Inhibition of cardiac sodium currents in adult rat myocytes by n-3 polyunsaturated fatty acids (original) (raw)
Abstract
- The acute effects of n-3 polyunsaturated fatty acids were determined on whole-cell sodium currents recorded in isolated adult rat ventricular myocytes using patch clamp techniques.
- The n-3 polyunsaturated fatty acids docosahexaenoic acid (22:6, n-3), eicosapentaenoic acid (20:5, n-3) and α-linolenic acid (18:3, n-3) dose-dependently blocked the whole-cell sodium currents evoked by a voltage step to −30 mV from a holding potential of −90 mV with EC50 values of 6.0 ± 1.2, 16.2 ± 1.3 and 26.6 ± 1.3 μM, respectively.
- Docosahexaenoic acid, eicosapentaenoic acid and α-linolenic acid at 25 μM shifted the voltage dependence of activation of the sodium current to more positive potentials by 9.2 ± 2.0, 10.1 ± 1.1 and 8.3 ± 0.9 mV, respectively, and shifted the voltage dependence of inactivation to more negative potentials by 22.3 ± 0.9, 17.1 ± 3.7 and 20.5 ± 1.0 mV, respectively. In addition, the membrane fluidising agent benzyl alcohol (10 mM) shifted the voltage dependence of activation to more positive potentials by 7.8 ± 2.5 mV and shifted the voltage dependence of inactivation to more negative potentials (by −24.6 ± 3.6 mV).
- Linoleic acid (18:2, n-6), oleic acid (18:1, n-9) and stearic acid (18:0) were either ineffective or much less potent at blocking the sodium current or changing the voltage dependence of the sodium current compared with the n-3 fatty acids tested.
- Docosahexaenoic acid, eicosapentaenoic acid, α-linolenic acid and benzyl alcohol significantly increased sarcolemmal membrane fluidity as measured by fluorescence anisotropy (steady-state, _r_ss, values of 0.199 ± 0.004, 0.204 ± 0.006, 0.213 ± 0.005 and 0.214 ± 0.009, respectively, compared with 0.239 ± 0.002 for control), whereas stearic, oleic and linoleic acids did not alter fluidity (the _r_ss was not significantly different from control).
- The potency of the n-3 fatty acids docosahexaenoic acid, eicosapentaenoic acid and α-linolenic acid to block cardiac sodium currents is correlated with their ability to produce an increase in membrane fluidity.
A number of epidemiological studies have shown that the consumption of a diet high in n-3 polyunsaturated fatty acids (PUFAs) can confer protection from coronary heart disease (Kromhout et al. 1985; Burr et al. 1989; Siscovick et al. 1995). In animal studies, dietary PUFAs have a similar protective action, particularly in relation to cardiac arrhythmias (McLennan et al. 1988, 1996; Hock et al. 1990; Pepe & McLennan, 1996). More recently, it has been reported that some PUFAs can exert powerful anti-arrhythmic actions when applied acutely, as an intravenous infusion in surgically manipulated animals (Billman et al. 1994, 1997). Studies using isolated neonatal cultured cardiac myocyte preparations have also demonstrated an anti-arrhythmic effect of acutely added PUFAs (Kang & Leaf, 1994, 1995, 1996).
The electrophysiological mechanism underlying the acute effect of fatty acids, at least in isolated cardiac myocytes, may involve an increase in the threshold for the generation of the action potential (Kang et al. 1995; McMurchie et al. 1999), suggesting that PUFAs may mediate their effects by interaction with sodium currents. In this context, n-3 PUFAs have been shown to have potent effects on sodium currents evoked in neurons (Vreugdenhil et al. 1996), in HEK293t cells transfected with the alpha subunit of the human cardiac sodium channel (Xiao et al. 1998) and in neonatal cardiac myocytes (Xiao et al. 1995). However, difficulties arise in the extrapolation of results obtained with cloned channels expressed in transfected cells to adult tissues, since the results are always subject to reservations as to whether channel properties have been modified by the expression system, and whether crucial subunits are missing or modified. Similarly, cultured neonatal cardiac myocytes exhibit important differences from the adult myocardium since many ionic currents are subject to considerable modulation during development (Wetzel et al. 1993; Matsubara et al. 1993; Nuss & Marban, 1994; Wang et al. 1996; Roden & George, 1997). An additional difficulty with neonatal cells is that sodium channels undergo substantial changes in their properties during development (Sada et al. 1995) which include changes in the action of class I antiarrhythmic agents (Xu et al. 1991, 1992). Due to these developmental changes, and of particular concern in the context of arrhythmias, neonatal cardiac myocytes have a fundamentally different electrophysiological profile from adult cells.
We have therefore investigated the effects of a variety of n-3 polyunsaturated fatty acids, differing in their degree of unsaturation and chain length, on sodium currents in isolated adult rat cardiac myocytes using whole-cell patch clamp recording techniques.
The shorthand notation for fatty acid structure is 'a:b, n-c'. The letter ‘a_’ represents the total number of carbon atoms in the fatty acyl chain; ‘b’, the number of double bonds separated by single methylene groups; and 'n-c', also written as ‘omega_-c_’ (or ω_-c), denotes the number of carbon atoms between the first double bond and the methyl end of the chain. The n-3 fatty acids are those in the linolenic acid family; n-6 fatty acids are in the linoleic acid family.
We have focused in particular on docosahexaenoic acid (DHA) (22:6, n-3), which occurs in relatively high concentrations in cardiac membrane phospholipids. In addition, we have investigated the effects of the n-3 PUFAs on sarcolemmal membrane fluidity in isolated cardiac myocytes using steady-state fluorescence anisotropy to determine whether changes in cell membrane physical properties correlate with the effects of PUFAs on sodium current activity. In order to investigate the idea that sodium current block by fatty acids is related to changes in membrane fluidity, we have also investigated the effects of benzyl alcohol, a known membrane fluidising agent, on both membrane fluidity and sodium currents.
METHODS
Animals used in these studies were cared for according to the Australian National Health and Medical Council Guidelines for the Care and Use of Animals. All experimental procedures were subject to prior approval by the University of Adelaide and CSIRO Human Nutrition Animal Ethics Committees.
