Ventral and dorsal pathways for language (original) (raw)
Abstract
Built on an analogy between the visual and auditory systems, the following dual stream model for language processing was suggested recently: a dorsal stream is involved in mapping sound to articulation, and a ventral stream in mapping sound to meaning. The goal of the study presented here was to test the neuroanatomical basis of this model. Combining functional magnetic resonance imaging (fMRI) with a novel diffusion tensor imaging (DTI)-based tractography method we were able to identify the most probable anatomical pathways connecting brain regions activated during two prototypical language tasks. Sublexical repetition of speech is subserved by a dorsal pathway, connecting the superior temporal lobe and premotor cortices in the frontal lobe via the arcuate and superior longitudinal fascicle. In contrast, higher-level language comprehension is mediated by a ventral pathway connecting the middle temporal lobe and the ventrolateral prefrontal cortex via the extreme capsule. Thus, according to our findings, the function of the dorsal route, traditionally considered to be the major language pathway, is mainly restricted to sensory-motor mapping of sound to articulation, whereas linguistic processing of sound to meaning requires temporofrontal interaction transmitted via the ventral route.
Keywords: DTI, extreme capsule, fMRI, language networks, arcuate fascicle, extreme capsule
Current theories on brain organization suggest that cognitive functions such as language are organized in widespread, segregated, and overlapping networks (1). Anatomically, such large-scale networks comprise specialized brain areas (network nodes) and their interconnecting white matter fiber tracts (network connections).
One of the first large-scale network models on language was elaborated by Wernicke (2) in the 19th century. Based on deficit-lesion correlations of postmortem dissections, he localized recognition of sound images of words to the posterior superior temporal lobe (later labeled Wernicke's area) and representations of motor images of words to the inferior frontal lobe (later labeled Broca's area). By reconciling the principle of localized functions with a connectionist framework, he hypothesized that words as supramodal linguistic units emerge from an interaction between these distant temporal and frontal areas (2). Lichtheim (3) later translated Wernicke's ideas into illustrative diagrams, formulating a three-component model of language in which Broca's and Wernicke's areas are interconnected via a hypothetical (not anatomically localized) “concept center” involved in semantic processing. This model became the standard reference for clinicians to predict aphasic syndromes from lesions of either a center or a connection (3).
Although anatomically and functionally underspecified, one important implication of these early network ideas was that sensory-based representations of speech must interface with at least two systems—a motor-articulatory system and a conceptual system. This observation, in turn, corresponds well to recent research on the functio-anatomical organization of other domains, particularly the visual system (4, 5) where sensory input interfaces with motor systems for visually guided reaching and grasping (dorsal “how” stream) and with conceptual systems for object recognition (ventral “what” stream).
Building on this analogy, Hickok and Poeppel (6, 7) and others (e.g., refs. 8 and 9) recently proposed a dual stream model for auditory language processing. From the superior temporal gyrus, which is engaged in early cortical stages of speech perception, the system diverges into two processing streams. The dorsal stream projects dorsoposteriorly toward inferior parietal and posterior frontal lobe regions and is involved in auditory-motor integration by mapping acoustic speech sounds to articulatory representations. The prototype task targeting this dorsal stream is repetition of speech (6, 7). The ventral stream projects ventrolaterally to the middle and inferior temporal cortices and serves as a sound-to-meaning interface by mapping sound-based representations of speech to widely distributed conceptual representations. Hence, the prototype task targeting this ventral stream is listening to meaningful speech (6, 7).
The goal of our study was to investigate the neuroanatomical basis of this dual stream model by defining cortical network nodes within both streams using functional MRI (fMRI) activations and tracking the white matter fibers linking these activated nodes using a novel method of DTI-based tractography (10).
Two fMRI language experiments were designed to functionally segregate both streams as predetermined by the model: overt repetition of aurally presented pseudowords versus real words was assumed to activate areas involved in auditory-motor mapping in the dorsal processing stream (11). In contrast, attentive listening to aurally presented sentences of meaningful speech versus meaningless pseudo speech was expected to activate areas associated with sentence comprehension in the ventral processing stream (12, 13).
The activated nodes then served as seed regions for a unique probabilistic fiber-tracking method (10). This method enables us to determine the most probable anatomical pathways linking two activated nodes. This is achieved by combining probabilistic maps generated from two seeds, which results in a voxelwise estimation of the probability that a voxel is part of the pathway connecting both seeds (see Methods). Here, we focused on investigating long-distance association tracts connecting temporal and frontal nodes within the dorsal and ventral processing streams in the left hemisphere.
