ISG15 inhibits Ebola VP40 VLP budding in an L-domain-dependent manner by blocking Nedd4 ligase activity (original) (raw)
Abstract
Ebola virus budding is mediated by the VP40 matrix protein. VP40 can bud from mammalian cells independent of other viral proteins, and efficient release of VP40 virus-like particles (VLPs) requires interactions with host proteins such as tsg101 and Nedd4, an E3 ubiquitin ligase. Ubiquitin itself is thought to be exploited by Ebola virus to facilitate efficient virus egress. Disruption of VP40 function and thus virus budding remains an attractive target for the development of novel antiviral therapies. Here, we investigate the effect of ISG15 protein on the release of Ebola VP40 VLPs. ISG15 is an IFN-inducible, ubiquitin-like protein expressed after bacterial or viral infection. Our results show that expression of free ISG15, or the ISGylation system (UbE1L and UbcH8), inhibits budding of Ebola virus VP40 VLPs. Addressing the molecular mechanism of this inhibition, we show that ISG15 interacts with Nedd4 ubiquitin ligase and inhibits ubiquitination of VP40. Furthermore, the L-domain deletion mutant of VP40 (ΔPT/PY), which does not interact with Nedd4, was insensitive to ISG15-mediated inhibition of VLP release. These data provide evidence of antiviral activity of ISG15 against Ebola virus and suggest a mechanism of action involving disruption of Nedd4 function and subsequent ubiquitination of VP40.
Keywords: Ebola virus, interferon, innate immunity, ubiquitin
The IFN pathway activates hundreds of cellular IFN-stimulated genes (ISGs), some of which have direct antiviral activity (1–5). For example, ISG15 was one of the first recognized ISG proteins whose expression was up-regulated not only by IFN but also by viral infection, LPS treatment, and retinoic acid (6–8). ISG15 has high homology to ubiquitin and can be detected in cells in both free and conjugated forms (9, 10). ISG15 conjugation (ISGylation) to proteins uses cascades of enzymatic reactions similar to those used in protein ubiquitination pathways (11). Some of these enzymes, like ubiquitin E1-like protein (UBE1L) (12, 13), are unique for ISG15, whereas two E2 enzymes, UbcH8 and UbcH6, are also used in the ubiquitination pathway (14–16)
The observation that ISG15 conjugation targets many components of the antiviral signaling pathway suggests that ISG15 may play a role in the innate antiviral response (12, 17). To this effect, it was shown that the NS1 protein of the Influenza B virus inhibits ISGylation (12), and ISG15 expression decreased Sindbis virus replication and provided protection against lethal infection (12, 18). Also, ISG15-null mice showed an increase susceptibility to both DNA- and RNA-containing viruses in vivo, including influenza, herpes simplex, and Sindbis viruses (19). Recently, inhibition of HIV-1 virion release in IFN-treated cells was shown to be mediated by ISG15 (20). Several reports have also linked induction and expression of ISG15 to inhibition of important viral pathogens of fish (21, 22). Together, these studies suggest that ISG15 has a potent and broad-based antiviral effect; however, the mechanism of action of ISG15 remains to be determined.
Ebola virus (Zaire; EBOZ) is a member of the Filoviridae family of negative-sense RNA viruses, and the VP40 matrix protein is a key structural protein critical for virion egress. Late-budding domains (L-domains) present in VP40 mediate interactions with host proteins to facilitate VLP and virus release (23–39).
For example, Ebola VP40 contains overlapping L-domains (7PTAP10 and 10PPEY13), which interact with members of the ESCRT pathway (e.g., tsg101) and members of the HECT family of WW-domain containing ubiquitin ligases (e.g., Nedd4) to facilitate budding (36, 40–48). Indeed, monoubiquitination of viral matrix proteins has been postulated to promote efficient release of virus and/or VLPs (36, 38, 39, 48–59).
The goal of this study was to determine whether expression of ISG15 can inhibit budding of Ebola VP40 VLPs, and if so, to elucidate the molecular mechanism of ISG15 activity. We found that expression of ISG15 inhibited release of VP40 VLPs in a dose-dependent manner. This inhibition was specific for ISG15, because down-regulation of ISG15 expression by ISG15-specific siRNAs rescued budding of VP40 VLPs. Regarding the mechanism of inhibition of budding, we demonstrate that ISG15 interacts with host ubiquitin ligase-Nedd4, and that ISG15 expression inhibits ubiquitination of Ebola VP40. Interestingly, expression of ISG15 did not inhibit release of an L-domain mutant of VP40, which does not interact with the Nedd4. Thus, our findings suggest that IFN-induced ISG15 inhibits budding of Ebola VP40 VLPs in an L-domain-dependent manner by a mechanism that involves disruption of Nedd4 function and subsequent ubiquitination of VP40. Our results implicate an important role for ISG15 in the enhancement of the innate antiviral response and suggest a mechanism of action whereby ISG15 disrupts Nedd4 ligase activity.
Results
Human ISG15 Inhibits Budding of Ebola VP40 VLPs.
We first sought to determine whether expression of human ISG15 would inhibit budding of Ebola VP40 VLPs. Human 293T cells were either mock-transfected or transfected with a constant amount of a plasmid-expressing VP40-WT together with increasing amounts of a plasmid-expressing human ISG15 (Fig. 1). Total plasmid DNA was held equivalent in all samples by using empty pCAGGS vector. At 48 h posttransfection, media from all samples were collected and centrifuged through a 20% sucrose cushion to pellet VLPs. Both cells and VLPs were lysed, and the indicated proteins were detected by SDS/PAGE and Western blotting (Fig. 1).
