Lesions of Endodontic Origin and Risk of Coronary Heart Disease (original) (raw)
. Author manuscript; available in PMC: 2008 Feb 6.
Abstract
A paucity of epidemiologic research exists regarding systemic health consequences of endodontic disease. This study evaluated whether incident radiographically evident lesions of endodontic origin were related to development of coronary heart disease (CHD) among 708 male participants in the VA Dental Longitudinal Study. At baseline and every three years for up to 32 years, participants (who were not VA patients) received complete medical and dental examinations, including full-mouth radiographs. Cox regression models estimated the relationship between incident lesions of endodontic origin and time to CHD diagnosis. Among those ≤ 40 years old, incident lesions of endodontic origin were significantly associated with time to CHD diagnosis (p < 0.05), after adjustment for covariates of interest, with hazard ratios decreasing as age increased. Among those > 40 years old, no statistically significant association was observed. These findings are consistent with research that suggests relationships between chronic periodontal inflammation and the development of CHD, especially among younger men.
Keywords: apical periodontitis, coronary heart disease, epidemiology, inflammation, longitudinal study
INTRODUCTION
A current research controversy centers around a hypothesized connection between the presence of chronic oral infections and the development of adverse systemic health conditions. Several epidemiologic investigations have uncovered relationships between chronic periodontal disease and coronary heart disease (Janket et al., 2003), stroke (Beck et al., 1996; Grau et al., 2004), premature birth and/or low birthweight (Jeffcoat et al., 2003), and respiratory disease (Scannapieco et al., 2003). However, other studies (Hujoel et al., 2002b) have not found significant relationships, sparking questions about the proposed association.
Apical periodontitis is “an acute or chronic inflammatory lesion around the apex of a tooth caused by bacterial infection of the pulp canal system” (Eriksen, 1998), and usually is subsequent to the presence or restoration of deep caries lesions or fractured teeth. Apical periodontitis can be acute and painful or chronic and asymptomatic, and though it can be treated (or prevented) by the elimination of bacteria via root canal therapy, it may persist or recur after treatment is completed. Histologically, it is represented by a periapical inflammatory response that arises after resorption of adjacent supporting bone and local infiltration of inflammatory cells. Clinically, it is diagnosed from patient symptoms, clinical signs, and radiographic images; chronic apical periodontitis, in particular, is confirmed through observation of periradicular radiolucencies on affected teeth.
Despite numerous differences between chronic inflammatory disease of periodontal and endodontic origins, there are notable similarities, primarily that: (1) both conditions share a common microbiota that often is associated with Gram-negative anaerobic bacteria (Sundqvist, 1992; Noiri et al., 2001), and (2) elevated systemic cytokine levels have been observed in conjunction with both disease processes (e.g., increased concentrations of inflammatory mediators have been detected both in gingival crevicular fluid of subjects with periodontal disease and in periapical tissues of endodontically involved teeth) (Barkhordar et al., 1999; Gamonal et al., 2000). To date, the role of chronic endodontic disease in the development of adverse systemic outcomes has not been thoroughly explored; thus, patients and practitioners lack potentially important knowledge about health risks to patients with apical periodontitis. This longitudinal investigation tested the hypothesis that men with more incident radiographically evident lesions of endodontic origin were more likely to develop coronary heart disease (CHD), and that, consistent with the periodontal literature, the effect would be more pronounced in younger men.
MATERIALS & METHODS
Prior to initiation, the project was approved by Institutional Review Boards at the University of North Carolina School of Dentistry and the VA Boston Healthcare System. Participants (who were not VA patients) were men enrolled in the VA Dental Longitudinal Study and the concurrent Normative Aging Study, ongoing epidemiologic studies of male adults in the Boston area. At baseline, in the late 1960s, 1158 systemically healthy dentate men were given complete physical examinations plus clinical and radiological dental examinations. Participants received all their dental and medical care from community practitioners outside the VA system, but agreed to return for study follow-up visits every 3 yrs.
