Ionic Currents Mediated by a Prokaryotic Homologue of CLC Cl− Channels (original) (raw)
Abstract
CLC-ec1 is an E. coli homologue of the CLC family of Cl− channels, which are widespread throughout eukaryotic organisms. The structure of this membrane protein is known, and its physiological role has been described, but our knowledge of its functional characteristics is severely limited by the absence of electrophysiological recordings. High-density reconstitution and incorporation of crystallization-quality CLC-ec1 in planar lipid bilayers failed to yield measurable CLC-ec1 currents due to porin contamination. A procedure developed to prepare the protein at a very high level of purity allowed us to measure macroscopic CLC-ec1 currents in lipid bilayers. The current is Cl− selective, and its pH dependence mimics that observed with a 36Cl− flux assay in reconstituted liposomes. The unitary conductance is estimated to be <0.2 pS. Surprisingly, the currents have a subnernstian reversal potential in a KCl gradient, indicating imperfect selectivity for anions over cations. Mutation of a conserved glutamate residue found in the selectivity filter eliminates the pH-dependence of both currents and 36Cl− flux and appears to trap CLC-ec1 in a constitutively active state. These effects correlate well with known characteristics of eukaryotic CLC channels. The E148A mutant displays nearly ideal Cl− selectivity.
Keywords: reconstitution, planar bilayer, CLC channel, liposome
INTRODUCTION
For the past 25 yr membrane reconstitution techniques have supplemented direct cellular electrophysiological recording in mechanistic investigations of ion channel proteins. The insertion of ion channels into an in vitro membrane system—whether based on biochemically accessible liposomes or electrically accessible planar lipid bilayers—provides advantages of chemical simplicity and low background, but suffers the loss of cellular complexity that often dictates physiological function. The recent arrival of high-resolution ion channel structures, however, defines ion channel reconstitution as an absolute imperative for the examination of structure-function relations in these proteins. This new role of reconstitution is a consequence of the nearly exclusive use of prokaryotic ion-channel homologues for structure determination, channels that are usually not expressible in eukaryotic cells used for cellular electrophysiological analysis. Direct functional observation of these channels requires that the bacterially expressed purified proteins be placed into a membrane in vitro. It is only by this kind of scrutiny that we can assess the mechanistic details by which the structurally visible prokaryotic channels differ from or conform to their eukaryotic relatives.
Among the growing collection of eukaryotic ion channels with prokaryotic homologues, the CLC family of Cl−-selective channels has recently attained structural status. CLC-ec1, a CLC homologue from E. coli originally named eriC (Maduke et al., 1999), was crystallized and its structure solved at 3.5 and 2.8 Å (Dutzler et al., 2002, 2003). The structure directly confirmed the basic CLC architecture deduced inferentially by electrophysiological analysis—the unusual double-barreled construction whereby the homodimeric channel carries two identical pores, one in each monomer (Middleton et al., 1994, 1996; Ludewig et al., 1996). More importantly, the crystallographic studies identified the conserved Cl−-coordinating residues at the “selectivity filter,” proposed a permeation pathway through which Cl− ions diffuse, and suggested a simple pH-dependent gating mechanism by which extracellular protons open the pore upon binding to a particular glutamate sidechain.
Since its identification, purification, and reconstitution, functional characterization of CLC-ec1 has relied exclusively on the measurement of Cl− fluxes in liposomes (Maduke et al., 1999; Iyer et al., 2002). Though lacking the high resolution of electrophysiological recordings, these studies showed CLC-ec1 to match expectations from the electrophysiological behaviors of eukaryotic CLC channels, at least in its basic characteristics. CLC-ec1–reconstituted liposomes are specifically permeable to Cl−, Br−, and several other monovalent inorganic anions and show no discernable permeability to Na+ or K+. Among anions, the CLC-ec1 selectivity sequence determined by 36Cl− fluxes is similar to electrophysiologically determined CLC selectivity. Moreover, CLC-ec1–mediated 36Cl− fluxes are accelerated by low pH, a trait linked to the protein's physiological role in extreme acid resistance in E. coli (Iyer et al., 2002), as well as reminiscent of proton-linked gating of several extensively characterized eukaryotic CLCs (Hanke and Miller, 1983; Rychkov et al., 1996; Chen and Chen, 2001).
Our previous attempts to obtain electrophysiological measurements from CLC-ec1 failed (unpublishable data). In this paper we describe a set of biochemical maneuvers that now allow us to record macroscopic currents of CLC-ec1 reconstituted in planar lipid bilayer membranes. We present an initial characterization of these currents, showing that their basic properties are roughly in harmony with those of 36Cl− fluxes in CLC-ec1-reconstituted liposomes.
MATERIALS AND METHODS
Chemicals
Lipids, 1-palmitoyl-2-oleoyl phosphatidylethanolamine (POPE), phosphatidylglycerol (POPG), and E. coli polar lipid extract were purchased from Avanti Polar Lipids. Detergents used for lipid solubilization and protein extraction were decylmaltoside (DM), Sol grade from Anatrace and 3-[(3-cholamido-propyl)dimethylammonio]-1-propanesulfonate (CHAPS) from Pierce Chemical Co. Endopeptidase LysC was obtained from Roche Chemicals. All other chemicals were reagent grade.
Recombinant DNA Manipulation and Protein Expression
The DNA sequence encoding CLC-ec1, the protein product of E. coli gene b0155 (now designated clcA in the E. coli genome database) was inserted into the pASK-IBA2 vector (Sigma-Aldrich) between the XbaI and HindII sites (Maduke et al., 1999). Appended to a deletion of the last five residues of the natural CLC-ec1 sequence was a linker containing LysC and thrombin sites followed by a hexahistine tag, to yield the final COOH-terminal sequence …LARSKAAKGSGTLVPRGSGLEHHHHHH. Mutations E148A and E148Q were generated using standard two-step PCR.
CLC-ec1 was expressed after plasmid transformation into in an adventitiously generated variant strain of commercially available DE3 cells derived from Stratagene lot # 0420399. This strain, which we denote Por-No, was used because it produces the lowest amounts of contaminating outer membrane porins we have observed from many strains tested. For a typical 3 liter prep, cells from the transformation plates were inoculated into 2-liter baffled flasks, each containing 1 liter Terrific broth (Sambrook et al., 1989) with ampicillin (100 μg/ml) and grown at 37°C. When OD600 reached 1.6–1.9, cells were induced 3 h with 0.2 mg/L anhydrotetracycline. Cells were collected by centrifugation and stored overnight at 4°C. The next morning they were resuspended in 50 ml TrisCl, 100 mM NaCl, pH 7.5, and disrupted by sonication on ice in presence of leupeptin (20 μg/ml), pepstatin (20 μg/ml), and PMSF (∼0.2 mg/ml). All further operations were performed at room temperature. Protein was extracted for 2 h at room temperature with gentle shaking in presence of 50 mM DM, and the extract was centrifuged at 16,000 rpm for 45 min. The supernatant was loaded onto a 3-ml Cobalt column (BD Biosciences), at a flow rate of 2 ml/min, which had been washed previously with 5 vol of 40 mM TrisCl, 200 mM NaCl, 10 mM DM, pH 7.5 (WB). After washing with 10–15 vol of WB, nonspecifically bound proteins were eluted with 10 vol of WB containing 30 mM imidazole. The protein was then eluted with WB containing 400 mM imidazole and concentrated to a final volume of ∼0.5 ml in a centrifugal membrane concentrator. The hexahistidine tag was cleaved by digestion with LysC (0.5 U) for 1 h. The resulting product was diluted 20-fold in 10 mM DM and loaded on a 1 ml Poros HQ high-capacity anion exchange column preequilibrated with 10 volumes of 30 mM NaCl, 10 mM TrisCl, 10 mM DM, pH 7.5 (LoQ). The column was washed with 30 volumes of LoQ and the protein was eluted applying a salt gradient between LoQ and HiQ Buffers (300 mM NaCl, 10 mM TrisCl, 10 mM DM, pH 7.5), 30 ml each. The resulting CLC-ec1 peak was fractioned into 5 separate aliquots (2 ml each). Only the aliquots corresponding to the rising phase of the elution peak were concentrated and used for bilayer work.
