Drug use in children: cohort study in three European countries (original) (raw)
Abstract
Objective To provide an overview of drug use in children in three European countries.
Design Retrospective cohort study, 2000-5.
Setting Primary care research databases in the Netherlands (IPCI), United Kingdom (IMS-DA), and Italy (Pedianet).
Participants 675 868 children aged up to 14 (Italy) or 18 (UK and Netherlands).
Main outcome measure Prevalence of use per year calculated by drug class (anatomical and therapeutic). Prevalence of “recurrent/chronic” use (three or more prescriptions a year) and “non-recurrent” or “acute” use (less than three prescriptions a year) within each therapeutic class. Descriptions of the top five most commonly used drugs evaluated for off label status within each anatomical class.
Results Three levels of drug use could be distinguished in the study population: high (>10/100 children per year), moderate (1-10/100 children per year), and low (<1/100 children per year). For all age categories, anti-infective, dermatological, and respiratory drugs were in the high use group, whereas cardiovascular and antineoplastic drugs were always in the low use group. Emollients, topical steroids, and asthma drugs had the highest prevalence of recurrent use, but relative use of low prevalence drugs was more often recurrent than acute. In the top five highest prevalence drugs topical inhaled and systemic steroids, oral contraceptives, and topical or systemic antifungal drugs were most commonly used off label.
Conclusion This overview of outpatient paediatric prescription patterns in a large European population could provide information to prioritise paediatric therapeutic research needs.
Introduction
Recent years have seen growing concerns about the incompleteness of the evidence relating to the efficacy and safety of drugs used in children. Almost all of the drugs prescribed to children are the same as those originally developed for adults. They are often prescribed on an unlicensed or “off label” basis (percentages ranging from 11-80%1) simply by extrapolating data for adults, without conducting any paediatric clinical, kinetic, dose finding, or formulation studies in children. Diseases in children, however, might be different from their adult equivalents, and the processes underlying growth and development might lead to a different effect or an adverse drug reaction unseen in adults (Reye’s syndrome is an outstanding example).
To provide legitimate and appropriate treatment for children’s diseases, new legislation was approved in the United States in 2003 and the European Union in 2007.2 Both the Food and Drug Administration (FDA) and the European Medicines Agency for the Evaluation of Medicinal Products (EMEA) now offer extensions of drug licences to companies who provide evidence concerning the efficacy and safety in children of new drugs or off label drugs.3 4 5 6 The World Health Organization underlines the need for these actions and in December 2007 launched a global campaign to “make medicines child size” to address the need for improved availability and access to safe child specific medicines for all children.7
We investigated the current use of paediatric drugs in children in three European countries, using population based data on primary care prescriptions.
Methods
Setting
The primary care of children is entrusted to general practitioners in the UK and the Netherlands and to paediatricians in Italy.8 9 Access to health care is free in Italy and the UK and fully covered by healthcare insurance in the Netherlands. In these countries, primary care physicians are responsible for children’s health care, which means that all clinical information concerning the patients (including summaries of specialist and hospital care) is kept in their medical records. As all children need to be registered with a general practitioner in the Netherlands and UK and with a family paediatrician in Italy, the databases are population based.9
Data collection
We used the same protocol to study prescription patterns in the three countries, making use of the Pedianet database (paediatric electronic medical records from 150 paediatricians since 2000) in Italy,10 the integrated primary care information (IPCI) database (comprising adult and paediatric electronic medical records from more than 400 doctors since 1996) in the Netherlands,8 11 12 and the IMS disease analyser database (IMS-DA: electronic medical records on adults and children from 670 doctors) in the UK.13 All of these databases include the complete automated medical records of primary care physicians and have been used and proved valid for pharmacoepidemiological research.9 The age and sex distribution in the various databases is representative for the country of origin.
Study population and drug prescriptions
The dynamic study population in each country consisted of all children aged 0-18 years (0-14 years in Italy) who had a database history of at least six months or who were born during the study period (1 January 2000 to 31 December 2005). We calculated the person time of follow-up for each child, stratified by calendar year and age group. Age was assessed on 1 January of each year and grouped according to the guidelines of the International Conference of Harmonization (ICH) as <2, 2-11, and 12-18.14 We could not further stratify the youngest age category into newborns (<1 month) and infants (1-24 months) as exact dates of birth were not available because of privacy regulations. Each child was followed from the start of the study period or the date of registration with the primary practice (whichever was the latest) until the cancellation of registration with the practice or the end of the study period. We used the person time accumulated in each calendar year as the denominator to calculate prevalence rates. Over the study period children could contribute to more than one age category.
All prescribed drugs in children during follow-up were retrieved from the prescription data in the database. The drug prescriptions were grouped on the basis of the WHO Anatomical Therapeutic Chemical (ATC) classification system, which made comparison between countries possible.
Statistical analysis
We estimated user prevalence rates (per 1000 person years) by counting the number of children using a specific drug in a specific calendar year. The prevalence rates were calculated by age and country to account for differences in distributions between populations and to allow for direct comparisons within groups. User prevalence rates should be interpreted as the number of children per 1000 who use a specific class of drug in one year. We could not calculate prevalence of drug use for children aged 15-18 in Italy because all of children were censored at the age of 15. We used person years rather than individuals as the denominator because of the dynamic nature of age and the population.
For each anatomical class of drug we assessed the age and country specific user prevalence rates for all individual drugs in 2005. We evaluated the five drugs with the highest prevalence per anatomical class in each country for off label status considering age only. A drug was considered to be off label for age if the child’s age at the time of use was below the lowest approved age mentioned in the summary of product characteristics of that drug in each country.15 Within each therapeutic drug level, we separately estimated the prevalence of children presenting “recurrent/chronic” (three or more prescriptions a year) versus “non-recurrent” or “acute” drug use (less than three prescriptions a year), and the ratio between them to identify the treatments more commonly used for chronic than acute paediatric diseases. We used χ2 test to compare user prevalence rates.
Results
Study population
Our population of 675 868 children generated 2 334 673 person years of follow-up (table 1); the mean individual follow-up was 3.5 years. Most of the children (66%) came from the IMS database in the UK, 19% from Italy, and 15% from the Netherlands. The databases recorded more than five million paediatric prescriptions. In all three countries the prescription rate was highest for the children aged under 2 and, in each age group, was significantly higher in the UK and Italy than in the Netherlands (P<0.001) (table 1).
Table 1.
Characteristics of study population
| Patients | No of children* | No (%) of person years | No of prescriptions | Prescriptions/ person year |
|---|---|---|---|---|
| Italy | ||||
| <2 years | 56 000 | 87 408 (22) | 286 597 | 3.3 |
| 2-11 years | 103 195 | 296 148 (73) | 690 688 | 2.3 |
| 12-14 years | 18 154 | 22 599 (6) | 35 883 | 1.6 |
| Females | 61 962 | 194 744 (48) | 462 580 | 2.4 |
| Males | 67 525 | 211 412 (52) | 550 588 | 2.6 |
| 2000 | 11 188 | 369 (0) | 1150 | 3.1 |
| 2001 | 73 364 | 45 330 (11) | 140 764 | 3.1 |
| 2002 | 95 712 | 78 850 (19) | 220 207 | 2.8 |
| 2003 | 103 987 | 94 131 (23) | 242 261 | 2.6 |
| 2004 | 106 555 | 96 388 (24) | 206 535 | 2.1 |
| 2005 | 102 911 | 91 086 (22) | 202 251 | 2.2 |
| Total | 129 487 | 406 156 (100) | 1 013 168 | 2.5 |
| UK | ||||
| <2 years | 95 060 | 106 250 (6) | 494 353 | 4.7 |
| 2-11 years | 262 306 | 855 678 (52) | 2 011 153 | 2.4 |
| 12-18 years† | 229 959 | 683 900 (42) | 1 549 372 | 2.3 |
| Females | 219 669 | 804 646 (49) | 2 047 616 | 2.5 |
| Males | 225 153 | 841 182 (51) | 2 007 262 | 2.4 |
| 2000 | 307 884 | 288 450 (18) | 659 067 | 2.3 |
| 2001 | 306 923 | 286 483 (17) | 677 373 | 2.4 |
| 2002 | 305 088 | 285 664 (17) | 670 690 | 2.3 |
| 2003 | 303 594 | 280 085 (17) | 679 216 | 2.4 |
| 2004 | 287 287 | 259 219 (16) | 674 389 | 2.6 |
| 2005 | 265 273 | 245 927 (15) | 694 143 | 2.8 |
| Total | 444 822 | 1 645 828 (100) | 4 054 878 | 2.5 |
| Netherlands | ||||
| <2 years | 25 694 | 36 601 (13) | 78 983 | 2.2 |
| 2-11 years | 62 326 | 159 010 (56) | 208 134 | 1.3 |
| 12-18 years | 40 364 | 87 078 (31) | 147 250 | 1.7 |
| Females | 49 709 | 138 262 (49) | 230 466 | 1.7 |
| Males | 51 850 | 144 427 (51) | 203 901 | 1.4 |
| 2000 | 56 423 | 48 752 (17) | 76 319 | 1.6 |
| 2001 | 53 274 | 46 822 (17) | 76 059 | 1.6 |
| 2002 | 57 998 | 50 219 (18) | 81 919 | 1.6 |
| 2003 | 62 216 | 49 279 (17) | 73 462 | 1.5 |
| 2004 | 60 315 | 50 882 (18) | 75 399 | 1.5 |
| 2005 | 52 252 | 36 735 (13) | 51 209 | 1.4 |
| Total | 101 559 | 282 689 (100) | 434 367 | 1.5 |
Drug use by anatomical class
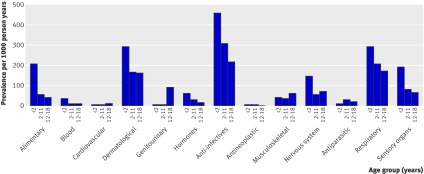
The highest prevalence rates among the children aged under 2 were for anti-infective drugs, respiratory drugs, and dermatological drugs, which were used by 48%, 30%, and 30% of the children, respectively (fig 1). The other common prescriptions were for gastrointestinal drugs (user prevalence of 20%), drugs for the nervous system (14%) and drugs for sensory organs (19%). Blood and blood forming organs, hormonal, and musculoskeletal system drugs were used in 1-10% of the children, and cardiovascular, genitourinary, antineoplastic, and antiparasitic drugs by less than 1%.
