HPV16 and BPV1 Infection Can Be Blocked by the Dynamin Inhibitor Dynasore (original) (raw)
. Author manuscript; available in PMC: 2008 Oct 1.
Abstract
The initial entry of papillomaviruses into their target cells has been shown to occur by clathrin-mediated endocytosis and caveolae-mediated endocytosis. These mechanisms entail the formation of nascent-coated vesicles at the plasma membrane. Such coated vesicles, clathrin or caveolin, form and pinch-off in a controlled mechanism that involves several proteins including dynamin. Dynamin is a GTPase that forms a dynamin ring at the stem connecting the nascent vesicle to the plasma membrane. In a still not fully characterized mechanism, dynamin’s contraction and twisting results in the scission of the vesicle. In an effort to better characterize the role and molecular mechanisms of dynamin’s function, researchers have identified dynasore, a dynamin GTPase inhibitor that prevents the scission of dynamin-dependent endocytic vesicles. Here, we have tested if infection by pseudovirus corresponding to the oncogenic human papillomavirus type 16 and bovine papillomavirus type 1 can be blocked by dynasore. We present data demonstrating that dynasore can block infection of human papillomavirus type 16 and bovine papillomavirus type 1 pseudovirions in a dose- and time-dependent manner with equal efficiency. Presently, there is no available therapy that can block infection by a wide range of papillomavirus regardless of species or genotypes. Targeting dynamin may lead to the rational design of drug able to prevent infection by papillomaviruses, and by other infectious agents dependent on this protein for initial internalization into target cells. Whether such an approach will prove successful needs further investigation.
Keywords: dynasore, papillomavirus endocytosis, dynamin inhibitor, viral infection, papillomavirus antiviral, dynamin-mediated endocytosis
INTRODUCTION
Our understanding of the processes of endocytosis in mammalian cells is increasing at a very rapid pace due to advances in molecular and cellular biology. Using fluorescent-labeled molecules, including transferrin, cholera toxin, and dextran, studies have defined various pathways leading to entry.1–5 Some of the best described mechanisms include clathrin-mediated endocytosis, caveolae-mediated endocytosis, phagocytosis, and macropinocytosis. The complexity of these mechanisms increases further when one tries to determine the role of auxiliary molecules involved in endocytosis such as dynamin.6,7
Dynamin is a 100-kd GTPase assisting endocytosis by helping to form the endocytic vesicle and, perhaps most importantly, by helping to pinch-off the newly formed vesicle from the cell membrane.8–10 The role of dynamin has been shown in the scission of clathrin-and caveolin-coated vesicles and in phagocytosis.11–13 The proposed mechanism of action involves the hexamerization of dynamin followed by its elongation, by cooperative GTP hydrolysis, at the stem connecting the newly formed vesicle and the plasma membrane.8–10 This elongation event ruptures the connective plasma membrane and releases the vesicle.
In an effort to further define the role of dynamin during endocytosis, a chemical screen searching for inhibitors of dynamin’s GTPase activity resulted in the finding of dynasore.14 Dynasore is a noncompetitive inhibitor of dynamin’s GTPase activity. Macia et al14 eloquently demonstrated that addition of dynasore to the culture of mammalian cells prevented the endocytosis of transferrin, low-density lipoprotein, and cholera toxin. The kinetics of action of dynasore are extremely fast, with an ability to block half of the new vesicle formation after 3–4 minutes, and to block almost all initiation and uncoating of vesicles by 6.5 minutes.14 The block of endocytosis had no effect on the endoplasmic reticulum–Golgi or Golgi–Golgi trafficking, suggesting that no indirect effects were attributed to dynasore and the inhibition of transferrin performed with 80 μM of dynasore in HeLa cells was reversible.14 Dynasore was subsequently used to block compensatory synaptic vesicle endocytosis.15 The block of synaptic vesicle endocytosis was reversible when dynasore was washed off before the next stimulation. The aforementioned studies showed by electron microscopy (EM) that the nascent endocytosis vesicles are prevented from forming completely or prevented from pinching off from the plasma membrane (no scission).