Reconstitution into Liposomes
E. coli polar lipids were dried under N2 and washed once with pentane. They were then suspended to a final concentration of 20 mg/ml in reconstitution buffer, RB (450 mM KCl, 25 mM citric acid, 25 mM phosphoric acid, adjusted to pH 7.0 with KOH), to which 35 mM CHAPS was added. The suspension was sonicated to clarity, and after a 2-h incubation CLC-ec1 protein was added to the desired concentration: 25–90 μg protein/mg lipid for planar bilayer reconstitution and 1.5 μg/mg for 36Cl− uptake. Detergent was dialyzed out overnight, and the resulting liposomes were fast frozen in ethanol/dry ice and stored at −80°C. A few experiments used liposomes made from POPE/POPG, with similar results.
Concentrative 36Cl− Uptake Assay
Influx of 36Cl− was assayed as described (Maduke et al., 1999). The reconstituted vesicles were thawed, and pH was adjusted to the desired value with H3PO4 and sonicating briefly to give unilamellar vesicles. External Cl− was removed by spinning 100 μl aliquots through a Sephadex G-50 columns equilibrated in uptake buffer at the desired pH, UB, (400 mM sorbitol, 25 mM citric acid, 25 mM phosphoric acid, pH adjusted with KOH). 36Cl− uptake was initiated by adding ∼800 μL of UB fortified with ∼0.1 μCi/ml 36Cl−. At the desired times samples were collected by adding 100 μl of vesicles to a Dowex-glutamate column and eluting into a scintillation vial with 1.5 ml of 400 mM sorbitol. 15 ml of scintillation fluid was added to the vial and radioactivity was counted.
Electrophysiological Recordings and Data Analysis
Liposomes containing CLC-ec1 at high protein density were incorporated under salt-gradient conditions into bilayers formed from POPE/POPG (7.5:2.5 mg/ml) in n-decane, using a horizontal planar lipid bilayer system (Chen and Miller, 1996; Nimigean and Miller, 2002). Briefly, two aqueous chambers (0.3–0.7 ml) are separated by a partition with a ∼50-μm hole where the bilayer is formed (35–100 pF). 1 μL of CLC-ec1–containing liposomes was added to the upper chamber to a preformed bilayer, and sometimes to favor vesicle fusion ∼1 μl of 1.5–3 M KCl was added right after the vesicles. The standard high-salt (HS) solution was: 290 mM KCl, 10 mM HCl, 5 mM histidine, 5 mM glutamic acid, pH 3 or 7 with KOH. The low-salt solution (LS) was: 35 mM KCl and 10 mM HCl containing the same buffers and adjusted to pH 3 or 7 with KOH. The low-Cl (LCL) solution was: 5 mM HCl, 285 mM K2SO4, 10 mM H2SO4, 5 mM histidine, 5 mM glutamate, pH 3 or 7 with KOH. For selectivity experiments 255 mM KCl in the HS solution was replaced with an equivalent amount of the potassium salt of the desired anion. All selectivity experiments were performed at symmetrical pH 3.
Currents were recorded using the Clampex 8.2 program (Axon Instruments, Inc.) with an Axopatch 200B amplifier (Axon Instruments, Inc.) sampled at 100 μs and filtered at 1 kHz. Data were analyzed using Clampfit (Axon Instruments, Inc.), Ana (written by Dr. Michael Pusch), and SigmaPlot 8.02. In all cases, voltages were corrected for liquid junction potentials (in most case <1 mV, and in no case >10 mV) and error bars represent the SEM of at least three separate experiments, each in a separate bilayer.
Electrophysiological Convention
The bilayer system consists of two aqueous solutions separated by a partition holding the membrane. By convention, we define the upper chamber to which vesicles are added as the “cis” side, and also define the opposite “trans” side as electrical ground, so that transmembrane voltage is reported as Vcis-Vtrans. This choice is analogous to the electrophysiological convention, where the extracellular side is defined as zero voltage. Likewise, we refer to positive current (positive charge flowing toward the trans side or negative charge toward the cis) as “outward”. We emphasize, however, that the ion channels are probably inserted in both directions in the reconstituted membranes, so the above sidedness is meaningful only for electrical convention, not for molecular orientation.
RESULTS
Our initial attempts to observe CLC-ec1 channels in lipid bilayers, aimed at single-channel behavior, all failed. We reconstituted the protein in lipid vesicles at low protein to lipid ratio, ∼1 μg/mg, equivalent to 1–5 channels per liposome (Maduke et al., 1999), and added the vesicles to lipid bilayers under fusion conditions routinely used for incorporation of other ion channels such as KcsA (Heginbotham et al., 1999). Moreover, we took care to use lipids and low pH conditions known to maintain robust CLC-ec1–mediated 36Cl− flux in the liposomes (Maduke et al., 1999; Iyer et al., 2002). Nonetheless, we never observed anion-selective unitary channel behavior.
This failure might mean that only a small fraction of the protein is functional, that the passage of ions through CLC-ec1 is very slow compared with conventional ion channels, or that CLC-ec1 opens so briefly, even under “fully activated” conditions at pH 3.0 (Iyer et al., 2002), that we cannot resolve single channels. The first hypothesis is undermined by previous work showing that the majority of reconstituted CLC-ec1 protein is active in mediating 36Cl− uptake (Maduke et al., 1999). If either of the latter two cases is operating, by simply increasing the number of CLC-ec1 molecules present in each vesicle we might be able to detect macroscopic currents. Accordingly, following the purification procedure of Dutzler et al. (2002) to obtain crystal-quality CLC-ec1, we reconstituted the protein at high protein density (∼30 μg/mg, ∼100 channels per vesicle) and incorporated the vesicles into planar lipid bilayers. Fig. 1 A shows a representative recording obtained at V = 0 with a 300/40 mM cis/trans KCl gradient at pH 3. Single channels with huge conductance (∼600 pS) reproducibly appear upon addition of these liposomes. The channels produce outward current (upward openings) at zero voltage and are thus cation selective, and they display long-lived openings with occasional brief partial closures of ∼1/3 the amplitude of the full current. Moreover, these channels can be driven into long closed states by high voltage (>150 mV) and by adding certain polyamines (unpublished data). These characteristics are strongly suggestive of outer membrane porins (Benz and Bauer, 1988; Iyer and Delcour, 1997). In these pilot experiments, we occasionally observed a few small anionic fusions, but after a single porin incorporation the bilayer current becomes dominated by the mildly cation-selective porin and is subsequently useless for our purposes.