In 2000-01, full-mouth dental radiographic series from participants at each visit were examined by one of two trained, calibrated second-year Boston University endodontic residents blinded to participants' CHD status. To achieve a random sample of participants, we first listed the 1158 records in random order, then the examiners reviewed charts in that order until their time commitment to the project expired, at which point 853 (74%) had been reviewed.
Examiners assessed all teeth with respect to endodontic therapy and chronic apical periodontitis. Teeth were classified as having undergone endodontic therapy if there was radio-opaque material evident in the root canal system, and as having chronic apical periodontitis if there were “Lesions of Endodontic Origin” (LEO) evident radiographically. Teeth were classified as having LEO if they exhibited periapical rarefaction contiguous with periodontal ligament space that was ≥ 2 mm wide, with absence of intact lamina dura. Inter-examiner reliability was calculated for assessment of endodontic therapy and LEO by Cohen's Kappa statistic (Cohen, 1960). Excellent inter-examiner reliability was expected, because the examiners were trained in the same endodontic residency program according to the criteria mentioned above.
The main exposure variable was “Lesion-Years”, which estimated the cumulative burden of chronic endodontic inflammation experienced by each subject. Lesion-Years was a time-dependent variable created by adding tooth-level observations within participants and across visits (e.g., 1 tooth with a LEO for 3 yrs added “3 lesion-years” to the participant-level total). For incident LEO, follow-up started midway between the visit at which the LEO was first observed and the preceding visit. The end date of follow-up differed, depending on the situation:
- If LEO were present at the participant's final visit, the end date was the final visit date.
- If LEO were present on the date of CHD diagnosis (see below), the end date was the CHD diagnosis date.
- If LEO resolved or were on an incident extracted tooth, the end date was midway between the visit at which LEO resolved (or tooth was extracted) and the preceding visit.
Only incident LEO were used in the calculation of lesion-years. Because we could not determine when LEO present at baseline first appeared, we instead chose to use “number of teeth with LEO at baseline” as a covariate in multivariable regression analyses.
The main outcome variable was “Time to First Diagnosis of CHD”. Participants were classified as having incurred this event if acute myocardial infarction (with/without hypertensive disease), chronic ischemic heart disease (with/without hypertensive disease), or angina pectoris (with/without hypertensive disease) was documented at any time after baseline. Regardless of whether the CHD event was fatal or non-fatal, the end of follow-up was the date of first report of the diagnosis (so, for fatal events, that date was the death date).
The newly created endodontic variables were merged with existing databases that contained demographic, medical, and dental variables. Covariates used in the analysis included baseline values of variables commonly used in modeling relationships between periodontal disease and CHD (education, income, body mass index [BMI], smoking, diabetes, hypertension, triglycerides, total cholesterol), plus dental variables of interest (total number of teeth, number of teeth with LEO, number of root-filled teeth, and mean alveolar bone loss measured by means of a Schei ruler [Beck et al., 1996]).
We used Cox regression modeling with time-dependent covariates to evaluate the relationship between lesion-years and time to CHD diagnosis, controlling for potential confounding variables. Because the periodontal literature suggests a more pronounced effect of chronic inflammation among younger than older individuals (see Mattila et al., 2000), we tested the interaction between lesion-years and age. It was not clear how age would interact with lesion-years if the interaction existed, so we used quadratic polynomial spline methodology (Witte and Greenland, 1997) to generate a cutpoint for age as follows: The age range was divided into 5 intervals defined by cutpoints at 40, 45, 50, and 55 yrs. Within each interval, a quadratic polynomial was used, and functions in 2 consecutive intervals were smoothed at their common boundary, allowing for a flexible shape in modeling the relationship.
