Regulation of Experimental Autoimmune Encephalomyelitis by Natural Killer (NK) Cells (original) (raw)
Abstract
In this report, we establish a regulatory role of natural killer (NK) cells in experimental autoimmune encephalomyelitis (EAE), a prototype T helper cell type 1 (Th1)-mediated disease. Active sensitization of C57BL/6 (B6) mice with the myelin oligodendrocyte glycoprotein (MOG)35-55 peptide induces a mild form of monophasic EAE. When mice were deprived of NK cells by antibody treatment before immunization, they developed a more serious form of EAE associated with relapse. Aggravation of EAE by NK cell deletion was also seen in β2-microglobulin−/− (β2m−/−) mice, indicating that NK cells can play a regulatory role in a manner independent of CD8+ T cells or NK1.1+ T cells (NK–T cells). The disease enhancement was associated with augmentation of T cell proliferation and production of Th1 cytokines in response to MOG35-55. EAE passively induced by the MOG35-55-specific T cell line was also enhanced by NK cell deletion in B6, β2m−/−, and recombination activation gene 2 (RAG-2)−/− mice, indicating that the regulation by NK cells can be independent of T, B, or NK–T cells. We further showed that NK cells inhibit T cell proliferation triggered by antigen or cytokine stimulation. Taken together, we conclude that NK cells are an important regulator for EAE in both induction and effector phases.
Experimental autoimmune encephalomyelitis (EAE)1 is a prototype autoimmune disease induced in laboratory animals, bearing significant similarities to multiple sclerosis in clinical and pathological aspects (1, 2). EAE is mediated by CD4+ T cells that recognize peptides derived from encephalitogenic proteins of the central nervous system in association with MHC class II molecules. The encephalitogenic T cells in EAE produce T helper cell type 1 (Th1) cytokines such as IL-2, IFN-γ, and TNF-α.
In principle, EAE induced by active challenge with encephalitogenic peptides represent monophasic or polyphasic clinical courses in which ascending paralysis is usually followed by spontaneous recovery. The recovery process probably depends on cellular interactions between encephalitogenic T cells and regulatory cells. In support of this concept, previous studies revealed that α/β T cells expressing CD4+ (3, 4), CD8+ (5–7), or CD4−CD8− (8, 9) phenotype can play a regulatory role in EAE. More recently, B cells (10) and γ/δ T cells (11) have also been identified as putative regulatory elements in EAE. Although encephalitogenic peptides (4) or TCR peptides (3, 9) in association with MHC molecules are recognized as the receptor ligands for some regulatory T cells, little is known about how other regulatory cells are triggered.
In this study, we examined whether NK cells could serve as a regulatory element in EAE. The possible role of NK cells in immunoregulation has been suggested in a number of studies (12–17). However, most of the previous works have focused on the role of NK cells in the immune response to foreign microbes, and did not investigate their role in the regulation of autoimmune response or autoimmune disease. Furthermore, the experimental results have not always been conclusive in proving the regulatory role of NK cells. In fact, early studies (12, 14) used antiasialo GM1 sera for NK cell deletion which can damage macrophages (18) or T cells (19), and the others (13, 15–17) did not distinguish NK cells from NK1.1+ T cells (NK–T cells) (20–22), a novel lymphocyte population that produces a large amount of IL-4 after TCR ligation (23). Because the decline of NK–T cells has been seen in the development of animal and human autoimmune diseases (24, 25) and in vivo deletion of NK–T cells can enhance the disease development (24), it is crucial to distinguish NK and NK–T cells in consideration for their regulatory functions.
To overcome the problems inherent in the previous studies, we selected a model of EAE induced in C57/BL6 (B6) with myelin oligodendrocyte glycoprotein (MOG)35-55 peptide (26). The B6 model is particularly useful, since the method of in vivo NK cell deletion is established and various gene knockout mice are available with the B6 background (27–29). We found that NK cell deletion in vivo results in enhancement of EAE in wild-type B6 mice. Using gene knockout mutant mice lacking β2-microglobulin (β2-m)−/− (27) or recombination activation gene (RAG)- 2−/− (28), we further proved that NK cells are qualified as a regulatory element in passive EAE, of which function can be independent of T, B, or NK–T cells. The results demonstrate that both induction and effector phases of EAE are subject to immunoregulation by NK cells. We also showed that cellular transfer can effectively compete with the disease enhancement by NK cell deletion. In vitro experiments indicate that the downregulation of EAE by NK cells may arise from their inhibitory effects on T cell proliferation.
Materials and Methods
Mice.
B6 mice were purchased from CLEA laboratory animal corporation (Tokyo, Japan). β2m−/− (27) and IFN-γ−/− (29) mice with the B6 background were purchased from The Jackson Laboratory (Bar Harbor, ME). RAG-2−/− mice (28) with the B6 background were purchased from Taconic Farms, Inc. (Germantown, NY). All the mice were kept under specific pathogen-free conditions and only female mice (8–14 wk) were used.
Reagents.
The rat MOG35-55 (MEVGWYRSPFSRVVHLYRNGK) was synthesized as previously described (30). IFA and heat-killed Mycobacterium tuberculosis H37Ra were purchased from Difco Laboratory (Detroit, MI). Hybridomas producing anti-NK1.1 mAb (PK136; reference 31) and isotype-matched control mAb (M-11; specific for human melanoma cell surface antigen) were obtained from American Type Culture Collection (Rockville, MD). The mAbs were purified from the supernatants using Protein A column chromatography. Anti–mouse CD3-FITC, NK1.1-PE, IgG2ab (clone 5.7), and Fc Block™® (anti–mouse FcRγ II/III mAb) were purchased from PharMingen (San Diego, CA). The 5.7 antibody reacts specifically with mouse IgG2a of Igh-Cb haplotype (e.g., B6, SJL/J).
Immunization.
For induction of active EAE, mice were injected in the hind foodpads bilaterally with a total of 200 μl of emulsion containing 200 μg MOG35-55 in IFA supplemented with 500 μg of M. tuberculosis. Booster immunization with an identical emulsion was given at both sides of the flank 1 wk later. They were intravenously injected with 500 ng of pertussis toxin (PT) (Seikagaku Kogyo, Tokyo, Japan) in 500 μl of PBS shortly after, and 48 h after first immunization. For the study of T cell response or generation of T cell line, mice were immunized only once with 200 μg MOG35-55 in IFA supplemented with 250 μg of M. tuberculosis without subsequent injection of PT.
In Vivo NK Cell Deletion.
To deplete NK cells in vivo, mice were intravenously injected with 500 μg of anti-NK1.1 mAb (PK136) one day before first immunization with MOG35-55 or passive transfer of ZB-1 T cell line. Control mice were treated either with 500 μg of control mAb (M-11) or PBS.
Culture Medium.
RPMI 1640 containing 5 × 10−5 M 2-mercaptoethanol (2-ME), 2 mM L-glutamine, and 100 U/μg/ml penicillin/streptomycin (referred to as the basic medium) were used after supplemented with syngeneic mouse serum or FCS alone or with the serum and IL-2 (supernatant of Con A–stimulated rat spleen cells).
T Cell Proliferative Responses.
To analyze the primary response, inguinal and popliteal LN cells (4 × 105/well) isolated from mice immunized with MOG35-55 were cultured in 96-well flat-bottomed plates with relevant peptides at 25 μg/ml in 0.2 ml of the basic medium supplemented with 1% syngeneic serum. The cultures were incubated for 72 h at 37°C in humidified air containing 5% CO2. Incorporation of [3H]thymidine (1 μCi/well) for the final 16 h of the incubation was counted with a beta-1205 counter (Pharmacia, Uppsala, Sweden). For assaying T cell line proliferation, T line cells (4 × 104/well) were cultured for 72 h in 96-well flat-bottomed plates in the presence of irradiated (3,300 rads) syngeneic spleen cells (8 × 105/well) as APCs in 0.2 ml of the basic medium supplemented with 5% FCS. Thymidine incorporation was determined as in the primary proliferation assay.
T Cell Line.
Long-term T cell lines specific for MOG35-55 were established with our modification of the split-well technique (9). The draining LNs were removed 11 d after immunization with MOG35-55 and the single-cell suspensions were prepared at 4 × 106/ml in the basic medium supplemented with 5% FCS. They were plated on to 96-well U-bottomed plates at 0.2 ml/ well and stimulated with 25 μg/ml of MOG35-55. The cells were fed with the basic medium supplemented with 10% IL-2 and 10% FCS every 3 d. On day 12, T line cells in each microwell were individually assessed for their antigen specificity. In brief, cells were restimulated with MOG35-55 (25 μg/ml) in another U-bottomed plate in the presence of X-irradiated (3,300 rads), syngeneic spleen cells as APCs (8 × 105/well). Rapidly growing lines were selected and further propagated with IL-2 in the U-bottomed plates. After several cycles of alternate stimulation with MOG35-55 peptide and propagation (every 10–14 d), the antigen specificity and encephalitogenicity of the lines were determined. The encephalitogenic T cell line ZB-1 was finally selected. The line cells (4 × 104/well) were stimulated with 25 μg/ml of MOG35-55 in the presence of spleen APCs (8 × 105/well) in the U-bottomed plates as in the T cell line proliferation assay. 3 d later, they were collected and injected via tail vein of recipients. Immediately after cell transfer, 500 ng of PT was intravenously injected. Except for RAG-2−/− mice, all the recipients had been irradiated with 450 rads of x rays shortly before cell transfer.
