A SQSTM1/p62 mutation linked to Paget’s disease increases the osteoclastogenic potential of the bone microenvironment (original) (raw)
Abstract
Paget’s disease of bone (PDB) is the second most common bone disease and is characterized by focal bone lesions which contain large numbers of abnormal osteoclasts (OCLs) and very active normal osteoblasts in a highly osteoclastogenic marrow microenvironment. The etiology of PDB is not well understood and both environmental and genetic causes have been implicated in its pathogenesis. Mutations in the SQSTM1/p62 gene have been identified in up to 30% of Paget’s patients. To determine if p62 mutation is sufficient to induce PDB, we generated mice harboring a mutation causing a P-to-L (proline-to-leucine) substitution at residue 394 (the murine equivalent of human p_62_P392L, the most common PDB-associated mutation). Bone marrow cultures from p62 P394L mice formed increased numbers of OCLs in response to receptor activator of NF-κB ligand (RANKL), tumor necrosis factor α (TNF-α) or 1α,25-(OH)2D3, similar to PDB patients. However, purified p62 P394L OCL precursors depleted of stromal cells were no longer hyper-responsive to 1α,25-(OH)2D3, suggesting effects of the p62 P394L mutation on the marrow microenvironment in addition to direct effects on OCLs. Co-cultures of purified p62 P394L stromal cells with either wild-type (WT) or p62 P394L OCL precursors formed more OCLs than co-cultures containing WT stromal cells due to increased RANKL production by the mutant stromal cells. However, despite the enhanced osteoclastogenic potential of both OCL precursors and marrow stromal cells, the p62 P394L mice had histologically normal bones. These results indicate that this PDB-associated p62 mutation is not sufficient to induce PDB and suggest that additional factors acting together with p62 mutation are necessary for the development of PDB in vivo.
INTRODUCTION
Paget’s disease of bone (PDB; OMIM 602080) is the second most common bone disease after osteoporosis, affecting up to 2–3% of adults over the age of 55, and can result in significant bone deformity, pain and other complications (1). It is a chronic bone disease characterized by focal regions of increased bone resorption accompanied by increased formation of new, highly disorganized bone (2). The etiology of PDB has been difficult to define, as it likely involves both genetic and environmental factors (3). Familial clustering is often observed, with between 15 and 40% of patients having at least one affected first-degree relative (4–6). In Paget’s families, the disease is inherited in an autosomal dominant fashion, although with highly variable penetrance (7,8). Recently, mutations associated with PDB or PDB-like disorders have been identified in four genes (8). However, mutations in only one of these genes, SQSTM1/p62, are generally associated with classical PDB (9,10), and up to 30% of familial PDB patients and 10% of sporadic patients have a p62 mutation (11–13). The p62 gene is ubiquitously expressed and encodes a 62 kDa protein that functions in multiple signal transduction pathways activated by receptor activator of NF-κB ligand (RANKL), tumor necrosis factor α (TNF-α) and IL-1, that promote osteoclast (OCL) formation at least in part through NF-κB activation (14). Essentially all of the PDB-associated p62 mutations identified to date affect the ubiquitin-associated domain in the carboxy-terminal region of the protein, with p62 P392L being the most commonly identified mutation (9–11,15). However, the role of p62 mutation in PDB is unclear, since the phenotype can be highly variable even among family members carrying the same mutation, and some individuals carrying p62 mutations fail to develop PDB even at an advanced age (16).
The primary cellular defect in PDB is generally thought to reside in the OCL, since the bone lesions are characterized by large numbers of hyper-multinucleated OCLs, and inhibition of OCL activity in PDB patients results in prolonged clinical and histological remissions (17). Although osteoblasts and other cells in the bone microenvironment also participate in the disease, the increased bone formation has been thought to represent a secondary response of osteoblasts to the elevated bone resorption. To determine if expression of mutant p62 in cells of the OCL lineage was sufficient to induce PDB, we previously generated transgenic mice which express human p62 P392L in OCLs and their precursors. These mice developed progressive osteopenia but did not form pagetic bone lesions (18).
Since mutant p62 protein is ubiquitously expressed in PDB patients carrying a p62 mutation, it is possible that expression of mutant p62 in osteoblasts or bone marrow stromal cells may contribute to the development of PDB by enhancing the capacity of these cells to support osteoclastogenesis as well as to increase new bone formation in response to the increased OCL activity. This hypothesis was supported by our previous observation that bone marrow stromal cells cultured from PDB patient lesions expressed elevated levels of RANKL (19). Thus, to generate a more accurate mouse model of PDB patients with inherited p62 mutations that would allow us to test this hypothesis, we introduced a P394L mutation (the murine equivalent of human p62 P392L) into the endogenous mouse p62 gene to create p62 P394L ‘knock-in’ (KI) mice, and determined if OCLs and stromal cells from these mice expressed the distinctive phenotypic characteristics of cells from PDB lesions, including increased OCL formation, hyper-multinucleated OCLs, increased responsivity of OCL precursors to RANKL, TNF-α and 1,25-(OH)2D3, and increased support of osteoclastogenesis by osteoblasts/stromal cells. We also examined the mice for development of pagetic bone lesions.
In this report, we show that although OCL precursors from the KI mice expressed some of the characteristics of OCL precursors from PDB patients, the OCLs that formed were not hyper-multinucleated or hyper-responsive to 1,25-(OH)2D3. Importantly, expression of p62 P394L in marrow stromal cells markedly increased RANKL production in response to 1,25-(OH)2D3, and increased OCL formation when KI stromal cells were co-cultured with either wild-type (WT) or KI OCL precursors. However, in spite of these cellular abnormalities, KI mice up to 18 months of age did not develop histologically detectable bone abnormalities. These results demonstrate for the first time that a p62 mutation linked to PDB has direct effects on cells in the bone microenvironment in addition to OCLs, and suggest that the p62 P392L mutation predisposes patients to PDB both by creating a permissive microenvironment that expresses elevated levels of RANKL, and OCL precursors that are hyper-responsive to RANKL. Nevertheless, additional factors must also contribute to the etiology of this disease, since p62 P394L mice do not develop PDB-like disease in vivo.
RESULTS
Generation of p62 P394L KI mice
To create a genetically accurate mouse model for PDB patients carrying the p62 P392L mutation, a line of KI mice with a targeted mutation in the endogenous p62 gene encoding a proline-to-leucine substitution at amino acid residue 394 (P394L, equivalent to the P392L substitution in the human gene) was generated by homologous recombination (Fig. 1; Supplementary Material, Fig. S1). In crosses between p62 P394L KI heterozygotes, offspring of each of the three possible genotypes (WT, p62+/P394L and p62 P394L/P394L) were generated in the expected ratio, indicating that this mutation has no discernable effect on normal embryonic development. Further, both KI heterozygotes and homozygotes were viable and had no grossly apparent phenotypic abnormalities up to 1.5 years of age. For the cell culture studies described later, we used OCLs and stromal cells primarily from p62+/P394L mice (referred to as KI mice), since these most closely model human PDB patients with a p62 mutation. However, similar results were obtained with cells isolated from p62 P394L/P394L mice.
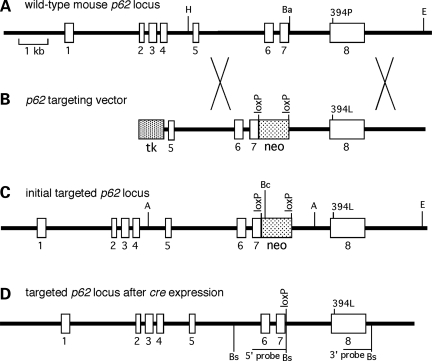
Figure 1.