RESULTS
Kappa values for presence vs. absence of endodontic therapy and LEO were 1.00 and 0.83, respectively. Data from 145 of the 853 participants were excluded from this analysis, due to CHD diagnosis prior to baseline (n = 16); no follow-up visits after baseline (n = 46); age outside the range of 31-65 yrs (n = 30); or incomplete data for ≥ 1 variable in the final regression model (n = 53, with 22, 17, and 14 participants missing values for triglycerides, income, and mean alveolar bone loss, respectively). For the 708 participants analyzed, mean age at baseline was 47.4 yrs; mean number of visits was 7.1 (range, 2-11); and median follow-up time was 24.0 yrs (maximum, 32.0). A total of 250 participants (35.3%) had ≥ 1 incident LEO, and 166 participants (23.4%) were subsequently diagnosed with CHD. The proportion of participants developing new LEO was higher among those who were relatively older, had more bone loss, had ≥ 1 root-filled tooth, had ≥ 1 tooth with a LEO, or had a greater BMI at baseline (Table 1).
Table 1.
Percent of Participants with ≥ 1 Incident Lesion of Endodontic Origin (LEO)a
| Characteristic at Baseline | Value | N | Percent with ≥ 1Incident LEO |
|---|---|---|---|
| Age (yrs) | ≤ 45 | 278 | 26.6 |
| > 45 | 430 | 40.9 | |
| Mean alveolar bone loss score | ≤ median (0.5) | 376 | 29.0 |
| > median (0.5) | 332 | 42.5 | |
| Number of endodontically treated teeth | 0 | 543 | 32.4 |
| ≥ 1 | 165 | 44.8 | |
| Number of teeth with LEO | 0 | 632 | 33.9 |
| ≥ 1 | 76 | 47.4 | |
| BMIb (kg/m2) | ≤ median (25.7) | 354 | 31.6 |
| > median (25.7) | 354 | 39.0 |
The spline methodology indicated that the logarithm of the hazard ratio for a one-unit increase in lesion-years decreased linearly as age increased, and that after age 45, the effect of lesion-years did not change with time. Among the 278 participants ≤ 45 yrs old at baseline, 74 (27%) had ≥ 1 incident LEO, while 176 (41%) of the 430 participants > 45 yrs old at baseline had ≥ 1 incident LEO (Table 2). The 2 age strata had approximately the same mean lesion-years accrued per participant with ≥ 1 incident LEO, with means of 8.8 and 8.6 lesion-years for younger and older participants, respectively. Among younger participants, the percentage with subsequent CHD diagnoses was greater for those with more lesion-years, while among older participants, that percentage was greater for those with fewer lesion-years.
Table 2.
Lesion-Years and Subsequent CHD Diagnosis, by Age
| Age (yrs) | Lesion-Yearsa | N (% of total participants) | Mean (SD) No. of Teeth with Incident LEO | Mean (SD) Lesion-Years | Mean (SD) Lesion-Years Attributable to Teeth without Endodontic Therapy | N (% in row) with Subsequent CHD Diagnosis | P-value (comparing % with subsequent CHD diagnosis within age stratum)b |
|---|---|---|---|---|---|---|---|
| ≤ 45 | > 8.8 | 21 (3.0) | 1.9 (0.9) | 18.8 (8.3) | 5.7 (8.5) | 5 (23.8) | |
| > 0-8.8 | 53 (7.5) | 1.1 (0.3) | 4.9 (2.0) | 1.6 (2.3) | 9 (17.0) | 0.444 | |
| 0 | 204 (28.8) | 0 | 0 | 0 | 33 (16.2) | ||
| > 45 | > 8.6 | 68 (9.6) | 1.7 (1.0) | 15.8 (8.3) | 6.6 (7.1) | 12 (17.7) | |
| > 0-8.6 | 108 (15.3) | 1.1 (0.3) | 4.1 (2.1) | 1.3 (2.1) | 32 (29.6) | 0.098 | |
| 0 | 254 (35.9) | 0 | 0 | 0 | 75 (29.5) |
We examined the proportional hazards assumption by checking log(-logS(t)) plots, and all variables met the conditions. The final multivariable Cox regression model showed an association between lesion-years and CHD risk among those ≤ 40 yrs old (p < 0.05) after adjustment for baseline values of important demographic, medical, and dental covariates (Fig.). Similar results were found after adjustment only for age, indicating minimal confounding effects by other covariates. The Fig. shows, for example, that after adjustment for covariates of interest, among 35-year-old participants, the time to CHD diagnosis was 1.4 times faster for every 3 lesion-years of exposure, with a 95% confidence interval from 1.1 to 1.8.