Fig 1 One year prevalence of drug prescriptions by age (<2, 2-11, 12-18 years), and anatomical class
Among the children aged 2-11, the prevalence of use of anti-infective, respiratory, and dermatological drugs decreased to 30%, 21%, and 17%, respectively. The prevalence was 1-10% for gastrointestinal, hormonal, musculoskeletal system, nervous system, antiparasitic, and sensory organ drugs; and less than 1% for blood and blood forming organs, cardiovascular, genitourinary, and antineoplastic drugs.
In adolescents (12-18 years), anti-infective, respiratory, and dermatological drugs were used by more than 10% per year. Most of the other drug classes were used by 1-10%, but the prevalence of use of cardiovascular and antineoplastic drugs was less than 1%.
Regarding sex differences, in the youngest age groups, most of the drugs were equally prescribed to both sexes or more commonly prescribed to boys than girls (rate ratio <1), particularly anti-infective and respiratory drugs. This pattern reversed in adolescence, when user prevalence for almost all drug classes (except non-sex hormones) was higher among girls than boys. This sex pattern, which was consistent across countries, was most pronounced for genitourinary drugs, with a user prevalence more than 60 times higher in girls because they include oral contraceptives, which accounted for 95% of the use of genitourinary drugs in girls. The use of drugs for blood and blood forming organs (mainly iron preparations) was also markedly higher among adolescent girls.
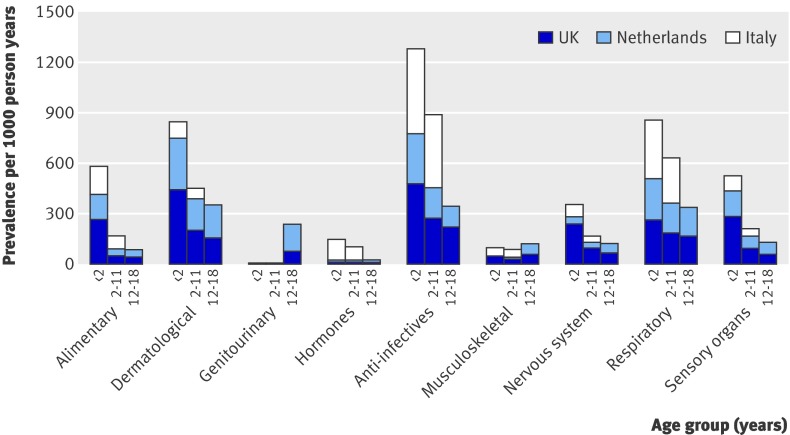
The age trend of prevalence of use was consistent across countries, although there were some variations in the age specific rates (fig 2). In particular, the UK showed the highest prevalence of alimentary drug use in children aged under 2, and the prevalence of prescriptions of dermatological drugs was threefold to fourfold higher in the UK and the Netherlands than in Italy (both P<0.001). The prevalence of genitourinary drug use (almost all oral contraceptives) was high in adolescent girls in the Netherlands (P<0.001). In Italy, the use of hormones (almost all systemic corticosteroids) was 10-fold higher in children aged <2 (P<0.001) and fivefold higher in those aged 2-11 (P<0.001); respiratory drug use was also greater in Italy than in the other two countries (P<0.001). The prevalence of the use of anti-infective drugs and drugs for musculoskeletal disorders was much lower in the Netherlands; the prevalence of prescriptions for drugs for the nervous system (including paracetamol, which can be prescribed in UK) was much higher in the UK; and the use of drugs for the sensory organs was much less in Italy.
Fig 2 Year prevalence of drug use (per 1000 person years) by age (<2, 2-11, 12-18), country, and anatomical class for most prevalently used drug classes (data for Italy excluded age category 12-18)
Prevalence of drug use in therapeutic class
Within the most commonly used anatomical drug classes, antibacterials accounted for most of the anti-infective drug use; and the therapeutic classes antiasthmatics, other respiratory products, and nasal preparations were the most commonly used drugs in the respiratory group (table 2). The therapeutic classes with the highest prevalence of use among the dermatological drugs were topical corticosteroids and emollients and barrier creams. Many therapeutic classes in the group of alimentary drugs (laxatives, antidiarrhoeal drugs, drugs for acid disorders) had a considerable prevalence of use. The most commonly prescribed drugs in the other classes were antianemia medications, cardiac drugs (mainly digoxin), sex hormones, oral corticosteroids, non-steroidal anti-inflammatory drugs, analgesics, and ophthalmological drugs.
Table 2.
Prevalence of acute use (<3 prescriptions per year) and recurrent use (≥3 prescriptions per year) by age and therapeutic level (prevalence per 1000 person years), ranked by the ratio of recurrent to acute use*
| Anatomical and therapeutic class (ATC) | Acute use | Recurrent use | Ratio recurrent/acute | Total prevalence | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| <2 | 2-11 | 12-18 | All ages | <2 | 2-11 | 12-18 | All ages | |||
| Gastrointestinal | ||||||||||
| Drugs used in diabetes (A10) | 0.0 | 0.2 | 0.3 | 0.2 | 0.0 | 0.9 | 2.5 | 1.3 | 7.0 | 1.5 |
| Digestives, including enzymes (A09) | 0.1 | 0.0 | 0.0 | 0.0 | 0.1 | 0.2 | 0.2 | 0.2 | 4.9 | 0.2 |
| Bile and liver therapy (A05) | 0.1 | 0.0 | 0.0 | 0.0 | 0.1 | 0.1 | 0.1 | 0.1 | 1.7 | 0.1 |
| Mineral supplements (A12 | 1.1 | 0.8 | 0.5 | 0.7 | 0.2 | 0.3 | 0.2 | 0.2 | 0.3 | 1.0 |
| Laxatives (A06) | 24.7 | 13.3 | 6.2 | 12.0 | 3.3 | 4.7 | 1.8 | 3.6 | 0.3 | 15.6 |
| Drugs for acid related disorders (A02) | 27.0 | 3.5 | 9.6 | 7.9 | 12.6 | 0.8 | 1.7 | 2.3 | 0.3 | 10.1 |
| Vitamins (A11) | 24.2 | 3.6 | 1.4 | 4.9 | 3.5 | 0.7 | 0.5 | 0.9 | 0.2 | 5.8 |
| Antiemetics and antinausea (A04) | 1.4 | 0.6 | 3.7 | 1.8 | 0.7 | 0.1 | 0.2 | 0.2 | 0.1 | 2.0 |
| Drugs for functional gastrointestinal disorders (A03) | 25.9 | 10.6 | 9.7 | 11.8 | 1.4 | 0.4 | 0.9 | 0.6 | 0.1 | 12.4 |
| Stomatological preparations (A01) | 56.3 | 6.6 | 4.2 | 10.7 | 3.2 | 0.2 | 0.2 | 0.5 | 0.0 | 11.2 |
| Antidiarrhoeal (A07) | 64.9 | 11.6 | 3.2 | 14.0 | 1.9 | 0.3 | 0.6 | 0.5 | 0.0 | 14.5 |
| Blood and blood forming organs | ||||||||||
| Antithrombotic agents (B01) | 0.2 | 0.2 | 0.3 | 0.3 | 0.2 | 0.3 | 0.3 | 0.3 | 1.1 | 0.5 |
| Antianaemic preparations (B03 | 20.8 | 3.6 | 6.8 | 6.4 | 2.9 | 0.5 | 1.2 | 1.0 | 0.2 | 7.4 |
| Antihaemorrhagics (B02) | 5.5 | 1.0 | 1.6 | 1.6 | 0.2 | 0.1 | 0.2 | 0.1 | 0.1 | 1.8 |
| Cardiovascular system | ||||||||||
| Agents acting on renin-angiotensin system (C09) | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.2 | 0.3 | 0.2 | 2.5 | 0.3 |