Targeting strategy for the generation of p62 P394L KI mice: (A) structure of the murine p62 gene, indicating the location of codon 394 in exon 8. Restriction sites used for construction of the targeting vector are shown – H, Hind III; Ba, BamHI; E, EcoRV; (B) structure of the targeting vector, including the location of the MC1-tk minigene, the _loxP_-flanked PGK-neo minigene in intron 7; (C) structure of the initial targeted p62 locus retaining the PGK-neo minigene. Restriction sites used for Southern blot analysis of ES cells are shown – A, AflIII; Bc, BclI; E, EcoRV; (D) structure of the final targeted p62 locus following transient cre expression in ES cells, indicating the location of the codon P394L substitution in exon 8 and the single remaining loxP site in intron 7. The locations of the BsoBI (Bs) restriction sites used for Southern blot analysis of KI mice are shown.
OCL formation and bone resorption by p62 P394L KI mouse OCL precursors
Cultures of unfractionated bone marrow cells from p62 P394L KI mice containing both OCL precursors and marrow stromal cells formed significantly more OCLs in response to RANKL, TNF-α and 1α,25-(OH)2D3 than WT marrow cultures (Fig. 2A), analogous to marrow cultures from PDB patients. However, unlike OCLs formed in PDB patient marrow cultures, the p62 P394L KI OCLs did not have an increased number of nuclei per OCL compared with WT (data not shown).
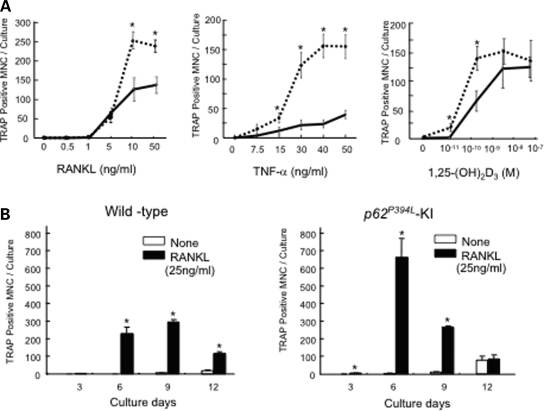
Figure 2.
OCL formation from KI or WT mice: (A) OCLs formed from marrow cultures. Non-adherent marrow cells from WT (solid lines) or KI mice (dotted lines) were cultured for 7 days in the presence of varying concentrations of RANKL, TNF-α or 1α,25-(OH)2D3. The cells were then fixed and stained for TRAP activity. Results are expressed as the mean ± SEM for quadruplicate cultures from a typical experiment. Asterisk: P < 0.01 compared with results from WT cell cultures. Similar results were obtained in five independent experiments; (B) time course for OCL formation in marrow cultures. OCL precursors from KI and WT mice were cultured in the presence of RANKL. After 3, 6, 9 or 12 days of culture, cells were fixed and the number of TRAP-positive OCLs counted. Results are expressed as the mean ± SEM for quadruplicate cultures from a typical experiment. Asterisk: P < 0.01 compared with results in WT cultures.
To characterize the mechanisms responsible for the increased levels of OCL formation in marrow cultures from KI mice, the time course for OCL formation in culture was first determined. Maximal numbers of OCLs formed in KI cultures after 6 days in the presence of RANKL, while maximal numbers were not reached until 9 days in WT cultures (Fig. 2B). OCL precursor proliferation was also significantly increased in marrow cultures from KI mice compared with WT cultures (data not shown). Further, KI marrow cultures expressed higher levels of phospho-IκB-α in response to TNF-α, while simultaneously showing increased degradation of total IκB-α compared with WT cultures, consistent with increased activation of the canonical NF-κB signaling pathway (Supplementary Material, Fig. S2). There was also a modest increase in the level of phospho-p38 in the KI cultures as compared with WT, but there were minimal effects on levels of phospho-MAPK or -JNK.
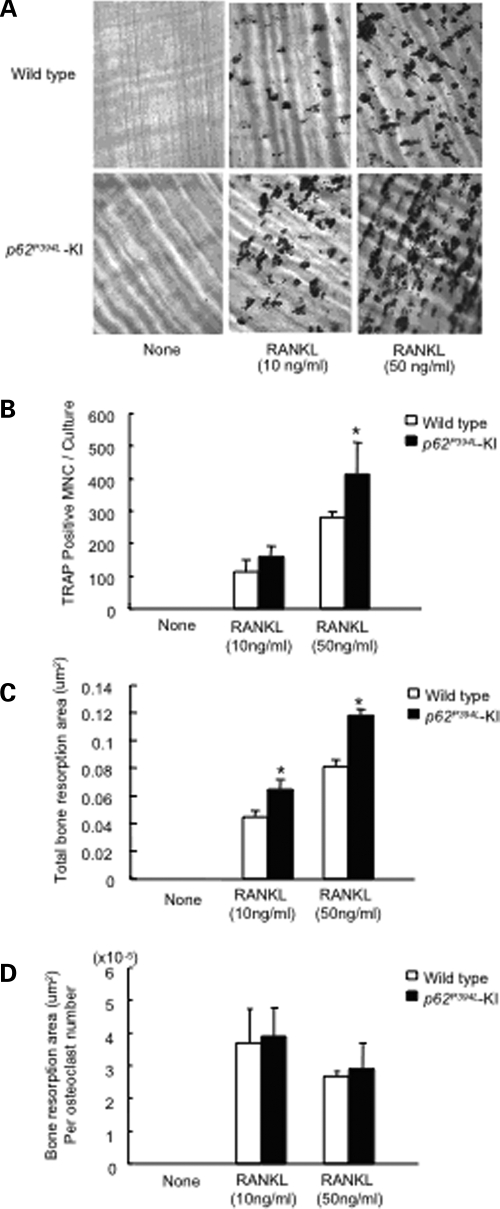
Bone resorption in marrow cultures from the KI mice was modestly increased compared with WT cultures (Fig. 3A–C). A 1.5-fold increase in bone resorption was observed when OCLs from KI mice were treated with RANKL (50 ng/ml) and cultured on dentin, as compared with RANKL-treated WT cultures. However, in contrast to OCLs from PDB patients which display increased bone resorption per OCL (3), the area resorbed per OCL was not different between KI and WT cultures (Fig. 3D).
Figure 3.
Resorption lacunae formed by OCLs from KI and WT OCL precursors: (A) resorption lacunae formed by OCLs on dentin. Non-adherent marrow cells were cultured on dentin slices for 14 days. The cells were then removed and the dentin slices were stained with acid hematoxylin. Original magnification, ×100; (B) TRAP-positive OCLs per dentin slice. After 9 days of culture, cells were fixed and stained for TRAP activity, and the number of TRAP-positive cells was quantitated. Results are expressed as the mean ± SEM for quadruplicate cultures from a typical experiment. Asterisk: P < 0.01 compared with results in WT cultures. Similar results were obtained in four independent experiments; (C) resorption area per dentin slice. Results represent the mean ± SEM for quadruplicate determinations for a typical experiment. Asterisk: P < 0.05 compared with results from WT cultures. Similar results were seen in five independent experiments; (D) bone resorption area per OCL number. Results represent the mean ± SEM for quadruplicate determinations of a typical experiment. Similar results were seen in four independent experiments.