Results
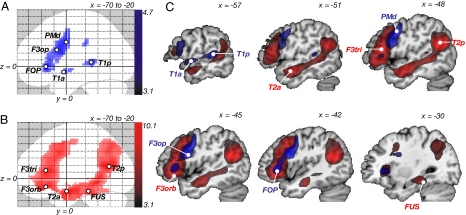
In the repetition experiment, the main effect of repetition versus rest resulted in strong bilateral temporofrontal activation with peaks in primary auditory and motor areas [supporting information (SI) Fig. S1_A_ _Upper Row_]. By contrasting repetition of pseudowords with real words, activation focused on the superior temporal gyrus in the left temporal lobe and shifted from primary motor to premotor and prefrontal areas in the left frontal lobe (Fig. S1_A_ Lower Row). This latter effect defined the network subserving auditory-motor mapping during sublexical pseudoword repetition. Within this network, the five peaks with the highest activation strengths were chosen as seed regions (Fig. 1 A and C and Table 1), which were located in the posterior and anterior parts of the superior temporal gyrus (T1a and T1p), the pars opercularis of the inferior frontal gyrus (F3op/BA 44), the dorsal premotor cortex (PMd/BA 6), and the ventrolateral prefrontal cortex [deep frontal operculum (FOP)].
Fig. 1.
fMRI results. Functional networks involved in (A) repetition (of pseudowords compared with words) and (B) comprehension (listening to normal sentences compared to meaningless pseudo sentences), analyzed in two random effects analyses (no. = 33). Activations are overlaid as maximum intensity projections (MIP; x, −70 to −20) on a canonical brain. Peak voxels within each cluster defined the nodes of the networks, which served as seed regions for the probabilistic fiber tracking. (C) Both contrasts (repetition, blue; comprehension, red) displayed along the x coordinate of the seed regions. Statistical threshold was set at P < 0.001, uncorrected. T1a/p, anterior/posterior superior temporal gyrus; T2a/p, anterior/posterior middle temporal gyrus; FUS, fusiform gyrus; F3orb/tri/op, pars orbitalis/triangularis and opercularis of the inferior frontal gyrus; FOP, deep frontal operculum; PMd, dorsal premotor cortex.
Table 1.
Repetition of pseudowords compared with real words
| Region | Seed | Coordinates | t |
|---|---|---|---|
| Left temporal | |||
| Posterior superior temporal gyrus | T1p | −57 −36 6 | 4.36 |
| Anterior superior temporal gyrus | T1a | −57 3 −9 | 3.39 |
| Left frontal | |||
| Frontal operculum/anterior insula | FOP | −42 27 0 | 4.75 |
| Dorsal premotor cortex (BA 6 ) | PMd | −48 0 36 | 4.25 |
| Inferior frontal gyrus, pars opercularis (BA 44), ventral premotor cortex | F3op | −45 9 24 | 4.20 |
| Supplementary motor area | No seed | −6 6 57 | 3.60 |
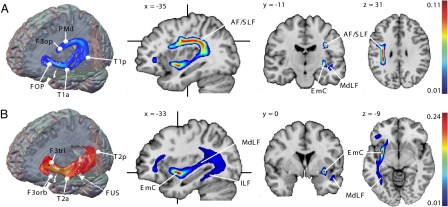
Long-distance association fibers connecting the activated nodes within this network were identified by permutatively combining the probability maps generated from both temporal with those from the three frontal seeds. All six pairwise region-to-region connections can be seen as group-mean maps in Fig. S2. These six connections constitute a fiber network subserving repetition of speech (Fig. 2A). Within this network, both temporal seeds (T1a/T1p) are connected with the premotor seeds (F3op and PMd) via a dorsal route along the AF/SLF system. Thereby, fibers from the anterior temporal seed (T1a) were collected from the middle longitudinal fascicle (MdLF) before entering into the AF/SLF system in the posterior superior temporal lobe. From there, fibers arch around the caudal end of the Sylvian fissure and course in the white matter of the rostral parietal lobe to the premotor cortex (Fig. 2A and Fig. S2). Thus a composite fiber bundle composed of MdLF and SLF/AF constitutes the dorsal pathway for language. Relating our findings to the proposed subdivision of the SLF into three components in monkeys (14), we detected what were most likely fibers of the SLF III in addition to the AF. In contrast, fibers from both the anterior and posterior temporal seed to the FOP run exclusively via the ventral route (Fig. 2A and Fig. S2), as described hereafter.
Fig. 2.
