Bisphenol A is released from polycarbonate drinking bottles and mimics the neurotoxic actions of estrogen in developing cerebellar neurons (original) (raw)
. Author manuscript; available in PMC: 2009 Jan 30.
Abstract
The impact of endocrine disrupting chemical (EDC) exposure on human health is receiving increasingly focused attention. The prototypical EDC bisphenol A (BPA) is an estrogenic high-production chemical used primarily as a monomer for production of polycarbonate and epoxy resins. It is now well established that there is ubiquitous human exposure to BPA. In the general population exposure to BPA occurs mainly by consumption of contaminated foods and beverages that have contacted epoxy resins or polycarbonate plastics. To test the hypothesis that bioactive BPA was released from polycarbonate bottles used for consumption of water and other beverages, we evaluated whether BPA migrated into water stored in new or used high-quality polycarbonate bottles used by consumers. Using a sensitive and quantitative competitive enzyme-linked immunosorbent assay, BPA was found to migrate from polycarbonate water bottles at rates ranging from 0.20 to 0.79 ng per hour. At room temperature the migration of BPA was independent of whether or not the bottle had been previously used. Exposure to boiling water (100°C) increased the rate of BPA migration by up to 55-fold. The estrogenic bioactivity of the BPA-like immunoreactivity released into the water samples was confirmed using an in vitro assay of rapid estrogen-signaling and neurotoxicity in developing cerebellar neurons. The amounts of BPA found to migrate from polycarbonate drinking bottles should be considered as a contributing source to the total “EDC-burden” to which some individuals are exposed.
Keywords: bisphenol A, BPA; competitive ELISA; endocrine disruption; estrogen; neurotoxicity; non-genomic; polycarbonate
Introduction
Bisphenol A (BPA, 2, 2-bis (4-hydroxyphenyl) propane; CAS RN 80-05-7) is a high production chemical used in the manufacture of numerous consumer goods and products. Bisphenol A has well characterized estrogenic and other endocrine disrupting activities that are mediated via multiple molecular mechanisms (Wetherill et al., 2007). In 2004, the estimated production volume of BPA in the United States was ∼2.3 billion pounds (CERHR, 2007). Of the 1.9 billion pounds of BPA used in the US in 2003, nearly 3/4 was used to manufacture polycarbonate resins that were in turn used to manufacture various consumer products including polycarbonate containers for storage of foods and beverages (CERHR, 2007).
Because of BPA’s high volume production and extensive use in plastics, there is widespread environmental contamination and well-documented human exposure to BPA. While not diminishing the importance of BPA pollutants in marine, aquatic, and soil ecosystems (for review see - Crain et al., 2007), recent studies have demonstrated that there is wide-spread BPA contamination of most individuals in industrialized human populations (Calafat et al., 2005; Vandenberg et al., 2007). The detection of adverse health effects in a number of laboratory animal models upon exposure to environmentally relevant doses of BPA that correspond to those observed in humans, strongly supports the idea that the endocrine disrupting activities of BPA contribute to adverse effects on human health (reviewed in Richter et al., 2007).
In the laboratory setting, biologically active and environmentally relevant levels of BPA were shown to leach from PC flasks upon autoclaving (Krishnan et al., 1993), from used PC rodent-housing containers (Howdeshell et al., 2003), and from various other forms of polycarbonate plastics and BPA-containing resins (Kang et al., 2006; Vandenberg et al., 2007). Because the major source of human exposure to BPA in the general population is likely through consumption of contaminated foods and beverages that have contacted epoxy or PC resins (Kang et al., 2006), we evaluated whether BPA migrated from new or used high-quality PC bottles that are commonly used by consumers for storage of water and other beverages. The exposures and mild treatments used in this study were designed to mimic conditions representative of normal consumer usage, including typical use in outdoor recreation settings. Along with characterizing the rate of BPA liberation into water at room temperature, the effect of short-term exposure to hot (100°C) water was determined. A sensitive and quantitative competitive-ELISA employing a BPA-specific monoclonal antibody was used to determine relative concentrations of BPA that were leached into water samples from newly purchased polycarbonate bottles and those subjected to normal use by consumers. The estrogenic bioactivity of the BPA-like immunoreactivity released into the water samples was demonstrated with an in vitro assay of rapid estrogen-signaling and neurotoxicity in immature cerebellar neurons (Belcher et al., 2005; Wong et al., 2003).
Materials and Methods
Reagents
Bisphenol A (CAS RN 80-05-7; purity grade > 99%; 23,965-8; lot no. Cl 03105ES) and dimethyl sulfoxide (Chromasolv Plus, for HPLC ≤ 99.7%; batch no. 00451HE) was purchased from Sigma-Aldrich (St. Louis, MO). HPLC grade water (W5sk, lot no. 056618) and methanol (A452sk lot no. 061495) were purchased from Fisher Scientific (Fairlawn, NJ) and used for all washes, dilutions, and sample preparations. New polycarbonate (PC) and high density polyethylene (HDPE) bottles (32 ounce loop-top; Nalgene, Rochester, NY) were obtained directly from a national recreation supply retailer (Campmor, Paramus, NJ). Used PC bottles were the same model as the new PC bottles that were collected from anonymous donors from Rockquest Climbing Gym (Cincinnati, OH). All donated bottles were described as having been used under normal conditions for between 1-9 years, although specific period of use was undetermined. All used bottles showed various degrees of visible external and internal wear including superficial scratches, pitting, and opaque discoloration. All bottles had an approximate internal surface area of 478 cm2 (bottle dimensions were length 17.2 cm; circumference 27.8 cm; diameter 8.9 cm). Water samples collected were stored in new 60 ml glass bottles (Fisher) fitted with Teflon® faced polyethylene lined caps.
