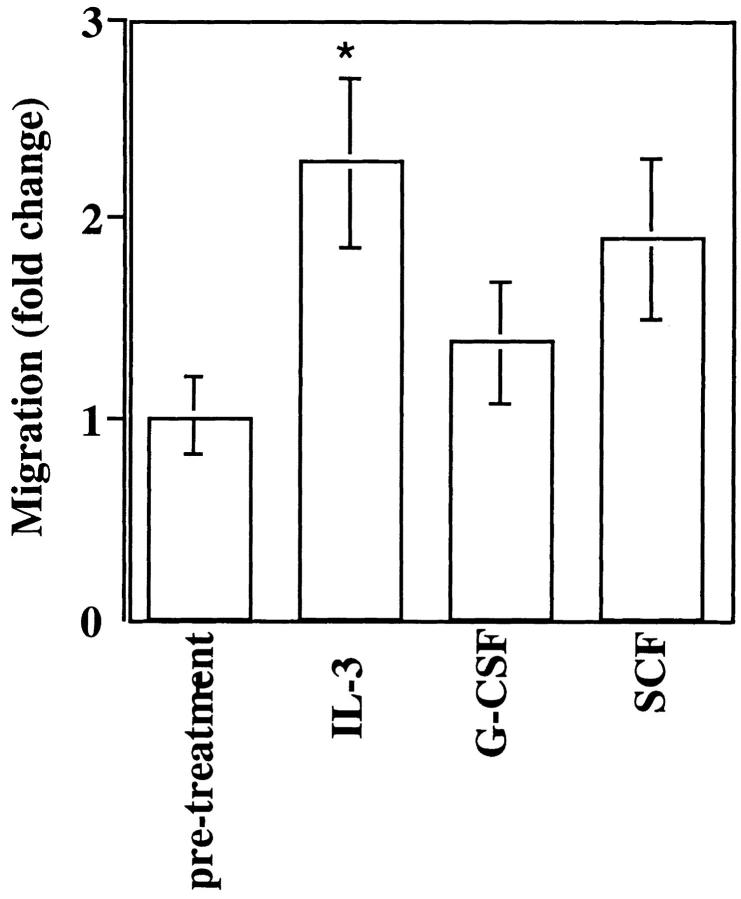
Several cytokines, including IL-3, SCF, G-CSF, and GM-CSF are known to mobilize hematopoietic progenitors to the peripheral blood (45). In view of the finding described above that G-CSF-mobilized CD34+ progenitors showed a reduced chemotactic response to SDF-1, we studied whether this and other cytokines were able to modify the response of CD34+ cells to SDF-1 in vitro. These experiments also possibly concern procedures such as the stimulation of CD34+ cells with cytokines in vitro, which is essential for ex vivo survival and/or expansion of HPC before transplantation (46–48), or the current use of cytokines for facilitating retroviral-mediated gene transfer into HPC (49). We tested whether an incubation for 18 h with either IL-3, SCF, or G-CSF affected the chemotaxis of human BM CD34+ cells to SDF-1 (Fig. 5). IL-3 induced a significant increase (2.2 fold) in the percentage of cells responding to SDF-1. Incubation of CD34+ cells with SCF or G-CSF resulted in increased chemotaxis of these cells to SDF-1 (1.4- to 1.9-fold), but which did not reach statistical significance. A similar increase in chemotactic activity after exposure to IL-3 was observed using PB CD34+ cells (data not shown).
Figure 5.
Cytokine modulation of CD34+ cell chemotaxis to SDF-1. CD34+ cells were incubated for 18 h in the presence of either 30 ng/ml IL-3, 50 ng/ml G-CSF or 100 ng/ml SCF and assayed for transendothelial chemotaxis in response to 300 ng/ml of SDF-1. Results show the mean fold change in the chemotactic response of CD34+ in three separate experiments performed in duplicate, with respect to chemotaxis before treatment (*P <0.05).