Fiber tracking results. Composite fiber networks subserving repetition (A) and comprehension (B) computed by averaging the pairwise connections of 33 subjects defined in the repetition and comprehension experiment, respectively. Three-dimensional tractography renderings visualize the spatial orientation of both networks. Crosshairs on sagittal sections indicate the orientation of the coronal and axial sections. Maximum PIBI (probability index forming part of the bundle of interest) values are given at the top of the color bar. EmC, extreme capsule; AF/SLF, arcuate and superior longitudinal fascicle; MdLF/ILF, middle and inferior longitudinal fascicle. Abbreviation of seed regions are as indicated in Fig. 1
In the comprehension experiment the main effect of listening to aurally presented normal and pseudo sentences evoked broad bilateral temporofrontal activation with a clear peak in primary auditory areas (Fig. S1_B_ Upper Row). By contrasting listening to meaningful sentences with listening to meaningless pseudo sentences, activation shifted to the middle and inferior temporal gyrus in the left temporal lobe and to the ventrolateral prefrontal cortex in the left frontal lobe (Fig. S1_B_ Lower Row). This activation defined the network subserving auditory sentence comprehension. Within this network, the five peaks with the highest activation strengths were chosen as seed regions (Fig. 1 B and C and Table 2), which were located in the posterior and anterior parts of the middle temporal gyrus (T2p and T2a), the fusiform gyrus (FUS), and the pars orbitalis (F3orb/BA 47) and pars triangularis (F3tri/BA 45) of the inferior frontal gyrus.
Table 2.
Listening to meaningful sentences compared with pseudo sentences
| Region | Seed | Coordinates | t |
|---|---|---|---|
| Left temporal | |||
| Posterior middle temporal gyrus | T2p | −48 −60 18 | 10.11 |
| Anterior middle temporal gyrus | T2a | −51 0 −18 | 9.70 |
| Fusiform gyrus | FUS | −30 −33 −18 | 8.37 |
| Angular gyrus | No seed | −36 −60 39 | 7.15 |
| Left frontal | |||
| Inferior frontal gyrus, pars triangularis (BA45) | F3tri | −48 27 12 | 8.95 |
| Inferior frontal gyrus, pars orbitalis (BA 47) | F3orb | −45 27 −12 | 8.83 |
| Frontal pole | No seed | −9 63 27 | 8.41 |
| Supplementary motor area | No seed | −3 18 54 | 7.24 |
| Middle frontal gyrus | No seed | −39 18 30 | 7.01 |
| Right temporal | |||
| Anterior middle temporal gyrus | No seed | 51 −3 −18 | 7.62 |
| Posterior middle temporal gyrus | No seed | 42 −54 18 | 5.77 |
| Fusiform gyrus | No seed | 33 −30 −21 | 5.40 |
| Right frontal | |||
| Inferior frontal gyrus, pars orbitalis | No seed | 48 24 −9 | 6.07 |
| Central sulcus | No seed | 42 −21 54 | 6.59 |
| Middle frontal gyrus | No seed | 51 30 30 | 5.24 |
To identify association pathways linking the activated nodes within this network for auditory comprehension, probability maps generated from the three temporal seeds were combined with those generated from both frontal seeds. All six pairwise region-to-region connections are displayed as group-mean maps in Fig. S3. These six connections form a fiber network involved in auditory sentence comprehension (Fig. 2B). Within this network, all temporal and frontal nodes are connected via a strong ventral pathway running through the extreme capsule (EmC) and entering medially to the insula into the orbitofrontal cortex. Starting from the anterior temporal node (T2a), fibers first run medially in posterior direction to join the MdLF before entering into the EmC. From the posterior temporal node (T2p), fibers join the MdLF in anterior direction and then continue into the EmC. From the fusiform gyrus (FUS), fibers first course posteriorly in the inferior longitudinal fascicle (ILF), then turn around in the caudal temporal lobe and join the MdLF in anterior direction to reach the EmC (Fig. 2B and Fig. S3). After entering into the frontal lobe, the EmC splits into two branches, an inferior branch running in the white matter on the floor of the orbital cortex which contacts the orbitofrontal seed (F3orb), and a superior branch running in the white matter of the inferior frontal gyrus, which terminates at the pars triangularis (F3tri). Thus the EmC is the ventral association pathway connecting the anterior temporal lobe with the ventrolateral prefrontal cortex.
Two temporal association tracts contribute fibers to the EmC: the MdLF and ILF, which run in the white matter of the superior and inferior temporal lobe while colleting fibers from adjacent cortices. The resulting composite fiber tract of EmC, MdLF, and ILF thus provides structural connectivity for language processing via a ventral route (Fig. 2B).