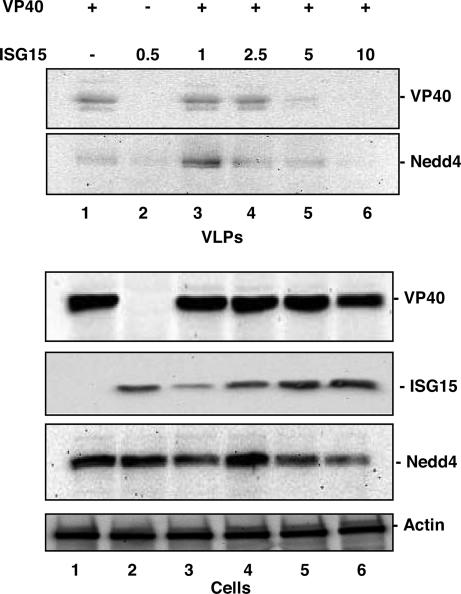
Fig. 1.
ISG15 inhibited Ebola VP40 VLP budding in a concentration-dependent manner. Human 293T cells were transfected as indicated. The total amount of plasmid DNA transfected was held equivalent in all samples by using empty pCAGGS vector. At 48 h posttransfection, VLPs and cell extracts were harvested and analyzed by Western blot with anti-VP40, -ISG15, or -Nedd4 antisera.
Western blotting of transfected cell extracts revealed that the levels of VP40 remained relatively constant (Fig. 1, Cells, lanes 1 and 3–6), as did levels of endogenous Nedd4 E3 ubiquitin ligase (Fig. 1, Cells, lanes 1–6). As expected, levels of ISG15 in cells increased with increasing amounts of ISG15 plasmid DNA transfected (Fig. 1, Cells, compare lanes 2–6). In contrast, the levels of VP40 detected in VLPs decreased significantly in response to increasing amounts of ISG15 (Fig. 1, VLPs, compare lanes 3–6). In correlation with the observed decrease in VP40 VLPs, the levels of endogenous Nedd4 incorporated into budding VP40 VLPs (36, 48, 61, 62) also decreased in response to increasing amounts of ISG15 (Fig. 1, VLPs, compare lanes 3–6). These findings indicate that expression of human ISG15 inhibits Ebola VLP release in a dose-dependent manner, as illustrated by a steady decrease of both VP40 and host Nedd4 in budding VLPs.
ISG15 Inhibits Ubiquitination of VP40.
Both the PTAP and overlapping PPEY motifs present in Ebola VP40 have been shown to possess L-domain activity and to interact physically and functionally with host proteins tsg101 and Nedd4, respectively (56, 58, 61, 62). Because ISG15 is a ubiquitin-like protein and can be conjugated to target proteins via lysine residues, we sought to determine whether ISG15 modulates the ubiquitination of VP40 and/or Nedd4 ligase activity to disrupt VP40 VLP budding.
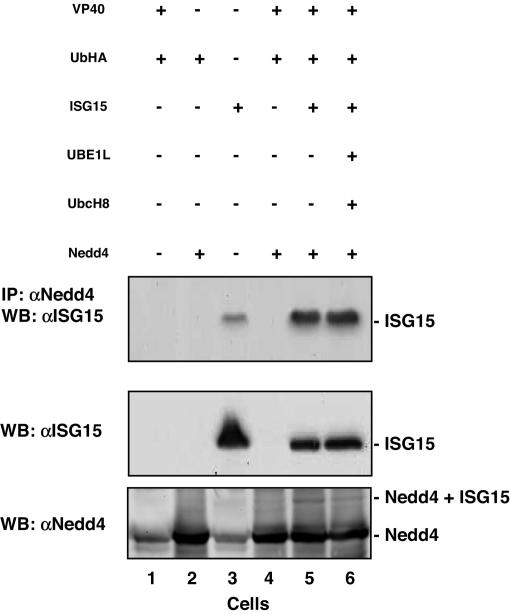
Human 293T cells were transfected with the indicated plasmids (Fig. 2), and cell extracts and VLPs were harvested at 24 h after transfection. The levels of Ebola VP40 were detected in both cell lysates and VLPs by immunoprecipitation with anti-VP40 antiserum, followed by Western blotting with anti-VP40 antiserum (Fig. 2A; H.C., IgG heavy chain). The level of VP40 present in appropriate cell extracts was equivalent (Fig. 2A, Cells, lanes 1 and 4–6). As expected, a basal level of VP40 was detected in VLPs (Fig. 2A, VLPs, lane 1), whereas enhanced budding of VP40 was evident when VP40 was coexpressed with Nedd4 (Fig. 2A, VLPs, lane 4). Interestingly, Nedd4-mediated enhancement of VP40 budding was abolished by coexpression of ISG15 alone (Fig. 2A, VLPs, lane 5), or the ISGylation system (Fig. 2A, VLPs, lane 6).
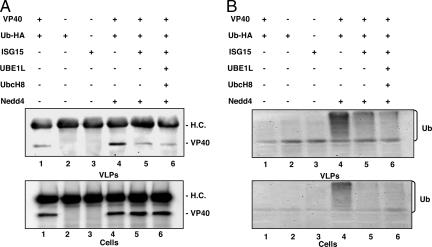
Fig. 2.
Expression of ISG15 affects Nedd4-mediated ubiquitination. Human 293T cells in 100-mm dishes were transfected with the indicated plasmids. VLPs and cell extracts were first immunoprecipitated with anti-VP40 antiserum, followed by Western blotting with (A) anti-VP40 antiserum (H.C., heavy chain), or (B) anti-HA antiserum.