Having demonstrated that dynasore was capable of blocking endocytosis that was mediated by dynamin, we sought out to test the ability of dynasore to block papillomavirus infection. Papillomavirus are the etiologic agents of disease including human cancers and skin and genital warts and can cause severe pain and discomfort in animals.16,17 Current vaccine effort to minimize infection in humans targets 4 of the over 100 infectious genotypes (Gardasil, Merck & Co., White-house Station, NJ). The targeted genotypes human papillomavirus types (HPV6, 11, 16, and 18 are the most common genotypes causing genital warts (HPV6 and 11) or cervical carcinoma (HPV 16 and 18). Gardasil has proven effective at preventing infection, but it is too soon to evaluate the effect on cervical cancer rates. Although Gardasil is effective, there are 9 other oncogenic types, and more that 100 other human genotypes whose infection is not targeted by Gardasil.18
Papillomavirus, including HPV16 and bovine papillomavirus type 1 (BPV1), have been shown to enter cells through clathrin-mediated endocytosis,19 whereas HPV31 has been shown to enter by clathrin-mediated endocytosis and caveolin-mediated endocytosis.20,21 A common feature of these 2 modalities of entry is the involvement of dynamin.22,23 In fact, HPV31 infection was described as dynamin 2 dependent because use of a dynamin dominant-negative molecule–blocked infection.20 In this article we show that dynasore is able to block HPV16 and BPV1 infection in a dose-dependent manner and that as with transferrin the block can be reversed, although neither completely nor after initial viral entry.
MATERIALS AND METHODS
Cells
Human epithelial cell HEK 293 were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (DMEM-10), 100 IU/mL penicillin, and 100 μg/mL streptomycin. Additional studies were carried out on HPV-negative human carcinoma cell line C33A lines cultured in the above media.
Pseudovirion production and purification
BPV1 and HPV16 pseudovirions were purified from 293TT as described.24 For BPV1 production, 293TT were transfected with a bicistronic BPV1 L1 and L2 plasmid pShell-BPV1 and the GFP cDNA–containing plasmid 8fwb. Similarly, for HPV16, 293TT were transfected with a bicistronic HPV16 L1 and L2 plasmid pShell-HPV16 and the GFP cDNA–containing plasmid 8fwb (plasmids and the 293TT viral packaging cell line were a generous gift from Drs Day and Schiller, National Cancer Institute/National Institutes of Health, Bethesda, MD). Transfected cells were split 1:2 after 24 hours. After high salt extraction, pseudovirions were purified on an optiprep gradient (27%–39%). Titer was determined by measuring the percentage of GFP-positive cells by FACS analysis on infected cells after 48 hours.
Addition of dynasore inhibitor
Dynasore was resuspended in DMSO at 80 mM and kept at 4°C as stock concentration. Working dilutions at varying concentrations of 20, 40, 80, and 100 μM were made by resuspending the stock solution in DMEM-10 before use. Control cells were incubated with an equal amount of DMSO in DMEM-10.
Pseudovirion infections in the presence or absence of dynasore
HEK 293 cells plated at 50,000 were grown on a 24-well plate to 100,000 at 37°C in DMEM-10. The next day, cells were washed with DMEM-10. Dynasore was added at the appropriate concentrations of 0, 20, 40, 80, or 100 μM/mL. DMSO was added to control cells at an equal concentration/amount. Cells were incubated at 37°C for 30 minutes with dynasore and then incubated on ice for 10 minutes before the addition of BPV1 or HPV 16. Pseudovirions were allowed to bind for 2 hours on ice to prevent endocytosis and then incubated at 37°C for 48 hours. Infection was measured as the percentage of GFP-transduced cells because the encapsidated plasmid 8fwb has the GFP cDNA. Infections were analyzed by FACS at Rosalind Franklin University of Medicine and Science (RFUMS, North Chicago, IL) flow core.
Dynasore washout (reversibility)
HEK 293 cells grown to 100,000 were first incubated with media containing 80 μM/mL dynasore at 37°C for 30 minutes. Media containing DMSO at an equal concentration/amount were used in control experiments. After incubating the cells with dynasore for 30 minutes, infections were performed by addition of BPV1 or HPV16 pseudovirions for 2 hours at 4°C to promote attachment but not internalization. For dynasore wash-off at time point −30 minutes, dynasore was removed by washing the cells 3 times before the addition of virus, and the media were replaced with DMEM-10 without dynasore. At 0 hours time point, dynasore was removed by washing the cells 3 times just before incubation at 37°C. During this wash-off postvirus attachment, any unbound virus is also removed. The cell’s media were replaced with DMEM-10 without dynasore. For +30 minutes, +2 hours, and +4 hours, cells were washed to remove any unbound virus postattachment before incubation at 37°C, but for these time points, the media were replaced with DMEM-10 containing the same amount of dynasore (80 μM/mL) and placed at 37°C to allow viral entry. These cells were incubated at 37°C for 30 minutes, 2 hours, and 4 hours at which point dynasore was removed by 3 washes in DMEM-10, and the media were replaced with regular DMEM-10 without dynasore. Cells were incubated for 48 hours at 37°C, and the infections with BPV1 and HPV16 pseudovirions were analyzed by FACS analysis for percentage of GFP expression.
