Plexin signaling selectively regulates the stereotyped pruning of corticospinal axons from visual cortex (original) (raw)
Abstract
Neurons in the developing CNS tend to send out long axon collaterals to multiple target areas. For these neurons to attain specific connections, some of their axon collaterals are subsequently pruned—a process called stereotyped axon pruning. One of the most striking examples of stereotyped pruning in the CNS is the pruning of corticospinal tract (CST) axons. The long CST collaterals from layer V neurons of the visual and motor cortices are differentially pruned during development. Here we demonstrate that select plexins and neuropilins, which serve as coreceptors for semaphorins, are expressed in visual cortical neurons at the time when CST axon collaterals are stereotypically pruned. By analyzing mutant mice, we find that the pruning of visual, but not motor, CST axon collaterals depends on plexin-A3, plexin-A4, and neuropilin-2. Expression pattern study suggests that Sema3F is a candidate local cue for the pruning of visual CST axons. Using electron microscopic analysis, we also show that visual CST axon collaterals form synaptic contacts in the spinal cord before pruning and that the unpruned collaterals in adult mutant mice are unmyelinated and maintain their synaptic contacts. Our results indicate that the stereotyped pruning of the visual and motor CST axon collaterals is differentially regulated and that this specificity arises from the differential expression of plexin receptors in the cortex.
Keywords: axon pruning, corticospinal tract, plexin
A functional nervous system depends on the precise wiring of neuronal connections with appropriate targets. During early development, neurons tend to send out axons with excessive branches to multiple target areas. When the neuronal targets become mature, the unnecessary branches are specifically pruned. Stereotyped axon pruning, or pruning of long axon collaterals in a predictable manner, is a major phenomenon in the developing CNS. This type of pruning has been observed in species ranging from Drosophila to mouse and is thought to be essential for the normal development of the CNS (1–5).
One classic example of stereotyped pruning in higher vertebrates is in the developing corticospinal tract (CST) (6–10). In developing rodents, CST axons originate from layer V cortical pyramidal neurons in all regions of the neocortex (9, 11). These axons are guided through the internal capsule, cerebral peduncle, and pyramidal tract and then turn dorsally to cross the midline at the pyramidal decussation before they reach the contralateral spinal cord (Fig. 1A). The targeting of primary CST axons to the spinal cord is followed by axon collateral branching to targets in the brainstem and spinal cord (Fig. 1B). This initial projection pattern of CST axons is later modified via stereotyped axon pruning as regions of the neocortex become specialized, and the rostral–caudal location of parent cells within the neocortex determines which axon collaterals are pruned (Fig. 1C). Thus, motor neurons in rostral cortex prune their axons from the superior and inferior colliculi, whereas visual neurons in caudal cortex prune their axons from the inferior colliculus and spinal cord (9, 12). Several neuronal cell-type specification genes such as Ctip2 and Fezl have been shown to play roles in the development of layer V cortical neurons, but these genes seem to be required irrespective of the locations of these neurons in the neocortex (13–15). The transcription factor Otx1 has been implicated in stereotyped pruning of the visual CST (16). However, whether the pruning of exuberant CST axon collaterals is directed by local signals or is preprogrammed is not known. Because the differential pruning of visual and motor CST axon collaterals occurs simultaneously, it is also important to know whether the two pruning processes are controlled by the same mechanisms.
Fig. 1.
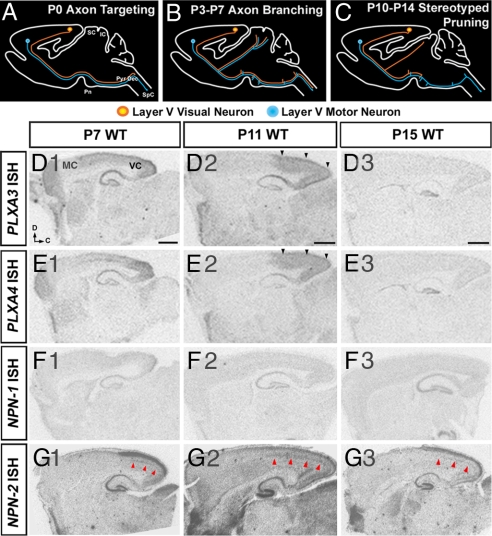
Plexin and neuropilin expression in the neocortex during CST pruning. (A–C) Diagrams of sagittal views of the brain representing different stages of CST development: axon targeting by P0, axon branching between P3 and P7, and stereotyped axon pruning between P10 and P14. (D and E) PLXA3 and PLXA4 mRNAs are expressed throughout the cortex at P7, and the expression becomes restricted to visual cortex by P11 (black arrowheads). (F) NPN-1 mRNA is not expressed in the neocortex between P7 and P15. (G) NPN-2 mRNA is expressed in the superficial and deep layers (red arrowheads) of the visual cortex between P7 and P15. D, dorsal; C, caudal; IC, inferior colliculus; MC, motor cortex; Pn, pons; Pyr Dec, pyramidal decussation; SC, superior colliculus; SpC, spinal cord; VC, visual cortex. (Scale bars: 1,000 μm.)
Here we report that semaphorin signaling through plexin-A3 (PLXA3) and plexin-A4 (PLXA4) regulates the stereotyped pruning of the visual CST. The plexins are a family of axon guidance molecules that serve as receptors for semaphorin ligands (17–19). Most secreted semaphorins (class 3) interact with plexins through the coreceptors neuropilin-1 (NPN-1) and neuropilin-2 (NPN-2), whereas membrane-bound semaphorins (classes 4–7) can interact directly with plexins (18). Semaphorin signaling through plexins has been associated with several aspects of neuronal development, including stereotyped pruning of the infrapyramidal bundle in the hippocampus (17–21). We find that PLXA3, PLXA4, and NPN-2 are required for the stereotyped removal of visual, but not motor, CST axon collaterals during postnatal development. We also find that Sema3F is expressed specifically in the dorsal spinal cord and inferior colliculus and may interact with the plexin and neuropilin coreceptors to initiate visual CST axon pruning.
