β1 integrins differentially control extravasation of inflammatory cell subsets into the CNS during autoimmunity (original) (raw)
Abstract
Inhibiting the α4 subunit of the integrin heterodimers α4β1 and α4β7 with the monoclonal antibody natalizumab is an effective treatment for multiple sclerosis (MS). However, the pharmacological action of natalizumab is not understood conclusively. Previous studies suggested that natalizumab inhibits activation, proliferation, or extravasation of inflammatory cells. To specify which mechanisms, cell types, and α4 heterodimers are affected by the antibody treatment, we studied MS-like experimental autoimmune encephalomyelitis (EAE) in mice lacking the β1-integrin gene either in all hematopoietic cells or selectively in T lymphocytes. Our results show that T cells critically rely on β1 integrins to accumulate in the central nervous system (CNS) during EAE, whereas CNS infiltration of β1-deficient myeloid cells remains unaffected, suggesting that T cells are the main target of anti-α4-antibody blockade. We demonstrate that β1-integrin expression on encephalitogenic T cells is critical for EAE development, and we therefore exclude α4β7 as a target integrin of the antibody treatment. T cells lacking β1 integrin are unable to firmly adhere to CNS endothelium in vivo, whereas their priming and expansion remain unaffected. Collectively, these results suggest that the primary action of natalizumab is interference with T cell extravasation via inhibition of α4β1 integrins.
Keywords: EAE, T lymphocyte, mouse genetics
Multiple sclerosis (MS) is a neurological autoimmune disease that is initiated by activated, self-reactive CD4+ T lymphocytes that recognize components of the myelin sheath, which surrounds and insulates nerve fibers. T cells enter the central nervous system (CNS) through postcapillary venules and are reactivated by antigen-presenting cells in the perivascular space. These steps are followed by the recruitment of additional inflammatory cells, such as macrophages, which cause inflammation, edema and, eventually, destruction of the myelin sheath (1).
The α4-integrin subunit can form a dimer with the β1 or the β7 subunit. In rodent experimental autoimmune encephalomyelitis (EAE), the widely used animal model for human MS, it was shown that antibodies directed against the α4-integrin subunit prevent the development of the disease (2). Subsequently, a beneficial effect of α4-integrin blockade has been demonstrated in many animal models as well as in clinical trials with MS patients. Based on these findings, natalizumab (marketed as Tysabri), a humanized monoclonal antibody directed against the α4-integrin subunit has been approved for treating relapsing-remitting MS (3).
Natalizumab is a very effective treatment for MS and significantly reduces the number and severity of clinical relapses (4). However, the exact mechanism of action of this drug remains unclear. Because α4 integrins can mediate both rolling and arrest of T cells on endothelial cells (5), it has been proposed that natalizumab prevents the interaction of α4 integrins on T cells with counterreceptors, such as vascular cell adhesion molecule-1 (VCAM-1), on inflamed endothelial cells, and thereby blocks the extravasation of encephalitogenic T lymphocytes into the CNS (6). It is also conceivable, however, that the antibody interferes with processes other than T cell extravasation. α4 integrins are expressed on most hematopoietic cells and play a role in several immunological tasks, including the activation of myeloid cells (7) and/or naïve T and B lymphocytes (8, 9), polarization of effector T cells into the TH1 or TH2 lineage (10), retention of memory T cells in their niches (11), and localization of hematopoietic stem cells (12). Recently, progressive multifocal leukoencephalopathy (PML), a rare but fatal demyelinating disease caused by JC virus infection in immunodeficient patients, has been associated with natalizumab use, raising the possibility that the antibody has a broader systemic immunosuppressive or immunomodulatory effect (13, 14). Finally, it is also not resolved whether both α4β1 and α4β7 integrins have a function during EAE development. Theoretically, natalizumab could block α4β1 as well as α4β7, and both integrins are expressed on encephalitogenic T cells (15). Studies addressing this question led to controversial results, with some suggesting that the β7 subunit has no role in EAE development (15, 16), whereas others identified a beneficial influence of the blockade of α4β7 or its counterreceptor mucosal addressin cell adhesion molecule-1 on the course of EAE (17, 18).
In the present paper we used mouse genetics to identify the role of α4β1 integrins during pathogenesis of EAE. The results of our study show that T lymphocytes require α4β1 integrins to elicit EAE. Furthermore, we demonstrate that the loss of α4β1 impairs T cell extravasation without affecting antigen-dependent proliferation and polarization.
Results
Integrin β1−/− Bone Marrow Chimeras (BMCs) Develop Normal EAE.
Treatment with blocking anti-α4 antibodies is neither specific for a certain cell type, nor specific for an α4-integrin heterodimer. Therefore, we undertook a genetic approach to test how the courses of EAE and MS might be affected by natalizumab treatment. We conditionally deleted the gene encoding the β1-integrin subunit (19). This approach restricts our findings to the loss of α4β1 integrins and excludes potential effects caused by abrogating α4β7 function.