Flow cytometric analysis of infection
Cells infected with pseudovirion in the presence or absence of dynasore for 48 hours at 37°C were harvested and washed 3 times in 1X PBS. Cells were processed by flow cytometric analysis (FACS-Calibur sorter, Becton-Dickinson, San Jose, CA) to determine infection efficiency based on GFP fluorescence.
Electron microscopy
HEK 293 cells grown to 4 million in a 10-cm2 dish were used in this experiment. Cells were harvested and fixed in 0.3% glutaraldehyde (PBS buffered) for 1 hour and then subsequently fixed in 3.0% glutaraldehyde (PBS buffered) overnight. Next day, specimens were washed in Sørensens phosphate buffer (pH 7.3) and postfixed with 1% buffered osmium tetraoxide. Specimens were dehydrated through an increasing series of ethanol washes, embedded in epon resin, and cured in a 70°C oven for 72 hours. Specimen blocks were sectioned to 70 nm using a Leica LIC-6 ultramicrotome and then contrast stained with uranyl acetate and lead citrate before viewing by transmission electron microscope. Micrographs were digitally acquired using a JEOL JEM-1230 transmission electron microscope at ×80,000 magnification. Specimen embedding, cutting, contrast staining, acquiring of images, and analysis were performed at the core EM center of RFUMS.
RESULTS
Dynasore blocks PV infection in a dose-dependent manner
To determine if a dynamin inhibitor would prevent viral infection, we infected cells with HPV16 or BPV1 pseudovirus in the presence of dynasore at different concentrations. The human epithelial cell HEK 293 was incubated with pseudovirions and increasing amounts of dynasore or with equal amount of DMSO control (Figure 1). Dynasore or DMSO was added onto HEK 293 cells 30 minutes before the addition of pseudovirus. Viral particles carrying the GFP cDNA as transgene in a “pseudogenome” were then allowed to bind to the cells for 2 hours on ice to block their endocytosis. Unbound viral particles were washed off, and cells were incubated for 48 hours at 37°C in media containing the corresponding concentration of dynasore. Using FACS analysis to detect GFP expression allowed us to determine the percentage of infected cells. We did not observe a drop in infection of either HPV16 or BPV1 using 20 μM of dynasore as compared with infections in the absence of inhibitor (DMSO control) (Figure 1: 0 and 20 μM, respectively, HPV16 line with rectangles, 0, BPV1 line with diamond ). BPV1 infection decreased by 19% and HPV16 by 33% at a dynasore concentration of 40 μM (Figure 1, 40 μM). A 45% decrease in infection was observed using 80 μM of dynasore for BPV1 and HPV16 (Figure 1, 80μM). We observed a further decrease in BPV1 infection in the presence of 100 μM of dynasore to 66%, but no significant decrease in infection with HPV16 in the presence of 100 μM of dynasore. The data points represent an average of 3 independent experiments, and error bars demonstrate the standard deviation between the experiments. These data suggest that dynasore blocks BPV1 and HPV16 infection in a dose-dependent manner. Similar dose-dependent inhibition of HPV16 infection by dynasore was observed using the HPV-negative human carcinoma cell line C33A lines (data not shown).
FIGURE 1.
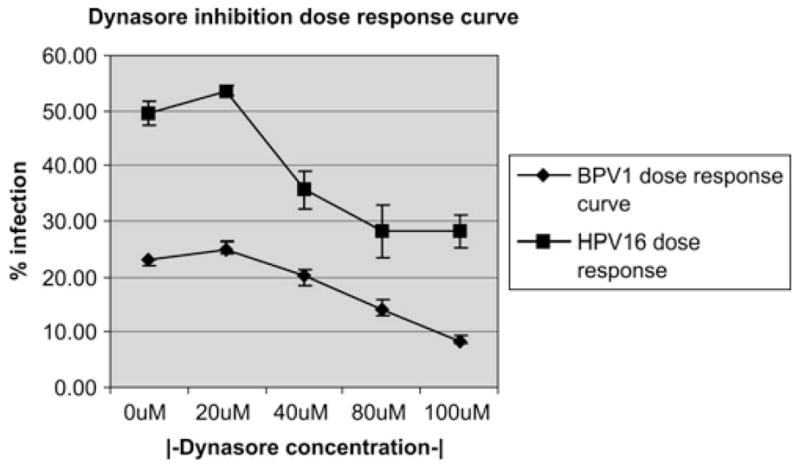
Dose-dependent inhibition of PV infection by dynasore inhibitor. HEK 293 cells were infected with BPV1 or HPV16 pseudovirions carrying the GFP cDNA, in the presence of 0, 20, 40, 80, and 100 μM dynasore. Infection was measured by FACS analysis of GFP-positive cells. Line with diamond is the BPV1 dose–response curve, and line with the rectangle is the HPV16 dose–response curve.