Discussion
Here, we present evidence that hematopoietic progenitor cells migrate in vitro and in vivo towards a gradient of a chemotactic factor produced by stromal cells. This stromal cell-derived chemotactic factor was identified as SDF-1 (23– 25) and is the first chemoattractant reported for human CD34+ progenitor cells. SDF-1, at the concentrations effective in chemotaxis assays, elicited a transient elevation in the concentration of cytoplasmic calcium in CD34+ cells. Pertussis toxin inhibited SDF-1-induced chemotaxis, suggesting that its signaling in CD34+ cells is mediated by G protein of the Gi class. SDF-1 was able to attract BFU-E, CFU-GM, and CFU-MIX progenitor cells from human BM, CB and mobilized PB. CD34+ cells migrating to SDF-1 included cells with a more primitive (CD34+/CD38− or CD34+/DR−) phenotype as well as CD34+ cells committed to the lymphoid and myeloid lineages. The chemotactic response of CD34+ cells to SDF-1 is increased by exposure to IL-3. Finally, the chemotactic response elicited by SDF-1 on PB CD34+ cells was lower than that found on their BM counterparts.
Our experiments revealed that mouse SDF-1 elicits transendothelial chemotaxis on human and mouse HPC (Fig. 2). That mouse SDF-1 has activity on human CD34+ progenitor cells is not surprising, because the amino acid sequences of this molecule are highly conserved, with only one amino acid difference between these two species (50). Although SDF-1 is not specific for hematopoietic progenitor cells, as it is also a chemoattractant for human lymphocytes and monocytes (17), it elicits rapid and direct chemotaxis of CD34+ HPC. Other molecules have been shown to elicit some chemotaxis and chemokinesis on mouse progenitor cells, such as SCF (51). However, the input population used for these experiments were total BM mononuclear cells, the percentage of migratory cells was very low (∼1%), and the maximum migration after exposure to SCF occurred much later (8–24 h) than in conventional chemotaxis assays (2–4 h). These considerations make it difficult to evaluate whether these effects were direct or indirect and thus their significance. In contrast, our results indicate that, (a) SDF-1 elicits maximal transendothelial migration of ∼25% of the CD34+ population 3 h after exposure (Fig. 2); (b) the same percentage of CD34+ cells flux Ca2+ rapidly (20 s) and transiently after exposure to SDF-1 (Fig. 3; data not shown); and (c) migration is completely blocked by pertussis toxin (Fig. 3).
Our data indicate that it is possible to increase significantly (2.5×) the number of mouse HPC lodged in the spleen after in vivo administration of SDF-1 in this organ (Fig. 2). There is increasing evidence that the homing of HPC into hematopoietic organs requires the specific interactions of selectins, integrins, and sugar receptors with their ligands, but the mechanisms underlying these events remain largely unclear. Regardless of the specific adhesion receptors involved in this process, we presume that activating chemotactic signals will be required, as they have been demonstrated to be necessary for the migration of mature leukocytes (11). In this context, it is conceivable that SDF-1 may facilitate and direct the migration of hematopoietic progenitor cells from BM blood sinusoid to the hematopoietic extravascular space. Although SDF-1 mRNA has been found in all tissues examined in humans and in mice, it was not found to be produced by hematopoietic cells (23, 25, 50).
During their maturation, hematopoietic progenitor cells have been postulated to occupy physically separated niches bordered with particular stromal cell types performing discrete functions (8). It is intriguing to speculate that the migration of progenitor cells from one niche to another within the BM microenvironment involves gradients of chemotactic factors, and that SDF-1 could be one of those factors involved in the local trafficking of human CD34+ cells. SDF-1 gradients could be generated by stromal cells producing different amounts of the chemokine and/or of chemokine-binding proteins, such as heparan sulfate. In fact, we have previously observed different levels of SDF-1 mRNA in different stromal cell clones (16), and different degrees of CD34 chemotaxis were also found in the conditioned media of different stromal lines (Fig. 1; data not shown).