Results
The Expression of Plexins and Neuropilins in Layer V Cortical Neurons Coincides with Visual CST Axon Pruning.
Previous studies have shown that Sema3F signaling through PLXA3, PLXA4, and NPN-2 regulates the stereotyped pruning of the infrapyramidal bundle in the hippocampus (20, 22–25). To address whether semaphorin signaling through PLXA3 and PLXA4 could also regulate the pruning of the CST, we analyzed the mRNA expression patterns of PLXA3 and PLXA4, as well as the neuropilins NPN-1 and NPN-2, in the neocortex. Because exuberant CST axon collaterals are pruned in the second week of postnatal development (4, 11, 12, 26), we focused our expression studies on this time window.
PLXA3 and PLXA4 were expressed throughout the cortex at postnatal day 7 (P7) (Fig. 1 D1 and E1). Between P7 and P11, over approximately the same time window at which stereotyped pruning of visual CST axons begins, their expression became restricted to the visual cortex (Fig. 1 D2 and E2). The levels of PLXA3 and PLXA4 expression were down-regulated thereafter (Fig. 1 D3 and E3). In addition, the expression of NPN-2 was elevated in layers II/III and V of the visual cortex after P7 [Fig. 1G and supporting information (SI) Fig. S1_O_], which coincides with the restricted expression of PLXA3 and PLXA4 in the visual cortex. In contrast, no expression of NPN-1 in the neocortex was detected in the second postnatal week (Fig. 1F).
CST projections arise predominantly from type I layer V cortical neurons (9, 27), which specifically express Ctip2 (13). PLXA3 and PLXA4 are broadly expressed in all layers of the visual cortex, and we found that a majority of Ctip2-immunopositive visual pyramidal neurons coexpressed mRNA for NPN-2 at P7 (Fig. S1_O_). These data suggest that plexins and neuropilins could regulate the pruning of visual CST collaterals. To test this, we investigated whether the pruning of CST axons was affected in mutant mice. As a control, we first explored whether regional as well as layer-specific patterning of the neocortex were altered in PLXA3 and PLXA4 mutant mice (throughout the text, PLXA3−/−, PLXA4−/−, and PLXA3/PLXA4−/− indicate PLXA3 and PLXA4 single and double knockouts, respectively), and we observed no patterning defects in PLXA3/PLXA4−/− mice compared with WT animals (n = 2; Fig. S1).
PLXA3 and PLXA4 Are Required for the Stereotyped Pruning of Visual CST Axon Collaterals.
Given the specific expression of PLXA3 and PLXA4 in the visual cortex after P7, we predicted that these two genes participate in the pruning of visual CST axons from the spinal cord and inferior colliculus, but not of motor CST axons from the superior and inferior colliculi. To test this hypothesis, we performed retrograde and anterograde tracing experiments in mutant mice. We injected the retrograde tracer cholera toxin subunit b (CTB) into the dorsal cervical spinal cord of P8 WT mice before pruning (n = 3) and P25 WT (n = 3) and PLXA3/PLXA4−/− (n = 4) mice after pruning to localize the distribution of retrogradely labeled layer V neurons in the cortex (Fig. 2A). CTB-labeled cells were visible in layer V of the entire neocortex of P8 WT mice but were absent from the visual cortex of P25 WT mice (Fig. 2 B, C, E, and F), demonstrating that layer V visual neurons prune their projections from the spinal cord by P25. In contrast, CTB-labeled neurons in layer V were visible throughout the cortex of P25 PLXA3/PLXA4−/− mice (Fig. 2 D and G), indicating that neurons in the visual cortex fail to prune their axons from the spinal cord in P25 PLXA3/PLXA4−/− mice.
Fig. 2.
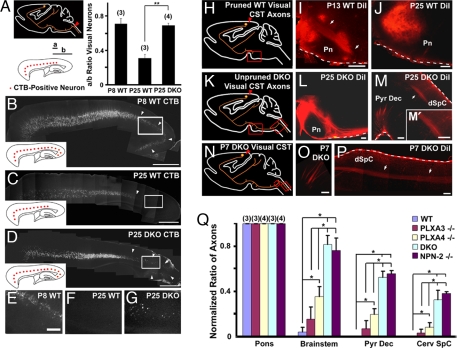
PLXA3 and PLXA4 are required for the stereotyped pruning of visual corticospinal axons from the spinal cord. (A–G) Layer V neurons in the visual cortex (orange) were retrogradely labeled by injecting CTB into the dorsal cervical spinal cord (red arrowhead in A). The distribution of labeled neurons in the visual cortex is normalized for comparison as diagramed in A (a/b ratio; see Methods). A Right shows that visual CST axons are not pruned from the spinal cord of P25 PLXA3/PLXA4−/− (DKO) mice (mean ± SEM; n values are indicated in parentheses). ∗∗, P < 0.01 (Student's t test). Representative images of retrogradely labeled neurons in the cortex for each set of experiments are shown in B–D. The Inset in each image summarizes the distribution of CTB-positive neurons (red) in the cortex, and the white arrowheads indicate the distribution of neurons in the visual cortex. Higher-magnification images taken from boxed regions in B–D are shown in E–G, respectively. (H–P) Anterograde tracing of axons from the visual cortex in WT and DKO mice. Red arrowheads in H, K, and N indicate the injection sites. DiI-labeled axons in WT mice are in the process of pruning at P13 (arrows in I), and at P25 axons terminate in the rostral pons (J). DiI-labeled axons in P25 DKO animals extend beyond the rostral pons (L), cross to the contralateral spinal cord at the pyramidal decussation, and terminate in the spinal cord (M). Unpruned axons in the DKO spinal cord (arrow) are shown in higher magnification in (M′). DiI-labeled axons in P7 DKO animals (arrows) grow normally into the pyramidal decussation (O) and dorsal spinal cord (P). White dashes in I, J, L, M, and P) indicate meninges and do not represent positive DiI labeling. (Q) A comparison of normalized ratios of BDA-labeled visual CST axons (mean ± SEM) present at the pons, brainstem, pyramidal decussation (Pyr Dec), and cervical spinal cord (Cerv SpC) in mice aged P30–P35 (see Methods). When compared with WT mice, significant pruning defects are found in PLXA4−/−, DKO, and NPN-2−/− mice [∗, P < 0.05 (ANOVA, Newman–Keuls test)], but not in PLXA3−/− mice. The defects in DKO and NPN-2−/− mice are also more severe than in PLXA3−/− or PLXA4−/− mice [∗, P < 0.05 (ANOVA, Newman–Keuls test)] at all levels beyond rostral pons. n values are indicated in parentheses. dSpC, dorsal spinal cord; Pn, pons. [Scale bars: 1,000 μm (B–D) and 200 μm (E–G, I, J, L, M, O, and P).]