Figure 1.
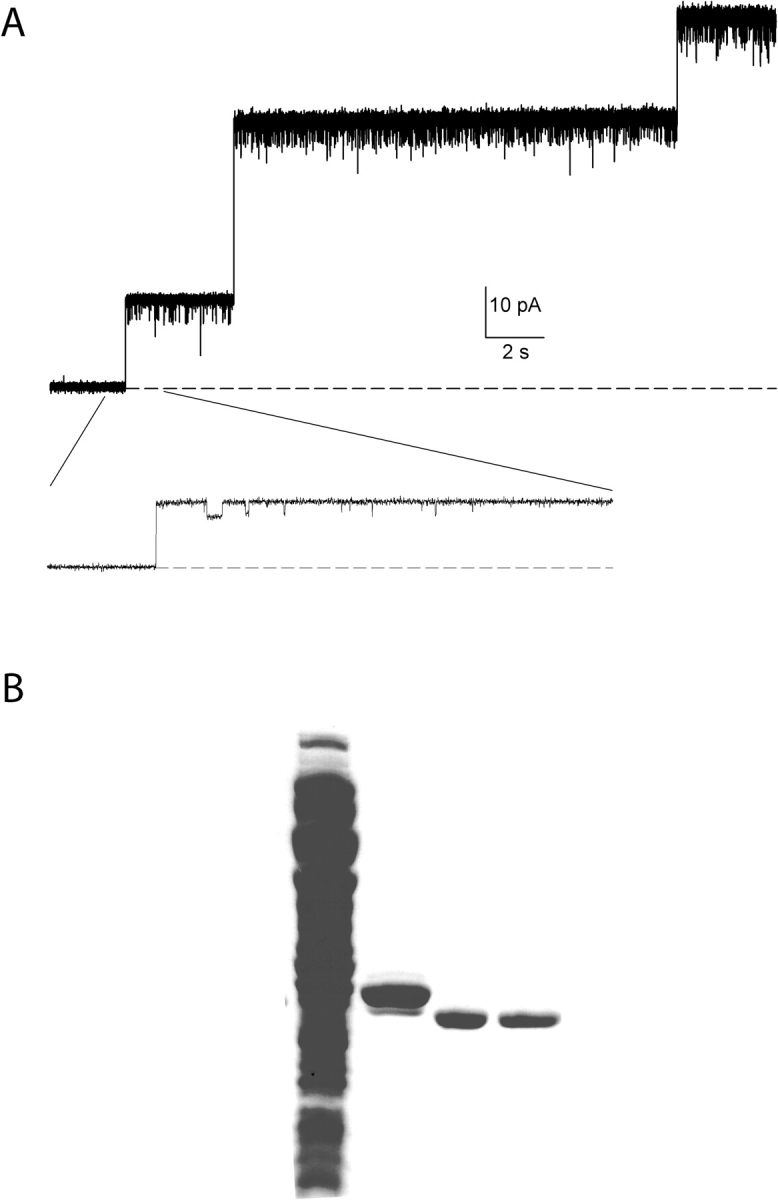
Minuscule, debilitating porin contamination in CLC-ec1 prep. (A) Electrophysiological recording of crystal-quality CLC-ec1 prep incorporated into planar lipid bilayers. The recording is at 0 mV holding potential and cis/trans salt gradient conditions: 300 mM/40 mM KCl, pH 3. Liposomes contained CLC-ec1 at 30 μg/mg density. Dashed line represents zero-current level. Inset shows part of the trace on an expanded time scale. (B) Coomassie blue stained SDS gel illustrating the various steps in the CLC-ec1 purification procedure. Lane 1, crude membrane extract; lane 2, elution from Co column; lane 3, after digestion with LysC; lane 4, final preparation after anion exchange step.
The prominence of putative porin in the bilayer assay exists side-by-side with a contamination in our CLC-ec1 preps undetectable on overloaded Coomassie-stained SDS gels. Indeed, the porin recording of Fig. 1 A was taken from a CLC-ec1 prep that crystallized in complex with a FAB fragment and diffracted beyond 3.0 Å resolution (unpublished data). Since we are attempting to observe macroscopic currents arising from many thousands of CLC-ec1 molecules, even a minuscule (∼0.1%) presence of a giant-conductance contaminant becomes disabling. For this reason, we require a level of purity even higher than that needed for high-quality diffraction.
We therefore modified the purification procedure by substituting the Superdex-200 size-exclusion step with an anion-exchange procedure. The rationale behind this choice is that gel filtration does not separate CLC-ec1, a dimer of 50-kD monomers, from porins, trimers of ∼30 kD monomers, as confirmed in “dummy” purifications performed on uninduced cells (unpublished data). Porins, generally more acidic than CLC-ec1, are expected to be retained more efficiently on an anion-exchanger. The protein gel of Fig. 1 B illustrates the two-step purification procedure involving a Co column followed by anion exchange on a Poros HQ column. Even after the Co column alone, the protein is quite pure (lane 2); no porin band is visible, although reconstitution of the prep at this point results in frequent incorporation of porin in bilayer recording as in Fig. 1 A. After removal of the histidine tag by digestion with LysC, apparent by the shift in molecular weight (lane 3), the prep is applied to the anion exchange column. Upon beginning a salt gradient, pure CLC-ec1 elutes early (lane 4) and produces porin channels only rarely upon reconstitution into planar bilayers.
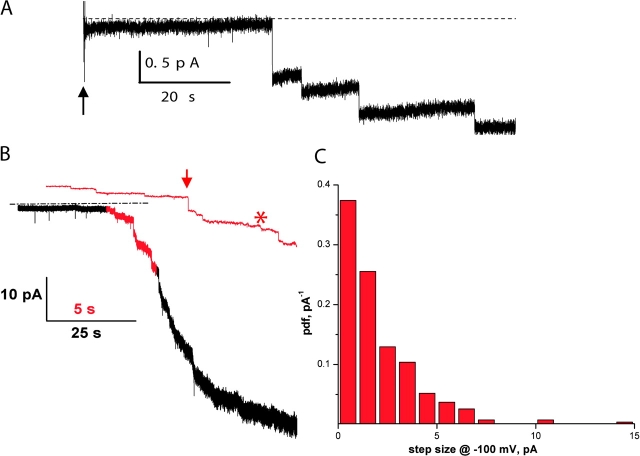
Using this improved purification procedure, we reconstituted CLC-ec1 at high density and incorporated the proteoliposomes into lipid bilayers. Fig. 2 A shows vesicle fusions at zero voltage under salt-gradient conditions as in Fig. 1 A (300/45 mM KCl cis/trans gradient at pH 3). After addition of the vesicles (arrow) current increases in small, well-defined steps. The steps are anion selective, as indicated by their inward-current polarity (downward at zero voltage). Even though each fusion event corresponds to the insertion of ∼200 CLC-ec1 molecules into the bilayer (Maduke et al., 1999), the jumps are very small, ∼0.5 pA, in harmony with our suspicion that the single-CLC-ec1 conductance might be small. Fig. 2 B shows a bilayer recording as in Fig. 2 A, but at a holding potential of −100 mV to visualize the current jumps, and using liposomes with ∼100 channels per vesicle. This recording involves >50 fusion events, unblemished by ∼50-pA steps that would reflect porin insertion. The red portion of the trace is shown on an expanded time scale. It is clear that the steps are of varied size; while some are as large as a few pA (arrow), other parts of the trace (star) show an apparently continuous creep of inward current that we ascribe to many sequential fusions too small to be resolved individually. To obtain a rough estimate of the single CLC-ec1 current we examined the step-size distribution at −100 mV holding voltage (Fig. 2 C). The step-size amplitude histogram is broad, as expected from the wide variation of liposome sizes (Heginbotham et al., 1998). The mean value for the observable steps is ∼2 pA, which represents an upper limit since we would miss most fusion steps below 0.3 pA. Assuming that the mean vesicle content is ∼100 CLC-ec1 dimers (Maduke et al., 1999) and that the open probability is close to unity at this acidic pH, this represents a unitary conductance of ∼0.1 pS. Electrical events with characteristics of these fusion-steps were never observed if CLC-ec1 protein was omitted from the reconstitution mix used to form the liposomes. Because of uncertainties arising from liposome size-heterogeneity (Maduke et al., 1999), the number of molecules and unitary conductance cited above should be considered only rough order of magnitude estimates, not precise values.