| Lipid modifying agents (C10) | 0.0 | 0.0 | 0.1 | 0.0 | 0.0 | 0.1 | 0.1 | 0.1 | 1.7 | 0.1 |
| Diuretics (C03) | 0.6 | 0.1 | 0.1 | 0.2 | 0.6 | 0.1 | 0.2 | 0.2 | 1.2 | 0.4 |
| Calcium channel blockers (C08 | 0.0 | 0.0 | 0.2 | 0.1 | 0.0 | 0.0 | 0.2 | 0.1 | 0.8 | 0.2 |
| β blocking agents (C07 | 0.1 | 0.2 | 2.2 | 0.8 | 0.1 | 0.2 | 0.7 | 0.3 | 0.4 | 1.2 |
| Cardiac therapy (C01) | 1.3 | 2.2 | 1.6 | 1.9 | 0.2 | 0.3 | 0.3 | 0.3 | 0.2 | 2.2 |
| Dermatological | ||||||||||
| Anti-acne preparations (D10) | 0.3 | 1.0 | 31.2 | 11.2 | 0.0 | 0.1 | 15.0 | 5.2 | 0.5 | 16.3 |
| Emollients and protectives (D02) | 98.8 | 45.1 | 25.6 | 43.8 | 48.5 | 21.8 | 8.1 | 19.8 | 0.5 | 63.6 |
| Antipsoriatics (D05) | 3.9 | 3.1 | 4.8 | 3.7 | 0.2 | 0.5 | 2.0 | 1.0 | 0.3 | 4.7 |
| Corticosteroids, dermatological preparations (D07) | 140.4 | 74.1 | 55.9 | 74.4 | 24.4 | 11.9 | 8.7 | 12.0 | 0.2 | 86.5 |
| Preparations for treatment of wounds and ulcers (D03) | 1.1 | 0.7 | 1.0 | 0.8 | 0.0 | 0.1 | 0.1 | 0.1 | 0.1 | 0.9 |
| Antiseptics and disinfectants (D08) | 3.9 | 2.4 | 2.8 | 2.7 | 0.1 | 0.1 | 0.2 | 0.1 | 0.1 | 2.8 |
| Antifungals for dermatological use (D01) | 50.8 | 18.4 | 19.6 | 22.0 | 1.6 | 0.6 | 1.5 | 1.0 | 0.0 | 23.0 |
| Antibiotics and chemotherapeutics (D06) | 43.6 | 36.4 | 23.6 | 32.8 | 0.8 | 0.9 | 0.9 | 0.9 | 0.0 | 33.7 |
| Other dermatological preparations (D11) | 5.3 | 8.9 | 9.8 | 8.9 | 0.2 | 0.1 | 0.4 | 0.2 | 0.0 | 9.1 |
| Genitourinary system and sex hormones | ||||||||||
| Sex hormones, modulators of genital system (G03) | 1.7 | 0.4 | 32.3 | 11.3 | 0.3 | 0.1 | 49.7 | 17.0 | 1.5 | 28.3 |
| Urologicals (G04) | 0.5 | 1.1 | 1.8 | 1.3 | 0.1 | 0.6 | 0.6 | 0.5 | 0.4 | 1.8 |
| Gynaecological anti-infectives and antiseptics (G01) | 1.1 | 1.3 | 9.2 | 4.0 | 0.0 | 0.0 | 0.5 | 0.2 | 0.0 | 4.2 |
| Systemic hormonal preparations, excluding sex hormones and insulins | ||||||||||
| Thyroid therapy (H03) | 0.3 | 0.2 | 0.3 | 0.2 | 0.4 | 0.5 | 1.1 | 0.7 | 3.1 | 0.9 |
| Pituitary and hypothalamic hormones (H01) | 0.1 | 2.2 | 1.5 | 1.7 | 0.0 | 1.3 | 1.4 | 1.2 | 0.7 | 3.0 |
| Corticosteroids for systemic use (H02) | 51.0 | 23.2 | 8.0 | 20.7 | 6.0 | 2.2 | 1.0 | 2.2 | 0.1 | 22.9 |
| Pancreatic hormones (H04) | 0.0 | 0.3 | 0.7 | 0.4 | 0.0 | 0.0 | 0.1 | 0.0 | 0.1 | 0.4 |
| Anti-infectives for systemic use | ||||||||||
| Antibacterials for systemic use (J01) | 340.0 | 241.4 | 166.3 | 225.6 | 95.2 | 47.0 | 27.6 | 45.2 | 0.2 | 270.7 |
| Antimycobacterials (J04) | 0.5 | 0.5 | 0.3 | 0.4 | 0.1 | 0.0 | 0.0 | 0.0 | 0.1 | 0.5 |
| Vaccines (excluding routine childhood vaccinations) (J07) | 11.8 | 10.6 | 14.3 | 12.0 | 0.8 | 0.4 | 1.0 | 0.6 | 0.1 | 12.6 |
| Antimycotics for systemic use (J02) | 1.1 | 0.6 | 3.7 | 1.7 | 0.0 | 0.0 | 0.2 | 0.1 | 0.0 | 1.8 |
| Antivirals for systemic use (J05) | 9.8 | 4.2 | 1.7 | 3.9 | 0.1 | 0.0 | 0.1 | 0.1 | 0.0 | 4.0 |
| Antineoplastic and immunomodulating drugs | ||||||||||
| Immunosuppressive agents (L04) | 0.0 | 0.0 | 0.1 | 0.1 | 0.0 | 0.1 | 0.4 | 0.2 | 3.8 | 0.3 |
| Antineoplastic agents (L01) | 0.0 | 0.1 | 0.1 | 0.1 | 0.0 | 0.1 | 0.1 | 0.1 | 0.9 | 0.2 |
| Musculoskeletal system | ||||||||||
| Muscle relaxants (M03) | 0.1 | 0.1 | 0.2 | 0.1 | 0.0 | 0.1 | 0.2 | 0.2 | 1.7 | 0.2 |
| Anti-inflammatory and antirheumatic products (M01) | 38.8 | 32.0 | 53.6 | 40.0 | 1.2 | 1.2 | 3.0 | 1.8 | 0.0 | 41.8 |
| Nervous system | ||||||||||
| Antiepileptics (N03) | 0.7 | 0.7 | 0.8 | 0.7 | 1.1 | 2.6 | 3.6 | 2.8 | 3.9 | 3.5 |
| Psychoanaleptics (N06) | 0.1 | 1.1 | 6.8 | 2.9 | 0.0 | 1.7 | 6.8 | 3.3 | 1.1 | 6.2 |
| Antiparkinsonian (N04) | 0.0 | 0.0 | 0.1 | 0.0 | 0.0 | 0.0 | 0.1 | 0.0 | 1.1 | 0.1 |
| Psycholeptics (N05) | 7.3 | 2.2 | 5.0 | 3.6 | 0.4 | 0.4 | 1.6 | 0.8 | 0.2 | 4.5 |
| Other nervous system drugs (N07) | 0.1 | 0.2 | 2.0 | 0.8 | 0.0 | 0.0 | 0.4 | 0.2 | 0.2 | 1.0 |
| Analgesics (N02) | 109.9 | 55.0 | 38.7 | 54.9 | 24.2 | 8.5 | 5.6 | 9.0 | 0.2 | 63.9 |
| Anaesthetics (N01) | 2.1 | 4.2 | 4.2 | 4.0 | 0.1 | 0.1 | 0.2 | 0.1 | 0.0 | 4.1 |
| Antiparasitic products | ||||||||||
| Ectoparasiticides (P03) | 2.9 | 14.9 | 10.6 | 12.2 | 0.1 | 1.5 | 0.8 | 1.1 | 0.1 | 13.4 |
| Antiprotozoals (P01) | 1.8 | 1.7 | 2.2 | 1.9 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 1.9 |
| Anthelmintics (P02) | 4.4 | 12.2 | 3.1 | 8.4 | 0.1 | 0.2 | 0.0 | 0.1 | 0.0 | 8.5 |
| Respiratory system | ||||||||||
| Drugs for obstructive airway diseases (R03) | 126.3 | 69.3 | 39.2 | 64.7 | 34.8 | 39.1 | 31.8 | 36.2 | 0.6 | 100.9 |
| Other respiratory system products (R07) | 45.4 | 55.1 | 53.0 | 53.4 | 2.8 | 8.1 | 14.1 | 9.6 | 0.2 | 63.0 |
| Antihistamines for systemic use (R06) | 50.4 | 29.1 | 17.4 | 27.3 | 3.5 | 2.1 | 2.6 | 2.4 | 0.1 | 29.7 |
| Nasal preparations (R01) | 79.1 | 36.2 | 43.7 | 43.0 | 3.9 | 2.1 | 4.4 | 3.1 | 0.1 | 46.1 |
| Cough and cold preparations (R05) | 4.1 | 2.2 | 1.7 | 2.2 | 0.2 | 0.1 | 0.0 | 0.1 | 0.0 | 2.3 |
| Throat preparations (R02) | 1.1 | 1.4 | 4.0 | 2.3 | 0.0 | 0.0 | 0.1 | 0.0 | 0.0 | 2.3 |
| Sensory system | ||||||||||
| Ophthalmological (S01) | 164.9 | 60.7 | 42.9 | 64.9 | 10.3 | 3.1 | 4.0 | 4.1 | 0.1 | 69.0 |
| Ophthalmological and otological preparations (S03) | 3.2 | 3.5 | 4.0 | 3.7 | 0.1 | 0.2 | 0.1 | 0.1 | 0.0 | 3.8 |
| Otological (S02) | 15.7 | 15.0 | 13.5 | 14.6 | 0.4 | 0.5 | 0.6 | 0.5 | 0.0 | 15.1 |
Ranking of user prevalence rates specific for age over the entire range of drugs showed that antibacterials are the most commonly prescribed drugs in all age groups (table 3) and are prescribed to at least twice as many children as the second most commonly used drug in each age category. The second most commonly used drug changed by age from ophthalmological drugs (<2 years) to drugs for obstructive airway disease (2-11) to sex hormones (12-18).
