Interleukin-15 and Natural Killer and NKT Cells Play a Critical Role in Innate Protection against Genital Herpes Simplex Virus Type 2 Infection (original) (raw)
Abstract
Interleukin-15 (IL-15), natural killer (NK) cells, and NK T (NKT) cells, components of the innate immune system, are known to contribute to defense against pathogens, including viruses. Here we report that IL-15−/− (NK− and NKT−/+) mice and RAG-2−/−/γc−/− (NK− and NKT−) mice that lack all lymphoid cells were very susceptible to vaginal infection with a low dose of herpes simplex virus type 2 (HSV-2). IL-15−/− and RAG-2−/−/γc−/− mice were 100-fold more susceptible and RAG-2−/−, CD-1−/− (NKT−), and gamma interferon (IFN-γ)−/− mice were 10-fold more susceptible to vaginal HSV-2 infection than control C57BL/6 mice. NK and/or NKT cells were the early source of IFN-γ in vaginal secretions following genital HSV-2 infection. This study demonstrates that IL-15 and NK-NKT cells are critical for innate protection against genital HSV-2.
The innate immune system plays a crucial role during the early phase of a viral infection. Innate cells, such as natural killer (NK) cells, neutrophils, macrophages, and dendritic cells, all contribute to innate protection against genital herpes simplex virus type 2 (HSV-2) infection (11, 13, 14, 26). Classical NK cells are large granular non-T-cell lymphocytes lacking expression of T-cell receptors (3). These cells are able to kill tumor and virus-infected cells or produce cytokines, particularly gamma interferon (IFN-γ), independently of each other (8). NK T (NKT) cells are a second set of NK cells which have been more recently identified and shown to express both T-cell receptors and NK cell markers (10). Although classical NK cells clearly play a role in the control of some mouse and human viruses, the relative contribution of NKT cell-mediated antiviral defense is not yet clear. Very recent data suggest that NKT cells play a role in the clearance of HSV-1 from skin lesions (6).
Interleukin-15 (IL-15) has a major impact on NK and NKT cell biology (16). This cytokine is produced by a variety of cell types, including monocytes/macrophages, dendritic cells, and bone marrow stromal cells (4, 7, 9, 19). Although IL-15 can bind to IL-2 receptors β and γ, it binds to the α chain of the IL-15 receptor with very high affinity (18, 20). The absence of IL-15 or IL-15Rα is associated with a complete lack of NK cells and a severe deficiency of NKT cells (24). The role of IL-15 in antiviral defense is not well documented. It has been shown that different viruses induce IL-15 production, which in turn leads to up-regulation of NK cytotoxic activity (5). An in vitro study has shown that enhanced NK cell activity via IL-15 induction led to innate protection against HSV-1 (1), and a protective role for IL-15 was shown in a mouse model of systemic HSV-2 (22, 23).
The general concept is that following a viral infection, IL-15 is rapidly produced by infected cells or monocytes/macrophages and then acts primarily on NK and NKT cells. Activation of these cells leads to rapid production of IFN-γ and/or enhanced NK cytotoxicity (2). Innate protection against genital HSV-2 in the absence of IL-15 and NK-NKT cells has not been studied. To learn more about the potential antiviral activity of IL-15 against genital HSV-2, we have studied the innate protection in IL-15−/− mice which also lack NK-NKT cells and in alymphoid mice which lack all lymphocytes, including NK-NKT cells, but have IL-15 cytokine.
NK-NKT cells, IL-15, and IFN-γ control susceptibility to genital HSV-2 infection.