Figure 2.
CLC-ec1 fusions in lipid bilayers. (A) Anionic fusion-steps. Vesicles containing CLC-ec1 at protein to lipid ratio 50 μg/mg were added at the arrow to a bilayer under KCl-gradient conditions and zero-voltage holding potential. Solutions are cis HS side trans LS at symmetrical pH 3. Dashed line represents the zero current level. The trace was filtered at 50 Hz for display purposes. (B) Fusions at −100 mV. A recording similar to that in A was collected, except that protein density was 25 μg/mg and holding potential was −100 mV and filtering was at 500 Hz. Red portion of the trace is also shown on expanded scale, with 50 Hz filtering.
Macroscopic CLC-ec1 Currents
Since the diminutive unitary conductance of CLC-ec1 precludes its characterization at the single-molecule level, this work must rely solely on macroscopic currents. Upon stimulating a bilayer containing thousands of CLC-ec1 molecules with a simple voltage pulse protocol in 300 mM symmetrical KCl at pH 3, we obtain currents like those shown in Fig. 3 A. The currents do not show voltage- or time-dependent gating; after relaxation of the capacitive transient, they are in steady-state. The I-V curve (Fig. 3 C) is almost ohmic, displaying only a slight rectification at positive voltages. Thus, CLC-ec1 does not share the characteristic voltage-dependent gating and macroscopic rectification extensively studied in numerous eukaryotic CLC channels (Jentsch et al., 2002).
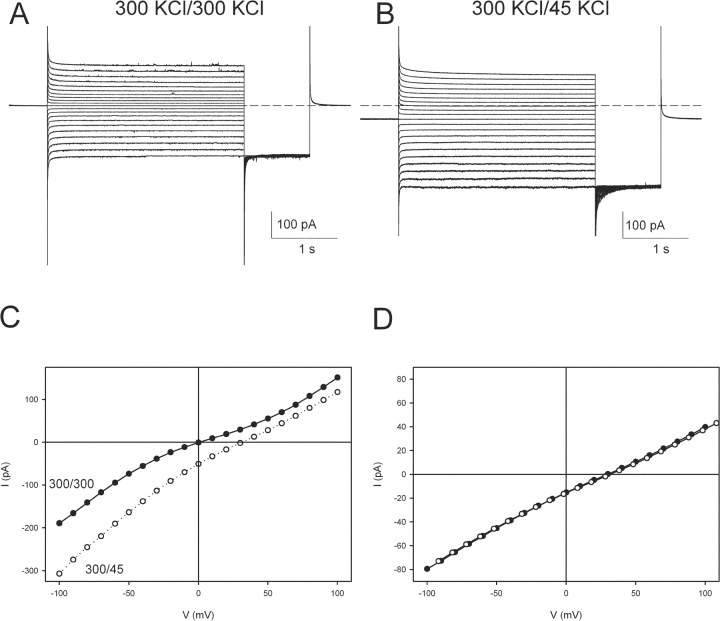
Figure 3.
Macroscopic CLC-ec1 currents. (A) Macroscopic CLC-ec1 currents elicited with the following protocol: from a holding potential of 0 mV the voltage is stepped to −100 to +100 mV in 10-mV steps for 3 s. After a 1-s tail pulse to −100 mV the voltage is then returned to 0 mV. The solutions are symmetrical HS at pH 3.0 on both sides. Dashed line represents the zero current level. (B) Currents from the same bilayer as in A, when the solution on the trans side is LS at pH 3. Dashed line represents the zero current level. The stimulation protocol is the same as in A. (C) I-V curves of the traces shown in A (filled circles) and B (empty circles). (D) An IV curve was taken in a 300/45 mM KCl gradient as in B (filled circles). K+ was then replaced by NMG+ on both sides of the bilayer, and an IV curve was recorded (empty circles).
When we apply a transmembrane salt gradient (300 mM KCl on the cis side and 45 mM KCl on the trans) the currents reverse at positive voltages (Fig. 3 B), indicating anion selectivity, in agreement with the inward current observed at zero voltage (Fig. 2 A). The reversal potential is +31 ± 1 mV (n = 13, Fig. 3 C), a value significantly below the ∼45 mV Nernst potential for Cl−. To test the possibility that permeability of K+ (the only other major ion in the system) accounts for this result, we substituted all the K+ on both sides of the membrane with equal amounts of the large organic cation N-methyl glucamine. Since this maneuver leaves the I-V curve unchanged (Fig. 3 D), the subnernstian reversal of CLC-ec1 cannot reflect K+ permeability.
pH Dependence of CLC-ec1
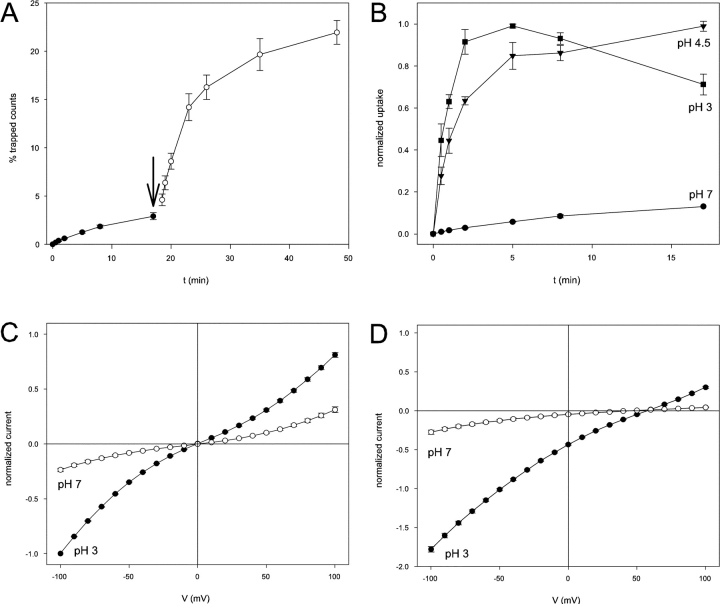
Recently Iyer et al. (2002) showed that CLC-ec1 is activated by acid, as expected from its physiological role in the extreme acid resistance response of enteric bacteria. Fig. 4 A illustrates this characteristic with a 36Cl− flux experiment in which pH is changed during the uptake time course. Initially with the CLC-ec1–reconstituted liposomes in symmetrical pH 7, uptake is slow and nearly linear over the first 15 min. Dropping the pH to 4.5 leads to an immediate 10-fold increase in initial uptake rate followed by an eventual attainment of steady-state after ∼30 min. Fig. 4 B compares uptake at symmetrical pH 7, 4.5, and 3. While at pH 3.0 and 4.5 uptake is rapid and reaches steady-state after ∼5 and 15 min, respectively, at pH 7.0 the process is still only at 20% of the equilibrium level after 1 h (unpublished data). These results are in good agreement with previous work from Iyer et al. (2002).