Table 3.
Top 10 most commonly used therapeutic classes in various age categories
| Therapeutic class (ATC) | Users/1000 person years |
|---|---|
| <2 years | |
| Antibacterials for systemic use (J01) | 435 |
| Ophthalmologicals (S01) | 175 |
| Corticosteroids, dermatological preparations (D07) | 165 |
| Drugs for obstructive airway diseases (R03) | 161 |
| Emollients and protectives (D02) | 147 |
| Analgesics (N02 | 134 |
| Nasal preparations (R01) | 83 |
| Antidiarrhoeals, intestinal anti-inflammatory/anti-infective agents (A07) | 67 |
| Stomatological preparations (A01) | 59 |
| Corticosteroids for systemic use (H02) | 57 |
| 2-11 years | |
| Antibacterials for systemic use (J01) | 288 |
| Drugs for obstructive airway diseases (R03) | 108 |
| Corticosteroids, dermatological preparations (D07 | 86 |
| Emollients and protectives (D02) | 67 |
| Ophthalmologicals (S01) | 64 |
| Analgesics (N02 | 63 |
| Other respiratory system products (R07) | 63 |
| Nasal preparations (R01) | 38 |
| Antibiotics and chemotherapeutics (D06) | 37 |
| Anti-inflammatory and antirheumatic products (M01) | 33 |
| 12-18 years | |
| Antibacterials for systemic use (J01) | 194 |
| Sex hormones and modulators of genital system (G03) | 82 |
| Drugs for obstructive airway diseases (R03) | 71 |
| Other respiratory system products (R07) | 67 |
| Corticosteroids, dermatological preparations (D07) | 65 |
| Anti-inflammatory and antirheumatic products (M01) | 57 |
| Nasal preparations (R01) | 48 |
| Ophthalmologicals (S01) | 47 |
| Anti-acne preparations (D10 | 46 |
| Analgesics (N02) | 44 |
When we ranked the therapeutic classes within each anatomical class on the basis of the ratio between recurrent (chronic) and non-recurrent (acute), we observed a different pattern (table 2). The drugs with a ratio of >1 (indicating mostly chronic/recurrent use) were often those with a low prevalence of use (except for sex hormones): antidiabetics, digestives, bile and liver therapy, antithrombotic agents, agents acting on the renin-angiotensin system, lipid lowering drugs, sex hormones, thyroid therapeutic agents, immunosuppressive agents, muscle relaxants, antiepileptics, and psychoanaleptics (table 2). In absolute terms, emollients, topical corticosteroids, sex hormones, anti-infectives, and drugs for obstructive airway disease showed the highest prevalence of recurrent use.
Most commonly used drugs in each anatomical class
In the most commonly used anatomical classes (dermatology, anti-infectives, and respiratory system), the most common individual dermatological drugs were fusidic acid (except for Italy), topical steroids, and topical imidazole/triazole derivatives (tables 4, 5, and 6) . The topical triazoles/imidazoles were off label in most countries for at least one or more age categories. In the anti-infectives group (J), penicillin derivatives (amoxicillin, co-amoxiclav, and phenoxymethylpenicillin) followed by macrolides (erythromycin, clarithromycin) were the most common, cefalexin (UK, <2 year) was the only off label drug. Oral aciclovir was one of the top five anti-infective drugs in Italy. Among the respiratory drugs, salbutamol and inhaled steroids (beclometasone, fluticasone, flunisolide), antihistamines (cetirizine, loratidine, clorpheniramine), and xylometazoline were most commonly prescribed. Beclometasone, xylometazoline, and cetirizine were off label in the youngest children (<2 years) in the UK and the Netherlands.
Table 4.
Most commonly used drugs (use per 1000 children per year) by anatomical level and age in 2005 plus paediatric licensing status in Netherlands
| Drug class and name | <2 years | 2-11 years | 12-18 years | Total users/1000 | |||
|---|---|---|---|---|---|---|---|
| No/1000 | % off label | No/1000 | % off label | No/1000 | % off label | ||
| Alimentary tract (A) | |||||||
| Lactulose | 92 | 0 | 332 | 0 | 58 | 0 | 482 |
| Domperidone | 79 | 0 | 222 | 0 | 73 | 0 | 374 |
| Miconazole | 200 | 0 | 30 | 0 | 8 | 0 | 238 |
| Nystatin | 130 | 0 | 11 | 0 | 3 | 0 | 144 |
| Laurilsulfate | 20 | 100 | 80 | 0 | 17 | 0 | 117 |
| Blood and blood forming organs (B) | |||||||
| Ferrous fumarate | 2 | 0 | 60 | 0 | 57 | 0 | 119 |
| Phytomenadione | 41 | 0 | 2 | 0 | 3 | 0 | 46 |
| Carbasalate calcium | 1 | 100 | 12 | 100 | 0 | NA | 13 |
| Cardiovascular (C) | |||||||
| Hydrocortisone (haemorrhoids) | 12 | 100 | 29 | 100 | 10 | 100 | 51 |
| Lidocaine | 3 | 100 | 30 | 0 | 13 | 0 | 46 |
| Propranolol | 0 | NA | 5 | 0 | 18 | 0 | 23 |
| Adrenaline (epinephrine) | 0 | NA | 17 | 0 | 4 | 0 | 21 |
| Enalapril | 0 | NA | 2 | 0 | 5 | 0 | 7 |
| Dermatological (D) | |||||||
| Fusidic acid | 194 | 100 | 1013 | 100 | 311 | 100 | 1518 |
| Hydrocortisone | 284 | 100 | 734 | 100 | 269 | 100 | 1287 |
| Miconazole | 273 | 0 | 337 | 0 | 204 | 0 | 814 |
| Triamcinolone | 36 | 100 | 360 | 100 | 292 | 100 | 688 |
| Ketoconazole | 48 | 100 | 168 | 100 | 139 | 100 | 355 |
| Genitourinary system and sex hormones (G) | |||||||
| Levonorgestrel/oestrogen | 1 | 100 | 3 | 100 | 1034 | 100 | 1038 |
| Cyproterone/oestrogen | 0 | NA | 4 | 100 | 321 | 100 | 325 |
| Norethisterone | 0 | NA | 2 | 100 | 98 | 100 | 100 |
| Miconazole | 4 | 100 | 14 | 100 | 58 | 100 | 76 |
| Lynestrenol | 0 | NA | 4 | 100 | 57 | 100 | 61 |
| Systemic hormonal preparations (H) | |||||||
| Desmopressin | 0 | NA | 94 | 0 | 49 | 0 | 143 |
| Prednisolone | 14 | 100 | 41 | 100 | 31 | 100 | 86 |
| Levothyroxine sodium | 1 | 0 | 13 | 0 | 16 | 0 | 30 |
| Prednisone | 0 | NA | 11 | 100 | 7 | 100 | 18 |
| Dexamethasone | 4 | 0 | 6 | 0 | 2 | 0 | 12 |
| Anti-infectives for systemic use (J) | |||||||
| Amoxicillin | 763 | 0 | 1870 | 0 | 302 | 0 | 2935 |
| Co-amoxiclav | 133 | 0 | 657 | 0 | 155 | 0 | 945 |
| Clarithromycin | 131 | 0 | 489 | 0 | 137 | 0 | 757 |
| Azithromycin | 47 | 0 | 246 | 0 | 111 | 0 | 404 |
| Pheneticillin | 22 | 0 | 211 | 0 | 161 | 0 | 394 |
| Antineoplastic and immunomodulating agents (L) | |||||||
| Fluorouracil | 0 | NA | 6 | 100 | 3 | 100 | 9 |
| Azathioprine | 0 | NA | 0 | 0 | 3 | 0 | 3 |
| Triptorelin | 0 | NA | 2 | 100 | 0 | 100 | 2 |
| Methotrexate | 0 | NA | 1 | 0 | 0 | 0 | 1 |
| Ciclosporin | 0 | NA | 1 | 0 | 0 | 0 | 1 |
| Musculoskeletal system (M) | |||||||
| Diclofenac | 0 | NA | 29 | 0 | 233 | 0 | 262 |
| Naproxen | 0 | NA | 10 | 0 | 171 | 0 | 181 |
| Ibuprofen | 0 | NA | 29 | 0 | 131 | 0 | 160 |
| Diclofenac, combinations | 0 | NA | 2 | 100 | 12 | 100 | 14 |
| Bufexamac | 3 | 100 | 8 | 100 | 3 | 100 | 14 |
| Nervous system (N) | |||||||
| Methylphenidate | 0 | NA | 125 | 0 | 140 | 0 | 265 |
| Paracetamol | 38 | 0 | 99 | 0 | 32 | 0 | 169 |
| Lidocaine-prilocaine | 3 | 0 | 110 | 0 | 14 | 0 | 127 |
| Carbasalate calcium | 0 | NA | 27 | 0 | 79 | 0 | 106 |
| Diazepam | 8 | 100 | 39 | 100 | 34 | 0 | 81 |
| Antiparasitic drugs, insecticides, and repellents (P) | |||||||
| Mebendazole | 1 | 0 | 87 | 0 | 14 | 0 | 102 |
| Metronidazole | 2 | 100 | 21 | 0 | 20 | 0 | 43 |
| Proguanil, combinations | 0 | NA | 4 | 0 | 10 | 0 | 14 |
| Permethrin | 1 | 0 | 8 | 0 | 3 | 0 | 12 |
| Respiratory system (R) | |||||||
| Salbutamol | 311 | 0 | 1053 | 0 | 448 | 0 | 1813 |
| Fluticasone | 159 | 0 | 702 | 0 | 201 | 0 | 1062 |
| Desloratadine | 14 | 0 | 447 | 0 | 366 | 0 | 827 |
| Xylometazoline | 154 | 100 | 356 | 0 | 143 | 0 | 654 |
| Levocetirizine | 0 | NA | 177 | 0 | 302 | 0 | 479 |
| Sensory organs (S) | |||||||
| Fusidic acid | 342 | 100 | 441 | 100 | 263 | 100 | 1049 |
| Levocabastine | 2 | 100 | 130 | 100 | 156 | 100 | 291 |
| Hydrocortisone/anti-infectives | 12 | 0 | 129 | 0 | 70 | 0 | 211 |
| Lidocaine | 33 | 100 | 135 | 0 | 16 | 0 | 185 |
Table 5 .