Bottle washing and rinsing
A standardized washing/rinsing procedure was used for all bottles tested. Initially, each piece was rinsed with at least 1 L of distilled water to remove any dust or residue, and then washed with a soft-nylon bristle laboratory bottle brush with a half-strength solution (5 g/l) of Alconox powdered precision cleaner (cat no.1104; Alconox White Plains, New York). This wash was followed by 6 rinses each with ∼1 L distilled water, and then two rinses with ∼100 ml HPLC grade water. Each piece was then rinsed 3 times with ∼100 ml of HPLC-grade methanol, inverted and allowed to air dry in a laminar flow hood before use. To ensure removal of superficial contaminants from previous incubations, prior to repeated analysis the complete washing/rinsing protocol was performed.
Autoclaved polypropylene pipet tips were used for all liquid transfers ≤ 1 ml. Larger volume liquid measurements were made in previously unused glass pipets or graduated cylinders that were washed and rinsed as described above. All additional lab ware that would directly or indirectly come into contact with samples was purchased new and prer-insed 3 times with 100% HPLC-grade methanol.
Exposure, experimental design and sample collection
Three replicate experiments for each bottle were conducted to determine if BPA was released into the water stored in new or used PC drinking bottles, or new HDPE bottles. For each sample, 100 ml of HPLC grade water was added to a bottle on day 0. Filled bottles were rotated on a cell culture roller bottle system (Wheaton) for indicated times up to 7 days (168 hours) at room temperature (22° C). Bottles were rotated with the purpose of mimicking the motion of water during normal usage, and to ensure that the bottle’s internal surface was in continuous contact with the water sample. The water contacted approximately 478 cm2 of the surface area in each of the bottles during rotation. At the same time as incubations were initiated, on day 1, 3, and 5, 1 ml water samples were collected and transferred to new glass collection bottles using polypropylene pipet tips. On day 7, a 50 ml water sample was collected for analysis by direct transfer into the collection bottle. Immediately following sample collection, all water bottles were washed and rinsed as described above.
The effects of hot water were assessed as described above except 100 ml of HPLC-grade water heated to 100°C was added to selected bottles. Those bottles were again rotated at room temperature for 24 hours during which time water samples cooled. Samples (50 ml) were then collected; bottles were washed, rinsed and dried immediately after collection as above. To assess the continued effect of heating on BPA release, 100 ml of room temperature HPLC grade water was then added to each of those bottles with sample collection following 24 hours of incubation at room temperature.
ELISA analysis of bisphenol A concentrations in water samples
A supersensitive BPA ELISA (Ecologiena, Japan Environchemicals, Tokyo) was used to determine the concentration of BPA in water samples collected from each bottle. With the exception of using additional known BPA standards to obtain a more accurate fit of the standard curve and performing triplicate, rather duplicate, determinations for each sample, the competitive-ELISA was performed according to a standard test protocol that results in minimum and maximum quantitative detection limits of 0.05 and 10 ng/ml, respectively. Relative cross-reactivity of the BPA ELISA for endocrine disruptors and chemicals structurally related to BPA (100% reactivity) has been determined; the most significant cross reactivity was observed for bisphenol B (15.6%) and bisphenol E (6%) percent. Cross reactivity for nonylphenol was 0.19% and <0.05% for diethylhexylphthalate, 17β-estradiol, estrone (http://www.abraxiskits.com/moreinfo/PN590023USER.pdf). In addition to the unknown samples, a standard curve from known concentrations of BPA was generated with non-linear regression analysis of each assay/analysis performed. Additional BPA standards were prepared for use as defined concentration standards for generation of each BPA standard curve, and as internal controls to confirm the performance of each assay. For each determination the standard curve generated for that experimental assay was used to calculate the concentration of BPA present in the known standards and each unknown sample. Additionally, control samples of HPLC water were included in each of the individual assays. For all control and experimental samples, triplicate measurements were made at 450 nm with a microprocessor controlled SPECTRAFluor PLUS microplate reader (Tecan). Data was directly exported to Excel (Microsoft, Corp), with non-linear regression/sigmoidal curve fitting and statistical analysis of data performed using Prism 5.0 (GraphPad).
Preparation of primary cerebellar neurons, treatment and cytotoxicity assay
Primary cerebellar cultures were prepared from neonatal female Sprague-Dawley rats (16-17 grams) without enzymatic-treatment. All animal procedures were done in accordance with protocols approved by the University of Cincinnati Institutional Animal Care and Use Committee and followed NIH guidelines. The resulting primary cerebellar cultures of maturing granule cell neurons were maintained free of serum and exogenous steroid hormones as previously described (Wong et al., 2001; Wong et al., 2003). Dissociated cerebellar cells were serially diluted in an appropriate volume of culture media and seeded at 2 × 105 granule cells per cm2 in 100 μL of media in 96-well culture plates (TPP; Basel, Switzerland) that were coated with poly-L-lysine (100 μg/mL; Sigma, St. Louis, MO). Cultures were incubated in 5% CO2 at 37°C for 4 hours to allow granule cell attachment. Treatments were performed immediately following cell attachment.
For each treatment, attached granule cells were exposed to controls (17β-estradiol, BPA, or appropriate vehicle) or water samples for 15 minutes and then washed twice with fresh medium following the exposure period to remove any residual concentrations of the test compounds. This 15-minute pulsed exposure was previously shown to mimic rapid non-transcriptional estrogenic mechanisms in maturing granule cells (Wong et al., 2003). Treated cultures were maintained in 5% CO2 at 37°C in a humidified incubator for 24 hours, with amounts of lactate dehydrogenase (LDH) released into the media determined as previously described (Wong et al., 2001; Wong et al., 2003). Briefly, the CytoTox 96® Non-Radioactive Cytotoxicity Assay (Promega; Madison, WI) was used to quantify LDH levels in accordance with the general guidelines of the manufacturer’s protocol (Promega, Technical Bulletin #TB163). Following treatments, culture plates were centrifuged at 250×g for 4 minutes, and 50 μL of the conditioned media supernatant from each well was collected and transferred to a new 96-well plate assay plate. Attached cells or cellular debris settled to the bottom of the wells was not disturbed during media collection. An equal 50 μL volume of CytoTox 96® Non-Radioactive Cytotoxicity Assay substrate mixture was added to each media sample. Sample/substrate solution was mixed and incubated in the dark room at temperature for 30 minutes. Visible wavelength absorbance data was collected at 492 nm immediately following termination of the colormetric reaction using a SPECTRAFluor PLUS microplate reader controlled by Genesis software (Tecan). The absorbance values from the conditioned media supernatant from cells exposed to the test compounds was normalized to mean absorbance values calculated from control samples.