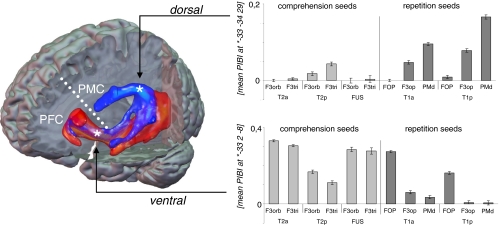
In Fig. 3, a composite fiber network subserving repetition and comprehension is displayed which consists of all 12 region-to-region connections. To illustrate the contribution of each region-to-region connection to either the ventral or the dorsal pathway, mean probability indices were calculated separately for each connection in the peak voxel within both pathways. Plots show that the ventral pathway is composed of all connections defined in the comprehension experiment and both connections to the FOP defined in the repetition experiment (Fig. 3 lower diagram), whereas the dorsal pathway consists of connections solely defined in the repetition experiment (Fig. 3 upper diagram).
Fig. 3.
Dual pathway network for language. Composite fiber network for repetition (blue) and comprehension (red). Three-dimensional tractography renderings visualize the spatial orientation of both networks to each other. Dashed white line illustrates the bisection of the frontal lobe into a ventral part, which is connected to the postrolandic brain via the ventral pathway and a dorsal part, which is connected to the postrolandic brain via the dorsal pathway. From peak voxels (indicated with an asterisk) within each pathway, mean PIBI values (y axis) were extracted to show the contribution of each temporofrontal connection to the respective pathway. On x axis, lower row indicates the temporal, upper row the frontal seed region of each pairwise connection. PIBI, probability index of forming part of the bundle of interest; PFC, prefrontal cortex; PMC, premotor cortex. Abbreviations of seed regions are as indicated in Fig. 1.
Discussion
Combining functional MRI and a unique probabilistic DTI-based fiber tracking method, we extracted the most probable anatomical pathways linking functionally specified language areas. Our data strongly support the dual stream model with two concurrent parallel anatomical pathways for language processing (6, 7, 9). We showed that in both streams, long-distance anatomical connectivity between functionally defined temporal and frontal nodes is mediated along different ventral and dorsal association fibers. Superior temporal and premotor regions, activated during repetition, interact via a dorsal pathway along the AF/SLF. In contrast, middle and inferior temporal regions and the ventrolateral prefrontal cortex, activated during auditory comprehension, interact via a ventral pathway that runs through the EmC.
In the following text we assert that the close correspondence between our findings, autoradiographic tracing studies in monkeys, and recent models of language processing strongly suggest the existence and functional significance of two association pathways for language in the human brain.
The dorsal pathway along the AF and SLF was described in numerous recent DTI studies of the human brain (15–17). There is no doubt that these broad fascicles connect the temporal and inferior parietal lobes with the frontal lobe. However, a remaining question is which areas in the frontal brain are actually reached by this dorsal tract. In our study, only the frontal seeds in the premotor cortices (F3op and PMd) are connected with the temporal lobe via the dorsal pathway. This closely corresponds to results from tracing studies in monkeys that show that neither the AF nor the SLF continues to the ventrolateral prefrontal cortex (14, 18).
The ventral pathway along the EmC is in line with DTI findings in humans (19) and autoradiographic tracing studies in monkeys (14, 18, 20). These studies strongly support that the ventrolateral prefrontal cortex, including the human homologues of BA 45 and BA 47, is connected with the temporal lobe mainly via the EmC. Importantly, the EmC must be distinguished from the uncinate fascicle, which is considered a limbic pathway (18) mainly connecting the amygdala and hippocampus in the medial temporal with the prefrontal lobe, and the external capsule, which is strictly a corticostriatal projection tract (18).
In the temporal lobe, the MdLF and ILF, two long-association tracts well defined in the monkey brain (14, 18) and recently also delineated in the human brain (21, 22), contribute fibers to both the AF/SLF and the EmC. This shows that functionally defined fiber tracking does not delineate anatomically defined fiber tracts as a whole but rather extracts composite pathways consisting of (portions of) different anatomically defined fiber bundles.
In the dorsal processing stream, repetition of pseudowords compared with real words revealed activation in left temporal and frontal areas. We ascribed activation of the superior temporal gyrus (T1a/T1p) to sublexical processing steps during speech perception (12, 23), whereas activation of prefrontal (FOP) and premotor areas (BA44, BA 6) is interpreted by the higher phonological processing demands necessary for preparing production of pseudowords compared with high-frequent normal words (8, 11, 24, 25). Anatomical connectivity between the temporal and premotor seeds is provided by the dorsal AF/SLF system. Functionally, this dorsal pathway has been proposed to map the phonemic representations onto motor representations for articulation (6, 8, 11), thereby constraining the planned motor output with the mental representations of the sound structure (26). In isolation, this stream may suffice to produce nonpropositional speech and echolalia. Repetition and imitation are hallmarks when children start speaking. Disruption of this network (e.g., after stroke) may relate to two aspects ascribed to conduction aphasia: relatively selective impairment of repetition with preserved comprehension and the production of phonological paraphasias (27).