Circulating PB HPC increase after chemotherapy and/or after exogenous cytokines are administrated. The use of PB CD34+ HPC for autologous and allogeneic transplantation has greatly increased in the past years (44). Mobilized PB CD34+ progenitor cells contain primitive progenitor cells and display similar capacities to survive, proliferate, and differentiate in vitro with respect to steady-state BM CD34+ cells (52–54). In addition, their proliferative and differentiation potential is demonstrated by the increasing number of successful transplantations using PB CD34+ HPC (3, 4, 41, 42). Therefore, the mobilization treatment does not impair the viability, response to exogenous cytokines, proliferation, and differentiation measured by the ability of CD34+ cells to form colonies in vitro or their ability to generate blood cells in vivo. Therefore, there is no indication that mobilized CD34+ cells are functionally compromised as a consequence of the mobilization treatment. Accordingly, the PB CD34+ cells used for our studies form colonies in methylcellulose assays in vitro at ratio comparable to their BM counterparts (see Materials and Methods; data not shown). Also, rapid neutrophil and platelet engraftment of PB CD34+ so far has been documented in three out of three patients receiving PB HPC infusions for hematopoietic reconstitution (see Materials and Methods; data not shown). Yet, these functionally competent CD34+ progenitors isolated from PB collections showed a fourfold reduction in their chemotactic activity to SDF-1 when compared with BM resident CD34+ cells. This finding was further confirmed in one experiment in which BM and PB CD34+ cells were collected from the same patient at the same timepoint after mobilization. Downregulation of responsiveness to SDF-1 may represent one mechanism by which CD34+ HPC are aided in their movement from BM extravascular space to the sinusoids and to the peripheral circulation.
G-CSF is the mobilizing agent most commonly used in the clinic (44). In fact, the PB CD34+ preparations used in this study were obtained from patients that were treated with G-CSF alone or in combination with chemotherapy. However, after 18 h of incubation in vitro with G-CSF, CD34+ cells did not reduce or increase significantly their chemotactic response to SDF-1 (Fig. 5). It is possible that the effect of G-CSF in vivo may require a longer exposure or it may involve the stromal microenvironment or other BM cells, such as macrophages, lymphocytes, or precursor cells (myelocytes, erythroblasts), which constituted only a minor contaminant (<5%) of our CD34+ cell preparations. However, preexposure of CD34+ cells to IL-3 induced a twofold increase in the chemotactic response of CD34+ cells to SDF-1. In the light of these results, it is of special interest that (1) a short (16-h) ex vivo incubation with IL-3 enhances the engraftment of mouse (55), sheep, and baboon HPC (56) into the BM of transplanted animals; (2) IL-3 has been shown to enhance mobilization when used in conjunction with G-CSF (45).
Although at present we do not know what the relevance is in vivo of SDF-1 in the homing of CD34+ to the marrow after transplantation or its involvement in the experimental mobilization of CD34+ cells, it is now clear that SDF-1 is a chemokine with unique properties on CD34+ hematopoietic progenitors, which may be of importance in the homing of BM CD34+ progenitor/stem cells to different organs during development and to different niches within the BM during differentiation and maturation of HPC. Recent experiments showing that mice that have been made genetically deficient in SDF-1 have normal (FL) myelopoiesis but drastically impaired BM myelopoiesis (57) suggest that, in fact, SDF-1 plays a critical role in the migration of HPC between the FL and the BM in vivo. Furthermore, manipulation of SDF-1 may offer promising ways to improve both transplantation and mobilization of hematopoietic cells.
Footnotes
The authors are indebted to Dr. Van Etten for critical reading of this manuscript and to Drs. Arman, Alvarez de Mon, and Alvarez for their efforts in locating invaluable PBPC and BM samples. We thank especially Dr. Anderson for helpful discussions and Drs. Mazo and Gerwin for their help.
This work has been funded by National Institutes of Health grants HL 148675-02 and HL94-10-B, and by the Aplastic Foundation of America grants CiCyT PB93-0317. J.C. Gutierrez-Ramos is the Amy C. Potter fellow. A. Aiuti's present address is TIGET, Instituto Scientifico San Raffaele, Via Olgettima 58, 20132, Milano, Italy.
1 Abbreviations used in this paper: BM, bone marrow; CFC, colony-forming cell; FL, fetal liver; G-CSF, granulocyte–colony-stimulating factor; HPC, hematopoietic progenitor cells; HS, Hepes-buffered saline; MCP, macrophage chemotactic protein; MIP, macrophage inflammatory protein; PBPC, peripheral blood progenitor cell; PI, propidium iodide; SDF, stromal cell-derived factor.