Isolation of cardiac myocytes
Enzymatic isolation of cardiac myocytes was performed as previously described (Saint et al. 1992). Briefly, male Sprague-Dawley rats (300–350 g) were injected with heparin (2000 i.u., i.p.). After 30 min, animals were anaesthetised with pentobarbitone sodium (60 mg kg−1) which was injected intraperitoneally. The heart was removed, washed in ice-cold, oxygenated, calcium-free Tyrode solution for 1 min, and then perfused, via an aortic cannula, with the same calcium-free Tyrode solution warmed to 37°C at a perfusion rate of between 9 and 10 ml min−1. This facilitated the removal of blood from both the coronary vasculature and ventricular chambers. The Tyrode solution contained (mM): NaCl, 134; Hepes, 10; KCl, 4; MgCl2, 1.2; NaH2PO4, 1.2; glucose, 11, and was adjusted to pH 7.4 with 1.0 M NaOH. After 5 min of perfusion the heart was subjected to enzymatic dissociation by perfusion with 25 μM calcium Tyrode solution containing collagenase (0.3 mg ml−1, Worthington CLS II), protease (0.1 mg ml−1, Sigma) and fetal calf serum (1 μg ml−1). After approximately 25 min when the heart became pale and flaccid, the ventricles were removed, cut into small pieces in fresh 25 μM calcium-Tyrode solution and triturated to dissociate the myocytes. Cell suspensions were then allowed to settle at room temperature and the Ca2+ concentration was increased gradually to 200 μM. Finally, cells were resuspended in Tyrode solution containing 1 mM calcium and plated onto glass coverslips. For fluidity measurements, cells were plated onto coverslips coated with 50 μg ml−1 laminin (Sigma). Except for the perfusion of the heart, all the preparation and maintenance of the cells was at room temperature (25°C).
Electrophysiological recording of cardiac Na+ current
Electrodes were prepared from borosilicate glass using a two-stage puller (Narishige Scientific Instruments, Tokyo, Japan) and resistances were typically between 1 and 3 MΩ when containing the pipette solution. Whole-cell currents were recorded 5 min after achievement of a whole-cell patch clamp configuration. Current recording was performed using an Axopatch 200A amplifier (Axon Instruments). Whole-cell capacitance and series resistance compensation was achieved using the controls on the amplifier; recording was only performed if series resistance compensation of at least 90 % could be achieved. Satisfactory voltage control was indicated by the following criteria: (1) the negative limb of the current-voltage curve spanned at least 25 mV; (2) there were no abnormal notches in the current-voltage curve; (3) there was no crossover between recordings at different voltages of the inactivation curve. Whole-cell sodium currents were evoked by voltage steps generated by a computer program which outputs the waveform via a digital to analogue converter connected to the command input of the amplifier. The resulting currents were filtered at 5 kHz and recorded through an analogue to digital converter operating at 20 kHz. Glass coverslips with adhering cardiac myocytes were transferred to a superfusion chamber (mounted on an inverted microscope) containing 0.5 ml of bath solution. Cardiac myocyte currents were recorded 5 min after achieving a whole-cell patch clamp configuration.
Membrane fluidity measurements
Membrane fluidity was determined by measuring the steady-state fluorescence anisotropy (_r_ss) of the probe _N_-((4-(6-phenyl-1,3,5-hexatrienyl)phenyl)propyl) trimethyl-ammonium _p_-toluenesulphonate (TMAP-DPH) (Molecular Probes, Eugene, OR, USA) according to a modification of a method described previously (de Jonge et al. 1996). This probe readily partitions into the cell membrane (Lentz, 1989). Isolated ventricular cardiac myocytes attached to laminin-coated glass coverslips were washed in Tyrode buffer. Cardiac myocytes were then loaded with 1 μM TMAP-DPH for 15 min at 37°C. The coverslips were placed in a glass cuvette containing 20 μM test fatty acids or 10 mM benzyl alcohol and _r_ss values were measured according to the following formula as described previously (Lentz, 1989):
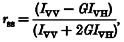
where _I_VV and _I_VH represent the fluorescence intensity parallel and perpendicular to the excitation plane (when set vertically), respectively. G is a correction factor for the difference in the transmission efficiency for vertically and horizontally polarised light, and is calculated by _I_HV/_I_HH, where _I_HH represents the fluorescence intensity parallel to the excitation plane when set horizontally. Measurements were obtained using a spectrofluorimeter (Hitachi 650–10S), provided with vertical and horizontal polarisation filters (Polaroid, Australia). The excitation and emission monochromators were positioned at wavelengths of 350 and 430 nm, respectively, with slit width set to 10 nm for both excitation and emission modes. Readings were corrected for both background fluorescence of TMAP-DPH and light scatter by the cardiac myocyte preparation itself.
Solutions and fatty acids
The standard external (bath) solution used for sodium current measurements contained (mM): NaCl, 20; _N_-tris-(hydroxy-methyl)-methyl-2-aminoethanesulphonic acid (Tes), 10; KCl, 5; MgCl2, 1; CaCl2, 2; CoCl2, 5; CsCl, 5; glucose, 10; choline chloride, 110; pH adjusted to 7.4 with 5.0 M NaOH. The pipette solution for all experiments contained (mM): CsF, 120; Tes, 10; MgCl2, 2; Na2-EGTA, 20; CaCl2, 2; pH adjusted to 7.4 with 5.0 M KOH. These solutions are designed to block all ionic currents other than sodium currents. In addition, the low extracellular sodium concentration (20 mM) is designed to reduce the peak sodium current and hence minimise series resistance errors in the clamp potential. Fatty acids (Sigma) were dissolved in ethanol at a final concentration of 50 mM containing 0.003 % (w/v) butylated-hydroxyanisole and stored at −80°C.
Statistical analysis
Appropriate equations were fitted to individual data sets using the algorithm built into the graphics program GraphPad Prizm version 2.00 (Graphpad Software Inc., San Diego, CA, USA). Data are presented as means ± standard error of the mean (s.e.m.) Significance between means was tested either using the two tailed Student's paired t test or one-way ANOVA with Dunnet's multiple comparisons test. The significance level was set at P < 0.05.