Figure 4.
pH dependence of CLC-ec1. (A) Lowering the pH increases the 36Cl− influx rate in CLC-ec1 containing liposomes. Uptake of 36Cl− was monitored at pH 7.0 (filled circles), and the pH was then lowered to 4.5 (empty circles) by addition of H3PO4. The uptake is normalized to the total number of counts per sample. (B) Time course of 36Cl− influx was followed at pH 7.0 (circles), 4.5 (triangles), and 3 (squares). Uptake is normalized to the steady-state uptake observed (equivalent to 20–30% of total counts, except at pH 3.0 where the uptake was roughly five- to tenfold lower). At pH 7.0 no steady-state was reached after as long as 4 h (not depicted). For normalization purposes the value at t = 17′ was assumed to be 13% of the steady-state value, in agreement with the results shown in A. (C) Normalized I-V curves of wildtype CLC-ec1 at pH 7.0 (empty circles) and pH 3.0 (filled circles) in symmetrical HS solutions. The I-Vs were normalized to the value of the currents at −100 mV in symmetrical HS solutions at pH 3.0 in the same bilayer. (D) Normalized I-V curves of wild-type CLC-ec1 at pH 7.0 (empty circles) and pH 3.0 (filled circles) with a salt gradient: HS solution on the cis side and LCL on the trans side. The I-V curves were normalized to the value of the currents at −100 mV in symmetrical HS solutions at pH 3.0 in the same bilayer.
In planar bilayers, CLC-ec1–mediated currents are similarly pH dependent. Fig. 4 C compares I-V curves in symmetrical 300 mM KCl at pH 7.0 and pH 3. Lowering pH from 7 to 3 produces a fourfold increase in zero-voltage slope conductance, a somewhat smaller pH activation than that seen in the 36Cl− uptake assay. We repeated this experiment (Fig. 4 D) with a transbilayer Cl− gradient so as to approximate more closely the conditions required for the liposome flux assay, reducing trans Cl− concentration from 300 to 5 mM while substituting it with SO4 2−. In these asymmetrical Cl− conditions, lowering the pH has a stronger (eightfold) effect on conductance at reversal, in respectable agreement with the flux results above. It is also worth noting that the Cl− selectivity of CLC-ec1, as indicated by the reversal potentials of Fig. 4 D, is similar at pH 3.0 and 7.
Electrophysiological Characteristics of Mutant E148A
Recent work from several labs (Dutzler et al., 2003; Estevez et al., 2003; Traverso et al., 2003) demonstrates that a conserved glutamate residue found in the selectivity filter of CLC channels plays a fundamental role in CLC gating. Mutation of this residue to Ala in CLC-0 and CLC-1 results in constitutively open channels. This effect in the eukaryotic channels is interpretable from the structure of the analogous CLC-ec1 mutant (E148A), which shows that the mutation frees the putative permeation pathway from the blocking position of the endogenous glutamate sidechain while preserving the polypeptide backbone almost perfectly (Dutzler et al., 2003). These structural studies further suggested that this carboxyl sidechain acts as a pH-dependent gate for the pore in wild-type CLC channels. According to this picture, at high extracellular pH the deprotonated sidechain occludes the Cl− permeation pathway, while upon protonation at low pH, the carboxyl group swings away from the pore and permits unimpeded access of Cl− to the extracellular solution; the known effect of lowered extracellular pH in opening CLC-0 (Chen and Chen, 2001; Dutzler et al., 2003) is thus specifically attributed to protonation of this glutamate sidechain.
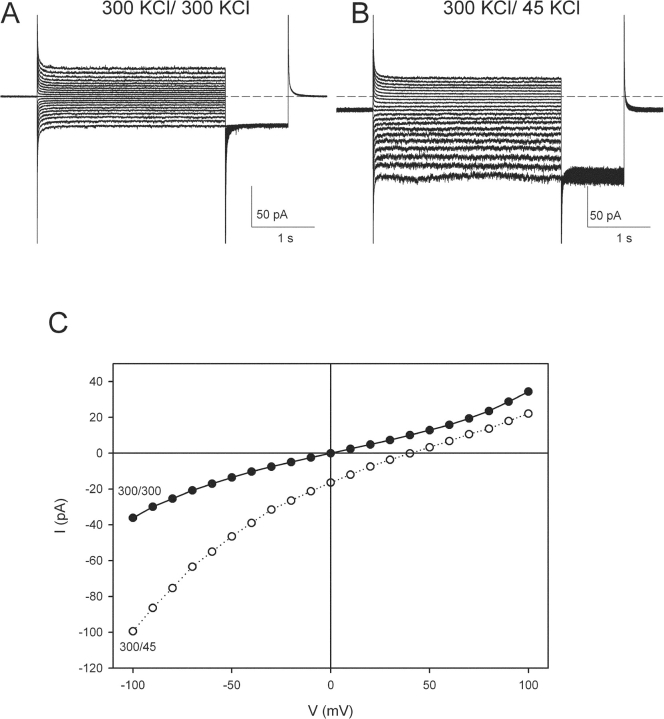
Since this idea has been tested only in eukaryotic CLC homologues, we considered it worthwhile to examine the functional consequences of mutating this critical glutamate in CLC-ec1 itself. Fig. 5 A shows typical traces recorded from the E148A mutant at pH 3.0 in symmetrical 300 mM KCl (Fig. 5 A) or in the presence of a 300/45 mM cis/trans KCl gradient (Fig. 5 B). In both cases, the I-V curves (Fig. 5 C) are similar to those of wild-type protein, with no apparent voltage or time dependence, although the mild rectification found in wild-type is absent in this mutant. A clear difference, however, is revealed in salt-gradient conditions; here the reversal potential (+41 ± 1 mV, n = 13) is close to the Cl− equilibrium potential, +44 mV, in contrast to wild-type (Fig. 3 C).
Figure 5.
Macroscopic currents of the E148A mutant. (A) Macroscopic E148A currents elicited with the same protocol as in 3A. The solutions are symmetrical HS at pH 3.0 on both sides. Dashed line represents the zero current level. (B) Currents from the same bilayer as in A, when the solution on the trans side is LS at pH 3. Dashed line represents the zero current level. The stimulation protocol is the same as in A. (C) I-V curves of the traces shown in A (filled circles) and B (empty circles).