References
- 1.Socinski MA, Cannistra SA, Elias A, Antman KH, Schnipper L, Griffin JD. Granulocyte–macrophage colony stimulating factor expands the circulating haemopoietic progenitor cell compartment in man. Lancet. 1988;1:1194–1198. doi: 10.1016/s0140-6736(88)92012-0. [DOI] [PubMed] [Google Scholar]
- 2.Siena S, Bregni M, Brando B, Ravagnani F, Bonadonna G, Gianni AM. Circulation of CD34+hematopoietic stem cells in the peripheral blood of high-dose cyclophosphamide-treated patients: enhancement by intravenous recombinant human granulocyte-macrophage colonystimulating factor. Blood. 1989;6:1905–1914. [PubMed] [Google Scholar]
- 3.Sheridan WP, Begley CG, Juttner CA, Szer J, Bik L, To, Maher D, McGrath KM, Morstyn G, Fox RM. Effect of peripheral-blood progenitor cells mobilised by filgrastim (G-CSF) on platelet recovery after high-dose chemotherapy. Lancet. 1992;339:640–644. doi: 10.1016/0140-6736(92)90795-5. [DOI] [PubMed] [Google Scholar]
- 4.Elias AD, Ayash L, Anderson KC, Hunt M, Wheeler C, Schwartz G, Tepler I, Mazanet R, Lynch C, Pap S, et al. Mobilization of peripheral blood progenitor cells by chemotherapy and granulocyte–macrophage colony-stimulating factor for hematologic support after high-dose intensification for breast cancer. Blood. 1992;79:3036–3044. [PubMed] [Google Scholar]
- 5.Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4:7–25. [PubMed] [Google Scholar]
- 6.Mason TM, Lord BI, Hendry JH. The development of spatial distributions of CFU-S and in-vitro CFC in femora of mice of different ages. Br J Haematol. 1989;73:455–461. doi: 10.1111/j.1365-2141.1989.tb00280.x. [DOI] [PubMed] [Google Scholar]
- 7.Lord BI, Testa NG, Hendry JH. The relative spatial distribution of CFU-S and CFU-C in the normal mouse femur. Blood. 1975;46:65–72. [PubMed] [Google Scholar]
- 8.Uchida N, Flaming WH, Alpern EJ, Weissman IL. Heterogeneity of hematopoietic stem cells. Curr Opin Immunol. 1993;5:177–184. doi: 10.1016/0952-7915(93)90002-a. [DOI] [PubMed] [Google Scholar]
- 9.Goodman JW, Hodgson GS. Evidence for stem cells in the peripheral blood of mice. Blood. 1962;19:702–709. [PubMed] [Google Scholar]
- 10.Storb R, Graham TC, Epstein RB, Sale GE, Thomas ED. Demonstration of hemopoietic stem cells in the peripheral blood of baboons by cross circulation. Blood. 1977;50:537–542. [PubMed] [Google Scholar]
- 11.Springer TA. Traffic signals on endothelium for lymphocyte recirculation and leukocyte emigration. Annu Rev Physiol. 1995;57:827–872. doi: 10.1146/annurev.ph.57.030195.004143. [DOI] [PubMed] [Google Scholar]
- 12.Butcher EC. Leukocyte–endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 1991;67:1033–1036. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- 13.Lasky LA. Selectins: interpreters of cell-specific carbohydrate information during inflammation. Science (Wash DC) 1992;258:964–969. doi: 10.1126/science.1439808. [DOI] [PubMed] [Google Scholar]
- 14.Webb IJ, Eickoff CE, Elias AD, Ayash LJ, Wheeler CA, Schwartz GN, Demetri GD, Anderson KC. Kinetics of peripheral blood mononuclear cell mobilization with chemotherapy and/or granulocyte–colonystimulating factor: implications for timing and yield of hematopoietic progenitor cell collections. Transfusion. 1996;36:160–167. doi: 10.1046/j.1537-2995.1996.36296181930.x. [DOI] [PubMed] [Google Scholar]
- 15.Itoh K, Tezuka H, Sakoda H, Konno M, Nagata K, Uchiyama T, Uchino H, Mori KJ. Reproducible establishment of hemopoietic supportive stromal cell lines from murine bone marrow. Exp Hematol. 1989;17:145–53. [PubMed] [Google Scholar]