Dynasore inhibition of PV infection is dependent on time of addition
We wanted to determine if the inhibition of infection by dynasore differs depending on the time at which the inhibitor was added. Dynasore was added to 293 cells at a final concentration of 80 μM at different times during infection. Percent inhibition of infection was measured by the changes in GFP-positive cells as measured by FACS analysis at 48 hours post infection. Addition of dynasore 30 minutes before pseudovirions resulted in a 50%–60% inhibition of infection by both BPV1 and HPV16 (Figure 2, −30 bars). We observed less inhibition when dynasore was added at time 0, which corresponded to the moment that cells were moved from the 2-hour infection on ice into the 37°C incubator (unbound virus was removed, and media with 80 μM were readded onto cells, Figure 2, 0 bars). Percent inhibition decreased further when dynasore was added 30 minutes, 2 hours, or 4 hours postinfection. Addition of dynasore after 4 hours resulted in 17% inhibition of infection for BPV1 and less than 10% inhibition of infection for HPV16. These data show that dynasore’s block of viral infection is highly dependent on the time it is added to the target cells.
FIGURE 2.
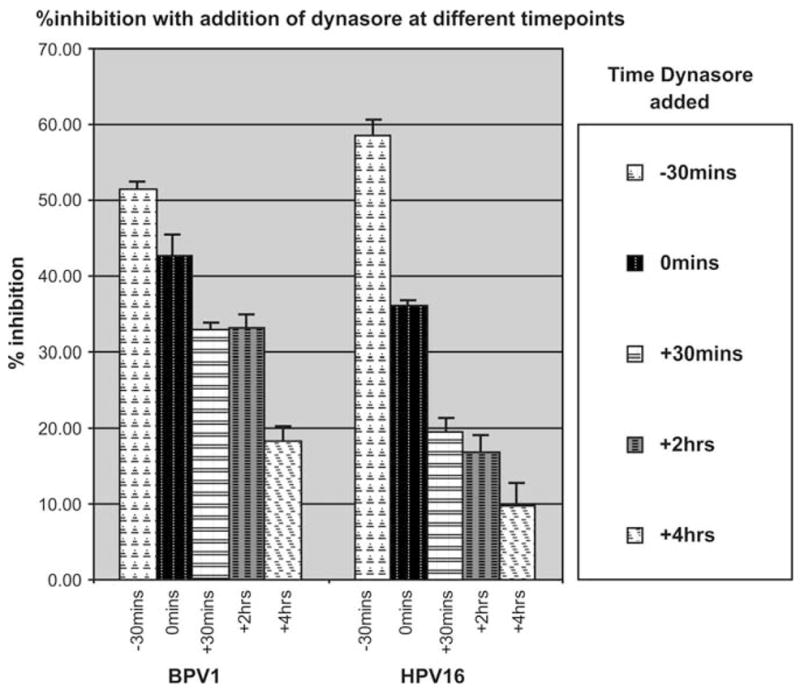
Dynasore inhibition of viral infection depends on the time of addition of the inhibitor. Dynasore (80 μM/mL) was added to 293 cells at different times during infection, and percent inhibition was measured by FACS analysis of GFP-positive cells. Bars: −30 minutes, dynasore was added 30 minutes before adding the virus; 0 minutes, dynasore was added just before incubating cells at 37°C; +30 minutes, dynasore was added 30 minutes after incubation at 37°C; +2 hours, dynasore was added 2 hours after infection; +4 hours, dynasore was added 4 hours after infection. Each bar is representative of independent experiments carried out in triplicates.
Inhibition of infection by dynasore is partly reversible
It has been shown that dynasore’s ability to block the endocytosis of transferrin is a reversible effect. We wanted to determine the effect of removing (washing off) dynasore at different times during infection. To confirm the previous published data and to show that in our system the effect of dynasore were reversible, we added alexa-fluor 594–labeled transferrin to control cells (Figure 3A, control), to cells in the presence of 80 μM dynasore (Figure 3A, dynasore), and to cells that were incubated with 80 μM dynasore for 30 minutes but whose media were replaced with dynasore-free media before the addition of labeled transferrin (Figure 3, washout). We confirmed that the internalization of labeled transferrin (Figure 3A, control panel) is blocked in the presence of 80 μM dynasore (Figure 3A dynasore panel) and that washing off of dynasore removes the block of internalization (Figure 3A, washout panel). Thus, we confirmed that the complete block of the internalization of transferrin is reversible under these conditions.
FIGURE 3.
