Basophils Produce IL-4 and Accumulate in Tissues after Infection with a Th2-inducing Parasite (original) (raw)
Abstract
Using mice in which the eGfp gene replaced the first exon of the Il4 gene (G4 mice), we examined production of interleukin (IL)-4 during infection by the intestinal nematode Nippostrongylus brasiliensis (Nb). Nb infection induced green fluorescent protein (GFP)pos cells that were FcɛRIpos, CD49bbright, c-kitneg, and Gr1neg. These cells had lobulated nuclei and granules characteristic of basophils. They were found mainly in the liver and lung, to a lesser degree in the spleen, but not in the lymph nodes. Although some liver basophils from naive mice express GFP, Nb infection enhanced GFP expression and increased the number of tissue basophils. Similar basophil GFP expression was found in infected Stat6−/− mice. Basophils did not increase in number in infected Rag2−/− mice; Rag2−/− mice reconstituted with CD4 T cells allowed significant basophil accumulation, indicating that CD4 T cells can direct both tissue migration of basophils and enhanced IL-4 production. IL-4 production was immunoglobulin independent and only partially dependent on IL-3. Thus, infection with a parasite that induces a “Th2-type response” resulted in accumulation of tissue basophils, and these cells, stimulated by a non-FcR cross-linking mechanism, are a principal source of in vivo IL-4 production.
Keywords: CD4, T cells, cytokine, green fluorescent protein, Nippostrongylus brasiliensis, liver
Introduction
IL-4 plays a crucial role in the development of Th2-type immune responses and the regulation of immunoglobulin isotype switching to IgE (1, 2). An analysis of the in vivo role of IL-4 and its congener IL-13 has been particularly important in understanding the pathogenesis of allergic diseases and of parasitic infections (1–3). Nonetheless, such experiments have been hampered by the difficulty of measuring cytokine production in situ. Current techniques that use in vitro stimulation (TCR dependent or independent) to detect cytokine production measure cytokine producing potential rather than actual in situ cytokine production. In an effort to overcome such limitations, mice that express surrogates for cytokines have been generated. Among the type I cytokines and the IFNs, mice that express GFP in lieu of IL-2 and IL-4 have been described previously (4,5), as have mice that make GFP in addition to IL-4 or yellow fluorescent protein in addition to IFNγ (6, 7). We have generated a mouse (the G4 mouse) in which the first exon and a portion of the first intron of the Il4 gene have been replaced by the gene encoding enhanced GFP. Cells from G4 mice possessing a Gfp allele of Il4 express GFP instead of IL-4 from that allele (5). Because GFP is not secreted, it is detectable by flow cytometry without need for restimulation; furthermore, GFP has a relatively long half-life and Gfp mRNA is more stable than Il4 mRNA. These characteristics make GFP a sensitive reporter of IL-4 production and, thus, its expression should be representative of in situ IL-4 production.
Infection of mice with the nematode Nippostrongylus brasiliensis (Nb) is a widely used experimental system to study in vivo Th2 immune responses. Nb infection is associated with a highly polarized Th2-type immune responses characterized by high levels of IgE, IL-4, and IL-13 production (3, 8–10). Differentiated Th2 cells producing those cytokines play an important role in the development of host protective immune responses (9, 10). Earlier studies have also demonstrated that IL-4 could be produced from splenic non–B, non–T cells and that such production was increased as a result of Nb infection as well as in the anti-IgD injection model (11). It was shown that FcɛRI+ cells stimulated by FcR cross-linkage or by treatment with ionomycin produced IL-4 and that this IL-4 production was enhanced by IL-3 (11–14). Phenotypic and electron microscopic examination indicated that the IL-4–producing cell population was enriched in basophils and purified FcɛRI+, c-kit− cells were shown to be excellent IL-4 producers (15–17). Human basophils have also been demonstrated to be IL-4 producers (18–22). Recent studies using 4get reporter mice were interpreted to indicate that eosinophils were the main IL-4 producers in the lungs of Nb-infected mice (23). 4get mice, unlike G4 mice, were generated by inserting the Gfp gene 3′ of the Il4 gene behind a highly efficient internal ribosomal entry sequence; therefore, the resulting cells produce both IL-4 and GFP (6). Although GFP expression in cells from these mice appears to Th2 specific, the frequency of GFP+ T cells is substantially greater than that of IL-4 positive cells. In addition, NKT cells express GFP constitutively, whereas in conventional mice NKT cells only express substantial amounts of IL-4 mRNA and protein after stimulation with anti-CD3 or αGalCer (24, 25). Thus, it is possible that GFP expression in 4get mice may reflect basal transcription of the IL-4 locus and may report the capacity of a cell to make IL-4 rather than actual IL-4 production.
To clarify the identification of IL-4–producing nonlymphocytes in response to parasite infection, we investigated IL-4 production using Nb-infected G4 mice. We observed that the principal non–T cells that produce IL-4 were FcɛRI+, CD49b+, c-kit−, and Gr1−. Electron microscopic analysis of these cells identified them as basophils, consistent with the aforementioned set of markers. Basophils were mainly found in tissues such as liver and lung, as well as in spleen and blood, but not in the lymph nodes. Basophil GFP expression was observed even in the liver in naive mice, indicating constitutive IL-4 expression by basophils. The extent of IL-4 production and, more importantly, their recruitment to the tissue was strikingly correlated with Nb infection. Furthermore, basophil responses were independent of IL-4, Stat6, and immunoglobulin, partially dependent on IL-3, but highly dependent on the presence of antigen-activated CD4 T cells. Our results demonstrate that basophils are the primary IL-4–producing non–T cells that accumulate in tissues such as liver and lung during parasitic infection, suggesting an immunoregulatory or effector function in Th2-related immune responses.
Materials and Methods
Mice.
The generation of GFP/IL-4 knockin (G4) mice has been reported previously (5). G4 mice were backcrossed to BALB/c mice and Rag2−/− 5C.C7 TCR transgenic mice for >10 generations; they were obtained from the National Institute of Allergy and Infectious Diseases contract facility at Taconic Farms. BALB/c G4 Stat6−/− mice were obtained by crossing G4 mice with BALB/c Stat6−/− mice. BALB/c mice were purchased from Division of Cancer Treatment, National Cancer Institute. BALB/c Rag2−/− mice were purchased from Taconic Farms. All mice were maintained under pathogen-free conditions.
Nb Infection.
Third stage infectious larvae (L3) of Nb were inoculated by subcutaneous injection. Lymph nodes (draining or nondraining), spleen, Peyer's patches, lung, and liver cells were harvested 10 d after infection, and GFP expression was measured by flow cytometry. In some experiments, infected mice were treated with 2 mg anti–IL-3 (8F8; reference 26) or control antibody (GL113) every 3 d after infection.
Liver and Lung Cell Preparation.
Isolation of cells from liver was performed as reported previously (27). Perfused lung was minced and incubated for 1 h in culture media containing 2.4 mg/ml collagenase type I (Invitrogen) and 0.1% DNase I (Roche). A single cell suspension was layered over a 30/70% Percoll gradient. Cells were spun at 2,000 revolutions/min for 20 min at room temperature. Cells at the interface were recovered and washed before counting. Processed cells were stained for surface markers and analyzed by FACS® analysis, or stimulated with 10 ng/ml PMA plus 1 μM ionomycin for 4 h. 2 μM monensin was added in the culture during the last 2 h of culture.
Flow Cytometry.
FITC or PE-labeled anti-FcɛRI (MAR-1) antibody was purchased from eBioscience. All the other antibodies used in this work were purchased from BD Biosciences. Data were acquired using a FACSCalibur™ (Becton Dickinson), and analyzed with Flowjo Software (Treestar, Inc.). In the cell sorting experiment, cells were sorted using a FACSVantage SE™ (Becton Dickinson).
Quantitative RT-PCR.
Total RNA was isolated using TRIzol (Invitrogen). First strand cDNAs were made using the SuperScriptTM Preamplification System (Invitrogen). Quantitative real-time PCR was performed on a 7900HT sequence detection system (Applied Biosystems). The sequences of the primers and MGB probe for GATA-3 are 5′-CAGAACCGGCCCCTTATCA-3′, 5′-CATTAGCGTTCCTCCTCCAGA-3′, and 5′-FAM-CGAAGGCTGTCGGCA-3′, respectively. GFP was detected by adding SYBR green to the PCR reaction. The primers for GFP are 5′-TGAACCGCATCGAGCTGAAG-3′ and 5′-GATGTTGTGGCGGATCTTGAAG-3′ (28). The primer and probe set for detecting murine IL-4 (FAM MGB Probe) and TaqMan® Ribosomal RNA Control Reagents for detecting the 18S ribosomal RNA (VIC MGB Probe) were purchased from Applied Biosystems.