- 16.Friedrich C, Zausch E, Sugrue SP, GutierrezRamos JC. Hematopoietic supportive functions of mouse bone marrow- and fetal liver-microenvironment: dissection of granulocyte-, B-lymphocyte-, and hematopoietic progenitorsupport at the stroma cell clone level. Blood. 1996;87:4596–4606. [PubMed] [Google Scholar]
- 17.Bleul CC, Fuhlbrigge RC, Casasnovas JM, Aiuti A, Springer TA. A highly efficacious lymphocyte chemoattractant. J Exp Med. 1996;184:1101–1109. doi: 10.1084/jem.184.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hahne M, Jäger U, Isenmann S, Hallmann R, Vestweber D. Five tumor necrosis factor-inducible cell adhesion mechanisms on the surface of mouse endothelioma cells mediate the binding of leukocytes. J Cell Biol. 1993;121:655–664. doi: 10.1083/jcb.121.3.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Verfaillie C, Blakolmer K, McGlave P. Purified primitive human hematopoietic progenitor cells with longterm in vitro repopulating capacity adhere selectively to irradiate bone marrow stroma. J Exp Med. 1990;172:509–520. doi: 10.1084/jem.172.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spooncer E, Heyworth CM, Dunn A, Dexter TM. Self renewal and differentiation of IL-3-dependent multipotential stem cells are modulated by stromal cells and serum factors. Differentiation. 1986;31:111–118. doi: 10.1111/j.1432-0436.1986.tb00391.x. [DOI] [PubMed] [Google Scholar]
- 21.Karasuyama H, Melchers F. Establishment of mouse cell lines which constitutively secrete large quantities of interleukin 2, 3, 4 or 5, using modified cDNA expression vectors. Eur J Immunol. 1988;18:97–104. doi: 10.1002/eji.1830180115. [DOI] [PubMed] [Google Scholar]
- 22.Suzuki J, Fujita J, Taniguchi S, Sugimoto K, Mori KJ. Characterization of murine hemopoietic-supportive (MS-1 and MS-5) and non-supportive (MS-K) cell lines. Leukemia. 1992;6:452–458. [PubMed] [Google Scholar]
- 23.Tashiro K, Tada H, Heiker R, Shirozu M, Nakano T, Honjo T. Signal sequence trap: a cloning strategy for secreted proteins and type I. Science (Wash DC) 1993;261:600–603. doi: 10.1126/science.8342023. [DOI] [PubMed] [Google Scholar]
- 24.Nagasawa T, Kikutani H, Kishimoto T. Molecular cloning and structure of a pre-B-cell growth-stimulating factor. Proc Nat Acad Sci USA. 1994;91:2305–2309. doi: 10.1073/pnas.91.6.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang W, Zhou P, Kahn SM, Tomita N, Johnson MD, Weinstein IB. Molecular cloning of TPAR1, a gene whose expression is repressed by the tumor promoter 12-O-tetradecanoylphorbol 13-acetate (TPA) Exp Cell Res. 1994;215:284–293. doi: 10.1006/excr.1994.1344. [DOI] [PubMed] [Google Scholar]
- 26.Graham GJ, Wright EG, Hewick R, Wolpe SD, Wilkie NM, Donaldson D, Lorimore S, Pragnell IB. Identification and characterization of an inhibitor of haemopoietic stem cell proliferation. Nature (Lond) 1990;344:442–444. doi: 10.1038/344442a0. [DOI] [PubMed] [Google Scholar]
- 27.Broxmeyer HE. Myelopoietic enhancing effects of murine macrophage inflammatory proteins 1 and 2 on colony formation in vitro by murine and human bone marrow granulocyte/macrophage progenitor cells. J Exp Med. 1989;170:1583–1594. doi: 10.1084/jem.170.5.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Avalos BR, Bartynski KJ, Elder PJ, Kotur MS, Burton WG, Wilkie NM. The active monomeric form of macrophage inflammatory protein-1a interacts with high and low affinity classes of receptors on human hematopoietic cells. Blood. 1994;94:1790–1801. [PubMed] [Google Scholar]
- 29.Johnston SC, Dustin ML, Hibbs ML, Springer TA. On the species specificity of the interaction of LFA-1 with intercellular adhesion molecules. J Immunol. 1990;145:1181–1187. [PubMed] [Google Scholar]