The blots in Fig. 2A were then stripped and reprobed with anti-HA antiserum to detect ubiquitinated VP40 (Fig. 2B). Thus, cell and VLP samples shown in Fig. 2B were first immunoprecipitated with anti-VP40 antiserum followed by Western blot by using anti-HA antiserum (Fig. 2B). In the absence of exogenous Nedd4 (Fig. 2B, cells plus VLPs, lane 1) and in control samples (lanes 2 and 3), ubiquitination of VP40 was not detectable. However, a strong ubiquitination profile of VP40 was observed in both cells and VLPs in the presence of Nedd4 (Fig. 2B, Cells and VLPs, lane 4). Strikingly, ubiquitination of VP40 was reduced significantly in the presence of free ISG15 (lane 5) or the ISGylation system (lane 6). These findings suggest that ubiquitination of VP40 is mediated by Nedd4, leading to an enhancement of VP40 VLP budding. Furthermore, coexpression of ISG15 counteracts the enhancing effect of Nedd4-mediated ubiquitination, leading to a reduction in VP40 VLP budding.
Based on the findings described above, we sought to determine whether ISG15 interacts with Nedd4, thus inhibiting its ubiquitin ligase activity and positive effect on VP40 VLP budding. Cell extracts were first immunoprecipitated with anti-Nedd4 antiserum followed by Western blot with anti-ISG15 antiserum (Fig. 3Top). As expected, ISG15 was not detected in control samples that did not receive the ISG15 expression plasmid (Fig. 3 Top, lanes 1, 2, and 4). In contrast, free ISG15 was detected in cells receiving only the ISG15 expression plasmid (Fig. 3 Top, lane 3). This weak signal is likely due to an interaction between endogenous Nedd4 and exogenous ISG15. A significantly stronger ISG15 signal was evident in cells receiving exogenous Nedd4 and ISG15 alone or ISG15 plus UBE1L and UbcH8 (Fig. 3 Top, lanes 5 and 6). These findings indicate that ISG15 physically interacts with endogenously and exogenously expressed Nedd4.
Fig. 3.
Interaction of Nedd4 and ISG15. Human 293T cells were transfected with the indicated plasmids, and cell extracts were harvested. (Top) Proteins were first immunoprecipitated with anti-Nedd4 antiserum, followed by Western blot with anti-ISG15 antiserum. (Middle and Bottom) Western blots of cell extracts using anti-ISG15 or anti-Nedd4 antisera as indicated. ISG15 detected in Top and Middle represents free ISG15.
In addition to the IP/Western blots described above, Western blots were performed to confirm expression of either ISG15 or Nedd4 in the appropriate samples (Fig. 3 Middle and Bottom). Free ISG15 was readily detected in cells receiving the ISG15 expression plasmid (Fig. 3 Middle, lanes 3, 5, and 6), but not in control samples (lanes 1, 2, and 4). A weak Nedd4 signal corresponding to endogenously expressed protein was detected by Western blot (Fig. 3 Lower, lanes 1 and 3), whereas a strong Nedd4 signal corresponding to endogenously and exogenously expressed Nedd4 was observed in lanes 2, 4, 5, and 6. A band corresponding in size to ISGylated-Nedd4 was observed in cell extracts coexpressing ISG15 and Nedd4 (Fig. 3 Bottom, lanes 5 and 6); however, whether ISG15 is conjugated to Nedd4 remains to be confirmed. In sum, these findings suggest that ISG15 and Nedd4 interact, leading to the possible ISGylation of Nedd4.
ISG15 Had No Effect on Budding of the VP40 L-Domain Mutant, VP40Δ_PTPY._
Because it appears that ISG15 inhibits budding of VP40 VLPs by disrupting Nedd4 activity, and because Nedd4 interacts with VP40 via the PPxY-type L-domain of VP40, we sought to determine whether expression of ISG15 would affect release of VP40-ΔPTPY. We know from previous studies that VP40-ΔPTPY does bud as a VLP, albeit at levels up to 100-fold less than that of VP40-WT. VLPs were isolated, and VP40-WT or VP40-ΔPTPY was detected by Western blot (Fig. 4). Consistent with results described above, budding of VP40-WT VLPs was enhanced in the presence of Nedd4 (Fig. 4A Top, compare lanes 1 and 4), and VP40-WT VLPs were not detected in control samples (lanes 2 and 3). As expected, budding of VP40-WT was reduced in the presence of free ISG15 alone or the ISGylation system (Fig. 4A Top, compare lane 4 with lanes 5 and 6).
Fig. 4.
ISG15 inhibits VP40 VLP budding in an L-domain-dependent manner. Human 293T cells were transfected with the indicated plasmids. At 48 h after transfection, media were concentrated and VLPs were harvested as indicated. (A) VP40-WT VLPs were analyzed by Western blot using anti-VP40 antiserum (Top) or anti-HA antiserum (Bottom). (B) VP40-ΔPTPY VLPs were analyzed by Western blot using anti-VP40 antiserum (Top), or anti-HA antiserum (Bottom).
In marked contrast, although VP40-ΔPTPY was detected in VLPs (Fig. 4B Top, lane 1), there was no enhancement of budding of VP40-ΔPTPY when coexpressed with Nedd4 (Fig. 4B Top, compare lanes 1 and 4). In addition, coexpression of free ISG15 (lane 5) or the ISGylation system (lane 6) did not inhibit release of VP40-ΔPTPY (Fig. 4B Top, compare lane 4 with lanes 5 and 6). These data indicate that the L-domain sequences of VP40 are required for both Nedd4-mediated enhancement and ISG15-mediated inhibition of VP40 VLP budding.