The prefrontal seed in the FOP, close to the anterior part of the insula, projects to the temporal lobe exclusively via the ventral pathway and thus differs from the premotor seeds. We speculate that the frontal operculum, with its tight structural connection to the superior temporal lobe via the EmC, might be involved in monitoring processes during repetition. In our experiment this might be particularly important to control correct sequencing of unknown combinations of phonemes when repeating pseudowords.
In the ventral stream, the contrast between listening to normal sentences and pseudo sentences activated middle and inferior temporal regions and the ventrolateral prefrontal cortex (BA 45, 47), areas that have previously been associated with lexical-semantic processing (28–31). The middle temporal lobe was shown to participate in accessing lexical, semantic, and conceptual information (6, 12, 23, 31). Contextual comprehension, however, requires an iterative exchange with the prefrontal cortex, which is involved in executive aspects of semantic processing (28)—e.g., controlled semantic retrieval (32), semantically based analysis of grammatical structures (30), and application of cognitive rules (33). Structurally, this interaction is provided by the EmC. With this pathway, it might be speculated, children learn to derive meaning and construct knowledge. Disturbance of this ventral pathway may lead to transcortical sensory aphasia, a syndrome characterized by poor comprehension but preserved repetition and production (34).
This functional interpretation of dorsal and ventral pathways with respect to repetition and comprehension is complementary to the account of Friederici et al. (35), who proposed that both pathways are implicated in specific types of syntactic operations. Processing of simple grammatical structures (e.g., computing local phrase structures) was found to involve the anterior superior temporal gyrus and the frontal operculum (36), areas linked by the ventral pathway (35). Processing of complex grammatical structures (e.g., computing hierarchical dependency relations), which is a particularly crucial aspect of human language, involves the posterior superior temporal gyrus/sulcus and BA 44/45 (36), areas connected via the dorsal pathway (35). However, variation of syntactic complexity may correlate with working memory demands (36), and thus, involvement of the dorsal stream for processing of complex syntactic operations might be partially explained as a result of an increase in syntactic working memory load.
It is noteworthy that Wernicke himself assumed a direct connection for a “temporal coincident activation of temporal and frontal speech regions,” which he located to the “fibrae propriae” between insula and claustrum (2), which is almost identical to the ventral pathway along the EmC described here. In fact, he postulated a dual pathway system for language processing with a direct route of “regulatory influence of the sensory centre on the motor centre” and another, indirect semantic route for conceptual understanding (37). However, the convictions of influential contemporary neurologists such as von Monakow (38), who favored the arcuate fascicle, led to the abandonment of the ventral language pathway in the scientific community.
Functional segregation into different processing streams is well described for the visual (5), auditory (39), and visuomotor (4) systems. In these domains, spatial stimulus processing and sensory-motor integration follows a dorsal stream, whereas stimulus perception and recognition are transmitted via a ventral stream. Thus, functionally and anatomically different streams were shown to subserve specific types of computations independent of the particular domain.
For language, our study shows how two task demands, prototypical for these computations, are preferentially mediated by one of two streams: sublexical pseudoword repetition by the dorsal stream and higher-level sentence comprehension by the ventral stream. Thus it seems that language, for all its human uniqueness and sophistication, adheres to the same anatomical principles that govern brain functions in other domains. This functional dichotomization into two segregated networks, however, is the result of an artificial experimental situation. We hypothesize that in naturally occurring speech (e.g., propositional speech), both networks interact closely to reach high proficiency in verbal communication.
By tracing the connections between seed regions that were functionally defined with a specific language task, our approach allows for an integrative, anatomically informed, and constrained investigation of brain networks of, in principle, any aspect of language processing. It will be of interest to investigate whether these two streams are sufficient to accommodate other levels of natural language processing.
Methods
Participants.
Thirty-three healthy volunteers (11 females, mean age 34 years, range 18–71 years, 18 right-handed) were recruited from the database of the Freiburg Brain Imaging Centre. All subjects were scanned with the approval of the local ethics committee and gave their written consent.
fMRI Event-Related Experiments.
Stimuli and experimental design.
In the repetition experiment, stimuli consisted of 60 German words (e.g., foto [photo in English], and tomate [tomato]), and 60 meaningless pseudowords (e.g., doso and losate), all of which were composed of two or three syllables). In the comprehension experiment, stimuli consisted of 90 well-formed German sentences (e.g., der pilot fliegt das flugzeug [the pilot flies the plane] and 90 meaningless pseudo sentences [e.g., ren simot plieft mas kugireug]. In both experiments, pseudo stimuli were derived from the original stimuli by substituting the phonemes on the basis of German phonotactical rules, which resulted in stimuli without any meaning but with a phonemic structure typical for German. Pseudo stimuli matched the original stimuli in length and phonemic complexity.