RESULTS
Block of voltage-dependent sodium currents in adult rat cardiac myocytes
The average capacitance of cardiac myocytes was 120.3 ± 5.2 pF (n = 51). Sodium currents in control cells were activated at approximately −55 mV and reached a maximum current at approximately −25 mV. The maximum sodium current densities elicited by a voltage step from −90 mV to −30 mV for extracellular Na+ concentrations of 20 mM and 70 mM were 8.9 ± 0.4 pA pF−1 (n = 51) and 13.8 ± 2.9 pA pF−1 (n = 7), respectively. Figure 1_A_ shows an example of typical sodium currents evoked in a single myocyte by a step in membrane potential from a holding potential of −90 mV to −30 mV, and the effect of 25 μM DHA on this current. In this cell, 25 μM DHA applied to the extracellular solution blocked the peak current amplitude by 42 %, after approximately 4 min of incubation. The time course of this block is shown in Fig. 1_B_. The block by DHA reached a plateau after approximately 3 min exposure and elimination of the effect of DHA could be achieved by a washout procedure by adding delipidated bovine serum albumin (BSA) to the extracellular solution for 2 min (in the absence of fatty acid).
Figure 1. Time course of sodium current inhibition by DHA in an adult rat ventricular myocyte.
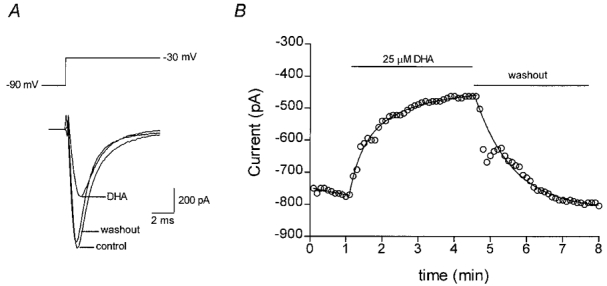
A, depiction of the voltage step (upper panel) and the current evoked by a step in membrane potential to −30 mV from a holding potential of −90 mV (lower panel) and following incubation in DHA (25 μM) and subsequent washout. B, time course of the inhibitory effect of DHA on the peak sodium current recorded from an adult rat ventricular myocyte. Sodium currents were evoked in whole-cell recording mode once every 6 s with 300 ms duration pulses to −30 mV from a holding potential of −90 mV. The horizontal bars indicate the period during which DHA was applied to the cell or the period of washout with a solution containing 1 mg ml−1 delipidated BSA.
Effect of fatty acids on sodium current activation
The effect of fatty acids on the voltage dependence of activation was determined by evoking current-voltage steps to various potentials between −90 mV and +30 mV from a holding potential of −140 mV (as depicted in upper panel of Fig. 2_A_). Representative sodium currents are shown in the lower panel of Fig. 2_A_. The maximum amplitude of the currents evoked was plotted against the test potential, as shown in Fig. 2_B_ for control cells and cells in the presence of 25 μM DHA. The data points were fitted by the equation:
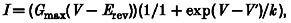 |
(1) |
|---|
using a least squares fitting algorithm, where _G_max is the maximum conductance, V′ is the membrane potential for half-activation of the channels, V is the test membrane potential, _E_rev is the reversal potential for the current and k is a slope factor. The control data were best fitted by _G_max = 19.3 nS, V′ = −37.2 mV, k = 4.17 mV−1 and _E_rev = 25.9 mV. In the presence of 25 μM DHA the maximum conductance, _G_max, was reduced by 50 % to 9.6 mV, V′ was −26.2 mV (i.e. shifted to more positive potentials by 11.0 mV), k was 6.43 mV−1 and _E_rev was 29.0 mV (not significantly different from control). The means of all the above parameters for the least squares fit of eqn (1) for the n-3 PUFAs DHA, eicosapentaenoic acid (EPA) and α-linolenic acid (ALA), all at 25 μM final concentration, are given in Table 1. Following treatment with DHA (n = 5), EPA (n = 10) or ALA (n = 6), _G_max values were significantly lower than controls (P < 0.01) and the I–V relation was significantly shifted to more positive potentials. This shift in membrane potential was similar for all n-3 PUFAs tested (9.2 ± 2.0, 10.0 ± 1.1 and 8.3 ± 0.9 mV for DHA, EPA or ALA-treated cardiac myocytes, respectively). However, DHA was more potent than either EPA or ALA at inhibiting _G_max in these cells. Linoleic acid (18:2, n-6), oleic acid (18:1, n-9) and the saturated fatty acid, stearic acid (18:0), did not significantly alter any of the sodium current activation parameters measured (Fig. 3 and Table 1). Benzyl alcohol at 10 mM (n = 3) significantly (P < 0.05) reduced the _G_max as shown in Table 1 and Fig. 3.
Figure 2. Effect of DHA on the voltage dependence of activation in an adult rat ventricular myocyte.
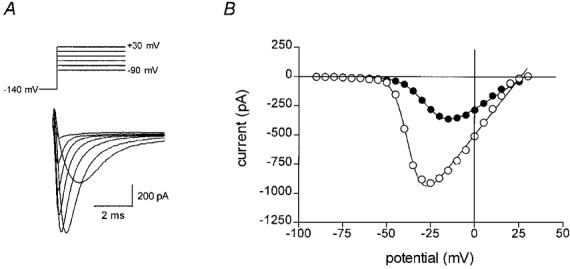
A, sodium currents were evoked by voltage steps from a holding potential of −140 mV to various voltages between −90 mV and +30 mV as depicted (upper panel). Plotted below are superimposed currents evoked at −40, −30, −20, −10, 0, 10 and 20 mV. B, the peak current amplitude was plotted against the pulse potential. Typical data are shown as points representing control (^) or in the presence of 25 μM DHA (•). The continuous line shows the least squares best fit of eqn (1). The parameters for the best fit in each case were: control, _G_max = 19.3 nS, V′ = −37.2 mV, k = 4.17 mV−1 and _E_rev = 25.9 mV; and in the presence of 25 μM DHA, _G_max = 9.6 nS, V′ = −26.2 mV, k = 6.43 mV−1 and _E_rev = 29.0 mV, where _G_max is the maximum conductance, V′ is the voltage at which 50 % of the channels are activated, k is the slope factor for the voltage dependence of activation and _E_rev is the reversal potential.
Table 1.