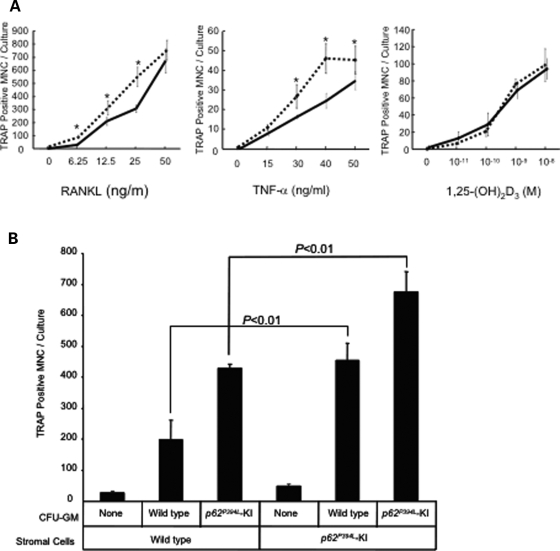
To determine if these effects were cell-autonomous in KI OCL precursors, as is the case in PDB, we cultured highly purified OCL precursors [CFU-GM (colony-forming units granulocyte-macrophage)] that lack marrow stromal cells, and marrow stromal cells from KI mice separately to determine their respective contribution to the enhanced OCL formation. CFU-GM from KI mice cultured in the absence of stromal cells were hyper-responsive to RANKL and TNF-α and formed increased numbers of OCLs compared with WT cultures. However, they were not hyper-responsive to 1α,25-(OH)2D3 (Fig. 4A).
Figure 4.
OCL formation by KI or WT OCL precursors in the absence or presence of KI or WT stromal cells: (A) OCLs formed from CFU-GM. CFU-GM from WT (solid lines) or KI mice (dotted lines) were cultured for 7 days in the presence of varying concentrations of RANKL, TNF-α or 1α,25-(OH)2D3. The cells were then fixed and stained for TRAP activity, and the number of TRAP-positive OCLs were counted. Results are expressed as the mean ± SEM for quadruplicate cultures from a typical experiment. Asterisk: P < 0.01 compared with results from WT cell cultures. Similar results were obtained in five independent experiments; (B) OCLs formed from CFU-GM in the presence of stromal cells. Stromal cells from KI or WT littermates were co-cultured with/without CFU-GM derived from KI or WT mouse bone marrow for 7 days in the presence of 10−8 m 1α,25-(OH)2D3. The cells were then fixed and stained for TRAP activity, and the number of TRAP-positive OCLs were counted. Results are expressed as the mean ± SEM for quadruplicate cultures from a typical experiment. Asterisk: P < 0.01 compared with results in WT cultures. Similar results were obtained from five independent experiments.
We then characterized bone marrow stromal cells from the KI mice for their capacity to support osteoclastogenesis. Marrow stromal cells and CFU-GM derived from KI or WT mice were co-cultured in the presence of 10−8 m 1α,25-(OH)2D3, and OCL formation was assessed after 9 days of culture. Both KI and WT stromal cells increased OCL formation from CFU-GM in response to 1α,25-(OH)2D3 as compared with cultures not containing stromal cells (Fig. 4B). However, KI stromal cells supported osteoclastogenesis to a much greater extent than WT stromal cells, and the greatest number of OCLs were formed in co-cultures of KI stromal cells with KI CFU-GM (Fig. 4B).
RANKL expression is increased in p62 P394L KI mouse stromal cells
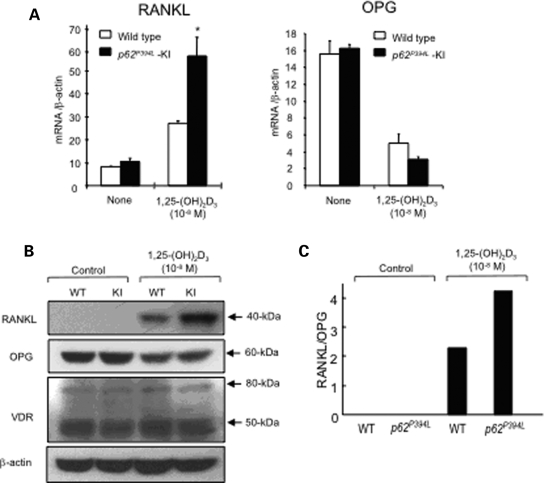
To determine the mechanism responsible for the increased osteoclastogenic capacity of KI stromal cells, WT and KI stromal cells were treated with 1α,25-(OH)2D3 for 10 days and their growth rate and expression levels of RANKL and osteoprotegerin (OPG) mRNA and protein were determined. Stromal cells derived from WT and KI mice had similar doubling times (data not shown). However, KI marrow stromal cells expressed higher levels of RANKL mRNA in response to 1α,25-(OH)2D3 than WT stromal cells, while OPG mRNA expression was decreased similarly in KI and WT stromal cells (Fig. 5A). Increased RANKL protein levels were also detected in lysates from 1α,25-(OH)2D3-treated KI stromal cells by western blotting, as compared with lysates from WT stromal cells (Fig. 5B). The ratio of RANKL/OPG levels in 1α,25-(OH)2D3-treated KI stromal cells was also significantly increased compared with WT stromal cells (Fig. 5C). Further, KI marrow stromal cells released RANKL into the conditioned media after treatment with 1α,25-(OH)2D3 [64 pg/ml, as measured by ELISA (enzyme-linked immunosorbent assay)], while no detectable RANKL was released from WT stromal cells. However, there were no differences in RANKL production between WT and KI stromal cells following treatment with IL-6 or PTHrP. There was also no difference in the level of vitamin D receptor in the WT and KI stromal cells, as determined by western blot analysis (Fig. 5B).
Figure 5.
RANKL and OPG expression in KI or WT stromal cells: (A) real-time RT–PCR. Stromal cells from KI and WT mice were cultured for 2 days with 10−8 m 1α,25-(OH)2D3. mRNA was then prepared and subjected to real time RT–PCR to quantitate RANKL (left) and OPG (right) mRNA levels. The values were normalized to β-actin mRNA levels; (B) western blot analysis. Stromal cells from KI and WT mice were cultured for 4 days with 10−8 m 1α,25-(OH)2D3 or vehicle. Cell lysates were then prepared and immunoblotted using anti-RANKL, anti-OPG, anti-VDR or anti-β-actin antibodies. The experiments were repeated three times with similar results; (C) ratio of RANKL/OPG. The RANKL and OPG expression levels from the western blot in (C) were quantitated by densitometry.
Activation of p38 MAPK is responsible for the increased RANKL production by stromal cells in response to 1α,25-(OH)2D3
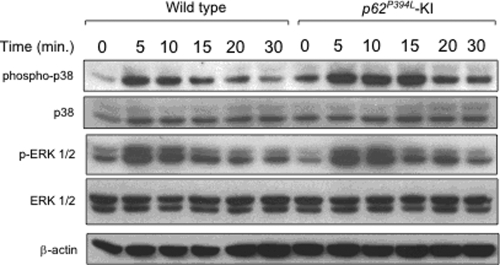
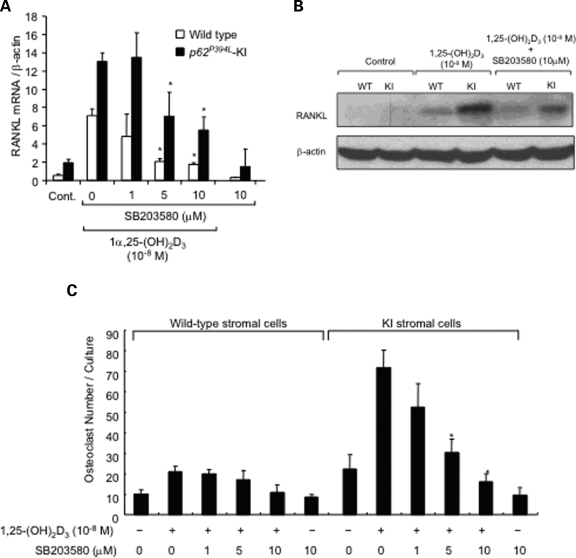
We next examined the effects of p62 P394L on activation of downstream signaling pathways induced by 1α,25-(OH)2D3 that resulted in the increased production of RANKL by stromal cells. As shown in Figure 6, both basal and 1α,25-(OH)2D3-induced phospho-p38 MAPK expression was elevated in KI marrow stromal cells compared with WT stromal cells, while no differences were seen in activation of ERK 1/2 in stromal cells of the two genotypes. Further, a highly specific p38 MAPK inhibitor (SB203580) dose-dependently decreased RANKL mRNA (Fig. 7A) and protein expression (Fig. 7B) in both WT and KI cells. Importantly, SB203580 inhibited the enhanced OCL formation that occurred in co-cultures of WT OCL precursors with KI stromal cells (Fig. 7C).