To gain insight into the innate mechanisms which contribute to protection and viral clearance in the genital tract, susceptibility to intravaginal (IVAG) HSV-2 infection was examined in various naïve knockout mouse strains, including RAG-2−/−/γc−/− mice (Tactonic, Germantown, N.Y.) that lack all lymphoid lineages, RAG-2−/− mice that lack T and B cells, IL-15−/− mice (kindly provided by Immunex, Amgen Inc., Thousand Oaks, Calif.) that lack NK and NKT cells, and CD-1−/−mice (Jackson Laboratories, Bar Harbor, Maine) that lack NKT cells or that have an IFN-γ−/− phenotype compared to the congenic control, C57BL/6 (B6) mice. Low doses (102 PFU) of HSV-2 strain 333 were initially chosen for this study, since the mice differ markedly in the components of their immune systems. Mice (6 to 8 weeks old) were inoculated with 2 mg of progesterone/mouse (Depo-Provera; UpJohn, Don Mills, Ontario, Canada) and 5 days later were anesthetized using ketamine-xylazine, placed on their backs, and infected IVAG with lethal doses (different for each strain) of HSV-2 in 10 μl of phosphate-buffered saline (PBS) for at least 45 min while being maintained under anesthetic. Vaginal washes were collected daily after infection by pipetting 2 × 30 μl of PBS into and out of the vagina six to eight times. Viral titers in vaginal washes were determined by plaque assay on Vero cell monolayers as described previously (12). Following IVAG HSV-2 infection, mice were monitored daily for up to 4 weeks. Scoring of the genital pathology was as follows: 0, no obvious genital pathology; 1, clear redness of the vaginal tissue; 2, redness and inflammation of the vaginal tissue and inflammation of the area between rectum and vagina; 3, severe inflammation of genital tissue and the surrounding area accompanied by hair loss; 4, extensive hair loss of external vaginal tissue and surrounding area with lesions of genital tract; and 5, extensive necrosis of vaginal or rectal tissue and surrounding areas. Mice were sacrificed upon reaching a score of 5 or demonstrating hind limb paralysis regardless of the vaginal pathology score.
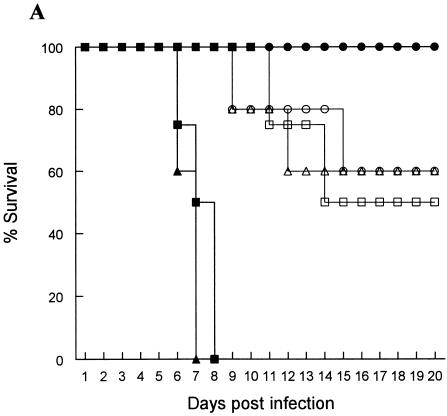
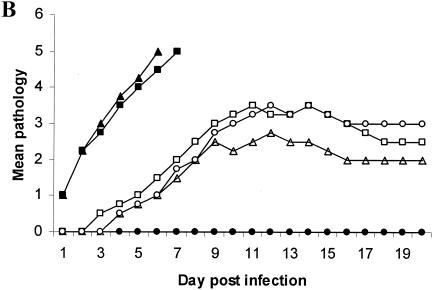
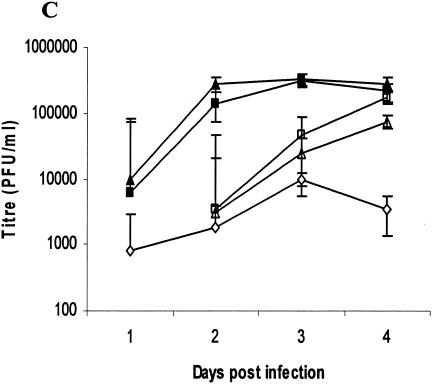
RAG-2−/−/γc−/− and IL-15−/− mice were the most susceptible (Fig. 1A) and had the most severe genital pathology (Fig. 1B); none of them survived infection, while B6 mice were totally resistant to the low-dose infection (Fig. 1A). RAG-2−/−, CD-1−/−, and IFN-γ−/− mice were also susceptible to the low-level infection dose (Fig. 1A). The highest viral shedding was detected in the vaginal washes of RAG-2−/−/γc−/− and IL-15−/− mice, while no viral shedding was detected in the washes from B6 mice (Fig. 1C). Viral titers in the vaginal washes from RAG-2−/−, CD-1−/−, and IFN-γ−/− mice were significantly lower than those of RAG-2−/−/γc−/− and IL-15−/− mice (Fig. 1C). These results clearly show that with NK and NKT cells, IL-15 and IFN-γ play a crucial role in innate defense against viral infection in the genital tract. This view was supported by the high degree of susceptibility of naïve IL-15−/− and RAG-2−/−/γc−/− mice, both of which lack NK and NKT cells, to very low doses (100 PFU) of HSV-2 following IVAG infection. These results also suggest that the killing ability of NK and/or NKT cells plays a more important role in controlling HSV-2 than their ability to produce IFN-γ. Our findings with NKT-deficient CD-1−/− mice support the findings of a very recent study that suggest that NKT cells play an important role in clearance of HSV-1 from skin lesions (6).