Figure.
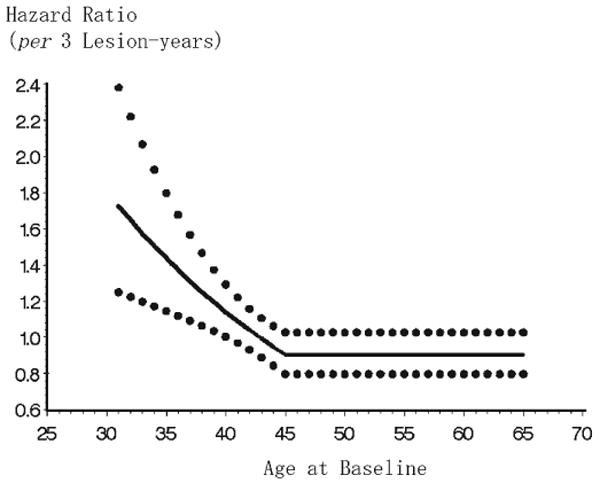
Effect of lesion-years on time to first CHD diagnosis, adjusted for demographic, medical, and dental covariates. Adjustment for baseline values of education, income, total cholesterol, triglycerides, diabetes, hypertension, smoking, BMI, mean alveolar bone loss, number of teeth, number of teeth with LEO, and number of endodontically treated teeth. Solid line = hazard ratio estimate; dotted lines = upper and lower 95% confidence intervals.
DISCUSSION
Mechanisms linking endodontic disease to CHD risk might be similar to those hypothesized for associations between periodontal disease and CHD, in which a localized inflammatory response to bacterial infection leads to release of cytokines into the systemic circulation and subsequent deleterious vascular effects (Beck et al., 1996). Links between endodontic inflammation and cardiovascular outcomes are biologically plausible, considering the predominance of Gram-negative anaerobes associated with endodontic infections (Baumgartner, 1991; Sundqvist, 1992), evidence of cytokine production in inflamed pulp and periapical granulomatous tissues (Miller et al., 1996; Kuo et al., 1998; Barkhordar et al., 1999), and observations of elevated systemic levels of inflammatory mediators in endodontic patients (Marton et al., 1988; Marton and Kiss, 1992). However, we know of only one published study that specifically examined the relationship between chronic endodontic disease and a systemic health outcome: A cross-sectional investigation of 1056 Swedish women aged 38-84 yrs reported no significant relationship between the number of teeth with LEO and the presence of CHD (Frisk et al., 2003).
Our findings of a significant association between incident LEO and subsequent CHD among younger, but not older, men are consistent with those of prior work related to periodontal inflammation and CHD risk (see Mattila et al., 2000). A relationship might truly exist among all age groups, but might be diluted in older adults, since they have other characteristics even more strongly associated with CHD development. Alternatively, this could represent the “healthy survivor” phenomenon, i.e., older people tend to be healthier than other members of their cohort who died earlier. This might be especially true in the present study: Enrollees were required to be systemically healthy at baseline, implying that older participants were unusually healthy compared with both deceased members of their cohort and also with their living peers. Additionally, acute endodontic inflammation might play a role in CHD risk. We could not assess acute inflammation using the present study design, but younger and older individuals might differ with respect to their acute disease experience, and this might contribute to observed differences between younger and older participants.