To directly compare the fate and the functional properties of control and β1-integrin-deficient T cells within the same animal, we established a genetic setting in which Cre-mediated deletion of the β1-integrin gene occurred in approximately half of the T cells. This was achieved by using the inducible Mx1 promoter-driven Cre recombinase transgene. Because Mx1-Cre-mediated gene deletion is efficient in hematopoietic stem cells and most of their progeny but also occurs in many other cell types, including vascular endothelial cells, we generated BMCs to restrict the ablation of the β1-integrin gene to hematopoietic cells. Bone marrow (BM) from mice carrying floxed β1-integrin alleles and an Mx1-Cre transgene (β1fl/fl/MxCre+) was transferred into lethally irradiated WT B6SJL mice. BM from β1fl/fl/MxCre− mice served as control. After reconstitution of the recipient mice with donor BM, polyinosinic-polycytidylic acid [poly(IC)] was injected, causing IFN-α and IFN-β production, which in turn promotes the expression of Mx1-Cre and the disruption of the β1 gene exclusively in the donor-derived hematopoietic cells (20). Recipient- and donor-derived cells were distinguished by the expression of Ly-5.2 (donor) or Ly-5.1 (recipient) surface antigens on all hematopoietic cells. Around 9 weeks after poly(IC) injection, Mx1-Cre-mediated deletion occurred in ≈60% of T cells and 95–98% of all other hematopoietic cells as determined by Southern blot analysis of genomic DNA (Fig. 1A). The presence of β1-integrin-positive T cells is likely due to absent Mx1-Cre transgene expression in thymic and long-lived peripheral T cells (21). To test the role of β1 expression on hematopoietic cells for EAE development, active EAE was induced in the BMCs by immunizing them with the myelin oligodendrocyte glycoprotein (MOG)-peptide MOG35–55.
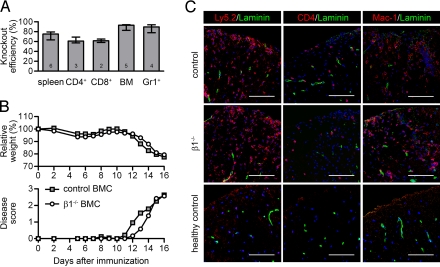
Fig. 1.
The clinical course of EAE is not altered in β1−/− BMCs. (A) Knockout efficiency for the indicated total and magnetically activated, cell-sorted populations was determined by Southern blotting. The number of samples for each population is given in each bar. Bars represent medians and interquartile ranges. (B) The relative weight normalized to day 0 and the clinical disease score of control and β1−/− BMCs with active EAE are shown. Data points indicate means of 13 mice from 3 independent experiments. Around day 16, mice were killed for histological and flow cytometric analyses. (C) Immunostaining of the spinal cord white matter of control and β1−/− BMCs with ongoing active EAE (clinical score 3) and healthy control mice. Infiltrating leukocytes were stained with Ly5.2, CD4, or Mac-1 antibodies (red), blood vessels were stained with a pan-laminin antibody (green), and nuclei were stained with DAPI (blue). (Scale bar, 100 μm.)
Actively immunized β1fl/fl/MxCre+ BMCs (called β1−/− BMCs) developed EAE with kinetics of weight loss and clinical scores similar to control BMCs (Fig. 1B). Histology of diseased animals revealed no qualitative difference in leukocyte infiltration in the CNS (Fig. 1C). Infiltrating inflammatory cells were stained with antibodies to Ly5.2 to highlight all hematopoietic cells, to CD4 to mark CD4+ T cells, or to Mac-1 to stain granulocytes and macrophages. In control and β1−/− BMCs, comparable amounts of inflammatory cells could be detected both subarachnoidal and in the parenchyma of the spinal cord white matter. These findings suggest that (i) loss of β1-integrin expression on the myeloid and B cell lineage did not influence the development of the disease, and that (ii) a halved pool of naïve T cells carrying β1 integrin was sufficient to trigger EAE.
β1-Deficient T Lymphocytes Do Not Accumulate in the CNS.
To assess the cell-autonomous roles of β1 integrins, we quantified the amount of β1-integrin-deficient immune cells within the diseased CNS tissue. To this end, we isolated the infiltrating leukocytes from the CNS of animals at the peak of EAE by density gradient centrifugation and determined the cellular composition of the infiltrates by flow cytometry. In line with the similar clinical course, the total number of isolated leukocytes was not altered between control and β1−/− BMCs (Fig. 2A). Furthermore, the population sizes of isolated CD4+ and CD8+ T cells, macrophages, and granulocytes were comparable in control and β1−/− BMCs (Fig. 2B). We next took advantage of our internally controlled system and determined the contribution of β1-deficient cells to the inflammatory infiltrates. In β1−/− BMC mice, Ly-5.1negGr-1medMac-1+ infiltrating macrophages and Ly-5.1negGr-1highMac-1+ granulocytes were uniformly β1-deficient (Fig. 2C), indicating that they were recruited into the CNS in a β1-integrin-independent manner. In contrast, CD4+ and CD8+ T cells isolated from the CNS of β1−/− BMC mice were almost entirely β1-integrin-positive (Fig. 2C). Thus, despite a 60:40 ratio of β1-integrin-negative to β1-integrin-positive peripheral T cells, an almost pure population of β1-positive cells was found in the inflamed CNS. These data strongly suggest that T cells but not myeloid cells require β1 integrins to infiltrate the CNS.
Fig. 2.