Serum IL-4 Measurement.
In vivo IL-4 production was measured by the in vivo cytokine capture assay as described previously (29). In brief, mice were injected i.v. with 10 μg of biotin-BVD4-1D11 antibody 10 d after infection. Mice were bled the next day, and serum levels of IL-4–biotin-anti–IL-4 complexes were determined by ELISA using microtiter plates coated with BVD6-24G2.3.
Electron Microscopy.
Cells in suspension were fixed for 2 h in a mixture of aldehydes containing 2.0% paraformaldehyde, 2.5% gluteraldehyde, and 0.025% CaCl2 in 0.1 M sodium cacodylate buffer, pH 7.4. Cells were washed in the same buffer, embedded in 2% agar, postfixed in 2% collidine-buffered osmium tetroxide for 2 h at room temperature, and stained en bloc with uranyl acetate for 2 h at room temperature. A graded series of alcohols was used for dehydration before infiltration and embedding in Eponate. Plastic 1-μm sections were stained with alkaline-Giemsa and studied by light microscopy. Thin sections were stained with lead citrate and examined by electron microscopy.
Online Supplemental Material.
The frequency of GFP expressing CD4 T cells and non-CD4 T cells in each lymphoid or nonlymphoid organ from Nb-infected G4 homozygous, heterozygous, and wild-type mice is illustrated in Fig. S1. In Fig. S2, kinetics of GFP expression from lung non-CD4 T cells after Nb infection is compared between G4 homozygous and heterozygous mice. Lung cells from Nb-infected G4 homozygous and heterozygous mice were in vitro stimulated. GFP and IL-4 expression of granulated (SSChigh) cells from homozygous, heterozygous, and wild-type mice were compared (Fig. S3). Adoptive transfer of wild-type CD4 T cells into G4 5C.C7 TCR transgenic mice at the time of Nb infection induces basophil accumulation and GFP expression (Fig. S4). Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20040590/DC1.
Results
GFP Expression by Cells from G4 Mice Faithfully Represents In Vitro and In Vivo IL-4 Production.
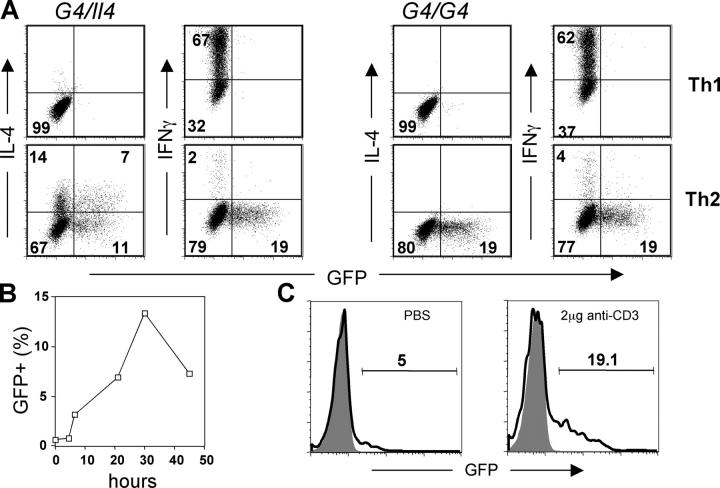
To test if GFP expression by cells of G4 mice is a surrogate for IL-4 production, CD4 T cells from G4 homozygous (G4/G4) and heterozygous (G4/Il4) mice were stimulated with plate-bound anti-CD3 plus anti-CD28 antibodies. Appropriate antibodies and cytokines were added in the culture to promote either Th1 or Th2 differentiation. When restimulated with immobilized anti-CD3/anti-CD28, both homozygous and heterozygous cells that had been cultured under Th2 conditions expressed GFP (5). In heterozygous cells, both IL-4 and GFP were produced as has been described previously (5); cells produced both IL-4 and GFP (7%), IL-4 only (14%), GFP only (11%), or neither (Fig. 1 A). Cells cultured under Th1 conditions expressed IFNγ, but not GFP or IL-4. Homozygous cells cultured under Th2 conditions produced GFP only (∼19%), consistent with these animals being knockouts for Il4, whereas those cultured under Th1 conditions produced only IFNγ (Fig. 1 A). Unlike 4get mice, developed by others (6), GFP expression was not detected until 48 h of Th2 priming of naive G4 CD4 T cells, and GFP was essentially undetectable by ∼36 h after removal from anti-CD3/anti-CD28 (unpublished data). Upon restimulation, these G4 Th2 cells reexpressed GFP (Fig. 1 B). It is important to note that the kinetics of GFP expression after restimulation is different from that of IL-4. This can be accounted for by the lack of the mRNA instability element in the 3′ untranslated region of the GFP gene and the stability of the GFP protein, which contrasts with the instability of IL-4 mRNA and with the rapid secretion of the IL-4 protein (30).
Figure 1.
GFP faithfully represents IL-4 expression in G4 knockin mice. (A) Lymph node CD4 T cells were purified from either G4 heterozygous (G4/Il4) or homozygous (G4/G4) mice and stimulated with plate-bound anti-CD3 plus anti-CD28 antibodies for 72 h. IL-12 and anti–IL-4, or IL-4, anti–IL-12, and anti-IFNγ were added in the culture to promote Th1 or Th2 differentiation, respectively. At the end of culture, cells were stimulated with plate-bound anti-CD3 plus anti-CD28 for 6 h. The representative result of intracellular IL-4 and IFNγ staining after stimulation is shown. (B) Resting Th2 CD4 T cells (from G4 homozygous mice) were restimulated with plate-bound antibodies and harvested at indicated times; GFP expresion was measured by flow cytometry. (C) G4 homozygous mice were injected intravenously with either PBS or 2 μg anti-CD3 antibody, and killed 90 min later. GFP expression by splenic NKT cells (NK1.1pos CD4pos) was measured by flow cytometry (bold line). GFP expression by CD4 T cells (NK1.1neg) was used to determine background levels of GFP (shaded area).
Very few (∼5%) NKT (CD4+, NK1.1+) cells isolated from untreated G4 mice were GFP+, but injection of 2 μg anti-CD3 caused ∼19% of these cells to be GFP positive 90 min later, without in vitro stimulation (Fig. 1 C). These results differ from those reported with 4get mice in which NKT cells were GFP+ before stimulation, but accord with the finding that NKT cells from untreated wild-type mice do not express IL-4 mRNA, yet IL-4 mRNA peaks at 90 min after injection of anti-CD3 (24). Therefore, our results suggest that GFP expression in G4 homozygous and heterozygous mice is a faithful surrogate for both in vitro and in vivo IL-4 production in contrast to 4get mice, in which GFP expression appears to correlate not with the actual production of IL-4 but with the capacity to make IL-4 upon subsequent stimulation.
Infection with Nb Induces GFP-expressing, non-CD4 T Cells in Liver and Lung.
To study in vivo Th2-type immune responses using G4 mice, 600 Nb L3 were subcutaneously injected into heterozygous or homozygous G4 mice, and GFP expression was measured ex vivo without restimulation. The frequency of GFP-expressing cells in draining lymph nodes and in the liver (Fig. 2 A) and lung (not depicted) peaked at ∼day 10 after infection. The majority of GFP-expressing CD4 T cells were CD44high (Fig. 2 A). Although the proportion of GFP+ T cells in different tissues varied, we did not find any differences between homozygous and heterozygous mice, indicating that the induction of the IL-4 surrogate (and thus presumably of IL-4) was IL-4 independent as has been reported previously (reference 31 and Fig S1, available at http://www.jem.org/cgi/content/full/jem.20040590/DC1).