Statistical analysis
Unless noted otherwise, all data presented are representative of at least 3 experiments or quantitative determinations, and are reported as the mean value ± SD or SEM. As appropriate for the experimental design, statistical analysis was conducted using an unpaired t test, or one-way analysis of variance (ANOVA) with post-test comparison between treatment groups using Tuckey-Kramer multiple comparison test. A minimal level of statistical significance for differences in values was considered to be p<0.05 and is indicated with an *. Data was analyzed with Excel (Microsoft) and GraphPad Prism® version 5.0 (GraphPad Software Inc.).
Results
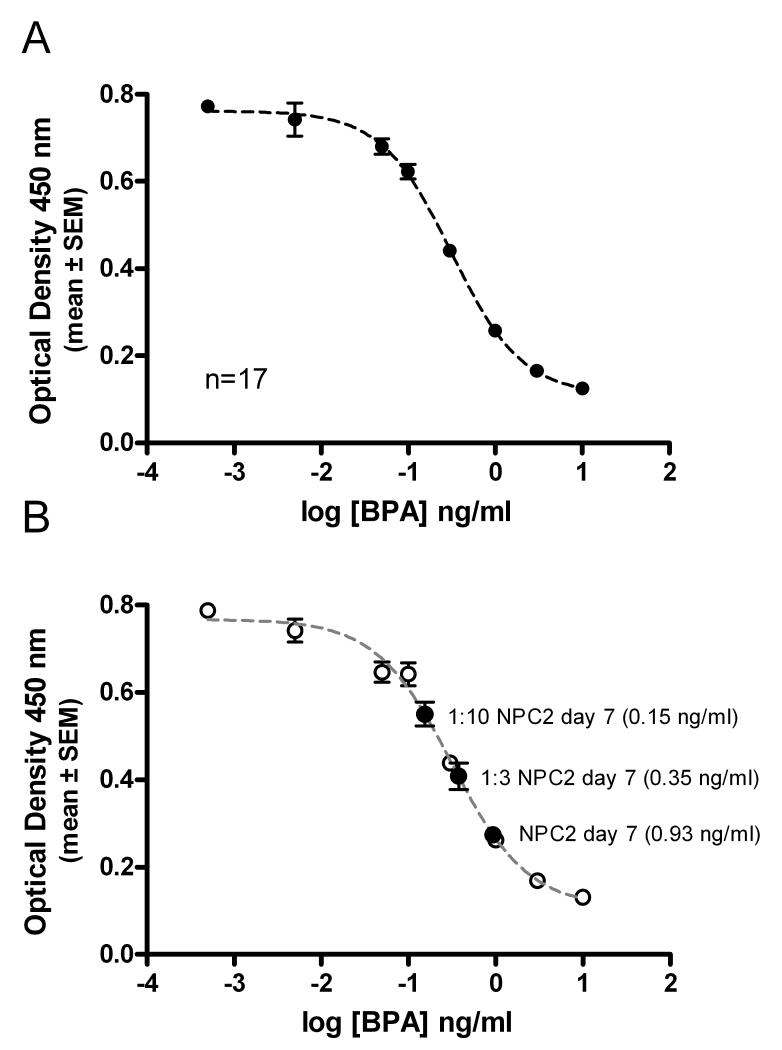
Over the course of these experiments, results of the BPA-specific ELISA were highly reproducible. Demonstrating the reproducibility of the assay, mean values and variance (SEM) for the combine standard curves generated from 17 different experiments are shown in Fig. 1A. Within that large data set, ANOVA analysis showed significant differences between mean values for BPA concentration standards at 0.005 ng/ml and 0.05 ng/ml even though they were outside the detection range of a typical assay. For each BPA standard of this assay curve the coefficient of variation ranged from 4.7% to a maximum of 11.5 %. The BPA ELISA limit of detection for an individual assay with duplicate sample measurements at each data point was 0.05 ng/ml (http://www.abraxiskits.com/moreinfo/PN590023USER.pdf). To confirm the ability to accurately detect reproducible differences in BPA concentration, a replicate analysis independent from those included in Table 1 was performed using undiluted day 7 sample from new PC bottle 2 (NPC2), and the same sample diluted 1:3, or 1:10 with HPLC water. The results from the BPA standards and each of the NPC2 samples for this analysis are shown in Figure 1B. The mean results of each diluted sample was calculated from the standard curve and corresponds well to those predicted from the initial calculated concentration of 0.98 ng/ml for this sample (Table 1).This representative experiment also demonstrates the typically low intra-assay variation of the assay. The maximum % coefficient of variance (CV) for triplicate measures of the standards was 7.2%; for the bottle-exposed water samples dilutions the maximal variation was observed in the 1:10 dilution (12.7%).
Figure 1. Bisphenol A (BPA) ELISA.