Even a small contribution to CHD development by endodontic disease might be important from a public health perspective. One review (Caplan, 2004) reported the presence of LEO in 14-70% of all participants and 0.6-8.5% of all teeth, with root-filled teeth evident in 22-78% of participants and 1.3-21.5% of teeth. LEO are more common in root-filled than non-root-filled teeth (Caplan, 2004), and poorer-quality treatment has been associated with LEO (Buckley and Spangberg, 1995; Ray and Trope, 1995). Overall quality of endodontic therapy is generally considered poor, with inadequate fillings reported in 44 to 86% of treated teeth or roots (Dugas et al., 2003; Caplan, 2004).
Enumerating one's cumulative endodontic infectious burden is not straightforward (Caplan, 2004). Endodontic disease can be acute or chronic, but acute endodontic inflammation is not evident radiographically; thus, the present study quantified only chronic endodontic inflammatory disease. The present longitudinal design allowed for such enumeration, given examinations approximately 3 yrs apart: Few databases would provide such richness of endodontic information. Yet teeth must be present to have LEO or endodontic therapy, so missing teeth pose a problem. In populations with limited resources or access to care, one might find either more endodontic disease (if people with chronic, asymptomatic apical periodontitis cannot afford treatment or extraction of affected teeth) or less endodontic disease (if people with acute episodes are more likely to have teeth extracted than endodontically treated). Thus, findings might be different if the study were replicated in populations that differed from this one regarding factors related to LEO, endodontic therapy, or CHD diagnosis.
It is likely that the amount of chronic apical periodontitis was underestimated here. First, due to attrition of participants or teeth, numerous LEO would go unrecorded if teeth were affected after one visit but extracted or successfully treated prior to the next visit. Second, radiographic evidence of the inflammatory process is delayed; substantial bony destruction must occur before the human eye can detect radiographic change (Lutwak, 1969; Manzke et al., 1975). Third, several different case definitions for LEO exist (see Caplan, 2004), and the present case definition was stringent. This strategy was intended to rule out false-positive calls but likely increased the number of false-negative calls. Additional false-negative calls could have resulted from obstruction of radiolucent periapical lesions by anatomic structures like the maxillary sinus, while false-positive calls could have resulted if other, non-inflammatory processes causing apical radiolucencies were counted as LEO (Nair et al., 1990). We would expect any such misclassifications to be non-differential across the subgroups (Table 2), thus biasing any observed associations toward the null.
Finally, several potentially important variables were not used in the present analysis, e.g., HDL cholesterol was not recorded at baseline, inflammatory mediators were not measured, and bacterial samples were not collected. Further, the variable “pack-year smoking history” had too many missing values, so control for smoking was limited to categories of “current smoker” and “non-smoker”. Inadequate control for smoking might be partly responsible for the observed association (Hujoel et al., 2002a; Spiekerman et al., 2003). Future research into the role of endodontic disease and the development of adverse systemic health outcomes should be based on prospective study designs that address these concerns, and, ultimately, the effect of eradication of LEO (through successful endodontic therapy or extraction) on CHD risk should be explored.
Acknowledgments
The authors thank Drs. Jeff Hutter, Martin Trope, and Ryan Yamanaka, without whose assistance this study would not have been possible. The VA Dental Longitudinal Study and the VA Normative Aging Study are components of the Massachusetts Veterans Epidemiology Research & Information Center, VA Boston Healthcare System, Boston, MA, USA. The studies are supported by the VA Cooperative Studies Program/ERIC, US Department of Veterans Affairs. Dr. Garcia was the recipient of a Career Development Award in Health Services Research from the VA HSR&D Service. He is supported by a VA Epidemiology Merit Review Award and by NIH grant K24-DE00419 from the National Institute of Dental and Craniofacial Research. The study also was supported by NIDCR Grant R01-DE13807. This paper is based on an abstract originally presented at the IADR/AADR meeting in Honolulu, HI, March, 2004.