Next, we examined the ubiquitination profile of VP40-WT VLPs and VP40-ΔPTPY VLPs in the presence or absence of ISG15 (Fig. 4 A and B Bottom). Consistent with results described above, ubiquitination of VP40-WT was readily evident in VLPs harvested from cells expressing VP40-WT, Ub-HA, and Nedd4 (Fig. 4A Bottom, lane 4), whereas no ubiquitination was detected in control samples (Fig. 4A Bottom, lanes 1–3). Ubiquitination was reduced in the presence of ISG15 alone (Fig. 4A Bottom, lane 5) or the ISGylation system (lane 6). In contrast to that of VP40-WT, a more diffuse and nonspecific pattern of ubiquitination was observed in VLPs from cells expressing VP40-ΔPTPY, Ub-HA, and Nedd4 (Fig. 4B Bottom, lane 4). Importantly, this observed pattern of ubiquitination of VP40-ΔPTPY did not change in the presence of free ISG15 (Fig. 4B Bottom, compare lanes 4 and 5) or the ISGylation system (compare lanes 4 and 6). Taken together, the data presented in Fig. 4 are consistent with our model that ISG15 disrupts Nedd4 activity leading to disruption of L-domain-mediated budding of VP40 VLPs.
ISG15-Specific siRNA Rescues Budding of VP40 VLPs.
To confirm that the inhibition of VP40 VLP budding is specific for expression of ISG15, control or ISG15-specific siRNAs were incorporated into the VLP budding assay. Human 293T cells were cotransfected with VP40 and ISG15 in the presence of nonspecific, scrambled siRNA (20 nmol) or ISG15-specific siRNA (20 nmol) (Fig. 5A). Proteins were radiolabeled at 24 h posttransfection with 100 μCi/ml of [35S]Met-Cys. Cell extracts and VLPs were harvested 6 hours later, and the indicated proteins were detected by immunoprecipitation (Fig. 5).
Fig. 5.
siRNAs targeting ISG15 rescue budding of VP40 VLPs. (A) Human 293T cells were transfected for 24 h with the indicated plasmids and with ISG15 siRNA (20 μM), or random siRNA (20 μM). VLPs and cell extracts (Cells) were harvested as described, and samples were immunoprecipitated with anti-VP40 (Top) or anti-ISG15 (Middle) antisera, as indicated. Actin (Bottom) is shown as a loading control. (B) Human 293T cells were transfected with the indicated plasmids or siRNAs for 24 h. Transfected cells were then treated with IFN-β (500 units/ml) for an additional 24 h. Cell extracts and VLPs were harvested as described and immunoprecipitated with either anti-VP40 (Top) or -ISG15 (Middle) antisera, as indicated. All numbers represent an average of three independent experiments.
Equivalent levels of expression of VP40 were observed in the appropriate cell extracts (Fig. 5A, cells, lanes 2 and 4–6). Expression of ISG15 in cell extracts cotransfected with ISG15 siRNA was abolished (Fig. 5A, cells, lane 6), whereas expression of ISG15 was unaffected in cell extracts transfected with random siRNA (lane 5). VP40 was readily detected in VLPs when expressed alone (Fig. 5A, VLPs, lane 2), and a 2-fold reduction in VP40 VLP budding was observed in the presence of ISG15 (1.0 μg of DNA transfected) (Fig. 5A, VLPs, compare lanes 2 and 4). This level of reduction remained in the presence of random siRNA (Fig. 5A, VLPs, lane 5), whereas the level of VP40 in VLPs harvested from cells receiving ISG15-specific siRNA increased close to that observed in the positive control sample (Fig. 5A, VLPs, compare lanes 1 and 6). These results confirm that inhibition of VP40 VLP budding is directly related to expression of ISG15.
In a complementary approach, expression of endogenous ISG15 was induced in VP40-transfected cells by treating with 500 units/ml of IFN-β (Fig. 5B). Cells expressing VP40 alone served as a positive control for VLP budding (Fig. 5B, VLPs, lane 1). The level of VP40 in VLPs from cells treated with IFN-β was reduced by ≈40% compared with that observed in the untreated positive control (Fig. 5B, VLPs, compare lanes 1 and 3). This reduction in VP40 VLP budding correlated with the IFN-β-mediated induction of endogenous ISG15 in these cells (Fig. 5B, Cells Middle, lane 3). Reduced levels of VP40 VLP budding were also evident in IFN-β-treated cells receiving random siRNA (Fig. 5B, VLPs, lane 4). In contrast, budding of VP40 VLP was rescued in IFN-β-treated cells receiving ISG15-specific siRNA (Fig. 5B, VLPs, compare lanes 1 and 5). Importantly, the level of endogenous ISG15 in these cells was virtually undetectable (Fig. 5B, Cells, lane 4), indicating that the ISG15-specific siRNA efficiently knocked down expression of ISG15. Taken together, the data presented in Fig. 5 indicate that inhibition of VP40 VLP budding is due specifically to expression (exogenous or endogenous) of host ISG15.
Discussion
Type 1 IFN plays a central role in the innate immune response to virus infection, in part by stimulating expression of a plethora of host genes, many of which possess antiviral functions (5, 63). One such gene, ISG15, is a ubiquitin-like protein that is conjugated to cellular proteins (9), and that has been shown recently to have antiviral activity (12, 17, 18, 64). In this study, we demonstrated that IFN treatment and/or expression of human ISG15 significantly inhibited release of Ebola VP40-WT VLPs. It is well documented that Ebola virus VP40 can bud independently as a VLP from mammalian cells (40, 46, 47, 61, 65). Efficient release of VP40 VLPs is known to depend on functional L-domain(s) of VP40, and at least two host proteins, tsg101 and Nedd4 (36, 48, 51, 61, 62, 66). Indeed, monoubiquitination of viral matrix proteins by WW-domain-containing E3 ligases such as Nedd4 plays a role in facilitating budding of rhabdoviruses, filoviruses, and retroviruses (24, 36, 37, 39, 61, 67).