Most commonly used drugs (use per 1000 children per year) by anatomical level and age in 2005 plus paediatric licensing status in UK
| Drug class and name | <2 years | 2-11 years | 12-18 years | Total users/1000 | |||
|---|---|---|---|---|---|---|---|
| No/1000 | % off label | No/1000 | % off label | No/1000 | % off label | ||
| Alimentary tract (A) | |||||||
| Lactulose | 797 | 0 | 2565 | 0 | 565 | 0 | 3927 |
| Miconazole | 566 | 0 | 134 | 0 | 31 | 0 | 731 |
| Ranitidine | 145 | 100 | 133 | 0 | 343 | 0 | 622 |
| Mebeverine | 0 | NA | 57 | 0 | 524 | 0 | 581 |
| Domperidone | 103 | 0 | 136 | 0 | 247 | 0 | 486 |
| Blood and blood forming organs (B) | |||||||
| Folic acid | 141 | 100 | 48 | 0 | 368 | 0 | 558 |
| Tranexamic acid | 0 | NA | 9 | 0 | 295 | 0 | 304 |
| Aspirin | 12 | 100 | 52 | 100 | 37 | 0 | 103 |
| Warfarin | 1 | 100 | 17 | 100 | 25 | 100 | 46 |
| Phytomenadione | 26 | 0 | 10 | 0 | 7 | 0 | 43 |
| Cardiovascular (C) | |||||||
| Adrenaline (epinephrine) | 6 | 100 | 580 | 0 | 383 | 0 | 970 |
| Propranolol | 4 | 0 | 27 | 0 | 262 | 0 | 293 |
| Furosemide | 18 | 100 | 38 | 0 | 19 | 0 | 76 |
| Atenolol | 2 | 100 | 31 | 100 | 42 | 100 | 78 |
| Enalapril | 0 | NA | 26 | 0 | 37 | 0 | 63 |
| Dermatological (D) | |||||||
| Hydrocortisone | 2425 | 0 | 7311 | 0 | 2574 | 0 | 12 310 |
| Fusidic acid | 880 | 0 | 3936 | 0 | 1457 | 0 | 6273 |
| Clobetasone butyrate | 232 | 0 | 1888 | 0 | 1080 | 0 | 3200 |
| Clotrimazole | 828 | 100 | 1617 | 0 | 627 | 0 | 3073 |
| Betamethasone | 74 | 0 | 967 | 0 | 1360 | 0 | 2401 |
| Genitourinary system and sex hormones (G) | |||||||
| Clotrimazole | 61 | 100 | 182 | 100 | 801 | 0 | 1046 |
| Norethisterone | 0 | NA | 4 | 100 | 1019 | 100 | 1025 |
| Levonorgestrel | 0 | NA | 0 | 0 | 946 | 0 | 946 |
| Medroxyprogestrogen | 0 | NA | 1 | 100 | 693 | 0 | 695 |
| Desogestrel | 1 | 100 | 0 | 100 | 268 | 100 | 272 |
| Systemic hormonal preparations (H) | |||||||
| Desmopressin | 0 | NA | 467 | 0 | 312 | 0 | 779 |
| Levothyroxine | 19 | 0 | 89 | 0 | 159 | 0 | 267 |
| Glucagon | 0 | NA | 77 | 0 | 108 | 0 | 185 |
| Dexamethasone | 19 | 100 | 44 | 0 | 8 | 0 | 72 |
| Somatropin | 0 | NA | 28 | 0 | 26 | 0 | 54 |
| Anti-infectives for systemic use (J) | |||||||
| Phenoxymethylpenicillin | 518 | 0 | 6057 | 0 | 5710 | 0 | 12285 |
| Flucloxacillin | 897 | 0 | 6043 | 0 | 4223 | 0 | 11 163 |
| Erythromycin | 1287 | 0 | 5265 | 0 | 3386 | 0 | 9938 |
| Trimethoprim | 351 | 0 | 2623 | 0 | 2122 | 0 | 5096 |
| Cefalexin | 345 | 100 | 1597 | 0 | 1098 | 0 | 3041 |
| Antineoplastic and immunomodulating agents (L) | |||||||
| Azathioprine | 0 | NA | 16 | 0 | 65 | 0 | 81 |
| Methotrexate | 0 | NA | 10 | 0 | 24 | 0 | 34 |
| Ciclosporin | 0 | NA | 13 | 100 | 8 | 0 | 22 |
| Tacrolimus | 0 | NA | 5 | 0 | 9 | 0 | 14 |
| Goserelin | 0 | NA | 2 | 100 | 2 | 100 | 6 |
| Musculoskeletal system (M) | |||||||
| Ibuprofen | 1085 | 0 | 5404 | 0 | 4251 | 0 | 10 740 |
| Diclofenac | 2 | 0 | 41 | 0 | 1247 | 0 | 1290 |
| Mefenamic acid | 0 | NA | 11 | 0 | 1278 | 0 | 1289 |
| Naproxen | 0 | NA | 4 | 0 | 143 | 0 | 147 |
| Ketoprofen | 0 | NA | 15 | 100 | 70 | 0 | 86 |
| Nervous system (N) | |||||||
| Paracetamol | 4292 | 0 | 11 085 | 0 | 2832 | 0 | 18 209 |
| Methylphenidate | 0 | NA | 286 | 0 | 433 | 0 | 719 |
| Pizotifen | 0 | NA | 207 | 0 | 430 | 0 | 637 |
| Fluoxetine | 0 | NA | 6 | 0 | 398 | 0 | 404 |
| Diazepam | 4 | 0 | 124 | 0 | 266 | 0 | 394 |
| Antiparasitic drugs, insecticides, and repellents (P) | |||||||
| Mebendazole | 24 | 100 | 1695 | 0 | 349 | 0 | 2069 |
| Phenothrin | 3 | 0 | 201 | 0 | 53 | 0 | 257 |
| Permethrin | 35 | 0 | 845 | 0 | 400 | 0 | 1280 |
| Malathion | 40 | 0 | 1088 | 0 | 372 | 0 | 1500 |
| Respiratory system (R) | |||||||
| Salbutamol | 1309 | 100 | 12 403 | 0 | 8321 | 0 | 22 034 |
| Beclometasone | 256 | 100 | 6332 | 0 | 3963 | 0 | 10 552 |
| Cetirizine | 24 | 100 | 3382 | 0 | 4145 | 0 | 7552 |
| Chlorphenamine | 578 | 0 | 3945 | 0 | 959 | 0 | 5482 |
| Loratadine | 1 | 0 | 1992 | 0 | 2261 | 0 | 4254 |
| Sensory organs (S) | |||||||
| Chloramphenicol | 4155 | 100 | 7161 | 0 | 2192 | 0 | 13 509 |
| Cromoglicic acid | 53 | 100 | 1875 | 0 | 2630 | 0 | 4559 |
| Fusidic acid | 1316 | 0 | 1951 | 0 | 540 | 0 | 3807 |
| Nedocromil | 0 | NA | 265 | 0 | 465 | 0 | 730 |
| Hydrocortisone | 101 | 100 | 236 | 0 | 57 | 0 | 395 |
Table 6.