EAE Score.
After immunization or cell transfer, mice were observed daily for clinical signs of EAE. The clinical grade was scored as follows: 0, no clinical signs; 0.5, partial loss of tail tonicity; 1, complete loss of tail tonicity; 2, flaccid tail and abnormal gait; 3, hind leg paralysis; 4, hind leg paralysis with hind body paresis; 5, hind and foreleg paralysis; and 6, death.
Cytokine ELISA.
11 d after immunization, the primed LN cells (4 × 105/well) were stimulated with MOG35-55 (25 μg/ml) in 96-well U-bottomed plates in the basic medium supplemented with 5% FCS. IFN-γ, IL-2, IL-4, and IL-10 in the culture supernatants (40 h after initiation of culture) were measured by Sandwich ELISA using a protocol from PharMingen. TNF-α was measured by a commercial kit (Quantikine M Mouse TNF-α Immunoassay®) from R&D Systems (Minneapolis, MN).
Immunofluorescence Analysis.
Peripheral blood or spleen cells were treated in ACK buffer to lyse erythrocytes, washed three times in PBS, and then suspended in PBS containing 0.1% BSA and 0.01% NaN3. The cells were first incubated with Fc Block™® for 15 min to block nonspecific binding of Ig to Fc receptor, washed in PBS, and then incubated with anti-CD3-FITC and/or anti-NK1.1-PE for 30 min on ice. After washing, they were suspended in PBS containing 0.5 μg/ml of propidium iodide (PI; Wako Pure Chemical Industries, Ltd., Osaka, Japan) and 10,000 cells were analyzed by FACSort® (Becton Dickinson, Mountain View, CA) with CellQuest® software. Dead cells were excluded by gating out PI-positive cells.
Results and Discussion
Augmentation of Active EAE by NK Cell Deletion.
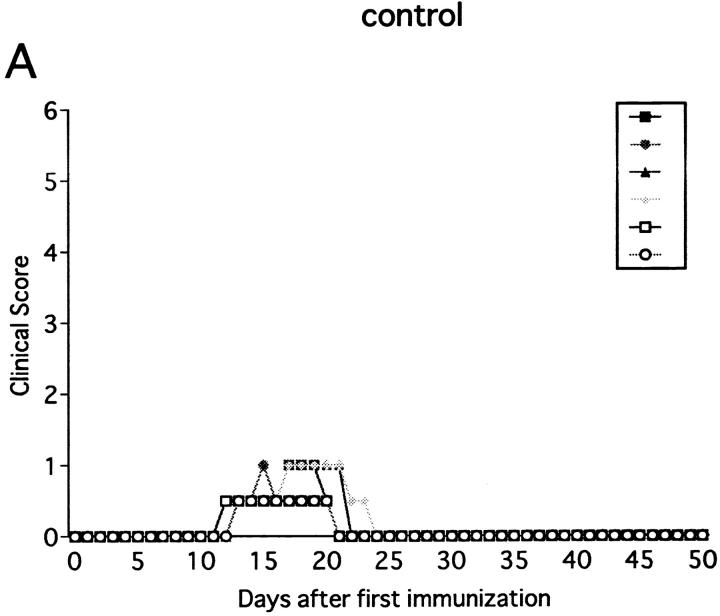
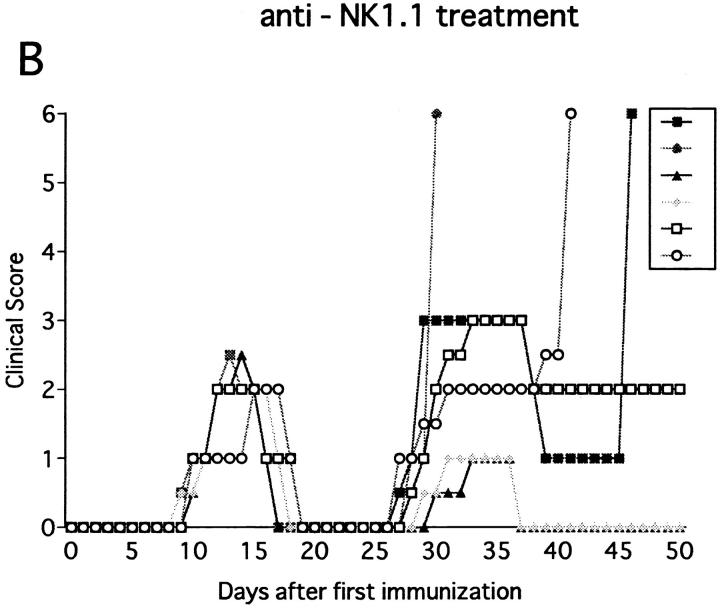
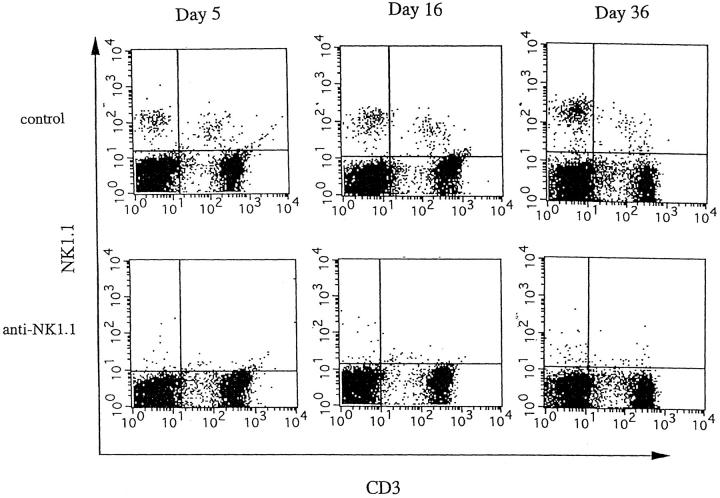
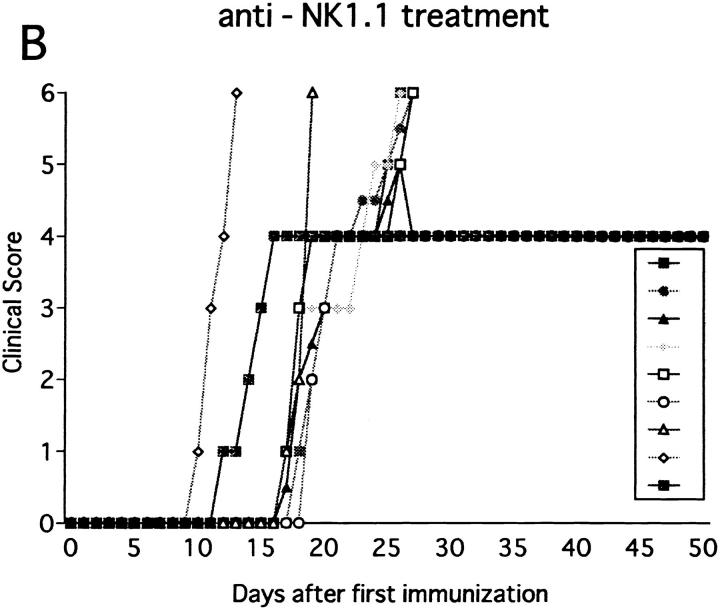
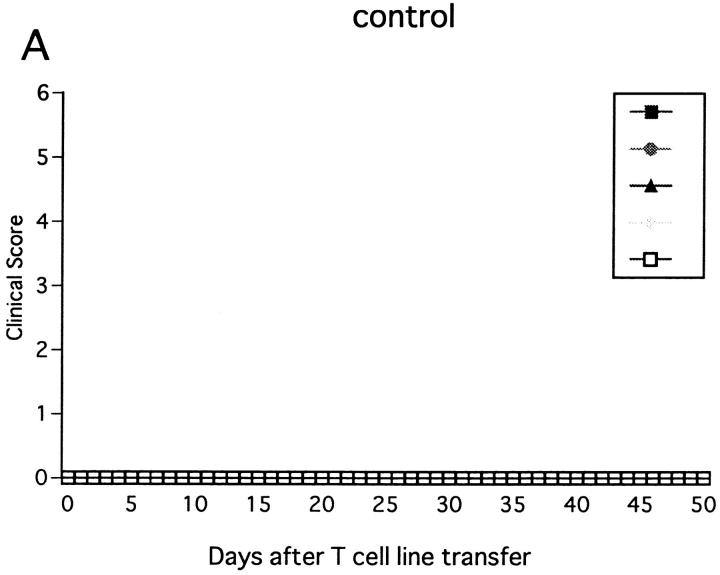
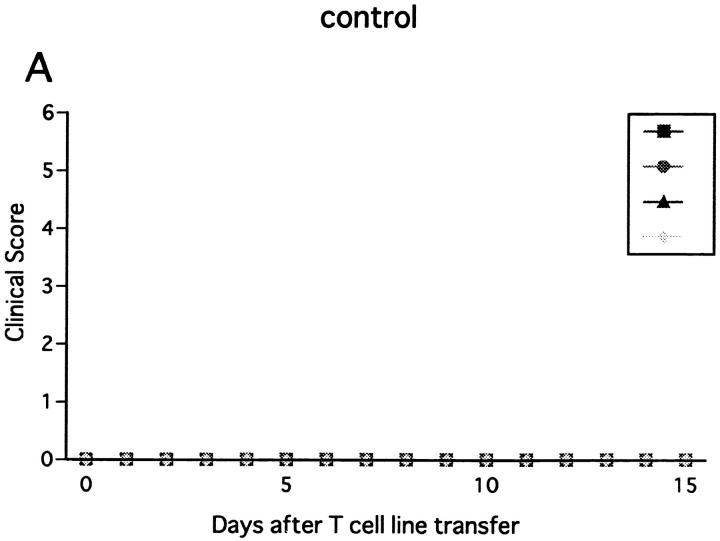
Immunization with MOG35-55 induced a mild form of monophasic EAE with transient loss of tail tone in untreated B6 mice and the mice injected with PBS or control mAb (Fig. 1 A, Table 1). Although the original report (26) described the induction of chronic, nonremitting EAE by this protocol, we have not seen such a serious form of EAE in any of the mice. The difference could be due to variances in mouse substrains or reagents used for immunization. To know the functional role of NK cells, EAE was induced in mice deprived of NK cells with anti-NK1.1 mAb. Control mice were pretreated with isotype-matched control mAb (M-11). Preparatory experiments revealed that the injection of anti-NK1.1 mAb completely deleted NK1.1+ population from the peripheral blood and the spleen within 24 h after antibody injection, which lasted for as long as 5 wk, whereas control mAb induced only a mild, transient loss of NK1.1+ cells (Fig. 2). This was consistent with the description that the mAb is effective in depleting NK cells in vivo (13, 15–17). In addition, we confirmed that mAbs reactive to the allotype of the anti-NK1.1 mAb (anti-IgG2a) did not stain spleen cells of the mice on 24 h, 72 h, 7 d, and 16 d after injection of anti-NK1.1. This excludes the possibility that antibody-coated NK1.1+ cells may remain but cannot be detected due to blocking of NK1.1 epitope.