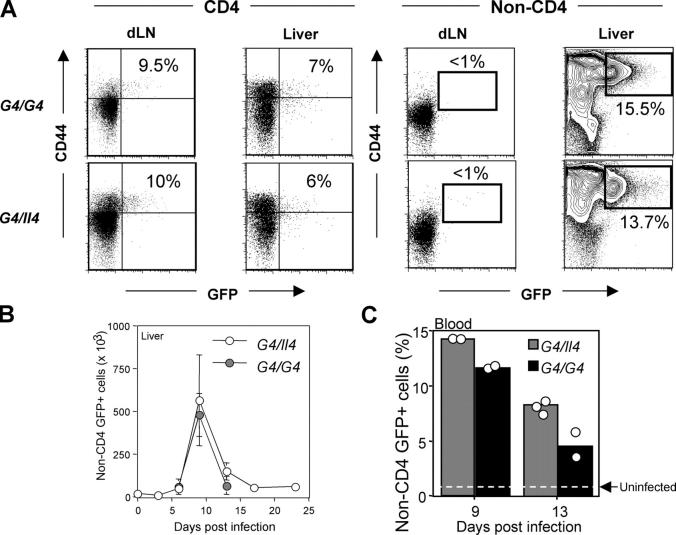
Figure 2.
Induction of GFP+ non-CD4 T cells in response to Nb infection. G4/Il4 and G4/G4 mice were subcutaneously inoculated with 600 Nb L3. (A) Nb-infected mice were killed 10 d later, and GFP expression from both CD4 and non-CD4 T cells was measured. Representative results of GFP expression from the draining lymph nodes and liver are shown. Indicated percentages represent the proportion of GFP+ cells among CD44bright CD4 T cells or among total non-CD4 T cells. Data are representative of more than three independent experiments. (B) Total numbers of non-CD4 GFP+ cells from liver cells were counted. The data were collected from three to four mice for each time point. (C) The percentage of blood GFP+ non-CD4 T cells from individual mice on the indicated date after infection is shown. The dotted line represents blood GFP+ non-CD4 T cells from uninfected mice.
We noted that a substantial population of the non-CD4 T cells (∼15%) expressed GFP, particularly in the liver (Fig. 2 A) and the lung (Fig. S1). Such cells were also found in the spleen (3–5%), but not in the lymph nodes (both draining and nondraining) or Peyer's patches (Fig. 2 A and Fig. S1). GFP expression in liver non–T cells peaked 9 d after infection, similar to that of CD4 T cells (Fig. 2 B). Approximately 104 cells (∼1% of total non-CD4 T cells) expressed low levels of GFP in the liver from naive G4 homozygous and heterozygous mice. The number of GFP+ liver non-CD4 cells increased >50-fold at the peak of the response. The infection also enhanced the intensity of GFP, indicating increased IL-4 production on a per cell basis. A similar response (both in terms of kinetics and degree of increase) was found in the lung after infection (Figs. S1 and S2, available at http://www.jem.org/cgi/content/full/jem.20040590/DC1). GFP+ non-CD4 T cells were also observed in the blood (Fig. 2 C). Similar to CD4 T cell responses, the level of GFP expression of non-CD4 T cells was similar in heterozygous and homozygous mice, indicating that the induction of GFP (IL-4) in these cells is also independent of IL-4 (Fig. 2 and Figs. S1 and S2).
The GFP+ Non-CD4 T Cells in Nb-infected Mice Are Basophils.
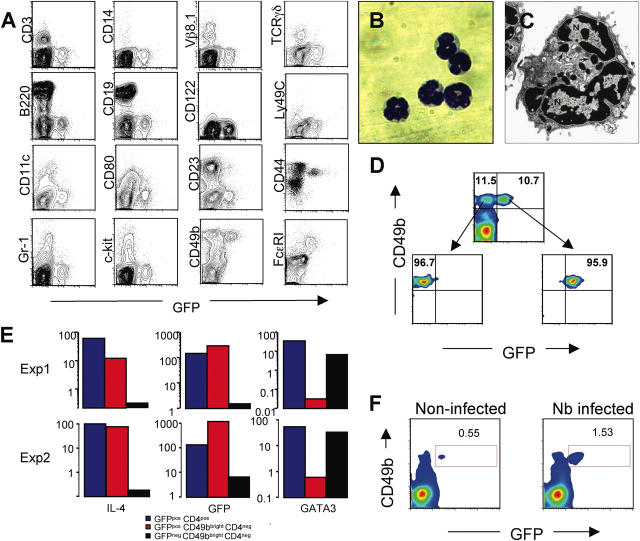
Non–B, non–T cells capable of producing IL-4 upon ex vivo stimulation have been reported previously (11–15, 32, 33). However, identification of such cells was difficult due to the lack of defined surface markers. Using 4get mice, Shinkai et al. have shown recently that Nb infection is associated with the appearance of GFP+ non–B, non–T cells (23). Their GFP+ population included cells with the morphology of eosinophils as well as cells with low side scatter, interpreted to be degranulated eosinophils. To identify the GFP-expressing non-CD4 T cells from Nb-infected G4 mice, we studied the cells by flow cytometry using a panel of antibodies. As shown in Fig. 3 A, GFP+ non-CD4 T cells were negative for CD3, γ/δ, and Vβ8.1, implying that they were not conventional, γ/δ, or NKT cells. In addition, they were negative for B220, CD19, CD11c, CD80, CD14, Gr-1, CD23, CD122, Ly49C, and c-kit. This indicates that they were not B cells, dendritic cells, macrophages, neutrophils, eosinophils, or NK cells. Virtually all the GFP+ cells show a low side scatter pattern and expressed high levels of CD49b (also known as DX5; reference 34) and of the high affinity IgE receptor, FcɛRI. Their expression of FcɛRI but not c-kit strongly suggests they are basophils, not mast cells. When cells were costained for CD49b and FcɛRI, most of double positive cells were GFP positive (unpublished data). More recently, the GFP+ degranulated non–T cells in Nb-infected 4get mice have been also identified as basophils (35).
Figure 3.
GFPpos non-CD4 T cells are basophils. (A) Non-CD4 T cells from liver cells from Nb-infected mice were stained for various surface markers (y axis). (B) GFP+CD49b+ non-CD4 T cells from Nb-infected liver were isolated by cell sorting. A cytospin preparation of sorted cells was stained with Wright-Giemsa (100×). (C) An electron micrograph of a GPF+ basophil reveals a multilobed nucleus (N) and characteristic granules (arrows; 25,000×). (D) Cell sorting profiles for GFP+CD49b+CD4− and GFP−CD49b+CD4− cells. (E) mRNA levels for IL-4, GFP, and Gata3 were measured by real-time PCR from sorted populations of basophils (GFP+CD49b+CD4−), of GFP−CD49b+CD4− cells, and of GFP+ CD4 T cells from Nb-infected mice. (F) The frequency of GFP+CD49b+ cells from bone marrow cells obtained from Nb-infected and noninfected G4 homozygous mice was compared.
Cells expressing both GFP and CD49b were isolated by cell sorting (Fig. 3 D), and subsequently analyzed by microscopy. As shown in Fig. 3 B, their nuclei were lobulated and they had a limited amount cytoplasm and few granules (36). Electron microscopic examination further confirmed their identification as basophils based on their lobulated nuclei and characteristic granules (Fig. 3 C). These cells, as well as purified GFP+CD4 T cells, contained mRNA for both IL-4 and GFP measured by real-time PCR (Fig. 3 E). However, unlike CD4 T cells, basophils were negative for Gata3 (Fig. 3 E) and c-maf mRNA (not depicted), implying that different mechanisms regulate IL-4 production in basophils than in T cells. GFP−CD49b+ cells, which are presumably NK cells, were positive for Gata3 mRNA, consistent with a previous paper (37). Electron microscopic examination of those populations confirmed an NK cell–like morphology (unpublished data).
The non-CD4 GFP+ cells found in the blood of Nb-infected mice exhibited similar characteristics (CD49b+ and FcɛRI+; unpublished data). A particularly interesting point was the presence of GFP/CD49b double positive cells in the bone marrow. These cells were ∼0.6% of the nucleated bone marrow cells. In Nb-infected mice, their frequency rose to ∼1.5% without any discernable change in GFP mean fluorescence intensity (Fig. 3 F). This implies that normal basophils are already producing IL-4 and that the major affect of Nb infection is to increase their numbers and their accumulation in the tissues.