References
- Barkhordar RA, Hayashi C, Hussain MZ. Detection of interleukin-6 in human dental pulp and periapical lesions. Endod Dent Traumatol. 1999;15:26–27. doi: 10.1111/j.1600-9657.1999.tb00744.x. [DOI] [PubMed] [Google Scholar]
- Baumgartner JC. Microbiologic and pathologic aspects of endodontics. Curr Opin Dent. 1991;1:737–743. [PubMed] [Google Scholar]
- Beck J, Garcia R, Heiss G, Vokonas PS, Offenbacher S. Periodontal disease and cardiovascular disease. J Periodontol. 1996;67 10:1123–1137. doi: 10.1902/jop.1996.67.10s.1123. [DOI] [PubMed] [Google Scholar]
- Buckley M, Spangberg LS. The prevalence and technical quality of endodontic treatment in an American subpopulation. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995;79:92–100. doi: 10.1016/s1079-2104(05)80081-2. [DOI] [PubMed] [Google Scholar]
- Caplan DJ. Epidemiologic issues in studies of association between apical periodontitis and systemic health. Endod Top. 2004;8:15–35. [Google Scholar]
- Cohen J. A coefficient of agreement for nominal scales. Educ Psychol Meas. 1960;20:37–46. [Google Scholar]
- Dugas NN, Lawrence HP, Teplitsky PE, Pharoah MJ, Friedman S. Periapical health and treatment quality assessment of root-filled teeth in two Canadian populations. Int Endod J. 2003;36:181–192. doi: 10.1046/j.1365-2591.2003.00640.x. [DOI] [PubMed] [Google Scholar]
- Eriksen HM. Epidemiology of apical periodontitis. In: Ørstavik D, Pitt Ford TR, editors. Essential endodontology: prevention and treatment of apical periodontitis. Oxford: Blackwell Science Ltd.; 1998. pp. 179–191. [Google Scholar]
- Frisk F, Hakeberg M, Ahlqwist M, Bengtsson C. Endodontic variables and coronary heart disease. Acta Odontol Scand. 2003;61:257–262. doi: 10.1080/00016350310005510. [DOI] [PubMed] [Google Scholar]
- Gamonal J, Acevedo A, Bascones A, Jorge O, Silva A. Levels of interleukin-1 beta, -8, and -10 and RANTES in gingival crevicular fluid and cell populations in adult periodontitis patients and the effect of periodontal treatment. J Periodontol. 2000;71:1535–1545. doi: 10.1902/jop.2000.71.10.1535. [DOI] [PubMed] [Google Scholar]
- Grau AJ, Becher H, Ziegler CM, Lichy C, Buggle F, Kaiser C, et al. Periodontal disease as a risk factor for ischemic stroke. Stroke. 2004;35:496–501. doi: 10.1161/01.STR.0000110789.20526.9D. [DOI] [PubMed] [Google Scholar]
- Hujoel PP, Drangsholt M, Spiekerman C, DeRouen TA. Periodontitis—systemic disease associations in the presence of smoking-causal or coincidental? Periodontol 2000. 2002a;30:51–60. doi: 10.1034/j.1600-0757.2002.03005.x. [DOI] [PubMed] [Google Scholar]
- Hujoel PP, Drangsholt M, Spiekerman C, DeRouen TA. Pre-existing cardiovascular disease and periodontitis: a follow-up study. J Dent Res. 2002b;81:186–191. [PubMed] [Google Scholar]
- Janket SJ, Baird AE, Chuang SK, Jones JA. Meta-analysis of periodontal disease and risk of coronary heart disease and stroke. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;95:559–569. doi: 10.1067/moe.2003.107. [DOI] [PubMed] [Google Scholar]
- Jeffcoat MK, Hauth JC, Geurs NC, Reddy MS, Cliver SP, Hodgkins PM, et al. Periodontal disease and preterm birth: results of a pilot intervention study. J Periodontol. 2003;74:1214–1218. doi: 10.1902/jop.2003.74.8.1214. [DOI] [PubMed] [Google Scholar]