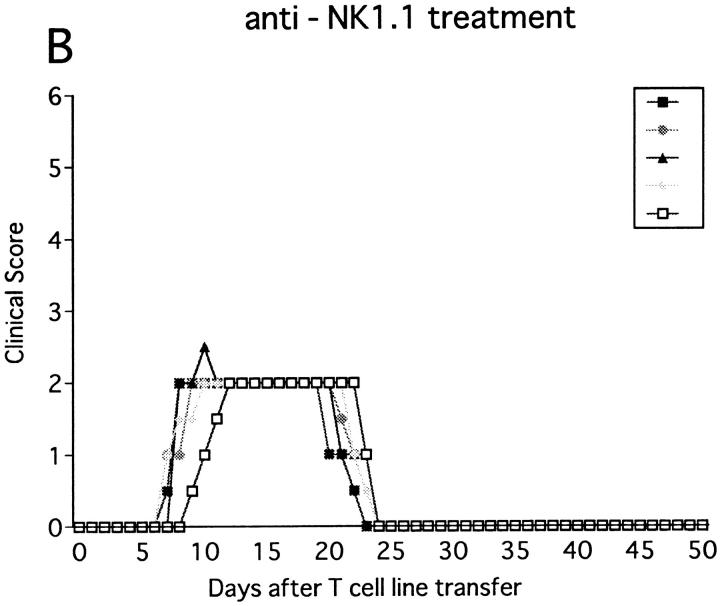
Figure 1.
Effect of anti-NK1.1 mAb on MOG35-55 induced EAE in wild-type B6 mice. Mice were immunized two times with the MOG35-55 for EAE induction. They were intravenously injected with PBS, 500 μg of control mAb (M-11; A), or 500 μg of anti-NK1.1 mAb (PK136; B) 1 d before first immunization with the MOG peptide. Clinical score of individual mice at each observation time point is shown by different marks. This is a representative of two experiments with similar results. The result of pretreatment with PBS did not differ significantly from that with control mAb.
Table 1.
Effect of NK Cell Deletion on EAE Actively Induced in Wild-type B6 and Induced Mutants
| Strain | Treatment | Incidence of EAE | Mortality | Maximum grade | Average disease onset |
|---|---|---|---|---|---|
| (d) | |||||
| Wild-type B6 | Control | 12/12 | 0/12 | 0.8 ± 0.2 | 12.3 ± 0.5 |
| Wild-type B6 | Anti-NK1.1 | 12/12 | 6/12* | 4.3 ± 1.8* | 9.5 ± 0.5‡ |
| β2m−/− | Control | 12/14 | 0/14 | 1.8 ± 0.8 | 18.8 ± 1.3 |
| β2m−/− | Anti-NK1.1 | 18/18 | 12/18‡ | 5.3 ± 0.9‡ | 16.1 ± 2.8 |
| IFN-γ−/− | Control | 6/6 | 5/6 | 5.9 ± 0.2 | 18.2 ± 1.6 |
| IFN-γ−/− | Anti-NK1.1 | 6/6 | 5/6 | 5.9 ± 0.2 | 17.5 ± 1.0 |
Figure 2.
NK cell deletion by anti-NK1.1 mAb. B6 mice were intraperitoneally injected with 500 μg of control mAb (M-11; top) or 500 μg of anti-NK1.1 mAb (PK136; bottom). On days 5, 16, and 36, the spleen cells were stained with anti-NK1.1-PE and anti-CD3-FITC mAbs. Note the persistent deletion of NK cells (NK1.1+CD3− cells) in the total spleen cells after anti-NK1.1 mAb treatment: 2.68–5.63% in mice treated with control mAb versus 0.3–0.6% in mice treated with anti-NK1.1 mAb.
The anti-NK1.1–treated mice developed EAE significantly earlier than control mice (P <0.001) as compared to control mice (Fig. 1 B, Table 1). Although all the mice completely recovered from the paralysis within 10 d regardless of the mAb treatments, only NK cell–deleted mice developed clinical relapse on days 26–29 after first immunization. The degree of paralysis in the relapse was variable, and mice with severe paralysis died of EAE. In summary, anti-NK1.1 mAb treatment significantly enhanced EAE in the mortality rate (P <0.001) and maximum clinical grade (P <0.01).
Augmentation of EAE in β2m− /− Mice by NK Cell Deletion.
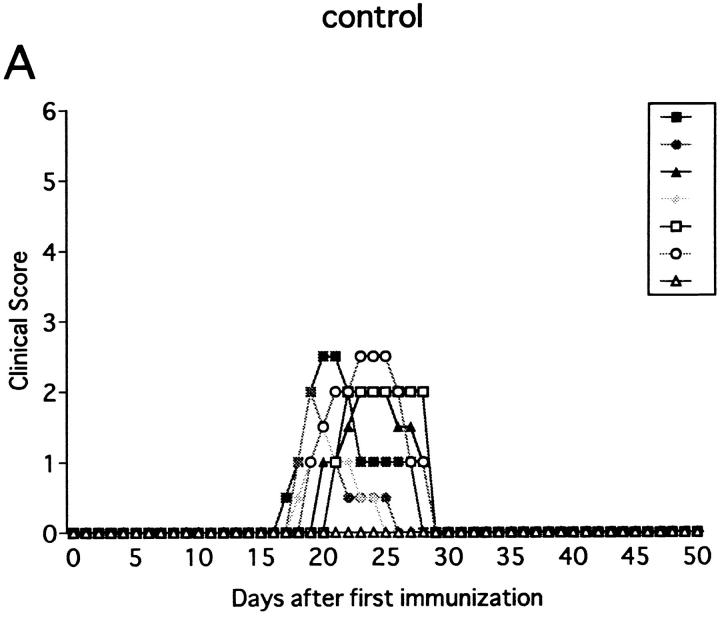
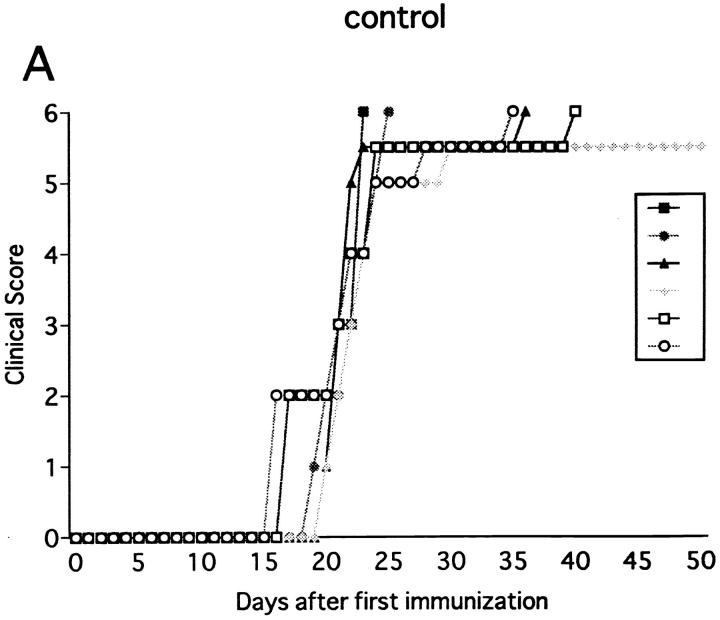
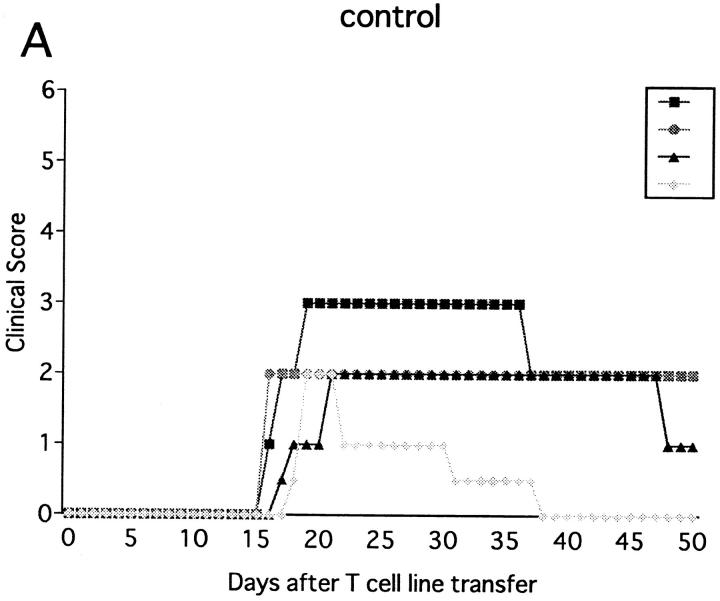
Injection of anti-NK1.1 mAb deletes both NK and NK–T cells. Since our primary target was NK cells, experimental systems were required in which involvement of NK–T cells can be entirely excluded. Because NK–T cells, positively selected against the CD1 molecule, are absent in β2m−/− mice defective for expression of CD1 (32, 33), we used this mutant strain for further analysis. Previous studies showed that β2m−/− mice are susceptible to some, but not all autoimmune diseases (34–36). We first examined whether immunization with MOG35-55 can induce EAE in this mutant. As shown in Fig. 3 A and Table 1, the mutant mice developed monophasic EAE, of which clinical course is comparable to that induced in wild-type B6 mice except for significantly later onset of illness (P <0.001). To our knowledge, this is the first experimental proof using knockout mice that shows that even when class I–restricted cells including CD8+ T cells, CD4−CD8− T cells, and NK–T cells are virtually absent, there is no alteration in EAE induction, spontaneous recovery, or resistance against relapses. Although a previous report has documented that there is a higher frequency of EAE relapses in CD8−/− mice (6), we have not seen any relapse in the mice lacking CD8+ T cells.