T lymphocytes depend on β1 integrins to enter the CNS. (A) Leukocytes and microglia cells were isolated from the brain and spinal cord of control or β1−/− BMCs with ongoing active EAE. Shown is the median number of isolated cells per CNS (average clinical score = 3, n = 5). (B and C) The isolated leukocytes were analyzed by flow cytometry. In B, the relative numbers of CD4+ T cells (CD4+), CD8+ T cells (CD8+), macrophages (Mac), and granulocytes (Gr) are shown (controls n = 8, β1−/− n = 9). (C) The β1 expression of the 4 leukocyte subsets was analyzed. Microglia cells are mainly host cell-derived and were excluded based on their cell surface expression of Ly-5.1. Each bar represents at least 5 mice. Bars in all panels represent medians and interquartile ranges.
EAE Development Is Impaired in β1fl/fl/CD4Cre+ Mice, and β1−/− T Cells Cannot Transfer EAE.
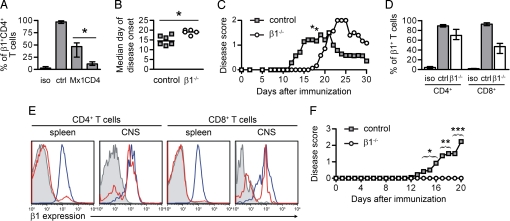
To further corroborate our findings, we used the CD4-Cre transgene (22) to specifically ablate the β1-integrin gene on T cells. Mice carrying floxed β1-integrin alleles and the CD4-Cre transgene (named β1fl/fl/CD4Cre+) had a 90% deletion efficiency on naïve splenic T cells as determined by flow cytometric analysis of β1 expression (Fig. 3A). Upon active immunization, these mice exhibited a significant delay in the onset of clinical EAE symptoms (Fig. 3 B and C). Interestingly, these animals eventually developed clinical symptoms, which were accompanied by an influx of β1-integrin-expressing T cells into the CNS that escaped CD4-Cre-mediated deletion (Fig. 3 D and E). These findings indicate that a loss of β1-integrin expression on 90% of the peripheral T lymphocyte population delays the development of EAE, but the presence of only 10% of β1-integrin-expressing T cells is sufficient to eventually trigger the disease. Because CD4-Cre does not ablate the β1-integrin gene in γδ T cells, however, it cannot be excluded that they are among the β1-integrin-expressing T cells contributing to EAE development in the β1fl/fl/CD4Cre+ mice.
Fig. 3.
β1 Integrin on T cells is important for EAE development. (A) Splenocytes from control (ctrl), β1fl/fl/MxCre+, or β1fl/fl/CD4Cre+ mice were stimulated unspecifically in vitro. Isotype control staining (iso) and β1-integrin expression of CD4+ T cells were analyzed by FACS. Shown are the medians and interquartile ranges of at least 5 animals. (B) The median day of disease onset of control and β1fl/fl/CD4Cre+ mice with active EAE is shown. (C) The clinical disease score of control and β1fl/fl/CD4Cre+ mice with active EAE is shown. Data points indicate means of 7 mice from 3 independent experiments. (D) Leukocytes and microglia cells were isolated by density gradient centrifugation from the CNS of control (n = 2) and β1fl/fl/CD4Cre+ (n = 3) mice with ongoing active EAE at day 31. The β1 expression of CD4+ and CD8+ T cells that were isolated from the CNS was quantified. Bars represent medians and interquartile ranges. (E) Shown are representative histograms of those T lymphocytes or of unspecifically stimulated splenocytes isolated from the same mice. Blue and red histograms represent control and β1−/− T cells, respectively. Isotype control stainings are shown in shaded gray histograms. (F) The clinical disease score of WT mice injected with encephalitogenic control or β1−/− MOG35–55-specific T cells is shown. Data points indicate means (controls n = 11, β1−/− n = 8; 4 independent experiments). The number of animals with EAE symptoms was compared by the Fisher exact test for each day (days 14–16: P < 0.05, days 17 and 18: P < 0.01, days 19 and 20: P < 0.005).
To assess the encephalitogenic potential of a homogenous population of β1-deficient T lymphocytes, we purified such cells and induced EAE by passive T cell transfer. To this end, we intercrossed β1fl/fl/MxCre+ mice with a transgenic strain expressing a MOG35–55 peptide-specific T cell receptor (TCR) (23) and isolated T cells from their offspring. These cells were restimulated in vitro with MOG35–55-loaded dendritic cells (DCs), all β1-integrin-positive cells were depleted, and the remaining encephalitogenic β1−/− T cell blasts were transferred into sublethally irradiated WT mice. Although transferred control T cells caused EAE symptoms in the recipients, none of the mice that received β1−/− T lymphocytes developed EAE (Fig. 3F) or contained inflammatory cell infiltrations in the spinal cord [supporting information (SI) Fig. S1]. Taken together, these experiments show that β1-integrin expression by encephalitogenic T cells is required for the pathogenesis of EAE, whereas β1 integrin is dispensable on inflammatory bystander cells.
β1−/− T Cells Show Normal Proliferation and Cytokine Responses.