Most commonly used drugs (use per 1000 children per year) by anatomical level and age in 2005 plus paediatric licensing status in Italy
| Drug class and name | <2 years | 2-11 years | Total users/1000 | ||
|---|---|---|---|---|---|
| No/1000 | % off label | No/1000 | % off label | ||
| Alimentary tract (A) | |||||
| Domperidone | 250 | 0 | 649 | 0 | 899 |
| Sodium fluoride | 571 | 0 | 192 | 0 | 763 |
| Cimetropium bromide | 341 | 0 | 124 | 0 | 465 |
| Nystatin | 139 | 0 | 133 | 0 | 272 |
| Lactitol | 45 | 0 | 168 | 0 | 213 |
| Blood and blood forming organs (B) | |||||
| Electrolytes | 124 | 0 | 151 | 0 | 275 |
| Tranexamic acid | 4 | 0 | 168 | 0 | 172 |
| Phytomenadione | 88 | 0 | 9 | 0 | 97 |
| Ferrous gluconate | 6 | 0 | 62 | 0 | 68 |
| Ferrous sulphate | 0 | NA | 48 | 0 | 48 |
| Cardiovascular (C) | |||||
| Epinephrine | 16 | 0 | 56 | 0 | 72 |
| Hydrocortisone | 0 | NA | 14 | 0 | 14 |
| Furosemide | 9 | 0 | 4 | 0 | 13 |
| Oxetacaine | 0 | NA | 8 | 0 | 8 |
| Disopyramide | 0 | NA | 1 | 0 | 1 |
| Dermatological (D) | |||||
| Betamethasone/antibiotics | 205 | 0 | 431 | 0 | 636 |
| Mometasone | 240 | 0 | 362 | 0 | 602 |
| Mupirocin | 90 | 0 | 313 | 0 | 403 |
| Clotrimazole | 175 | 100 | 118 | 100 | 293 |
| Econazole | 90 | 100 | 83 | 100 | 173 |
| Genitourinary system and sex hormones (G) | |||||
| Conjugated oestrogens | 57 | 0 | 26 | 0 | 83 |
| Oxybutynin | 0 | NA | 37 | 0 | 37 |
| Benzydamine | 2 | 100 | 19 | 100 | 21 |
| Povidone-iodine | 1 | 100 | 14 | 100 | 15 |
| Estriol | 9 | 100 | 5 | 100 | 14 |
| Systemic hormonal preparations (H) | |||||
| Betamethasone | 1430 | 0 | 2064 | 0 | 3494 |
| Prednisone | 5 | 0 | 240 | 0 | 245 |
| Desmopressin | 0 | NA | 120 | 0 | 120 |
| Dexamethasone | 18 | 0 | 6 | 0 | 24 |
| Levothyroxine | 5 | 0 | 17 | 0 | 22 |
| Anti-infectives for systemic use (J) | |||||
| Amoxicillin | 2573 | 0 | 3603 | 0 | 6176 |
| Co-amoxiclav | 1760 | 0 | 4210 | 0 | 5970 |
| Azithromycin | 666 | 0 | 2616 | 0 | 3282 |
| Clarithromycin | 683 | 0 | 2385 | 0 | 3068 |
| Aciclovir | 309 | 0 | 739 | 0 | 1048 |
| Antineoplastic and immunomodulating agents (L) | |||||
| Pidotimod | 11 | 0 | 80 | 0 | 91 |
| Leuprorelin | 0 | NA | 7 | 100 | 7 |
| Triptorelin | 0 | NA | 6 | 100 | 6 |
| Methotrexate | 0 | NA | 5 | 100 | 5 |
| Ciclosporin | 0 | NA | 3 | 100 | 3 |
| Musculoskeletal system (M) | |||||
| Ibuprofen | 508 | 100 | 1399 | 100 | 1907 |
| Morniflumate | 118 | 100 | 446 | 100 | 564 |
| Ketoprofen | 8 | 0 | 354 | 0 | 362 |
| Flurbiprofen | 19 | 0 | 220 | 0 | 239 |
| Niflumic acid | 62 | 0 | 168 | 0 | 232 |
| Nervous system (N) | |||||
| Paracetamol | 603 | 0 | 491 | 0 | 1094 |
| Paracetamol, combinations | 255 | 0 | 506 | 0 | 761 |
| Niaprazine | 158 | 0 | 39 | 0 | 197 |
| Diazepam | 41 | 0 | 85 | 0 | 126 |
| Valproic acid | 4 | 0 | 39 | 0 | 43 |
| Antiparasitic drugs, insecticides, and repellents (P) | |||||
| Mebendazole | 38 | 0 | 479 | 0 | 517 |
| Pyrantel | 11 | 0 | 145 | 0 | 156 |
| Mefloquine | 8 | 0 | 16 | 0 | 24 |
| Albendazole | 1 | 0 | 22 | 0 | 23 |
| Permethrin | 0 | NA | 13 | 0 | 13 |
| Respiratory system (R) | |||||
| Beclometasone | 1584 | 0 | 2849 | 0 | 4433 |
| Salbutamol | 1202 | 0 | 1932 | 0 | 3134 |
| Flunisolide | 615 | 0 | 1256 | 0 | 1871 |
| Cetirizine | 234 | 0 | 1435 | 0 | 1669 |
| Salbutamol combinations | 537 | 0 | 725 | 0 | 1262 |
| Sensory organs (S) | |||||
| Tobramycin | 441 | 0 | 515 | 0 | 956 |
| Anti-infectives, combinations | 117 | 0 | 256 | 0 | 373 |
| Dexamethasone and anti-infectives | 42 | 100 | 229 | 100 | 271 |
| Nedocromil | 43 | 0 | 156 | 0 | 199 |
| Combinations of different antibiotics | 90 | 0 | 75 | 0 | 165 |
In the moderately used drugs (gastrointestinal, genitourinary, nervous system, and sensory system drugs), the most commonly prescribed alimentary tract drugs (A) were laxatives (lactulose), miconazole, domperidone, and mebeverine. Only ranitidine and laurilsulfate were off label in children <2 years. For the genitourinary drugs, the top five in the Netherlands and UK were oral contraceptives and topical antifungals (miconazole), whereas in Italy (up to age 12) oestrogens, drugs to treat incontinence, and antiseptics were the most commonly prescribed. The percentage of off label use of oral contraceptives and antifungals was high in the Netherlands and the UK. Among drugs for the nervous system, paracetamol is clearly the most used (but probably underestimated because of high over the counter use); methylphenidate (Netherlands and UK), lidocaine (Netherlands), pizotifen (UK), fluoxetine (UK) diazepam, niaprazine (Italy), and valproic acid (Italy) were also in the top five of at least one country. None of them was used off label, except diazepam for children under 12 in the Netherlands. In the group of sensory organ drugs many different drugs were used in the various countries, the most commonly prescribed drugs in the Netherlands (fusidic acid, levocabastine) and the UK (chloramphenicol) were off label.
The low prevalence drugs comprised many classes (groups blood, cardiovascular, hormonal, antineoplastic, musculoskeletal, antiparastic). In the blood forming organs group (B), phytomenadione, iron, tranexamic acid, platelet inhibitors, and vitamin K antagonists were most commonly prescribed. Salicylic acid derivatives were off label. In the cardiovascular drug group topical steroids (antihaemorrhoid creams), topical anaesthetics (lidocaine, oxetacaine), β blockers (propranolol, atenolol), furosemide, disopyramide, adrenaline (epinephrine), and enalapril were most common. Furosemide, β blockers, adrenaline, and topical (antihaemorrhoidal) steroids were off label in at least one country. For the non-sex hormones, desmopressin, oral steroids (dexamethasone, prednisolone and prednisone), levothyroxine and glucagons) were the most commonly prescribed drugs. Only the oral steroids were off label (Netherlands and UK only). The most commonly prescribed antineoplastic and immunomodulating drugs differed substantially between countries but were almost always off label. In the musculoskeletal drug group non-steroidal anti-inflammatory drugs were the most commonly prescribed, with important sequence differences between countries but little off label use except in Italy, where the number one and two drugs (ibuprofen and morniflumate) were off label. In all countries the number one antiprotozoal drug was mebendazole, with little off label drug use.
Discussion
We have provided a unique overview of primary care prescription patterns in a large multinational European paediatric population. The data could be used to improve the prioritisation of research into long term safety of paediatric drugs, as well as efficacy and effectiveness studies in paediatric medicine. Off label use in some of the most commonly and recurrently used drugs is high (such as oral contraceptives) and these should be considered for prioritisation.
Prioritisation of research on drug safety in paediatrics
We recommend two important assessments in prioritising research needs in medicines for children: public health assessment,16 comprising the severity and prevalence of disease and the availability of treatment alternatives; and assessment of use. This may comprise the frequency or volume of use and the licensing/labelling status of medicines for children. The use of off label and unlicensed medicines implies that there are no proper labelling and dosing recommendations, which can potentially be harmful to children.17 18 19 20 Therefore off label and unlicensed medicines should be a higher priority for research than licensed/on label medications, especially if no data on safety and efficacy in children are available. We focused on assessing the volume and labelling status to provide knowledge to experts and facilitate research prioritisation that includes both the public health as well as the assessment of use.