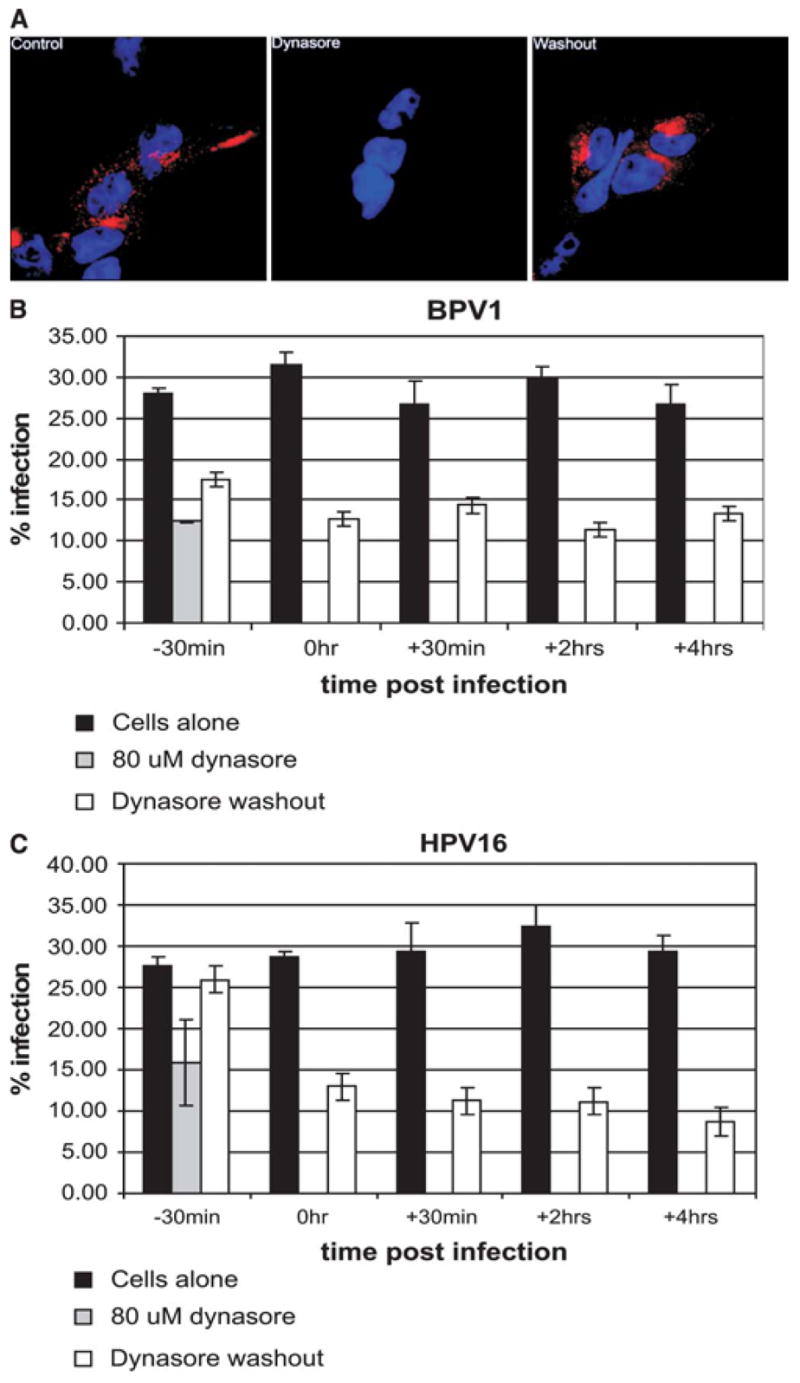
The inhibitory effect of dynasore is partly reversible. (A) Control panel: HEK 293 cells incubated with alexa-fluor 594–labeled transferrin; dynasore panel: cells were incubated with 80 μM/mL dynasore for 30 minutes and then labeled transferrin was added to media; washout panel: cells were incubated with 80 μM/mL dynasore for 30 minutes, media were replaced with dynasore-free fresh media, and labeled transferrin was added after 20 minutes. All steps were incubated at 37°C. Remaining surface transferrin was removed by acid wash before fixation and imaging of cells. Transferrin in red, and nuclei in blue (DAPI). (B andC)Black bars: percent infection of cells incubated with DMSO for 30 minutes and infected with BPV1 in B, HPV16 in C virus; gray bars: percent infection of cells incubated with dynasore for 30 minutes and infected with BPV1 virus; white bars: percent infection of cells incubated with dynasore for 30 minutes and infected with BPV1 virus. Dynasore was washed off (white bars) at −30 minutes, 0 hour, +30 minutes, +2 hours, and +4 hours, in relation addition of virus.