Our results indicate that IFN treatment and/or ISG15 expression significantly inhibits release of VP40 VLPs [supporting information (SI) Fig. 6], and that the IFN-induced inhibition of VP40 VLP budding is mediated by ISG15, because ISG15-specific siRNA reversed the IFN inhibition of VLP release. These data extend those described for ISG15 modulation of HIV-1 budding (20) by addressing the molecular mechanism of inhibition of VP40 VLP budding by ISG15. We show here that ISG15 disrupts ubiquitin ligase activity of Nedd4 and its ability to ubiquitinate VP40. The extent of Nedd4-mediated ubiquitination of VP40 in cells and VLPs was reduced dramatically in the presence of ISG15; however, it should be noted that the presence of ubiquitinated forms of VP40 in VLP samples from ISG15-transfected cannot be ruled out completely because of the paucity of VP40 present in these VLP samples (Fig. 4A, lanes 5 and 6). The ISG15-mediated inhibition of ubiquitination and subsequent budding were L-domain-dependent. Indeed, expression of ISG15 and Nedd4 had no effect on budding of the L-domain mutant, VP40-ΔPTPY. In addition, VP40 VLP budding was not inhibited by ISG15 in the presence of inactive, dominant-negative Nedd4 (SI Fig. 7). These findings reveal a mechanism of antiviral activity of ISG15 that involves blocking E3 ligase activity of host Nedd4.
Based on these data, we hypothesize that inactivation of Nedd4 ligase by ISG15 may also lead to inefficient release of other viruses containing L-domains in their matrix proteins. As part of this study, we have used a transfection/infection approach to examine the effect of ISG15 on release of VSV. Like Ebola VP40, VSV M protein possesses a functional PPxY type L-domain that interacts with host Nedd4 (24, 36, 48, 61, 62, 67, 68). We show that expression of ISG15 resulted in titers of VSV-WT that were ≈10-fold lower than those measured in the absence of ISG15 at 4 and 6 h postinfection (SI Table 1). Importantly, expression of ISG15 had little to no effect on titers of recombinant virus PY>A4; a PPxY-type L-domain-defective mutant of VSV (SI Table 1).
Future experiments need to determine whether ISG15 expression can also inhibit budding of live Ebola virus and clearly establish the role of Type I IFN-mediated antiviral response to Ebola virus infection in vivo. To this effect, it has been shown that mice lacking the Type I IFN response (IRFR1 knockout mice) are highly susceptible to Ebola virus infection (69). Also the transcription profile of blood samples from non-human primates infected with Ebola virus reflects both the IFN and cytokine response. However, a common feature of filovirus infection is to suppress the antiviral response, and a correlation between the virulence of highly pathogenic Ebola viruses (Zaire and Sudan strains) and their ability to antagonize the Type I IFN response has been observed (60).
Additional experiments to test the role of ISG15 in the IFN-mediated inhibition of virus budding by using ISG15-knockout mice are currently underway. A better understanding of these virus–host interactions and the mechanism of action of innate immune proteins such as ISG15 is fundamental for the future development of novel and specific antiviral therapeutics.
Materials and Methods
Cells and Plasmids.
Human 293T cells and BHK-21 cells were cultured in DMEM with 10% FBS. Plasmids VP40-WT and VP40-ΔPT/PY have been described in ref. 48, and pISG15, UBE1L and the histidine-tagged ISG15 (ISG15-His) was obtained from Ian Pitha-Rowe (Dartmouth Medical School, Hanover, NH) and are described (20). UbcH8 was provided by Bret A. Hassel (University of Maryland, Baltimore). The HA-tagged tsg101 is described in ref. 48.
VLP Budding Assay.
293T cells were transfected with the indicated amount of plasmid DNA by using Lipofectamine (Invitrogen) in OptiMem (Invitrogen/Life Technologies). Transfected cells were metabolically labeled 24 h posttransfection with 100 μCi/ml of [35S] Met-Cys (Perkin–Elmer). Six hours later, medium was harvested, clarified, and layered over a 20% sucrose cushion in STE buffer (0.01 M Tris·HCl, pH 7.5; 0.01 M NaCl; 0.001 M EDTA, pH 8.0), then centrifuged at 36,000 rpm for 2 h at 4°C. The pellet was suspended in sodium chloride/Tris/EDTA buffer and lysed with lysis buffer (50 mM Tris, pH 8; 150 mM NaCl; 1.0% Nonidet P-40; proteinase inhibitor mixture). Cells were washed twice with 1× PBS and then lysed in lyses buffer. Both cell and VLP samples were immunoprecipitated with the appropriate antiserum and analyzed by SDS/PAGE.
Antiserum, Immunoprecipitation, and Western Blotting.
Antiserum against human tsg101 and the HA epitope tag were purchased from Santa Cruz Biotechnology. ISG15 antiserum was purchased from Cell Signaling Technology. Western blotting and immunoprecipitations were performed as described in ref. 20.
VSV Infection.
Human 293T cells were transfected with pISG15 (2.0 μg) or pcDNA3.1 empty vector by using Lipofectamine (Invitrogen). Twenty-four hours after transfection, the cells were infected with VSV (Indiana, Serotype) at a multiplicity of infection of 10. At the indicated times postinfection, supernatant samples were harvested and titered by standard plaque assay on BHK-21 cells.
Transfection of ISG15 siRNA.
ISG15-synthetic siRNA (G1P2) and scrambled siRNA (used as a negative control) were purchased from Ambion. Human 293T cells were cotransfected with ISG15 siRNA (20 nmol) or scrambled siRNA (20 nmol), and VP40-WT by using Lipofectamine 2000 (Invitrogen). To express ISG15, cells were either transfected with pISG15 or treated with IFN-β (1,000 units/ml) for 24 h. Transfected cells were then metabolically labeled with 100 μCi/ml of [35S]Met-Cys, and media samples were harvested 6 hours later for use in the VLP budding assay, as described above. Levels of ISG15 synthesized in cell lysates were determined by Western blot analysis.
Supplementary Material
Supporting Information
ACKNOWLEDGMENTS.