(A) The combined standard curve resulting from a non-linear sigmoidal curve fit from all BPA standard curves used in the 17 experiments presented in this study were averaged and are shown graphically. Data points are mean optical density values ± SEM at each concentration. (B) Shown are the results for a single replicate dilution analysis of sample NPC2 in which the concentration of undiluted and diluted sample NPC2 was calculated independently from the results shown in Table 1 (i.e. this result was not included in Table 1). Standard curve data points for this assay are indicated with open circles and NPC2 sample values are indicated with filled circles (mean ± SEM; n=3). The standard curve used to calculate the indicated BPA concentrations is shown as a gray dashed curve.
Table 1.
Bisphenol A concentrations in drinking bottle water
| Concentration of BPA released into 100 ml water at RT (mean ng/ml ± SD; (n)) | BPA released into 100°C water (24 hr) | BPA released from heated bottle at RT (24 hr) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Bottle description; # | Day 1 | Day 3 | Day 5 | Day 7 | rate (ng hr-1) | [BPA] ng/ml | rate (ng hr-1) | [BPA] ng/ml | rate (ng hr-1) |
| New Polycarbonate | |||||||||
| 1 | 0.08 ± 0.06 | 0.25 ± 0.07 | 0.28 ± 0.08 | 0.73 ± 0.05 (4) | 0.44 | ||||
| 2 | 0.21 ± 0.10 (2) | 0.35 ± 0.10 (2) | 0.61 ± 0.05 | 0.98 ± 0.15 (6) | 0.58 | 7.67 ± 0.57 | 32.0 | 4.6 ± 0.59 | 19.2 ng hr-1 |
| 3 | 0.36 ± 0.17 | 0.79 ± 0.17 | 0.68 ± 0.04 | 1.33 ± 0.09 (2) | 0.79 | 3.84 ± 0.12 | 16.0 | 2.3 ± 0.44 | 9.6 ng hr-1 |
| Used Polycarbonate | |||||||||
| 1 | 0.28 ± 0.16 (2) | 0.33 ± 0.20 (2) | 0.29 ± 0.10 (6) | 0.34 ± 0.06 (6) | 0.20 | ||||
| 2 | 0.21 ± 0.05 | 0.39 ± 0.14 | 0.39 ± 0.12 | 0.88 ± 0.19 | 0.52 | ||||
| 3 | ND | ND | ND | 0.93 ± 0.02 | 0.55 | 1.92 ± 0.40 | 8.0 | 0.66 ± 0.01 | 2.8 ng hr-1 |
| 4 | 0.29 ± 0.05 | 0.46 ± 0.06 | 0.58 ± 0.14 (5) | 0.62 ± 0.12 | 0.37 | ||||
| 5 | ND | 0.76 ± 0.06 | 0.72 ± 0.13 (2) | 0.80 ± 0.14 (4) | 0.48 | ||||
| New high density polyethylene | |||||||||
| 1 | 0.01 ± 0.02 | 0.08 ± 0.03 | 0.09 ± 0.04 | 0.15 ± 0.04 | |||||
| 2 | 0.08 ± 0.03 | 0.16 ± 0.07 | 0.08 ± 0.04 | 0.19 ± 0.13 (6) | |||||
| 3 | ND | ND | ND | 0.08 ± 0.09 (2) |
Analysis of control HPLC-grade water samples was included for each experiment revealed levels of BPA at the limit of the assay detection (0.06 ng/ml; SD ± 0.06, n=28). The concentrations of BPA-like immunoreactivity present in all samples collected from HDPE bottles were very low (Table 1); the mean value of BPA estimated for water samples collected following 7 days of incubation from the HDPE bottles was 0.14 ng/ml (SD ± 0.09, n=11). In contrast to those control values, detectable levels of BPA were identified in all water samples collected from either the new or used PC bottles (Table 1).
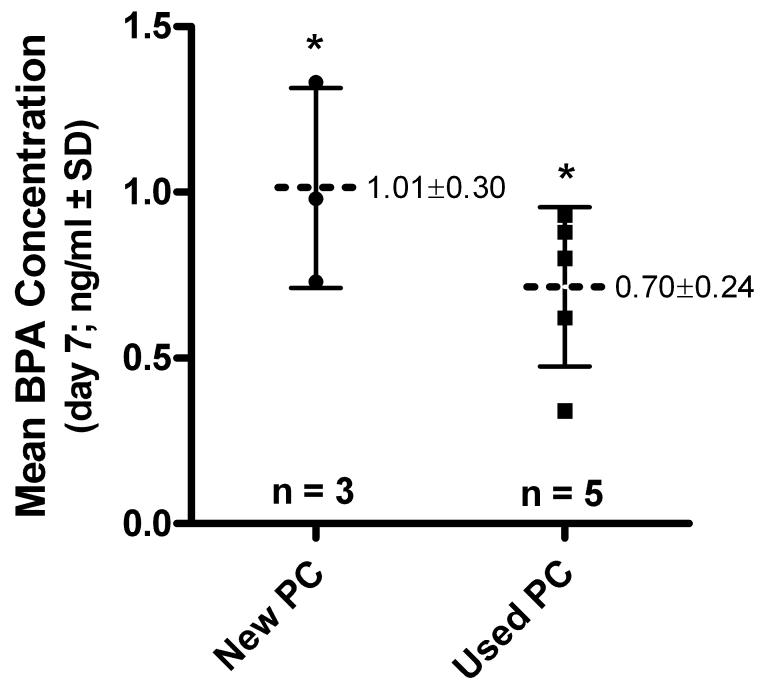
In general, the concentration of BPA released from polycarbonate bottles into water at room temperature increased with time (Table 1). Following 7 days of room temperature incubation, the concentration of BPA released from new PC bottles was 1.0 ng/ml, and 0.7 ng/ml from used PC bottles (Fig. 1C). Mean concentrations of BPA in water samples from new and used bottles were significantly higher than the detection limit of the assay, but not different from one another. Thus, the amount of BPA released from the new PC water bottles and ones previously used by consumers was found to be the same. Based on the concentrations of BPA at the end of the 168 hour incubation period the estimated rate of BPA migration at room temperature averaged from new bottles was 0.60 ng/hr (SD ± 0.18) and 0.42 ng/hr (SD ± 0.14) from used bottles (Table 1). Because the values calculated for rates of migration from new or used bottles were not significantly different from each other, the average rate of migration from all bottles was calculated as 0.49 ng/hr (SD ± 0.17).