To study this aberrant CST projection in PLXA3/PLXA4−/− mice further, we performed anterograde tracing experiments in WT and mutant mice during and after pruning. When we injected 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI) into the visual cortex of WT mice (Fig. 2H), we found that CST axons were in the process of pruning at P13 (n = 4; Fig. 2I) and that pruning had been completed by P25 (n = 4; Fig. 2J). In contrast, when we analyzed P25 PLXA3/PLXA4−/− mice with DiI injections in visual cortex (n = 4; Fig. 2K), we observed a large bundle of axons that extended beyond the rostral pons (Fig. 2L) and terminated at cervical and upper thoracic spinal cord levels (Fig. 2M). In addition, we observed numerous unpruned axons in the inferior colliculus of all P25 PLXA3/PLXA4−/− mice analyzed (n = 4; data not shown). However, in P25 WT control mice, some axonal labeling was also noted in the inferior colliculus (n = 2 of four mice). The number of labeled axons in the inferior colliculus of WT mice was visibly less than that in mutant mice. Because of the anatomical proximity of the visual and auditory cortices, the required amount of tracer injection into the visual cortex for quantification often resulted in a significant spillover of the tracer to the neighboring auditory cortex, which normally targets the inferior colliculus. Because of this limitation, we were unable to reach a quantitative conclusion, but the analysis is indicative of a pruning defect from the inferior colliculus of PLXA3/PLXA4−/− mice as well.
We next analyzed the visual CST axons in PLXA3/PLXA4−/− mice before pruning to assess whether the growth and pruning of the axons was simply delayed. At P7, PLXA3/PLXA4−/− mice with injections of DiI into the visual cortex have visual CST axons in the pyramidal decussation and spinal cord as seen in WT mice (Fig. 2 N–P). This indicates that the growth and pruning of visual CST axons are not simply delayed, but that pruning is truly defective in PLXA3/PLXA4−/− mice.
To analyze the respective contributions of PLXA3 and PLXA4 for the pruning of visual CST axons from the spinal cord in vivo, we injected biotinylated dextran amine (BDA) into the visual cortex of WT (n = 3), PLXA3−/− (n = 3), PLXA4−/− (n = 4), and PLXA3/PLXA4−/− (n = 3) mice older than P25 to anterogradely label the CST axons. Because the visual CST axons in WT adult animals are pruned back to the rostral pons, we quantified the severity of the defect by obtaining counts for the relative number of axons that progressed beyond the rostral pons in mutant mice (Fig. 2Q; see Methods). No significant differences were observed between WT and PLXA3−/− mice. However, PLXA4−/− mice exhibited significantly higher relative numbers of axons beyond the rostral pons compared with WT mice. In addition, the pruning defect was more severe in PLXA3/PLXA4−/− mice (Fig. 2Q), indicating that both PLXA3 and PLXA4 contribute to the pruning of visual CST axons from the spinal cord but that PLXA4 is preferentially required in vivo.
PLXA3 and PLXA4 Are Not Required for the Stereotyped Pruning of Motor CST Axon Collaterals.
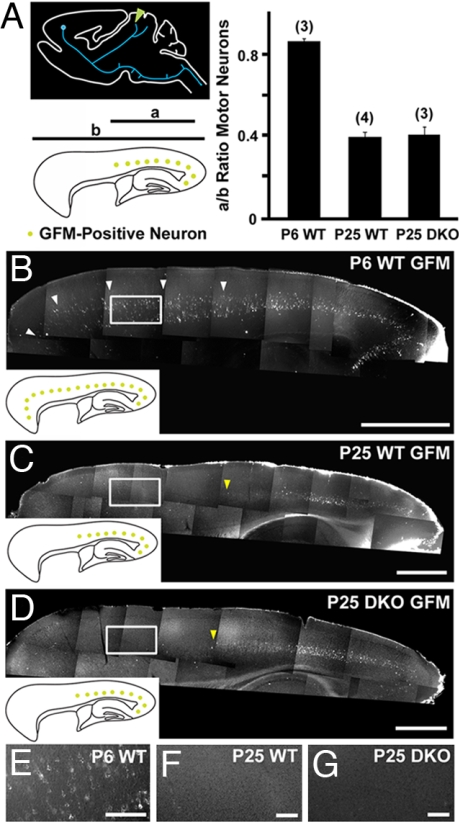
To test whether PLXA3 and PLXA4 regulate the stereotyped pruning of motor CST axons, we injected green fluorescent microsphere (GFM) retrograde tracers into the superior colliculus to examine the distribution of layer V motor neurons that extend transient axon collaterals to the superior colliculus (Fig. 3A). In WT mice, layer V neurons in rostral and caudal regions of the cortex were retrogradely labeled with GFM at P6 (n = 3; Fig. 3 B and E). However, at P25 (n = 4) only layer V cells in the caudal half of the cortex were labeled (Fig. 3 C and F). In PLXA3/PLXA4−/− mice (n = 3), GFM-labeled layer V cells were absent from more rostral regions of the cortex at P25, as we had observed in WT mice (Fig. 3 D and G). In addition, we carried out anterograde injections of BDA in the motor cortex of WT (n = 3) and PLXA3/PLXA4−/− (n = 3) mice at P20 and observed no BDA-labeled axons in the colliculi in these mice (data not shown). Thus, both anterograde and retrograde labeling results confirmed that stereotyped pruning of motor CST axon collaterals from the midbrain was normal in PLXA3/PLXA4−/− mice.