Together, our results indicate that Nb infection induces accumulation of IL-4–producing basophils in tissues (liver and lung). Because most of GFP+ cells are CD49b+ and FcɛRI+, but c-kit−, it appears that basophils are the main population of nonlymphocytes in G4 mice that express GFP and that produce IL-4 in response to Nb infection.
Basophils Are Main IL-4 Producers after Restimulation.
To test if GFP expression by basophils from Nb-infected mice and their possession of IL-4 mRNA indicate that they have the capacity to produce IL-4 in vitro, liver cells from Nb-infected G4 homozygous or heterozygous mice were stimulated in vitro with PMA plus ionomycin, and tested for IL-4 production by intracellular staining. As shown in Fig. 4 A, essentially all GFP-expressing cells from heterozygous mice also were positive for intracellular IL-4, and there were a small portion of IL-4–producing non-GFP cells. Most FcɛRI+ cells produced IL-4 upon stimulation (unpublished data). We did not detect cells containing cytosolic IL-5, IL-13, or IFNγ in these populations (unpublished data). The level of FcɛRI expressed on basophils was slightly higher in GFP homozygous mice than in heterozygous mice. Although it has been shown that IgE regulates FcɛRI expression on murine basophils (38), the difference in levels of detected FcɛRI most likely represents competition by IgE bound to FcɛRI in heterozygous G4 mice, which does not occur in homozygous G4 mice; the latter, being IL-4 knockout, would not be expected to produce IgE.
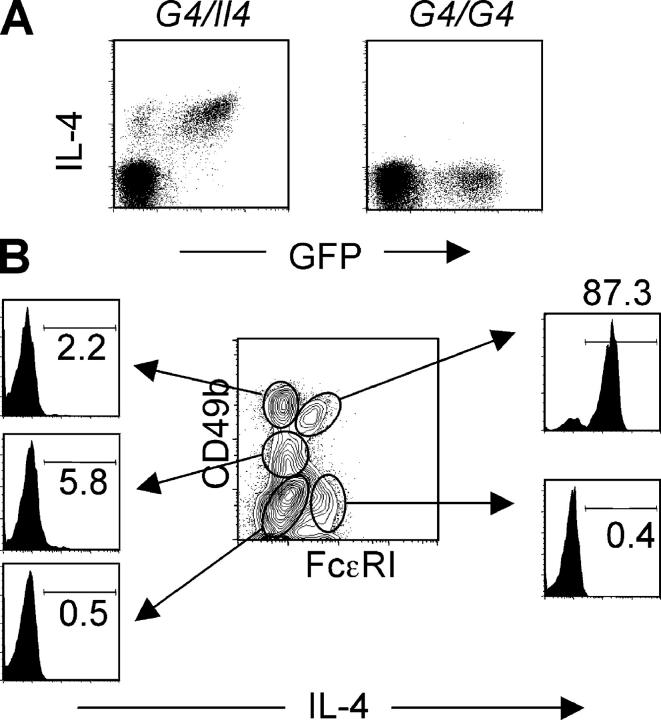
Figure 4.
Basophils are major IL-4 producers. (A) Liver cells from Nb-infected G4 heterozygous and homozygous mice were stimulated in vitro with PMA and ionomycin for 4 h. Cells were stained for intracellular IL-4. The FACS® profile for GFP and IL-4 expression from non-CD4 T cells is shown. (B) Liver cells from Nb-infected BALB/c (non-GFP) mice were stimulated in vitro as mentioned previously. Cells were stained for FcɛRI, CD49b, IL-4, and CD4. The IL-4 staining from each gated population of non-CD4 T cells is shown. Percentages of IL-4 positive cells are indicated. Experiments were repeated three times with similar results.
Because the majority of basophils from G4 mice express GFP ex vivo and can produce IL-4 after stimulation, we tested normal BALB/c mice to see if a comparable situation held for basophils from non-GFP mice. Liver cells from BALB/c mice infected with Nb were stimulated with PMA plus ionomycin and stained for CD49b, FcɛRI, and IL-4. As shown in Fig. 4 B, 87% of basophils (cells expressing both CD49b and FcɛRI) are positive for IL-4. Among CD49b+FcɛRI−, mainly NK-type cells, few (∼2%) produce IL-4. Only 5% of FcɛRI− CD49b+/− (presumably including eosinophils) produced IL-4 upon stimulation with PMA and ionomycin. We also tested granulated cells (SSChigh) from lungs of Nb-infected G4 homozygous and heterozygous mice and from wild-type mice to see if they express GFP ex vivo and are capable of making IL-4 upon stimulation. We did not detect any GFP expression ex vivo (Fig. S3, available at http://www.jem.org/cgi/content/full/jem.20040590/DC1) or IL-4 after stimulation with PMA and ionomycin (unpublished data).
Basophil IL-4 (GFP) Expression Is Stat6 Independent.
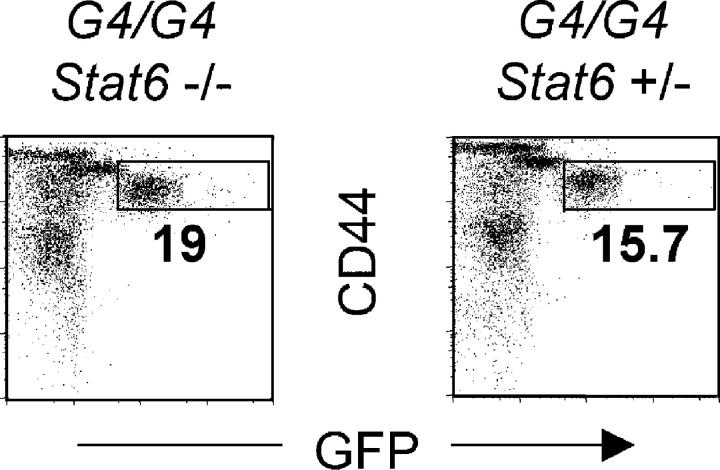
To investigate the mechanisms involved in IL-4 production, we tested whether basophil GFP expression is dependent on Stat6. Stat6−/− G4 homozygous mice (which are thus both IL-4 and Stat6 deficient) were infected with Nb. Littermate mice (Stat6+/−, G4 homozygous) were used as controls. At the peak of the response, GFP expression by non-CD4 CD44bright liver or lung cells from Stat6−/− and Stat6+/− mice was comparable (Fig. 5). These results indicate that in vivo IL-4 expression by basophils is independent of both Stat6 and IL-4. It has been reported previously that serum levels of IL-4 production were similar in wild-type and Stat6−/− Nb-infected mice (39).
Figure 5.
Basophil GFP induction is independent of IL-4 and Stat6. Liver cells from Nb-infected G4 homozygous (IL-4 deficient) Stat6−/− or littermates Stat6+/− mice were stained for CD44 and CD4. The GFP profile from non-CD4 T cells is shown. Experiments were repeated three times with similar results.
CD4 T Cells Are Required for the Basophil Recruitment to the Liver, but Basophil GFP Expression Is B Cell Independent.