The impact of elevated water temperature on BPA release was determined for two of the new, and one used PC bottle by addition of 100 ml of 100°C HPLC grade water to each bottle and incubation for 24 hours, during which time the water samples slowly cooled. The concentration of BPA in each sample was found to be at least double the value accumulated in unheated samples during the entire 7 day incubation period. The markedly higher amounts of BPA that leached into the water indicate that exposure of polycarbonate to heated water greatly increased the rates of BPA migration. The increase in the rate of release following exposure to 100°C water and slow cooling ranged from 15- to 55-fold (Table 1). While the concentrations of BPA from the heated water samples were within the range of assay detection, the concentrations estimated for both new PC bottles (3.84 and 7.67 ng/ml) are at the extreme limit of linearity of the assay. As result, the reported concentrations may modestly underestimate the concentrations of BPA actually present. An elevated rate of BPA liberation was detectable following subsequent incubation with room temperature water suggesting that the effects of heating on BPA migration were not limited to acute effects of heated water, but that there were longer term effects upon migration rate.
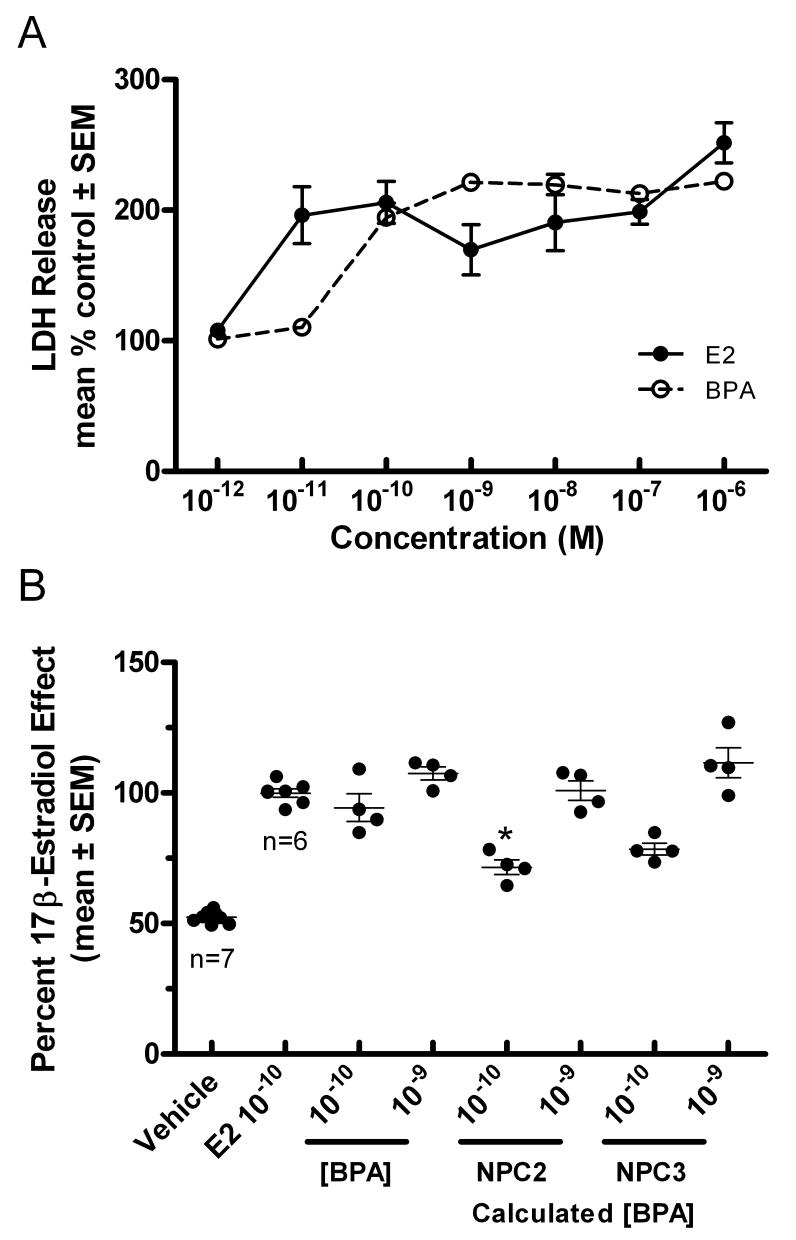
The biological activity of liberated BPA in water samples was examined using a an oncotic cell-death assay for rapid-estrogenic signaling effects in developing cerebellar granule cells (Wong et al., 2003). Serially diluted water samples from two new polycarbonate bottles calculated to range in BPA concentration from about 0.01 nM to 1 nM (0.0023 to 0.23 ng/ml) were found to significantly increase estrogen-like LDH-release from immature granule cells (Fig. 3A). No detectable increase in LDH-release was observed following exposure of granule cells to parallel dilutions of water samples from HDPE bottles (not shown). Effects could be observed at an estimated BPA concentration of approximately 0.1 nM and 1 nM. Compared to the maximal low-dose effects of estrogen (0.1 nM), the estrogenic-effects induced by diluted water samples were similar to those observed for BPA. At the low doses, the estrogen-like effects of sample NPC2 (72 ± 6%) were significantly reduced compared to those observed for 1 nM BPA (89 ± 5% of maximal estradiol effect). The effects induced with the low dose of sample NPC3 (79 ± 5%), while slightly less than predicted, were not different from those induced by BPA. At 1 nM, the BPA positive control and each diluted water sample elicited maximal estradiol-like responses.