Fig. 3.
PLXA3 and PLXA4 are not required for the stereotyped pruning of motor corticospinal axons from the superior colliculus. (A) Layer V neurons in the motor cortex (blue) were retrogradely labeled by injection of GFM in the superior colliculus (green arrowhead). The distribution of labeled neurons in the cortex is normalized for comparison as diagramed (a/b ratio; see Methods). The bar graph indicates no significant motor CST pruning defects from the superior colliculus of PLXA3/PLXA4−/− (DKO) mice (mean ± SEM; n values are indicated in parentheses). Representative images of retrogradely labeled neurons in the cortex for each set of experiments are shown in B–D. The Inset in each image summarizes the distribution of CTB-positive neurons (green) in the cortex. Layer V motor neurons that extend transient projections to the superior colliculus at P6 are indicated by white arrowheads (B). Yellow arrowheads (C and D) mark the border of GFM-positive neurons. Higher-magnification images taken from boxed regions in B–D are shown in E–G, respectively. [Scale bars: 1,000 μm (B–D) and 200 μm (E–G).]
NPN-2 Is Required for the Stereotyped Pruning of Visual CST Axon Collaterals.
An increase in NPN-2 expression in the visual cortex after P7 (Fig. 1G and Fig. S1_O_) implies that NPN-2 may interact with PLXA3 and PLXA4 to mediate visual CST axon pruning through semaphorin signals. An analysis of BDA-injected NPN-2 mutant (NPN-2−/−) mice at P25 and older (n = 4) revealed a large percentage of visual CST axons that extended beyond the rostral pons and into the spinal cord (Fig. 4A). NPN-2−/− pruning defects were as severe as those observed in PLXA3/PLXA4−/− mice, suggesting that NPN-2 regulated stereotyped pruning by serving as a coreceptor for both plexin receptors (Fig. 2Q).
Fig. 4.
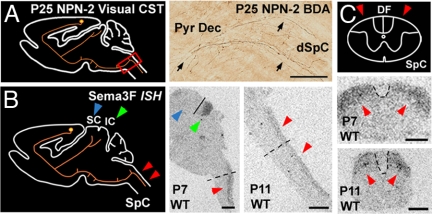
Stereotyped pruning of the visual CST is likely to be initiated by Sema3F signaling through PLXA3 and PLXA4 and their coreceptor, NPN-2. (A) Diagram and image showing unpruned BDA-positive visual CST axons (black arrows) in the pyramidal decussation (Pyr Dec) and dorsal cervical spinal cord (dSpC) of P25 NPN-2−/− mice. (B) Sema3F mRNA expression is observed in the transient targets of visual CST axon collaterals at the inferior colliculus (green arrowheads) and dorsal spinal cord (red arrowheads) of P7 and P11 WT mice. A line is shown separating the superior and inferior colliculus. Sema3F mRNA is absent from the superior colliculus (blue arrowhead), which retains its visual CST axon collaterals. Dashed lines indicate the regions from which transverse sections in C are taken. (C) Sema3F mRNA expression in the dorsal regions of transverse sections of the spinal cord at P7 and P11 (red arrowheads). Dashed lines outline the dorsal funiculus, where CST axons are located. DF, dorsal funiculus; IC, inferior colliculus; SC, superior colliculus; SpC, spinal cord. [Scale bars: 100 μm (A), 1,000 μm (B), and 500 μm (C).]
We next examined the expression patterns of all class 3 semaphorins that could potentially interact with NPN-2 in the dorsal spinal cord. We found that only Sema3F mRNA was expressed in the superficial one-third of the dorsal spinal cord from P3–P15 in WT mice, suggesting that Sema3F is involved in initiating the pruning of visual CST axons (Fig. 4 B and C and data not shown). We also discovered that Sema3F was expressed in the inferior colliculus, another transient target of visual CST axon collaterals (Fig. 4B and data not shown). In contrast, Sema3F is not expressed in the superior colliculus where visual CST axons are retained (Fig. 4B). Taken together, our results suggest that NPN-2 serves as a coreceptor to PLXA3 and PLXA4 in CST axon remodeling in the spinal cord and suggest a possible role of Sema3F as a candidate ligand for these receptors.
Unpruned Visual CST Axon Collaterals in PLXA3/PLXA4−/− Mice Are Unmyelinated and Retain Their Synaptic Contacts in the Spinal Cord.