FIG. 1.
Susceptibility to vaginal HSV-2 infection in mice lacking all or some of the lymphocyte lineages, IL-15, or IFN-γ−/−. Naïve mice were infected IVAG with a low dose (100 PFU) of HSV-2 and monitored daily for vaginal pathology, survival, and vaginal virus titer. Statistical differences in the viral titers were determined by analysis of variance followed by Tukey's test. The statistical significance of the survival rates was determined by log rank statistic (P of <0.05 was considered statistically significant). (A and B) RAG-2−/−/γc−/− (▪) and IL-15−/− (▴) mice (n = 24) were extremely susceptible to low-dose challenge and showed the most severe pathology compared to B6 control mice (•), which showed 100% survival (n = 20). RAG-2−/− (□), CD-1−/− (▵) and IFN-γ−/− (○) mice (n = 10) were significantly (P < 0.01) more susceptible to vaginal HSV-2 than B6 mice but significantly (P < 0.01) less susceptible than RAG-2−/−/γc−/− and IL-15−/− mice. (C) Vaginal HSV-2 titers (n = 8 to 10/group) were examined on days 1 to 4 after viral challenge. While no viral shedding was detected in B6 control mice (♦), RAG-2−/−/γc−/− (▪) and IL-15−/− (▴) mice had the highest viral titers compared to the mice of all other groups. RAG-2−/− (□), CD-1−/− (Δ), and IFN-γ−/− (⋄) mice were significantly more susceptible to vaginal HSV-2 than B6 mice but less susceptible than RAG-2−/−/γc−/− and IL-15−/− mice.
RAG-2−/−/γc−/− and IL-15−/− mice are 100-fold more susceptible to IVAG HSV-2 infection than control B6 mice.
We then determined the lethal dose of HSV-2 for each knockout mouse strain and the congenic B6 control mice. Where applicable, mice were infected IVAG with 102, 103, 5 × 103, or 104 PFU of HSV-2. A dose was considered lethal when 100% of mice succumbed to infection at up to 9 to 11 days postinfection. The lethal IVAG HSV-2 dose for RAG-2−/−/γc−/− and IL-15−/− mice was 10-fold lower than that for RAG-2−/−, CD-1−/−, and IFN-γ−/− mice and 100-fold lower than that for B6 mice (Table 1). The relative levels of severity of the genital pathology observed following a lethal dose of IVAG HSV-2 infection in knockout and B6 mice were as follows: IL-15−/− > RAG-2−/−/γc−/− > IFN-γ−/− > RAG-2−/−> CD-1−/− > B6 mice. Even with a 100-fold lower lethal dose, IL-15−/− and RAG-2−/−/γc−/− mice succumbed to infection 4 to 5 days earlier than B6 control mice (data not shown). This suggests the importance of IL-15 in protection against IVAG HSV-2. Whether IL-15 directly provides protection against HSV-2 infection or acts through activation of NK-NKT cells has yet to be examined.
TABLE 1.
Survival (%) of knockout and B6 mice following infection with different doses of IVAG HSV-2 for detection of lethal infectious doses
| Mouse strain | % Survival (no. of mice) at an infectious dose (PFU/mouse) of: | |||
|---|---|---|---|---|
| 102 | 103 | 5 × 103 | 104 | |
| B6 | 100 (20) | 60 (10) | 25 (10) | 0 (20) |
| IL-15−/− | 0 (24) | NAa | NA | NA |
| RAG-2−/−/γc−/− | 0 (24) | NA | NA | NA |
| RAG-2−/− | 50 (10) | 0 (10) | NA | NA |
| CD-1−/− | 60 (10) | 0 (10) | NA | NA |
| IFN-γ−/− | 40 (10) | 0 (10) | NA | NA |
NK and/or NKT cells are the early (days 1 to 3) source of IFN-γ in vaginal secretions following IVAG HSV-2 infection.