Stimuli were distributed into two sessions in the repetition and into three sessions in the comprehension experiment; order of stimuli within a session was pseudorandomized, with pairs of normal and pseudo stimuli never occurring in the same session. The interstimulus interval varied between 6,000 and 11,000 ms in the repetition and 3,000 and 6,000 ms in the comprehension experiment. Stimuli were presented binaurally with the software Presentation (http://nbs.neurobs.com) using MR-compatible headphones.
Task.
In the comprehension experiment, subjects were asked to listen carefully to all stimuli and press a button at the end of each stimulus, irrespective of whether they had heard a normal sentence or a pseudo sentence. This simple task was chosen to keep executive demands as low as possible while ensuring that participants were alert. In the repetition experiment, subjects were asked to overtly repeat words and pseudowords immediately after presentation.
MRI Data Acquisition.
Functional and structural MRI data from all 33 subjects were acquired on a 3T Siemens TIM Trio scanner (see SI Methods)
fMRI Data Analysis.
fMRI data were analyzed with SPM5 (www.fil.ion.ucl.ac.uk/spm) using standard procedures for preprocessing, single-subject, and random-effects group analyses (see SI Methods).
Definition of Seed Regions.
The seed regions for the probabilistic fiber tracking were extracted from the _t_-maps of the fMRI random effects analyses of both experiments. Within the major activation clusters the peak voxels were identified, resliced to the native space of each subject's DTI data, and enlarged to a sphere with a radius of 4 mm, each containing 33 seed voxels.
Probabilistic DTI-Based Fiber Tracking.
DTI data were analyzed using a recently developed method of pathway extraction (10), which is implemented in the Matlab-based DTI and Fiber Toolbox (www.uniklinik-freiburg.de/mr/live/arbeitsgruppen/diffusion_en.html).
First, the diffusion tensor (DT) was computed (40) from the movement- and distortion-corrected diffusion-weighted imaging dataset. Second, a Monte Carlo simulation of random walks (MCRW) similar to the probabilistic index of connectivity (PICo) method (41) was used to calculate probabilistic maps separately for each seed region. In these maps, the visiting frequency of a voxel reflects the degree of connectivity to the seed region. The number of random walks was set to 105 and maximum fiber length to 150 voxels. The tracking area was restricted to a white-matter mask to avoid tracking across anatomical borders. To ensure contact of the cortical seed regions with white matter, a rim of gray matter was included in the mask. Third, region-to-region anatomical connectivity between two seed regions (A and B) was computed using a newly developed combination of probability maps (10). On a computational level, this combination implies a multiplication, which takes the main traversing direction of the random walk into account. Walks starting from seed regions A and B may face in opposing directions (connecting fibers) or merge and face in the same direction (merging fibers; see Fig. S4). Within the pathway connecting A and B, the proportion of connecting fibers should exceed the proportion of merging fibers. Using the directional information during the multiplication, merging fibers are suppressed and connecting fibers are preserved (10). This method enables the extraction of the most probable direct pathway between two seed regions without using a priori knowledge about the presumed course. The resulting values represent a voxel-wise estimation of the probability index that a voxel is part of the connecting fiber bundle of interest (probability index forming part of the bundle of interest [PIBI]).
To identify the most probable temporofrontal association tracts, all temporal maps were combined permutatively with all frontal maps. This resulted in six combined maps defined in the repetition and six combined maps defined in the comprehension experiment, respectively.
Postprocessing of Probability Maps.
The combined maps were scaled to the range between 0 and 1, spatially normalized into the standard MNI space, and smoothed with an isotropic 3 mm Gaussian kernel.
Group maps for each region-to-region connection were computed by averaging the combined maps from all subjects, resulting in 12 mean maps. Composite networks for repetition and comprehension were computed by averaging the six mean maps defined in each experiment, respectively. Consequently, voxels represent the arithmetic mean of the PIBI from all contributing probability maps. To remove random artifacts, only voxels with PIBI values >0.0148 were displayed, which excludes 95% of the voxels with PIBI >10−6. This value was generated empirically from the distribution observed in a large collection of preprocessed combined probability maps (see Fig. S5).
Supplementary Material
Supporting Information
Acknowledgments.