We thank Dr. Ian Pitha-Rowe for the UBEL1 and ISG15 plasmids. We also thank Drs. Oxana Malakhova, Dong-Er Zhang, and Serge Fuchs for critical review of our manuscript. This work was supported by National Institutes of Health Grants R01 AI-46499 (to R.N.H.) and AI R01 19737-23 (to P.M.P.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Samuel CE. Antiviral actions of interferons. Clin Microbiol Rev. 2001;14:778–809. doi: 10.1128/CMR.14.4.778-809.2001. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paun A, Pitha PM. The innate antiviral response: new insights into a continuing story. Adv Virus Res. 2007;69:1–66. doi: 10.1016/S0065-3527(06)69001-5. [DOI] [PubMed] [Google Scholar]
- 3.Gantier MP, Sadler AJ, Williams BR. Fine-tuning of the innate immune response by microRNAs. Immunol Cell Biol. 2007;85:458–462. doi: 10.1038/sj.icb.7100091. [DOI] [PubMed] [Google Scholar]
- 4.Bose S, Banerjee AK. Innate immune response against nonsegmented negative strand RNA viruses. J Interferon Cytokine Res. 2003;23:401–412. doi: 10.1089/107999003322277810. [DOI] [PubMed] [Google Scholar]
- 5.de Veer MJ, et al. Functional classification of interferon-stimulated genes identified using microarrays. J Leukoc Biol. 2001;69:912–920. [PubMed] [Google Scholar]
- 6.Kitareewan S, et al. UBE1L is a retinoid target that triggers PML/RARalpha degradation and apoptosis in acute promyelocytic leukemia. Proc Natl Acad Sci USA. 2002;99:3806–3811. doi: 10.1073/pnas.052011299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Labrada L, Liang XH, Zheng W, Johnston C, Levine B. Age-dependent resistance to lethal alphavirus encephalitis in mice: analysis of gene expression in the central nervous system and identification of a novel interferon-inducible protective gene, mouse ISG12. J Virol. 2002;76:11688–11703. doi: 10.1128/JVI.76.22.11688-11703.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ritchie KJ, Zhang DE. ISG15: the immunological kin of ubiquitin. Semin Cell Dev Biol. 2004;15:237–246. doi: 10.1016/j.semcdb.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 9.Haas AL, Ahrens P, Bright PM, Ankel H. Interferon induces a 15-kilodalton protein exhibiting marked homology to ubiquitin. J Biol Chem. 1987;262:11315–11323. [PubMed] [Google Scholar]
- 10.Loeb KR, Haas AL. The interferon-inducible 15-kDa ubiquitin homolog conjugates to intracellular proteins. J Biol Chem. 1992;267:7806–7813. [PubMed] [Google Scholar]
- 11.Pickart CM, Cohen RE. Proteasomes and their kin: proteases in the machine age. Nat Rev Mol Cell Biol. 2004;5:177–187. doi: 10.1038/nrm1336. [DOI] [PubMed] [Google Scholar]
- 12.Yuan W, Krug RM. Influenza B virus NS1 protein inhibits conjugation of the interferon (IFN)-induced ubiquitin-like ISG15 protein. EMBO J. 2001;20:362–371. doi: 10.1093/emboj/20.3.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malakhova OA, et al. Protein ISGylation modulates the JAK-STAT signaling pathway. Genes Dev. 2003;17:455–460. doi: 10.1101/gad.1056303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim KI, Giannakopoulos NV, Virgin HW, Zhang DE. Interferon-inducible ubiquitin E2, Ubc8, is a conjugating enzyme for protein ISGylation. Mol Cell Biol. 2004;24:9592–9600. doi: 10.1128/MCB.24.21.9592-9600.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao C, et al. The UbcH8 ubiquitin E2 enzyme is also the E2 enzyme for ISG15, an IFN-alpha/beta-induced ubiquitin-like protein. Proc Natl Acad Sci USA. 2004;101:7578–7582. doi: 10.1073/pnas.0402528101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malakhova O, Malakhov M, Hetherington C, Zhang DE. Lipopolysaccharide activates the expression of ISG15-specific protease UBP43 via interferon regulatory factor 3. J Biol Chem. 2002;277:14703–14711. doi: 10.1074/jbc.M111527200. [DOI] [PubMed] [Google Scholar]
- 17.Zhao C, Denison C, Huibregtse JM, Gygi S, Krug RM. Human ISG15 conjugation targets both IFN-induced and constitutively expressed proteins functioning in diverse cellular pathways. Proc Natl Acad Sci USA. 2005;102:10200–10205. doi: 10.1073/pnas.0504754102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lenschow DJ, et al. Identification of interferon-stimulated gene 15 as an antiviral molecule during Sindbis virus infection in vivo. J Virol. 2005;79:13974–13983. doi: 10.1128/JVI.79.22.13974-13983.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lenschow DJ, et al. IFN-stimulated gene 15 functions as a critical antiviral molecule against influenza, herpes, and Sindbis viruses. Proc Natl Acad Sci USA. 2007;104:1371–1376. doi: 10.1073/pnas.0607038104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okumura A, Lu G, Pitha-Rowe I, Pitha PM. Innate antiviral response targets HIV-1 release by the induction of ubiquitin-like protein ISG15. Proc Natl Acad Sci USA. 2006;103:1440–1445. doi: 10.1073/pnas.0510518103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Das BK, Collet B, Snow M, Ellis AE. Expression of interferon type I, II, Mx and gammaIP genes in the kidney of Atlantic salmon, Salmo salar, is induced during smolting. Fish Shellfish Immunol. 2007;23:825–830. doi: 10.1016/j.fsi.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 22.Kileng O, Brundtland MI, Robertsen B. Infectious salmon anemia virus is a powerful inducer of key genes of the type I interferon system of Atlantic salmon, but is not inhibited by interferon. Fish Shellfish Immunol. 2007;23:378–389. doi: 10.1016/j.fsi.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 23.Gottwein E, et al. The Mason-Pfizer monkey virus PPPY and PSAP motifs both contribute to virus release. J Virol. 