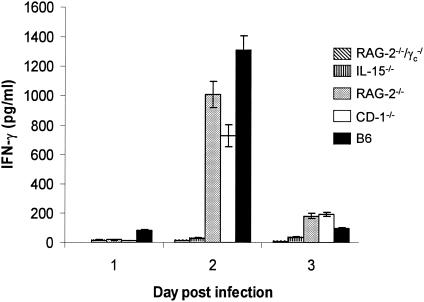
Vaginal HSV-2 infection induces production of IFN-γ (13, 17). Milligan and Bernstein showed that following IVAG HSV-2 infection, there was an early (days 1 to 3) and late (days 6 to 8) IFN-γ peak in vaginal washes (13). They went on to use antisera in experiments suggesting that the early peak of IFN-γ was likely produced by NK cells. To identify the source of IFN-γ in the vaginal mucosa following IVAG HSV-2 infection, the concentrations of IFN-γ in the vaginal washes of the knockout (lacking NK and/or NKT cells) and B6 control mice were measured by enzyme-linked immunosorbent assay (R&D Systems; detection limit = 10 ng/ml) (Fig. 2). There was no IFN-γ above the background level in the washes from RAG-2−/−/γc−/− or IL-15−/− mice, both strains lacking NK and NKT cells. In contrast, significant amounts of IFN-γ were detected 2 days after IVAG HSV-2 infection in mice positive for NK and/or NKT cells. Although macrophages, neutrophils, and NK, NKT, and activated T cells are sources of IFN-γ (2, 15, 21, 25), our results clearly show that NK and/or NKT cells were the source of IFN-γ in genital secretions at early times (days 1 to 3) after HSV-2 infection. During this time, no IFN-γ was detected in the vaginal washes from IVAG HSV-2-infected IL-15−/− and RAG-2−/−/γc−/− mice, which lack NK and NKT cells but have macrophages and neutrophils. Additionally, this early production of IFN-γ cannot be from T cells, since they are present in IL-15−/− mice. RAG-2−/−, CD-1−/−, and normal B6 mice, all positive for NK cells or NKT cells or both, had high levels of IFN-γ in vaginal washes 2 days after IVAG infection. These results support those of an earlier report that suggested NK cells as the main source of the early peak of IFN-γ following IVAG HSV-2 infection (13). However, our results suggest that IL-15 plays a more crucial role than IFN-γ in defense against IVAG HSV-2, since IL-15−/− mice were 10-fold more sensitive to HSV-2 infection than IFN-γ−/− mice.
FIG. 2.
NK and/or NKT cells are the early source of IFN-γ in vaginal washes following IVAG HSV-2 infection. Significant levels of IFN-γ were detected on day 2 postinfection only in the vaginal washes from mice having NK and/or NKT cells (B6, RAG-2−/−, and CD-1−/− mice), whereas no or very low levels of IFN-γ were detected in the washes from mice lacking NK and NKT cells (RAG-2−/−/γc−/− and IL-15−/− mice). Vaginal washes from IFN-γ−/− mice were used as a negative control and had values no higher than those of the PBS control, showing the sensitivity of the test for the vaginal fluids.
In the absence of IL-15, viruses might have a higher level of replication and spread in the genital epithelium at early times postinfection. Further, lack of NK and NKT cells in these mice and, as a result, lack of early cytokine (particularly IFN-γ) might impair the innate response against HSV-2. Although RAG-2−/−/γc−/− mice might be able to have induction of IL-15 following infection, there are no NK or NKT cells to be activated following IL-15 induction. This may suggest that following HSV-2 infection, induction of IL-15 from infected epithelial cells or macrophages in the submucosa leads to activation of NK and NKT cells, which can then provide innate protection against genital HSV-2 infection.
Acknowledgments
We thank Immunex for provision of the IL-15−/− mice and B. Anne Croy (University of Guelph) for providing some of the IL-15−/− and IFN-γ−/− mice.
This work was supported by grants from the Canadian Institutes of Health Research (CIHR) and the Canadian Network on Vaccines & Immunotherapeutics (CANVAC). Kenneth L. Rosenthal is a Career Scientist from the Ontario HIV Treatment Network (OHTN).