- 30.Becker-Andre M, Van-Huijsduinen R, Losberger C, Whelan J, Delamarter J. Murine endothelial/leukocyte adhesion molecule 1 is a close functional homologue of the human protein. Eur J Biochem. 1992;206:401–411. doi: 10.1111/j.1432-1033.1992.tb16940.x. [DOI] [PubMed] [Google Scholar]
- 31.Taub D, Longo D, Murphy W. IP-10 induces mononuclear infiltration in mice and promotes the migration of T lymphocytes in peripheral tissues of hu-SCID mice. Blood. 1996;87:1423–1431. [PubMed] [Google Scholar]
- 32.Miyaura C, Onozaki K, Akiyama Y, Taniyama T, Hirano T, Kishimoto T, Suda T. Recombinant human interleukin 6 (B-cell stimulatory factor 2) is a potent inducer of differentiation of mouse myeloid leukemia cells (M1) FEBS Lett. 1988;234:17–21. doi: 10.1016/0014-5793(88)81293-6. [DOI] [PubMed] [Google Scholar]
- 33.Huang S, Terstappen LW. Lymphoid and myeloid differentiation of single human CD34+, HLA-DR+, CD38− hematopoietic stem cells. Blood. 1994;83:1515–1526. [PubMed] [Google Scholar]
- 34.Andrews RG, Singer JW, Bernstein ID. Precursors of colony-forming cells in humans can be distinguished from colony-forming cells by expression of the CD33 and CD34 antigens and light scatter properties. J Exp Med. 1989;169:1721–1731. doi: 10.1084/jem.169.5.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shah VO, Civin CI, Loken MR. Flow cytometric analysis of human bone marrow. IV. Differential quantitative expression of T-200 common leukocyte antigen during normal hemopoiesis. J Immunol. 1988;140:1861–1867. [PubMed] [Google Scholar]
- 36.Tjonnfjord GE, Veiby EP, Steen R, Egeland T. T lymphocyte differentiation in vitro from adult human prethymic CD34+bone marrow cells. J Exp Med. 1993;177:1531–1539. doi: 10.1084/jem.177.6.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sutherland IJ, Eaves CJ, Eaves AC, Dragowska N, Lansdorp P. Characterization and partial purification of human bone marrow cells capable of initiating long-term hematopoiesis in vitro. Blood. 1989;74:1563–1570. [PubMed] [Google Scholar]
- 38.Murphy PM. The molecular biology of leukocyte chemoattractant receptors. Annu Rev Immunol. 1994;12:593–633. doi: 10.1146/annurev.iy.12.040194.003113. [DOI] [PubMed] [Google Scholar]
- 39.Katz A, Wu D, Simon MI. Subunits beta gamma of heterotrimeric G protein activate beta 2 isoform of phopholipase C. Nature (Lond) 1992;360:686–689. doi: 10.1038/360686a0. [DOI] [PubMed] [Google Scholar]
- 40.Gianni AM, Siena S, Bregni M, Tarella C, Stern AC, Pileri A, Bonadonna G. Granulocyte-macrophage colony-stimulating factor to harvest circulating haemopoietic stem cells for autotransplantation. Lancet. 1989;2:580–585. doi: 10.1016/s0140-6736(89)90711-3. [DOI] [PubMed] [Google Scholar]
- 41.Chao NJ, Schriber JR, Grimes K, Long GD, Negrin RS, Raimondi CM, Horning SJ, Brown SL, Miller L, Blume KG. Granulocyte colony-stimulating factor “mobilized” peripheral blood progenitor cells accelerate granulocyte and platelet recovery after high-dose chemotherapy. Blood. 1993;81:2031–2035. [PubMed] [Google Scholar]
- 42.Peters WP, Rosner G, Ross M, Vredenburgh J, Meisenberg B, Gilbert C, Kurtzberg J. Comparative effects of granulocyte-macrophage colony-stimulating factor (GM-CSF) and granulocyte colony-stimulating factor (G-CSF) on priming peripheral blood progenitor cells for use with autologous bone marrow after high-dose chemotherapy. Blood. 1993;81:1709–1719. [PubMed] [Google Scholar]