2003;77:9474–9485. doi: 10.1128/JVI.77.17.9474-9485.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harty RN, et al. Rhabdoviruses and the cellular ubiquitin-proteasome system: a budding interaction. J Virol. 2001;75:10623–10629. doi: 10.1128/JVI.75.22.10623-10629.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martin-Serrano J, Zang T, Bieniasz PD. Role of ESCRT-I in retroviral budding. J Virol. 2003;77:4794–4804. doi: 10.1128/JVI.77.8.4794-4804.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ott DE, et al. Equine infectious anemia virus and the ubiquitin-proteasome system. J Virol. 2002;76:3038–3044. doi: 10.1128/JVI.76.6.3038-3044.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perez OD, et al. Leukocyte functional antigen 1 lowers T cell activation thresholds and signaling through cytohesin-1 and Jun-activating binding protein 1. Nat Immunol. 2003;4:1083–1092. doi: 10.1038/ni984. [DOI] [PubMed] [Google Scholar]
- 28.Strack B, Calistri A, Craig S, Popova E, Gottlinger HG. AIP1/ALIX is a binding partner for HIV-1 p6 and EIAV p9 functioning in virus budding. Cell. 2003;114:689–699. doi: 10.1016/s0092-8674(03)00653-6. [DOI] [PubMed] [Google Scholar]
- 29.von Schwedler UK, et al. The protein network of HIV budding. Cell. 2003;114:701–713. doi: 10.1016/s0092-8674(03)00714-1. [DOI] [PubMed] [Google Scholar]
- 30.Gottlinger HG, Dorfman T, Sodroski JG, Haseltine WA. Effect of mutations affecting the p6 gag protein on human immunodeficiency virus particle release. Proc Natl Acad Sci USA. 1991;88:3195–3199. doi: 10.1073/pnas.88.8.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jayakar HR, Murti KG, Whitt MA. Mutations in the PPPY motif of vesicular stomatitis virus matrix protein reduce virus budding by inhibiting a late step in virion release. J Virol. 2000;74:9818–9827. doi: 10.1128/jvi.74.21.9818-9827.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Noda T, et al. Ebola virus VP40 drives the formation of virus-like filamentous particles along with GP. J Virol. 2002;76:4855–4865. doi: 10.1128/JVI.76.10.4855-4865.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wills JW, et al. An assembly domain of the Rous sarcoma virus Gag protein required late in budding. J Virol. 1994;68:6605–6618. doi: 10.1128/jvi.68.10.6605-6618.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Accola MA, Strack B, Gottlinger HG. Efficient particle production by minimal Gag constructs, which retain the carboxy-terminal domain of human immunodeficiency virus type 1 capsid-p2 and a late assembly domain. J Virol. 2000;74:5395–5402. doi: 10.1128/jvi.74.12.5395-5402.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Craven RC, Harty RN, Paragas J, Palese P, Wills JW. Late domain function identified in the vesicular stomatitis virus M protein by use of rhabdovirus-retrovirus chimeras. J Virol. 1999;73:3359–3365. doi: 10.1128/jvi.73.4.3359-3365.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harty RN, Brown ME, Wang G, Huibregtse J, Hayes FP. A PPxY motif within the VP40 protein of Ebola virus interacts physically and functionally with a ubiquitin ligase: implications for filovirus budding. Proc Natl Acad Sci USA. 2000;97:13871–13876. doi: 10.1073/pnas.250277297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harty RN, Paragas J, Sudol M, Palese P. A proline-rich motif within the matrix protein of vesicular stomatitis virus and rabies virus interacts with WW domains of cellular proteins: implications for viral budding. J Virol. 1999;73:2921–2929. doi: 10.1128/jvi.73.4.2921-2929.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schubert U, et al. Proteasome inhibition interferes with gag polyprotein processing, release, and maturation of HIV-1 and HIV-2. Proc Natl Acad Sci USA. 2000;97:13057–13062. doi: 10.1073/pnas.97.24.13057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Strack B, Calistri A, Accola MA, Palu G, Gottlinger HG. A role for ubiquitin ligase recruitment in retrovirus release. Proc Natl Acad Sci USA. 2000;97:13063–13068. doi: 10.1073/pnas.97.24.13063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoenen T, et al. VP40 octamers are essential for Ebola virus replication. J Virol. 2005;79:1898–1905. doi: 10.1128/JVI.79.3.1898-1905.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Irie T, Harty RN. L-domain flanking sequences are important for host interactions and efficient budding of vesicular stomatitis virus recombinants. J Virol. 2005;79:12617–12622. doi: 10.1128/JVI.79.20.12617-12622.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kallstrom G, et al. Analysis of Ebola virus and VLP release using an immunocapture assay. J Virol Methods. 2005;127:1–9. doi: 10.1016/j.jviromet.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 43.Neumann G, et al. Ebola virus VP40 late domains are not essential for viral replication in cell culture. J Virol. 2005;79:10300–10307. doi: 10.1128/JVI.79.16.10300-10307.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hartlieb B, Weissenhorn W. Filovirus assembly and budding. Virology. 2006;344:64–70. doi: 10.1016/j.virol.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 45.Ye L, et al. Ebola virus-like particles produced in insect cells exhibit dendritic cell stimulating activity and induce neutralizing antibodies. Virology. 2006;351:260–270. doi: 10.1016/j.virol.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 46.McCarthy SE, Johnson RF, Zhang YA, Sunyer JO, Harty RN. Role for amino acids 212KLR214 of Ebola virus VP40 in assembly and budding. J Virol. 2007;81:11452–11460. doi: 10.1128/JVI.00853-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Noda T, et al. Assembly and budding of Ebolavirus. PLoS Pathog. 2006;2:e99. doi: 10.1371/journal.ppat.0020099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Licata JM, et al. Overlapping motifs (PTAP and PPEY) within the Ebola virus VP40 protein function independently as late budding domains: involvement of host proteins TSG101 and VPS-4. J Virol. 2003;77:1812–1819. doi: 10.1128/JVI.77.3.1812-1819.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Freed EO. HIV-1 gag proteins: diverse functions in the virus life cycle. Virology. 1998;251:1–15. doi: 10.1006/viro.1998.9398. [DOI] [PubMed] [Google Scholar]