Activation parameters from least squares fit of eqn (1)
| Structure | _G_max (nS) | _V_′ (mV) | k (mV−1) | _V_rev (mV) | |
|---|---|---|---|---|---|
| Control | 20.5 ± 1.7 | −42.9 ± 2.9 | 2.7 ± 0.5 | 23.9 ± 1.1 | |
| DHA 25 μm | (22:6, n-3) | 13.4 ± 1.4† | −33.7 ± 2.1† | 5.0 ± 0.5† | 25.7 ± 2.0 |
| Control | 22.2 ± 1.3 | −39.2 ± 2.1 | 3.2 ± 0.4 | 37.8 ± 2.3 | |
| EPA 25 μm | (20:5, n-3) | 15.6 ± 1.0† | −29.2 ± 2.0‡ | 4.8 ± 0.4‡ | 39.8 ± 2.2 |
| Control | 22.1 ± 1.7 | −33.6 ± 2.5 | 4.1 ± 0.3 | 31.9 ± 2.2 | |
| ALA 25 μm | (18:3, n-3) | 18.2 ± 1.2† | −25.3 ± 2.1‡ | 5.3 ± 0.4† | 40.9 ± 3.7* |
| Control | 18.4 ± 2.1 | −41.7 ± 1.6 | 2.9 ± 0.8 | 27.4 ± 0.4 | |
| LA 25 μm | (18:2, n-6) | 17.4 ± 2.0 | −39.9 ± 1.5 | 2.5 ± 0.9 | 32.9 ± 2.6 |
| Control | 16.6 ± 2.7 | −46.6 ± 2.9 | 2.4 ± 0.5 | 32.6 ± 1.9 | |
| OA 25 μm | (18:1, n-9) | 16.2 ± 2.5 | 46.2 ± 2.3 | 2.3 ± 0.7 | 34.1 ± 1.4 |
| Control | 17.9 ± 2.2 | −47.8 ± 2.8 | 2.2 ± 0.4 | 32.2 ± 1.9 | |
| SA 25 μm | (18:0) | 17.9 ± 2.1 | −49.0 ± 2.8 | 1.4 ± 0.2* | 30.3 ± 3.0 |
| Control | 23.7 ± 0.5 | −46.0 ± 1.5 | 5.1 ± 0.9 | 10.5 ± 1.4 | |
| Benzyl alcohol(10 mm) | 7.7 ± 3.0* | −38.2 ± 1.8 | 4.8 ± 0.3 | 9.1 ± 1.1 |
Figure 3. Effect of various fatty acids on the voltage dependence of activation in adult rat ventricular myocytes.
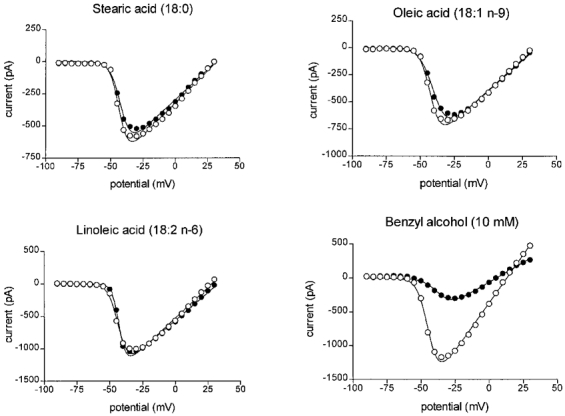
Sodium currents were evoked by voltage steps to various potentials from a holding potential of −140 mV to various voltages between −90 mV and +30 mV as depicted in Fig. 2_A_. The peak current amplitude was plotted against the pulse potential. Points represent typical control data (^) or in the presence of 25 μM fatty acid or benzyl alcohol (•) as indicated.
Effect of fatty acids on sodium current inactivation
The effect of the fatty acids on the voltage dependence of inactivation of the sodium current was investigated by stepping the membrane potential to a test potential of −30 mV from holding potentials which varied between −140 and −30 mV (Fig. 4_A_). The peak amplitude of the evoked current was plotted against the holding potential for both control cells and cells in the presence of 25 μM DHA, and the data points fitted with the Boltzmann equation:
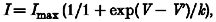 |
(2) |
|---|
where _I_max is the maximum current, V is the test membrane potential, V′ is the membrane potential at which half of the channels are inactivated, and k is a slope factor. In this cell, 25 μM DHA induced a shift to more hyperpolarised potentials in the voltage dependence of inactivation by 24.7 mV and _I_max was reduced from a control value of −746 pA to −320 pA in the presence of DHA. The mean values for the parameters _I_max, V′ and k, for the least squares fit of eqn (2) for the n-3 polyunsaturated fatty acids DHA (n = 6), EPA (n = 10) and ALA (n = 6) all at 25 μM final concentration, as well as benzyl alcohol (n = 3) at 10 mM, are given in Table 2. In addition to the n-3 PUFAs, the following fatty acids were also tested: linoleic acid (n = 5), oleic acid (n = 7) and the saturated fatty acid, stearic acid (n = 8) (Table 2). A significant reduction in _I_max occurred following treatment with either DHA (P < 0.05), EPA (P < 0.01) or ALA (P < 0.01). Benzyl alcohol also significantly reduced _I_max (n = 3, P < 0.01). A shift of V′ to more hyperpolarised potentials was also observed following treatment with DHA (22.3 ± 0.9 mV; P < 0.001), EPA (17.1 ± 3.7 mV; P < 0.01) or ALA (20.5 ± 1.0 mV; P < 0.001). The fatty acids linoleic acid, oleic acid and stearic acid did not significantly alter _I_max, although addition of linoleic acid and oleic acid was associated with a small shift of V′ to more hyperpolarised potentials (6.3 ± 0.6 and 3.5 ± 1.2 mV, respectively). Nevertheless, the shift in voltage dependence was considerably less with these three fatty acids than that observed by any of the n-3 PUFAs (Table 2).
Figure 4. Effect of DHA on the voltage dependence of inactivation in adult rat ventricular myocytes.