Figure 3.
Effect of anti-NK1.1 mAb on MOG35-55 induced EAE in β2m−/− mice. The mice were immunized two times with MOG35-55 peptide for EAE induction. On the day before first immunization, control mAb (A) or anti-NK1.1 mAb (B) was intravenously injected. This is a representative of three experiments with similar results.
Although NK cell–deleted wild-type B6 consistently recovered from the first episode (Fig. 1 B), β2m−/− mice, deprived of NK cells, developed a more serious form of acute progressive or chronic, nonremitting EAE (Fig. 3 B). Although the days of onset were not significantly altered, the maximum clinical score was dramatically enhanced in NK cell–deleted β2m−/− as compared to control β2m−/− (P <0.001). Although the total mortality rate evaluated on day 50 was not much different between NK-deleted B6 and NK-deleted β2m−/− (6/12 versus 12/18), the latter tended to die at an earlier time point. If mortality rate is assessed on day 30, NK cell–deleted β2m−/− showed a higher rate compared with NK cell–deleted wild-type mice (1/12 versus 12/18, P <0.001). Taking it into consideration that the clinical courses of control wild-type B6 and β2m−/− mice are comparable, we reason that β2m−/− mice are more dependent on regulatory NK cells than wild-type B6 in order to compensate for the absence of class I–restricted regulatory cells.
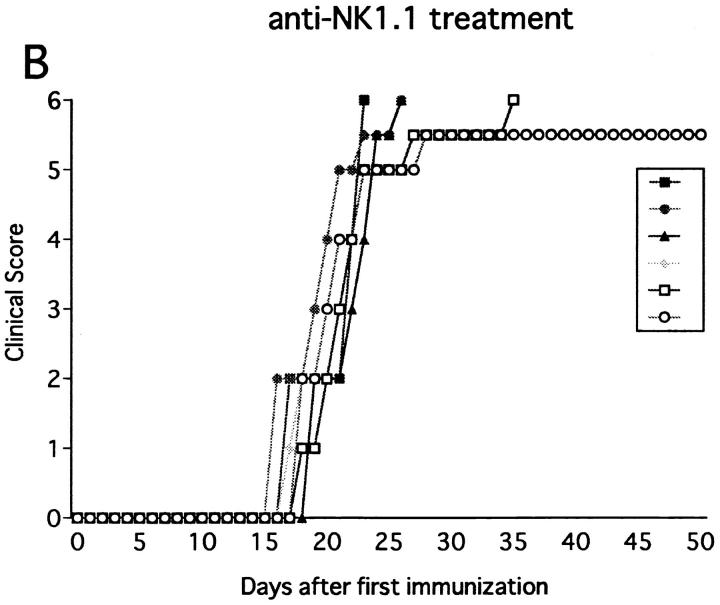
Anti-NK1.1 mAb on EAE Induced in IFN-γ− /− Mice.
NK cell activity is known to be impaired in IFN-γ−/− mice (29). This information prompts us to study the effect of NK cell deletion on EAE in this mutant strain. Interestingly, the spleen in the mutant was only one-fourth the size of that of wild-type mice. In addition, the proportion of NK cells (NK1.1+CD3− cells) in the total spleen cells was markedly reduced (1.0% in IFN-γ−/− versus 5.0% in wild-type), indicating that the development of NK cells is impaired in the mutant due to the genetic defect. As shown in Fig. 4, IFN-γ−/− mice immunized with MOG35-55 developed monophasic EAE which is more serious and uniform than wild-type B6 mice. Notably, pretreatment with anti-NK1.1 mAb showed no obvious effects on the clinical course (Fig. 4, Table 1). These results allowed us to interpret that the regulation mediated by NK cells is reduced in IFN-γ−/− mice, probably due to the decreased number as well as impaired regulatory function of NK cells. It remains unclear how the gene defect leads to the NK cell dysfunction.
Figure 4.
Effect of anti-NK1.1 mAb on MOG35-55 induced EAE in IFN-γ−/− mice. The mice were immunized two times with MOG35-55 peptide for EAE induction. 1 d before first immunization, the mice were intravenously injected with control mAb (A) or anti-NK1.1 mAb (B). The result of pretreatment with PBS did not differ from results shown in A or B.
Correlation of EAE Severity with Th1 Cytokine Production.
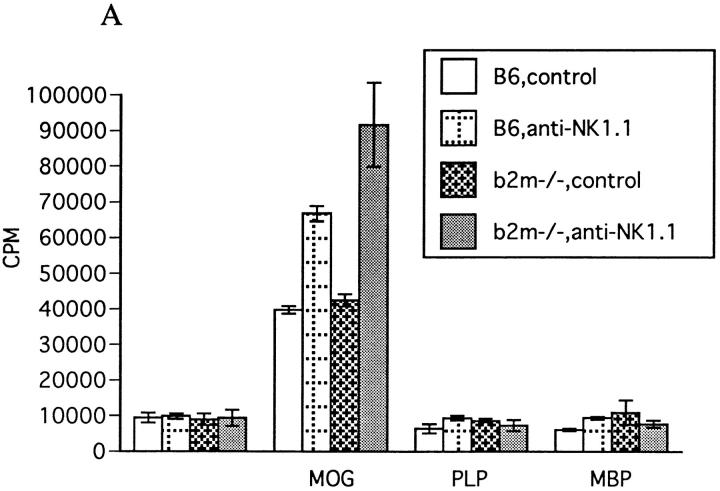
To know the immunological basis for the enhancement of EAE by NK cell deletion, we measured the proliferation of LN cells and their Th1 (IFN-γ, IL-2) and Th2 (IL-4, IL-10) cytokine production in response to the MOG peptide. As shown in Fig. 5 A, the proliferative response to MOG35-55 was significantly enhanced with anti-NK1.1 mAb treatment in both wild-type and βm−/− mice. Production of IFN-γ by the LN cells was also enhanced by NK deletion in vivo (Fig. 5 B). We also measured the cytokine levels in the sera on day 12 after first immunization. As shown in Table 2, the levels of IFN-γ and TNF-α in the sera were dramatically elevated in NK cell–deleted mice compared with controls in both wild-type and β2m−/− mice. It was interesting to note that the cytokine levels roughly correlated with clinical severity of EAE induced in each group of mice. In contrast, the level of IL-4 was not altered by NK cell deletion. The association of EAE enhancement with enhanced production of Th1 cytokines implied that NK cell deletion leads to the augmentation of MOG35-55–specific Th1 induction, whereas Th2 induction is unaltered or relatively inhibited.
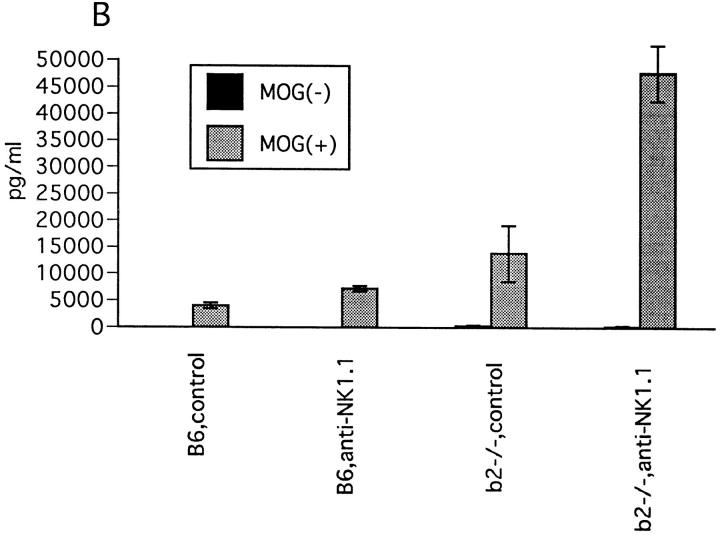
Figure 5.