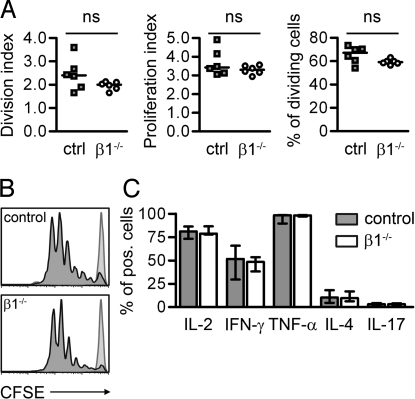
The lack of β1-deficient T cells in the brain of β1−/− BMCs and their failure to transfer EAE could be due to impaired activation, proliferation, or extravasation into the CNS. To test T cell activation and proliferation, we intercrossed β1fl/fl/MxCre+ mice with transgenic animals expressing MHC II-restricted TCRs specific for the ovalbumin (OVA) peptide OVA323–339 (OT-II.2) (24). Two to three months after knockout induction by poly(IC) injection, naïve CD4+ T cells were purified, labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE), and injected intravenously into WT recipients. LPS-matured, OVA323–339-loaded DCs were intravenously coinjected, and after 3 days cells were isolated from the spleen and their proliferative response tracked by dye dilution. β1−/− and control T cells showed comparable proliferation and division indices (Fig. 4 A and B). The percentage of cells that divided was not altered between control and β1−/− T cells. These findings indicate that T cells do not require β1 integrins for priming and proliferation.
Fig. 4.
β1 Deficiency does not influence T cell proliferation and polarization. (A) Proliferation of control (ctrl) or β1−/− T cells was analyzed by flow cytometry. The division and proliferation index and the percentage of dividing cells were calculated from the generation sizes (n = 6). (B) CFSE stainings from 1 representative control and β1−/− sample are shown. Light gray-shaded histograms represent control animals that received DCs without OVA323–339 peptide. (C) Shown are the percentages of cytokine-expressing T cells. The intracellular cytokine expression of control and β1−/− T lymphocytes was analyzed by FACS. Bars represent medians and interquartile ranges (controls n = 4, β1−/− n = 5). ns, not significant.
Antibodies directed against integrin α4 affect the T helper cell TH1/TH2 polarization of the T cell response (10). Because the cytokine polarization of T helper cells, particularly a shift toward IL-17-expressing TH17 cells, is crucial for the generation of autoimmune responses (25), we tested whether a lack of β1 expression on T cells affects their cytokine secretion pattern. Intracellular cytokine staining and subsequent flow cytometric analysis of in vivo antigen-stimulated OT-II.2 cells revealed similar cytokine responses between β1-deficient and control cells (Fig. 4C). Together, these findings indicate that the lack of β1-integrin-deficient T cells in the EAE lesions is not due to impaired activation.
β1−/− T Cells Cannot Firmly Adhere to CNS Endothelium in Vivo.
To investigate whether β1 integrins are required for T cell infiltration into the CNS, we used both an in vitro and an in vivo approach to assess the interaction of β1-integrin-deficient T cells with inflamed CNS endothelium. We isolated T cells from poly(IC)-induced, OT-II.2-transgenic β1fl/fl/MxCre+ or control BMCs and triggered their proliferation by coculturing them with OVA323–339-loaded DCs. For further analysis, T cell blasts were sorted into β1-integrin-positive and β1-integrin-negative populations. Flow cytometry of the surface integrins showed high expression of α4, β1, and β7 subunits on control cells. On knockout cells, expression of the β1-integrin subfamily was lost, whereas expression of the αLβ2 integrin and the β7 subunit was slightly increased (Fig. 5A). First, we tested the static adhesion of β1-integrin-deficient T cell blasts to the TNF-α-stimulated brain endothelioma cell line bEnd5 and observed no difference in binding between β1-positive and β1-negative T cells at room temperature (Fig. 5B). Because the adhesion was likely due to a predominant interaction between αLβ2 integrin and endothelial intercellular adhesion molecule-1 (ICAM-1), we also performed adhesion assays on the ICAM-1−/− endothelioma cell line bEndI1.1, which revealed a significant impairment of β1−/− T cell adhesion (Fig. 5B). The adhesion of β1−/− T cells was even further impaired when the assays were performed at 4°C (Fig. S2), which selectively abrogates αLβ2-mediated adhesion (26). These findings suggest that T cells adhere to activated endothelial cells by binding VCAM-1 with α4β1 integrin and ICAM-1 with αLβ2 integrin.
Fig. 5.
In vivo firm adhesion of β1−/− T lymphocytes to the spinal cord microvasculature is dramatically reduced. (A) Integrin expression of proliferating control (blue) and β1−/− T lymphocytes (red) was analyzed by FACS. Isotype control stainings are shown in shaded gray histograms. Histograms are representative of 3 independent experiments. (B) Adhesion of T cell blasts to the endothelioma cell lines bEnd5 (WT) and bEndI1.1 (ICAM-1−/−) at room temperature. Graphs show medians and interquartile ranges of adherent T cells per field of view (fov) (n = 4). (C) Firm adhesion of control and β1−/− T cell blasts to the spinal cord microvascular wall was analyzed by IVM in WT mice with ongoing active EAE. Firm adhesion was analyzed 10 min, 30 min, and 1 h after infusion of T cells. Bars represent medians and interquartile ranges (n = 6). (D) Initial contact events of T cell blasts with endothelial cells were analyzed by IVM. From 6 experiments with control and β1−/− T cells, 46 and 30 vessels were analyzed, respectively. Shown is the percentage of rolling or captured T cells among the total number of T cells passing through a given venule during a 1-min observation period. Each dot represents one vessel, and the red line indicates the median. ns, not significant.