REFERENCES
- 1.Ahmad, A., E. Sharif-Askari, L. Fawaz, and J. Menezes. 2000. Innate immune response of the human host to exposure with herpes simplex virus type 1: in vitro control of the virus infection by enhanced natural killer activity via interleukin-15 induction. J. Virol. 74**:**7196-7203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biron, C. A., and L. Brossay. 2001. NK cells and NKT cells in innate defense against viral infections. Curr. Opin. Immunol. 13**:**458-464. [DOI] [PubMed] [Google Scholar]
- 3.Biron, C. A., K. B. Nguyen, G. C. Pien, L. P. Cousens, and T. P. Salazar-Mather. 1999. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu. Rev. Immunol. 17**:**189-220. [DOI] [PubMed] [Google Scholar]
- 4.Blauvelt, A., H. Asada, V. Klaus-Kovtun, D. J. Altman, D. R. Lucey, and S. I. Katz. 1996. Interleukin-15 mRNA is expressed by human keratinocytes Langerhans cells, and blood-derived dendritic cells and is downregulated by ultraviolet B radiation. J. Investig. Dermatol. 106**:**1047-1052. [DOI] [PubMed] [Google Scholar]
- 5.Fawaz, L. M., E. Sharif-Askari, and J. Menezes. 1999. Up-regulation of NK cytotoxic activity via IL-15 induction by different viruses: a comparative study. J. Immunol. 163**:**4473-4480. [PubMed] [Google Scholar]
- 6.Grubor-Bauk, B., A. Simmons, G. Mayrhofer, and P. G. Speck. 2003. Impaired clearance of herpes simplex virus type 1 from mice lacking CD1d or NKT cells expressing the semivariant V alpha 14-J alpha 281 TCR. J. Immunol. 170**:**1430-1434. [DOI] [PubMed] [Google Scholar]
- 7.Jonuleit, H., K. Wiedemann, G. Muller, J. Degwert, U. Hoppe, J. Knop, and A. H. Enk. 1997. Induction of IL-15 messenger RNA and protein in human blood-derived dendritic cells: a role for IL-15 in attraction of T cells. J. Immunol. 158**:**2610-2615. [PubMed] [Google Scholar]
- 8.Kurago, Z. B., C. T. Lutz, K. D. Smith, and M. Colonna. 1998. NK cell natural cytotoxicity and IFN-gamma production are not always coordinately regulated: engagement of DX9 KIR+ NK cells by HLA-B7 variants and target cells. J. Immunol. 160**:**1573-1580. [PubMed] [Google Scholar]
- 9.Liu, T., H. Nishimura, T. Matsuguchi, and Y. Yoshikai. 2000. Differences in interleukin-12 and -15 production by dendritic cells at the early stage of Listeria monocytogenes infection between BALB/c and C57 BL/6 mice. Cell. Immunol. 202**:**31-40. [DOI] [PubMed] [Google Scholar]
- 10.MacDonald, H. R. 2002. Development and selection of NKT cells. Curr. Opin. Immunol. 14**:**250-254. [DOI] [PubMed] [Google Scholar]
- 11.Malmgaard, L., S. R. Paludan, S. C. Mogensen, and S. Ellermann-Eriksen. 2000. Herpes simplex virus type 2 induces secretion of IL-12 by macrophages through a mechanism involving NF-κB. J. Gen. Virol. 81**:**3011-3020. [DOI] [PubMed] [Google Scholar]
- 12.McDermott, M. R., C. H. Goldsmith, K. L. Rosenthal, and L. J. Brais. 1989. T lymphocytes in genital lymph nodes protect mice from intravaginal infection with herpes simplex virus type 2. J. Infect. Dis. 159**:**460-466. [DOI] [PubMed] [Google Scholar]
- 13.Milligan, G. N., and D. I. Bernstein. 1997. Interferon-gamma enhances resolution of herpes simplex virus type 2 infection of the murine genital tract. Virology 229**:**259-268. [DOI] [PubMed] [Google Scholar]