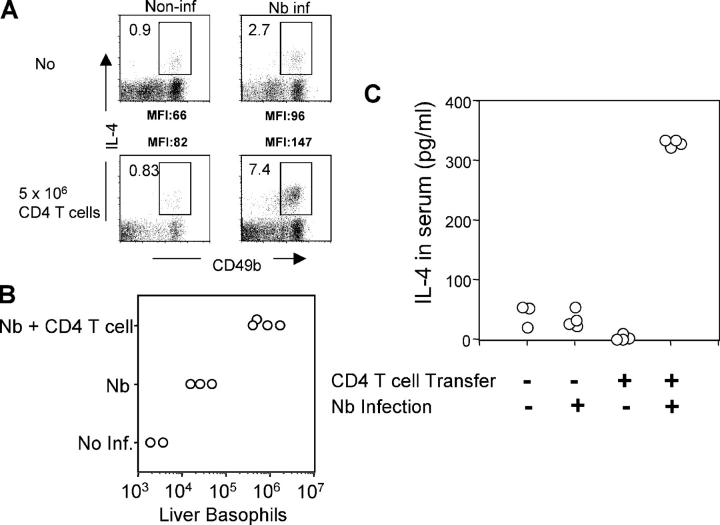
To test if the presence of CD4 T cells affects IL-4 production and/or recruitment of basophils, Rag2−/− mice were infected with Nb; on the day of infection, some animals received 5 × 106 purified CD4 T cells. At day 10, liver cells were harvested and stimulated with PMA and ionomycin. As shown in Fig. 6 A, <1% of non-CD4 CD49b+ cells from noninfected Rag2−/− mice produced IL-4 upon stimulation. Nb infection increased the frequency of IL-4–producing cells to 2.7% and there was an ∼10-fold increase in the absolute number of liver basophils, measured by expression of both CD49b and FcɛRI among non-CD4 T cells (Fig. 6 B). Transfer of purified CD4 T cells into Rag2−/− mice infected with Nb increased the proportion of IL-4–producing non-CD4 CD49b+ cells to 7–8%, and the total number of basophils increased >10-fold compared with infected nonreconstituted mice (Fig. 6 B). Furthermore, there was an increase in the mean fluorescence intensity of IL-4 staining, after stimulation, in the T cell–reconstituted infected group compared with the nonreconstituted infected group, indicating that CD4 T cells both recruit basophils to the tissues and increase IL-4–producing capacity on a per cell level in infected mice. To test whether T cell reconstitution also increased in situ IL-4 production, Rag2−/− mice were reconstituted with CD4 T cells from G4 homozygous donors; some were infected with Nb. On day 11, serum IL-4 levels were measured with the in vivo cytokine capture assay. Reconstitution with T cells increased IL-4 production in Nb-infected animals >10-fold (Fig. 6 C). Because the only source of IL-4 in these mice is non–T cells (the transferred T cells are IL-4 deficient) and basophils are the dominant non–T cells producing GFP in G4 mice without restimulation, this result strongly argues that these cells are also producing IL-4 in situ in response to Nb infection.
Figure 6.
CD4 T cell–dependent basophil responses. Rag2−/− mice (non-GFP) were infected with Nb. At the time of infection, 5 × 106 purified CD4 T cells from G4 homozygous mice (IL-4–deficient) were transferred into Nb-infected Rag2−/− mice. (A and B) 10 d later, mice were killed, and liver cells were isolated and in vitro stimulated with PMA plus ionomycin. Cells were stained for CD49b, CD4, and IL-4. IL-4 staining from CD49b+ non-CD4 T cells is shown. Indicated is the percentage of IL-4+ cells among non-CD4 T cells (A) or the total number of liver basophils (B, CD49b+FcɛRI+CD4−). Experiments were repeated three times with similar results. (C) Rag2−/− mice were infected with Nb, and CD4 T cells were simultaneously administered as mentioned previously. 10 μg of biotin anti–IL-4 antibody was injected intravenously 10 d after infection. The next day, serum was collected from the mice, and serum IL-4 was measured by ELISA as described in Materials and Methods. Each circle represents an individual mouse.
Basophil accumulation in the tissues was not observed after Nb infection of 5C.C7 Rag2−/− G4 homozygous T cell receptor transgenic mice, which express a TCR specific for cytochrome c peptide presented on I-Ek class II molecule. Their failure to support the mobilization of basophils in response to Nb infection suggests it is not simply T cell number that is important, but the capacity of those T cells to recognize Nb-associated antigens, indicating that antigen-driven T cell activation is necessary for the optimal function of CD4 T cells in recruiting basophils (Fig. S4, available at http://www.jem.org/cgi/content/full/jem.20040590/DC1). IL-4 is not critical for this recruitment because the G4 homozygotes are IL-4 knockouts. When 5C.C7 Rag2−/− G4 homozygous T cell receptor transgenic mice were reconstituted with CD4 T cells from normal B10.A mice at the time of infection, there was an ∼10-fold increase in liver basophils and essentially all those cells expressed GFP (Fig. S4). Because these mice lack B cells and, thus, cannot produce immunoglobulins, including IgE, their in situ production of GFP, as an IL-4 surrogate, implies that FcɛRI cross-linkage through antigen-specific interaction with bound IgE (or other Igs) is not required for the production of the cytokine.
Is IL-3 Production the Mechanism through which T Cells Control Basophil Accumulation and IL-4 Production?
Both in vitro and in vivo evidence indicated that IL-3, presumably derived from T cells, plays a crucial role of regulating basophil responses (12–14, 33). Studies in IL-3–deficient mice further demonstrate impaired basophil development after parasite infection (40). To test whether IL-3 is important in the IL-4/Stat6/IgE-independent accumulation of basophils, Rag2−/− mice were infected with Nb and IL-3 was administered using a mini-osmotic pump. The frequency of basophils rose from 2 to 13%; their absolute number increased >100-fold (unpublished data).
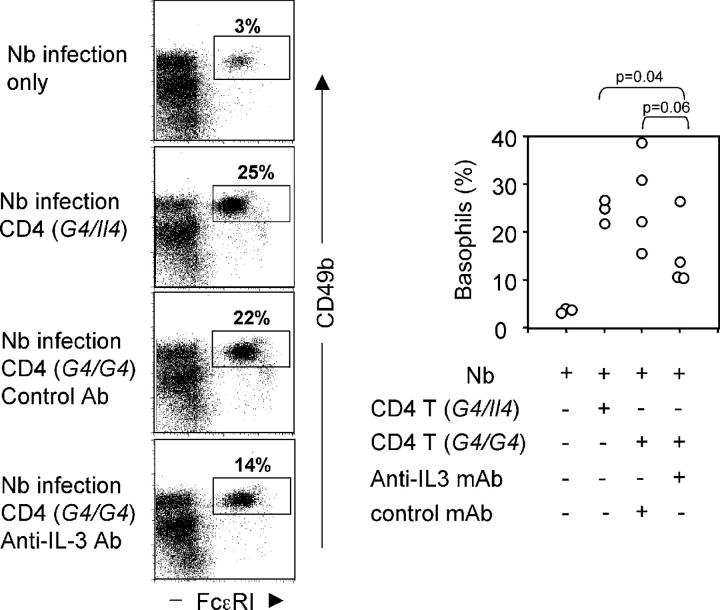
To test whether IL-3 production by activated CD4 T cells was responsible for basophil mobilization in Nb infection, CD4 T cells from G4 homozygous or heterozygous mice were transferred into Rag2−/− mice at the time of infection. A neutralizing anti–IL-3 mAb (MP2-8F8) or an isotype-matched control antibody (GL113) was injected throughout the period. The presence of CD4 T cells (either from G4 homozygous or heterozygous) significantly enhanced basophil recruitment on day 10 (Fig. 7). This recruitment was only partially inhibited by anti–IL-3 mAb (Fig. 7); serum IL-4 levels showed a similar pattern: 385 ± 284 pg from control antibody treated group and 169 ± 143 pg from anti–IL-3 mAb treated group. However, the p-values for these inhibitions were 0.06 and 0.1, respectively, suggesting that if IL-3 was important in this process, it was not the only factor that played a role or that the antibody-mediated inhibition was incomplete.
Figure 7.
Neutralization of IL-3 partially inhibits basophil responses. 5 × 106 CD4 T cells (from either G4 heterozygous or homozygous mice) were transferred into Rag2−/− mice infected with Nb. The infected mice were treated with 2 mg anti–IL-3 (MP2-8F8) or control (GL113) antibody every 3 d. On day 11, liver cells were harvested and analyzed for basophil frequency. The left panel represents flow cytometric data for one individual mouse in each group. The right panel presents data from each mouse with a circle representing results from an individual mouse.
Discussion
Here, we have examined IL-4 production during the course of infection with the intestinal nematode Nb using G4 mice. Nb infection induced a substantial population of GFP+ cells displaying the phenotypic and morphologic characteristics of basophils. Basophil responses reached a peak at ∼day 10 after infection. They were found mainly in the liver and lung, to a lesser extent in spleen and blood, but not in lymph nodes. Approximately 90% of the basophils found in liver and lung of Nb-infected mice expressed GFP. If these cells were obtained from G4 heterozygous mice, the majority of the basophils produced IL-4 upon in vitro stimulation with PMA and ionomycin. In situ GFP expression by basophils from Nb-infected mice did not require IL-4 or Ig-mediated FcR cross-linkage. Recruitment of basophils into the tissues failed to occur in Nb-infected Rag2−/− mice; reconstituting these mice with CD4 T cells resulted in appearance of basophils in the tissues, implying that T cells were critical to this accumulation. Voehringer et al. have also observed that basophil accumulation in the lung of Nb-infected animals was CD4 T cell dependent, as was eosinophil accumulation (35). Using a sensitive cytokine capture assay, we detected significant amounts of serum IL-4 in Nb-infected Rag2−/− mice that had been reconstituted with G4 homozygous CD4 T cells. Because the T cells in this model are incapable of producing IL-4, these results indicate that basophils from Nb-infected mice produce IL-4 in vivo.