The E148A mutation annihilates the pH dependence of CLC-ec1 activity, as seen with both flux and electrical assays (Fig. 6). In E148A-reconstituted liposomes, 36Cl− uptake timecourses are virtually superimposable over the range pH 3–7 (Fig. 6 A), in striking contrast to their pH sensitivity with wild-type protein (Fig. 4 B). Moreover, the absolute uptake rates for E148A are close to that of “fully activated” wild-type CLC-ec1 at acid pH, in support of the idea that this mutation promotes a constitutively active conformation. Similar results were obtained for the E148Q mutant (unpublished data). The CLC-ec1–mediated currents also lose their pH sensitivity in the E148A mutant (Fig. 6 B), mirroring the flux results. Normalized I-V curves for E148A in symmetrical 300 mM Cl− are nearly identical at pH 7.0 and pH 3, and similar results were obtained at lower Cl− concentrations (unpublished data).
Figure 6.
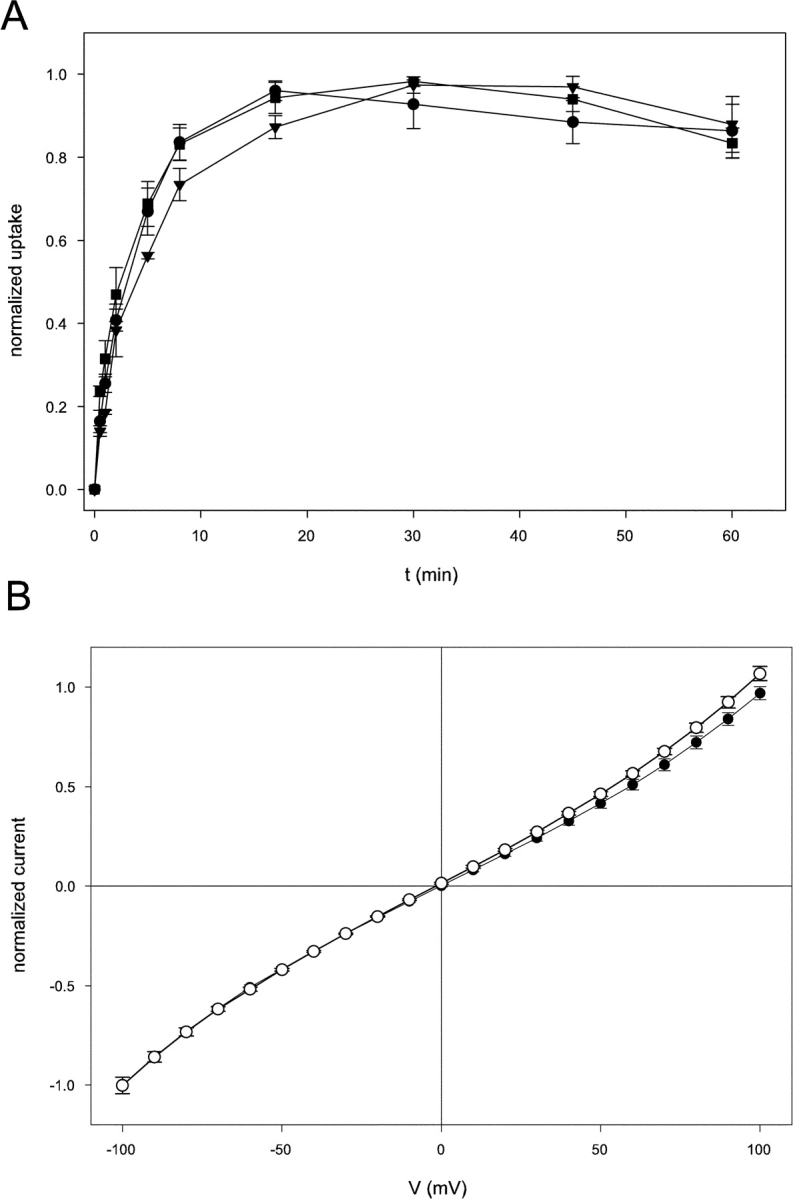
pH dependence of the E148A mutant. (A) Time course of 36Cl− influx for the E148A mutant was followed at pH 7.0 (circles), 4.5 (triangles), and 3 (squares). Uptake is normalized to the steady-state uptake observed (equivalent to 20–30% of total counts, except at pH 3.0 where the uptake was roughly five- to tenfold lower). (B) Normalized I-V curves of E148A CLC-ec1 at pH 7.0 (empty circles) and pH 3.0 (filled circles) in symmetrical HS solutions. The I-V curves were normalized to the value of the currents at −100 mV in symmetrical HS solutions at pH 3.0 in the same bilayer.
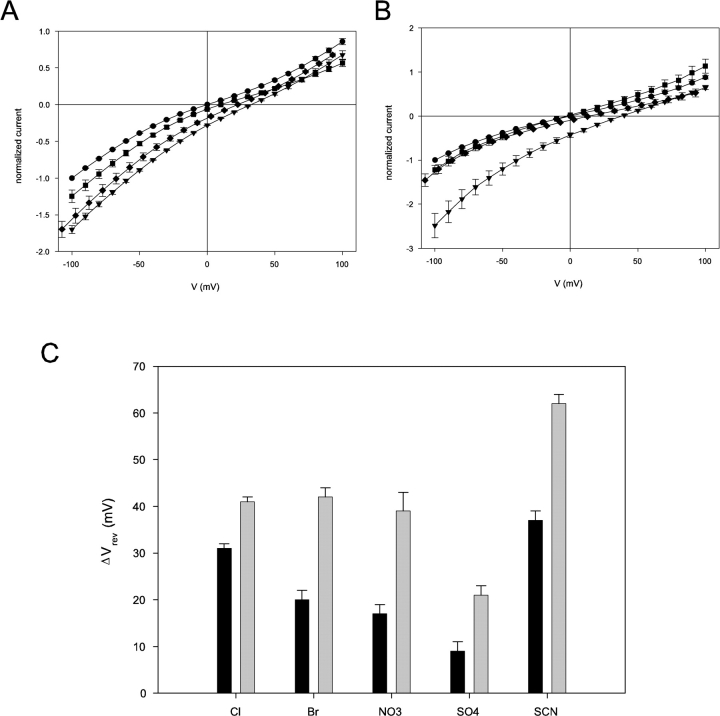
Interanionic Selectivity of CLC-ec1
We have seen above (Fig. 3 C) that the CLC-ec1 reversal potential in a Cl− gradient is subnernstian. The mechanism underlying this deviation from ideal Cl−-selective behavior is currently under investigation. For this reason, instead of expressing the selectivity among anions in terms of intrinsically model-dependent permeability ratios, we report only reversal potentials as indicators of selectivity. To investigate selectivity for various anions, we first collected I-V curves under reference conditions, with a 300/45 mM cis/trans KCl gradient at pH 3. We then added 255 mM of various test anions to the trans side (as potassium salts) and again recorded the reversal potential. The induced shift in reversal potential, a direct indicator of selectivity relative to Cl−, is simply the difference:
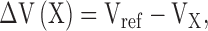
where Vref is the reversal potential in reference conditions and VX is that measured after addition of 255 mM anion X− to the trans side. A shift smaller than that induced by Cl− indicates a lower permeability, while a larger shift means higher permeability. Fig. 7 A shows the normalized I-V curve of CLC-ec1 in the reference condition, as well as after addition of various anions, all of which shift the reversal potential leftward while leaving the overall shape of the I-V curve unchanged. The induced shift for Br− (20 ± 2 mV) is not as large as for Cl− (31 ± 1 mV), but is larger than that elicited by SO4 = (9 ± 2 mV). This experiment was repeated with the E148A mutant (Fig. 7 B). Here, Cl− and Br− induce similar shifts in reversal potential, while SO4 = shows higher permeation than in wild-type, as if the glutamate mutation alters the interanionic selectivity of CLC-ec1. Fig. 7 C summarizes values of the induced shift for wild-type CLC-ec1 and E148A. Overall, wildtype CLC-ec1 shows weak selectivity among the small inorganic anions tested, with a selectivity sequence: SCN− > Cl− > Br− ∼ NO3 − > SO4 =. Apart from the position of SCN− this selectivity sequence agrees tolerably with that previously obtained (Maduke et al., 1999) with a flux method. The mutant E148A almost completely loses its ability to discriminate among different anions, allowing easy passage even to the doubly charged SO4 =, which is poorly permeant in wild-type. Moreover, the selectivity sequence is changed to SCN− > Br− ∼ Cl− ∼ NO3 − > SO4 =.