Because the β1-deficient T cell blasts express αLβ2 but cannot accumulate in the CNS in vivo, we performed intravital fluorescence video microscopy (IVM) of the spinal cord white matter microvasculature to test directly whether binding of integrin αLβ2 to ICAM-1 plays a compensatory role for the adhesion of β1-deficient T cells to inflamed brain endothelium in vivo. Control or β1-deficient, OVA323–339-specific T cell blasts were injected via a carotis catheter into WT mice with mild EAE symptoms (clinical score of 1 to 2). The EAE causes an up-regulation of adhesion molecules on the endothelial cells, which enables control T cell blasts to adhere readily to the microvasculature. We observed that adhesion of OVA323–339-specific, β1-deficient T cells to white matter microvessels of the cervical spinal cord was significantly diminished in comparison with control T cells (Fig. 5C). Because it has been shown that besides firm adhesion, α4 integrins can mediate the events during the initial contact of T cells to the CNS endothelium, we analyzed T cell rolling and capture; that is, their abrupt transient stop (5, 27). Both events were not significantly impaired, suggesting that this α4-integrin function can be compensated by (an)other, unknown adhesion molecule(s) (Fig. 5D). We obtained similar results when we performed the experiment with β1-deficient, MOG35–55-specific T cells (23) (Fig. S3). These data indicate that the dramatic reduction of T cell adhesion is due to their inability to establish a firm contact with the endothelial cells of the blood–brain barrier without β1 integrins (see Movie S1 and Movie S2). The data further suggest that the interaction of α4β1 integrin with endothelial VCAM-1 is the critical molecular interaction for the stable adhesion of activated T cells to the brain vasculature, because increased expression of α4β7 integrins on the β1-deficient T lymphocytes could not rescue the adhesion defect.
Discussion
Inhibition of α4 integrins with a blocking antibody (natalizumab) has become a powerful therapy for MS. Almost all hematopoietic cells express the 2 α4 integrins (α4β1 and α4β7) (15). Both integrins can potentially regulate blood cell extravasation into inflamed tissues, as well as differentiation, priming, and proliferation of T cells. To elucidate how the anti-α4 integrin blockade exerts its beneficial effect on the MS course, we deleted the β1-integrin gene in specific hematopoietic cell populations of mice and subsequently studied the development of EAE, a widely used model for MS. Because deletion of the β1 gene abrogates α4β1 but not α4β7 integrin expression, our studies permit us to draw several important conclusions.
First, our genetic experiments suggest that β1-integrin-expressing T cells are the main target of natalizumab treatment. β1 integrins are expressed on almost all mammalian cell types, including the hematopoietic lineages that are crucial for the pathogenesis of EAE and MS (11). We observed that deletion of the β1-integrin gene in antigen-presenting cells or granulocytes and macrophages affected neither the development of the disease nor the ability of these cells to accumulate in the CNS, indicating that they can be excluded as targets of the natalizumab treatment. In contrast, β1-deficient T cells failed to accumulate in the CNS when EAE was induced in mice possessing both β1-positive and β1-negative T cells, indicating that T lymphocytes depend on β1-integrin expression to infiltrate the brain and spinal cord of diseased animals. Furthermore, deletion of the β1-integrin gene on ≈90% of the T cells delayed but did not abolish development of active EAE, whereas deletion of β1 integrins on the entire encephalitogenic T lymphocyte population prevented their ability to induce disease development. These findings suggest that (i) the beneficial therapeutic effects of natalizumab are due to a direct effect on the T cell lineage and that (ii) the efficacy of natalizumab therapy critically depends on a complete blockade of α4 integrins on encephalitogenic T cells. The latter findings may explain the results of a recent study reporting that blocking antibodies against α4 integrins failed to inhibit EAE development in a mouse model (28). Furthermore, they also show that an efficient and selective inhibition of T cell accumulation in the brain of MS patients is likely sufficient to retard or halt the disease course, and thus underscore the notion that autoreactive CD4+ T cells within the CNS represent the trigger of the disease (1). We did not directly investigate the reason for the development of PML as a rare side effect of natalizumab treatment, but it has been shown that that the cellular immune response by CD4+ and CD8+ T cells is critically required to contain JC virus infection (29). Therefore, we speculate based on our results that inefficient immune surveillance of the CNS by T cells facilitates the replication of JC virus.
Second, our investigations resolve the controversy over whether the anti-α4 antibodies exert their main effect through integrin α4β1, α4β7, or both. Antibody-blocking studies against the α4 subunit cannot distinguish between the 2 integrin heterodimers, and studies in which β7-integrin protein was inhibited or the β7-integrin gene was ablated revealed opposing results (15–18). Our system of deleting the β1-integrin gene, and therefore the α4β1-heterodimer, offered the possibility to address this question. We found that β1-deficient T cell blasts were incapable of accumulating in the CNS of EAE mice, although their integrin α4β7 expression levels were even higher than on control cells. These results indicate that the beneficial effects of the anti-α4 antibody on T cells are largely, if not exclusively, mediated by interfering with α4β1 and not with α4β7 binding. Because loss of β1-integrin subunit expression results in the ablation of several αxβ1-integrin heterodimers in addition to α4β1, it is possible that in our mouse model, their absence also contributes to the T cell adhesion defect.