- 43.Lane TA, Law P, Maruyama M, Young D, Burgess J, Mullen M, Mealiffe M, Terstappen LWMM, Hardwick A, Moubayed M, et al. Harvesting and enrichment of hematopoietic progenitor cells mobilized into the peripheral blood of normal donors by granulocyte–macrophage colonystimulating factor (GM-CSF) or G-CSF: potential role in allogeneic marrow transplantation. Blood. 1995;85:275–282. [PubMed] [Google Scholar]
- 44.Lee JH, Klein HG. Collection and use of circulating hematopoietic progenitor cells. Hematol Oncol Clin North Am. 1995;9:1–22. [PubMed] [Google Scholar]
- 45.Guillaume T, D'Hondt V, Symann M. IL-3 and peripheral blood stem cell harvesting. Stem Cells. 1993;11:173–181. doi: 10.1002/stem.5530110303. [DOI] [PubMed] [Google Scholar]
- 46.Sato N, Sawada K, Koizumi K, Tarumi T, Leko M, Yasukouchi T, Yamaguchi M, Takahashi TA, Sekiguchi S, Koike T. In vitro expansion of human peripheral blood CD34+ cells. Blood. 1993;82:3600–3609. [PubMed] [Google Scholar]
- 47.Brugger HW, Luft T, Frey T, Mertelsmann R, Kanz L. Maintenance of transplantation potential in ex vivo expanded CD34+-selected human peripheral blood progenitor cells. Blood. 1994;84:2898–2903. [PubMed] [Google Scholar]
- 48.Migliaccio G, Migliaccio AR, Druzin ML, Giardina PJ, Zsebo KM, Adamson JW. Long-term generation of colony-forming cells in liquid culture of CD34+cord blood cells in the presence of recombinant human stem cell factor. Blood. 1992;79:2620–2627. [PubMed] [Google Scholar]
- 49.Flasshove M, Banerjee D, Mineishi S, Li MX, Bertino JR, Moore MAS. Ex vivo expansion and selection of human CD34+peripheral blood progenitor cells after introduction of a mutated dihydrofolate reductase cDNA via retroviral gene transfer. Blood. 1995;85:566–574. [PubMed] [Google Scholar]
- 50.Shirozu M, Nakano T, Inazawa J, Tashiro K, Tada H, Shinohara T, Honjo T. Structure and chromosomal localization of the human stromal cell-derived factor 1 (SDF1) gene. Genomics. 1995;28:495–500. doi: 10.1006/geno.1995.1180. [DOI] [PubMed] [Google Scholar]
- 51.Okumura N, Tsuji K, Ebihara Y, Tanaka I, Sawai N, Koike K, Komiyama A, Nakahata T. Chemotactic and chemokinetic activities of stem cell factor on murine hematopoietic progenitor cells. Blood. 1996;87:4100–4108. [PubMed] [Google Scholar]
- 52.Healy LE, Nirsimloo N, Scott M, Apperley JF, Gordon MY. In vitro proliferation by cells mobilized into the peripheral blood for collection and autologous transplantation. Exp Hematol. 1994;22:1278–1282. [PubMed] [Google Scholar]
- 53.Haylock DN, Dowse TL, To LB, Juttner CA, Simmons PJ. Ex vivo expansion and maturation of peripheral blood CD34+cells into the myeloid lineage. Blood. 1992;80:1405–1412. [PubMed] [Google Scholar]
- 54.To LB, Haylock DN, Dowse T, Simmons PJ, Trimboli S, Ashman LK, Juttner CA. A comparative study of the phenotype and proliferative capacity of peripheral blood (PB) CD34+ cells mobilized by four different protocols and those of steady-phase PB and bone marrow CD34+cells. Blood. 1994;84:2930–2939. [PubMed] [Google Scholar]
- 55.Tavassoli M, Konno M, Shiota Y, Omoto E, Minguell JJ, Zanjani ED. Enhancement of the grafting efficiency of transplanted marrow cells by preincubation with IL-3 and GM-CSF. Blood. 1991;77:1599–1606. [PubMed] [Google Scholar]
- 56.Zanjani ED, Ascensao JL, Tavassoli M. Liverderived fetal hematopoietic stem cells selectively and preferentially home to the fetal bone marrow. Blood. 1993;81:399–404. [PubMed] [Google Scholar]
- 57.Nagasawa T, Hirota S, Tachibana K, Takakura N, Nishikawa S, Kitamura Y, Yoshida N, Kikutani H, Kishimoto T. Defects of B-cell lymphopoiesis and bonemarrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature (Lond) 1996;382:635–638. doi: 10.1038/382635a0. [DOI] [PubMed] [Google Scholar]