Effect of NK cell deletion on T cell response to MOG35-55. (A) LN cell proliferative response. 11 d after immunization with MOG35-55, draining LN cells were prepared and their proliferative responses to MOG35-55, PLP136-150 (PLP), and rat myelin basic protein89-101 (MBP) (reference 9) were assayed by a standard method. 1 d before immunization, mice were injected intravenously either with control mAb or with anti-NK1.1 mAb. Data represent mean ± SD of the mean cpm obtained by triplicate cultures in four independent experiments. Each column shows the data of wild-type B6 mice pretreated with control M-11 mAb (B6,control), B6 pretreated with anti-NK1.1 mAb (B6,anti-NK1.1), β2m−/− mice pretreated with control M-11 mAb (b2m−/−, control), and β2m−/− mice pretreated with anti-NK1.1 mAb (b2m−/−, anti-NK1.1). (B) IFN-γ production by LN cells. 11 d after immunization with MOG35-55, the LN cells from control mAb– (control) or anti-NK1.1 mAb– treated (anti-NK1.1) B6 or β2m−/− mice were cultured for 40 h with (MOG+) or without MOG35-55 (MOG−) and the supernatants were collected for measurement of IFN-γ, IL-2, IL-4, and IL-10 by ELISA. Although IL-2, IL-4, and IL-10 were not detectable in this experimental setting, significant production of IFM-γ was measured as shown here. Data represent mean ± SD of the mean value obtained by duplicate assays in four independent experiments.
Table 2.
Cytokine Levels in the Sera of Mice on Day 12 after Immunization
| Strain | Treatment | IFN-γ | TNF-α | IL-4 |
|---|---|---|---|---|
| Wild-type B6 | – | 46.8 ± 2.2 | 46.3 ± 2.8 | 62.3 ± 2.3 |
| Wild-type B6 | Control | 80.2 ± 2.8 | 48.2 ± 1.8 | 65.4 ± 4.1 |
| Wild-type B6 | Anti-NK1.1 | 348.6 ± 15.1‡ | 114.7 ± 4.3‡ | 66.8 ± 3.4 |
| β2m−/− | – | 114.4 ± 5.1 | 118.2 ± 6.9 | 79.6 ± 2.7 |
| β2m−/− | Control | 263.7 ± 17.6 | 117.3 ± 11.0 | 67.9 ± 3.6 |
| β2m−/− | Anti-NK1.1 | 1043.7 ± 28.7‡ | 283.8 ± 8.3* | 72.1 ± 6.2 |
Augmentation of Passive EAE by NK Cell Deletion.
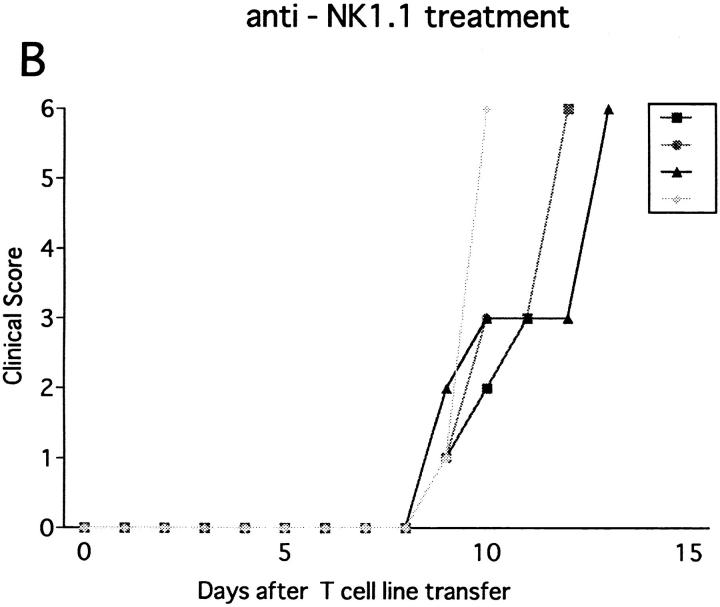
To learn whether NK cells have a regulatory effect on the effector phase of EAE as well, we studied the effect of NK cell deletion on EAE passively induced with the ZB-1 line. The line T cells were CD4+ and specifically recognized MOG35-55 in the context of MHC class II (data not shown). Adoptive transfer of 3 × 106 of the line cells did not induce EAE in the B6 mice pretreated with PBS or with control mAb. In contrast, mice pretreated with anti-NK1.1 mAb developed EAE on days 8–10 after cell transfer (Fig. 6 B). The clinical sign of EAE was relatively mild, but persisted for longer than 2 wk. The result proved that NK1.1+ cells inhibit the effector phase of EAE.
Figure 6.
Effect of NK cell deletion on passive EAE in wild-type B6 mice. After activation with MOG35-55 for 3 d, ZB-1 line cells (3 × 106) were intravenously transferred into wild-type B6 mice pretreated on day −1 with control mAb (A) or with anti-NK1.1 mAb (B). The mice had been x irradiated (450 rad) shortly before cell transfer, and received 500 ng of PT immediately after cell transfer.
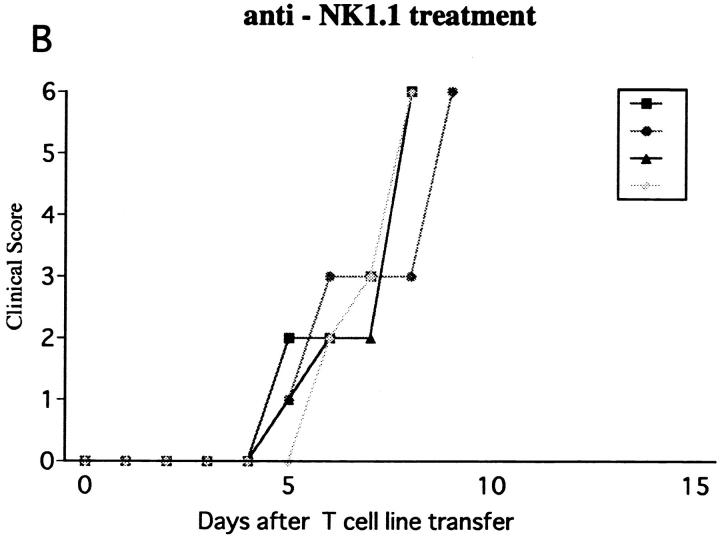
Using the same line cells, we next induced EAE in β2m−/− mice. To ensure the induction of disease, 107 cells were transferred to the mutant strain. The first sign of EAE was on days 16–17 after transfer in control mice (β2m−/− nonpretreated or pretreated with control mAb). Although the clinical course was variable, none of the mice died of EAE in the observation period 50 d after cell transfer (Fig. 7 A). In contrast, NK cell–deleted β2m−/− mice developed earlier onset of hyperacute EAE and all the mice died within 1 wk after onset (Fig. 7 B) proving that the regulatory role of NK cells in passive EAE can be independent of NK–T cells or CD8+ T cells.
Figure 7.
Effect of NK cell deletion on passive EAE in β2m−/− mice. After activation with MOG35-55 for 3 d, ZB-1 line cells (107) were intravenously transferred into β2m−/− mice pretreated on day −1 with control mAb (A) or with anti-NK1.1 mAb (B). The mice had been x irradiated (450 rad) shortly before cell transfer, and received 500 ng of PT immediately after cell transfer.