Figure 7.
Selectivity of wild-type and E148A CLC-ec1. (A) Normalized I-Vs of wildtype CLC-ec1 in different ionic conditions in symmetrical pH 3. The solution on the cis side was HS; the trans side contained LS solution to which was added 255 mM KCl (circles), KBr (squares), K2SO4 (diamonds), or nothing (triangles). (B) Normalized I-Vs of E148A CLC-ec1 in the same conditions as in A (symbols are also the same). (C) Shifts in reversal potentials induced by test anions (Cl−, Br−, NO3 −, SCN− and SO4 =) in CLC-ec1 wildtype (black bars) and E148A (gray bars). The shifts, Vrev(X), were calculated for test anion X according to definition given in the text.
DISCUSSION
Though discovered only 5 yr ago (Maduke et al., 1999), CLC-ec1, a prokaryotic member of the CLC family of Cl− channels, has now joined the select group of structurally known ion channel homologues. Moreover, CLC-ec1 is currently the only prokaryotic relative of a eukaryotic ion channel for which a role in bacterial physiology has been identified (Iyer et al., 2002). It is ironic, therefore, that this unique structural and biological information about CLC-ec1 has not been accompanied by electrophysiological recordings. Instead, its functional characterization has relied solely on low-resolution Cl−-flux methods. We have now recorded macroscopic currents from CLC-ec1 reconstituted into planar bilayer membranes. The currents are anion selective, voltage independent, and acid activated. In addition, mutation of a glutamate residue found in the selectivity filter, E148, abolishes the pH-dependence of Cl− currents in bilayers and fluxes in liposomes.
The Currents Are Carried by CLC-ec1
An essential task facing the experimenter for the first time reconstituting a protein in planar bilayers is to establish that the currents observed actually arise from the protein purified (Heginbotham et al., 1999). Five separate sets of evidence establish that the currents recorded here are mediated by the CLC-ec1 in our preparations, which we estimate are >99% pure. First, the currents arise from distinct “fusion-steps” wholly dependent on liposomes reconstituted with many CLC-ec1 molecules functionally active as judged by 36Cl− flux. Second, the Cl− selectivity of the currents is an indicator of the specificity expected from a CLC family member, especially in the negatively charged planar bilayers used here, where artifactual conductances tend to be cation selective. Third, the selectivity of the current among inorganic anions is reminiscent of eukaryotic CLC channels. Fourth, the currents are activated by low pH, as expected from both the demonstrated acid activation of CLC-ec1-catalyzed Cl− flux and the involvement of CLC-ec1 in the physiology of strong acid resistance in E. coli (Iyer et al., 2002). Finally, the mutation E148A eliminates the pH dependence of both 36Cl− uptake and the currents. For these reasons we will henceforth refer without apology to these as CLC-ec1 currents.
CLC-ec1 Has a Low Turnover Rate
The principal obstacle we encountered in trying to record CLC-ec1 electrophysiologically was the undetectably low current carried by a single molecule. This problem necessitated reconstitution at very high protein density, a requirement which, together with the voltage independence of CLC-ec1 currents, denies us direct measurement of single-channel currents through conventional techniques such as single-channel recording or nonstationary noise analysis. To gain a rough estimate of the unitary conductance, we analyzed the step-size distribution of the fusions of vesicles containing many CLC-ec1 molecules (Fig. 2 C). This analysis yielded an upper limit on unitary conductance of ∼0.1 pS, equivalent to a turnover rate of ∼105 ions/s at 100 mV. This is a borderline value, which could indicate either an unusually slow channel or an unusually fast transporter.
Effects of Mutation E148A
Mutation of the glutamate found in the selectivity filter of CLC-ec1 has three major effects. The currents lose their pH dependence, become almost ideally anion-selective, and fail to discriminate among inorganic anions. The complete loss of pH dependence in E148A, which remains active even at neutral pH, supports the idea (Dutzler et al., 2003) that this residue is the extracellular pH sensor in CLC-ec1. This result is analogous to the finding (Dutzler et al., 2003; Estevez et al., 2003; Traverso et al., 2003) that mutation of equivalent residues in CLC-0 (E166) and CLC-1 (E232) greatly increases channel open probability in these eukaryotic homologues. Although we lack the unambiguous evidence of single channels, two facts argue that the E148A mutant is maximally active. First, the uptake rate of radioactive 36Cl− for the mutant is close to that of wild-type at acidic pH, where wild-type CLC-ec1 is fully activated. Second, the fusion step-size observed for the mutant is similar to that of wild-type CLC-ec1 at pH 3.0 (unpublished data). We conclude, therefore, that the E148 sidechain and its counterparts in the eukaryotic channels share a common function: occluding extracellular access to the Cl− selectivity region in a pH-dependent manner. However, the mutation E148A also drastically alters the selectivity properties of CLC-ec1; while actually enhancing general anion selectivity, the currents lose the ability to discriminate among different anions. This effect contrasts with that found in CLC-0, where mutation E166A preserves channel selectivity (Traverso et al., 2003). These selectivity changes thus raise doubts that the crystal structure of the mutant is a precise model for Cl− conduction through wild-type CLC-ec1. Rather, they hint at the possibility that this mutation traps the protein in a state which, while allowing passage of Cl− ions, is not the naturally occurring conductive state of CLC-ec1.
Anion Selectivity
All previous investigations indicate that CLC channels display a nearly ideal preference for anions over cations. In contrast, CLC-ec1 presents a surprising and troubling exception. In a 6.7-fold KCl gradient, the reversal potential of CLC-ec1 is ∼15 mV below the Nernst potential for Cl−, a result that can be interpreted with the certainty provided by thermodynamics: some other ion is also carrying current in the system. One possibility is that together with perfectly Cl−-selective CLC-ec1 we are inadvertently reconstituting a spurious nonselective conductance that degrades the reversal potential of the total current toward zero voltage. This possibility is unlikely since the reversal potential is strikingly consistent among different preparations and among experiments with current amplitudes varying from 10 pA to 1 nA. Furthermore, reversal for the E148A mutant is very close to the ideal Nernstian value, indicating that the process underlying this behavior is mediated by CLC-ec1 itself. This result also shows that whatever the other permeating ion, removal of this glutamate residue eliminates its ability to move through CLC-ec1.