Nonlymphocyte populations that are capable of producing IL-4 were initially reported almost 15 yr ago (12). These experiments relied on ex vivo stimulation with either ionomycin or by cross-linkage of FcɛRI or an FcγR. Conrad et al. demonstrated that injection of anti-IgD antibodies or Nb infection increased the production of IL-4 by splenic non–B, non–T cells upon in vitro stimulation (11). Injection of IL-3 also increased IL-4 production by such cells (13). Subsequent studies identified these cells as basophils (15).
Mouse basophils have been difficult to study due to lack of specific surface markers; now they can be detected by their unique expression of CD49b and FcɛRI and their failure to express c-kit, clearly distinguishing them from mast cells and eosinophils. Basophils from normal naive G4 mice constitutively express GFP, as do basophils from 4get mice (35). Approximately 1% of non-CD4 liver cells from naive G4 heterozygous and homozygous mice display the phenotype of basophils, are GFP+, and produce IL-4 upon in vitro stimulation, indicating that IL-4 expression in resting basophils is constitutive and does not require IL-4 (unpublished data). Indeed, comparable levels of basophils that are capable of producing IL-4 after in vitro stimulation were observed in Nb-infected IL-4Rα−/− mice (unpublished data).
The frequency of CD49b/FcɛRI double positive non-CD4 T cells in G4 homozygous or heterozygous mice is similar to that in normal mice, indicating that the presence of GFP does not alter the development of these cells. Moreover, 60–80% of basophils from livers of naive G4 heterozygotes or wild-type mice express IL-4 upon stimulation with PMA and ionomycin, as detected by intracellular staining, further supporting the concept that the expression of GFP does not distort the biology of the basophils. Nb infection increased the numbers of basophils in the liver or lung ∼50-fold, the proportion of basophils that are GFP+, and the mean fluorescence intensity of GFP in positive cells. Similar basophil responses were found after infection of G4 mice with Schistosoma mansoni or Heligosomoides polygyrus, but not with Toxoplasma gondii (reference 41 and unpublished data), suggesting that basophil response appear in other helminth induced Th2 type immune responses. Human basophils have been reported to produce IL-4 after stimulation with S. mansoni egg antigen (18).
How is IL-4 induced in basophils in vivo? Stimulation of basophil IL-4 production in vitro can occur as a result of FcR cross-linkage by aggregation of bound IgE or IgG (12, 14). A correlation has been reported between the frequency of human basophils that release IL-4 in response to challenge with a parasite antigen and the concentration of specific IgE in those individuals (19). However, other basophil stimulants, including C5a (42), have been reported to cause IL-4 secretion. Whether such stimulants are responsible for in situ GFP expression in the immunoglobulin-independent basophil IL-4 production in G4 mice needs further investigation. However, the finding that the majority of liver basophils in naive G4 homozygous and heterozygous mice are GFP+ and that a substantial proportion of bone marrow basophils are GFP+ raises the possibility that a “noninflammatory” stimulant may exist. Although it has already been reported that 4get non-CD4 T cells are GFP+ in noninfected mice (23), it must be pointed out that the expression of GFP in that system correlates well with the potential to produce high levels of IL-4 rather than with actual production of IL-4. Thus, our results are the first to demonstrate that basophils are very likely to produce IL-4 without overt antigenic stimulation.
Cytokines are important factors in basophil activation. IL-3 has been shown to enhance histamine release from and IL-4 production by basophils (12, 13, 16, 22, 33). However, IL-3 is not essential for basophil generation, but it does contribute to enhanced basophil accumulation upon parasite infection (40). The fact that neutralization of IL-3 only partially inhibits basophil recruitment and IL-4 production in Nb-infected animals suggests either than anti–IL-3 did not fully inhibit the IL-3 or that other factors are involved in basophil responses that may be lost in mice chronically deprived of IL-3.
IL-3 and IL-18, used together, have been shown to stimulate IL-4 production by bone marrow–generated murine basophils in vitro without a requirement for FcR cross-linkage (16). However, treatment with anti–IL-18 antibody did not reduce frequency of or degree of GFP expression in basophils from Nb-infected mice (unpublished data). Similarly, it has been observed that caspase-1 knockout mice, which are severely deficient in IL-18, normally recruit basophils when Nb infected (unpublished data). It may very well be that cytokines other than IL-18 play a role in basophil IL-4 responses in response to Nb infection. Alternatively, those Nb-associated molecules that act as Th2-driving adjuvants may directly activate and trigger basophil responses (43, 44). We are currently investigating whether such mechanisms could induce basophil responses.
What is particularly striking is that CD4 T cells are necessary for optimal migration of, and IL-4 production by, basophils. The fact that Nb-infected 5C.C7 TCR transgenic CD4 T cells failed to induce basophil responses strongly suggests that T cell activation by Nb antigen is required. How the help from CD4 T cells is linked to basophil recruitment is not yet clear at this moment. IL-3 produced by T cells may enhance basophil production/responses (33), but it is not absolutely required for IL-4 production (unpublished data). How, then, could T cells induce basophil responses? CD4 T cells responsive to parasite antigens could produce other cytokines or chemokines that recruit and/or activate basophils (43, 45).
What is the role of IL-4 made by basophils? Basophil IL-4, or alternatively IL-13, could have important effector functions. Particularly in the lung where Th2 responses are associated with airway hyperresponsiveness, endothelial metaplasia, and mucus production, which are mediated largely by IL-13, the rich source of this cytokine provided by basophils could be of particular pathogenic significance.
Because tissue basophils in Nb-infected mice are GFP+ without overt stimulation and these cells contain IL-4 mRNA, they appear to provide a source of IL-4 even without antigen-driven stimulation. However, upon stimulation, they very likely increase their production. Indeed, basophils may produce more total IL-4 in the tissues in response to antigenic challenge than any other cell type, including CD4 T cells (19, 32, 46).
Although in Nb infection, the mobilization of IL-4–producing basophils may well be too slow for them to act sufficiently early in the response to bias the first cohort of responding CD4 T cells in the Th2 direction, they may have a very important role in propagating Th2 responses by providing a source of IL-4 to prime newly emerging or newly recruited naive CD4 T cells to become IL-4 producers (46).
Earlier studies have shown basophil and eosinophil migration to sites of inflammation in tick infection and that depleting basophils diminished eosinophil recruitment, suggesting that secretion of IL-4 or other type 2 cytokines at these sites may be important as immune modulators (47). Other examples of basophil responses include increased blood basophil levels in Nb-infected rat (48), basophils in skin reactions after infestation with Dermacentor varibilis (49), and, more recently, basophil IL-4 production in a mouse model of allergic pulmonary inflammation (50).
In vitro–cultured basophils have been reported to induce IgE synthesis by B cells (51, 52). Thus, basophils might promote and/or amplify antibody responses associated with parasite infection, but the degree to which basophils can act as class-specific helper cells for IgE production in vivo has still to be determined.
In summary, our results using G4 mice reveal evidence that murine basophils are the primary, if not sole, IL-4–producing nonlymphocytes during parasitic infection. Constitutive production of IL-4 by these cells and their tissue localization patterns implies that they play important roles in the development of adaptive immune responses and/or in host effector function.
Acknowledgments
The authors thank S. Tanksley, T. Moyer, and C. Henry for their excellent cell sorting; R. Monahan-Earley for helping to prepare EM samples; Dr. T. Yoshimoto for helpful discussions, particularly about the possible role of IL-18; Dr. A. Sher for critical review of the manuscript, Dr. S. Galli for sharing unpublished data; and members of the Paul laboratory for helpful discussion.
M. Prout and G. LeGros are supported by the Health Research Council of New Zealand. The ultrastructural studies were supported by National Institutes of Health grant AI33372 (to A.M. Dvorak).
The authors have no conflicting financial interests.
Abbreviation used in this paper: Nb, Nippostrongylus brasiliensis.