Figure 6.
Activation of the p38 MAPK and ERK 1/2 signaling pathways in KI or WT stromal cells induced by 1α,25-(OH)2D3: stromal cells from KI or WT mice were cultured with IMDM + 10% FCS for 14 days. Cells were then cultured with IMDM + 2% FCS for 24 h as a means of starvation. Cells were then exposed to 1α,25-(OH)2D3 for the indicated times, and were then lysed, fractionated by SDS–PAGE, and analyzed by immunoblot using antibodies recognizing phosphorylated and total p38 MAPK and ERK 1/2. β-actin served as the loading control.
Figure 7.
Effect of a p38 MAPK inhibitor on RANKL expression in KI stromal cells: (A) RANKL mRNA levels. Stromal cells from KI and WT mice were cultured for 2 days in the presence of 10−8m 1α,25-(OH)2D3 with varying concentrations of SB203580, a p38 MAPK inhibitor. mRNA was then prepared and subjected to real-time RT–PCR using primers for RANKL. The values were normalized to β-actin mRNA levels; (B) western blot analysis. Stromal cells from KI and WT mice were cultured for 4 days with or without 10−8m 1α,25-(OH)2D3 and 10 µM SB203580. Cell lysates were then prepared and immunoblotted with an anti-RANKL or anti-β-actin antibody; (C) OCL formation from WT CFU-GM cultured in the presence of WT or KI stromal cells and SB203580. Confluent bone marrow stromal cells from WT or KI mice were pretreated with H2O or SB203580 (10 μM) for 1 h. CFU-GM from WT mice were then added and OCL formation was induced by the addition of 10−8m 1α,25-(OH)2D3 in the presence or absence of SB203580. After 3 days of co-culture, the cells were fixed and stained for TRAP, and the number of TRAP-positive OCLs was counted. Results are expressed as the mean ± SEM for quadruplicate cultures from a typical experiment. Asterisk: P < 0.01 compared with results from WT cell cultures. Similar results were obtained from three independent experiments.
Bone phenotype of p62 P394L KI mice
Since both OCL precursors and marrow stromal cells from KI mice were highly osteoclastogenic, we assessed the bone phenotype of both p62+/P394L and p62 P394L/P394L mice compared with WT mice. Histological evaluation of either heterozygous or homozygous KI mice up to 18 months of age revealed no obvious bone abnormalities. Further, histomorphometric evaluation of vertebral bones from mice of each genotype at 4, 8 and 12 months of age revealed no significant differences in cancellous bone structure, OCL perimeter and both static and dynamic indices of bone formation among the three groups (Table 1).
Table 1.
Cancellous bone structure and turnover variables in p62 P394L mice
| Group | An.N (F:M) | Oc.Pm (%) | Ob.Pm (%) | Md.Pm (%) | MAR (µm/d) | BFR (µm2/µm/d) | BV/TV (%) | Tb.N (#/mm2) | Tb.Wi (µm) | Tb. Sp (mm2) |
|---|---|---|---|---|---|---|---|---|---|---|
| 4 Months | ||||||||||
| +/+ | 9 (7:2) | 25.2 ± 2.0 | 23.5 ± 1.7 | 33.2 ± 3.8 | 1.5 ± 0.1 | 0.5 ± 0.1 | 29.6 ± 2.2 | 7.5 ± 0.6 | 39.4 ± 1.8 | 99.6 ± 10.9 |
| +/p62_M_ | 13 (9:4) | 28.9 ± 1.9 | 21.1 ± 2.4 | 33.3 ± 3.1 | 1.6 ± 0.1 | 0.6 ± 0.1 | 24.8 ± 2.2 | 6.5 ± 0.4 | 37.7 ± 1.5 | 124.6 ± 11.1 |
| p62_M_/p62_M_ | 4 (1:3) | 22.1 ± 3.2 | 18.7 ± 3.8 | 28.8 ± 4.5 | 1.3 ± 0.2 | 0.4 ± 0.1 | 20.4 ± 1.8 | 5.7 ± 0.2 | 35.4 ± 2.8 | 139.4 ± 6.4 |
| 8 Months | ||||||||||
| +/+ | 4 (2:2) | 27.4 ± 2.9 | 12.5 ± 1.7 | 21.3 ± 7.6 | 1.1 ± 0.4 | 0.3 ± 0.1 | 22.6 ± 1.0 | 5.8 ± 0.2 | 39.4 ± 2.4 | 134.5 ± 4.9 |
| +/p62_M_ | 10 (4:6) | 24.7 ± 2.2 | 10.3 ± 1.5 | 28.7 ± 2.3 | 1.5 ± 0.1 | 0.4 ± 0.1 | 20.3 ± 1.4 | 6.0 ± 0.3 | 33.6 ± 1.2 | 136.1 ± 9.3 |
| P62_M_/p62_M_ | 5 (3:2) | 28.6 ± 2.2 | 11.3 ± 1.3 | 30.2 ± 3.2 | 1.3 ± 0.3 | 0.4 ± 0.1 | 19.0 ± 2.8 | 5.3 ± 0.6 | 35.8 ± 3.8 | 165.2 ± 28.5 |
| 12 Months | ||||||||||
| +/+ | 3 (1:2) | 28.6 ± 2.3 | 10.7 ± 1.8 | 28.1 ± 7.1 | 1.4 ± 0.1 | 0.4 ± 0.1 | 19.3 ± 3.3 | 6.1 ± 0.5 | 31.2 ± 3.0 | 134.9 ± 16.2 |
| +/p62_M_ | 4 (2:2) | 28.1 ± 3.6 | 16.0 ± 6.1 | 26.8 ± 3.9 | 1.7 ± 0.2 | 0.5 ± 0.1 | 16.7 ± 0.4 | 4.9 ± 0.2 | 34.4 ± 0.8 | 172.7 ± 7.5 |
| p62_M_/p62_M_ | 4 (1:3) | 36.8 ± 7.9 | 20.7 ± 2.0 | 33.9 ± 2.5 | 1.9 ± 0.1 | 0.7 ± 0.1 | 20.9 ± 4.1 | 6.0 ± 1.1 | 34.6 ± 1.2 | 145.3 ± 26.5 |
| _P_-values | ||||||||||
| Genotype | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| Age | NS | NS | <0.01a | NS | =0.01b | NS | <0.05c | NS | NS | NS |
DISCUSSION
Increased OCL formation and resorption play a major pathogenic role in the bone changes that result in PDB of bone. Large numbers of OCLs form in long-term cultures of pagetic bone marrow and in cultures of monocytes from patients with PDB (20,21). Further, OCL precursors from both sporadic and familial PDB patients are hyper-responsive to 1α,25-(OH)2D3, RANKL and TNF-α, forming OCLs at low concentrations of these factors (19,21,22). In addition, the OCLs that form in marrow cultures and in co-cultures of peripheral blood mononuclear cells with bone stromal cells from PDB patients are increased in size, hyper-multinucleated and have an increased bone resorbing capacity (21). In the present study, purified marrow OCL precursors depleted of stromal cells from p62 P394L KI mice were similarly found to be hyper-responsive to RANKL and TNF-α, and formed increased numbers of OCLs compared with WT cultures. However, the purified p62 P394L KI OCL precursors were not hyper-responsive to 1α,25-(OH)2D3 and did not form hyper-multinucleated OCLs or show increased bone resorption per OCL. These findings are similar to our previous results with OCL precursors from transgenic mice expressing the human p62 P392L gene under the control of the tartrate resistant acid phosphatase (TRAP) gene promoter (18). However, the transgenic mouse model did not allow us to assess the potential effects of p62 P392L on stromal or other cell types in the bone microenvironment, since transgene expression was restricted to cells in the OCL lineage. In contrast, the KI mouse model allows us to address this question.