To determine the ability to reverse block of infection, we infected cells incubated with 80 μM dynasore and washed off the dynasore at various time points in relation to addition of pseudovirions. In Figures 3B and C black bars represent the percent infection in the absence of dynasore, gray bar shows the percent infection in the presence of dynasore, and white bars represent the percent infection that results with washing off of dynasore at the described time point in relation to addition of virus. For every time point at which dynasore was washed off (Figures 3B and C, white bars) we had a corresponding control infection, that is, infections in which no dynasore was added but cells were subjected to the same washes (Figures 3B and C, white bars). BPV1 infection of cells preincubated with dynasore for 30 minutes was blocked (compare Figure 3B black bar versus gray bar at −30 minutes). We partially restored infection of these cells by washing off of dynasore just before the addition of pseudovirions (Figure 3B, bars at −30 minutes). Surprisingly, washing off of dynasore at the moment that cells were moved from the 2-hour infection on ice into the 37°C incubator did not reverse the block of infection (Figure 3B, 0 hour). We also were not able to reverse the block of infection by washing off of dynasore at 30 minutes, 2 hours, or 4 hours post infection (Figure 3B, bars at 30 minutes, 2 hours, and 4 hours). HPV16 infection of cells preincubated with dynasore for 30 minutes was blocked but was completely restored by washing off of dynasore just before the addition of pseudovirions (Figure 3C, compare black and gray bars at −30 minutes). And much like BPV1 infections, we were unable to reverse the block of infection by washing off of dynasore after the 2-hour infection on ice (Figure 3C, bars at 0 hour, +30 minutes, 2 hours, and 4 hours). These data demonstrate that the block of infection by dynasore can be reversed before the addition of virus, but not after the addition of virus.
Attached endocytotic vesicles are observed in the presence of dynasore
Dynasore’s block of dynamin’s GTPase function results in the inability of vesicles to bud off from the cells surface.14 We observed the presence of U-type vesicles at the plasma membrane in HEK 293 cells in the presence of 80 μM dynasore using EM (Figure 4A). The dense staining surrounding the vesicle suggests that it is a clathrin-coated vesicle. The lumen (or open lumen) of the U-type vesicle is not densely stained, suggesting that the vesicle is empty. EM analysis of BPV1-infected cells for 1 hour in the absence of dynasore shows internalized densely stained vesicles, suggesting that the vesicles are filled with cargo (Figures 4B–E, arrow indicates vesicles). Analysis of cells infected with BPV1 for 1 hour in the presence of 80 μM dynasore shows densely stained vesicles attached to the plasma membrane (Figures 4F–I), again suggesting that the vesicles are filled with cargo. These data suggest that indeed endocytosis has been blocked at an early point during entry and that the vesicles that are attached to the membrane may contain viral particles.
FIGURE 4.
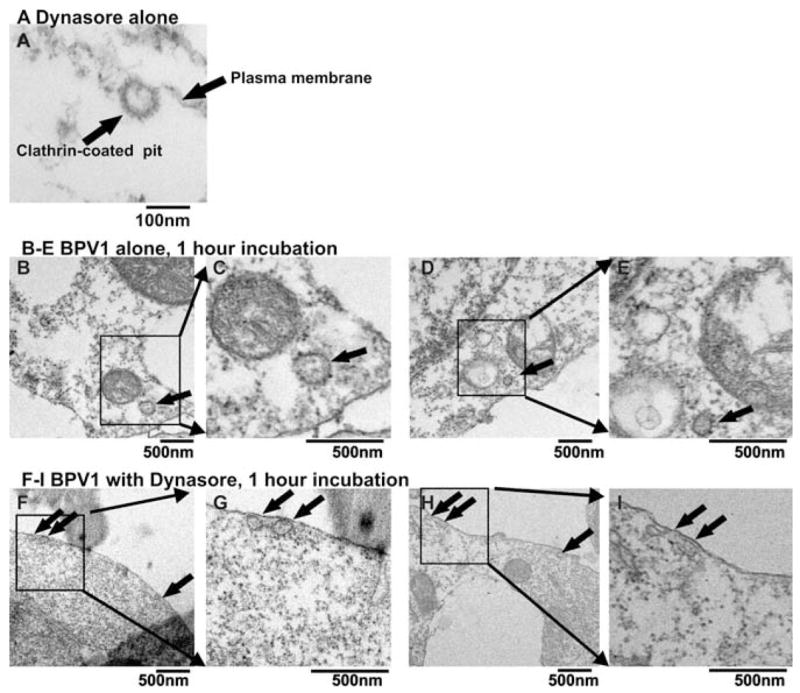
Electron microscopic analysis of the effect of dynasore inhibitor. (A) HEK 293 cells incubated for 30 minutes with 80 μM dynasore. (B–E) HEK 293 cells infected with BPV1 pseudovirus for 1 hour in the absence of dynasore. (F–I) HEK 293 cells infected with BPV1 pseudovirus for 1 hour in the presence of 80 μM dynasore. Scale bar represents 100 nm; ×80,000 magnification.