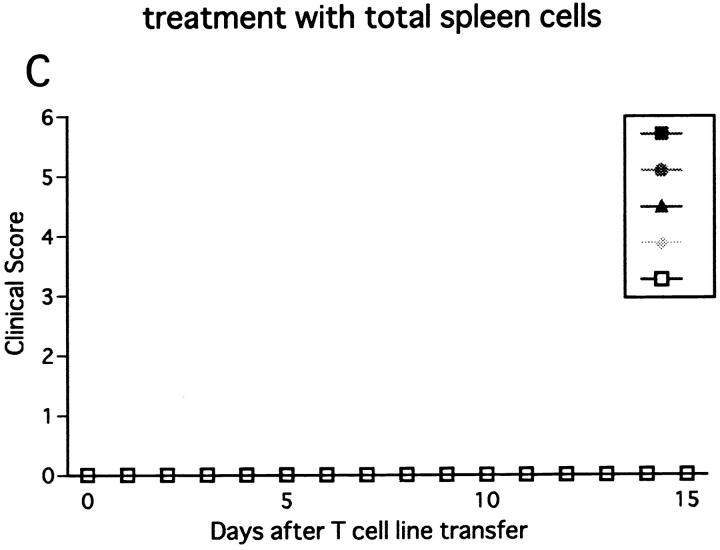
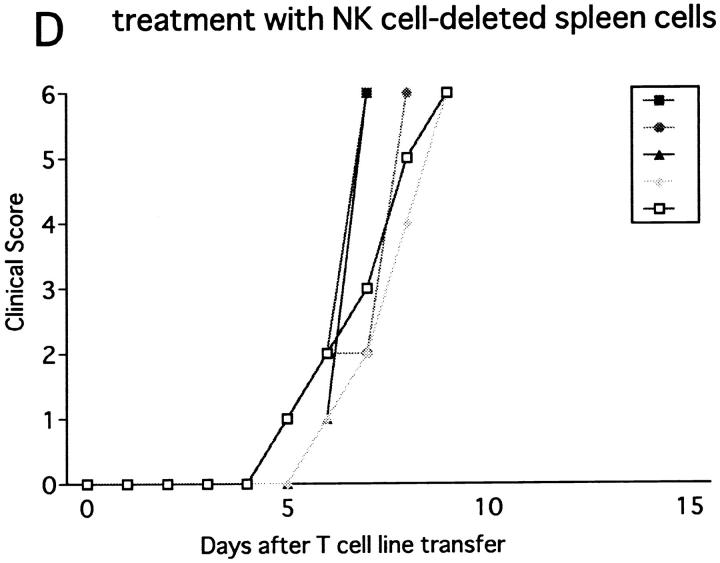
Next we injected the line cells into RAG-2−/− mice, which lack T, B, and NK–T cells due to the defect in the recombination machinery. Transfer of 107 or 3 × 106 cells induced a hyperacute, lethal form of EAE in untreated RAG-2−/− mice (data not shown). This indicates that the RAG-2−/− mice are more prone to passive EAE than wild-type or β2m−/− mice, probably due to the absence of regulatory cells bearing clonotypic receptors. In contrast, when the number of transferred cells was reduced to 5 × 105, EAE was not induced in control RAG-2−/− mice. However, if NK cells were deleted in vivo, the same number of cells induced hyperacute EAE within 5–6 d after cell transfer (Fig. 8 B). Notably, this lethal disease was completely prevented by transfer of 2 × 107 spleen cells of normal RAG-2−/− mice, which contain 4 × 106 NK cells (Fig. 8 C). In contrast, the same number of spleen cells of RAG-2−/− mice deprived of NK cells with anti-NK1.1 mAb showed no ameliorating effects (Fig. 8 D). These results indicate that NK cells of RAG-2−/− mice play a regulatory role, whereas non–NK cells from the mutant strain do not.
Figure 8.
Effect of NK cell deletion on passive EAE in RAG-2−/− mice and the treatment with spleen cells. 5 × 105 of activated ZB-1 line cells were intravenously transferred into (A) RAG-2−/− mice pretreated on day −1 with control mAb (A) or with anti-NK1.1 mAb (B, C, and D). The mice were injected with 500 ng PT after cell transfer. Although mice in B did not receive any further manipulation, 2 × 107 of spleen cells from RAG-2−/− mice were intravenously transferred to mice in C on day 2 and the same number of spleen cells from RAG-2−/− mice which had been pretreated with anti-NK1.1 mAb on day −1, were intravenously transferred to mice in D. This is a representative of two experiments with similar results.
In additional experiments, 5 × 105 of ZB-1 line cells were transferred into wild-type or β2m−/− mice that had been pretreated with anti-NK1.1 mAb (4 mice/group). None of the mice developed EAE, contrasting the fatal outcome seen in RAG-2−/− mice. This indicates that RAG-2−/− mice are more dependent on NK cells than wild-type or β2m−/− in the protection against passive EAE. Taken together, we conclude that NK cells can inhibit the effector phase of EAE in a manner independent of T, B, or NK–T cells.
Inhibition of T Cell Proliferation by NK Cells.
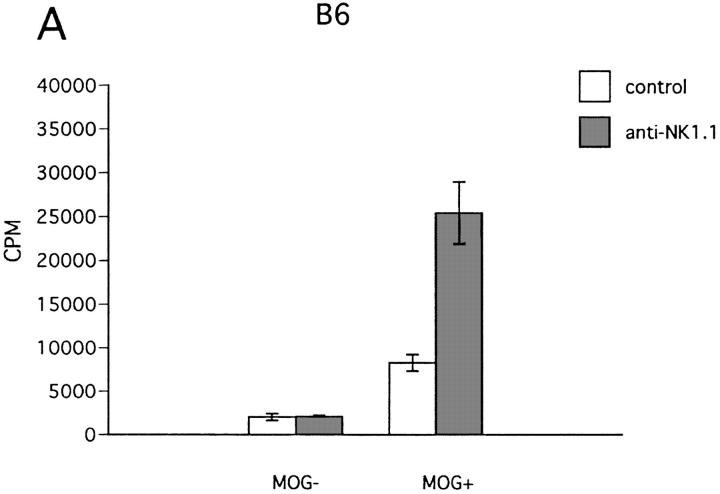
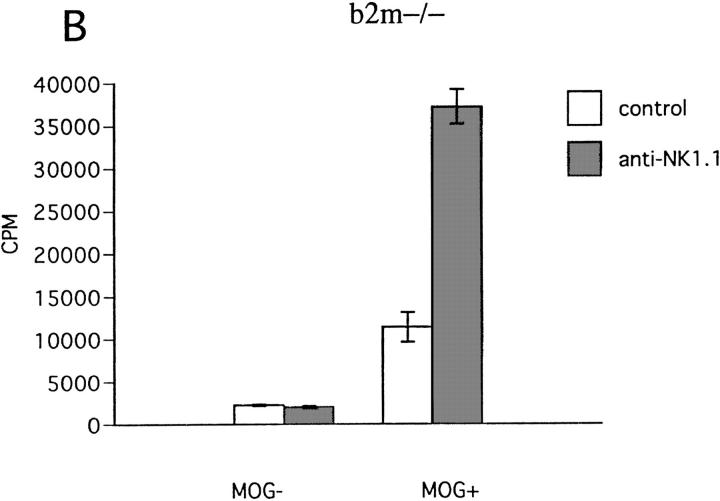
To obtain insights into the mechanism of NK cell–mediated regulation, we conducted in vitro experiments, assaying antigen-induced proliferation of ZB-1 cells in the presence of irradiated spleen cells as APCs. First, spleen cells from NK cell–deleted mice were compared with those from control mice as accessory cells for ZB-1 line cells. The experiment showed that antigen-induced ZB-1 cell proliferation is enhanced when spleen cells were derived from NK-deleted mice. We obtained similar results not only with spleen cells from wild-type B6 mice, but also from β2m−/− mice (Fig. 9, A and B). These indicate that irradiated, spleen NK cells would inhibit antigen-induced Th1 proliferation. Notably, the proliferative responses obtained by using different sources of spleen APCs roughly correlated with clinical grade of EAE and Th1 cytokine levels in the sera (Table 2), supporting the relevance of the in vitro system for estimating in vivo conditions.
Figure 9.
Effect of NK cell deletion on antigen-induced proliferation of T line cells. ZB-1 T line cells (4 × 104 cells/well) were stimulated with MOG35-55 in the presence of x irradiated spleen cells (8 × 105 cells/well) from wild-type B6 (A) or β2m−/− mice (B). In each experiment, spleen cells from mice pretreated with control mAb (control) and those pretreated with anti-NK1.1 mAb (anti-NK1.1) were compared in their accessory function. Data represent mean cpm ± SD of triplicate cultures. This is a representative of three experiments with similar results.
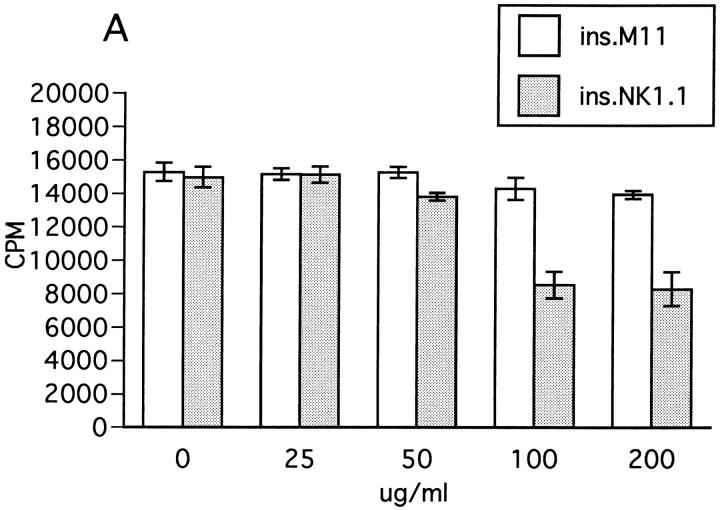
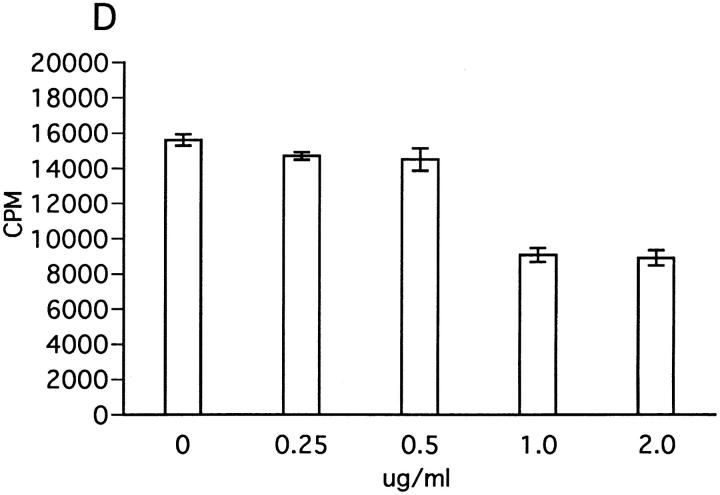
We next examined the effect of insolubilized anti-NK1.1 mAb which can trigger activation of NK cells via cross-linking NKR-P1 molecule (37). In a reciprocal manner with the results in Fig. 9, ZB-1 cell proliferation in response to MOG was significantly inhibited when spleen NK cells were stimulated by anti-NK1.1 mAb (Fig. 10 A). In contrast, insolubilized control mAb had no effect, further supporting the inhibitory role of NK cells against T cell proliferation. The background proliferation of ZB-1 cells was not inhibited by insolubilized anti-NK1.1 (Fig. 10 B). We also explored the possible involvement of IFN-γ as a downregulatory factor, since this Th1 cytokine is the major product of NK cells. However, with the addition of exogenous IFN-γ, the proliferation of ZB-1 line cells was slightly but significantly enhanced (Fig. 10 C), whereas anti– IFN-γ mAb inhibited the cell proliferation (Fig. 10 D). In addition, depressed proliferative response induced by insolubilized anti-NK1.1 mAb was further inhibited by anti– IFN-γ mAb (data not shown). These results indicate that NK cell–mediated T cell suppression is not mediated by IFN-γ.