Stromal cells from p62 P394L KI mice expressed significantly higher levels of RANKL in response to 1α,25-(OH)2D3 than WT stromal cells, analogous to patients with PDB (19). This increased RANKL expression was not due to changes in the distribution of the cell types present, since stromal cell cultures of the two genotypes were morphologically identical. Also, the increased RANKL expression by KI stromal cells appeared to be restricted to 1α,25-(OH)2D3 stimulation, since effects of PTHrP and IL-6 on RANKL production were similar in WT and KI stromal cells. KI but not WT marrow stromal cells also released RANKL into their conditioned media. These findings may in part explain the enhanced RANKL production present in the marrow microenvironment of pagetic lesions (19).
Previous studies have demonstrated that p38 MAPK signaling plays a major role in the induction of RANKL expression in bone marrow stromal cells in response to various cytokines, including IL-1β, TNF-α and TGF-β (23,24). Similarly, we found that the induction of RANKL by 1α,25-(OH)2D3 was dependent upon p38 MAPK in both WT and KI stromal cells, since addition of a p38 MAPK inhibitor, SB203580, to co-cultures of either WT or KI stromal cells with WT OCL precursors decreased RANKL expression and inhibited the 1α,25-(OH)2D3-induced OCL formation. However, RANKL expression was higher in KI than WT stromal cells under all treatment conditions, even in the presence of SB203580, suggesting that enhanced activation of p38 MAPK signaling is not the sole mechanism by which RANKL expression is elevated in stromal cells expressing p62 P394L.
The enhanced osteoclastogenic microenvironment caused by expression of mutant p62 in stromal cells may explain the increased bone remodeling found in uninvolved bone from patients with PDB (25). These authors ascribed this increased bone turnover to 2° hyperparathyroidism, although this seems unlikely since hyperparathyroidism is observed in <16% of PDB patients (26). Alternatively, PDB patients harboring a mutant p62 gene may have a more osteoclastogenic bone microenvironment throughout their skeleton than normal individuals. However, it remains unclear how this change in the marrow microenvironment could contribute to the development of the highly localized lesions found in PDB patients.
Although the p62 P394L mutation increased the osteoclastogenic potential of both OCL precursors and the marrow microenvironment in the KI mice, these changes were not sufficient to induce the development of pagetic OCLs or bone lesions. These results suggest that p62 P392L is a predisposing mutation but is not sufficient to induce PDB, and that additional factors, either genetic or environmental, may also be required. Several other loci in addition to the p62 gene mapping to human chromosome 5q35 have been reported to be linked to familial PDB (8), and Lucas et al. (27) recently reported that the locus at 10p13 appears to account for the majority of PDB in families of British descent who do not carry p62 mutations. Thus, it is possible that mutations or polymorphisms in more than one gene may contribute to the development of PDB in individual patients, which could account for the variable penetrance and disease severity seen even within PDB families. However, this would still not account for the focal nature of the disease within individual patients. One intriguing possibility for which there is preliminary evidence is that somatic mutations in bone cells may contribute to the pathogenesis of pagetic lesions, much as somatic mutations of oncogenes and tumor suppressor genes do in cancer (28).
There is also strong support for an environmental component to the etiology of PDB (3). For example, a significant reduction in the prevalence of PDB over the past 2–3 decades has been reported, which would be consistent with changes in the environment that might affect PDB prevalence (29). We and others have detected measles virus nucleocapsid transcripts in OCLs from PDB patients and suggested that MVNP may be an environmental factor that contributes to the development of PDB (3,30,31). To test this, we targeted MVNP to the OCL lineage in transgenic mice, which resulted in formation of bone lesions characteristic of PDB, although these were only detected in a subset of the mice and not all bones were affected (32). Further, when OCL precursors from TRAP-p62 P392L transgenic mice were transduced with MVNP, the OCL precursors became hyper-responsive to 1α,25-(OH)2D3, which they were not previously (33).
Taken together, these results demonstrate that the murine equivalent of the human p62 P392L mutation has major effects on both OCL precursors and the bone microenvironment, but is not sufficient to induce PDB. Further, this unique mouse model can serve as a valuable research tool for identifying environmental and other genetic factors that may, in combination with mutant p62, contribute to the development of PDB.
MATERIALS AND METHODS
Chemicals
Fetal calf serum (FCS) was purchased from JRH Biologicals (Lenexa, KS, USA). All other chemicals and media were purchased from Sigma-Aldrich (St Louis, MO, USA) unless otherwise noted. RANKL, TNF-α and macrophage colony-stimulating factor (M-CSF) were purchased from R&D Systems (Minneapolis, MN, USA). 1α,25-(OH)2D3 was generously provided by Teijing Corp. (Tokyo). Polyclonal anti-IκB-α, anti-phospho-IκB-α, anti-p38, anti-phospho-p38, anti-ERK, and anti-phospho-ERK antibodies were from Cell Signaling Technology (Danvers, MA, USA). Protease inhibitor mixtures and SB203580 were from Calbiochem (San Diego, CA, USA).
Generation of p62 P394L KI mice
All animal studies were approved by the Institutional Animal Care and Use Committees at Virginia Commonwealth University, University of Pittsburgh School of Medicine, and the VA Pittsburgh Healthcare System, and were conducted in accordance with the Animal Welfare Act, the PHS Policy on Humane Care and Use of Laboratory Animals, and the U.S. Government Principles for the Utilization and Care of Vertebrate Animals Used in Testing, Research, and Training.