DISCUSSION
Papillomaviruses have been identified as the etiologic agent of cervical and anal cancers and of tumors and disease in animals.16,17 There is no available therapy that can block infection by a wide range of papillomavirus genotypes regardless of species. Such therapy or preventative mean would interfere with a common pathway or event during papillomavirus infection. Our goal was to determine if the newly identified and characterized specific dynamin inhibitor dynasore would block infection of both an oncogenic human papillomavirus (HPV16) and of an animal papillomavirus (BPV1). Dynasore is a GTPase involved in the scission of nascent clathrin-coated or caveolin-1–coated endocytic vesicles at the plasma membrane. Papillomaviruses have been shown to use either clathrin-or caveolin-mediated endocytosis, and we show here that dynasore is capable of blocking HPV16 and BPV1 infection in a dose-dependent manner.
Our data demonstrated that addition of dynasore is capable of blocking HPV16 and BPV1 infection with equal efficiency. We showed that dynasore’s effect is at the very early stages of infection; presumably at the initial internalization stages because addition of dynasore at times post infection results in a smaller block of infection. Our data confirmed the ability to reverse the effect of dynasore by removing dynasore before the addition of transferrin, but our data indicated that washing cells to try to remove dynasore’s block in cells after addition of virus does not restore infection levels. Using EM, we observed what appeared to be viral particle–filled vesicles unable to pinch-off from the plasma membrane in the presence of dynasore.
The process of papillomavirus infection has been shown to occur by clathrin-mediated endocytosis and/or caveolin 1.19–21 These mechanisms are different in the molecules coating the nascent vesicles but are both dependent on the cleavage event that ensues with the hydrolysis of GTP by dynamin.6,7 Characterizing of the precise model of papillomavirus endocytosis has not been clearly defined. Our experiments described in this article showed that the ability to block dynamin’s function is sufficient to block infection of HPV16 and BPV1. Using a dominant-negative dynamin, Smith et al21 had shown that HPV31 infection drops by approximately 60% (Figure 3, p 9926), a level comparable to our data; HPV16 infection was not analyzed in that study.
We added dynasore at different time points during infection and determined that the level of infection increased as we delayed the addition of dynasore. In fact, addition of dynasore 4 hours postinfection resulted in a 50% drop in infection block, suggesting that 50% of the infectious virions had already entered by 4 hours. This 4-hour and 50% block supports the finding by FACS analysis that after 4 hours, 50% of the viral particles had internalized.19 Thus, our dynasore data have confirmed the kinetics regarding papillomavirus infection. Surprisingly though, we could not reverse the infection block by dynasore by washing off of dynasore when virus was present in our cultures. Our experiments showed that the incubation of dynasore on cells before the pseudovirus addition did not result in a nonreversible block of infection, that is, it could be washed off and infection could occur. But our data consistently showed that if virus was incubated with dynasore during our 2-hour infections, washing off of the cells did not restore the high levels of infection. Our EM images suggest that dynasore does freeze endocytic vesicles at the plasma membrane and thus prevents the proper internalization of PV pseudovirions. More work is needed and ongoing to determine where dynasore has secluded or perhaps redirected the bound viral particles.
In summary, in this article we present evidence that HPV16 and BPV1 enter cells in a dynamin-dependent mechanism. Our data suggest that targeting the internalization step of papillomaviruses with dynasore results in a nonreversible block of infection. This approach may lead to rational drug design targeting dynamin as a broad-impact strategy at preventing infection of papillomaviruses or other infectious agents whose initial cellular internalization is dependent on dynamin function. We are currently exploring the effect of dynasore on various other papillomavirus genotypes infection, and we are attaining similar results as presented in this article.
Acknowledgments
This work was supported by the H. M. Bligh Cancer Research Laboratory of RFUMS, North Chicago, IL, by the National Cancer Institute/National Institutes of Health grant K22:CA117971 to P.I.M., by the American Cancer Society, IL, grant #07-34 to P.I.M., and by a Cystic Fibrosis Foundation grant to N.A.B. EM, FACS, and confocal microscopy analysis work were performed in the core facilities of RFUMS. We thank Ms Seiler for support with EM (RFUMS).