- Kuo ML, Lamster IB, Hasselgren G. Host mediators in endodontic exudates. I. Indicators of inflammation and humoral immunity. J Endod. 1998;24:598–603. doi: 10.1016/S0099-2399(98)80118-0. [DOI] [PubMed] [Google Scholar]
- Lutwak L. Symposium on osteoporosis. Nutritional aspects of osteoporosis. J Am Geriatr Soc. 1969;17:115–119. doi: 10.1111/j.1532-5415.1969.tb03166.x. [DOI] [PubMed] [Google Scholar]
- Manzke E, Chesnut CH, III, Wergedal JE, Baylink DJ, Nelp WB. Relationship between local and total bone mass in osteoporosis. Metabolism. 1975;24:605–615. doi: 10.1016/0026-0495(75)90140-7. [DOI] [PubMed] [Google Scholar]
- Marton IJ, Kiss C. Influence of surgical treatment of periapical lesions on serum and blood levels of inflammatory mediators. Int Endod J. 1992;25:229–233. doi: 10.1111/j.1365-2591.1992.tb01154.x. [DOI] [PubMed] [Google Scholar]
- Marton I, Kiss C, Balla G, Szabo T, Karmazsin L. Acute phase proteins in patients with chronic periapical granuloma before and after surgical treatment. Oral Microbiol Immunol. 1988;3:95–96. doi: 10.1111/j.1399-302x.1988.tb00091.x. [DOI] [PubMed] [Google Scholar]
- Mattila KJ, Asikainen S, Wolf J, Jousimies-Somer H, Valtonen V, Nieminen M. Age, dental infections, and coronary heart disease. J Dent Res. 2000;79:756–760. doi: 10.1177/00220345000790020901. [DOI] [PubMed] [Google Scholar]
- Miller GA, DeMayo T, Hutter JW. Production of interleukin-1 by polymorphonuclear leukocytes resident in periradicular tissue. J Endod. 1996;22:346–351. doi: 10.1016/S0099-2399(96)80215-9. [DOI] [PubMed] [Google Scholar]
- Nair PN, Sjogren U, Krey G, Kahnberg KE, Sundqvist G. Intra-radicular bacteria and fungi in root-filled, asymptomatic human teeth with therapy-resistant periapical lesions: a long-term light and electron microscopic follow-up study. J Endod. 1990;16:580–588. doi: 10.1016/S0099-2399(07)80201-9. [DOI] [PubMed] [Google Scholar]
- Noiri Y, Li L, Ebisu S. The localization of periodontal-disease-associated bacteria in human periodontal pockets. J Dent Res. 2001;80:1930–1934. doi: 10.1177/00220345010800101301. [DOI] [PubMed] [Google Scholar]
- Ray HA, Trope M. Periapical status of endodontically treated teeth in relation to the technical quality of the root filling and the coronal restoration. Int Endod J. 1995;28:12–18. doi: 10.1111/j.1365-2591.1995.tb00150.x. [DOI] [PubMed] [Google Scholar]
- Scannapieco FA, Bush RB, Paju S. Associations between periodontal disease and risk for nosocomial bacterial pneumonia and chronic obstructive pulmonary disease. A systematic review. Ann Periodontol. 2003;8:54–69. doi: 10.1902/annals.2003.8.1.54. [DOI] [PubMed] [Google Scholar]
- Spiekerman CF, Hujoel PP, DeRouen TA. Bias induced by self-reported smoking on periodontitis-systemic disease associations. J Dent Res. 2003;82:345–349. doi: 10.1177/154405910308200504. [DOI] [PubMed] [Google Scholar]
- Sundqvist G. Ecology of the root canal flora. J Endod. 1992;18:427–430. doi: 10.1016/S0099-2399(06)80842-3. [DOI] [PubMed] [Google Scholar]
- Witte JS, Greenland S. A nested approach to evaluating dose-response and trend. Ann Epidemiol. 1997;7:188–193. doi: 10.1016/s1047-2797(96)00159-7. [DOI] [PubMed] [Google Scholar]