References
- 1.Seder, R.A., and W.E. Paul. 1994. Acquisition of lymphokine-producing phenotype by CD4+ T cells. Annu. Rev. Immunol. 12:635–673. [DOI] [PubMed] [Google Scholar]
- 2.Finkelman, F.D., J. Holmes, I.M. Katona, J.F. Urban Jr., M.P. Beckmann, L.S. Park, K.A. Schooley, R.L. Coffman, T.R. Mosmann, and W.E. Paul. 1990. Lymphokine control of in vivo immunoglobulin isotype selection. Annu. Rev. Immunol. 8:303–333. [DOI] [PubMed] [Google Scholar]
- 3.Finkelman, F.D., T. Shea-Donohue, J. Goldhill, C.A. Sullivan, S.C. Morris, K.B. Madden, W.C. Gause, and J.F. Urban Jr. 1997. Cytokine regulation of host defense against parasitic gastrointestinal nematodes: lessens from studies with rodent models. Annu. Rev. Immunol. 15:505–533. [DOI] [PubMed] [Google Scholar]
- 4.Naramura, M., R.J. Hu, and H. Gu. 1998. Mice with a fluorescent marker for interleukin 2 gene activation. Immunity. 9:209–216. [DOI] [PubMed] [Google Scholar]
- 5.Hu-li, J., C. Pannetier, L. Guo, M. Lohning, H. Gu, C. Watson, M. Assenmacher, A. Radbruch, and W.E. Paul. 2001. Regulation of expression of IL-4 alleles: analysis using a chimeric GFP/IL-4 gene. Immunity. 14:1–11. [DOI] [PubMed] [Google Scholar]
- 6.Mohrs, M., K. Shinkai, K. Mohrs, and R.M. Locksley. 2001. Analysis of type 2 immunity in vivo with a bicistronic IL-4 reporter. Immunity. 15:303–311. [DOI] [PubMed] [Google Scholar]
- 7.Stetson, D.B., M. Mohrs, R. Lee Reinhardt, J.L. Baron, Z. Wang, L. Gapin, M. Kronenberg, and R.M. Locksley. 2003. Constitutive cytokine mRNAs mark natural killer (NK) and NK T cells poised for rapid effector function. J. Exp. Med. 198:1069–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gause, W.C., J.F. Urban Jr., and M.J. Stadecker. 2003. The immune response to parasitic helminths: insights from murine models. Trends Immunol. 24:269–277. [DOI] [PubMed] [Google Scholar]
- 9.McKenzie, G.J., C.L. Emson, S.E. Bell, S. Anderson, P. Fallon, G. Zurawski, R. Murrays, R. Grencis, and A.N.J. McKenzie. 1998. Impaired development of Th2 cells in IL-13-deficient mice. Immunity. 9:423–432. [DOI] [PubMed] [Google Scholar]
- 10.Urban, J.F., Jr., N. Noben-Trauth, D.D. Donaldson, K.B. Madden, S.C. Morris, M. Collins, and F.D. Finkelman. 1998. IL-13, IL-4Rα, and Stat6 are required for the expulsion of the gastrointestinal nematode parasite Nippostrongylus brasiliensis. Immunity. 8:255–264. [DOI] [PubMed] [Google Scholar]
- 11.Conrad, D.H., S.Z. Ben-Sasson, G. LeGros, F.D. Finkelman, and W.E. Paul. 1990. Infection with Nippostrongylus brasiliensis or injection of anti-IgD antibodies markedly enhances Fc-receptor–mediated interleukin 4 production by non–B, non–T cells. J. Exp. Med. 171:1497–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ben-Sasson, S.Z., G. LeGros, D.H. Conrad, F.D. Finkelman, and W.E. Paul. 1990. Cross-linking Fc receptors stimulate splenic non-B, non-T cells to secrete interleukin 4 and other lymphokines. Proc. Natl. Acad. Sci. USA. 87:1421–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.LeGros, G., S.Z. Ben-Sasson, D.H. Conrad, I. Clark-Lewis, F.D. Finkelman, M. Plaut, and W.E. Paul. 1990. IL-3 promotes production of IL-4 by splenic non-B, non-T cells in response to Fc receptor cross-linkage. J. Immunol. 145:2500–2506. [PubMed] [Google Scholar]
- 14.Seder, R.A., M. Plaut, S. Barbieri, J. Urban Jr., F.D. Finkelman, and W.E. Paul. 1991. Purified FcɛR+ bone marrow and splenic non-B, non-T cells are highly enriched in the capacity to produce IL-4 in response to immobilized IgE, IgG2a, or ionomycin. J. Immunol. 147:903–909. [PubMed] [Google Scholar]
- 15.Seder, R.A., W.E. Paul, A.M. Dvorak, S.J. Sharkis, A. Kagey-Sobotka, Y. Niv, F.D. Finkelman, S.A. Barbieri, S.J. Galli, and M. Plaut. 1991. Mouse splenic and bone marrow cell populations that express high-affinity Fcɛ receptors and produce interleukin 4 are highly enriched in basophils. Proc. Natl. Acad. Sci. USA. 88:2835–2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoshimoto, T., H. Tsutsui, K. Tominaga, K. Hoshino, H. Okamura, S. Akira, W.E. Paul, and K. Nakanishi. 1999. IL-18, although antiallergic when administered with IL-12, stimulates IL-4 and histamine release by basophils. Proc. Natl. Acad. Sci. USA. 96:13962–13966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paul, W.E. 1991. Interleukin-4 production by Fc epsilon R+ cells. Skin Pharmacol. 4:8–14. [PubMed] [Google Scholar]
- 18.Falcone, F.H., C.A. Dahinden, B.F. Gibbs, T. Noll, U. Amon, H. Hebestreit, O. Abrahamsen, J. Klaucke, M. Schlaak, and H. Haas. 1996. Human basophils release interleukin-4 after stimulation with Schistosoma mansoni egg antigen. Eur. J. Immunol. 26:1147–1155. [DOI] [PubMed] [Google Scholar]
- 19.Mitre, E., R.T. Taylor, J. Kunofcik, and T.B. Nutman. 2004. Parasite antigen-driven basophils are a major source of IL-4 in human filarial infections. J. Immunol. 172:2439–2445. [DOI] [PubMed] [Google Scholar]
- 20.Philips, Clair, W.R. Coward, D.I. Pritchard, and C.R.A. Hewitt. 2003. Basophils express a type 2 cytokine profile on exposure to protease from helminthes and house dust mites. J. Leukoc. Biol. 73:165-171. [DOI] [PubMed] [Google Scholar]
- 21.Dahinden, C.A., S. Rihs, and B. Ochsensberger. 1997. Regulation of cytokine expression by human blood basophils. Int. Arch. Allergy Immunol. 113:134–137. [DOI] [PubMed] [Google Scholar]
- 22.MacGlashan, D., Jr., J.M. White, S.-K. Huang, S.J. Ono, J.T. Schroeder, and L.M. Lichtenstein. 1994. Secretion of IL-4 from human basophils. The relationship between IL-4 mRNA and protein in resting and stimulated basophils. J. Immunol. 152:3006–3016. [PubMed] [Google Scholar]
- 23.Shinkai, K., M. Mohrs, and R.M. Locksley. 2002. Helper T cells regulate type-2 innate immunity in vivo. Nature. 420:825–829. [DOI] [PubMed] [Google Scholar]
- 24.Yoshimoto, T., and W.E. Paul. 1994. CD4pos, NK1.1pos T cells promptly produce interleukin 4 in response to in vivo challenge with anti-CD3. J. Exp. Med. 179:1285–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsuda, J.L., L. Gapin, J.L. Baron, S. Sidobre, D.B. Stetson, M. Mohrs, R.M. Locksley, and M. Kronenberg. 2003. Mouse Vα14_i_ natural killer T cells are resistant to cytokine polarization in vivo. Proc. Natl. Acad. Sci. USA. 100:8395–8400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abrams, J.S., and M.K. Pearce. 1988. Development of rat anti-mouse interleukin 3 monoclonal antibodies which neutralize bioactivity in vitro. J. Immunol. 140:131–137. [PubMed] [Google Scholar]