References
- 1.Ehrlich M, Boll W, Van Oijen A, et al. Endocytosis by random initiation and stabilization of clathrin-coated pits. Cell. 2004;118:591–605. doi: 10.1016/j.cell.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 2.Gaidarov I, Santini F, Warren RA, et al. Spatial control of coated-pit dynamics in living cells. Nat Cell Biol. 1999;1:1–7. doi: 10.1038/8971. [DOI] [PubMed] [Google Scholar]
- 3.Mayor S, Pagano RE. Pathways of clathrin-independent endocytosis. Nat Rev Mol Cell Biol. 2007;8:603–612. doi: 10.1038/nrm2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pelkmans L, Helenius A. Insider information: what viruses tell us about endocytosis. Curr Opin Cell Biol. 2003;15:414–422. doi: 10.1016/s0955-0674(03)00081-4. [DOI] [PubMed] [Google Scholar]
- 5.van der Goot FG, Gruenberg J. Intra-endosomal membrane traffic. Trends Cell Biol. 2006;16:514–521. doi: 10.1016/j.tcb.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 6.Lajoie P, Nabi IR. Regulation of raft-dependent endocytosis. J Cell Mol Med. 2007;11:644–653. doi: 10.1111/j.1582-4934.2007.00083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ungewickell EJ, Hinrichsen L. Endocytosis: clathrin-mediated membrane budding. Curr Opin Cell Biol. 2007;19:417–425. doi: 10.1016/j.ceb.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 8.Liu J, Kaksonen M, Drubin DG, et al. Endocytic vesicle scission by lipid phase boundary forces. Proc Natl Acad Sci USA. 2006;103:10277–10282. doi: 10.1073/pnas.0601045103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roux A, Cuvelier D, Nassoy P, et al. Role of curvature and phase transition in lipid sorting and fission of membrane tubules. Embo J. 2005;24:1537–1545. doi: 10.1038/sj.emboj.7600631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roux A, Uyhazi K, Frost A, et al. GTP-dependent twisting of dynamin implicates constriction and tension in membrane fission. Nature. 2006;441:528–531. doi: 10.1038/nature04718. [DOI] [PubMed] [Google Scholar]
- 11.Damke H, Baba T, Warnock DE, et al. Induction of mutant dynamin specifically blocks endocytic coated vesicle formation. J Cell Biol. 1994;127:915–934. doi: 10.1083/jcb.127.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muro S, Wiewrodt R, Thomas A, et al. A novel endocytic pathway induced by clustering endothelial ICAM-1 or PECAM-1. J Cell Sci. 2003;116:1599–1609. doi: 10.1242/jcs.00367. [DOI] [PubMed] [Google Scholar]
- 13.Schlunck G, Damke H, Kiosses WB, et al. Modulation of Rac localization and function by dynamin. Mol Biol Cell. 2004;15:256–267. doi: 10.1091/mbc.E03-01-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Macia E, Ehrlich M, Massol R, et al. Dynasore, a cell-permeable inhibitor of dynamin. Dev Cell. 2006;10:839–850. doi: 10.1016/j.devcel.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 15.Newton AJ, Kirchhausen T, Murthy VN. Inhibition of dynamin completely blocks compensatory synaptic vesicle endocytosis. Proc Natl Acad Sci USA. 2006;103:17955–17960. doi: 10.1073/pnas.0606212103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campo MS. Animal models of papillomavirus pathogenesis. Virus Res. 2002;89:249–261. doi: 10.1016/s0168-1702(02)00193-4. [DOI] [PubMed] [Google Scholar]
- 17.Howley P, Lowy P. Papillomavirus and their replication. Fundam Virol. 2001:1019–1052. [Google Scholar]
- 18.Meneses P, Robertson E. Papillomavirus biology and therapeutic approaches. Gene Ther Mol Biol. 2005;9:217–228. [Google Scholar]
- 19.Day PM, Lowy DR, Schiller JT. Papillomaviruses infect cells via a clathrin-dependent pathway. Virology. 2003;307:1–11. doi: 10.1016/s0042-6822(02)00143-5. [DOI] [PubMed] [Google Scholar]
- 20.Hindmarsh PL, Laimins LA. Mechanisms regulating expression of the HPV 31 L1 and L2 capsid proteins and pseudovirion entry. Virol J. 2007;4:19. doi: 10.1186/1743-422X-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith JL, Campos SK, Ozbun MA. Human papillomavirus type 31 uses a caveolin 1-and dynamin 2-mediated entry pathway for infection of human keratinocytes. J Virol. 2007;81:9922–9931. doi: 10.1128/JVI.00988-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Merrifield CJ, Feldman ME, Wan L, et al. Imaging actin and dynamin recruitment during invagination of single clathrin-coated pits. Nat Cell Biol. 2002;4:691–698. doi: 10.1038/ncb837. [DOI] [PubMed] [Google Scholar]
- 23.Pelkmans L, Helenius A. Endocytosis via caveolae. Traffic. 2002;3:311–320. doi: 10.1034/j.1600-0854.2002.30501.x. [DOI] [PubMed] [Google Scholar]
- 24.Buck CB, Pastrana DV, Lowy DR, et al. Efficient intracellular assembly of papillomaviral vectors. J Virol. 2004;78:751–757. doi: 10.1128/JVI.78.2.751-757.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]