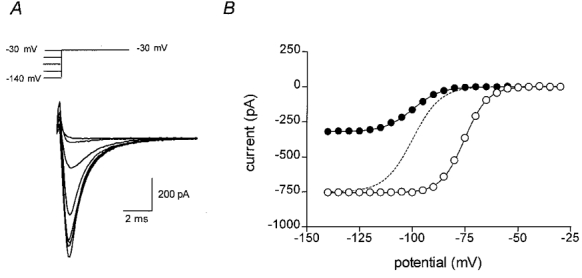
A, sodium currents were evoked by voltage steps to −30 mV from various holding potentials, as depicted (upper panel). Plotted below are currents evoked at −130, −120, −110, −100, −90, −80, −70, −60 and −50 mV, shown superimposed. B, the peak current amplitude is shown plotted against the holding potential. Points represent typical control data (^) or in the presence of 25 μM DHA (•). The continuous line shows the least squares best fit of eqn (2) for control or DHA. The parameters for the best fit in each case were: control, _I_max = −746 pA, V′ = −75.2 mV and k = 5.28 mV−1; and in the presence of 25 μM DHA, _I_max = −320 pA, V′ = −99.9 mV and k = 6.60 mV−1. The dashed line shows the data points for DHA scaled to the same maximum as the control data.
Table 2.
Inactivation parameters from least squares fit of eqn (2)
| Structure | _I_max (pA) | _V_′(mV) | k (mV−1) | |
|---|---|---|---|---|
| Control | −850 ± 92 | −74.3 ± 1.5 | 5.2 ± 0.1 | |
| DHA 25 μm | (22:6, n-3) | −544 ± 88* | −96.6 ± 1.3‡ | 6.5 ± 0.2‡ |
| Control | −1258 ± 107 | −69.3 ± 2.1 | 6.5 ± 0.2 | |
| EPA 25 μm | (20:5, n-3) | −816 ± 106† | −86.5 ± 3.5† | 7.4 ± 0.3‡ |
| Control | −988 ± 103 | −68.4 ± 2.0 | 5.8 ± 0.3 | |
| ALA 25 μm | (18:3, n-3) | −740 ± 83† | −88.9 ± 2.0‡ | 7.0 ± 0.2† |
| Control | −869 ± 105 | −71.0 ± 2.8 | 6.3 ± 0.4 | |
| LA 25 μm | (18:2, n-6) | −898 ± 119 | −77.3 ± 3.0‡ | 6.9 ± 0.4 |
| Control | −901 ± 128 | −76.0 ± 2.9 | 6.2 ± 0.3 | |
| OA 25 μm | (18:1, n-9) | −902 ± 156 | −79.5 ± 2.3* | 6.4 ± 0.1 |
| Control | −974 ± 121 | −73.7 ± 1.7 | 6.2 ± 0.2 | |
| SA 25 μm | (18:0) | −919 ± 105 | −73.6 ± 2.2 | 6.9 ± 0.4 |
| Control | −716 ± 63 | −86.0 ± 1.8 | 8.4 ± 0.4 | |
| Benzyl alcohol (10 mm) | −140 ± 77† | −110.6 ± 3.8* | 6.8 ± 1.4 |
Concentration dependence of sodium current block by n-3 polyunsaturated fatty acids
When whole-cell sodium currents were evoked from a holding potential of −90 mV (close to the resting potential of the cells in vivo), DHA, EPA and ALA produced a concentration-dependent block of peak sodium current amplitude over the concentration range 1–50 μM. The degrees of block by DHA, EPA and ALA over this concentration range in several cells are shown in Fig. 5_A_. The EC50 for each of the fatty acids was estimated from the least squares fit of the Hill equation:
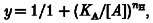 |
(3) |
|---|
where y is the fractional block, _K_A is the apparent affinity constant, [_A_] is the concentration of fatty acid and n_H is the Hill coefficient. The most potent polyunsaturated fatty acid was DHA (EC50 = 6.0 ± 1.2 μM, n = 5), followed by EPA (EC50 = 16.2 ± 1.3 μM, n = 5), and ALA (EC50 = 26.6 ± 1.3 μM, n = 6). The potency for each of the fatty acids DHA, EPA and ALA to shift the voltage dependence of inactivation was also determined. Figure 5_B_ shows the dose-response relationship for the shift in inactivation (Δ_V′) plotted against log concentration for each of the fatty acids. In this case there was no obvious difference in potency between DHA, EPA and ALA. The other fatty acids examined, linoleic acid, oleic acid and stearic acid, did not produce a measurable shift in voltage dependence up to concentrations of 25 μM.
Figure 5. Concentration-dependent block of n-3 polyunsaturated fatty acids in adult rat ventricular myocytes.
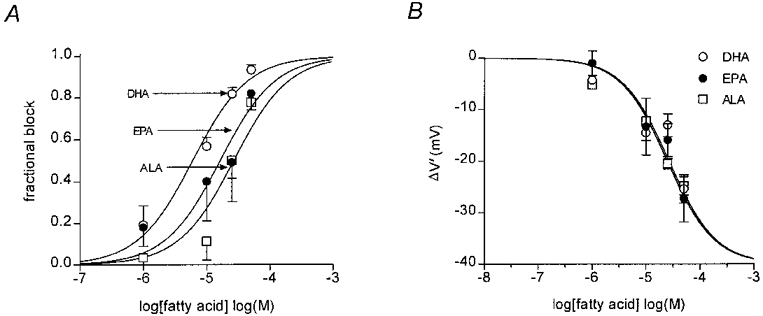
A, concentration-response curves for the n-3 PUFAs, DHA, EPA and ALA. The degree of block of the sodium current for each cell was measured as a fraction of the control current, to compensate for differences in peak current amplitude due to differences in cell size. The lines show the least squares fit using the Hill equation (eqn (3)), which gave EC50 values of 6.0 ± 1.2 μM (mean ±s.e.m., n = 5), 16.2 ± 1.3 μM (n = 5) and 26.6 ± 1.3 μM (n = 6) for DHA (^), EPA (•) and ALA (□), respectively. B, dose-response relationship for shift in inactivation (Δ_V′_) and fatty acid concentration.
Adult rat cardiac myocyte membrane fluidity
The effect of acute addition of fatty acids on cardiac myocyte membrane fluidity was determined by steady-state fluorescence anisotropy using the probe TMAP-DPH. The fluorescence anisotropy (_r_ss) following incubation in stearic acid (20 μM; n = 6), oleic acid (20 μM; n = 10) or linoleic acid (20 μM; n = 7) was not significantly different from control (no fatty acid additions, n = 23, Fig. 6). However, _r_ss was significantly decreased following incubation with (20 μM) DHA (0.199 ± 0.004, n = 21, P < 0.01), EPA (0.204 ± 0.006, n = 9, P < 0.01), or ALA (0.213 ± 0.005, n = 11, P < 0.01) compared with control (0.239 ± 0.003). These data indicate that cardiac myocyte membrane fluidity was increased following acute addition of DHA, EPA and ALA. In addition, the fluorescence anisotropy of TMAP-DPH was significantly decreased to 0.214 ± 0.009 (indicative of an increase in membrane fluidity) following addition of the membrane fluidising agent benzyl alcohol as shown in Fig. 6.