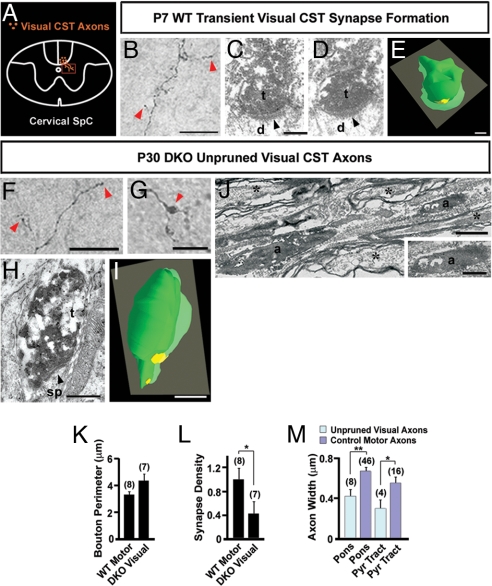
We have previously shown that hippocampal mossy fibers form synaptic contacts with their targets before pruning (24). However, it is still unclear whether transient CST axons in the spinal cord of WT mice form synaptic contacts with their targets. Using BDA axon tracing combined with EM, we analyzed the development of transient CST axons from the visual cortex of WT mice (Fig. 5A). BDA-labeled CST axons from the visual cortex were easily visible in the dorsal spinal cord by P7 (Fig. 5B). These transient CST axons were unmyelinated (data not shown), as are the majority of CST axons from motor cortex at this time (6, 9), and exhibited branches that terminated in bouton-like structures in the gray matter of the spinal cord (Fig. 5B). Serial section analysis of these boutons revealed that several (n = 5 of nine boutons) established asymmetric synaptic contacts with their targets (Fig. 5 C and D). Three-dimensional EM reconstruction of these boutons (n = 3) demonstrated that the perimeter of transient boutons and the length of synapses were consistent with those of motor CST axons in the gray matter at comparable ages (Fig. 5E). The formation of synaptic contacts suggests that transient axons from the visual cortex have the potential to communicate with these targets before stereotyped axon pruning.
Fig. 5.
EM analysis of visual CST axons in the spinal cord of WT and PLXA3/PLXA4−/− mice. (A) Diagram of a transverse cervical spinal cord section showing visual CST axons branching ventrally into the gray matter. The box indicates the location where the images in B, F, and G are taken. (B) A BDA-labeled transient visual CST axon in P7 WT mouse branches into the cervical spinal cord gray matter and exhibits bouton-like structures (red arrowheads). (C and D) Serial electron micrographs showing a BDA-labeled visual CST axon terminal (t) forming an asymmetric synapse (black arrowhead) with a dendrite (d) in the gray matter of the cervical spinal cord of P7 WT mouse. (E) Three-dimensional EM reconstruction of serial sections from a BDA-labeled terminal shown in C and D demonstrating an axon terminal (green) adjacent to a postsynaptic density (yellow). (F and G) Unpruned BDA-labeled visual CST axons are found in the dorsal CST of the spinal cord of P30 PLXA3/PLXA4−/− (DKO) mice. Axon branches within the gray matter contain bouton-like structures (red arrowheads). (H) An electron micrograph showing a BDA-positive visual CST axon terminal (t) in the gray matter of the spinal cord of a P30 DKO mouse. The BDA-labeled terminal contains vesicles that cluster adjacent to a postsynaptic density (black arrowhead) within a dendritic spine (sp). (I) Three-dimensional reconstruction of the axon terminal in H (green) adjacent to a postsynaptic density (yellow). (J) An electron micrograph showing unmyelinated, unpruned BDA-positive visual CST axons (a) adjacent to unlabeled myelinated axons (asterisks) within the brainstem. (K) A comparison of average bouton sizes (mean ± SEM) of adult WT motor and adult DKO visual axons in the spinal cord indicates no significant differences. (L) A comparison of average synapse number per bouton (mean ± SEM) for adult WT motor and adult DKO visual axonal boutons in the spinal cord indicates significantly fewer synapses made by unpruned adult DKO visual axonal boutons [∗, P ≤ 0.05 (Student's t test)]. (M) A comparison of average axon diameter (mean ± SEM) of BDA-positive unpruned visual axons in DKO animals and surrounding motor axons indicates that in the caudal pons and pyramidal tract (Pyr Tract) DKO unpruned visual axons are significantly smaller in size [∗, P < 0.05; ∗∗, P < 0.01 (Student's t test)]. n values are indicated in parentheses. [Scale bars: 50 μm (B), 0.25 μm (C–E), 25 μm (F and G), 0.5 μm (H, I, and Inset in J), and 1 μm (J).]
To examine the development of unpruned visual CST axons in mutant mice, we analyzed BDA-labeled visual CST axons in the caudal pons and pyramidal tract of P30 PLXA3/PLXA4−/− mice (n = 3) using EM. All BDA-labeled unpruned visual CST fibers were found to be unmyelinated (n = 8 BDA-labeled axons) (Fig. 5J) and were surrounded by large numbers of BDA-immunonegative, myelinated axons. A majority of motor CST axons were found to be myelinated in WT (>90%) and PLXA3/PLXA4−/− (>90%) mice, suggesting that a large percentage of the unpruned visual CST axons failed to develop normally. The diameter of the BDA-labeled, unmyelinated axons was significantly smaller than that of surrounding BDA-negative, myelinated axons in both the pons and pyramidal tract (Fig. 5M). Despite the unusual absence of myelin in unpruned axons, several were found to extend branches within the gray matter of the spinal cord and terminate in bouton-like expansions (Fig. 5 F and G). Three-dimensional EM reconstruction of these boutons and quantification of electron micrographs (n = 8 boutons) demonstrated that they were comparable in size to motor CST terminations in the spinal cord (Fig. 5K). Furthermore, we found that nearly half (n = 3 of seven boutons) of the unpruned visual CST boutons still made asymmetric synaptic contacts on dendritic profiles (Fig. 5 H and I). The number of synaptic contacts per bouton was significantly lower for unpruned visual CST axons in PLXA3/PLXA4−/− mice compared with motor CST boutons in WT animals (Fig. 5L). In summary, our results demonstrate that although nearly 50% of unpruned visual CST axons retain synapses in the spinal cord, these unpruned axons are likely to be physiologically impaired because they are unmyelinated.