Figure 3. Comparative analysis of BPA-like bioactivity of water samples in developing cerebellar neurons.
(A) Concentration response analysis of LDH-released from granule cells for known concentrations of 17β-estradiol and BPA. Results are expressed as means ± SEM (n=8). (B) Dilutions of water samples from two polycarbonate bottles stimulate BPA-like increases in LDH release following brief exposure. After 15′ exposure to 17β-estradiol, BPA, or concentrations of diluted water samples corresponding to approximately 10-11, 10-10, or 10-9 M BPA-like immunoreactivity, the amount of LDH-released from granule cells into culture media at 24 hrs was determined and compared to LDH levels released from vehicle treated controls cultures. Levels of LDH released were normalized to the maximal estrogenic effect induced by 10-10 M 17β-estradiol (E2) and compared to the effect induced by known concentrations of BPA. Results are expressed as means ± SEM (n=4 for BPA and water samples n=7 for vehicle and n=6 for estradiol controls). Levels of significance difference between values calculated fo reach group was assessed with a one-way ANOVA. In panel B, * indicates a significant difference in the values between treatment groups exposed to the same concentration of BPA (0.1 nM) and the estimated concentration of BPA in the experimental water sample.
Discussion
Detectable levels of BPA-like immunoreactivity were observed in all room temperature samples of water following incubation in polycarbonate water bottles, regardless of whether or not the bottle was new or previously used by a consumer. By contrast, water samples collected after identical exposure to negative control water bottles made of HDPE, a polymer consisting of long chains of the monomer ethylene, contained much lower levels of BPA-like immunoreactivity. The water samples from two of HDPE bottles were estimated to contained concentrations of BPA significantly above the theoretical mean for the 0.05 ng/ml detection limit of the assay (bottle no.1 p=0.049; bottle no. 2 p=0.046 as calculated using a one sample t-test). The differences between the mean values for untreated water and water samples incubated in the HDPE bottles were reproducible and highly significant (p=0.0025). Neither the HDPE polymers, nor the polypropylene plastic loop-tops common to tested HDPE and PC bottles, are expected to contain BPA which could account for the observed elevation in BPA-like immunoreactivity. However, the presence of non-polymer additives or coating can not be ruled out as a source of the apparently elevated levels of BPA relative to untreated water.
The bioequivalence of water samples from the PC drinking bottles and BPA was demonstrated using a rapid and sensitive in vitro assay that detects “non-genomic” rapid estrogenic signaling effects in developing neurons (Wong et al., 2001; Wong et al., 2003). Specifically, the endpoint of this assay (LDH release due to oncotic cell death) is dependent on activation of a defined estrogen signaling mechanism that requires activation of two signaling pathways; activation of extracellular-signal regulated kinase (ERK) dependant signaling at cell surface and a distinct signaling pathway dependant on intracellular activation of protein phosphatase 2A (PP2A) activity (Belcher et al., 2005; Wong et al., 2003). Rather than using the traditional MCF7 growth assay to demonstrate estrogen-like activity of BPA and the BPA-like immunoreactivity present in the collected water samples, we employed this non-genomic assay because rapid non-genomic actions are typically more sensitive for detection of estrogen-like EDC activity (Wetherill et al., 2007). Additionally this assay benefits from being more rapid than the MCF7 growth assay. Using the granule cell/LDH system, results from multiple samples can be treated, collected and analyzed in <36 hours, avoiding the much longer incubation periods required to detect estrogen-like increases in MCF7 cell population growth. The use of primary cerebellar neurons under defined conditions also avoids many of the complexities associated with using transformed MCF7 cells. Potentially confounding factors with the MCF7 growth assay include more complicated culture conditions, requirements of serum/hormone withdrawal following culture expansion, and variability resulting from population heterogeneity of MCF7 sub-lines which results in differential ER expression and variable estrogen growth response characteristics.
The results presented here confirm and extend previous studies that demonstrated migration of BPA from PC plastics. In the seminal study of Krishnan and coworkers, biologically active and environmentally relevant levels of BPA (minimal concentration of ∼2.85 ng/ml) were shown to leach into water from PC flasks used for growth of bakers yeast (Saccharomyces cerevisiae) upon autoclaving at 120-125°C. While those studies established clearly that biologically active BPA could migrate from PC into water at elevated temperatures, a comparison of the presented results with subsequent studies analyzing migration of BPA under less extreme temperature conditions could be considered more relevant.
The studies of Howdershell et al (2003) evaluated whether BPA was released from plastic animal caging at room temperature and found that BPA was released into water incubated in new or used PC cages. From a surface area of PC caging comparable to that of the water bottles analyzed here (478 cm2) low concentrations (∼0.27 ng/ml) of BPA were detected in the 250 ml water samples following a 7 day exposure period. In contrast, exposed samples collected from used cages contained large amounts of BPA which resulted in BPA concentrations up to 310 ng/ml of water (Howdeshell et al., 2003). This stands in apparent contradiction to the findings reported here that demonstrate no difference in BPA-liberation between new and used PC bottles. While a number of factors might account for those apparently different findings, differences in previous treatment of polycarbonate animal caging and the used water bottles studied here are a likely explanation for the observed differences in BPA migration. The used PC cages were acquired following use in an animal care facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (Howdeshell et al., 2003). As a result, sanitization of cages would have followed the minimal standards outline in the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Research, 1996), which would entail frequent washing with hot water of at least 180° F (82.2 °C) and soap or detergent. While not stated explicitly, these mouse cages were likely used in a pathogen-free barrier facility and would have also undergone repeated rounds of autoclaving at temperatures of 120° to 122°C. In comparison to the used PC drinking bottles, which would have been washed by consumers at a typical household hot water temperature of ∼50°C, it is likely that the elevated temperatures of autoclaving would result in a greater extent of PC polymer hydrolysis, which could account for increased migration of monomeric BPA. This suggestion is supported by the finding that brief exposure to 100°C water greatly increased the rate of BPA migration from the water bottles analyzed here.