To generate KI mice carrying the p62 P394L mutation in the endogenous mouse SQSTM1/p62 gene (Entrez GeneID: 18412), a targeting vector was generated by subcloning 5′ and 3′ regions of the p62 gene into the multiple cloning sites flanking the PGK-neo minigene in the pKO Scrambler NTKV-1901 vector (Stratagene, La Jolla, CA, USA), which had first been modified to contain loxP sites at both ends of PGK-neo minigene. This vector also provides an MC1-tk minigene as a negative selectable marker. Both the 5′ and 3′ homology arms of the p62 gene were amplified from 129/SV embryonic stem (ES) cell deoxyribonucleic acid (DNA) by high-fidelity polymerase chain reaction (PCR), and were fully sequenced. The 3.9 kb 5′ homology arm extends from the Hind III site in intron 4 to the BamHI site in intron 7, while the 4.4 kb 3′ homology arm extends from the same BamHI site to an EcoRV site 3′ of the p62 gene (Fig. 1A and B). The P394L mutation (a C-to-T transition) was introduced into exon 8 by PCR-based site-directed mutagenesis. The targeting vector was linearized by Ase I digestion, and was introduced into 129/SvEv ES cells by electroporation. The cells were plated in selective medium containing 250 µg/ml G418 and 2 µm gancyclovir, and ∼450 G418r/gancyclovirr clones were picked and expanded for analysis. Genomic DNA was prepared and analyzed by long-range PCR and Southern blot analysis to identify clones in which the targeting vector had integrated into the endogenous p62 locus by homologous recombination and possessed the C→T mutation. Three correctly targeted ES cell clones were identified (Supplementary Material, Fig. S1A). To excise the PGK-neo minigene which was still present in intron 7 (Fig. 1C), each of the three clones was expanded and electroporated with an MC1-cre gene and grown initially in the absence of selection. Colonies were then picked, trypsinized, and divided into duplicate plates, one grown with and one without G418. G418-sensitive colonies were further expanded and deletion of the PGK-neo gene was confirmed by PCR analysis. Two independent correctly targeted and _neo_-deleted clones (Fig. 1D) were further expanded and microinjected into day 3.5 blastocysts derived from C57BL/6 mice, which were then reimplanted into the uteri of pseudopregnant foster females. Chimeric offspring were identified by coat color chimerism (agouti/black). A total of 17 chimeras were obtained from the two targeted ES cell lines, and those showing the highest degree of chimerism were bred to WT C57BL/6 females. Germline transmission of the targeted gene in the resulting pups was assessed both by coat color (agouti) and by genotyping of DNA isolated from a small tail biopsy. Numerous agouti pups were obtained from these matings, and ∼50% carried the P394L KI allele, as expected. Heterozygotes were interbred to obtain homozygous KI mice. The mice are genotyped by PCR using a pair of primers that flank the introduced loxP site: sense (exon 7): 5′-GAA CAG ATG GAG TCG GGA AAC TGC-3′ and antisense (intron 7): 5′-GTT GCC AAG ACT AGA CAG GAC AGG-3′, yielding a product of 268 bp for the WT allele and 312 for the p62 P394L KI allele.
OCL formation
Non-adherent marrow cells (1 × 105 cells/well: 96-well plate) were prepared as previously described (34) and cultured in α-MEM + 10% FCS for 9 days in the presence of varying concentrations of TNF-α, RANKL or 1,25-(OH)2D3. In experiments in which RANKL and TNF-α were used to stimulate OCL formation, the cultures also contained 10 ng/ml of M-CSF. M-CSF was not added to cultures treated with 1α,25-(OH)2D3. For studies using highly purified OCL precursors, CFU-GM-derived cells were generated in marrow cultures as previously described (18) and then cultured (1 × 105 cells/well: 96-well plate) for 9 days. Cells were then stained for TRAP using a leukocyte acid phosphatase kit (Sigma), and TRAP-positive cells were quantitated.
Bone resorption assay
Non-adherent marrow cells were cultured on mammoth dentin slices (Wako, Osaka, Japan) in α-MEM + 10% FCS containing 10 ng/ml M-CSF and either RANKL (10 or 50 ng/ml) or TNF-α (50 ng/ml). After 14 days of culture, the cells were stained for TRAP and the number of TRAP-positive cells was counted. The cells were then removed, the dentin slices were stained with acid hematoxylin, and areas of dentin resorption were determined using image analysis techniques (NIH Image System).
Stromal cell culture
Long-term Dexter-type marrow cultures were established to obtain marrow stromal cells as previously described (35). Briefly, 107 marrow cells were incubated in IMDM with 10% FCS in T-25 tissue culture flasks and the cultures fed weekly. At the end of 3 weeks of culture, non-adherent cells were removed, and the cultures were used as a source of marrow stromal cells.
Quantitative PCR analysis
Mouse marrow stromal cells were cultured for 2 days and subjected to reverse transcription PCR (RT–PCR) analysis for expression of RANKL and OPG mRNA. Total RNA was extracted using RNAzol B solution (Tel-Test Inc., Griendswood, TX, USA) and cDNAs were synthesized using a RNA PCR Kit (Applied Biosystems, Foster City, CA, USA). RANKL and OPG mRNA levels were then quantitated using a Bio-Rad ICYCLER and SYBR Green, and were normalized to β-actin. The gene-specific primers for mouse RANKL were 5′-CAG CAT CGC TCT GTT CCT GTA-3′ (sense) and 5′-CTG CGT TTT CAT GGA GTC TCA-3′ (antisense). The gene-specific primers for mouse OPG were 5′-CCC TTG CCC TGA CCA CTC-3′ (sense) and 5′-TCC TCA CAC TCA CAC ACT CG-3′ (antisense). The gene-specific primers for mouse β-actin were 5′-GGC CGT ACC ACT GGC ATC GTG ATG-3′ (sense) and 5′-CTT GGC CGT CAG GCA GCT CGT AGC-3′ (antisense).
Co-culture of marrow stromal cells with OCL precursors
Stromal cells (3 × 103 cells/well; 96-well plates) from KI or WT littermates were co-cultured with CFU-GM derived from KI or WT mouse bone marrow (1 × 105 cells/well) for 7 days in α-MEM + 10% FCS with or without 10−8 m 1α,25-(OH)2D3. The OCL number was then determined by TRAP staining.
Immunoblotting of OCL precursors or stromal cells from KI or WT mice
Cytokine-treated or Control OCL precursors (CFU-GM) or stromal cells from KI or WT mice were washed twice with ice-cold PBS (phosphate buffered saline). Cells were lysed in buffer containing 20 mm Tris, pH 7.5, 150 mm NaCl, 1 mm ethylenediaminetetraacetic acid (EDTA), 1 mm EGTA [ethylene glycol-bis-(2-aminoethyl)-N,N,N′, N′-tetraacetic acid], 1% Triton X-100, 2.5 mm sodium pyrophosphate, 1 mm β-glycerophosphate, 1 mm Na3VO4, 1 mm NaF, and ×1 protease inhibitor mixture. Fifty micrograms of cell lysate was boiled in the presence of sodium dodecyl sulfate (SDS) sample buffer [0.5 M Tris–HCl, pH 6.8, 10% (w/v) SDS, 10% glycerol, 0.05% (w/v) bromphenol blue] for 5 min and subjected to electrophoresis on 7.5% SDS–PAGE (polyacrylamide gel electrophoresis). Proteins were transferred to nitrocellulose membranes using a semi-dry blotter (Bio-Rad, CA, USA) and incubated in blocking solution (5% non-fat dry milk in TBS containing 0.1% Tween-20) for 1 h to reduce non-specific binding. Membranes were then exposed to primary antibodies overnight at 4°C, washed three times, and incubated with secondary goat anti-mouse or rabbit IgG HRP-conjugated antibody for 1 h. Membranes were washed extensively, and an enhanced chemiluminescence detection assay was performed following the manufacturer’s directions (Bio-Rad). All blots were densitometrically quantitated and the results expressed relative to Control and normalized to β_-_actin.
RANKL ELISA assay
Conditioned media from stromal cell cultures was harvested 7 days after the addition of 1α,25-(OH)2D3 and fresh media. The concentration of RANKL present was determined using an ELISA kit for mouse RANKL (R&D Systems, Inc.), according to the manufacturer’s instructions.
MTT assay
Stromal cells (3 × 103 cells/well) seeded in 96-well plates were treated with vehicle or 10−8 m 1α,25-(OH)2D3 for 1, 5 and 10 days. MTT reagent (Sigma) was added and metabolic activity was assayed at OD570.