Third, adhesion assays and IVM revealed that α4β1 integrins are essential for T cell adhesion to inflamed brain endothelial cells, whereas priming and proliferation of T cells is unaffected by loss of α4β1. The ability of α4β1 integrins to mediate T cell adhesion to endothelial cells is well-established. However, it has also been reported that α4β1 integrins are recruited into the immunological synapse, where they control T cell priming and proliferation (10). We observed that antigen-specific T cell proliferation was unaffected by the lack of β1 expression on encephalitogenic T cells, suggesting that immunosuppressive effects of the treatment are unlikely.
Finally, we also excluded a defect in the induction of TH17 cells. There is increasing evidence that the development of EAE and MS depends on the induction of TH17 cells (30, 31), which represents a T cell subset that differs from classical TH1 T cells and is characterized by the expression of cytokines, such as IL-17 and TNF-α (32). The expression of a normal cytokine profile by antigen-stimulated, β1-deficient T lymphocytes excludes such a possibility.
In summary, our findings indicate that treatment of MS patients with natalizumab compromises α4β1-mediated T cell adhesion to inflamed brain endothelial cells. Consequently, an antibody specifically targeting α4β1 integrins on encephalitogenic T lymphocytes should be sufficient to inhibit EAE and, presumably, MS development.
Materials and Methods
Animals.
Mice carrying a floxed β1-integrin gene (19) were intercrossed with mice carrying an Mx1 promoter (20) or a CD4 promoter-driven Cre recombinase transgene (22). To obtain TCR-transgenic, β1-deficient T cells, β1fl/fl/MxCre+ mice were intercrossed with OVA-specific (referred to as OT-II.2) (24) or MOG-specific (referred to as 2D2) TCR transgenic mice (23). BMCs were made as described previously (33). Mice were kept on a mixed 129Sv/C57BL/6 genetic background and bred in the animal facilities at the Max Planck Institute of Biochemistry. All animal studies were performed with the license of the government of Oberbayern (permission number 68/05). Female C57BL/6 mice (8–10 weeks) for IVM were purchased from Harlan. All IVM experiments were performed in accordance with the requirements of the local government in Bern, Switzerland (permission numbers 55/04 for EAE experiments and 104/04 for IVM).
Induction and Evaluation of Active and Transfer EAE.
Active EAE was induced exactly as described previously (33). Transfer EAE was induced by transferring 2 × 106 encephalitogenic T cells intravenously into WT sex-matched C57BL/6 mice irradiated with 3.5 Gy. A detailed description of the generation of encephalitogenic T cells and information regarding all used antibodies are supplied as SI Materials and Methods and Table S1.
Southern Blot Analysis.
Cre-mediated gene ablation efficiency was assessed by Southern blotting as described previously (12, 21).
Histological Analysis.
The spinal cord was rapidly dissected and in part frozen unfixed in optimal cutting temperature compound. Cryosections (10 μm) were stained for immunofluorescence according to standard protocols. After blocking unspecific binding with a streptavidin/biotin blocking kit (Vector Laboratories), Cy3-conjugated streptavidin was used to detect biotinylated primary antibodies. Images were taken with a DMIRE2 confocal microscope (Leica).
Flow Cytometry.
Mononuclear cells were isolated from the CNS by Percoll density gradient centrifugation of the dissected brain and spinal cord (33). Single-cell suspensions of hematopoietic organs and staining for FACS analysis were prepared as described previously (33). Biotinylated antibodies were detected with Cy5-conjugated streptavidin (Jackson ImmunoResearch). Dead cells or residual host cells were excluded from the analysis by staining with propidium iodide (2.5 μg/mL; Sigma) or Ly-5.1 CyChrome, respectively. Measurements were done on a FACSCalibur (BD Biosciences) and analyzed using FlowJo software (TreeStar).
Cell Culture.
DCs were generated from murine BM cells as described previously (33). Activated T cells for adhesion assays and IVM experiments were generated in vitro from β1fl/fl/OT-II.2+/MxCre+ mice as described in the SI Materials and Methods. For unspecific stimulation, single-cell suspensions of splenocytes were stimulated after ACK-lysis for 2 days with 50 ng/mL phorbol-12-myristate-13-acetate (PMA) and 500 ng/mL ionomycin (both Calbiochem).
Proliferation Assays.
Sorting of T cells, in vivo proliferation assays, and FACS analysis were performed as described previously (33). Proliferation parameters were analyzed by using the proliferation platform of the FlowJo software. The proliferation index indicates the average number of divisions that a dividing cell undergoes, and the division index indicates the average number of divisions all cells undergo. For intracellular cytokine stainings, splenocytes were stimulated after isolation for 4 h with 50 ng/mL PMA, 500 ng/mL ionomycin, and 10 μg/mL brefeldin A (Sigma). The stainings were performed with the Leucoperm kit according to the manufacturer's guidelines (AbD Serotec).
Adhesion Assays.
Adhesion assays to the endothelioma cell lines bEnd5 and bEndI1.1 were carried out as described previously (34). A total of 2 × 104 endothelioma cells per well were plated. A total of 1 × 105 cultured, MACS-sorted, OT-II.2 transgenic T cell blasts were allowed to adhere per well, either at room temperature or at 4°C. All conditions were performed in duplicates. After fixation, 2 pictures were acquired from each well with an Axiovert 200M microscope (Zeiss), and the number of adherent T cells was quantified by using the MetaMorph software (Molecular Devices).
IVM.