This research was funded by the Bundesministerium für Bildung und Forschung (Research Collaborations Grant 01GW0661 for “Mechanisms of Brain Reorganisation in the Language Network”) and the Deutsche Forschungsgemeinschaft Grant WE 1352/14–1.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Mesulam MM. Large-scale neurocognitive networks and distributed processing for attention, language, and memory. Ann Neurol. 1990;28(5):597–613. doi: 10.1002/ana.410280502. [DOI] [PubMed] [Google Scholar]
- 2.Wernicke C. The aphasic symptom-complex. A psychological study on an anatomical basis. Breslau, Germany: Translated from German). (Cohn and Weigert; 1874. [Google Scholar]
- 3.Lichtheim L. On aphasia. Brain. 1885;7:433–485. [Google Scholar]
- 4.Goodale MA, Milner AD. Separate visual pathways for perception and action. Trends Neurosci. 1992;15(1):20–25. doi: 10.1016/0166-2236(92)90344-8. [DOI] [PubMed] [Google Scholar]
- 5.Ungerleider L, Mishkin M. Two cortical visual pathways. In: Ingle DJ, Goodale MA, Mansfield RJW, editors. Analysis of Visual Behavior. Cambridge, MA: MIT Press; 1982. pp. 549–586. [Google Scholar]
- 6.Hickok G, Poeppel D. Dorsal and ventral streams: A framework for understanding aspects of the functional anatomy of language. Cognition. 2004;92(1–2):67–99. doi: 10.1016/j.cognition.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 7.Hickok G, Poeppel D. The cortical organization of speech processing. Nat Rev Neurosci. 2007;8(5):393–402. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- 8.Warren JE, Wise RJ, Warren JD. Sounds do-able: Auditory-motor transformations and the posterior temporal plane. Trends Neurosci. 2005;28(12):636–643. doi: 10.1016/j.tins.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 9.Wise RJ. Language systems in normal and aphasic human subjects: Functional imaging studies and inferences from animal studies. Br Med Bull. 2003;65:95–119. doi: 10.1093/bmb/65.1.95. [DOI] [PubMed] [Google Scholar]
- 10.Kreher BW, et al. Connecting and merging fibres: Pathway extraction by combining probability maps. Neuroimage. 2008;43(1):81–89. doi: 10.1016/j.neuroimage.2008.06.023. [DOI] [PubMed] [Google Scholar]
- 11.Wise RJ, Greene J, Buchel C, Scott SK. Brain regions involved in articulation. Lancet. 1999;353(9158):1057–1061. doi: 10.1016/s0140-6736(98)07491-1. [DOI] [PubMed] [Google Scholar]
- 12.Binder JR, et al. Human temporal lobe activation by speech and nonspeech sounds. Cereb Cortex. 2000;10(5):512–528. doi: 10.1093/cercor/10.5.512. [DOI] [PubMed] [Google Scholar]
- 13.Scott SK, Blank CC, Rosen S, Wise RJ. Identification of a pathway for intelligible speech in the left temporal lobe. Brain. 2000;123(Pt 12):2400–2406. doi: 10.1093/brain/123.12.2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmahmann JD, et al. Association fibre pathways of the brain: Parallel observations from diffusion spectrum imaging and autoradiography. Brain. 2007;130(Pt 3):630–653. doi: 10.1093/brain/awl359. [DOI] [PubMed] [Google Scholar]
- 15.Catani M, et al. Symmetries in human brain language pathways correlate with verbal recall. Proc Natl Acad Sci USA. 2007;104(43):17163–17168. doi: 10.1073/pnas.0702116104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Catani M, Jones DK, Ffytche DH. Perisylvian language networks of the human brain. Ann Neurol. 2005;57(1):8–16. doi: 10.1002/ana.20319. [DOI] [PubMed] [Google Scholar]
- 17.Glasser MF, Rilling JK. DTI tractography of the human brain's language pathways. Cereb Cortex. 2008;18(11):2471–2482. doi: 10.1093/cercor/bhn011. [DOI] [PubMed] [Google Scholar]
- 18.Schmahmann JD, Pandya DN. Fiber Pathways of the Brain. New York: Oxford Univ Press; 2006. [Google Scholar]
- 19.Anwander A, Tittgemeyer M, von Cramon DY, Friederici AD, Knosche TR. Connectivity-based parcellation of Broca's area. Cereb Cortex. 2007;17(4):816–825. doi: 10.1093/cercor/bhk034. [DOI] [PubMed] [Google Scholar]
- 20.Petrides M, Pandya DN. Efferent association pathways from the rostral prefrontal cortex in the macaque monkey. J Neurosci. 2007;27(43):11573–11586. doi: 10.1523/JNEUROSCI.2419-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Catani M, Jones DK, Donato R, Ffytche DH. Occipito-temporal connections in the human brain. Brain. 2003;126(Pt 9):2093–2107. doi: 10.1093/brain/awg203. [DOI] [PubMed] [Google Scholar]