Histomorphometric analysis of p62 P394L vertebral bones
The first through fifth lumbar vertebrae from 4-, 8- and 12-month-old p62 P394L homozygote, heterozygote and WT littermates were fixed in 10% buffered formalin for 24–48 h. The first through fourth lumbar vertebrae were then completely decalcified in 10% EDTA at 4°C, processed through graded alcohols and embedded in paraffin. The fifth lumbar vertebra was processed without decalcification and embedded in methyl methacrylate. Longitudinal sections (5 µm thick) of both decalcified and undecalcified vertebral bodies were cut and mounted on glass slides. The decalcified sections were stained for TRAP as described by Liu et al. (36). The undecalcified sections were either stained with 1% toluidine blue for visualization of osteoblasts or left unstained for visualization of calcein labels. Histomorphometry was performed on the region of cancellous bone between the cranial and caudal growth plates of the third and the fifth lumbar vertebral bodies under bright field or fluorescent light at a magnification of ×200, using the OsteoMeasure 4.00C morphometric program (OsteoMeasure; OsteoMetrics, Atlanta, GA, USA). OCL perimeter (Oc.Pm) was defined as the length of bone surface covered with TRAP-positive mono-or multinuclear cells. Osteoblast perimeter (Ob.Pm), cancellous bone volume (BV/TV), trabecular width (Tb.Wi), number (Tb.N) and separation (Tb.Sp), mineralized perimeter (Md.Pm), mineral apposition rate (MAR) and bone formation rate (BFR) were also measured. All variables were expressed and calculated according to the recommendations of the American Society for Bone and Mineral Research Nomenclature Committee (37,38).
Statistical analysis
For all cell culture studies, significance was evaluated using a two-tailed, unpaired Student’s _t_-test, with P < 0.05 considered to be significant. For the histological analysis of bones, two-way analysis of variance (ANOVA) was performed to compare the differences in all histomorphometric variables among genotype and across age. One-way ANOVA was performed to compare the differences among age groups of each genotype of variables in which an age-related change was detected. Statistical analysis was performed using NCSS 2004 and PASS programs (NCSS, Kaysville, UT, USA).
SUPPLEMENTARY MATERIAL
Supplementary Material is available at HMG Online.
FUNDING
This work was supported by research funds from the National Institutes of Health (R01 AR053537 to J.J.W. and PO1 AR049363 to G.D.R.), the Paget Foundation, funds received from National Institutes of Health/NCRR/CTRC Grant # 1 UL1 RR024153-01, and the VCU/Massey Cancer Center Transgenic/Knock-out Mouse Core, which is supported by National Institutes of Health Grant P30 CA16059.
Supplementary Material
[Supplementary Data]
ACKNOWLEDGEMENTS
The materials are the result of work supported with resources and the use of facilities at the VA Pittsburgh Healthcare System, Research and Development.
Conflict of Interest statement. None declared.
REFERENCES
- 1.Kanis J.A. Pathophysiology and Treatment of Paget’s Disease of Bone. 2nd edn. London: Martin Dunitz; 1998. [Google Scholar]
- 2.Hosking D.J. Paget’s disease of bone. Br. Med. J. (Clin. Res. Ed.) 1981;283:686–688. doi: 10.1136/bmj.283.6293.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roodman G.D., Windle J.J. Paget disease of bone. J. Clin. Invest. 2005;115:200–208. doi: 10.1172/JCI24281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siris E.S., Ottman R., Flaster E., Kelsey J.L. Familial aggregation of Paget’s disease of bone. J. Bone Miner. Res. 1991;6:495–500. doi: 10.1002/jbmr.5650060511. [DOI] [PubMed] [Google Scholar]
- 5.Morales-Piga A.A., Rey-Rey J.S., Corres-Gonzalez J., Garcia-Sagredo J.M., Lopez-Abente G. Frequency and characteristics of familial aggregation of Paget’s disease of bone. J. Bone Miner. Res. 1995;10:663–670. doi: 10.1002/jbmr.5650100421. [DOI] [PubMed] [Google Scholar]
- 6.Seton M., Choi H.K., Hansen M.F., Sebaldt R.J., Cooper C. Analysis of environmental factors in familial versus sporadic Paget’s disease of bone – the New England Registry for Paget’s Disease of Bone. J. Bone Miner. Res. 2003;18:1519–1524. doi: 10.1359/jbmr.2003.18.8.1519. [DOI] [PubMed] [Google Scholar]
- 7.Leach R.J., Singer F.R., Roodman G.D. The genetics of Paget’s disease of the bone. J. Clin. Endocrinol. Metab. 2001;86:24–28. doi: 10.1210/jcem.86.1.7112. [DOI] [PubMed] [Google Scholar]
- 8.Daroszewska A., Ralston S.H. Genetics of Paget’s disease of bone. Clin. Sci. (Lond.) 2005;109:257–263. doi: 10.1042/CS20050053. [DOI] [PubMed] [Google Scholar]
- 9.Hocking L.J., Lucas G.J., Daroszewska A., Mangion J., Olavesen M., Cundy T., Nicholson G.C., Ward L., Bennett S.T., Wuyts W., et al. Domain-specific mutations in sequestosome 1 (SQSTM1) cause familial and sporadic Paget’s disease. Hum. Mol. Genet. 2002;11:2735–2739. doi: 10.1093/hmg/11.22.2735. [DOI] [PubMed] [Google Scholar]
- 10.Laurin N., Brown J.P., Morissette J., Raymond V. Recurrent mutation of the gene encoding sequestosome 1 (SQSTM1/p62) in Paget disease of bone. Am. J. Hum. Genet. 2002;70:1582–1588. doi: 10.1086/340731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morissette J., Laurin N., Brown J.P. Sequestosome 1: mutation frequencies, haplotypes, and phenotypes in familial Paget’s disease of bone. J. Bone Miner. Res. 2006;21(Suppl. 2):38–44. doi: 10.1359/jbmr.06s207. [DOI] [PubMed] [Google Scholar]
- 12.Chung P.Y.J., Beyens G., Guanabens N., Boonen S., Papapoulos S., Karperien M., Eekhoff M., Van Wesenbeeck L., Jennes K., Geusens P., et al. Founder effect in different European countries for the recurrent P392L SQSTM1 mutation in Paget’s disease of bone. Calcif. Tissue Int. 2008;83:34–42. doi: 10.1007/s00223-008-9137-2. [DOI] [PubMed] [Google Scholar]
- 13.Rhodes E.C., Johnson-Pais T.L., Singer F.R., Ankerst D.P., Bruder J.M., Wisdom J., Hoon D.S.B., Lin E., Bone H.G., Simsic K.J., et al. Sequestosome 1 (SQSTM1) mutations in Paget’s disease of bone from the United States. Calcif. Tissue Int. 2008;82:271–277. doi: 10.1007/s00223-008-9114-9. [DOI] [PubMed] [Google Scholar]
- 14.Moscat J., Diaz-Meco M.T., Wooten M.W. Signal integration and diversification through the p62 scaffold protein. Trends Biochem. Sci. 2007;32:95–100. doi: 10.1016/j.tibs.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 15.Layfield R., Ciani B., Ralston S.H., Hocking L.J., Sheppard P.W., Searle M.S., Cavey J.R. Structural and functional studies of mutations affecting the UBA domain of SQSTM1 (p62) which cause Paget’s disease of bone. Biochem. Soc. Trans. 2004;32:728–730. doi: 10.1042/BST0320728. [DOI] [PubMed] [Google Scholar]