Using a protocol of repeated washing and exposure to 100°C water for 1 hr Brede et al. (2003) demonstrated that BPA from new PC baby bottles leached into boiling water (0.23 ± 0.12 ng/ml). Following simulated use that included dishwashing 51 times, brushing and boiling, the concentrations of BPA liberated was elevated to 8.4 ± 4 ng/ml, a 36.5-fold increase. However, an additional 118 wash cycles did not change the amount of BPA released which was reported to be 6.7 ± 4 ng/ml. From these results it was concluded that there was a link between elevated temperatures and increased BPA migration. It is notable that the concentration of BPA liberated from the PC baby bottles, and the increases in migration rate resulting from exposure to boiling water were similar to the values reported here. However, because the additional variables of washing and brushing were included in the treatments of the baby bottles (Brede et al., 2003), it is only possible to link increased BPA migration with simulated use. In contrast, the data presented here clearly uncouple the independent variables of new and used, from exposure to elevated water temperature. As a result, in the presented analysis, the study’s treatment design has isolated elevated temperature of water as a single variable. Thus, the linkage of BPA liberation and elevated temperatures is clearly established. While the exposure to elevated temperatures of boiling water is much higher than the typical home wash water temperatures used by consumers, the storage of higher temperature (boiling) water or beverages in these PC bottles is a common practice in cold weather outdoor activities such as alpine snow sports, climbing, and mountaineering. Such practices would likely result in increased levels of BPA in beverages stored and consumed from those bottles.
The studies investigating BPA migration from scientific-grade PC labware (Krishnan et al., 1993) and animal caging (Howdeshell et al., 2003) directly address potential confounding factors in the laboratory environment. Those studies also suggest that BPA released from PC plastic could be one point source of BPA contamination in humans. Studies investigating migration of BPA from infant feeding bottles have been especially influential and raised much concern within the general public (Biles et al., 1997; Brede et al., 2003; Sun Y, 2000; Wong et al., 2005). To date, however, few epidemiological studies have been conducted to investigate the relationship between BPA exposure and human health. Although, as reviewed by Vandenberg and colleagues, a fair number of studies have been conducted that have identified sources or levels of BPA in humans (Vandenberg et al., 2007). The aim of the majority of those studies has been to estimate levels of BPA migrating from packaging into foods, with most studies focusing on BPA leaching from epoxy resin lining into canned food. As mentioned above, a subset of studies more directly relevant to those presented here, investigated the release of BPA from commercial infant formula bottles. In most studies, significant release of BPA from PC food and beverage containers was detected, yet based on current acceptable exposure levels the potential adverse heath effects were concluded to be minimal (Brede et al., 2003; D’Antuono et al., 2001; Wong et al., 2005). However, there is evidence demonstrating that mixtures of different estrogenic chemicals can have much greater disruptive effects than those predicted from exposure to low concentrations of an individual compound (Rajapakse et al., 2002). Studies of rapid actions of BPA in developing neurons of the rat cerebellum have also demonstrated that BPA alone acts as a highly potent and efficacious estradiol-mimetic; yet, when combine at very low concentrations (1 pM) with estradiol, BPA acts as a potent antagonist of estrogen signaling (Zsarnovszky et al., 2005). Those finding and others have highlighted the complex, and often unanticipated nature of EDC action. It is now clear that conclusions from single-compound studies, or association of health-risks with a single EDC, may not fully reflect the effects of environmental exposures to EDCs. Thus, the contribution of the concentrations of BPA that contaminate drinking water and foods stored in PC bottles should be considered as a single, though important component of the total mixture of EDCs to which humans are acutely and chronically exposed through out their life-time.
In summary, in this study it was found that bioactive BPA was liberated from both new and used PC drinking bottles and migrated at a similar rate into water at room temperature. Exposure to elevated temperatures above those typically used for washing by consumers, but not outside normal household practice (e.g. boiling to sterilize infant feeding bottles), or outdoor applications (addition of very hot or boiling water or beverages to drinking bottles) greatly elevated the rate of BPA migration. The exposures anticipated from the BPA drinking bottles are likely one of many sources of BPA contamination that contributes to the total amount of endocrine disrupting compounds to which some individuals are exposed.
Figure 2. Comparison of bisphenol A (BPA) migration into water from new and used polycarbonate bottles.
Individual values calculated following room temperature incubation for 7 days in new or used polycarbonate bottles are show. The mean values calculate are indicated and graphically represented with a horizontal dashed line. Error bars represent the standard deviation. * indicates that values are significantly different from the 0.05 ng/ml detection limit as calculated with a one sample t-test using a theoretical mean of 0.05. Values from new and used samples were not significantly different from one another (unpaired t test; p = 0.1688).
Abbreviations
BPA
2,2-bis (4-hydroxyphenyl) propane
EDC
endocrine disrupting chemical
ELISA
enzyme-linked immunosorbent assay
HDPE
high-density polyethylene
HPLC
high-performance liquid chromatography
LDH
lactate dehydrogenase
PC
polycarbonate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Belcher SM, Le HH, Spurling L, Wong JK. Rapid estrogenic regulation of extracellular signal- regulated kinase 1/2 signaling in cerebellar granule cells involves a G protein- and protein kinase A-dependent mechanism and intracellular activation of protein phosphatase 2A. Endocrinology. 2005;146:5397–406. doi: 10.1210/en.2005-0564. [DOI] [PubMed] [Google Scholar]
- Biles JE, McNeal TP, Begley TH, Hollifield HC. Determination of Bisphenol-A in Reusable Polycarbonate Food-Contact Plastics and Migration to Food-Simulating Liquids. J. Agric. Food Chem. 1997;45:3541–3544. [Google Scholar]
- Brede C, Fjeldal P, Skjevrak I, Herikstad H. Increased migration levels of bisphenol A from polycarbonate baby bottles after dishwashing, boiling and brushing. Food Additives & Contaminants. 2003;20:684–689. doi: 10.1080/0265203031000119061. [DOI] [PubMed] [Google Scholar]
- Calafat AM, Kuklenyik Z, Reidy JA, Caudill SP, Ekong J, Needham LL. Urinary concentrations of bisphenol A and 4-nonylphenol in a human reference population. Environ Health Perspect. 2005;113:391–5. doi: 10.1289/ehp.7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CERHR Interm NTP-CERHR report on the reproductive and developmental toxicity of bisphenol A. 2007 http://cerhr.niehs.nih.gov/chemicals/bisphenol/BPA_Interim_DraftRpt.pdf.