Our data on use support the conclusions of the recently published EMEA consensus/expert derived list of research priorities concerning off patent medicinal products,16 which emphasised the need for paediatric studies of the safety of topical, systemic, and inhaled steroids. Steroids are associated with impaired growth,21 abnormalities in glucose metabolism,22 and adrenal suppression.23 24 Of these, growth retardation is the most common and is of particular concern in children. The extent of growth suppression varies with the method of administration (such as inhaled or oral) and the duration of treatment, as well as with the type and dose of glucocorticoid used.21 25 EMEA also lists topical and systemic antifungals (imidazoles/triazoles), acid reducing drugs, and antineoplastic drugs as research priorities. These drugs are often or recurrently used and are mostly off label. Many other drugs listed did not appear as commonly used drugs in our study and, on the basis of frequency of use in primary care alone, would not be considered as priorities but apparently were considered priorities for other reasons. On the other hand, sex hormones are not listed on the priority list, whereas they are commonly and recurrently prescribed, mostly off label. Few long term safety studies on the use of sex hormones in adolescents are available and to our knowledge there are no randomised controlled trials on their safety and efficacy in this age group. The use of oral contraceptives in adolescents has been associated with an increased risk of lower bone mineral density, higher serum cholesterol concentrations, triglyceridaemia,26 27 28 cardiovascular events (such as myocardial infarction and stroke), and venous thromboembolism.29 30 31 32 33 As the use of sex hormones in young adolescents is relatively high, leading to a long duration of use, further studies on the efficacy and long term safety effects of these drugs in young women are warranted.
Although patterns of drug use and labelling status can inform decisions on prioritisation of research, these data inform also us about suboptimal use and might even uncover undesirable prescribing practices. For example, fusidic acid and chloramphenicol are often used and often off label (tables 4-6). In the Netherlands, fusidic acid is prescribed for the treatment of conjunctivitis, similar to chloramphenicol in the UK. The beneficial effect of antibiotics in the treatment of this condition, however, has not been proved.34 35 Indeed acute bacterial conjunctivitis is often a self limiting condition, and topical antibiotic use offers only marginal benefit in improving clinical outcomes; hence the emphasis should be on educating clinicians not to prescribe such treatment rather than a call for more research.36 37 Another example underlining the need for education rather than research is the cough and cold medications. These drugs are not only available over the counter but are also often prescribed, which should be strongly discouraged because of reports of death and lack of efficacy.38
Patterns of drug use
We found that the prevalence of the most commonly prescribed drugs in primary care is highest in children aged under 2, that the most commonly used drugs (anti-infectives, dermatologicals, and respiratory drugs) are the same in all three age categories, and that almost all other drugs are used by less than 10% of children a year. In general, we can categorise three groups of drug use: drugs used by more than 10% of children a year, those used by 1-10%, and those used by less than 1%. The use of the high prevalence drug classes decreases with age but remains high, whereas the use of the lowest prevalence drug groups increases to a moderate prevalence rate in adolescence, except in the case of cardiovascular and antineoplastic agents. Only a few therapeutic drug classes accounted for most use in a specific anatomical class: antibacterials, topical corticosteroids, antiasthma and antianaemia medications, cardiac drugs, sex hormones, oral corticosteroids, non-steroidal anti-inflammatory drugs, analgesics, and ophthalmological drugs. Relatively speaking, the high prevalence drugs were more often used for acute use. Only 12 drug classes (antidiabetics, digestives, bile and liver therapy, antithrombotic agents, drugs affecting the renin-angiotensin system, lipid lowering drugs, sex hormones, thyroid therapeutic agents, immunosuppressive agents, muscle relaxants, antiepileptics, and psychoanaleptics) were prescribed more often for recurrent than acute use.
We observed an age related sex reversal: prevalence rates for drug use were consistently higher in adolescents girls than in adolescent boys (except in the case of non-sex hormones), whereas the opposite was true in the younger age categories. This agrees with findings from previous Dutch and Danish studies.39 40
Interestingly, the percentage of off label use varied highly between countries, and similar drugs differed in off label status between countries. This confirms that the differences in the paediatric status of the drugs, instead of the different prescription habits or medical cultures as postulated by many authors, represent the real reason for the variability reported by years and from many European studies and surveys on the off label use in children.41
Previous studies
Our study was population based, had a large sample size, and covered different European countries. Previous European studies have been country or region specific and have concentrated on specific conditions, except for studies from Sweden, the Netherlands, and Denmark in the late 1990s and a recent Italian study covering data from 2000-6.40 42 43 44 These studies took all types of drugs into account but the methods to calculate prevalence and ranking (on the basis of number of dispensed boxes or user prevalence) and age ranges varied largely, which complicates direct comparisons. The overall results—highest drug use in lowest age category, ranking of the most commonly used drugs (anti-infectives, respiratory, and dermatological drugs), and sex pattern (more prescriptions for girls than boys after the age of 10)—are consistent with our findings.39 40 45
Potential of multi-country database studies
We have shown the potential of studying the primary care prescribing of a wide range of drugs using multiple databases. As all databases include outcome data, such as morbidity and mortality, they can also be used for studies of paediatric drug safety. The country specific estimates provide insights into prescription differences and allow a search for high prevalence countries regarding drug prescribing.
Limitations
We captured only outpatient, primary care drug prescriptions and not use of over the counter drugs (which resulted in a substantial underestimation of the use of paracetamol and phytomenadione, and potentially other drugs such as cough and cold medications). In the Netherlands, the UK, and Italy, most health problems are dealt with in primary care,8 and as drug prescriptions by a specialist for a chronic disease are often continued by general practitioners or paediatricians, most of them are picked up. Drugs given in hospital and the monitoring of chemotherapeutic and biological drugs are unlikely to be fully captured by our databases. Despite differences in the absolute prevalence rates of drug prescribing and the types of drugs prescribed, age and sex patterns were consistent in the three countries. As the UK accounted for 60% of the study population, however, the pooled results are inevitably dominated by UK prescription patterns so we conducted stratified analyses as much as possible. Because of the nature of the databases, we studied drug prescriptions rather than drug intake, and so the prevalence of actual drug exposure might be lower than estimated here.
What is already known on this topic
- Most previous research on drug use in children has focused on specific high use areas such as antibiotics and respiratory and neuropsychiatric drugs, therefore most of these drugs have a paediatric licensing status
- Paediatric expert groups have been established by the European Medicines Evaluation Board (EMEA) to identify those drugs that are important for the paediatric community and that require additional efficacy and safety data
What this study adds
- Data on frequency of prescriptions and off label status of drugs could provide objective evidence for the prioritisation of research in paediatric drugs
- Information on the safety and efficacy of some of the most commonly used drugs in children (such as oral contraceptives, steroids, and triazoles/imidazoles) is lacking, and not all such drugs are on the list of research needs
We thank Peter Stephens of IMS Health for providing the IMS-DA database and all the physicians contributing data to the databases.
Contributors: All authors conceived the idea for the study, designed the study, and analysed and interpreted the data. MCJMS and KMCV drafted the manuscript, which was revised by AN and EFS. AC and ICKW supervised the study. MCJMS is guarantor.
Funding: The study was funded by the European Community’s 6th Framework Programme, project No LSHB-CT-2005-005216: TEDDY: Task force in Europe for Drug Development for the Young. The funding agency had no role in the collection of data, the analysis or interpretation of the data, or of the decision to submit. ICKW’s post was funded by a UK Department of Health Public Health Career Scientist Award.
Competing interests: MCJMS has received various unconditional research grants from pharmaceutical companies (Merck, Pfizer, Johnson and Johnson, Amgen, Roche, Altana, GSK) and is consultant to Pfizer, Celgene, Servier, and Sanofi Aventis. AN has been reimbursed by Pfizer for attending several conferences. GP has received unconditional research grants from Merck and BMS). CG has received fees for speaking, consulting, and research from Sanofi Pasteur, GSK, Abbott, BMS, Gilead, Abbott, Tibotec, Boheringer Ingelheim, GSK-Biologicals). LC has received research grants from GSK, Abbott, Merck, and BMS.
Ethical approval: The use of IMS data for this study has been reviewed by an independent scientific and ethics committee.
Provenance and peer review: Not commissioned; externally peer reviewed.
Cite this as: BMJ 2008;337:a2245
References
- 1.Pandolfini C, Bonati M. A literature review on off-label drug use in children. Eur J Pediatr 2005;164:552-8. [DOI] [PubMed] [Google Scholar]
- 2.Regulation (EC) No 1901/2006 of the European Parliament and of the Council of 12 December 2006 on medicinal products for paediatric use. Paediatric regulation. Official Journal of the European Union 2006;18:L378/1. [Google Scholar]
- 3.Sutcliffe A. Testing new pharmaceutical products in children. BMJ 2003;326:64-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.US Food and Drug Administration. Pediatric exclusivity labeling changes as of January 5, 2005. Rockville, MD: 2005.