Figure 6. Effect of fatty acids on adult rat ventricular myocyte sarcolemmal membrane fluidity.
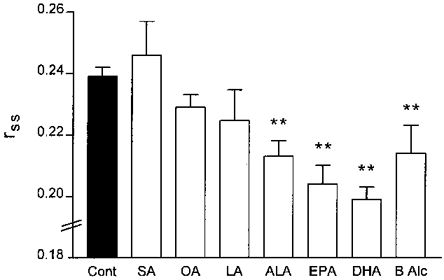
Ventricular myocytes plated on coverslips were incubated for 15 min with 1 μM TMAP-DPH at 37 °C. Cardiac myocytes were then transferred to a glass cuvette containing 20 μM fatty acids and the steady-state fluorescence anisotropy of TMAP-DPH was measured (as described in Methods). Data are presented as means ±s.e.m.**P < 0.01 compared with control. Abbreviations: Cont, control (no additions); SA, stearic acid (18:0); OA, oleic acid (18:1, n-9); LA, linoleic acid (18:2, n-6); ALA, α-linolenic acid (18:3, n-3); EPA, eicosapentaenoic acid (20:5, n-3); DHA, docosahexaenoic acid (22:6, n-3); B Alc, benzyl alcohol.
DISCUSSION
Polyunsaturated fatty acids, particularly of the n-3 class, are known to exert an anti-arrhythmic effect in a variety of in vitro and in vivo preparations (Leifert et al. 1999). This may in part be a consequence of an alteration in the electrophysiology of the myocardium, since a reduction in excitability of the myocardium (either in the intact heart or in single cardiac myocytes) is the most often observed effect. While it has been shown that PUFAs can influence a range of ionic currents in cardiac myocytes, for example, Ca2+ current (Pepe et al. 1994) and transient outward K+ current (Bogdanov et al. 1998), one of the most powerful means of producing a reduction in cell excitability is by block of voltage-dependent sodium channels, which produces an increase in threshold for action potential generation. Indeed, voltage-dependent sodium channel blockade is the principal mechanism of action of class I anti-arrhythmic agents such as lidocaine (lignocaine).
This study shows that acute addition of micromolar concentrations of n-3 PUFAs to adult rat ventricular myocytes rapidly blocks the sodium current and shifts the voltage dependence of inactivation to more hyperpolarised potentials. In the presence of 25 μM DHA, the most potent of the n-3 PUFAs tested, the maximum conductance was reduced by 50 %, and the voltage dependence of inactivation shifted by about 20 mV. These effects were also apparent for the other n-3 PUFAs tested, EPA and to a lesser extent, ALA. The non n-3 fatty acids stearic, linoleic and oleic acid were without effect on most of the activation and inactivation parameters associated with the sodium current. The fatty acids presumably exert their effects by dissolving in the cell membrane, since washing with control solution failed to reverse their effects, but perfusion with medium containing delipidated BSA did reverse the effects (presumably by removing membrane-associated PUFAs because of the high affinity of BSA for fatty acids).
The mechanism(s) by which n-3 PUFAs may effect cardiac sodium currents are unclear. The spectrum of changes produced by the n-3 PUFAs (a reduction in maximum current, a slight shift in the voltage dependence of activation, and a substantial shift in the voltage dependence of inactivation), is similar to that observed with certain general anaesthetic agents (Saint, 1998). It has been suggested that the effects of general anaesthetics on ion channel activity may be induced by alterations in membrane physical properties, particularly fluidity (Haydon & Urban, 1983), leading to the hypothesis that fatty acids may similarly affect sodium channels via a change in membrane fluidity. This effect seems plausible if, as has been suggested by others (Tan & Weaver, 1997), it is a change in the physical properties at the interface between the channel protein and the surrounding membrane lipid environment which is responsible for the change in channel properties. Consistent with this notion, the fatty acids increased cardiac myocyte membrane fluidity in this study, as measured by steady-state fluorescence anisotropy, in a similar rank order potency to their effect on the sodium current. The n-3 PUFAs were the most potent inhibitors of the sodium current while fatty acids of other classes were much less potent. Within the n-3 PUFAs examined, DHA was the most potent and ALA the least potent with regards to effects on both membrane fluidity and sodium current block. The results therefore suggest that the n-3 PUFAs induce a block of ventricular myocyte sodium currents which may in part be associated with changes in membrane lipid physical properties, since one would anticipate that protein molecules embedded in the membrane would be influenced by the properties of the membrane lipids which surround them (McMurchie, 1988; McMurchie et al. 1997; Tan & Weaver, 1997). This conclusion was supported by the data on the well known membrane fluidising agent benzyl alcohol (Staiman & Seeman, 1975; Sinicrope et al. 1992), a molecule unrelated in structure to fatty acids, which increased membrane fluidity and produced similar effects on the sodium currents. Our conclusion is also consistent with results which reported that changing membrane lipid content, and thus membrane fluidity, can modify the function of ion channels such as Ca2+-activated K+ channels in smooth muscle cells (Bregestovski & Bolotina, 1989).
As noted above, the original impetus for work on the effects of fatty acids in the heart came from epidemiological and dietary studies. Dietary fatty acids are incorporated into the phospholipid structure of the membrane, rather than being free in solution, but nevertheless appear to exert an anti-arrhythmic effect. A possible mechanism is that the PUFAs incorporated into the membrane phospholipids may provide a pool of n-3 PUFAs that are made available by the action of phospholipases activated in ischaemia or in other circumstances. It is known that the accumulation of free fatty acids which occurs early during myocardial ischaemia depends upon activation of phospholipase A2, and it has been estimated that the free fatty acid concentration following their release from membrane phospholipids may result in a concentration of 20 μM in the rat heart after 1 h of ischaemia (Van der Vusse et al. 1997). Hence, the concentrations of PUFAs used in this study (1–50 μM) are similar to those found in the ischaemic myocardium, and the release of fatty acids from the membrane by phospholipases may explain the anti-arrhythmic effects of dietary PUFAs.