Surgical preparation of the spinal cord window, IVM, and quantitative analysis of the spinal cord white matter microcirculation were performed exactly as described previously (27, 35). A detailed description is provided in the SI Materials and Methods.
Statistical Analysis.
All statistical analysis was performed by using the GraphPad Prism software (version 5.00). Data are presented as medians with interquartile ranges. Mann–Whitney U statistics were used for comparisons between different datasets. Asterisks in Figs. 3 and 5 indicate significant differences (*, P < 0.05; **, P < 0.01; and ***, P < 0.005). For analysis of adherent T cells in the IVM analysis, mean values were calculated from the values in each animal, and the 2 groups were compared by using a Mann–Whitney U test.
Supplementary Material
Supporting Information
Acknowledgments.
We thank Christopher B. Wilson (University of Washington, Seattle) for the CD4Cre mice, Francis R. Carbone (University of Melbourne, Australia) for the OT-II.2 mice, Vijay K. Kuchroo (Harvard Medical School, Boston) for the 2D2 mice, Roy Zent and Zena Werb for carefully reading the manuscript, and Ursula Kuhn and Heidi Tardent for technical assistance. C.C. is supported by a fellowship from the French Multiple Sclerosis Society (ARSEP), and M.S. is supported by the Peter-Hans Hofschneider Foundation. The work was funded by the Max Planck Society, German Research Council Grant DFG SFB571, the National MS Society of the United States, and the Swiss MS Society.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.McFarland HF, Martin R. Multiple sclerosis: A complicated picture of autoimmunity. Nat Immunol. 2007;8:913–919. doi: 10.1038/ni1507. [DOI] [PubMed] [Google Scholar]
- 2.Yednock TA, et al. Prevention of experimental autoimmune encephalomyelitis by antibodies against α4β1 integrin. Nature. 1992;356:63–66. doi: 10.1038/356063a0. [DOI] [PubMed] [Google Scholar]
- 3.Ransohoff RM. Natalizumab for multiple sclerosis. N Engl J Med. 2007;356:2622–2629. doi: 10.1056/NEJMct071462. [DOI] [PubMed] [Google Scholar]
- 4.Polman CH, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2006;354:899–910. doi: 10.1056/NEJMoa044397. [DOI] [PubMed] [Google Scholar]
- 5.Berlin C, et al. α4 integrins mediate lymphocyte attachment and rolling under physiologic flow. Cell. 1995;80:413–422. doi: 10.1016/0092-8674(95)90491-3. [DOI] [PubMed] [Google Scholar]
- 6.von Andrian UH, Engelhardt B. α4 integrins as therapeutic targets in autoimmune disease. N Engl J Med. 2003;348:68–72. doi: 10.1056/NEJMe020157. [DOI] [PubMed] [Google Scholar]
- 7.Dasgupta S, Jana M, Liu X, Pahan K. Role of very-late antigen-4 (VLA-4) in myelin basic protein-primed T cell contact-induced expression of proinflammatory cytokines in microglial cells. J Biol Chem. 2003;278:22424–22431. doi: 10.1074/jbc.M301789200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carrasco YR, Batista FD. B-cell activation by membrane-bound antigens is facilitated by the interaction of VLA-4 with VCAM-1. EMBO J. 2006;25:889–899. doi: 10.1038/sj.emboj.7600944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nguyen K, Sylvain NR, Bunnell SC. T cell costimulation via the integrin VLA-4 inhibits the actin-dependent centralization of signaling microclusters containing the adaptor SLP-76. Immunity. 2008;28:810–821. doi: 10.1016/j.immuni.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 10.Mittelbrunn M, et al. VLA-4 integrin concentrates at the peripheral supramolecular activation complex of the immune synapse and drives T helper 1 responses. Proc Natl Acad Sci USA. 2004;101:11058–11063. doi: 10.1073/pnas.0307927101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sixt M, Bauer M, Lämmermann T, Fässler R. β1 integrins: Zip codes and signaling relay for blood cells. Curr Opin Cell Biol. 2006;18:482–490. doi: 10.1016/j.ceb.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 12.Bungartz G, et al. Adult murine hematopoiesis can proceed without β1 and β7 integrins. Blood. 2006;108:1857–1864. doi: 10.1182/blood-2005-10-007658. [DOI] [PubMed] [Google Scholar]
- 13.Berger JR, Koralnik IJ. Progressive multifocal leukoencephalopathy and natalizumab–unforeseen consequences. N Engl J Med. 2005;353:414–416. doi: 10.1056/NEJMe058122. [DOI] [PubMed] [Google Scholar]
- 14.Ransohoff RM. “Thinking without thinking” about natalizumab and PML. J Neurol Sci. 2007;259:50–52. doi: 10.1016/j.jns.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 15.Engelhardt B, et al. The development of experimental autoimmune encephalomyelitis in the mouse requires α4-integrin but not α4β7-integrin. J Clin Invest. 1998;102:2096–2105. doi: 10.1172/JCI4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leone DR, et al. An assessment of the mechanistic differences between two integrin α4β1 inhibitors, the monoclonal antibody TA-2 and the small molecule BIO5192, in rat experimental autoimmune encephalomyelitis. J Pharmacol Exp Ther. 2003;305:1150–1162. doi: 10.1124/jpet.102.047332. [DOI] [PubMed] [Google Scholar]