Discussion
The development of the CST has served as a classical example for studying the stereotyped pruning of long-range axon collaterals (3, 4, 9). Our analysis of this system in mutant mice has identified specific roles for plexin/neuropilin signaling in controlling the stereotyped pruning of visual CST axons. We found that the pruning of visual CST collaterals from the spinal cord depends directly on PLXA3, PLXA4, and NPN-2 signaling. Sema3F is the only class 3 semaphorin expressed in the targets of visual CST axons before and during stereotyped axon pruning. Thus, an increased sensitivity to Sema3F within local targets of visual CST axon collaterals could initiate pruning (Fig. S2). This finding corroborates previous reports showing that this same set of ligand and receptors is required for the stereotyped pruning of hippocampal mossy fiber collaterals (20, 22–25). Quantitative analysis of the CST pruning defect in mutants revealed that PLXA3 and PLXA4 are differentially required in vivo and that PLXA4 plays a larger role in this system than PLXA3. We have identified similar preferential uses of these two plexins in regulating the in vivo guidance of peripheral sensory and sympathetic axons (28, 29), implying that differential usages of coexisting plexins are common in vivo. With the specific and diverse expression patterns of members of the semaphorin ligand and receptor families in the developing nervous system, our results further support the notion that semaphorin signaling plays essential roles in mediating stereotyped pruning throughout the CNS.
We also demonstrate that the local signals for the pruning of axon collaterals from visual and motor CST are distinct, even though the early projection patterns from the two cortical regions are indistinguishable. This specificity depends on the spatial and temporal expression patterns of plexins and neuropilins in the visual cortex, as well as semaphorin in the relevant subcortical regions during stereotyped axon pruning. The homeodomain transcription factor Otx1 was previously found to be expressed within all layer V cortical projection neurons during the pruning of CST axon collaterals (16). Because loss of Otx1 expression in mutant mice results in visual CST pruning defects within the spinal cord and midbrain, it will be interesting to determine whether Otx1 directly regulates the expression of PLXA3, PLXA4, and NPN-2 in the visual cortex. In addition, what factors could account for the pruning of motor CST axon collaterals from the superior and inferior colliculi? One possibility is that transcription factors related to Otx1 may determine the expression of other plexins or axon guidance receptors that trigger the specific pruning of motor CST axon collaterals. Alternatively, these motor collaterals may lack the receptors that allow axons to sense stabilizing trophic factors in the colliculi.
Visual CST axons form transient synaptic contacts with their targets in the spinal cord before pruning. When axonal remodeling is impaired in mutant mice, the synapses are retained. Unpruned visual CST axons in the spinal cords of plexin mutants are smaller in size and make fewer synaptic contacts than motor CST axons. In addition, these unpruned axons are unmyelinated even though CST axons from motor cortex are generally myelinated in adult mice (6, 9). This unexpected finding suggests that other factors in addition to synaptic contacts, and thus neuronal activity, may be required for the proper myelination of the CST axons. It is plausible that several of these abnormal axonal branches and synaptic contacts in plexin mutants may be physiologically impaired, which might result in pathological changes later in adulthood. Certainly, more studies will be needed to address how the absence of plexin signaling affects the physiology of visual and motor responses and what pathological changes will occur because of the existence of the abnormal connections. Abnormal pruning of axonal branches has been implied in neurodevelopmental disorders; it will be of interest to explore whether changes that occur to the unpruned axon collaterals throughout the life of the plexin/neuropilin mutants are similar to the pathological findings observed in patients with neuropsychiatric developmental disorders such as schizophrenia and autism.
Methods
Mouse Breeding.
Animal protocols were approved by the Institutional Animal Care and Use Committee at the University of California, Davis. Genotyping on knockout mice was carried out as described previously (23, 29, 30).
Mouse Tracer Injections.
WT and mutant mice were injected with various tracers at different postnatal ages (P0–P45). DiI (Molecular Probes) and BDA (Molecular Probes) anterograde tracing was performed as described previously (31, 32). Mice were injected blindly before determining genotype. Briefly, focal injections of DiI (20% in N,_N_-dimethylformamide) or BDA (10–20% in PBS) were made in either the motor or occipital cortex of WT and mutant mice in vivo and allowed to trace for a minimum of 3 days. Locations of the injection sites were confirmed in sagittal sections of the cortex to ensure that tracers were injected in the appropriate regions of the cortex.
For retrograde tracing studies, GFM (Lumafluor) and Alexa Fluor 594-conjugated CTB (Molecular Probes) were injected into the superior colliculus or cervical spinal cord of WT and mutant mice and allowed to trace in vivo for a minimum of 2 days. Mice injected with anterograde or retrograde tracers were perfused with 4% paraformaldehyde, and their brains were cut sagittally or coronally at 50–100 μm as described previously (24).
Immunohistochemistry and in Situ Hybridization (ISH).
Immunohistochemistry was performed on free-floating sections as described previously (24). Ctip2 was used as an antibody in the study (1:1,000; Abcam). The probes for ISH and the procedures for radioactive α-33P ISH were as described previously (33). Combined immunohistochemical and ISH colocalization experiments were performed on tissue sections mounted on slides. Briefly, nonradioactive ISH was performed on 12- to 14-μm cryosectioned brains by using digoxigenin-labeled probes for NPN-2 as described previously (23). Sections were then developed for Ctip2 immunohistochemical colocalization by using the methods described (24).
Analysis of Retrogradely Labeled Layer V Cortical Neurons.