- Crain DA, Eriksen M, Iguchi T, Jobling S, Laufer H, LeBlanc GA, Guillette LJ., Jr An ecological assessment of bisphenol-A: Evidence from comparative biology. Reproductive Toxicology. 2007;24:225–239. doi: 10.1016/j.reprotox.2007.05.008. [DOI] [PubMed] [Google Scholar]
- D’Antuono A, Dall’Orto VC, Balbo AL, Sobral S, Rezzano I. Determination of Bisphenol A in Foo d-Simulating Liquids Using LCED with a Chemically Modified Electrode. Journal of Agricultural and Food Chemistry. 2001;49:1098–1101. doi: 10.1021/jf000660n. [DOI] [PubMed] [Google Scholar]
- Howdeshell KL, Peterman PH, Judy BM, Taylor JA, Orazio CE, Ruhlen RL, vom Saal FS, Welshons WV. Bisphenol A is released from used polycarbonate animal cages into water at room temperature. Environ. Health Perspect. 2003;111:1180–1187. doi: 10.1289/ehp.5993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Research. National Research Council . Guide for the Care and Use of Laboratory Animals. National Academy Press; Washington, D.C.: 1996. [Google Scholar]
- Kang J-H, Kondo F, Katayama Y. Human exposure to bisphenol A. Toxicology. 2006;226:79–89. doi: 10.1016/j.tox.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Krishnan AV, Stathis P, Permuth SF, Tokes L, Feldman D. Bisphenol-A: an estrogenic substance is released from polycarbonate flasks during autoclaving. Endocrinology. 1993;132:2279–2286. doi: 10.1210/endo.132.6.8504731. [DOI] [PubMed] [Google Scholar]
- Mountfort K, Kelly J, Jickells S, Castle L. Investigations into the potential degradation of polycarbonate baby bottles during sterilization with consequent release of bisphenol A. Food Addit Contam. 1997;14:737–40. doi: 10.1080/02652039709374584. [DOI] [PubMed] [Google Scholar]
- Richter CA, Birnbaum LS, Farabollini F, Newbold RR, Rubin BS, Talsness CE, Vandenbergh JG, Walser-Kuntz DR, vom Saal FS. In vivo effects of bisphenol A in laboratory rodent studies. Reproductive Toxicology. 2007;24:199–224. doi: 10.1016/j.reprotox.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y,WM, Al-Dirbashi O, Kuroda N, Nakazawa H, Nakashima K. High-performance liquid chromatography with peroxyoxalate chemiluminescence detection of bisphenol A migrated from polycarbonate baby bottles using 4-(4,5-diphenyl-1H-imidazol-2-yl)benzoyl chloride as a label. J Chromatogr B Biomed Sci Appl. 2000;749:49–56. doi: 10.1016/s0378-4347(00)00387-x. [DOI] [PubMed] [Google Scholar]
- Rajapakse N, Silva E, Kortenkamp A. Combining xenoestrogens at levels below individual no-observed-effect concentrations dramatically enhances steroid hormone action. Environ Health Perspect. 2002;110:917–921. doi: 10.1289/ehp.02110917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV. Human exposure to bisphenol A (BPA) Reproductive Toxicology. 2007;24:139–177. doi: 10.1016/j.reprotox.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Wetherill YB, Akingbemi BT, Kanno J, McLachlan JA, Nadal A, Sonnenschein C, Watson CS, Zoeller RT, Belcher SM. In vitro molecular mechanisms of bisphenol A action. Reproductive Toxicology. 2007;24:178–198. doi: 10.1016/j.reprotox.2007.05.010. [DOI] [PubMed] [Google Scholar]
- Wong JK, Kennedy PR, Belcher SM. Simplified serum- and steroid-free culture conditions for the high-throughput viability analysis of primary cultures of cerebellar neurons. J Neurosci Methods. 2001;110:45–55. doi: 10.1016/s0165-0270(01)00419-8. [DOI] [PubMed] [Google Scholar]
- Wong JK, Le HH, Zsarnovszky A, Belcher SM. Estrogens and ICI182,780 (Faslodex) modulate mitosis and cell death in immature cerebellar neurons via rapid activation of p44/p42 mitogen-activated protein kinase. J Neurosci. 2003;23:4984–95. doi: 10.1523/JNEUROSCI.23-12-04984.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong KO, Leo LW, Seah HL. Dietary exposure assessment of infants to bisphenol A from the use of polycarbonate baby milk bottles. Food Additives & Contaminants. 2005;22:280–288. doi: 10.1080/02652030500077502. [DOI] [PubMed] [Google Scholar]
- Zsarnovszky A, Le HH, Wong H-S, Belcher SM. Ontogeny of rapid estrogen-mediated ERK1/2 signaling in the rat cerebellar cortex in vivo: potent non-genomic agonist and endocrine disrupting activity of the xenoestrogen bisphenol A. Endocrinology. 2005;146:5388–5396. doi: 10.1210/en.2005-0565. [DOI] [PubMed] [Google Scholar]