Figure 10.
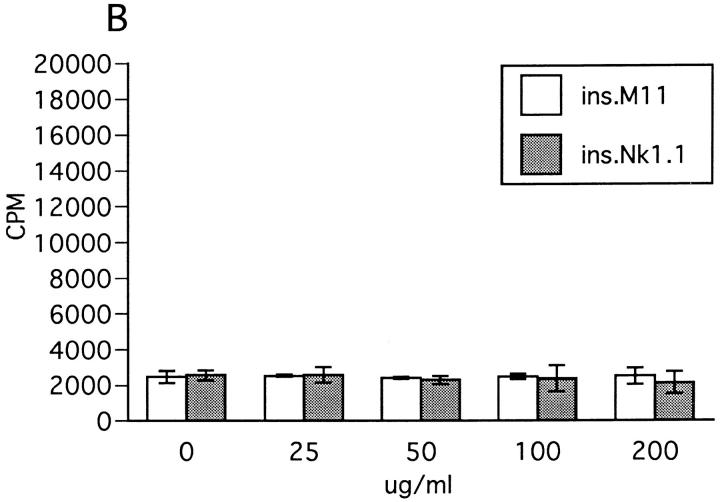
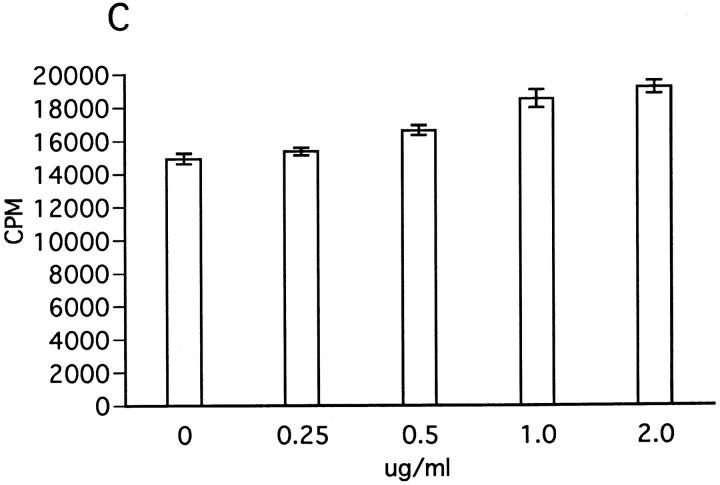
Effect of insolubilized anti-NK1.1 mAb, IFN-γ, and anti–IFN-γ on ZB-1 line proliferation. (A) Anti-NK1.1 and control M-11 mAb dissolved in PBS were added into relevant wells at various concentrations (shown in micrograms per milliliter), incubated overnight, and then washed with PBS intensively. ZB-1 line cells (4 × 104/well) were stimulated with MOG35-55 (25 μg/ml) in the presence of irradiated spleen APCs (8 × 105/well) in the wells coated with M-11 (ins. M11) or with anti-NK1.1 mAb (ins.NK1.1). (B) ZB-1 line cells were cultured with spleen cell APCs in the absence of the MOG peptide in the antibody-coated wells in parallel with experiment A. (C) ZB-1 line cells were stimulated with MOG35-55 using spleen APCs in the presence of different concentrations of recombinant mouse IFN-γ (PharMingen). (D) ZB-1 line cells were stimulated with MOG35-55 using spleen APCs in the presence of different concentrations of anti–mouse IFN-γ mAb (PharMingen). All the data represent mean cpm ± SD of triplicate cultures.
Cytotoxic killing of B7-1+ macrophages has been suggested as a mechanism for regulation by NK cells (12, 38). We have not yet excluded the possibility that NK cell– mediated suppression of ZB-1 line cells may arise from cytotoxic killing of B7-1+ APCs by NK cells. However, we feel that this is not very likely because the NK/target cell ratio in the assay condition is far from an optimal one, and the number of B7-1+ cells in the spleen or LNs did not decrease in mice deprived of NK cells and then challenged for EAE. Furthermore, we recently found that T cell proliferation independent of antigen presentation process (recombinant IFN-γ or anti-CD3 mAb triggered T cell proliferation) can also be inhibited by NK cells. Experiments are now in progress to analyze the interaction between NK cells and encephalitogenic T cells.
Concluding Remarks.
The present study demonstrates that NK cells are involved in the regulation of EAE. Using β2m−/− mice, we proved that NK cells can regulate EAE in a manner independent of NK–T cells. The function of NK cells in immune response sometimes depends on other immune elements such as CD8+ T cells (39). However, using induced mutants, we excluded the requirement of other populations such as CD8+ T cells in the NK cell–mediated regulation of EAE. We also indicated that the gene knockout mice for β2m and RAG-2 can be more susceptible to EAE, particularly when NK cells are deleted. This raises a possibility that depression of NK activity to the degree that would have little effect on otherwise healthy individuals, may lead to enhancement or induction of autoimmune disease in those with a defect in the regulatory system.
In summary, we show how NK cells are deeply involved in the resistance against EAE, a representative Th1-mediated autoimmune disease. Although NK cells collaborate with adaptive immunity and enhance Th1 immunity through producing IFN-γ in the protection against microbes (40– 42), they would rather inhibit Th1 activity in the protection against autoimmune disease. Functions of NK cells are modulated by a number of endogenous and exogenous stimuli, and modulation of NK function has been suggested in autoimmune diseases such as multiple sclerosis (43). Further studies on the regulatory interaction between NK and T cells may lead to identification of key molecules involved in the regulation of autoimmune diseases.
Acknowledgments
This work was supported by grants from the Science and Technology Agency, the Ministry of Health and Welfare, grant-in-aid for Scientific Research 07457159 from the Ministry of Education, Science and Culture, and the grant provided by the Ichiro Kanehara Foundation.
Footnotes
1
Abbreviations used in this paper: β2m; β2-microglobulin; EAE, experimental autoimmune encephalomyelitis; MOG, myelin oligodendrocyte glycoprotein; PT, pertussis toxin; RAG, recombination activation gene.
References
- 1.Experimental allergic encephalomyelitis: a useful model for multiple sclerosis. 1984. E.C. Alvord, Jr., M.W. Kies, and A.J. Suckling, editors. Alan R. Liss, New York.
- 2.Tabira, T., and J. Kira. 1992. Strain and species differences of encephalitogenic determinants of myelin basic protein and proteolipid apoprotein. In Myelin: Biology and Chemistry. R.E. Martenson, editor. CRC Press Inc., Boca Raton, FL. 783–799.