- 22.Makris N, et al. Delineation of the middle longitudinal fascicle in humans: A quantitative, in vivo, DT-MRI study. Cereb Cortex. 2008 Jul 31; doi: 10.1093/cercor/bhn124. doi: 10.1093/cercor/bhn124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scott SK, Johnsrude IS. The neuroanatomical and functional organization of speech perception. Trends Neurosci. 2003;26(2):100–107. doi: 10.1016/S0166-2236(02)00037-1. [DOI] [PubMed] [Google Scholar]
- 24.Bookheimer SY, Zeffiro TA, Blaxton TA, Gaillard PW, Theodore WH. Activation of language cortex with automatic speech tasks. Neurology. 2000;55(8):1151–1157. doi: 10.1212/wnl.55.8.1151. [DOI] [PubMed] [Google Scholar]
- 25.Price CJ, et al. Hearing and saying. The functional neuro-anatomy of auditory word processing. Brain. 1996;119(Pt 3):919–931. doi: 10.1093/brain/119.3.919. [DOI] [PubMed] [Google Scholar]
- 26.Indefrey P, Levelt WJ. The spatial and temporal signatures of word production components. Cognition. 2004;92(1–2):101–144. doi: 10.1016/j.cognition.2002.06.001. [DOI] [PubMed] [Google Scholar]
- 27.Anderson JM, et al. Conduction aphasia and the arcuate fasciculus: A reexamination of the Wernicke-Geschwind model. Brain Lang. 1999;70(1):1–12. doi: 10.1006/brln.1999.2135. [DOI] [PubMed] [Google Scholar]
- 28.Bookheimer S. Functional MRI of language: New approaches to understanding the cortical organization of semantic processing. Annu Rev Neurosci. 2002;25:151–188. doi: 10.1146/annurev.neuro.25.112701.142946. [DOI] [PubMed] [Google Scholar]
- 29.Demonet JF, et al. The anatomy of phonological and semantic processing in normal subjects. Brain. 1992;115(Pt 6):1753–1768. doi: 10.1093/brain/115.6.1753. [DOI] [PubMed] [Google Scholar]
- 30.Dapretto M, Bookheimer SY. Form and content: Dissociating syntax and semantics in sentence comprehension. Neuron. 1999;24(2):427–432. doi: 10.1016/s0896-6273(00)80855-7. [DOI] [PubMed] [Google Scholar]
- 31.Vigneau M, et al. Meta-analyzing left hemisphere language areas: Phonology, semantics, and sentence processing. Neuroimage. 2006;30(4):1414–1432. doi: 10.1016/j.neuroimage.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 32.Thompson-Schill SL, D'Esposito M, Aguirre GK, Farah MJ. Role of left inferior prefrontal cortex in retrieval of semantic knowledge: A reevaluation. Proc Natl Acad Sci USA. 1997;94(26):14792–14797. doi: 10.1073/pnas.94.26.14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Musso M, et al. Broca's area and the language instinct. Nat Neurosci. 2003;6(7):774–781. doi: 10.1038/nn1077. [DOI] [PubMed] [Google Scholar]
- 34.Boatman D, et al. Transcortical sensory aphasia: Revisited and revised. Brain. 2000;123(Pt 8):1634–1642. doi: 10.1093/brain/123.8.1634. [DOI] [PubMed] [Google Scholar]
- 35.Friederici AD, Bahlmann J, Heim S, Schubotz RI, Anwander A. The brain differentiates human and non-human grammars: Functional localization and structural connectivity. Proc Natl Acad Sci USA. 2006;103(7):2458–2463. doi: 10.1073/pnas.0509389103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grodzinsky Y, Friederici AD. Neuroimaging of syntax and syntactic processing. Curr Opin Neurobiol. 2006;16(2):240–246. doi: 10.1016/j.conb.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 37.Wernicke C. The Aphasic Symptom-Complex. Berlin: Translated from German). (Urban and Schwarzenberg; 1906. [Google Scholar]
- 38.Monakow V. Novel experimental contributions to the anatomy of the loop: Preliminary communication (Translated from German) Neurologisches Centralblatt. 1885;12:265–268. [Google Scholar]
- 39.Kaas JH, Hackett TA. ‘What’ and ‘where’ processing in auditory cortex. Nat Neurosci. 1999;2(12):1045–1047. doi: 10.1038/15967. [DOI] [PubMed] [Google Scholar]
- 40.Basser PJ, Mattiello J, LeBihan D. Estimation of the effective self-diffusion tensor from the NMR spin echo. J Magn Reson B. 1994;103(3):247–254. doi: 10.1006/jmrb.1994.1037. [DOI] [PubMed] [Google Scholar]
- 41.Parker GJ, Haroon HA, Wheeler-Kingshott CA. A framework for a streamline-based probabilistic index of connectivity (PICo) using a structural interpretation of MRI diffusion measurements. J Magn Reson Imaging. 2003;18(2):242–254. doi: 10.1002/jmri.10350. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information