- 5.Davies A, Bateman M, Yates A, Bruno M. Pediatric regulations in Europe & the US. Regulatory Affairs Focus 2005;10:18-22. [Google Scholar]
- 6.Watson R. EU offers incentives to firms to produce medicines for children. BMJ 2006;332:1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization. Make medicines child size. Geneva: WHO, 2007. www.who.int/childmedicines/en/.
- 8.Van der Lei J, Duisterhout J, Westerhof H, van der Does E, Cromme P, Boon W, et al. The introduction of computer-based patient records in the Netherlands. Ann Intern Med 1993;119:1036-41. [DOI] [PubMed] [Google Scholar]
- 9.Sturkenboom M. Other European databases for pharmacoepidemiology. In: Mann RD AE, ed. Pharmacovigilance. 2nd ed. London: Wiley, 2007.
- 10.Sturkenboom M, Nicolosi A, Cantarutti L, Mannino S, Picelli G, Scamarcia A, et al. Incidence of mucocutaneous reactions in children treated with nilfumic acid, other nonsteroidal antiinflammatory drugs, or nonopioid analgesics. Pediatrics 2005;116:e26-33. [DOI] [PubMed] [Google Scholar]
- 11.Vlug A, van der Lei J, Mosseveld B, van Wijk M, van der Linden P, Sturkenboom M, et al. Postmarketing surveillance based on electronic patient records: the IPCI project. Methods Inf Med 1999;38:339-44. [PubMed] [Google Scholar]
- 12.‘T Jong G, Eland I, Sturkenboom M, Anker Jv, Stricker B. Unlicensed and off-label prescription of drugs to children: a population based cohort study. BMJ 2002;324:1314-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wong I, Murray M. The potential of UK clinical databases in enhancing paediatric medication research. Br J Clin Pharmacol 2005;59:750-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rose K, Stotter H. ICH E 11: clinical investigation of medicinal products in the paediatric population. In: Rose K, van den Anker JN, eds. Guide to paediatric clinical research. Basel: Karger, 2007:33-37.
- 15.Neubert A, Bonifazi A, Catapano M, Baiardi P, Guiaquinto C, Knibbe C, et al. Defining off-label and unlicensed use of medicines for children: results of a Delphi survey. Pharmacol Res (in press). [DOI] [PubMed]
- 16.European Medicines Agency. Priority list of off-patent medicinal products for pediatric studies. London: EMEA, 2006. (EMEA/49677/2006.)
- 17.European Medicines Agency. Evidence of harm from off-label or unlicensed medicines in children. European Medicines Agency pre-authorisation evaluation of medicines for human use. London: EMA, 2004. (EMEA/126327/2004.)
- 18.Horen B, Montastruc J, Lapeyre-Mestre M. Adverse drug reactions and off-label drug use in paediatric outpatients. Br J Clin Pharmacol 2002;54:665-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neubert A, Dormann H, Weiss J, Egger T, Criegee-Rieck M, Rascher W, et al. The impact of unlicensed and off-label drug use on adverse drug reactions in paediatric patients. Drug Saf 2004;27:1059-67. [DOI] [PubMed] [Google Scholar]
- 20.Choonara I, Conroy S. Unlicensed and off-label drug use in children: implications for safety. Drug Saf 2002;25:1-5. [DOI] [PubMed] [Google Scholar]
- 21.Allen D. Growth suppression by glucocorticoid therapy. Endocrinol Metab Clin North Am 1996;25:699-717. [DOI] [PubMed] [Google Scholar]
- 22.Eigen H, Rosenstein B, FitzSimmons S, Schidlow D. Cystic Fibrosis Foundation Prednisone Trial Group. A multicenter study of alternate-day prednisone therapy in patients with cystic fibrosis. J Pediatr 1995;126:515-23. [DOI] [PubMed] [Google Scholar]
- 23.Todd G, Dunlop K, McNaboe J, Ryan M, Carson D, Shields M. Growth and adrenal suppression in asthmatic children treated with high-dose fluticasone propionate. Lancet 1996;348:27-9. [DOI] [PubMed] [Google Scholar]
- 24.Gulliver T, Eid N. Effects of glucocorticoids on the hypothalamic-pituitary-adrenal axis in children and adults. Immunol Allergy Clin North Am 2005;25:541-55. [DOI] [PubMed] [Google Scholar]
- 25.Allen D. Effects of inhaled steroids on growth, bone metabolism, and adrenal function. Adv Pediatr 2006;53:101-10. [DOI] [PubMed] [Google Scholar]
- 26.Cromer B, Scholes D, Berenson A, Cundy T, Clark M, Kaunitz A. Depot medroxyprogesterone acetate and bone mineral density in adolescents—the black box warning: a position paper of the society for adolescent medicine. J Adolesc Health 2006;39:296-301. [DOI] [PubMed] [Google Scholar]
- 27.Hartard M, Kleinmond C, Kirchbichler A, Jeschke D, Wiseman M, Weissenbacher E, et al. Age at first oral contraceptive use as a major determinant of vertebral bone mass in female endurance athletes. Bone 2004;35:836-41. [DOI] [PubMed] [Google Scholar]
- 28.Lloyd T, Lin H, Matthews A, Bentley C, Legro R. Oral contraceptive use by teenage women does not affect body composition. Obstet Gynecol 2002;100:235-9. [DOI] [PubMed] [Google Scholar]
- 29.Heinemann L, Lewis M, Spitzer W, Thorogood M, Guggenmoos-Holzmann I, Bruppacher R. Thromboembolic stroke in young women. A European case-control study on oral contraceptives. Transnational research group on oral contraceptives and the health of young women. Contraception 1998;57:29-37. [DOI] [PubMed] [Google Scholar]
- 30.Heinemann L, Lewis M, Thorogood M, Spitzer W, Guggenmoos-Holzmann I, Bruppacher R. Case-control study of oral contraceptives and risk of thromboembolic stroke: results from international study on oral contraceptives and health of young women. BMJ 1997;315:1502-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lewis M. Myocardial infarction and stroke in young women: what is the impact of oral contraceptives? Am J Obstet Gynecol 1998;179:S68-77. [DOI] [PubMed] [Google Scholar]
- 32.Samuelsson E, Hagg S. Incidence of venous thromboembolism in young Swedish women and possibly preventable cases among combined oral contraceptive users. Acta Obstet Gynecol Scand 2004;83:674-81. [DOI] [PubMed] [Google Scholar]
- 33.Samuelsson E, Hedenmalm K, Persson I. Mortality from venous thromboembolism in young Swedish women and its relation to pregnancy and use of oral contraceptives—an approach to specifying rates. Eur J Epidemiol 2005;20:509-16. [DOI] [PubMed] [Google Scholar]
- 34.Rietveld R, ter Riet G, Bindels P, Schellevis F, van Weert H. Do general practitioners adhere to the guideline on infectious conjunctivitis? Results of the second Dutch national survey of general practice. BMC Fam Pract 2007;8:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rietveld R, ter Riet G, Bindels P, Bink D, Sloos J, van Weert H. The treatment of acute infectious conjunctivitis with fusidic acid: a randomised controlled trial. Br J Gen Pract 2005;55:924-30. [PMC free article] [PubMed] [Google Scholar]
- 36.Hamerlynck J, Rietveld R, Hooft L. From the Cochrane Library: marginally higher chance of cure by antibiotic treatment in acute bacterial conjunctivitis. Ned Tijdschr Geneeskd 2007;151:594-6. [PubMed] [Google Scholar]
- 37.Rose P. Management strategies for acute infective conjunctivitis in primary care: a systematic review. Expert Opin Pharmacother 2007;12:1903-21. [DOI] [PubMed] [Google Scholar]
- 38.Sharfstein J, North M, Serwint J. Over the counter but no longer under the radar—pediatric cough and cold medications. N Engl J Med 2007;357:2321-4. [DOI] [PubMed] [Google Scholar]
- 39.Madsen H, Andersen M, Hallas J. Drug prescribing among Danish children: a population-based study. Eur J Clin Pharmacol 2001;57:159-65. [DOI] [PubMed] [Google Scholar]
- 40.Schirm E, van den Berg P, Gebben H, Sauer P, De Jong-van den Berg L. Drug use of children in the community assessed through pharmacy dispensing data. Br J Clin Pharmacol 2000;50:473-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pandolfini C, Bonati M. A literature review on off-label drug use in children. Eur J Pediatr 2005;164:552-8. [DOI] [PubMed] [Google Scholar]
- 42.Madsen H, Andersen M, Hallas J. Drug prescribing among Danish children: a population-based study. Eur J Clin Pharmacol 2001;57:159-65. [DOI] [PubMed] [Google Scholar]
- 43.Clavenna A, Berti A, Gualandi L, Rossi E, De Rosa M, Bonati M. Drug utilisation profile in the Italian pediatric population. Eur J Pediatr 2008. Apr 30 [epub head of print]. [DOI] [PubMed]
- 44.Thrane N, Sørensen H. A one-year population-based study of drug prescriptions for Danish children. Acta Paediatr 1999;88:1131-6. [DOI] [PubMed] [Google Scholar]
- 45.Silwer L, Lundborg C. Patterns of drug use during a 15 years period: data from a Swedish Country 1998-2002. Pharmacoepidemiol Drug Saf 2005;14:813-20. [DOI] [PubMed] [Google Scholar]