Acknowledgments
This work was supported by the National Health and Medical Research Council of Australia (D.A.S.) and the CSIRO Human Nutrition/University of Adelaide Collaborative Grants Program (E.J.M. and D.A.S.). W.R.L. was supported by a National Heart Foundation of Australia Research Scholarship.
References
- Billman GE, Hallaq H, Leaf A. Prevention of ischemia-induced ventricular fibrillation by omega 3 fatty acids. Proceedings of the National Academy of Sciences of the USA. 1994;91:4427–4430. doi: 10.1073/pnas.91.10.4427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billman GE, Kang JX, Leaf A. Prevention of ischemia-induced cardiac sudden death by n-3 polyunsaturated fatty acids in dogs. Lipids. 1997;32:1161–1168. doi: 10.1007/s11745-997-0149-2. [DOI] [PubMed] [Google Scholar]
- Bogdanov KY, Spurgeon HA, Vinogradova TM, Lakatta EG. Modulation of the transient outward current in adult rat ventricular myocytes by polyunsaturated fatty acids. American Journal of Physiology. 1998;274:H571–579. doi: 10.1152/ajpheart.1998.274.2.H571. [DOI] [PubMed] [Google Scholar]
- Bregestovski PD, Bolotina VM. Membrane fluidity and kinetics of Ca2+-dependent potassium channels. Biomedica et Biochimica Acta. 1989;48:S382–387. [PubMed] [Google Scholar]
- Burr ML, Fehily AM, Gilbert JF, Rogers S, Holliday RM, Sweetnam PM, Elwood PC, Deadman NM. Effects of changes in fat, fish, and fibre intakes on death and myocardial reinfarction: diet and reinfarction trial (DART) Lancet. 1989;334:757–761. doi: 10.1016/s0140-6736(89)90828-3. [DOI] [PubMed] [Google Scholar]
- de Jonge HW, Dekkers DH, Bastiaanse EM, Bezstarosti K, van der Laarse A, Lamers JM. Eicosapentaenoic acid incorporation in membrane phospholipids modulates receptor-mediated phospholipase C and membrane fluidity in rat ventricular myocytes in culture. Journal of Molecular and Cellular Cardiology. 1996;28:1097–1108. doi: 10.1006/jmcc.1996.0101. [DOI] [PubMed] [Google Scholar]
- Haydon DA, Urban BW. The action of alcohols and other non-ionic surface active substances on the sodium current of the squid giant axon. The Journal of Physiology. 1983;341:411–427. doi: 10.1113/jphysiol.1983.sp014813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hock CE, Beck LD, Bodine RC, Reibel DK. Influence of dietary n-3 fatty acids on myocardial ischemia and reperfusion. American Journal of Physiology. 1990;259:H1518–1526. doi: 10.1152/ajpheart.1990.259.5.H1518. [DOI] [PubMed] [Google Scholar]
- Kang JX, Leaf A. Effects of long-chain polyunsaturated fatty acids on the contraction of neonatal rat cardiac myocytes. Proceedings of the National Academy of Sciences of the USA. 1994;91:9886–9890. doi: 10.1073/pnas.91.21.9886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JX, Leaf A. Prevention and termination of beta-adrenergic agonist-induced arrhythmias by free polyunsaturated fatty acids in neonatal rat cardiac myocytes. Biochemical and Biophysical Research Communications. 1995;208:629–636. doi: 10.1006/bbrc.1995.1385. [DOI] [PubMed] [Google Scholar]
- Kang JX, Leaf A. Protective effects of free polyunsaturated fatty acids on arrhythmias induced by lysophosphatidylcholine or palmitoylcarnitine in neonatal rat cardiac myocytes. European Journal of Pharmacology. 1996;297:97–106. doi: 10.1016/0014-2999(95)00701-6. [DOI] [PubMed] [Google Scholar]
- Kang JX, Xiao YF, Leaf A. Free, long-chain, polyunsaturated fatty acids reduce membrane electrical excitability in neonatal rat cardiac myocytes. Proceedings of the National Academy of Sciences of the USA. 1995;92:3997–4001. doi: 10.1073/pnas.92.9.3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kromhout D, Bosschieter EB, de Lezenne Coulander C. The inverse relation between fish consumption and 20-year mortality from coronary heart disease. New England Journal of Medicine. 1985;312:1205–1209. doi: 10.1056/NEJM198505093121901. [DOI] [PubMed] [Google Scholar]
- Leifert WR, Jahangiri A, McMurchie EJ. Antiarrhythmic fatty acids and antioxidants in animal and cell studies (Review) Journal of Nutritional Biochemistry. 1999;10:252–267. doi: 10.1016/s0955-2863(99)00011-x. [DOI] [PubMed] [Google Scholar]
- Lentz BR. Membrane ‘fluidity’ as detected by diphenylhexatriene probes. Chemistry and Physics of Lipids. 1989;50:171–190. [Google Scholar]
- McLennan PL, Abeywardena MY, Charnock JS. Dietary fish oil prevents ventricular fibrillation following coronary artery occlusion and reperfusion. American Heart Journal. 1988;116:709–717. doi: 10.1016/0002-8703(88)90328-6. [DOI] [PubMed] [Google Scholar]
- McLennan P, Howe P, Abeywardena M, Muggli R, Raederstorff D, Mano M, Rayner T, Head R. The cardiovascular protective role of docosahexaenoic acid. European Journal of Pharmacology. 1996;300:83–89. doi: 10.1016/0014-2999(95)00861-6. [DOI] [PubMed] [Google Scholar]
- McMurchie EJ. Dietary lipids and the regulation of membrane fluidity and function. In: Aloia RC, Curtain CC, Gordon LM, editors. Physiological Regulation of Membrane Fluidity. New York: Alan R. Liss Inc.; 1988. pp. 189–237. [Google Scholar]
- McMurchie EJ, Leifert WR, Head RJ. Antiarrhythmic properties of polyunsaturated fatty acids in adult rat cardiac myocytes: a role for membrane fluidity. In: Riemersma RA, Armstrong RA, Kelly RW, Wilson R, editors. Essential Fatty Acids and Eicosanoids. Champaign, Illinois, USA.: AOCS Press; 1999. pp. 284–289. [Google Scholar]
- McMurchie EJ, Leifert WR, McLennan PL, Head RJ. Antiarrhythmic properties of polyunsaturated fatty acids in adult rat cardiac myocytes: a role for membrane fluidity? Prostaglandins Leukotrienes and Essential Fatty Acids. 1997;57:193. [Google Scholar]