Sagittal sections of the brains of WT and mutant mice were used for analysis. In mice injected with CTB in the cervical spinal cord or GFM in the superior colliculus, raw images of the cortex were taken with a CCD camera (Zeiss) and imported into PhotoShop (Adobe Systems). Montaged images of the sagittally cut brain were created in PhotoShop and analyzed with NIH Image J. For CTB-injected mice, the rostral blade of the dentate gyrus of the hippocampus was used as an arbitrary landmark for analysis (because most layer V neurons caudal to this region extend transient projections to the spinal cord). The length of cortex occupied by CTB-positive neurons caudal to the rostral blade of the dentate gyrus was normalized to the entire length of the occipital cortex (also measured from the rostral blade of the dentate gyrus) (Fig. 2A). For GFM-injected mice, the rostral–caudal length of cortex occupied by GFM-positive neurons was normalized to the entire length of the cortex (Fig. 3A).
Analysis of Pruning Defects for BDA-Labeled Visual Corticospinal Axons.
BDA was injected into the visual cortex of WT and mutant mice. BDA-labeled axons were scored at different locations along the visual CST: within the rostral half of the pons, at the brainstem rostral to the inferior olive, at the pyramidal decussation, and within the cervical spinal cord. Axon counts were performed on all 50-μm serial sagittal sections by using an eyepiece reticule as described in ref. 34. Labeled axons were scored only if their axon segments were >15 μm to minimize counting axons more than once in adjacent sections. Because the visual CST is pruned back from the spinal cord to the rostral pons in WT mice by P20, the severity of the pruning defect was determined by normalizing the absolute counts of axons at different locations along the visual CST to the total number of axons accessing the rostral pons.
EM Processing and Quantification.
Sections that contained BDA-labeled CST axons were preserved for ultrastructural analysis with EM as described (24). The perimeters of all boutons were measured in each serial section, and then the largest measured perimeter for each bouton was used in calculating the average bouton perimeter (Fig. 5K). Synapses per bouton were counted in serial sections such that each synapse was counted only once even if it appeared in multiple serial sections and was reported as synapse density (Fig. 5L). For axon width measurements, axon diameters for single axons were measured in serial sections and then the average axon diameter for that axon was recorded. Axon diameters were measured from electron micrographs containing BDA-labeled axons from visual cortex of PLXA3/PLXA4−/− animals. Surrounding BDA-negative axon diameters from the same electron micrographs were also measured as controls for comparison (Fig. 5M). All measurements were done using NIH Image J.
Statistics for all data were obtained from Statistica 6.0 (Statsoft).
Supplementary Material
Supporting Information
Acknowledgments.
We thank P. Nguyen, M. Chen, S. Mikula, A. Graziano, and K. Murray for technical assistance and E. G. Jones and members of the H.-J.C. and E. G. Jones laboratories for valuable discussions and comments. This research was supported by grants from the Klingenstein Fund, Autism Speaks/National Alliance for Autism Research, the March of Dimes Birth Defects Foundation, and National Institutes of Health Grant HD045757 (to H.-J.C.).
Footnotes
The authors declare no conflict of interest.
References
- 1.Kantor DB, Kolodkin AL. Curbing the excesses of youth: Molecular insights into axonal pruning. Neuron. 2003;38:849–852. doi: 10.1016/s0896-6273(03)00364-7. [DOI] [PubMed] [Google Scholar]
- 2.Low LK, Cheng HJ. A little nip and tuck: Axon refinement during development and axonal injury. Curr Opin Neurobiol. 2005;15:549–556. doi: 10.1016/j.conb.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 3.Low LK, Cheng HJ. Axon pruning: An essential step underlying the developmental plasticity of neuronal connections. Philos Trans R Soc London B. 2006;361:1531–1544. doi: 10.1098/rstb.2006.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luo L, O'Leary DD. Axon retraction and degeneration in development and disease. Annu Rev Neurosci. 2005;28:127–156. doi: 10.1146/annurev.neuro.28.061604.135632. [DOI] [PubMed] [Google Scholar]
- 5.Innocenti GM, Price DJ. Exuberance in the development of cortical networks. Nat Rev Neurosci. 2005;6:955–965. doi: 10.1038/nrn1790. [DOI] [PubMed] [Google Scholar]
- 6.Jones EG, Schreyer DJ, Wise SP. Growth and maturation of the rat corticospinal tract. Prog Brain Res. 1982;57:361–379. doi: 10.1016/S0079-6123(08)64137-0. [DOI] [PubMed] [Google Scholar]
- 7.Martin JH. The corticospinal system: From development to motor control. Neuroscientist. 2005;11:161–173. doi: 10.1177/1073858404270843. [DOI] [PubMed] [Google Scholar]
- 8.O'Leary DD, Koester SE. Development of projection neuron types, axon pathways, and patterned connections of the mammalian cortex. Neuron. 1993;10:991–1006. doi: 10.1016/0896-6273(93)90049-w. [DOI] [PubMed] [Google Scholar]
- 9.Stanfield BB. The development of the corticospinal projection. Prog Neurobiol. 1992;38:169–202. doi: 10.1016/0301-0082(92)90039-h. [DOI] [PubMed] [Google Scholar]
- 10.Terashima T. Anatomy, development and lesion-induced plasticity of rodent corticospinal tract. Neurosci Res. 1995;22:139–161. doi: 10.1016/0168-0102(95)00895-9. [DOI] [PubMed] [Google Scholar]
- 11.Stanfield BB, O'Leary DD. The transient corticospinal projection from the occipital cortex during the postnatal development of the rat. J Comp Neurol. 1985;238:236–248. doi: 10.1002/cne.902380210. [DOI] [PubMed] [Google Scholar]