- 16.Leach R.J., Singer F.R., Ench Y., Wisdom J.H., Pina D.S., Johnson-Pais T.L. Clinical and cellular phenotypes associated with sequestosome 1 (SQSTM1) mutations. J. Bone Miner. Res. 2006;21(Suppl. 2):45–50. doi: 10.1359/jbmr.06s208. [DOI] [PubMed] [Google Scholar]
- 17.Langston A.L., Ralston S.H. Management of Paget’s disease of bone. Rheumatology. 2004;43:955–959. doi: 10.1093/rheumatology/keh243. [DOI] [PubMed] [Google Scholar]
- 18.Kurihara N., Hiruma Y., Zho H., Subler M.A., Dempster D.W., Singer F.R., Reddy S.V., Gruber H.E., Windle J.J., Roodman G.D. Mutation of the sequestosome 1 (p62) gene increases osteoclastogenesis but does not induce Paget disease. J. Clin. Invest. 2007;117:133–142. doi: 10.1172/JCI28267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Menaa C., Reddy S.V., Kurihara N., Maeda H., Anderson D., Cundy T., Cornish J., Singer F.R., Bruder J.M., Roodman G.D. Enhanced RANK ligand expression and responsivity of bone marrow cells in Paget’s disease of bone. J. Clin. Invest. 2000;105:1833–1838. doi: 10.1172/JCI9133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kukita A., Chenu C., McManus L.M., Mundy G.R., Roodman G.D. Atypical multinucleated cells form in long-term marrow cultures from patients with Paget’s disease. J. Clin. Invest. 1990;85:1280–1286. doi: 10.1172/JCI114565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neale S.D., Smith R., Wass J.A., Athanasou N.A. Osteoclast differentiation from circulating mononuclear precursors in Paget’s disease is hypersensitive to 1,25-dihydroxyvitamin D3 and RANKL. Bone. 2000;27:409–416. doi: 10.1016/s8756-3282(00)00345-8. [DOI] [PubMed] [Google Scholar]
- 22.Menaa C., Barsony J., Reddy S.V., Cornish J., Cundy T., Roodman G.D. 1,25-Dihydroxyvitamin D3 hypersensitivity of osteoclast precursors from patients with Paget’s disease. J. Bone Miner. Res. 2000;15:228–236. doi: 10.1359/jbmr.2000.15.2.228. [DOI] [PubMed] [Google Scholar]
- 23.Nguyen A.N., Stebbins E.G., Henson M., O’Young G., Choi S.J., Quon D., Damm D., Reddy M., Ma J.Y., Haghnazari E., et al. Normalizing the bone marrow microenvironment with p38 inhibitor reduces multiple myeloma cell proliferation and adhesion and suppresses osteoclast formation. Exp. Cell Res. 2006;312:1909–1923. doi: 10.1016/j.yexcr.2006.02.026. [DOI] [PubMed] [Google Scholar]
- 24.Rossa C., Ehmann K., Liu M., Patil C., Kirkwood K.L. MKK3/6-p38 MAPK signaling is required for IL-1beta and TNF-alpha-induced RANKL expression in bone marrow stromal cells. J. Interferon Cytokine Res. 2006;26:719–729. doi: 10.1089/jir.2006.26.719. [DOI] [PubMed] [Google Scholar]
- 25.Meunier P.J., Coindre J.M., Edouard C.M., Arlot M.E. Bone histomorphometry in Paget’s disease. Quantative and dynamic analysis of pagetic and nonpagetic bone tissue. Arthritis Rheum. 1980;23:1095–1103. doi: 10.1002/art.1780231005. [DOI] [PubMed] [Google Scholar]
- 26.Siris E.S., Clemens T.P., McMahon D., Gordon A., Jacobs T.P., Canfield R.E. Parathyroid function in Paget’s disease. J. Bone Miner. Res. 1989;4:75–79. doi: 10.1002/jbmr.5650040111. [DOI] [PubMed] [Google Scholar]
- 27.Lucas G.J.A., Riches P.L., Hocking L.J., Cundy T., Nicholson G.C., Walsh J.P., Ralston S.H. Identification of a major locus for Paget’s disease on chromosome 10p13 in families of British descent. J. Bone Miner. Res. 2008;23:58–63. doi: 10.1359/jbmr.071004. [DOI] [PubMed] [Google Scholar]
- 28.Layfield R. The molecular pathogenesis of Paget disease of bone. Expert Rev. Mol. Med. 2007;9:1–13. doi: 10.1017/S1462399407000464. [DOI] [PubMed] [Google Scholar]
- 29.Cooper C., Harvey N.C., Dennison E.M., van Staa T.P. Update on the epidemiology of Paget’s disease of bone. J. Bone Miner. Res. 2006;21(Suppl. 2):3–8. doi: 10.1359/jbmr.06s201. [DOI] [PubMed] [Google Scholar]
- 30.Reddy S.V., Singer F.R., Roodman G.D. Bone marrow mononuclear cells from patients with Paget’s disease contain measles virus nucleocapsid messenger ribonucleic acid that has mutations in a specific region of the sequence. J. Clin. Endocrinol. Metab. 1995;80:2108–2111. doi: 10.1210/jcem.80.7.7608263. [DOI] [PubMed] [Google Scholar]
- 31.Friedrichs W.E., Reddy S.V., Bruder J.M., Cundy T., Cornish J., Singer F.R., Roodman G.D. Sequence analysis of measles virus nucleocapsid transcripts in patients with Paget’s disease. J. Bone Miner. Res. 2002;17:145–151. doi: 10.1359/jbmr.2002.17.1.145. [DOI] [PubMed] [Google Scholar]
- 32.Kurihara N., Zhou H., Reddy S.V., Garcia Palacios V., Subler M.A., Dempster D.W., Windle J.J., Roodman G.D. Expression of measles virus nucleocapsid protein in osteoclasts induces Paget’s disease-like bone lesions in mice. J. Bone Miner. Res. 2006;21:446–455. doi: 10.1359/JBMR.051108. [DOI] [PubMed] [Google Scholar]
- 33.Kurihara N., Hiruma Y., Zho H., Subler M.A., Dempster D.W., Singer F.R., Reddy S.V., Gruber H.E., Windle J.J., Roodman G.D. Mutation of the sequestosome 1 (p62) gene increases osteoclastogenesis but does not induce Paget disease. J. Clin. Invest. 2007;117:133–142. doi: 10.1172/JCI28267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hentunen T.A., Jackson S.H., Chung H., Reddy S.V., Lorenzo J., Choi S.J., Roodman G.D. Characterization of immortalized osteoclast precursors developed from mice transgenic for both bcl-X(L) and simian virus 40 large T antigen. Endocrinol. 1999;140:2954–2961. doi: 10.1210/endo.140.7.6867. [DOI] [PubMed] [Google Scholar]
- 35.Mori K.J., Fujitake H., Ohkubo H., Ito Y., Dexter T.M. Development of stromal cell colonies in bone marrow cell culture. Gann. 1978;69:689–693. [PubMed] [Google Scholar]
- 36.Liu C., Sherrard D.J., Maloney N.A., Howard G.A. Reactivation of inhibited bone acid phosphatase and its significance in bone histomorphometry. J. Histochem. Cytochem. 1987;35:1355–1363. doi: 10.1177/35.12.3680930. [DOI] [PubMed] [Google Scholar]
- 37.The American Society for Bone and Mineral Research President’s Committee on Nomenclature. Proposed standard nomenclature for new tumor necrosis factor family members involved in the regulation of bone resorption. J. Bone Miner. Res. 2000;15:2293–2296. doi: 10.1359/jbmr.2000.15.12.2293. [DOI] [PubMed] [Google Scholar]
- 38.Parfitt A.M., Drezner M.K., Glorieux F.H., Kanis J.A., Malluche H., Meunier P.J., Ott S.M., Recker R.R. Bone histomorphometry: standardization of nomenclature symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J. Bone Miner. Res. 1987;2:595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
[Supplementary Data]