- Matsubara H, Suzuki J, Inada M. Shaker-related potassium channel, Kv1.4, mRNA regulation in cultured rat heart myocytes and differential expression of Kv1.4 and Kv1.5 genes in myocardial development and hypertrophy. Journal of Clinical Investigation. 1993;92:1659–1666. doi: 10.1172/JCI116751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuss HB, Marban E. Electrophysiological properties of neonatal mouse cardiac myocytes in primary culture. The Journal of Physiology. 1994;479:265–279. doi: 10.1113/jphysiol.1994.sp020294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepe S, Bogdanov K, Hallaq H, Spurgeon H, Leaf A, Lakatta E. Omega 3 polyunsaturated fatty acid modulates dihydropyridine effects on L-type Ca2+ channels, cytosolic Ca2+, and contraction in adult rat cardiac myocytes. Proceedings of the National Academy of Sciences of the USA. 1994;91:8832–8836. doi: 10.1073/pnas.91.19.8832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepe S, McLennan PL. Dietary fish oil confers direct antiarrhythmic properties on the myocardium of rats. Journal of Nutrition. 1996;126:34–42. doi: 10.1093/jn/126.1.34. [DOI] [PubMed] [Google Scholar]
- Roden DM, George AL., Jr Structure and function of cardiac sodium and potassium channels. American Journal of Physiology. 1997;273:H511–525. doi: 10.1152/ajpheart.1997.273.2.H511. [DOI] [PubMed] [Google Scholar]
- Sada H, Ban T, Fujita T, Ebina Y, Sperelakis N. Developmental change in fast Na channel properties in embryonic chick ventricular heart cells. Canadian Journal of Physiology and Pharmacology. 1995;73:1475–1484. doi: 10.1139/y95-205. [DOI] [PubMed] [Google Scholar]
- Saint DA. The effects of propofol on macroscopic and single channel sodium currents in rat ventricular myocytes. British Journal of Pharmacology. 1998;124:655–662. doi: 10.1038/sj.bjp.0701876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint DA, Ju Y-K, Gage PW. A persistent sodium current in cardiac myocytes. The Journal of Physiology. 1992;453:219–231. doi: 10.1113/jphysiol.1992.sp019225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinicrope FA, Dudeja PK, Bissonnette BM, Safa AR, Brasitus TA. Modulation of P-glycoprotein-mediated drug transport by alterations in lipid fluidity of rat liver canalicular membrane vesicles. Journal of Biological Chemistry. 1992;267:24995–25002. [PubMed] [Google Scholar]
- Siscovick DS, Raghunathan TE, King I, Weinmann S, Wicklund KG, Albright J, Bovbjerg V, Arbogast P, Smith H, Kushi LH, Cobb LA, Copass MK, Psaty BM, Lemaitre R, Retzlaff B, Childs M, Knopp RH. Dietary intake and cell membrane levels of long-chain n-3 polyunsaturated fatty acids and the risk of primary cardiac arrest. Journal of the American Medical Association. 1995;274:1363–1367. doi: 10.1001/jama.1995.03530170043030. [DOI] [PubMed] [Google Scholar]
- Staiman AL, Seeman P. Different sites of membrane action for tetrodotoxin and lipid-soluble anesthetics. Canadian Journal of Physiology and Pharmacology. 1975;53:513–524. doi: 10.1139/y75-073. [DOI] [PubMed] [Google Scholar]
- Tan CY, Weaver DF. Molecular pathogenesis of alcohol withdrawal seizures: the modified lipid-protein interaction mechanism. Seizure. 1997;6:255–274. doi: 10.1016/s1059-1311(97)80073-8. [DOI] [PubMed] [Google Scholar]
- Van der Vusse GJ, Reneman RS, Van Bilsen M. Accumulation of arachidonic acid in ischemic/reperfused cardiac tissue: possible causes and consequences. Prostaglandins Leukotrienes and Essential Fatty Acids. 1997;57:85–93. doi: 10.1016/s0952-3278(97)90497-x. [DOI] [PubMed] [Google Scholar]
- Vreugdenhil M, Bruehl C, Voskuyl RA, Kang JX, Leaf A, Wadman WJ. Polyunsaturated fatty acids modulate sodium and calcium currents in CA1 neurons. Proceedings of the National Academy of Sciences of the USA. 1996;93:12559–12563. doi: 10.1073/pnas.93.22.12559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Feng ZP, Kondo CS, Sheldon RS, Duff HJ. Developmental changes in the delayed rectifier K+ channels in mouse heart. Circulation Research. 1996;79:79–85. doi: 10.1161/01.res.79.1.79. [DOI] [PubMed] [Google Scholar]
- Wetzel GT, Chen F, Klitzner TS. Ca2+ channel kinetics in acutely isolated fetal, neonatal, and adult rabbit cardiac myocytes. Circulation Research. 1993;72:1065–1074. doi: 10.1161/01.res.72.5.1065. [DOI] [PubMed] [Google Scholar]
- Xiao YF, Kang JX, Morgan JP, Leaf A. Blocking effects of polyunsaturated fatty acids on Na+ channels of neonatal rat ventricular myocytes. Proceedings of the National Academy of Sciences of the USA. 1995;92:11000–11004. doi: 10.1073/pnas.92.24.11000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao YF, Wright SN, Wang GK, Morgan JP, Leaf A. Fatty acids suppress voltage-gated Na+ currents in HEK293t cells transfected with the alpha-subunit of the human cardiac Na+ channel. Proceedings of the National Academy of Sciences of the USA. 1998;95:2680–2685. doi: 10.1073/pnas.95.5.2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu YQ, Pickoff AS, Clarkson CW. Evidence for developmental changes in sodium channel inactivation gating and sodium channel block by phenytoin in rat cardiac myocytes. Circulation Research. 1991;69:644–656. doi: 10.1161/01.res.69.3.644. [DOI] [PubMed] [Google Scholar]
- Xu YQ, Pickoff AS, Clarkson CW. Developmental changes in the effects of lidocaine on sodium channels in rat cardiac myocytes. Journal of Pharmacology and Experimental Therapeutics. 1992;262:670–676. [PubMed] [Google Scholar]