- 14.Milligan, G. N., N. Bourne, and K. L. Dudley. 2001. Role of polymorphonuclear leukocytes in resolution of HSV-2 infection of the mouse vagina. J. Reprod. Immunol. 49**:**49-65. [DOI] [PubMed] [Google Scholar]
- 15.Munder, M., M. Mallo, K. Eichmann, and M. Modolell. 1998. Murine macrophages secrete interferon gamma upon combined stimulation with interleukin (IL)-12 and IL-18: a novel pathway of autocrine macrophage activation. J. Exp. Med. 187**:**2103-2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ohteki, T. 2002. Critical role for IL-15 in innate immunity. Curr. Mol. Med. 2**:**371-380. [DOI] [PubMed] [Google Scholar]
- 17.Parr, M. B., and E. L. Parr. 1999. The role of gamma interferon in immune resistance to vaginal infection by herpes simplex virus type 2 in mice. Virology 258**:**282-294. [DOI] [PubMed] [Google Scholar]
- 18.Pereno, R., J. Giron-Michel, A. Gaggero, E. Cazes, R. Meazza, M. Monetti, E. Monaco, Z. Mishal, C. Jasmin, F. Indiveri, S. Ferrini, and B. Azzarone. 2000. IL-15/IL-15Rα intracellular trafficking in human melanoma cells and signal transduction through the IL-15Rα. Oncogene 19**:**5153-5162. [DOI] [PubMed] [Google Scholar]
- 19.Puzanov, I. J., M. Bennett, and V. Kumar. 1996. IL-15 can substitute for the marrow microenvironment in the differentiation of natural killer cells. J. Immunol. 157**:**4282-4285. [PubMed] [Google Scholar]
- 20.Smith, X. G., E. M. Bolton, H. Ruchatz, X. Wei, F. Y. Liew, and J. A. Bradley. 2000. Selective blockade of IL-15 by soluble IL-15 receptor alpha-chain enhances cardiac allograft survival. J. Immunol. 165**:**3444-3450. [DOI] [PubMed] [Google Scholar]
- 21.Smyth, M. J., N. Y. Crowe, D. G. Pellicci, K. Kyparissoudis, J. M. Kelly, K. Takeda, H. Yagita, and D. I. Godfrey. 2002. Sequential production of interferon-gamma by NK1.1+ T cells and natural killer cells is essential for the antimetastatic effect of alpha-galactosylceramide. Blood 99**:**1259-1266. [DOI] [PubMed] [Google Scholar]
- 22.Tsunobuchi, H., H. Nishimura, F. Goshima, T. Daikoku, Y. Nishiyama, and Y. Yoshikai. 2000. Memory-type CD8+ T cells protect IL-2 receptor alpha-deficient mice from systemic infection with herpes simplex virus type 2. J. Immunol. 165**:**4552-4560. [DOI] [PubMed] [Google Scholar]
- 23.Tsunobuchi, H., H. Nishimura, F. Goshima, T. Daikoku, H. Suzuki, I. Nakashima, Y. Nishiyama, and Y. Yoshikai. 2000. A protective role of interleukin-15 in a mouse model for systemic infection with herpes simplex virus. Virology 275**:**57-66. [DOI] [PubMed] [Google Scholar]
- 24.Williams, N. S., J. Klem, I. J. Puzanov, P. V. Sivakumar, J. D. Schatzle, M. Bennett, and V. Kumar. 1998. Natural killer cell differentiation: insights from knockout and transgenic mouse models and in vitro systems. Immunol. Rev. 165**:**47-61. [DOI] [PubMed] [Google Scholar]
- 25.Yeaman, G. R., J. E. Collins, J. K. Currie, P. M. Guyre, C. R. Wira, and M. W. Fanger. 1998. IFN-gamma is produced by polymorphonuclear neutrophils in human uterine endometrium and by cultured peripheral blood polymorphonuclear neutrophils. J. Immunol. 160**:**5145-5153. [PubMed] [Google Scholar]
- 26.Zhao, X., E. Deak, K. Soderberg, M. Linehan, D. Spezzano, J. Zhu, D. M. Knipe, and A. Iwasaki. 2003. Vaginal submucosal dendritic cells, but not Langerhans cells, induce protective Th1 responses to herpes simplex virus-2. J. Exp. Med. 197**:**153-162. [DOI] [PMC free article] [PubMed] [Google Scholar]