- 12.Thong IG, Dreher B. The development of the corticotectal pathway in the albino rat: Transient projections from the visual and motor cortices. Neurosci Lett. 1987;80:275–282. doi: 10.1016/0304-3940(87)90467-8. [DOI] [PubMed] [Google Scholar]
- 13.Arlotta P, et al. Neuronal subtype-specific genes that control corticospinal motor neuron development in vivo. Neuron. 2005;45:207–221. doi: 10.1016/j.neuron.2004.12.036. [DOI] [PubMed] [Google Scholar]
- 14.Chen B, Schaevitz LR, McConnell SK. Fezl regulates the differentiation and axon targeting of layer 5 subcortical projection neurons in cerebral cortex. Proc Natl Acad Sci USA. 2005;102:17184–17189. doi: 10.1073/pnas.0508732102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Molyneaux BJ, Arlotta P, Hirata T, Hibi M, Macklis JD. Fezl is required for the birth and specification of corticospinal motor neurons. Neuron. 2005;47:817–831. doi: 10.1016/j.neuron.2005.08.030. [DOI] [PubMed] [Google Scholar]
- 16.Weimann JM, et al. Cortical neurons require Otx1 for the refinement of exuberant axonal projections to subcortical targets. Neuron. 1999;24:819–831. doi: 10.1016/s0896-6273(00)81030-2. [DOI] [PubMed] [Google Scholar]
- 17.He Z, Wang KC, Koprivica V, Ming G, Song HJ. Knowing how to navigate: Mechanisms of semaphorin signaling in the nervous system. Sci STKE. 2002;2002:RE1. doi: 10.1126/stke.2002.119.re1. [DOI] [PubMed] [Google Scholar]
- 18.Tran TS, Kolodkin AL, Bharadwaj R. Semaphorin regulation of cellular morphology. Annu Rev Cell Dev Biol. 2007;23:263–292. doi: 10.1146/annurev.cellbio.22.010605.093554. [DOI] [PubMed] [Google Scholar]
- 19.Waimey KE, Cheng HJ. Axon pruning and synaptic development: How are they per-plexin? Neuroscientist. 2006;12:398–409. doi: 10.1177/1073858406292631. [DOI] [PubMed] [Google Scholar]
- 20.Faulkner RL, Low LK, Cheng HJ. Axon pruning in the developing vertebrate hippocampus. Dev Neurosci. 2007;29:6–13. doi: 10.1159/000096207. [DOI] [PubMed] [Google Scholar]
- 21.Pasterkamp RJ, Kolodkin AL. Semaphorin junction: Making tracks toward neural connectivity. Curr Opin Neurobiol. 2003;13:79–89. doi: 10.1016/s0959-4388(03)00003-5. [DOI] [PubMed] [Google Scholar]
- 22.Bagri A, Cheng HJ, Yaron A, Pleasure SJ, Tessier-Lavigne M. Stereotyped pruning of long hippocampal axon branches triggered by retraction inducers of the semaphorin family. Cell. 2003;113:285–299. doi: 10.1016/s0092-8674(03)00267-8. [DOI] [PubMed] [Google Scholar]
- 23.Cheng HJ, et al. Plexin-A3 mediates semaphorin signaling and regulates the development of hippocampal axonal projections. Neuron. 2001;32:249–263. doi: 10.1016/s0896-6273(01)00478-0. [DOI] [PubMed] [Google Scholar]
- 24.Liu XB, Low LK, Jones EG, Cheng HJ. Stereotyped axon pruning via plexin signaling is associated with synaptic complex elimination in the hippocampus. J Neurosci. 2005;25:9124–9134. doi: 10.1523/JNEUROSCI.2648-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sahay A, Molliver ME, Ginty DD, Kolodkin AL. Semaphorin 3F is critical for development of limbic system circuitry and is required in neurons for selective CNS axon guidance events. J Neurosci. 2003;23:6671–6680. doi: 10.1523/JNEUROSCI.23-17-06671.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stanfield BB, O'Leary DD, Fricks C. Selective collateral elimination in early postnatal development restricts cortical distribution of rat pyramidal tract neurones. Nature. 1982;298:371–373. doi: 10.1038/298371a0. [DOI] [PubMed] [Google Scholar]
- 27.Molnar Z, Cheung AF. Towards the classification of subpopulations of layer V pyramidal projection neurons. Neurosci Res. 2006;55:105–115. doi: 10.1016/j.neures.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 28.Waimey KE, Huang PH, Chen M, Cheng HJ. Plexin-A3 and plexin-A4 restrict the migration of sympathetic neurons but not their neural crest precursors. Dev Biol. 2008;315:448–458. doi: 10.1016/j.ydbio.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yaron A, Huang PH, Cheng HJ, Tessier-Lavigne M. Differential requirement for plexin-A3 and -A4 in mediating responses of sensory and sympathetic neurons to distinct class 3 semaphorins. Neuron. 2005;45:513–523. doi: 10.1016/j.neuron.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 30.Chen H, et al. Neuropilin-2 regulates the development of selective cranial and sensory nerves and hippocampal mossy fiber projections. Neuron. 2000;25:43–56. doi: 10.1016/s0896-6273(00)80870-3. [DOI] [PubMed] [Google Scholar]
- 31.Brosamle C, Schwab ME. Cells of origin, course, and termination patterns of the ventral, uncrossed component of the mature rat corticospinal tract. J Comp Neurol. 1997;386:293–303. doi: 10.1002/(sici)1096-9861(19970922)386:2<293::aid-cne9>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 32.O'Leary DD, Terashima T. Cortical axons branch to multiple subcortical targets by interstitial axon budding: Implications for target recognition and “waiting periods.”. Neuron. 1988;1:901–910. doi: 10.1016/0896-6273(88)90147-x. [DOI] [PubMed] [Google Scholar]
- 33.Liu XB, Murray KD, Jones EG. Switching of NMDA receptor 2A and 2B subunits at thalamic and cortical synapses during early postnatal development. J Neurosci. 2004;24:8885–8895. doi: 10.1523/JNEUROSCI.2476-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kingsbury MA, Graf ER, Finlay BL. Altered development of visual subcortical projections following neonatal thalamic ablation in the hamster. J Comp Neurol. 2000;424:165–178. doi: 10.1002/1096-9861(20000814)424:1<165::aid-cne12>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information