- 17.Kanwar JR, Kanwar RK, Wang D, Krissansen GW. Prevention of a chronic progressive form of experimental autoimmune encephalomyelitis by an antibody against mucosal addressin cell adhesion molecule-1, given early in the course of disease progression. Immunol Cell Biol. 2000;78:641–645. doi: 10.1046/j.1440-1711.2000.00947.x. [DOI] [PubMed] [Google Scholar]
- 18.Kanwar JR, et al. β7 integrins contribute to demyelinating disease of the central nervous system. J Neuroimmunol. 2000;103:146–152. doi: 10.1016/s0165-5728(99)00245-3. [DOI] [PubMed] [Google Scholar]
- 19.Brakebusch C, et al. Skin and hair follicle integrity is crucially dependent on β1 integrin expression on keratinocytes. EMBO J. 2000;19:3990–4003. doi: 10.1093/emboj/19.15.3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuhn R, Schwenk F, Aguet M, Rajewsky K. Inducible gene targeting in mice. Science. 1995;269:1427–1429. doi: 10.1126/science.7660125. [DOI] [PubMed] [Google Scholar]
- 21.Brakebusch C, et al. β1 integrin is not essential for hematopoiesis but is necessary for the T cell-dependent IgM antibody response. Immunity. 2002;16:465–477. doi: 10.1016/s1074-7613(02)00281-9. [DOI] [PubMed] [Google Scholar]
- 22.Lee PP, et al. A critical role for Dnmt1 and DNA methylation in T cell development, function, and survival. Immunity. 2001;15:763–774. doi: 10.1016/s1074-7613(01)00227-8. [DOI] [PubMed] [Google Scholar]
- 23.Bettelli E, et al. Myelin oligodendrocyte glycoprotein-specific T cell receptor transgenic mice develop spontaneous autoimmune optic neuritis. J Exp Med. 2003;197:1073–1081. doi: 10.1084/jem.20021603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barnden MJ, Allison J, Heath WR, Carbone FR. Defective TCR expression in transgenic mice constructed using cDNA-based α- and β-chain genes under the control of heterologous regulatory elements. Immunol Cell Biol. 1998;76:34–40. doi: 10.1046/j.1440-1711.1998.00709.x. [DOI] [PubMed] [Google Scholar]
- 25.Steinman L. A brief history of TH17, the first major revision in the TH1/TH2 hypothesis of T cell-mediated tissue damage. Nat Med. 2007;13:139–145. doi: 10.1038/nm1551. [DOI] [PubMed] [Google Scholar]
- 26.Laschinger M, Engelhardt B. Interaction of α4-integrin with VCAM-1 is involved in adhesion of encephalitogenic T cell blasts to brain endothelium but not in their transendothelial migration in vitro. J Neuroimmunol. 2000;102:32–43. doi: 10.1016/s0165-5728(99)00156-3. [DOI] [PubMed] [Google Scholar]
- 27.Vajkoczy P, Laschinger M, Engelhardt B. α4-integrin-VCAM-1 binding mediates G protein-independent capture of encephalitogenic T cell blasts to CNS white matter microvessels. J Clin Invest. 2001;108:557–565. doi: 10.1172/JCI12440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kerfoot SM, et al. Reevaluation of P-selectin and α4 integrin as targets for the treatment of experimental autoimmune encephalomyelitis. J Immunol. 2006;176:6225–6234. doi: 10.4049/jimmunol.176.10.6225. [DOI] [PubMed] [Google Scholar]
- 29.Koralnik IJ. Progressive multifocal leukoencephalopathy revisited: Has the disease outgrown its name? Ann Neurol. 2006;60:162–173. doi: 10.1002/ana.20933. [DOI] [PubMed] [Google Scholar]
- 30.Chen Y, et al. Anti-IL-23 therapy inhibits multiple inflammatory pathways and ameliorates autoimmune encephalomyelitis. J Clin Invest. 2006;116:1317–1326. doi: 10.1172/JCI25308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vaknin-Dembinsky A, Balashov K, Weiner HL. IL-23 is increased in dendritic cells in multiple sclerosis and down-regulation of IL-23 by antisense oligos increases dendritic cell IL-10 production. J Immunol. 2006;176:7768–7774. doi: 10.4049/jimmunol.176.12.7768. [DOI] [PubMed] [Google Scholar]
- 32.Iwakura Y, Ishigame H. The IL-23/IL-17 axis in inflammation. J Clin Invest. 2006;116:1218–1222. doi: 10.1172/JCI28508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Montanez E, et al. Analysis of integrin functions in peri-implantation embryos, hematopoietic system, and skin. Methods Enzymol. 2007;426:239–289. doi: 10.1016/S0076-6879(07)26012-4. [DOI] [PubMed] [Google Scholar]
- 34.Reiss Y, Hoch G, Deutsch U, Engelhardt B. T cell interaction with ICAM-1-deficient endothelium in vitro: essential role for ICAM-1 and ICAM-2 in transendothelial migration of T cells. Eur J Immunol. 1998;28:3086–3099. doi: 10.1002/(SICI)1521-4141(199810)28:10<3086::AID-IMMU3086>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 35.Stein JV, et al. L-selectin-mediated leukocyte adhesion in vivo: Microvillous distribution determines tethering efficiency, but not rolling velocity. J Exp Med. 1999;189:37–50. doi: 10.1084/jem.189.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information