- 50.Katzmann DJ, Babst M, Emr SD. Ubiquitin-dependent sorting into the multivesicular body pathway requires the function of a conserved endosomal protein sorting complex, ESCRT-I. Cell. 2001;106:145–155. doi: 10.1016/s0092-8674(01)00434-2. [DOI] [PubMed] [Google Scholar]
- 51.Martin-Serrano J, Perez-Caballero D, Bieniasz PD. Context-dependent effects of L domains and ubiquitination on viral budding. J Virol. 2004;78:5554–5563. doi: 10.1128/JVI.78.11.5554-5563.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Demirov DG, Ono A, Orenstein JM, Freed EO. Overexpression of the N-terminal domain of TSG101 inhibits HIV-1 budding by blocking late domain function. Proc Natl Acad Sci USA. 2002;99:955–960. doi: 10.1073/pnas.032511899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sundquist WI, et al. Ubiquitin recognition by the human TSG101 protein. Mol Cell. 2004;13:783–789. doi: 10.1016/s1097-2765(04)00129-7. [DOI] [PubMed] [Google Scholar]
- 54.Hoeller D, et al. Regulation of ubiquitin-binding proteins by monoubiquitination. Nat Cell Biol. 2006;8:163–169. doi: 10.1038/ncb1354. [DOI] [PubMed] [Google Scholar]
- 55.VerPlank L, et al. Tsg101, a homologue of ubiquitin-conjugating (E2) enzymes, binds the L domain in HIV type 1 Pr55(Gag). Proc Natl Acad Sci USA. 2001;98:7724–7729. doi: 10.1073/pnas.131059198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Garrus JE, et al. Tsg101 and the vacuolar protein sorting pathway are essential for HIV-1 budding. Cell. 2001;107:55–65. doi: 10.1016/s0092-8674(01)00506-2. [DOI] [PubMed] [Google Scholar]
- 57.Martin-Serrano J, Zang T, Bieniasz PD. HIV-1 and Ebola virus encode small peptide motifs that recruit Tsg101 to sites of particle assembly to facilitate egress. Nat Med. 2001;7:1313–1319. doi: 10.1038/nm1201-1313. [DOI] [PubMed] [Google Scholar]
- 58.Pornillos O, Alam SL, Davis DR, Sundquist WI. Structure of the Tsg101 UEV domain in complex with the PTAP motif of the HIV-1 p6 protein. Nat Struct Biol. 2002;9:812–817. doi: 10.1038/nsb856. [DOI] [PubMed] [Google Scholar]
- 59.Goff A, Ehrlich LS, Cohen SN, Carter CA. Tsg101 control of human immunodeficiency virus type 1 Gag trafficking and release. J Virol. 2003;77:9173–9182. doi: 10.1128/JVI.77.17.9173-9182.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kash JC, et al. Global suppression of the host antiviral response by Ebola- and Marburg viruses: increased antagonism of the type I interferon response is associated with enhanced virulence. J Virol. 2006;80:3009–3020. doi: 10.1128/JVI.80.6.3009-3020.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yasuda J, Nakao M, Kawaoka Y, Shida H. Nedd4 regulates egress of Ebola virus-like particles from host cells. J Virol. 2003;77:9987–9992. doi: 10.1128/JVI.77.18.9987-9992.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Timmins J, et al. Ebola virus matrix protein VP40 interaction with human cellular factors Tsg101 and Nedd4. J Mol Biol. 2003;326:493–502. doi: 10.1016/s0022-2836(02)01406-7. [DOI] [PubMed] [Google Scholar]
- 63.Basler CF, Garcia-Sastre A. Viruses and the type I interferon antiviral system: induction and evasion. Int Rev Immunol. 2002;21:305–337. doi: 10.1080/08830180213277. [DOI] [PubMed] [Google Scholar]
- 64.Pitha-Rowe IF, Pitha PM. Viral defense, carcinogenesis and ISG15: novel roles for an old ISG. Cytokine Growth Factor Rev. 2007;18:409–417. doi: 10.1016/j.cytogfr.2007.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Martinez O, Valmas C, Basler CF. Ebola virus-like particle-induced activation of NF-kappaB and Erk signaling in human dendritic cells requires the glycoprotein mucin domain. Virology. 2007;364:342–354. doi: 10.1016/j.virol.2007.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Panchal RG, et al. In vivo oligomerization and raft localization of Ebola virus protein VP40 during vesicular budding. Proc Natl Acad Sci USA. 2003;100:15936–15941. doi: 10.1073/pnas.2533915100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Irie T, Licata JM, McGettigan JP, Schnell MJ, Harty RN. Budding of PPxY-containing rhabdoviruses is not dependent on host proteins TGS101 and VPS4A. J Virol. 2004;78:2657–2665. doi: 10.1128/JVI.78.6.2657-2665.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yasuda J, Hunter E, Nakao M, Shida H. Functional involvement of a novel Nedd4-like ubiquitin ligase on retrovirus budding. EMBO Rep. 2002;3:636–640. doi: 10.1093/embo-reports/kvf132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bray M. The role of the Type I interferon response in the resistance of mice to filovirus infection. J Gen Virol. 2001;82:1365–1373. doi: 10.1099/0022-1317-82-6-1365. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information