An obvious guess for the identity of the other permeating ion is K+, since this is the only ion in the system comparable to Cl− in concentration. But K+ is ruled out as the culprit by two observations. First, replacement of K+ with a large organic cation does not alter reversal potential. Second, the assay of concentrative 36Cl− uptake, whereby CLC-ec1 shows robust, full activity, is based on impermeability of K+ (Goldberg and Miller, 1991; Maduke et al., 1999); if the CLC-ec1–containing liposomes were even slightly permeable to K+, the salt gradient supporting 36Cl− uptake would be swiftly dissipated and no radioactivity would be trapped inside the vesicles.
So what other ion is going through? We do not yet have a definite answer. The only other prominent ions in solutions are the buffers glutamate and histidine. These are unlikely candidates because of the low concentrations involved (5 mM) and because we have obtained similar results with citrate and phosphate as buffers (unpublished data). To account for the observed deviation from ideal behavior, the permeabilities of these large organic ions through CLC-ec1 would need to be >10-fold that of Cl−, an unlikely occurrence through the narrow and winding pathway revealed by the crystal structure of CLC-ec1. The only other possibilities are H+ and OH− ions, and these are even less suitable as explanatory candidates. At pH 3, these ions are at concentrations of 1 and 10−8 mM, respectively, so their permeabilities would have to be orders of magnitude greater than that of Cl− to account for the shift. We can, moreover, strictly rule out OH− and H+ as electrodiffusive contributors to a Cl−-dominated reversal potential, since increasing pH symmetrically from 3 to 7 in the presence of a KCl gradient does not change the anionic reversal (Fig. 4 D); if either of these ions were diffusing through CLC-ec1 along with Cl−, a change in concentration by four orders of magnitude should induce a shift of reversal potential, in contrast to the null effect we observe.
We are thus left with a perplexing situation. The CLC-ec1 currents display a subnernstian reversal potential whose properties cannot be explained in the familiar terms of electrodiffusive mechanisms. We currently are in the process of investigating this puzzling behavior; preliminary results suggest that the mechanism of ion permeation is not electrodiffusive but rather involves direct coupling between Cl− and H+.
Acknowledgments
We are grateful to R. MacKinnon for hosting CM for an extended lab visit, where initial experiments were carried out. We also thank R. Iyer, C. Nimigean, M. Walden, and W. Nguitragool for discussions and castigations on the manuscript.
Olaf S. Andersen served as editor.
Abbreviations used in this paper: DM, decylmaltoside; POPE, 1-palmitoyl-2-oleoyl phosphatidylethanolamine; POPG, phosphatidylglycerol.
References
- Benz, R., and K. Bauer. 1988. Permeation of hydrophilic molecules through the outer membrane of gram-negative bacteria. Review on bacterial porins. Eur. J. Biochem. 176:1–19. [DOI] [PubMed] [Google Scholar]
- Chen, M.F., and T.Y. Chen. 2001. Different fast-gate regulation by external Cl− and H+ of the muscle-type ClC chloride channels. J. Gen. Physiol. 118:23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, T.-Y., and C. Miller. 1996. Nonequilibrium gating and voltage dependence of the ClC-0 Cl− channel. J. Gen. Physiol. 108:237–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutzler, R., E.B. Campbell, M. Cadene, B.T. Chait, and R. MacKinnon. 2002. X-ray structure of a ClC chloride channel at 3.0 Å reveals the molecular basis of anion selectivity. Nature. 415:287–294. [DOI] [PubMed] [Google Scholar]
- Dutzler, R., E.B. Campbell, and R. MacKinnon. 2003. Gating the selectivity filter in ClC chloride channels. Science. 300:108–112. [DOI] [PubMed] [Google Scholar]
- Estevez, R., B.C. Schroeder, A. Accardi, T.J. Jentsch, and M. Pusch. 2003. Conservation of chloride channel structure revealed by an inhibitor binding site in ClC-1. Neuron. 38:47–59. [DOI] [PubMed] [Google Scholar]
- Goldberg, A.F.X., and C. Miller. 1991. Solubilization and functional reconstitution of a chloride channel from Torpedo californica electroplax. J. Membr. Biol. 124:199–206. [DOI] [PubMed] [Google Scholar]
- Hanke, W., and C. Miller. 1983. Single chloride channels from Torpedo electroplax: activation by protons. J. Gen. Physiol. 82:25–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heginbotham, L., L. Kolmakova-Partensky, and C. Miller. 1998. Functional reconstitution of a prokaryotic K+ channel. J. Gen. Physiol. 111:741–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heginbotham, L., M. LeMasurier, L. Kolmakova-Partensky, and C. Miller. 1999. Single K+ channels from Streptomyces lividans: Functional asymmetries and sidedness of proton activation. J. Gen. Physiol. 114:551–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer, R., and A.H. Delcour. 1997. Complex inhibition of OmpF and OmpC bacterial porins by polyamines. J. Biol. Chem. 272:18595–18601. [DOI] [PubMed] [Google Scholar]
- Iyer, R., T.M. Iverson, A. Accardi, and C. Miller. 2002. A biological role for prokaryotic ClC chloride channels. Nature. 419:715–718. [DOI] [PubMed] [Google Scholar]
- Jentsch, T.J., V. Stein, F. Weinreich, and A.A. Zdebik. 2002. Molecular structure and physiological function of chloride channels. Physiol. Rev. 82:503–568. [DOI] [PubMed] [Google Scholar]
- Ludewig, U., M. Pusch, and T.J. Jentsch. 1996. Two physically distinct pores in the dimeric ClC-0 chloride channel. Nature. 383:340–343. [DOI] [PubMed] [Google Scholar]
- Maduke, M., D.J. Pheasant, and C. Miller. 1999. High-level expression, functional reconstitution, and quaternary structure of a prokaryotic ClC-type chloride channel. J. Gen. Physiol. 114:713–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton, R.E., D.J. Pheasant, and C. Miller. 1994. Reconstitution of detergent-solubilized Cl− channels and analysis by concentrative uptake of 36Cl− and planar lipid bilayers. Methods. 6:28–36. [Google Scholar]
- Middleton, R.E., D.J. Pheasant, and C. Miller. 1996. Homodimeric architecture of a ClC-type chloride ion channel. Nature. 383:337–340. [DOI] [PubMed] [Google Scholar]
- Nimigean, C.M., and C. Miller. 2002. Na+ block and permeation in a K+ channel of known structure. J. Gen. Physiol. 120:323–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rychkov, G.Y., M. Pusch, D.S.J. Astill, M.L. Roberts, T.J. Jentsch, and A.H. Bretag. 1996. Concentration and pH dependence of skeletal muscle chloride channel ClC-1. J. Physiol. 497:423–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook, J., E.F. Fritsch, and T. Maniatis. 1989. Molecular Cloning: A Laboratory Manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. A.2–A.3.
- Traverso, S., L. Elia, and M. Pusch. 2003. Gating competence of constitutively open CLC-0 mutants revealed by the interaction with a small organic inhibitor. J. Gen. Physiol. 122:295–306. [DOI] [PMC free article] [PubMed] [Google Scholar]