- 27.Matsui, K., T. Yoshimoto, H. Tsutsui, Y. Hyodo, N. Hayashi, K. Hiroishi, N. Kawada, H. Okamura, K. Nakanishi, and K. Higashino. 1997. Propionibacterium acnes treatment diminishes CD4+NK1.1+ T cells but induces type I T cells in the liver by induction of IL-12 and IL-18 production from Kupffer cells. J. Immunol. 159:97–106. [PubMed] [Google Scholar]
- 28.Guo, L., J. Hu-Li, J. Zhu, C.J. Watson, M.J. Difilippantonio, C. Pannetier, and W.E. Paul. 2002. In Th2 cells the Il4 gene has a series of accessibility states associated with distinctive probabilities of IL-4 production. Proc. Natl. Acad. Sci. USA. 99:10623–10628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Finkelman, F.D., and S.C. Morris. 1999. Development of an assay to measure in vivo cytokine production in the mouse. Int. Immunol. 11:1811–1818. [DOI] [PubMed] [Google Scholar]
- 30.Guhaniyogi, J., and G. Brewer. 2001. Regulation of mRNA stability in mammalian cells. Gene. 265:11–23. [DOI] [PubMed] [Google Scholar]
- 31.Noben-Trauth, N., L.D. Shultz, F. Brombacher, J.F. Urban Jr., H. Gu, and W.E. Paul. 1997. An interleukin 4 (IL-4)-independent pathway for CD4+ T cell IL-4 production is revealed in IL-4 receptor-deficient mice. Proc. Natl. Acad. Sci. USA. 94:10838–10843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aoki, I., C. Kinzer, A. Shirai, W.E. Paul, and D.M. Klinman. 1995. IgE receptor-positive non-B/non-T cells dominate the production of interleukin 4 and interleukin 6 in immunized mice. Proc. Natl. Acad. Sci. USA. 92:2534–2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kullberg, M.C., J.A. Berzofsky, D. Jankovic, S. Barbieri, M.E. Williams, P. Perlmann, A. Sher, and M. Troye-Blomberg. 1996. T cell-derived IL-3 induces the production of IL-4 by non-B, non-T cells to amplify the Th2-cytokine response to a non-parasite antigen in _Schistosoma mansoni_-infected mice. J. Immunol. 156:1482–1489. [PubMed] [Google Scholar]
- 34.Arase, H., T. Saito, J.H. Phillips, and L.L. Lanier. 2001. Cutting edge: the mouse NK cell-associated antigen recognized by DX5 monoclonal antibody is CD49b (alpha 2 integrin, very late antigen-s). J. Immunol. 167:1141–1144. [DOI] [PubMed] [Google Scholar]
- 35.Voehringer, D., K. Shinkai, and R. Locksley. 2004. Type 2 immunity reflects orchestrated recruitment of cells committed to IL-4 production. Immunity. 20:267–277. [DOI] [PubMed] [Google Scholar]
- 36.Dvorak, A.M., G. Nabel, K. Pyne, H. Cantor, H.F. Dvorak, and S.J. Galli. 1982. Ultrastructural identification of the mouse basophil. Blood. 59:1279–1285. [PubMed] [Google Scholar]
- 37.Samson, S.I., O. Richard, M. Tavian, T. Ranson, C.A.J. Vosshenrich, F. Colucci, J. Buer, F. Grosveld, I. Godin, and J.P. Di Santo. 2003. Gata-3 promotes maturation, IFN-γ production, and liver-specific homing of NK cells. Immunity. 19:701–711. [DOI] [PubMed] [Google Scholar]
- 38.Lantz, C.S., M. Yamaguchi, H.C. Oettgen, I.M. Katona, I. Miyajima, J.-P. Kinet, and S.J. Galli. 1997. IgE regulates mouse basophil FcɛRI expression in vivo. J. Immunol. 158:2517–2521. [PubMed] [Google Scholar]
- 39.Finkelman, F.D., S.C. Morris, T. Orekhova, M. Mori, D. Donaldson, S.L. Reiner, N.L. Reilly, L. Schopf, and J.F. Urban Jr. 2000. Stat6 regulation of in vivo IL-4 responses. J. Immunol. 164:2303–2310. [DOI] [PubMed] [Google Scholar]
- 40.Lantz, C.S., J. Boesiger, C.H. Song, N. Mach, T. Kobayashi, R.C. Mulligans, Y. Nawa, G. Dranoff, and S.J. Galli. 1998. Role for interleukin-3 in mast-cell and basophil development and in immunity to parasite. Nature. 392:90–93. [DOI] [PubMed] [Google Scholar]
- 41.Williams, M.E., M.C. Kullberg, S. Barbieri, P. Caspar, J.A. Berzofsky, R.A. Seder, and A. Sher. 1993. Fc epsilon receptor-positive cells are a major source of antigen-induced interleukin-4 in spleens of mice infected with Schistosoma mansoni. Eur. J. Immunol. 23:1910-1916. [DOI] [PubMed] [Google Scholar]
- 42.Ochensberger, B., S. Rihs, T. Brunner, and C.A. Dahinden. 1995. IgE-independent interleukin-4 expression and induction of a late phase of leukotriene C4 formation in human blood basophils. Blood. 86:4039–4049. [PubMed] [Google Scholar]
- 43.Maizels, R.M., and M. Yazdanbakhsh. 2003. Immune regulation by helminth parasites: cellular and molecular mechanisms. Nat. Rev. Immunol. 3:733–744. [DOI] [PubMed] [Google Scholar]
- 44.Holland, M.J., Y.M. Harcus, P.L. Riches, and R.M. Maizels. 2000. Proteins secreted by the parasitic nematode Nippostrongylus brasiliensis act as adjuvant for Th2 responses. Eur. J. Immunol. 30:1977–1987. [DOI] [PubMed] [Google Scholar]
- 45.Devouassoux, G., D.D. Metcalfe, and C. Prussin. 1999. Eotaxin potentiates antigen-dependent basophil IL-4 production. J. Immunol. 163:2877–2882. [PubMed] [Google Scholar]
- 46.Poorafshar, M., H. Helmby, M. Troye-Blomberg, and L. Hellman. 2000. MMCP-8, the first lineage-specific differentiation marker for mouse basophils. Elevated numbers of potent IL-4-producing and MMCP-8-positive cells in spleens of malaria-infected mice. Eur. J. Immunol. 30:2660–2668. [DOI] [PubMed] [Google Scholar]
- 47.Brown, S.J., S.J. Galli, G.J. Gleich, and P.W. Askenase. 1982. Ablation of immunity to Amblyomma americanum by anti-basophil serum: cooperation between basophils and eosinophils in expression of immunity to ectoparasites (ticks) in guinea pigs. J. Immunol. 129:790–796. [PubMed] [Google Scholar]
- 48.Ogilvie, B.M., P.W. Askenase, and M. Elaine Rose. 1979. Basophils and eosinophils in three strains of rats and in athymic (nude) rats following infection with the nematodes Nippostrongylus brasiliensis or _Trichinella spiralis_Immunology. 39:385–389. [PMC free article] [PubMed] [Google Scholar]
- 49.Steeves, E.B.T., and J.R. Allen. 1990. Basophils in skin reactions of mast cell-deficient mice infested with Dermacentor variabilis. Int. J. Parasitol. 20:655–667. [DOI] [PubMed] [Google Scholar]
- 50.Luccioli, S., D.T. Brody, S. Hasan, A. Keane-Myers, C. Prussin, and D.D. Metcalfe. 2002. IgE+, Kit-, I-A/I-E- myeloid cells are the initial source of IL-4 after antigen challenge in a mouse model of allergic pulmonary inflammation. J. Allergy Clin. Immunol. 110:117–124. [DOI] [PubMed] [Google Scholar]
- 51.Gauchat, J.-F., S. Henchoz, G. Mazzei, J.-P. Aubry, T. Brunner, H. Blasey, P. Life, D. Talabot, L. Flores-Romo, J. Thompson, et al. 1993. Induction of human IgE synthesis in B cells by mast cells and basophils. Nature. 365:340–343. [DOI] [PubMed] [Google Scholar]
- 52.Yanagihara, Y., K. Kajiwara, Y. Basaki, K. Ikizawa, M. Ebisawa, C. Ra, H. Tachimoto, and H. Saito. 1998. Cultured basophils but not cultured mast cells induce human IgE synthesis in B cells after immunologic stimulation. Clin. Exp. Immunol. 111:136–143. [DOI] [PMC free article] [PubMed] [Google Scholar]