- 3.Kumar V, Sercarz EE. The involvement of T cell receptor peptide-specific regulatory CD4+T cells in recovery from antigen-induced autoimmune disease. J Exp Med. 1993;178:909–916. doi: 10.1084/jem.178.3.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen Y, Kuchroo VK, Inobe J-I, Hafler DA, Weiner HL. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science (Wash DC) 1994;265:1237–1240. doi: 10.1126/science.7520605. [DOI] [PubMed] [Google Scholar]
- 5.Sun D, Ben-Nun A, Wekerle H. Regulatory circuits in autoimmunity: recruitment of counter-regulatory CD8+ T cells by encephalitogenic CD4+T line cells. Eur J Immunol. 1988;18:1993–1999. doi: 10.1002/eji.1830181219. [DOI] [PubMed] [Google Scholar]
- 6.Koh D-R, Fung-Leung W-P, Ho A, Gray D, Acha-Orbea H, Mak T-W. Less mortality but more relapses in experimental allergic encephalomyelitis in CD8−/−mice. Science (Wash DC) 1992;256:1210–1213. doi: 10.1126/science.256.5060.1210. [DOI] [PubMed] [Google Scholar]
- 7.Jiang H, Zhang SI, Pernis B. Role of CD8+T cells in murine experimental allergic encephalomyelitis. Science (Wash DC) 1992;256:1213–1215. doi: 10.1126/science.256.5060.1213. [DOI] [PubMed] [Google Scholar]
- 8.Sun D, Wekerle H, Rapper K, Gold DP. CD4−CD8−splenic T cells from Lewis rats recovered from experimental autoimmune encephalomyelitis respond to encephalitogenic T cells that mediate this disorder. Cell Immunol. 1991;137:292–302. doi: 10.1016/0008-8749(91)90080-u. [DOI] [PubMed] [Google Scholar]
- 9.Kozovska MF, Yamamura T, Tabira T. T–T cellular interaction between CD4−CD8−regulatory T cells and T cell clones presenting TCR peptide. Its implication for TCR vaccination against experimental autoimmune encephalomyelitis. J Immunol. 1996;157:1781–1790. [PubMed] [Google Scholar]
- 10.Wolf SD, Dittel BN, Hardardottir F, Janeway CA., Jr Experimental autoimmune encephalomyelitis induction in genetically B cell–deficient mice. J Exp Med. 1996;184:2271–2278. doi: 10.1084/jem.184.6.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kobayashi Y, Kawai K, Ito K, Honda H, Sobue G, Yoshikai Y. Aggravation of murine experimental allergic encephalomyelitis by administration of T-cell receptor γδ–specific antibody. J Neuroimmunol. 1997;73:169–174. doi: 10.1016/s0165-5728(96)00187-7. [DOI] [PubMed] [Google Scholar]
- 12.Gilbertson SM, Shah PD, Rowley DA. NK cells suppress the generation of Lyt-2+cytolytic T cells by suppressing or eliminating dendritic cells. J Immunol. 1986;136:3567–3571. [PubMed] [Google Scholar]
- 13.Takeda K, Dennert G. The development of autoimmunity in C57BL/6 lprmice correlates with the disappearance of natural killer type 1–positive cells: evidence for their suppressive action on bone marrow stem cell proliferation, B cell immunoglobulin secretion, and autoimmune symptoms. J Exp Med. 1993;177:155–164. doi: 10.1084/jem.177.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scharton TM, Scott P. Natural killer cells are a source of interferon γ that drives differentation of CD4+ T cell subsets and induces early resistance to Leishmania majorin mice. J Exp Med. 1993;178:567–577. doi: 10.1084/jem.178.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kos FJ, Engelman EG. Requirement for natural killer cells in the induction of cytotoxic T cells. J Immunol. 1995;155:578–584. [PubMed] [Google Scholar]
- 16.Orange JS, Wang B, Terhorst C, Biron CA. Requirement for natural killer cell–produced interferon γ in defense against murine cytomegalovirus infection and enhancement of this pathway by interleukin 12 administration. J Exp Med. 1995;182:1045–1056. doi: 10.1084/jem.182.4.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilder JA, Koh CY, Yuan D. The role of NK cells during in vivo antigen-specific antibody responses. J Immunol. 1996;156:146–152. [PubMed] [Google Scholar]
- 18.Mercurio AM, Schwarting GA, Robbins PW. Glycolipids of the mouse peritoneal macrophages. J Exp Med. 1984;160:1114–1125. doi: 10.1084/jem.160.4.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suttles J, Schwarting GA, Stout RD. Flow cytometric analysis reveals presence of asialo GM1 on the surface membrane of autoimmune cytotoxic T cells. J Immunol. 1986;136:1586–1591. [PubMed] [Google Scholar]
- 20.Bix M, Locksley RM. Natural T cells. Cells that co-express NKRP-1 and TCR. J Immunol. 1995;155:1020–1022. [PubMed] [Google Scholar]
- 21.Bendelac A. Mouse NK1+T cells. Curr Opin Immunol. 1995;7:367–374. doi: 10.1016/0952-7915(95)80112-x. [DOI] [PubMed] [Google Scholar]
- 22.Taniguchi M. V alpha 14+NK T cells: a novel lymphoid cell lineage with regulatory function. J Allergy Clin Immunol. 1996;98:S263–S269. doi: 10.1016/s0091-6749(96)70074-x. [DOI] [PubMed] [Google Scholar]
- 23.Yoshimoto T, Paul WE. CD4pos, NK1.1posT cells promptly produce interleukin 4 in response to in vivo challenge with anti-CD3. J Exp Med. 1994;179:1285–1295. doi: 10.1084/jem.179.4.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mieza MA, Itoh T, Cui JQ, Makino Y, Kawano T, Tsuchida K, Koike T, Shirai T, Yagita H, Matsuzawa A, et al. Selective reduction of Vα14+NK T cells associated with disease development in autoimmune-prone mice. J Immunol. 1996;156:4035–4040. [PubMed] [Google Scholar]
- 25.Sumida T, Sakamoto A, Murata H, Makino Y, Takahashi H, Yoshida S, Nishioka K, Iwamoto I, Taniguchi M. Selective reduction of T cells bearing Vα24JαQ antigen receptor in patients with systemic sclerosis. J Exp Med. 1995;182:1163–1168. doi: 10.1084/jem.182.4.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mendel I, Kerlero de Rosbo N, Ben-Nun A. A myelin oligodendrocyte glycoprotein peptide induces typical chronic experimental autoimmune encephalomyelitis in H-2bmice: fine specificity and T cell receptor Vb expression of encephalitogenic T cells. Eur J Immunol. 1995;25:1951–1959. doi: 10.1002/eji.1830250723. [DOI] [PubMed] [Google Scholar]
- 27.Koller BH, Marrack P, Kappler JW, Smithies O. Normal development of mice deficient in beta-2M MHC class I proteins, and CD8+T cells. Science (Wash DC) 1990;248:1227–1229. doi: 10.1126/science.2112266. [DOI] [PubMed] [Google Scholar]
- 28.Shinkai Y, Rathbun G, Lam K-P, Oltz EM, Stewart V, Mendelsohn M, Charron J, Datta M, Young F, Stall AM, Alt FW. RAG-2–deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 1992;68:855–867. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- 29.Dalton DK, Pitts-Meek S, Keshav S, Figari IS, Bradley A, Stewart TA. Multiple defects of immune cell function in mice with disrupted interferon-gamma genes. Science (Wash DC) 1993;259:1739–1745. doi: 10.1126/science.8456300. [DOI] [PubMed] [Google Scholar]
- 30.Tokuchi F, Nishizawa M, Nihei J, Motoyama K, Nagashima K, Tabira T. Lymphokine production by encephalitogenic and non-encephalitogenic T-cell clones reactive to the same antigenic determinant. J Neuroimmunol. 1990;30:71–79. doi: 10.1016/0165-5728(90)90054-q. [DOI] [PubMed] [Google Scholar]
- 31.Koo GC, Peppard JR. Establishment of monoclonal anti-NK1.1 antibody. Hybridoma. 1984;3:301–303. doi: 10.1089/hyb.1984.3.301. [DOI] [PubMed] [Google Scholar]
- 32.Bendelac A, Killeen N, Littman DR, Schwartz RH. A subset of CD4+thymocytes selected by MHC class I molecules. Science (Wash DC) 1994;263:1774–1778. doi: 10.1126/science.7907820. [DOI] [PubMed] [Google Scholar]
- 33.Adachi Y, Koseki H, Zijlstra M, Taniguchi M. Positive selection of invariant Vα14+T cells by non-major histocompatibility complex–encoded class I–like molecules expressed on bone marrow–derived cells. Proc Natl Acad Sci USA. 1995;92:1200–1204. doi: 10.1073/pnas.92.4.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mozes E, Kohn LD, Hakim F, Singer DS. Resistance of MHC class I–deficient mice to experimental systemic lupus erythematosus. Science (Wash DC) 1993;261:91–93. doi: 10.1126/science.8316860. [DOI] [PubMed] [Google Scholar]
- 35.Shenoy M, Kaul R, Goluszko E, David C, Christadoss P. Effect of MHC class I and CD8 cell deficiency on experimental autoimmune myasthenia gravis pathogenesis. J Immunol. 1994;152:5330–5335. [PubMed] [Google Scholar]
- 36.Sumida T, Furukawa M, Sakamoto A, Namekawa T, Maeda T, Zijlstra M, Iwamoto I, Koike T, Yoshida S, Tomioka H, Taniguchi M. Prevention of insulitis and diabetes in β2-microglobulin–deficient non-obese diabetic mice. Int Immunol. 1994;6:1445–1449. doi: 10.1093/intimm/6.9.1445. [DOI] [PubMed] [Google Scholar]
- 37.Arase H, Arase N, Saito T. Interferon γ production by natural killer (NK) cells and NK1.1+T cells upon NKR-P1 cross-linking. J Exp Med. 1996;183:2391–2396. doi: 10.1084/jem.183.5.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chambers BJ, Salcedo M, Ljunggren H-G. Triggering of natural killer cells by the costimulatory molecule CD80 (B7-1) Immunity. 1996;5:311–317. doi: 10.1016/s1074-7613(00)80257-5. [DOI] [PubMed] [Google Scholar]
- 39.Gray JD, Hirokawa M, Horwitz DA. The role of transforming growth factor beta in the generation of suppression: an interaction between CD8+T cells and NK cells. J Exp Med. 1994;180:1937–1942. doi: 10.1084/jem.180.5.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Janeway CA., Jr The immune system evolved to discriminate infectious nonself from noninfectious self. Immunol Today. 1992;13:11–16. doi: 10.1016/0167-5699(92)90198-G. [DOI] [PubMed] [Google Scholar]
- 41.Fearon DT, Locksley RM. The instructive role of innate immunity in the acquired immune response. Science (Wash DC) 1996;272:50–53. doi: 10.1126/science.272.5258.50. [DOI] [PubMed] [Google Scholar]
- 42.Garside P, Mowat AM. Polarization of Th-cell responses: a phylogenetic consequence of nonspecific immune defense? . Immunol Today. 1995;16:220–223. doi: 10.1016/0167-5699(95)80162-6. [DOI] [PubMed] [Google Scholar]
- 43.Trinchieri G. Biology of natural killer cells. Adv Immunol. 1989;47:187–376. doi: 10.1016/S0065-2776(08)60664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]